Abstract
Background:
The goal of this study was to assess the impact of deep local hyperthermia on oxygen (O2) saturation and infected volumes of lungs on coronavirus disease 2019 (COVID-19) cancer patients.
Materials and Methods:
Fifty patients who suffered from COVID-19 (according to their computed tomography (CT) images and laboratory findings) were included in this study. The mentioned patients were divided into two groups (I and II) with thirty-five participants. The infected volumes and COVID-19 infectious locations were diagnosed using their CT images, and deep local hyperthermia was performed for group II. After three consequent days, the SPO2, D-dimer, and infected volumes of lung parenchyma of both groups were compared to each other.
Results:
For group II, the mean ± SD (standard deviation) of O2 pressure saturation (SPO2) before/after hyperthermia was 85 ± 0.0/91.3 ± 0.5, respectively, while for group I, the mean ± SD of SPO2 before/after 3 days was 85 ± 0.0/88 ± 0.2, respectively. For infected volumes of lungs before/after hyperthermia in group II, the mean ± SD was 31.36 ± 3.13/4 ± 1.53, respectively. Nonetheless, the infected volumes of lungs for group I were 34.21 ± 3.41/10 ± 2.12 before/after three days. For group II, the amount of D-dimer before/after hyperthermia was 3200 ± 106/510 ± 121, respectively. However, for group I, it was 3100/740 before/after the consequent three days, respectively.
Conclusion:
Deep local lung hyperthermia for COVID-19 cancer patients is suggested, as a result of its positive impacts on SPO2 improvement and also D-dimer serum level, C-reactive protein, and Lactate dehydrogenaze reduction for the mentioned patients.
Keywords: Cancer, COVID-19, D-dimer, hyperthermia, lung, SPO2
INTRODUCTION
Based on literature and studies, coronaviruses are a diverse group of viruses that may pose a threat to infect several animals, and according to a large amount of evidence, they may cause mild-to-severe respiratory infections in the human body.[1] At the end of 2019, a novel type of coronavirus emerged known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China.[1] A vast number of investigations showed that the mentioned virus may cause an outbreak of unusual viral pneumonia. As a result of the highly transmissible nature of the virus and the fast spread of it around the world, the disease is known as coronavirus disease 2019 (COVID-19). Nowadays, many types of COVID-19 including delta (delta coronavirus) and lambda (γ coronavirus) emerged in many countries. To the best of our knowledge, it may cause infection to the lung and decrease the oxygen (O2) saturation of blood cells. From the beginning of its spread to now, several studies have evaluated the effects of different methods on COVID-19 and discussed the clinical modalities, which may lead to decreased lung infections. COVID-19 may inject its genome into lung cells and pose to infect lung parenchyma, which may lead to some devastating impacts on the human body. Therefore, it would seem that trying to shape the deformation of lung cell receptors or creating some defects in intracellular enzyme may have considerable positive impacts on treating COVID-19 patients.
According to reading different studies, hyperthermia has a prominent role in treating lung cancer patients using radiofrequency (RF) waves.[2,3,4] David et al. have discussed the tolerance of the lung to hyperthermia and found that using 44.9 degrees of Celsius may increase lung perfusion without any adverse effects.[5,6] Hyperthermia can lead to some effects on extra- and intracellular proteins, which may be useful for treating the lung cells of COVID-19 patients.
Therefore, the purpose of this work was to evaluate the impact of deep local hyperthermia of the lung on the O2 saturation of COVID-19 cancer patients.
MATERIALS AND METHODS
This study was performed at Sayed-Al-Shohada Hospital (Isfahan, Iran) from June to November 2021. The protocol of this cross-sectional study was approved by the ethical board of Isfahan University of Medical Sciences, Isfahan, Iran (IR. MUI. REC. 1399. 684).
Patient selection
Fifty patients who suffer from moderate-to-severe COVID-19 were included in this study. The patients are included in this work based on the following criteria: positive nasopharynx or oropharynx Polymeraze chain reaction (PCR), highly suggestive COVID-19 evidence on the computed tomography (CT) images, non-autoimmune disease, non-diabetic, non-lung cancer (non-primary or metastatic tumors) patients, and O2 saturation higher than 85% (SPO2 > 85%). Patients who aged higher than 75 years, suffer from autoimmune disease, and had decreased SAO2 after hyperthermia were excluded from this study [Figure 1]. In addition, patients who had decreased SPO2 (48 hours after hyperthermia) were excluded from this work. In this work, the studied patients were divided into two groups (I and II) as follows.
Figure 1.
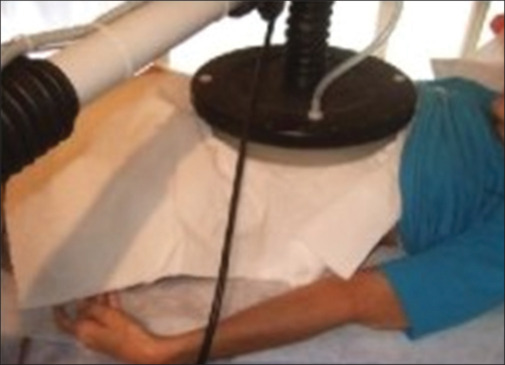
Deep local hyperthermia of lung
Patients who just received the routine modalities of COVID-19 treatment (such as remdesivir, corticosteroid, and actemra) were included in study group I, and patients who were treated with the mentioned routine modalities plus deep local lung hyperthermia were included in control group II.
Deep local hyperthermia
For group II, the whole lungs were contoured on CT images of the patients using a treatment planning system (TPS) (TiGRT, LinaTech, China). Therefore, a three-dimensional volumetric map guide of lesions based on the patient's geometry was provided. The Anterior Posterior- Posterior Anterior (AP-PA) diameter of the chest and the chest wall–lung interface thickness were measured using patient CT scan data. Moreover, the lungs were irradiated by a maximum of 41 degrees of Celsius RF waves (Novin Teb, Isfahan, Iran), which are located at the chest wall surface [Figure 1]. The mentioned patients were subjected to deep local hyperthermia (7–10 MHz RF waves) two times per day for three subsequent days. Each fraction time was 20 minutes. In this study, lower frequencies (7–8.7 MHz) were used to penetrate RF waves inside the lung. The SPO2 of patients was evaluated before using an O2 saturation measurement device, during and after 1 hour, 6 hours, and then daily after hyperthermia. A daily chest X-ray (CXR) was performed for the stated patients, and a second CT scan was applied for patients who had suitable clinical situations for CT.
RESULTS
Table 1 illustrates the clinical features of the studied patients. Table 2 indicates the impact of lung hyperthermia on the SPO2 of COVID-19 patients. The table also compares D-dimer, ferritin, and LDH for the patients.
Table 1.
Clinical, biological, and demographic characteristics of the studied patients
| Group I | Group II (hyperthermia) | |
|---|---|---|
| Sex | Male: 12 | Male: 14 |
| Female: 13 | Female: 11 | |
| Age (the minimum–maximum, mean±SD/range) *are expressed in years | 42.71-68.23, 61.27±2.36 | 39.46-68.56, 63.40±3.25 |
| Autoimmune disease | None | None |
| Anemia | None | None |
| Hospitalization days | 7 | 4 |
| Vaccination | All | All |
| Addicted smoke | None | None |
| Laboratory findings | ||
| Oropharynx/nasopharynx PCR | Positive | Positive |
| Lung CT images (COVID-19 infection diagnosis) | All | All |
Table 2.
SPO2, infected volumes of lungs, and laboratory findings of studied patients before and after hyperthermia
| Group I (before/after treatment) | Group II (before/after hyperthermia) | |
|---|---|---|
| SPO2 | ||
| Maximum | 88/90 | 89/92 |
| Minimum | 85/87 | 85/89 |
| Mean | 85±0.0/88±0.2 | 85±0.0/91.3±0.5 |
| Infected lung volumes | ||
| Mean | 34.21±3.41/10±2.12 | 31.36±3.13/4±1.53 |
| LDH | ||
| Mean | 857±115/569.7±13.3 | 851±23.6/541.4±12.4 |
| Ferritin | ||
| Mean | 1006±112/302±41 | 1011±143/281±47 |
| D-dimer | ||
| Mean | 3100±117/740±126 | 3200±106/510±121 |
Based on our findings, for group II, the mean ± SD of SPO2 before hyperthermia was 85 ± 0.0, while it was 91.3 ± 0.5 after local hyperthermia. For the other patients (group I), the mean ± SD of SPO2 before and after their treatment was 85 ± 0.0 and 88 ± 0.2, respectively.
Furthermore, for group II, the maximum and minimum SPO2 before/after hyperthermia was 89/92 and 85/89, respectively. However, for group I, the maximum and minimum SPO2 before/after their treatment was 88/90 and 85/87, respectively. In this study, it was found that, although the SPO2 of the patients increased with hyperthermia, it decreased 1 hour after hyperthermia. In addition, our data showed a sharp increase in their SPO2 6 hours after hyperthermia ended.
For the effect of hyperthermia on the infected volumes of lungs (Figure 2 shows lung CT of 74 years old patients), it was found that the mean ± SD of the infected volume of the lung for group II before/after hyperthermia was 31.36 ± 3.13 and 4 ± 1.53, respectively. Nevertheless, the infected volumes of lungs for group I were 34.21 ± 3.41/10 ± 2.12 before/after their treatment.
Figure 2.
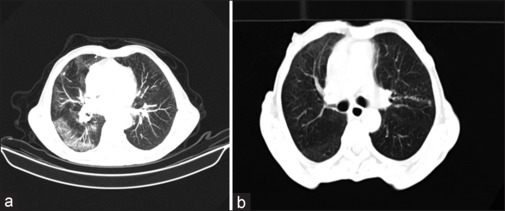
Lung CT of a 74-year-old patient before (a) and after three consequent days of hyperthermia (b)
According to our analysis, D-dimer, ferritin, and LDH before/after hyperthermia were 3200 ± 106/510 ± 121, 1011 ± 143/281 ± 47, and 851 ± 23.6/541.4 ± 12.4 for group II, but for group I, they were 3100 ± 117/740 ± 126, 1006 ± 112/302 ± 41, and 857 ± 115/569.7 ± 13.3 before/after hyperthermia, respectively.
Moreover, it is considered that group II treatment time (three consequent days) was lower than group (I). Table 3 indicates the impacts of hyperthermia on the SPO2.
Table 3.
Implication of hyperthermia on the SPO2 of group II (n=35)
| Before | During | 1 hour after hyperthermia | 6 hours after hyperthermia | |
|---|---|---|---|---|
| SPO2 | ||||
| Maximum | 89 | 91 | 87 | 92 |
| Minimum | 85 | 86 | 84 | 89 |
DISCUSSION
Nowadays, COVID-19 and other types of the virus (delta, lambda, and mu), which result from different mutations, are spread around the world. A wealth of studies have investigated different ideas and modalities, which may reduce or eliminate the toxicity of COVID-19 in the human body. According to different studies, it is considered that there is no experimental study about the impact of deep local lung hyperthermia on O2 saturation of COVID-19 patients. Moreover, a large number of studies have shown that hyperthermia is a safe method, which applies non-ionizing RF waves. Therefore, this study was performed to evaluate the advantages and disadvantages of hyperthermia with regard to the adverse effects of COVID-19 on critical organs such as lungs.
Based on the results, hyperthermia may lead to an increase in SPO2 in all patients who were included in group II. Moreover, although the SPO2 of the patients was increased during hyperthermia, it decreased about 1 hour after hyperthermia. In addition, our data showed a sharp increase in the SPO2 6 hours after hyperthermia ended. Furthermore, the amount of D-dimer in patients who underwent hyperthermia decreased after hyperthermia.
In this study, it was found that hyperthermia may cause a considerable decrease in the infected volumes of lungs for the studied patients.
It is considered that deep local hyperthermia takes its toll on infected lungs using short RF waves, which may lead to higher transmittance inside the lung parenchyma.
These results are highly due to the high temperature of the lungs, which resulted from hyperthermia, and also the increased kinetic energy of the organ, leading to consuming more O2, as a result of the increasing metabolic rate of the cells and growing blood flows to the lung parenchyma. In addition, the higher temperatures of the lung parenchyma and lower levels of O2 in the cells may cause damage to intra- and extracellular proteins including membrane proteins, receptors, nucleoid proteins, and cytoplasmic enzymes resulting in a vast number of defects in the genome synthesis of the virus in the nucleus. Furthermore, damaged proteins of the cytoplasm and membrane may lead to some delays in interphasic cycles and cell death, respectively, as a result of many defects in the mineral transmitters in the cell.[6,7] Moreover, the shape deformation of receptors, which stems from the higher temperature of the lung parenchyma, may lead to the disability of antigens to bind them.
It would seem that high temperatures may damage COVID-19 in an in vitro situation, as a result of denaturation of virus proteins or its capsid,[8] but it is considered that the effect of RF waves and high temperature in an in vivo situation may be different, because the virus merely injects its genome into the lung cells without any capsid.[9] Therefore, intracellular enzymes may be used to produce virus proteins (pneumocyte type 2).[8] Also, this would seem that the Golgi device may produce a new capsid for COVID-19.[8] According to the results of Sarah et al.,[9] temperature may have a substantial role in the immune system of the body and phagocytose process, which may have an impact on coronavirus. Moreover, deep local hyperthermia may have a prominent role in increasing the immune system response including increased macrophage, lymphocytes, Tumour necrose factor (TNF-α), interleukin II, and cytotoxicity effects on antigens.[10] Hyperthermia may excite the adaptive immune system and natural killer cells against COVID-19 lung cells [Figure 3]. Baronzia et al.[10] have evaluated the impact of local and whole-body hyperthermia on the immune system and found that the effect of local hyperthermia on the immune system is time-dependent and it is considered that the local hyperthermia may not increase the cytokines (except TNF-α and interleukin II). Based on the results of David et al.,[11] using 44.9 degrees of Celsius may increase lung blood perfusion without any significant damage to the lungs.
Figure 3.
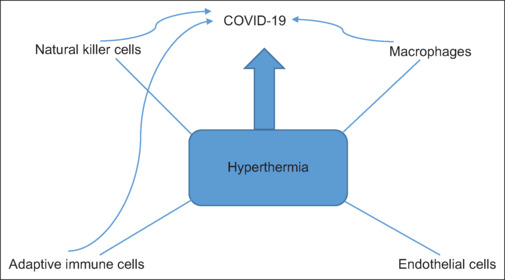
Schematic effect of hyperthermia on COVID-19
Furthermore, ribonucleicacide (RNA) templates, which are known as messenger RNA (mRNA), are commonly employed by cell ribosomes to produce amino acids. Moreover, ribosomes may link to each other to assemble polyribosomes, which can enhance protein synthesis. Nakamura and Hall have found that temperature-dependent reductions in polyribosomes may lead to a reduction in protein synthesis. McCormick and Penman have reported that using 42 degrees of Celsius may disaggregate polyribosomes, which can cause lower protein synthesis.[12] Also, using 42 degrees of Celsius may decrease rRNA production to only 3% after 1 hour[13,14] and it can cause a significant reduction in protein translation, which may be useful to preserve energy. Moreover, 42 degrees of Celsius can alter other mechanisms of certain mRNA production[15] and also polyadenylation.[16] Some studies have shown that polyadenylation of the coronavirus RNA may promote virus survival through translation enhancement and replication.[17] This would seem that the inhibition of protein synthesis and polyadenylation of viral RNA may significantly dampen COVID-19 infectivity. Cortese et al. have concluded that the cellular organelles may be remodeled in the mentioned virus-infected cells, and the inhibition of cytoskeletal rearrangements in the infected cells may lead to suppression of production of viral particles.[18] Therefore, it seems that hyperthermia[19] may alter the cytoskeleton dynamics and decrease the infectivity of COVID-19. In addition, the stated virus can alter lysosomal trafficking, because it can release new virions. Chosh et al.[20] stated that the deacidification of lysosomes (which may lead to the inactivation of lysosomal enzymes) during the COVID-19 infection can impair antigen processing and presentation in the cells. Mao et al.[21] have shown that the acidification of lysosomes may be done by increasing temperatures, and thus, hyperthermia could limit the release of new viral particles and also enhance antigen recognition by cells of the immune system.
Hyper-inflammatory state (also known as cytokine storm) is one of the hallmarks of severe COVID-19 patients who progress to severe forms of the disease, characterized by increased serum levels of Interlukine IL-1b, IL-6, IL-8, and TNF-a, which have been associated with a decreased survival rate.[22,23] Increasing fibrinogen production (via IL-6) and promoting rapid clot formation (via IL-8, IL-1b, and IL-6)[24,25] may contribute pro-inflammatory cytokines to thrombosis for a reason, which may lead to identifying hypercoagulability in COVID-19 as “thrombo inflammation.”[26] In this sense, hyperthermia could be a suitable choice, because the downregulation of pro-inflammatory gene expression through exposition of the stated cells to heat (41 degrees of Celsius for as short as 10 min) can result in inhibition of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-jB) and mitogen-activated protein kinase (MAPK) for up to 20 h.[27] Furthermore, after exposing mononuclear blood cells from healthy donors to 39 degrees of Celsius, lymphocytes are increased with no predilection of lymphocyte subtype as determined through flow cytometry, and levels of IL-1b, IL-6, and Interfron (IFN-c) are decreased.[28,29,30,31,32,33]
In this study, there was not any evidence of coagulation in our patients, which could be a suitable choice for applying hyperthermia to treat COVID-19. Based on our findings, deep local hyperthermia could be a good choice to treat COVID-19 patients.
CONCLUSION
In this paper, the impacts of deep local lung hyperthermia on COVID-19 patients were investigated.
According to our findings, applying deep local lung hyperthermia for COVID-19 patients is suggested, as a result of its prominent effects on SPO2 increase in the patients. Furthermore, hyperthermia may lead to a decrease in D-dimer and ferritin (CRP and D-DIMER) in the mentioned patients. Further research about the effects of deep local lung hyperthermia with larger sample sizes and longer-time follow-up is suggested.
List of abbreviations
CT = Computed tomography
CXR = Chest X-ray
O2 sat = O2 saturation.
Ethics approval and consent to participate
This study was approved by the Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.MED.REC.1399.684).
Financial support and sponsorship
Isfahan University of Medical Sciences, Isfahan, Iran, has supported this study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors acknowledge Isfahan University of Medical Sciences, Isfahan, Iran, for financial support of this study.
REFERENCES
- 1.Hu B, Guo H, Zhou P, Li SZ. Characteristics of SARS-CoV-2 and COVID-19. Nat Microbiol. 2021;19:141–54. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: A prospective, randomized, multicenter trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119–25. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 3.Yang WH, Xie J, Lai ZY, Yang MD, Zhang GD. Radiofrequency deep hyperthermia combined with chemotherapy in the treatment of advanced non-small cell lung cancer. Chin Med J. 2019;132:922–27. doi: 10.1097/CM9.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oei AL, Vriend LE, Crezee J, Franken NA, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat Oncol. 2015;10:165. doi: 10.1186/s13014-015-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 6.David AR, Jeffrey FF, Michael RJ, Christopher AD, et al. Tolerance of the isolated perfused lung to hyperthermia. J Thorac Cardiovasc Surg. 1991;101:732–9. [PubMed] [Google Scholar]
- 7.Draelos KZ, Levine N. Capacitive radiofrequency hyperthermia in the treatment of cutaneous murine melanoma. J Invest Dermatol. 1987;89:818–22. doi: 10.1111/1523-1747.ep12461034. [DOI] [PubMed] [Google Scholar]
- 8.Storm FK. Clinical radiofrequency hyperthermia: A review. Natl Cancer Inst Mongor. 1982;61:343–50. [PubMed] [Google Scholar]
- 9.Hoffmann M, Kleine-Weber H, Schroeder S. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beachy SH, Repasky EA. Toward establishment of temperature thresholds for immunological impact of heat exposure in humans. Int J Hyperthermia. 2011;27:344–52. doi: 10.3109/02656736.2011.562873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baronzio GF, Della Seta R, D’Amico M. Effects of Local and Whole Body Hyperthermia on Immunity. Landes Bioscience. 2000-2013 [Google Scholar]
- 12.Rickaby DA, Fehring JF, Johnston MR, Dawson CA. Tolerance of the isolated perfused lung to hyperthermia. J Thorac Cardiovasc Surg. 1991;101:732–9. [PubMed] [Google Scholar]
- 13.McCormick W, Penman S. Regulation of protein synthesis in HeLa cells: Translation at elevated temperatures. J Mol Biol. 1969;39:315–33. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- 14.Sadis S, Hickey E, Weber LA. Effect of heat shock on RNA metabolism in HeLa cells. J Cell Physiol. 1988;135:377–86. doi: 10.1002/jcp.1041350304. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Z, Dammert MA, Hoppe S, Bierhoff H, Grummt I. Heat shock represses rRNA synthesis by inactivation of TIF-IA and lncRNA-dependent changes in nucleosome positioning. Nucleic Acids Res. 2016;44:8144–52. doi: 10.1093/nar/gkw496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalgi R, Hurt JA, Lindquist S, Burge CB. Widespread inhibition of posttranscriptional splicing shapes the cellular transcriptome following heat shock. Cell Rep. 2014;7:1362–70. doi: 10.1016/j.celrep.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Di Giammartino DC, Shi Y, Manley JL. PARP1 Represses PAP and Inhibits Polyadenylation during Heat Shock. Mol Cell. 2013;49:7–17. doi: 10.1016/j.molcel.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortese M, Lee J-Y, Cerikan B, Burge CB. Integrative imaging reveals SARS-CoV-2 induced reshaping of subcellular morphologies. Cell Host Microbe. 2020;28:853–66. doi: 10.1016/j.chom.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pawlik A, Nowak JM, Grzanka D, Gackowska L, Michalkiewicz J, Grzanka A. Hyperthermia induces cytoskeletal alterations and mitotic catastrophe in p53-deficient H1299 lung cancer cells. Acta Histochem. 2013;115:8–15. doi: 10.1016/j.acthis.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Jasinska-Konior K, Wieche CO, Sarna M, Panek A, Swakoń J, Michalik M, et al. Increased elasticity of melanoma cells after low-LET proton beam due to actin cytoskeleton rearrangements. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-43453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh S, Dellibovi-Ragheb TA, Kerviel A, Pak E, Qiu Q, Fisher M, et al. β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell. 2020;183:1520–35.e14. doi: 10.1016/j.cell.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao G-J, Liang Z-Z, Gao G-Q, Wang Y-Y, Guo X-Y, Su L, et al. A photostable Si-rhodaminebased near-infrared fluorescent probe for monitoring lysosomal pH during heat stroke. Anal Chim Acta. 2019;1092:117–25. doi: 10.1016/j.aca.2019.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–43. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Channappanavar R, Kanneganti TD. Coronaviruses: Innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020;41:1083–99. doi: 10.1016/j.it.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43–62. doi: 10.1007/s00281-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 26.Bester J, Matshailwe C, Pretorius E. Simultaneous presence of hypercoagulation and increased clot lysis time due to IL-1b, IL–6 and IL-8. Cytokine. 2018;110:237–42. doi: 10.1016/j.cyto.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–61. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuhlmeier KM. Short term hyperthermia prevents the activation of mitogen-activated protein kinase p38. Exp Gerontol. 2009;44:406–12. doi: 10.1016/j.exger.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Kappel M, Diamant M, Hansen MB, Klokker M, Klokker M, Pedersen BK. Effects of in vitro hyperthermia on the proliferative response of blood mononuclear cell subsets, and detection of interleukins 1 and 6, tumour necrosis factor-alpha and interferon-gamma. Immunology. 1991;73:304–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Mancilla-Galindo J, Galindo-Sevilla N. Exploring the rationale for thermotherapy in COVID-19. Int J Hyperthermia. 2021;38:202–12. doi: 10.1080/02656736.2021.1883127. [DOI] [PubMed] [Google Scholar]
- 31.Hall EJ. 7th. Wolters Klower; 2020. Radiobiology for the Radiologist. [Google Scholar]
- 32.Bushong J. The Basic Principles of Medical Imaging Instruments. 20201. [Google Scholar]
- 33.Bushberg J. The Physical Principles of Medical Thermotherapy. 2017 [Google Scholar]


