Abstract
Background
Full-endoscopic microvascular decompression (fE-MVD) is an emerging treatment option for trigeminal neuralgia (TN). However, the risk factors associated with postoperative recurrence of TN after fE-MVD procedure remain controversial. The aim of the present study was to summarize the surgical technique of fE-MVD for the treatment of TN and to develop a predictive model for recurrence at 1 year postoperatively based on independent risk factors.
Methods
A total of 124 consecutive patients with TN who underwent fE-MVD procedure were enrolled in this study between December 2008 and July 2022. Imaging data such as the area of cerebellopontine angle (CPA), the length of trigeminal cisternal segment, and the angle of trigeminal nerve (TGN) were measured from preoperative magnetic resonance imaging (MRI). Patients were randomly divided into a training set and a validation set according to the 7:3 ratio, respectively. Variables that were significant in the univariate logistic analyses were, subsequently, included in the multivariate logistic regression analyses in training set. Then, we developed a predictive nomogram for the 1-year recurrence of TN for patients who treated with fE-MVD.
Results
All 124 patients experienced clinically significant pain relief (Barrow Neurology Institute (BNI) I–II) after fE-MVD. 124 patients had a follow-up time of more than 1 year, with 14 cases of recurrence. In the univariate analysis, the patients’ responsible vessels of non-arterial, clinical features of atypical, and CPA area ratio (healthy/affected side) >1 were found to be significantly associated with recurrence of TN after fE-MVD. Multivariate logistic regression analyses result showed that the patients' responsible vessels of non-arterial (odds ratio (OR) = 21.067, 95% confidence interval (CI): 1.942–228.575), clinical features of atypical (OR = 9.027, 95% CI: 1.135–71.777), and CPA area ratio >1 (OR = 19.522, 95% CI: 2.906–131.160) were independent predictors of TN recurrence. Based on the independent predictive factors, we developed a predictive nomogram that predicts the 1-year recurrence of TN after fE-MVD. In the receiver operating characteristic (ROC) curve analysis, the area under the curve (AUC) of the nomograms for 1-year recurrence associated with optimal candidates prediction was 0.910 in the training set and 0.859 in the validation set.
Conclusions
FE-MVD for the treatment of TN is a safe, reliable and effective procedure. Patients' responsible vessels of non-arterial, clinical features of atypical, and CPA area ratio (healthy/affected side) >1 are key risk factors associated with 1 year postoperative recurrence of TN after fE-MVD. Finally, we have developed a nomogram to predict the 1-year recurrence of TN for patients who treated with fE-MVD, which can be used to provide advice for patients after fE-MVD.
Keywords: Neuroendoscopy, Surgery, Microvascular decompression, Neurovascular compression syndrome, Trigeminal neuralgia, Neuropathic pain, Recurrence, Nomogram, Predictive model
Background
Trigeminal neuralgia (TN), a severe neuropathic pain disorder affecting the distribution area of the trigeminal nerve (TGN) in the face, is a common neurovascular compression (NVC) syndrome, predominantly occurs in middle-aged women. Its annual incidence ranges from 4.5 to 28.9/100 000 [1, 2]. According to the proposal of the 2018 International Classification of Headache Disorders, Third Edition (ICHD-3), the diagnosis of TN should be based on clinical symptoms. Typical symptoms are characterized by recurrent, unilateral, transient, electric shock-like pain, localized to the distribution of one or more branches of the TGN. In contrast, atypical symptoms involve persistent or intermittent dull pain, often without a clear trigger point, and may be accompanied by facial numbness or hyperalgesia [2, 3]. The exact pathogenesis of TN is not fully understood, but it is generally hypothesized that vascular compression of the TGN by peripheral vessels leads to demyelinating lesions along the nerve.
Treatments options for TN include medication, percutaneous microballoon compression, Gamma Knife radiosurgery, radiofrequency thermocoagulation and microvascular decompression (MVD), but the first three treatments are generally effective and have significantly high recurrence rates [4, 5]. In contrast, radiofrequency thermocoagulation often causes abrupt changes in cardiovascular responses system and a potential permanent damage to the TGN, leading to complications such as sudden bradycardia or facial numbness [6]. Most studies now consider MVD as the first-line surgical treatment for medically refractory TN [7, 8]. Emerging evidence demonstrate that neural pathology occurs at the root entry/exit zone (REZ) due to compression by a responsible vessel [9, 10]. This zone is associated with the transition of peripheral Schwann cell myelination to central oligodendroglial myelination, which is thought to make the entry zone particularly vulnerable to pressure [11]. MVD is a non-destructive surgical procedure that decompresses the TGN from conflicting blood vessels during open posterior fossa surgery [7]. With the application and development of neuroendoscopic surgical techniques in clinical practice, full-endoscopic microvascular decompression (fE-MVD) for the treatment of TN has become more acceptable [12, 13]. However, how to predict postoperative recurrence and select patients suitable for fE-MVD remains a critical step in decision-making. The purpose of this retrospective study was to summarize the surgical technique of fE-MVD for the treatment of TN, and to develop a predictive model for recurrence at 1 year postoperatively based on independent risk factors.
Methods
Patient recruitment
This was a single-centre, retrospective study and conducted in accordance with the tenets of the Helsinki Declaration of 1975 as revised in 2000. The study was approved by the Institutional Review Board of Beijing Shijitan Hospital, Capital Medical University. All patients or their legal guardians in this study authorized the use of their medical records and information.
Between December 2008 and July 2022, 124 fE-MVD procedures were performed in patients with facial pain and a clinical diagnosis of TN at Beijing Shijitan Hospital, Capital Medical University. Adequate follow-up data were available for 124 patients. Magnetic resonance imaging (MRI) was performed preoperatively, and the imaging manifestations were consistent with the clinical symptoms and physical examination in all patients.
Patient inclusion criteria were as follows: (1) Aged > 18 years; (2) Met the clinical diagnostic criteria of the World Health Organization (WHO) for TN [14]; (3) Absence of or diminished efficacy of drug treatment; (4) Existence of significant adverse effects of oral drugs limiting strict adherence to the medication over extended period of time; (5) Patients were examined preoperatively with MRI and proved or strongly suspected the presence of NVC; (6) Patients underwent fE-MVD by the same team of neurosurgeons of the Beijing Shijitan Hospital; (7) Patients were followed up for more than 1 year.
Patient exclusion criteria were as follows: (1) Diagnosed with secondary TN; (2) Diagnosed with idiopathic TN; (3) History of cranio-cerebral surgery, percutaneous microballoon compression, Gamma Knife radiosurgery, or radiofrequency thermocoagulation; (4) Patients with serious diseases who could not tolerate anaesthesia and surgery.
The nomogram was based on a retrospective cohort study of 124 patients with TN underwent fE-MVD. 88 patients were randomly divided into a training set (88/124, 70%), and 36 patients into a validation set (36/124, 30%). The predictive accuracy and discriminative ability of the nomograms were determined by receiver operating characteristic (ROC), decision curve analysis (DCA), and clinical impact curve (CIC) analysis. The model was internal validated by validation set of 36 patients. Predictive value of the model based on AUC (0.5–0.7 is fair, 0.7–0.9 is good, > 0.9 is excellent).
Imaging data
Patients underwent preoperative MRI routine sequences and thin 3D enhancement scanning sequences, including 3D-FFE, 3D-MRA, mDixon, and 3D-mDixon enhanced sequences (PHILIPS INGENIA 3.0 T high-field MRI system). The MRI scan was centered on the trigeminal cisternal segment for localization, ensuring full coverage of the intracranial segment of the TGN (Fig. 1A). To facilitate image cross-referencing analysis, the same central positioning was used for the 3D-FFE, mDixon, and 3D-mDixon enhanced sequences. 3D-FFE images were reconstructed in multivariate layers, ensuring that the entire bilateral trigeminal cisternal segment in the axial image was displayed in the same layer. Measurements were recorded using a physician's workstation on the reconstructed images as follows: (1) Area ratio of cerebellopontine angle (CPA) (healthy side/affected side): the areas of the CPA on both sides were measured with reference to the measurement method described by Obata et al. [15]. The same scan level of the REZ of the TGN was selected for measurement, with the arachnoid membrane of the prepontile cistern as the anterolateral boundary, and the vertical angle formed by the pons and the flocculus as the posterolateral boundary. The line between the anterior median fissure and the median sulcus of rhomboid fossa at the base of the fourth ventricle served as the median line, dividing the CPA into left and right parts. We manually sketched the area of the CPA on the axial position sequence that would show the full length of the TGN on both sides, and the computer automatically calculated the values of the area for analysis. Finally, the area ratio of healthy/affected CPA was calculated (Fig. 1B); (2) Length ratio of trigeminal cisternal segment (healthy side/affected side): The length of the trigeminal cisternal segment was measured at the level where both TGNs were clearly visualized in the axial image, and length ratio (healthy side/affected side) was calculated (Fig. 1C); (3) Angle ratio of TGN (healthy side/affected side): The angle between the TGN and the pons was measured at the level where both TGNs were clearly visualized in the axial image, and the angle ratio (healthy side/affected side) was calculated (Fig. 1D); (4) Type of responsible vessel: Based on the intraoperative findings and imaging data, the responsible vessel was identified and classified as either arterial or non-arterial (venous or a combination of arteries and veins).
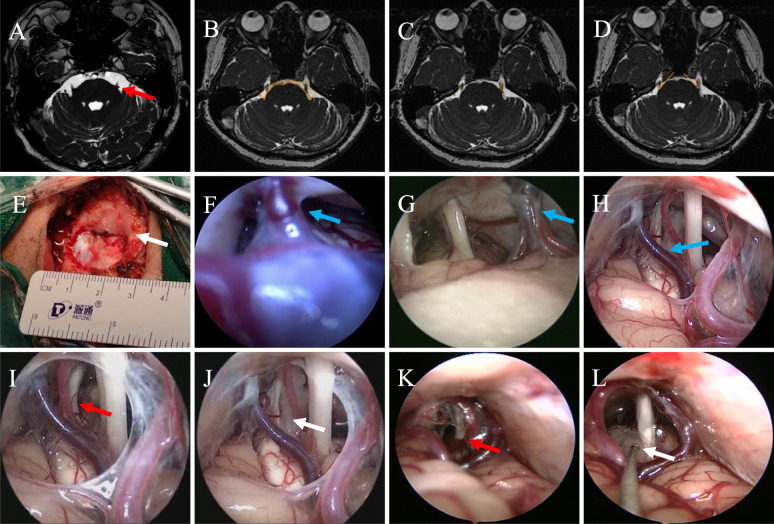
Fig. 1.
Preoperative MRI and surgery of patients with TN underwent fE-MVD. A Picture shows the patient's preoperative 3D FIESTA MRI imaging, with the red arrow pointing to the responsible vessel, which forms a compression in the REZ of TGN; B Area values of bilateral CPA in 3D-FFE sequence axial slices (shown by the yellow line), the area of the healthy (left) side CPA is larger than that of the affected (right) side, and the ratio of CPA area (healthy/affected side) = 1.67/0.91 = 1.84 > 1; C Length values of bilateral TGN in 3D-FFE sequence axial slices (shown by the yellow line), it can be seen that the length of the TGN of the healthy (left) side is longer than that of the affected (right) side, and the ratio of the length of the TGN (healthy/affected side) = 0. 82/0.45 = 1.82 > 1; D TGN pinch angle value in 3D-FFE sequence axial slices (shown by the yellow line), it can be seen that the pinch angle on the healthy (left) side is larger than that on the affected (right) side, and the TGN pinch angle ratio (healthy/affected side) = 51.8/42.4 = 1.22 > 1. E Surgical incision and bone window. Small bone window craniotomy was performed to form an elliptical bone window with a diameter of 2.0 to 3.0 cm, which can clearly expose the junction of transverse sinus and sigmoid sinus (indicated by the white arrow). F–H Neuro-endoscopic classification of PV (indicated by the blue arrow). F Type I: length < 5 mm and the tension is high; G Type II: length between 5 and 10 mm and the tension is moderate; H Type III: length > 10 mm and the tension is low. I–J Pictures show that the responsible artery (indicated by the red arrow) forms a contact compression on TGN from REZ to Meckel's cave, and that the neurovascular decompression is adequate and definitive with the aid of the neuroendoscope with a Teflon cotton placed under direct vision (indicated by the white arrow). K–L Artery and vein are compressed in parallel above the TGN. The red arrow indicates artery and vein, and the white arrow indicates cotton. MRI, magnetic resonance imaging; TN, trigeminal neuralgia; fE-MVD, full-endoscopic microvascular decompression; REZ, root entry/exit zone; TGN, trigeminal nerve; CPA, cerebellopontine angle
Surgical procedure
After a satisfactory general anaesthesia, all patients were placed in lateral decubitus position. The retromastoid craniotomy was performed by making a straight incision behind the mastoid process and within the hairline. The incision was approximately 4 cm long and starting 1 cm above the transverse sinus. Subsequently, small bone window craniotomy was performed to form an elliptical bone window with a diameter of 2.0 to 3.0 cm, clearly exposing the junction of transverse sinus and sigmoid sinus (Fig. 1E). The endocranium was cut in a “K” shape and the free edge was suspended and fixed. Adequate release of cerebrospinal fluid (CSF) was conducted during surgery to allow natural collapse of the cerebellar hemispheres. The 30° endoscope was placed slowly along the side of the cerebellum after the cerebellum was coated with brain cotton. The operator made the endoscopic lens enter the CPA along the petrosal bone or the junction between the petrosal bone and the canopy to initially understand the relationship of each nerve and vessels in the visual field. The whole process from REZ to Meckel's cave should be observed during TGN decompression. The endoscopic view allows the operator to reveal the nerve and vessels, correctly identify the responsible vessel, and release the arachnoid around the nerve and vessels fully. The operator applied the “pre-placed” technique, i.e., through the “lever principle”, pre-pad 1 to 2 small Teflon cotton pieces at the proximal end of the responsible vessel to lift it appropriately to relieve the pressure at the point of NVC, and then place Teflon cotton pieces at the point, and withdraw the pre-pad after the position is satisfactorily adjusted. In cases where the vertebrobasilar artery is collaterally compressing or pushing other vessels to compress the nerve, the operator applied the “set up bridge” technique, in which two small pads of cotton are placed on each side of the vertebrobasilar collaterals to properly elevate them away from the brainstem, and then Teflon cotton is placed sequentially at the point of NVC to relieve the compression. Finally, the operator applied the “diving” technique, which stimulates brain pulsations under physiological conditions, to assess the efficacy of decompression and to ensure that the pad cotton did not move, while keeping the surgical field clean and replacing bloody CSF and air to avoid postoperative adhesions. Before the end of the operation, the operator should observe the operating area in multivariate directions and comprehensively to prevent the responsible vessels from being missed.
Clinical evaluations
In this study, the Barrow Neurological Institute (BNI) Facial Pain Score was used for assessing the postoperative outcomes [16]. In short, the BNI pain scores are graded as follows: BNI I, complete pain relief without medications; BNI II, some pain but not requiring medications; BNI III, some pain but adequately controlled with medications; BNI IV, some pain not adequately controlled with medications; BNI V, continued severe pain or no pain relief. In this study, BNI I and II were defined as clinically significant outcome, whereas BNI III to V were defined as ineffective treatment outcomes. Recurrence was defined as a postoperative BNI I–II which transitioned to BNI III–V after at least a 1 year period and the clinical symptoms met the diagnostic criteria for TN.
Follow-up after fE-MVD
This study used outpatient visits, inpatient observation, and telephone follow-up to monitor patients' follow-up. Follow-up details included the patients' surgical outcome, whether and when the pain recurred, medication use, and whether there were complications such as postoperative cerebral infarction, intracranial infection, or CSF leakage. In the follow-up results, pain relief was assessed by two neurosurgeons in a double-blind design, and if the results were inconsistent, the results were determined by a third neurosurgeon.
Statistical analyses
SPSS 25.0 software and R software, version 4.1.1, were used for all statistical calculations. Chi-square or Fisher's exact tests were used to analyze dichotomous variables. Patients were randomly divided into a training set and a validation set according to the 7:3 ratio. Variables that were significant in the univariate logistic analyses were subsequently included in the multivariate logistic regression analyses in training set. A two-sided test was used with a test level of α = 0.05.
Results
Clinical data
A total of 124 patients were included in this study, including 56 males and 68 females, with 74 patients aged ≤ 60 years and 50 patients aged > 60 years, 44 patients with disease duration ≤ 3 years and 80 patients with disease duration > 3 years. For clinical features, 65 cases were typical TN, 59 cases were atypical TN. The responsible vessels were found intraoperatively in all patients, including 77 cases of arterial compression, 47 cases of non-arterial compression (19 cases of venous compression and 28 cases of arteriovenous compression). 124 patients had a clinically significant outcome after operation (i.e., BNI I–BNI II). All patients were followed up over 1 year, with an average follow-up time of 16.5 ± 2.4 months, and 14 patients experienced a recurrence. These variables were statistically significant in patients with different endpoints, including type of responsible vessels, clinical features, and CPA area ratio (healthy/affected side) (P < 0.05, Table 1).
Table 1.
Baseline characteristics and comparison of postoperative recurrence in patients with TN treated with fE-MVD
| Variables | Patients with TN who underwent fE-MVD | χ2 value | P value | |
|---|---|---|---|---|
| Recurrence (n = 14) | Recurrence-free (n = 110) | |||
| Sex, n (%) | 0.149 | 0.699* | ||
| Female | 7 (50.00) | 61 (55.45) | ||
| Male | 7 (50.00) | 49 (44.55) | ||
| Age (years), n (%) | 0.042 | 0.837* | ||
| ≤ 60 | 8 (57.14) | 66 (60.00) | ||
| > 60 | 6 (42.86) | 44 (40.00) | ||
| Disease duration (years), n (%) | 3.098 | 0.078* | ||
| ≤ 3 | 2 (14.29) | 42 (38.18) | ||
| > 3 | 12 (85.71) | 68 (61.82) | ||
| Clinical features, n (%) | 6.077 | 0.014* | ||
| Typical | 3 (21.43) | 62 (56.36) | ||
| Atypical | 11 (78.57) | 48 (43.64) | ||
| Type of responsible vessels, n (%) | 20.249 | < 0.001* | ||
| Arterial | 1 (7.14) | 76 (69.09) | ||
| Non-arterial | 13 (92.86) | 34 (30.91) | ||
| CPA area ratio, n (%) | 0.014# | |||
| ≤ 1 | 5 (35.71) | 78 (70.91) | ||
| > 1 | 9 (64.29) | 32 (29.09) | ||
| Angle ratio of TGN, n (%) | 1.820 | 0.177* | ||
| ≤ 1 | 3 (21.43) | 44 (40.00) | ||
| > 1 | 11 (78.57) | 66 (60.00) | ||
| Length ratio of TGN (cisternal segment), n (%) | 0.224 | 0.636* | ||
| ≤ 1 | 6 (42.86) | 40 (36.36) | ||
| > 1 | 8 (57.14) | 70 (63.64) | ||
TN: Trigeminal neuralgia; fE-MVD: full-endoscopic microvascular decompression; CPA: cerebellopontine angle; TGN: trigeminal nerve; * Chi-square test; # Fisher's exact test
Neuro-endoscopic classification of petrosal vein (PV)
Through the fE-MVD procedure in 124 patients, we found that the anatomy of the PV in the endoscopic view has its unique characteristics, and we further classified PV into 3 types from type I to type III according to length and tension of PV. The tension of the PV is judged mainly by the morphology and freedom of movement of the PV in the endoscopic view. That is, if the PV is taut and has little or no free mobility, it is judged to have high tension; if the PV is tortuous and has relatively good free mobility, it is judged to have low tension; and in between, it is judged to have medium tension. The typology is as follows: Type I, length < 5 mm and the tension is high (Fig. 1F); Type II, length between 5 and 10 mm and the tension is moderate (Fig. 1G); Type III, length > 10 mm and the tension is low (Fig. 1H). Among the 124 patients in our group, there were 12 patients whose PV was classified as type I, 26 patients as type II and 86 patients as type III.
Clinical characteristics of the training and validation sets
In this study, we randomly divided all patients into a training set (88/124, 70%) and a validation set (36/124, 30%). The database included patients' sex, age, disease duration, clinical features, type of responsible vessel, CPA area ratio, angle ratio of TGN, and length ratio of TGN (cisternal segment), a total of 8 variables.
Risk factors for postoperative recurrence
In the univariate analysis, the patients' responsible vessels of non-arterial (odds ratio (OR) = 17.500, 95% confidence interval (CI): 2.127–143.972), clinical features of atypical (OR = 6.000, 95% CI: 1.215–29.637), and CPA area ratio (healthy/affected side) > 1(OR = 8.140, 95% CI: 1.959–33.828) were found to be significantly associated with the recurrence of TN for patients who underwent fE-MVD (P < 0.05, Table 2). Additionally, the multivariate logistic regression analyses results showed that, the patients' responsible vessels of non-arterial (OR = 21.067, 95% CI: 1.942–228.575), clinical features of atypical (OR = 9.027, 95% CI: 1.135–71.777), and CPA area ratio > 1 (OR = 19.522, 95% CI: 2.906–131.160) were identified as independent predictors for the recurrence of TN following fE-MVD procedure (P < 0.05, Table 2).
Table 2.
Univariate and multivariate logistic regression analyses of risk factors for postoperative recurrence of TN in training set
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | P value | |
| Type of responsible vessels | ||||
| Arterial | Reference | Reference | ||
| Non-arterial | 17.500 (2.127–143.972) | 0.008 | 21.067 (1.942–228.575) | 0.012 |
| Clinical features | ||||
| Typical | Reference | Reference | ||
| Atypical | 6.000 (1.215–29.637) | 0.028 | 9.027 (1.135–71.777) | 0.038 |
| CPA area ratio | ||||
| ≤ 1 | Reference | Reference | ||
| > 1 | 8.140 (1.959–33.828) | 0.004 | 19.522 (2.906–131.160) | 0.002 |
| Disease duration (years) | ||||
| ≤ 3 | Reference | |||
| > 3 | 7.111 (0.866–58.361) | 0.068 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 0.766 (0.215–2.733) | 0.681 | ||
| Age (years) | ||||
| ≤ 60 | Reference | |||
| > 60 | 1.172 (0.329–4.175) | 0.807 | ||
| Angle ratio of TGN | ||||
| ≤ 1 | Reference | |||
| > 1 | 1.611 (0.395–6.564) | 0.506 | ||
| Length ratio of TGN (cisternal segment) | ||||
| ≤ 1 | Reference | |||
| > 1 | 0.766 (0.215–2.733) | 0.681 | ||
TN: trigeminal neuralgia; CPA: cerebellopontine angle; TGN: trigeminal nerve; OR: odds ratio; CI: confidence interval
Developing and validating the predictive model
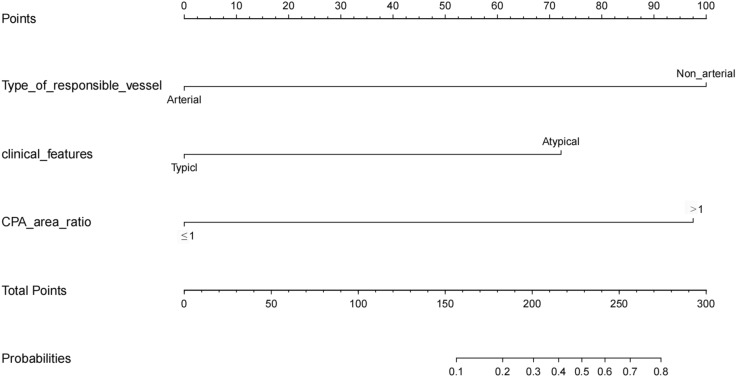
Based on the independent predictive factors, we developed a predictive model and generated a nomogram that predicts the 1-year recurrence of TN for patients who underwent fE-MVD procedure. Each clinical factor was assigned a specific score, and a straight line was drawn from this score to the point axis to calculate the total score. The total scores were then used to determine the 1-year recurrence probability, which is represented on the corresponding axis (Fig. 2).
Fig. 2.
Nomogram used to predict 1-year recurrence of TN for patients who underwent fE-MVD procedure. Clinical factor corresponds to a specific point by drawing a line straight upward to the axis of points. After the sum of the points is located on the axis of total points, the sum represents the probability of 1-year recurrence rate by drawing straight down to the axis of probabilities. TN, trigeminal neuralgia; fE-MVD, full-endoscopic microvascular decompression; CPA, cerebellopontine angle
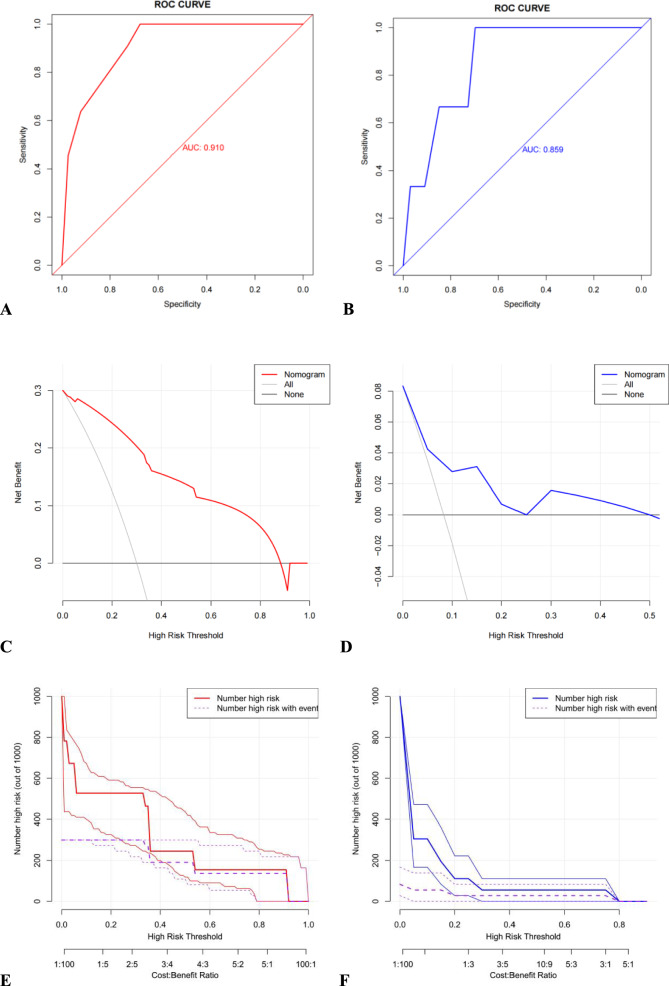
The model's performance in identifying and calibrating the factors were assessed through internal validation. In the ROC curve analysis, the area under the curve (AUC) of the nomograms for 1-year recurrence associated with optimal candidates prediction was 0.910 in the training set and 0.859 in the validation set (Fig. 3A–B). The clinical effectiveness of the nomograms were validated using the DCA and CIC analyses: the DCA showed that the net benefit of the prediction model was significantly higher than that of extremes of complete intervention (All) and no intervention at all (None) in both the training and validation sets (Fig. 3C–D). The CIC results showed the number of individuals classified as high risk by the prediction model at various threshold probabilities compared to the actual outcomes, suggesting the column-line diagrams had a strong clinical applicability (Fig. 3E–F).
Fig. 3.
Multiple evaluation methodologies of predictive nomogram. ROC curves for predicting 1-year recurrence of TN for patients who underwent fE-MVD in training set (A AUC = 0.910) and validation set (B AUC = 0.859). The DCA curve for predicting 1-year recurrence of TN for patients who underwent fE-MVD in the training set (C) and validation set (D). The CIC curve for predicting 1-year recurrence of TN for patients who underwent fE-MVD in the training set (E) and validation set (F). ROC, receiver operating characteristic; TN, trigeminal neuralgia; fE-MVD, full-endoscopic microvascular decompression; AUC, area under curve; DCA, decision curve analysis; CIC, clinical impact curve
Discussion
FE-MVD for the treatment of TN may be able to improve the detection rate of the responsible vessel, the efficacy and safety of the procedure, while reducing the complication rate and recurrence rate. Since Gardner performed the first MVD on a patient with TN in 1959, MVD has gradually developed and matured in its theoretical basis and technique [17]. The detection rate of responsible vessels in microscopic MVD is about 89.0%–95.0%, the surgical efficiency is 81.0%–86.0%, and the recurrence rate is 5.0%–14.0%. The reasons for poor surgical efficacy and high postoperative recurrence rate are primarily intraoperative failure to completely or accurately identifying responsible vessels, omission of responsible vessels, and ineffective decompression [12, 18, 19]. Most of these missing responsible vessels are located in the REZ or Meckel's cave area, or are blocked by the petrosal bone thereby becoming an anatomical blind area when using microscopic MVD. It is often necessary to over-pull the brain tissue or grind away the developed petrosal bone ridge to barely reveal the responsible vessels, increasing the incidence of complications [20]. In recent years, some neurosurgeons have found that endoscopic-assisted microscopic MVD has more advantages in the detection of responsible vessels, thus gradually carrying out endoscopic-assisted microscopic MVD for the treatment of TN. In 2002, Jarrahy first reported the treatment of TN by fE-MVD [21]. Subsequently, in his retrospective study of 255 patients with TN, Kabil reported a perfect intraoperative detection rate of 100.0% for the responsible vessels when using fE-MVD, with postoperative and 3-year facial pain relief rates of 95.0% and 93.0%, respectively [22]. Consistent results have been reported in recent studies [12, 18, 23].
FE-MVD for the treatment of TN has advantages and techniques not available with microscopic MVD. (1) The endoscope provides excellent visualization and comprehensive evaluation of the NVC in patients with TN [18]. The fE-MVD procedure allows the operator to have a panoramic view of the CPA, and by adjusting the depth and angle of the lens, the operative field is revealed completely and clearly, avoiding the blind spots of microscopic MVD. In 56 patients (56/124, 45.2%) of our group, the responsible vessel's compression was found to be ventral to the TGN, a location that is often difficult to detect during microscopic MVD. In contrast, endoscopy provides a better view to detect NVC hidden ventral to the TGN without pulling the brain tissue and nerves. The operator and assistant can clearly visualize the entire intracranial segment of the TGN, increasing the detection rate of the responsible vessel. (2) During the fE-MVD procedure, once the NVC has been identified, the operator can place the Teflon cotton in the ideal position under direct vision and assess whether the ideal position of the Teflon cotton can be achieved to achieve sufficient decompression, consolidate the surgical effect and reduce the recurrence rate. (3) FE-MVD procedure utilizes the space between the vessels, nerves and surrounding tissues in the CPA without grinding away the petrosal tubercle and overstretching the cerebellar hemispheres, resulting in less injury and bleeding, reducing the incidence of complications. (4) The “pre-placed” technique applied during the operation can effectively prevent accidental injury in the process of sharp neurovascular separation, and it is convenient to adjust the position of Teflon cotton when the operator holding the endoscope in one hand and operating with the other (Fig. 1I–J). (5) The “set up bridge” technique can effectively exposes the blocked portion of the vertebrobasilar artery and fully decompresses the NVC, ensuring that the vertebral artery or basilar artery does not rebound after decompression, ensuring adequate MVD and reducing the recurrence rate. (6) The “diving” technique stimulates the pulsation of the brain under physiological conditions, assessing the effectiveness of decompression and ensuring that the padded cotton did not move. The technique also displaces bloody CSF and air in the cranial cavity, which can reduce complications such as postoperative headache and fever.
The PV in the endoscopic view during surgery has its unique anatomical features, and the surgical strategy is different for the different anatomical features of the patient's PV. Intraoperative bleeding in MVD is most often caused by injury to the PV during surgical manipulation [24]. The PV has a close anatomical relationship with the TGN and is an extremely important draining vein in the posterior cranial fossa, which often becomes a roadblock vein, obstructing the surgical field and interfering with surgical manipulation in MVD [25]. Injury to the PV can lead to serious complications such as cerebellar and brainstem oedema or infarction, so the operator should avoid damaging the PV as much as possible during surgery [25, 26]. In this study, we have further classified PV into 3 types based on the length, morphology and tension of the PV in the endoscopic field of view, with the aim of adapting the surgical strategy to the individual situation of the PV during surgery and avoiding damage to the PV. In this study, the operator damaged 2 cases of type I PV at an earlier stage, and fortunately no serious complications such as cerebral haemorrhage or cerebral infarction occurred postoperatively. In the later stage, with our accumulated surgical experience and improved surgical techniques, there was no further injury to the PV. In this group, neither type II nor type III PVs were injured during surgery. Therefore, for both type I and type II PVs, especially type I, the operator should be careful when inserting and withdrawing the endoscope and minimize the lateral adjustment of the endoscope to avoid damage to the PV due to endoscopic “behind-the-scope blindness”. The classification of PV is not yet perfect and needs to be consolidated and improved by more cases in surgical practice.
The results of this study suggest that the primary factors associated with postoperative recurrence of TN for patients who underwent fE-MVD include clinical features, type of responsible vessel, and CPA area ratio (healthy/affected side): (1) Clinical features of atypical: After fE-MVD procedure, patients with typical clinical features may have relatively better outcomes compared to those with atypical features. In this study, there were 3 recurrences in patients with clinical features of typical, 11 recurrences in patients with clinical features of atypical. Previous studies have shown extremely poor outcomes in patients with atypical TN. Some patients previously had typical TN, but over the years this has evolved into atypical TN. These patients may have gone through a stage called transitional TN, which has features of both typical and atypical TN [27, 28]. (2) Type of responsible vessels: This study showed that the postoperative recurrence rate was lower when the type of responsible vessel was arterial. Postoperative recurrence rate was higher when the type of responsible vessel was venous or combination of arteries and veins. Arterial compression alone is often easy to identify intraoperatively, resulting in more adequate decompression. Non-arterial compression is easily overlooked and missed, compromising intraoperative decompression and leading to postoperative recurrence (Fig. 1K, L). (3) CPA area ratio (healthy/affected side): Patients with a smaller CPA area on the affected side relative to the healthy side were more likely to have postoperative recurrence. In the present study, there were 8 recurrence cases in which the CPA area of the affected side was smaller than that of the healthy side. A reduced CPA area leads to increased vascular nerve contact in this area and also increases the difficulty of intraoperative manipulation of the fE-MVD [29]. (4) Other factors: Age, gender, degree of compression of the responsible vessel, and disease duration have been reported in existing literature to be insignificant in assessing the risk of TN recurrence after surgery [30]. Some scholars hypothesize that patients over 60 years of age have superior long-term outcomes, which is associated with the presence of cerebral atrophy and the relatively large room for the posterior fossa [31]. Existing studies report that the postoperative recurrence rate of TN is higher in female patients due to the smaller volume of the posterior fossa and the higher probability of vascular–neurological contact of the CPA compared to male patients [32]. When the TGN is compressed for an extended period of time, the nerves are susceptible to irreversible damage, and even if the NVC is relieved by surgery, the clinical symptoms are not easy to restore. However, our study fails to support these findings due to the limited sample size used and inclusion of older patients.
The recurrence rate varies among patients with similar risk factors. When an fE-MVD fails due to a multi-factorial causes, single-factor analysis frequently overlooks other significant contributing factors, leading to an inaccurate assessment of the patients' prognosis. Nomogram is a useful tool for estimating the likelihood that a patient will survive; it integrates the effects of multiple factors to estimate patient survival probability, has been widely used in cancer survival analysis, and is progressively taking the place of the traditional prediction models. It is unfortunate that TN patients treated with fE-MVD hardly ever employ this predictive model.33 Currently, only Zhang et al. have developed a nomogram based on the prognosis of 1054 patients with TN who underwent microscopic MVD procedure in 2020 [33]. While the model was a robust predictive model for long-term remission probability for microscopic MVD, it is non-applicable for patients treated with fE-MVD. Therefore, due to the improvement of technology and the change of operation concept, we should update different predictors to build a new model. In the present study, we included 124 patients into the model and developed the first nomogram model based on fE-MVD for TN. Based on the results of multivariate logistic regression analyses, we included the patients' responsible vessels of non-arterial, clinical features of atypical, and CPA area ratio (healthy/affected side) > 1, a total of three factors, as nomogram score points. The results also support our view that typical pain, arterial compression, and patients with CPA area ratio (healthy/affected side) < 1 have a better prognosis [27, 31, 34]. The model has good prediction ability. In the ROC curve analysis, the AUC of the nomograms (Fig. 3A, B) for 1-year recurrence associated with optimal candidates prediction was 0.910 in the training set and 0.859 in the validation set. Additionally, the clinical efficacy of the nomograms was validated by DCA and CIC analyses.
Study limitations
However, the study still has several shortcomings. Firstly, this study is based on a single-centre study. Although this research centre is one of the most frequently operated institutions for TN, it is unclear whether ethnicity, climate, and other factors affect the prognosis. In the future further research, we will adopt multi-centre cooperation and randomly select patients with TN from other centres as external validation. Secondly, this study is a retrospective cohort study, and the bias generated during the follow-up is unavoidable.
Conclusions
FE-MVD for the treatment of TN is a safe, reliable and effective procedure. Patients' responsible vessels of non-arterial, clinical features of atypical, and CPA area ratio (healthy/affected side) > 1 are key predictors that influence postoperative recurrence of TN for patients who undergo fE-MVD procedure. At last, we have developed a nomogram to predict the 1-year recurrence of TN for patients who treated with fE-MVD, which can be used to provide advice for patients after MVD.
Acknowledgements
We are grateful to all the patients recruited for this study.
Abbreviations
- fE-MVD
Full-endoscopic microvascular decompression
- TN
Trigeminal neuralgia
- CPA
Cerebellopontine angle
- TGN
Trigeminal nerve
- MRI
Magnetic resonance imaging
- BNI
Barrow Neurological Institute
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- NVC
Neurovascular compression
- ICHD-3
International Classification of Headache Disorders, Third Edition
- MVD
Microvascular decompression
- DCA
Decision curve analysis
- CIC
Clinical impact curve
- CSF
Cerebrospinal fluid
- PV
Petrosal vein
- OR
Odds ratio
- CI
Confidence interval
Author contributions
Study conception and design: WCP, FG, ZQH; neuroendoscopic surgical implementation: WCP, FG, ZQH; data collection: RZ, LX; data and statistical analysis: WCP, XLX, ZHL; manuscript preparation: WCP, RZ. ZQH and FG take responsibility for the integrity of the work as a whole from inception to published article. All authors approved the final version of the paper.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC), No. 82171858 (to ZQH), and Science and Technology Research and Development Program of China National Railway Group Co., Ltd, No. J2023Z607 (to FG). The funders had no role in study design; data collection, analysis, and interpretation; writing of the paper; or decision to submit the paper for publication.
Availability of data and materials
The data presented in this study are available in article and supplementary material. Further inquiries can be directed to the corresponding authors.
Declarations
Ethics approval and consent to participate
This single-centre, retrospective study was conducted in accordance with the tenets of the Helsinki Declaration of 1975 as revised in 2000. The study was approved by the Institutional Review Board of Beijing Shijitan Hospital, Capital Medical University (Approval No: sjtkyll-lx-2022-52). All patients or their legal guardians in this study authorized the release of their medical records and information.
Consent for publication
Informed consent was obtained from all individual participants included in the study and alive at follow-up.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Feng Guan, Email: guanfengdr@126.com.
Zhiqiang Hu, Email: neuro7@163.com.
References
- 1.Spina A, Mortini P, Alemanno F, Houdayer E, Iannaccone S. Trigeminal neuralgia: toward a multimodal approach. World Neurosurg. 2017;103:220–30. [DOI] [PubMed] [Google Scholar]
- 2.Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 2020;383(8):754–62. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018 Jan;38(1):1-211. 10.1177/0333102417738202. [DOI] [PubMed]
- 4.Gupta M, Sagi V, Mittal A, Yekula A, Hawkins D, Shimizu J, Duddleston PJ, Thomas K, Goetsch SJ, Alksne JF, et al. Results of three or more Gamma Knife radiosurgery procedures for recurrent trigeminal neuralgia. J Neurosurg. 2021;135(6):1789–98. [DOI] [PubMed] [Google Scholar]
- 5.Huo X, Sun X, Zhang Z, Guo W, Guan N, Luo J. Dyna-CT-assisted percutaneous microballoon compression for trigeminal neuralgia. J Neurointerv Surg. 2014;6(7):521–6. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Park JH, Hong JH. Analysis of the hemodynamic response during radiofrequency thermocoagulation in Trigeminal Neuralgia. Pain Physician. 2022;25(7):E1057–62. [PubMed] [Google Scholar]
- 7.Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T, Obermann M, Cruccu G, Maarbjerg S. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020;19(9):784–96. [DOI] [PubMed] [Google Scholar]
- 8.Andersen ASS, Heinskou TB, Rochat P, Springborg JB, Noory N, Smilkov EA, Bendtsen L, Maarbjerg S. Microvascular decompression in trigeminal neuralgia—a prospective study of 115 patients. J Headache Pain. 2022;23(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonini G, Di Pasquale A, Cruccu G, Truini A, Morino S, Saltelli G, Romano A, Trasimeni G, Vanacore N, Bozzao A. Magnetic resonance imaging contribution for diagnosing symptomatic neurovascular contact in classical trigeminal neuralgia: a blinded case-control study and meta-analysis. Pain. 2014;155(8):1464–71. [DOI] [PubMed] [Google Scholar]
- 10.Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L. Significance of neurovascular contact in classical trigeminal neuralgia. Brain. 2015;138(Pt 2):311–9. [DOI] [PubMed] [Google Scholar]
- 11.Peker S, Kurtkaya O, Uzun I, Pamir MN. Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery. 2006;59(2):354–9. [DOI] [PubMed] [Google Scholar]
- 12.Zagzoog N, Attar A, Takroni R, Alotaibi MB, Reddy K. Endoscopic versus open microvascular decompression for trigeminal neuralgia: a systematic review and comparative meta-analysis. J Neurosurg. 2018;131:1532. [DOI] [PubMed] [Google Scholar]
- 13.Bohman LE, Pierce J, Stephen JH, Sandhu S, Lee JY. Fully endoscopic microvascular decompression for trigeminal neuralgia: technique review and early outcomes. Neurosurg Focus. 2014;37(4):E18. [DOI] [PubMed] [Google Scholar]
- 14.Bendtsen L, Zakrzewska JM, Abbott J, Braschinsky M, Di Stefano G, Donnet A, Eide PK, Leal PRL, Maarbjerg S, May A, et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019;26(6):831–49. [DOI] [PubMed] [Google Scholar]
- 15.Obata Y, Kawano Y, Tanaka Y, Maehara T. Prognostic impact and post-operative evaluation of volumetric measurement of the cerebellopontine cistern in trigeminal neuralgia using 3 tesla magnetic resonance imaging. Neurol Med Chir (Tokyo). 2018;58(2):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the barrow neurological institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013–9. [DOI] [PubMed] [Google Scholar]
- 17.Gardner WJ, Miklos MV. Response of trigeminal neuralgia to decompression of sensory root; discussion of cause of trigeminal neuralgia. J Am Med Assoc. 1959;170(15):1773–6. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Mao F, Cheng F, Peng C, Guo D, Wang B. A meta-analysis of endoscopic microvascular decompression versus microscopic microvascular decompression for the treatment for cranial nerve syndrome caused by vascular compression. World Neurosurg. 2019;126(647–655): e647. [DOI] [PubMed] [Google Scholar]
- 19.Lee JYK, Pierce JT, Sandhu SK, Petrov D, Yang AI. Endoscopic versus microscopic microvascular decompression for trigeminal neuralgia: equivalent pain outcomes with possibly decreased postoperative headache after endoscopic surgery. J Neurosurg. 2017;126(5):1676–84. [DOI] [PubMed] [Google Scholar]
- 20.Chen F, Niu Y, Meng F, Xu P, Zhang C, Xue Y, Wu S, Wang L. Recurrence rates after microvascular decompression in patients with primary trigeminal neuralgia and its influencing factors: a systematic review and meta-analysis based on 8,172 surgery patients. Front Neurol. 2021;12: 738032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarrahy R, Cha ST, Eby JB, Berci G, Shahinian HK. Fully endoscopic vascular decompression of the glossopharyngeal nerve. J Craniofac Surg. 2002;13(1):90–5. [DOI] [PubMed] [Google Scholar]
- 22.Kabil MS, Eby JB, Shahinian HK. Endoscopic vascular decompression versus microvascular decompression of the trigeminal nerve. Minim Invasive Neurosurg. 2005;48(4):207–12. [DOI] [PubMed] [Google Scholar]
- 23.Dubey A, Yadav N, Ratre S, Parihar VS, Yadav YR. Full endoscopic vascular decompression in trigeminal neuralgia: experience of 230 patients. World Neurosurg. 2018;113:e612–7. [DOI] [PubMed] [Google Scholar]
- 24.Flanders TM, Blue R, Roberts S, McShane BJ, Wilent B, Tambi V, Petrov D, Lee JYK. Fully endoscopic microvascular decompression for hemifacial spasm. J Neurosurg. 2018;131(3):813–9. [DOI] [PubMed] [Google Scholar]
- 25.Matsushima K, Matsushima T, Kuga Y, Kodama Y, Inoue K, Ohnishi H, Rhoton AL Jr. Classification of the superior petrosal veins and sinus based on drainage pattern. Neurosurgery. 2014;10(Suppl 2):357–67. [DOI] [PubMed] [Google Scholar]
- 26.Liebelt BD, Barber SM, Desai VR, Harper R, Zhang J, Parrish R, Baskin DS, Trask T, Britz GW. Superior petrosal vein sacrifice during microvascular decompression: perioperative complication rates and comparison with venous preservation. World Neurosurg. 2017;104:788–94. [DOI] [PubMed] [Google Scholar]
- 27.Tyler-Kabara EC, Kassam AB, Horowitz MH, Urgo L, Hadjipanayis C, Levy EI, Chang YF. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. J Neurosurg. 2002;96(3):527–31. [DOI] [PubMed] [Google Scholar]
- 28.Li ST, Pan Q, Liu N, Shen F, Liu Z, Guan Y. Trigeminal neuralgia: what are the important factors for good operative outcomes with microvascular decompression. Surg Neurol. 2004;62(5):400–4. [DOI] [PubMed] [Google Scholar]
- 29.Peng WC, Zhao R, Guan F, Liang X, Jing B, Zhu GT, Mao BB, Hu ZQ. Fully endoscopic microvascular decompression for the treatment of hemifacial spasm, trigeminal neuralgia, and glossopharyngeal neuralgia: a retrospective study. BMC Surg. 2023 Oct 27;23(1):331. 10.1186/s12893-023-02214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng J, Meng J, Liu W, Zhang H, Hui X, Lei D. Nerve atrophy in trigeminal neuralgia due to neurovascular compression and its association with surgical outcomes after microvascular decompression. Acta Neurochir (Wien). 2017;159(9):1699–705. [DOI] [PubMed] [Google Scholar]
- 31.Bick SK, Huie D, Sneh G, Eskandar EN. Older patients have better pain outcomes following microvascular decompression for trigeminal neuralgia. Neurosurgery. 2019;84(1):116–22. [DOI] [PubMed] [Google Scholar]
- 32.Hardaway FA, Holste K, Ozturk G, Pettersson D, Pollock JM, Burchiel KJ, Raslan AM. Sex-dependent posterior fossa anatomical differences in trigeminal neuralgia patients with and without neurovascular compression: a volumetric MRI age- and sex-matched case-control study. J Neurosurg. 2019;132(2):631–8. [DOI] [PubMed] [Google Scholar]
- 33.Zhang WB, Zeng YY, Chang BW, Min LZ, Sun QY, Bin L, Tao BB, Wang XQ. Prognostic nomogram for microvascular decompression-treated trigeminal neuralgia. Neurosurg Rev. 2021;44(1):571–7. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Levy EI, Scarrow AM, Kassam A, Jannetta PJ. Recurrent trigeminal neuralgia attributable to veins after microvascular decompression. Neurosurgery. 2000;46(2):356–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in article and supplementary material. Further inquiries can be directed to the corresponding authors.