Abstract
Objective
This study aimed to explore the association between dietary niacin intake and rheumatoid arthritis (RA) in American women through the National Health and Nutrition Examination Survey (NHANES) database.
Methods
A retrospective analysis was conducted based on NHANES 2003–2016 data. Dietary niacin intake was stratified using weighted quartiles and association of dietary niacin intake with RA was explored using weighted logistic regression models and restricted cubic splines (RCS). Subgroup analysis was conducted, adjusting for all confounding factors, and a likelihood ratio test was utilized to determine significant covariates for the interaction term. Stratified analysis was conducted on significant covariates to determine their impact on the association of dietary niacin intake with RA.
Results
Fourteen thousand five hundred and thirty-nine American women were selected according to inclusion and exclusion criteria, among whom 845 (4.4%) had RA. Compared with American women without RA, American women with RA had significantly lower dietary niacin intake (18.90 vs 21.22, P<0.001). Logistic regression models and RCS analysis reported a significant linear negative correlation between dietary niacin intake and prevalence of RA (Odds Ratio (OR) < 1, P < 0.05, P-non-linear >0.05). The interaction-term P-values showed that this association was significantly influenced by poverty income ratio (PIR), education level, Body Mass Index (BMI), and smoking (P for interaction < 0.05). Stratified analysis unveiled that this association was particularly significant in individuals aged ≥ 40 years (OR: 0.98, 95% Confidence Interval (CI): 0.97–0.99, P < 0.05), PIR > 3.5 (OR: 0.96, 95% CI: 0.93–0.99, P < 0.05), with a college education or higher (OR: 0.97, 95% CI: 0.94–0.99, P < 0.01), BMI ≥ 30kg/m² (OR: 0.98, 95% CI: 0.96–0.99, P < 0.05), non-smokers (OR: 0.97, 95% CI: 0.95–0.99, P < 0.01), or former smokers (OR: 0.95, 95% CI: 0.95–0.99, P < 0.05).
Conclusion
Increased dietary niacin intake was associated with a reduced prevalence of RA, especially in women aged ≥40, PIR > 3.5, with at least a college education, BMI ≥ 30kg/m², and currently non-smokers.
Keywords: rheumatoid arthritis, dietary niacin intake, women, NHANES
Introduction
A systemic autoimmune illness, rheumatoid arthritis (RA), can impact the heart, kidneys, lungs, digestive system, eyes, skin, and nervous system in addition to joints.1,2 Globally, there were an estimated 17.6 million (95% UI: 15.8–20.3) RA patients in 2020, translating to an age-standardized global prevalence rate of 208.8 cases per 100,000 persons.3 RA is more prevalent in women, affecting them more significantly than men at all ages.3 Furthermore, the incidence rates for females and males reach their peaks at ages 70–74 and 75–79, respectively.4 RA has an insidious onset and progresses slowly.5,6 If not treated promptly, it may cause progressive joint damage and deformity, chronic pain, long-term disability, and premature death.3,4,7 Unfortunately, RA is incurable, leading to increased burden on individuals and society.8,9 However, if the disease is diagnosed early, many patients can achieve complete remission or significantly reduce disease activity.10 Therefore, early identification of RA is crucial for taking preventive measures and establishing the correct treatment plan.
Dietary factors are linked to RA risk and progression.11,12 Dietary interventions (such as the Mediterranean diet or dietary supplements) can improve RA outcomes to some extent.13,14 According to the nutritional pattern of RA population (anti-inflammatory, antioxidant, n-3 polyunsaturated fatty acids, vitamins and minerals, weight control, etc).,15 vitamins with antioxidant activity have attracted attention due to their potential to alleviate oxidative stress and inflammatory response in RA.16–21 Niacin (Vitamin B3) is a dietary vitamin that contains two vitamin isomers, niacin and niacinamide. Niacin is the nutritional precursor of the bioactive molecules nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate, playing important roles in mitochondrial energy metabolism and cellular redox reactions.22,23 An animal experiment found that niacin can reduce cell apoptosis and promote follicular development to rescue female ovarian premature aging, improving female quality of life.24 Mirzaaghasi et al25 found that the combination of niacin and prednisolone may have potential benefits for managing RA. However, there is currently no relevant research report in the existing literature on the association of dietary niacin intake with RA in women. Given the higher prevalence of RA in women, we believe it is necessary to investigate the association of dietary niacin intake with RA in women to improve RA management.
Based on this, we assumed that RA patients had a lower dietary intake of niacin and evaluated it using the National Health and Nutrition Examination Survey (NHANES) database. Subgroup analysis was to identify possible factors that may affect the association of dietary niacin intake with RA.
Methods
Data Source and Study Population
NHANES database is an ongoing program in the United States that uses a multi-stage stratified complex design to collect and evaluate the health and nutritional status of adults and children. Participants first receive survey interviews at home and then proceed to the Mobile Examination Center (MEC) for various clinical and laboratory examinations. NHANES was authorized by the Ethics Review Committee of the National Center for Health Statistics (NCHS) in the United States, and all participants provided informed consent. Our study only used it for secondary analysis and did not require further approval from the institutional review board.
We merged the surveys from 2003 to 2016, covering seven consecutive cycles, into one analysis sample. Seventy-one thousand fifty-eight participants were included. We excluded participants with missing medical questionnaire data or dietary niacin intake data (n = 11,016), missing covariate data (n = 31,332), and male participants (n = 14,171). Fourteen thousand five hundred and thirty-nine participants were finally selected for analysis. Detailed inclusion and exclusion processes are shown in Figure 1.
Figure 1.
Flowchart of participant selection process.
Niacin
Data collection of dietary niacin intake was based on two 24-hour dietary recall interviews in NHANES. The Mobile Examination Center (MEC) hosted the initial in-person dietary recall interview, which was followed by a phone conversation for a second interview three to 10 days later. Dietary data assessment included records of types and quantities of food and beverages (including all types of water) consumed in the past 24 hours, and estimated energy, nutrients, and other components obtained from these foods and beverages. Dietary niacin intake (mg/day) was calculated based on the average value from participants’ two dietary recalls.26,27
Ra
The diagnosis of arthritis was obtained through self-reported questionnaires. Participants were asked that “Has a doctor or other health professional ever told you that you have arthritis?” The response options are “yes” or “no”. Participants who answered “yes” were further asked that “What type of arthritis is this?” Participants who answered “RA” were confirmed to have RA.28
Variables
Variables included age, race, poverty income ratio (PIR), education level, Body Mass Index (BMI), alcohol consumption, smoking, diabetes, hypertension, white blood cell count (WBC), red blood cell count (RBC), mean platelet volume (MPV), systemic immune inflammation index (SII), and energy intake.29 Since RA is common in women over 40 years old,30 the age was divided into <40 years old and ≥40 years old. Education level was divided into did not graduate from high school, graduated from high school, and college education or above.31 PIR was classified as low income (≤1.3), middle income (1.3–3.5), and high income (>3.5).32 According to the World Health Organization standards, participants’ BMI was calculated based on weight/height2 (kg/m²), divided into <25, 25–30, and ≥30.33 Alcohol drinking was categorized as yes or no.34 Smoking status was defined based on the following questions:35 for “Do you now smoke cigarettes”, answering “Every day” or “Some days” was defined as “now smoking”; for “Smoked at least 100 cigarettes in life”, answering “Yes” was defined as “former smoking”; the rest were defined as “never smoking”. Hypertension36 was defined as meeting one of the following criteria: (a) previously diagnosed with hypertension; (b) self-reported use of antihypertensive medication; (c) in the NHANES examination component, average systolic blood pressure greater than or equal to 130 mmHg or diastolic blood pressure greater than or equal to 80 mmHg.37 Diabetes38 was defined as meeting one of the following criteria: (a) being informed by a doctor of having diabetes; (b) taking antidiabetic medication; (c) having glycated hemoglobin >6.5%; (d) having fasting blood glucose >126 mg/dL. WBC, RBC, MPV, and SII were parameters in the complete blood cell count (CBC). Using Beckman Coulter equipment, analysis was performed using a single-beam spectrophotometer with hemoglobin measurement, along with counting, sizing, grading methods, and automated dilution and mixing devices for sample processing. The WBC difference was detected using volume scattering technology (Volume, Conductivity, and Scatter Technology, VCS). The formula for SII was platelet count × neutrophil count/lymphocyte count.30 Relevant variables could be defined to corresponding modules through variable names (https://wwwn.cdc.gov/Nchs/Nhanes/).
Statistical Analysis
All statistical analyses were done by R (V4.2.2) software. Total population was assigned into RA group and non-RA group according to the definition of RA, and baseline tables were generated using the tableone package. Categorical variables were presented as sample size and proportion (n(%)), while continuous variables were shown as mean and standard deviation (mean(SD)) (n: unweighted sample size; n(%): weighted proportion; mean: weighted mean; SD: weighted standard deviation). Dietary niacin intake was stratified using weighted quartiles and a weighted logistic regression model of dietary niacin intake and RA was constructed using the survey package, adjusting for various confounding factors. In the weighted logistic regression model after adjusting for all confounding factors, restricted cubic splines (RCS) were utilized to explore the association of dietary niacin intake with RA. Stratified analysis was performed on categorical variables, and likelihood ratio test was conducted on interaction terms of stratified logistic regression models adjusted for all confounding factors. P<0.05 indicates a significant difference. In the weighted logistic regression model, subgroup analysis was done for confounding factors with interaction term P<0.05.
Results
Baseline Characteristics
This study included 14,539 female participants, and clinical and biochemical characteristics of the participants are presented in Table 1. The overall population consisted of 63.4% individuals aged 40 years and above, 10.9% participants had diabetes, 45.4% were hypertensive, and the average dietary niacin intake was 21.12 mg/d. The dietary niacin intake was significantly lower in RA population than in non-RA population (18.90 mg/d vs 21.22 mg/d, P<0.001). Age, race, PIR, education level, BMI, smoking, drinking, diabetes, hypertension, and RBC showed significant statistical differences between groups (P<0.05).
Table 1.
Characteristics of NHANES Participants from 2003 to 2016
| Characteristics | Total | Non-Rheumatoid Arthritis | Rheumatoid Arthritis | P value |
|---|---|---|---|---|
| Overall | 14539 | 13,694 (95.6) | 845 (4.4) | |
| Age | <0.001 | |||
| <40 | 5183 (36.6) | 5103 (37.8) | 80 (10.8) | |
| ≥40 | 9356 (63.4) | 8591 (62.2) | 765 (89.2) | |
| Race | <0.001 | |||
| Mexican American | 2356 (7.8) | 2231 (7.9) | 125 (6.0) | |
| Other Hispanic | 1290 (4.8) | 1204 (4.7) | 86 (5.7) | |
| Non-Hispanic White | 6777 (69.8) | 6428 (70.1) | 349 (65.0) | |
| Non-Hispanic Black | 2972 (11.4) | 2722 (11.0) | 250 (18.4) | |
| Other race | 1144 (6.3) | 1109 (6.3) | 35 (4.9) | |
| PIR | <0.001 | |||
| ≤1.3 | 4800 (23.4) | 4415 (22.9) | 385 (35.5) | |
| 1.3–3.5 | 5455 (36.2) | 5154 (36.1) | 301 (39.0) | |
| >3.5 | 4284 (40.4) | 4125 (41.1) | 159 (25.5) | |
| Education | <0.001 | |||
| Did not graduate from high school | 3508 (16.5) | 3216 (16.1) | 292 (26.0) | |
| Graduated from high school | 3372 (22.7) | 3171 (22.6) | 201 (26.3) | |
| College education or above | 7659 (60.8) | 7307 (61.4) | 352 (47.7) | |
| BMI (kg/m2) | <0.001 | |||
| <25 | 4454 (35.0) | 4282 (35.4) | 172 (25.8) | |
| 25–30 | 4157 (28.0) | 3930 (28.1) | 227 (25.8) | |
| ≥30 | 5928 (37.0) | 5482 (36.5) | 446 (48.4) | |
| Smoking | <0.001 | |||
| Never smoking | 9109 (59.7) | 8660 (60.3) | 449 (46.5) | |
| Former smoking | 2861 (21.0) | 2663 (20.9) | 198 (23.3) | |
| Now Smoking | 2569 (19.3) | 2371 (18.8) | 198 (30.2) | |
| Alcohol drinking | 0.002 | |||
| No | 5802 (32.2) | 5412 (31.8) | 390 (39.8) | |
| Yes | 8737 (67.8) | 8282 (68.2) | 455 (60.2) | |
| Diabetes | <0.001 | |||
| No | 12416 (89.1) | 11,818 (89.7) | 598 (76.0) | |
| Yes | 2123 (10.9) | 1876 (10.3) | 247 (24.0) | |
| Hypertension | <0.001 | |||
| No | 7403 (54.6) | 7167 (55.6) | 236 (32.9) | |
| Yes | 7136 (45.4) | 6527 (44.4) | 609 (67.1) | |
| White blood cell (103/μL) | 7.39 (2.30) | 7.39 (2.30) | 7.49 (2.37) | 0.431 |
| Red blood cell (106/μL) | 4.45 (0.40) | 4.45 (0.39) | 4.40 (0.42) | 0.011 |
| MPV (fL) | 8.17 (0.94) | 8.17 (0.94) | 8.17 (0.96) | 0.869 |
| SII | 580.20 (339.20) | 578.22 (333.70) | 622.90 (439.60) | 0.060 |
| Energy (Kcal) | 1789.65 (625.47) | 1796.47 (625.75) | 1642.81 (601.22) | <0.001 |
| Dietary niacin intake (mg/d) | 21.12 (9.32) | 21.22 (9.35) | 18.90 (8.29) | <0.001 |
Correlation Between Dietary Niacin Intake and RA
There was a significant negative correlation between dietary niacin intake and prevalence of RA (Odds Ratio (OR)<1, P<0.05) (Table 2). Further stratification of dietary niacin intake into quartiles was performed, with lower dietary niacin intake Q1 (≤14.95) as the reference, to construct a weighted logistic regression model. Compared with Q1, there was a trend of reducing RA prevalence with elevating dietary niacin intake (Q2 (14.95–19.65), Q3 (19.65–25.63), Q4 (>25.63)), with a significant P value for trend (P for trend <0.05).
Table 2.
Associations Between Dietary Niacin Intake and Odds Ratios (95% Confidence Intervals) for Rheumatoid Arthritis, NHANES 2003–2016
| OR (95% CI) | |||
|---|---|---|---|
| Participants | Crude | Model I | Model II |
| All participants | 0.97 (0.95–0.98)*** | 0.98 (0.97–0.99)* | 0.98 (0.97–0.99)* |
| Dietary niacin intake (mg/day) | |||
| Q1 (≤14.95) | Ref. | Ref. | Ref. |
| Q2 (14.95–19.65) | 0.82 (0.64–1.05) | 0.95 (0.73–1.23) | 0.94 (0.72–1.22) |
| Q3 (19.65–25.63) | 0.65 (0.47–0.89) | 0.78 (0.56–1.10) | 0.78 (0.56–1.09) |
| Q4 (>25.63) | 0.46 (0.34–0.63) | 0.63 (0.44–0.88) | 0.63 (0.45–0.89) |
| P for trend | <0.001 | 0.036 | 0.047 |
Note: Crude: Unadjusted; Model I: Adjusted for age, race, PIR, education level, BMI, alcohol consumption, smoking; Model II: Adjusted for age, race, PIR, education level, BMI, alcohol consumption, smoking, diabetes, hypertension, white blood cell count, red blood cell count, mean platelet volume, systemic immune-inflammatory index.*P-value<0.05, ***P-value< 0.001.
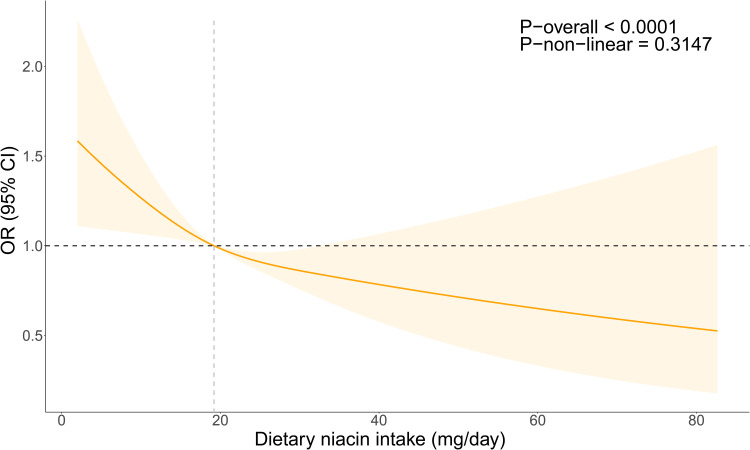
Overall, after adjusting for all confounding factors, a significant overall trend was seen between dietary niacin intake and RA (P-overall < 0.05), and there was a linear relationship between them in the RCS analysis (P-non-linear = 0.3147 > 0.05) (Figure 2).
Figure 2.
OR of dietary niacin intake concerning RA adjusted for covariates in NHANES 2003–2016 The RCS line is adjusted for various factors including age, race, PIR, education, BMI, alcohol consumption, smoking, diabetes, hypertension, white blood cell count, red blood cell count, mean platelet volume, and systemic immune-inflammatory index. The OR is visualized by the Orange line, and the shaded region signifies the 95% CI.
Abbreviations: OR, Odds Ratio; CI, Confidence Interval.
Subgroup Analysis
Interaction test unveiled that association between dietary niacin intake and RA prevalence was influenced by PIR (P for interaction = 0.008), education level (P for interaction = 0.002), BMI (P for interaction = 0.027), and smoking (P for interaction = 0.001) (Table 3).
Table 3.
Association Between Dietary Niacin Intake and Rheumatoid Arthritis in Categorical Variables
| Participants | OR | 95% CI1 | P-value | P for interaction |
|---|---|---|---|---|
| Age | 0.855 | |||
| <40 | 0.98 | 0.94–1.02 | 0.300 | |
| ≥40 | 0.97 | 0.96–0.99 | 0.001 | |
| Race | 0.225 | |||
| Mexican American | 0.96 | 0.93–0.99 | 0.007 | |
| Other Hispanic | 0.96 | 0.92–0.99 | 0.009 | |
| Non-Hispanic White | 0.97 | 0.95–0.99 | <0.001 | |
| Non-Hispanic Black | 0.97 | 0.96–0.99 | 0.008 | |
| Other race | 1.00 | 0.96–1.03 | 0.800 | |
| PIR | 0.008 | |||
| ≤1.3 | 0.98 | 0.97–0.99 | 0.042 | |
| 1.3–3.5 | 0.97 | 0.95–0.99 | 0.017 | |
| >3.5 | 0.95 | 0.92–0.99 | 0.007 | |
| Education | 0.002 | |||
| Did not graduate from high school | 0.98 | 0.96–1.00 | 0.088 | |
| Graduated from high school | 0.99 | 0.97–1.02 | 0.500 | |
| College education or above | 0.95 | 0.93–0.97 | <0.001 | |
| BMI (kg/m2) | 0.027 | |||
| <25 | 0.96 | 0.93–0.98 | 0.002 | |
| 25–30 | 0.98 | 0.96–1.01 | 0.200 | |
| ≥30 | 0.97 | 0.95–0.99 | <0.001 | |
| Smoking | 0.001 | |||
| Never smoking | 0.96 | 0.94–0.98 | <0.001 | |
| Former smoking | 0.96 | 0.94–0.98 | <0.001 | |
| Now Smoking | 0.99 | 0.97–1.01 | 0.400 | |
| Alcohol drinking | 0.614 | |||
| No | 0.97 | 0.95–0.99 | 0.002 | |
| Yes | 0.97 | 0.95–0.99 | 0.002 | |
| Diabetes | 0.247 | |||
| No | 0.97 | 0.96–0.99 | <0.001 | |
| Yes | 0.96 | 0.93–0.99 | 0.004 | |
| Hypertension | 0.874 | |||
| No | 0.97 | 0.94–0.99 | 0.011 | |
| Yes | 0.97 | 0.96–0.99 | 0.004 |
Note: Interaction term p-values adjusted for age, race, PIR, education level, BMI, alcohol consumption, smoking, diabetes, hypertension, white blood cell count, red blood cell count, mean platelet volume, systemic immune-inflammatory index.
Subgroup analysis was conducted (Table 4). It was found that in individuals aged ≥40 years (Crude: OR: 0.97, 95% Confidence Interval (CI): 0.96–0.99, P<0.01; Model I, Model II: OR: 0.98, 95% CI: 0.97–0.99, P<0.05), PIR>3.5 (Crude: OR: 0.95, 95% CI: 0.92–0.99, P<0.01; Model I, Model II: OR: 0.96, 95% CI: 0.93–0.99, P<0.05), and college or above (Crude: OR: 0.95, 95% CI: 0.93–0.97, P<0.001; Model I, Model II: OR: 0.97, 95% CI: 0.94–0.99, P<0.01), BMI≥30kg/m² (Crude: OR: 0.97, 95% CI: 0.95–0.99, P<0.001; Model I, Model II: OR: 0.98, 95% CI: 0.96–0.99, P<0.05) and never smoked (Crude: OR: 0.96, 95% CI: 0.94–0.98, P<0.001; Model I, Model II: OR: 0.97, 95% CI: 0.95–0.99, P<0.01) or former smokers (Crude: OR: 0.96, 95% CI: 0.94–0.98, P<0.001; Model I: OR: 0.97, 95% CI: 0.95–0.99, P<0.05; Model II: OR: 0.98, 95% CI: 0.95–0.99, P<0.05), there was a significant negative correlation between dietary niacin intake and RA prevalence.
Table 4.
Association Between Dietary Niacin Intake and Rheumatoid Arthritis by Age, PIR, Education Level, BMI, Smoking (95% Confidence Interval), NHANES, 2003–2016
| OR (95% CI) | |||
|---|---|---|---|
| Participants | Crude | Model I | Model II |
| Age | |||
| <40 | 0.98 (0.94–1.02) | 0.98 (0.94–1.02) | 0.98 (0.94–1.02) |
| ≥40 | 0.97 (0.96–0.99)** | 0.98 (0.97–0.99)* | 0.98 (0.97–0.99)* |
| PIR | |||
| ≤1.3 | 0.98 (0.97–0.99)* | 1.00 (0.98–1.01) | 1.00 (0.98–1.01) |
| 1.3–3.5 | 0.97 (0.95–0.99)* | 0.98 (0.96–1.01) | 0.98 (0.96–1.01) |
| >3.5 | 0.95 (0.92–0.99)** | 0.96 (0.93–0.99)* | 0.96 (0.93–0.99)* |
| Education | |||
| Did not graduate from high school | 0.98 (0.96–1.00) | 0.99 (0.97–1.01) | 0.99 (0.97–1.01) |
| Graduated from high school | 0.99 (0.97–1.02) | 1.00 (0.98–1.03) | 1.00 (0.98–1.03) |
| College education or above | 0.95 (0.93–0.97)*** | 0.97 (0.94–0.99)** | 0.97 (0.94–0.99)** |
| BMI (kg/m2) | |||
| <25 | 0.96 (0.93–0.98)** | 0.97 (0.94–1.00) | 0.97 (0.94–1.00) |
| 25–30 | 0.98 (0.96–1.01) | 1.00 (0.97–1.02) | 1.00 (0.97–1.03) |
| ≥30 | 0.97 (0.95–0.99)*** | 0.98 (0.96–0.99)* | 0.98 (0.96–0.99)* |
| Smoking | |||
| Never smoking | 0.96 (0.94–0.98)*** | 0.97 (0.95–0.99)** | 0.97 (0.95–0.99)** |
| Former smoking | 0.96 (0.94–0.98)*** | 0.97 (0.95–0.99)* | 0.98 (0.95–0.99)* |
| Now Smoking | 0.99 (0.97–1.01) | 1.00 (0.98–1.03) | 1.00 (0.98–1.03) |
Note: Crude: Unadjusted; Model I: Adjusted for age, race, PIR, education level, BMI, alcohol consumption, smoking; Model II: Adjusted for age, race, PIR, education level, BMI, alcohol consumption, smoking, diabetes, hypertension, white blood cell count, red blood cell count, mean platelet volume, systemic immune-inflammatory index.*P-value<0.05, **P-value<0.01, ***P-value< 0.001.
Discussion
To our knowledge, this is the first study to investigate the correlation between dietary niacin intake and RA in American women using the NHANES database. Our research results showed that compared to non-RA individuals, RA patients had a lower dietary niacin intake (18.90 mg/d vs 21.22 mg/d, P<0.05). Additionally, there was a negative correlation between dietary niacin intake and prevalence of RA, especially in individuals aged ≥40 years, with PIR > 3.5, with a college degree or higher, with a BMI ≥ 30kg/m², and in those who had never smoked or previously smoked.
A rat experiment showed that the combination of niacin and prednisolone can activate immune regulation in rats, promote a decrease in certain hematological and RA parameters (such as neutrophil iron absorption, myeloperoxidase, nitric oxide, C-reactive protein, etc)., and may be a useful method for treating RA.25 However, there have been no studies reporting the impact of dietary niacin on RA patients. Niacin plays a role in the most common RA extra-articular manifestations, such as interstitial lung disease.39 Jia et al40 showed that niacin can alleviate pulmonary arterial hypertension through hematopoietic prostaglandin D synthase (H-PGDS) in macrophages. Lohani et al revealed that niacin deficiency leads to genetic instability in normal fetal lung fibroblasts and increases genetic instability caused by nitrosamine ketone, a carcinogen in cigarette smoke.41 Thus, niacin is critical in RA occurrence and development. Therefore, analyzing the impact of dietary niacin on RA through the NHANES database has certain clinical translational value.
Currently, the recommended dietary intake of niacin for adult women is 14 mg/d, with a tolerable upper intake level of 35 mg/d.42,43 Our research unraveled that when dietary intake of niacin was >14.95 mg/d, the prevalence of RA was significantly reduced. Following dietary guidelines has a positive impact on lowering RA risk. Nonetheless, characteristics of the Western diet include a high intake of red meat, saturated fat, trans fat, and refined carbohydrates, mainly increasing RA risk through inflammation, obesity induction, and insulin resistance.44,45 Foods rich in niacin include fish, meat, milk, liver, peanuts, and legumes.22 The Mediterranean diet may be rich in niacin as it contains varying fruits, vegetables, nuts, seafood, fish, whole grains, legumes, olive oil, and moderate amounts of wine.22,46 Based on this, American women can make dietary adjustments related to niacin to maintain a healthier lifestyle, which may be helpful in preventing RA.
According to reports, NAD levels decrease with age,47,48 meanwhile, the incidence of RA is also increasing,4 and RA patients are more prone to complications such as cardiovascular diseases.49 Sufficient dietary niacin intake can prevent dyslipidemia and cardiovascular diseases.50–52 Therefore, intake of niacin should also be increased with age. In addition, smoking and obesity (BMI≥30kg/m²) are recognized environmental factors for RA.53,54 Reduced dietary niacin intake is associated with an elevated risk of obesity.55 Niacin deficiency also increases the genetic instability risk caused by smoking41 and is vital in triggering specific RA subtypes.56,57 Additionally, higher levels of education and income often represent healthier dietary patterns.58,59 However, statistics show that only 0.1% of Americans maintain a healthy diet.60 Therefore, in the population of American women studied, there may also be insufficient dietary niacin intake due to excessive reliance on processed foods, fast food, etc. In conclusion, attention should be paid to supplementation of dietary niacin intake in these populations, which may be beneficial for the prevention of RA.
Pathogenesis of RA is complex, mainly manifested as bone destruction, synovitis, immune cell dysfunction, and inflammatory reactions.10,61 Immune system of RA patients is typified by systemic inflammation and generation of autoantibody, causing activation and release of immune cells, resulting in joint damage and functional impairment.62–66 Niacin is crucial for physiological functions of varying immune cells, including macrophage-specific loss of hematopoietic prostaglandin D synthase, homeostasis of circulating monocyte subsets, and neutrophil migration in inflammation,40,67,68 and it also has anti-inflammatory effects.69,70 Therefore, niacin may have an impact on the regulation of the immune system to some extent, thereby alleviating RA symptoms. In addition, RA patients often suffer from pain, inflammation, and neurologic-related issues.71–73 Niacin is a key mediator for neuronal development and survival in the central nervous system, and it helps alleviate pain and symptoms related to the nervous system.42,74 Although niacin is not a therapeutic drug for RA, this evidence suggests its potential benefits in RA. Therefore, much research is needed to clarify the exact effects and safety of niacin in RA.
This work demonstrated a negative association between dietary niacin and prevalence of RA, based on a nationally representative survey of American women. It does, however, have certain deficiencies. Firstly, our data does not include the intake of dietary niacin supplements, so it cannot accurately reflect the total amount of dietary niacin in individuals. Furthermore, dietary habits and nutritional needs undergo dynamic changes throughout the lifespan, which cannot be assessed by cross-sectional studies. In addition, our study results are based on a survey of American women, and research is needed to determine if they can be generalized to other populations. Finally, this study is a cross-sectional study and cannot make causal inferences.
Funding Statement
This study was supported by funds from Zhejiang Provincial Natural Science Foundation (LGF18H090013).
Data Sharing Statement
The data and materials in the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethical Committee of Affiliated Jinhua Hospital, Zhejiang University School of Medicine. Informed consent was waived by the committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Conforti A, Di Cola I, Pavlych V, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021;20:102735. doi: 10.1016/j.autrev.2020.102735 [DOI] [PubMed] [Google Scholar]
- 2.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular Manifestations in Rheumatoid Arthritis. Maedica. 2010;5:286–291. [PMC free article] [PubMed] [Google Scholar]
- 3.GBDRA C, Cross M, Haile LM. Global, regional, and national burden of rheumatoid arthritis, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e594–e610. doi: 10.1016/S2665-9913(23)00211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78:1463–1471. doi: 10.1136/annrheumdis-2019-215920 [DOI] [PubMed] [Google Scholar]
- 5.Stack RJ, Nightingale P, Jinks C, et al. Delays between the onset of symptoms and first rheumatology consultation in patients with rheumatoid arthritis in the UK: an observational study. BMJ Open. 2019; 9:e024361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert BTP, Lamacchia C, Mongin D, et al. Cohort profile: SCREEN-RA: design, methods and perspectives of a Swiss cohort study of first-degree relatives of patients with rheumatoid arthritis. BMJ Open. 2021; 11:e048409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: a Review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103 [DOI] [PubMed] [Google Scholar]
- 8.Roodenrijs NMT, van der Goes MC, Welsing PMJ, et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology. 2021;60:3778–3788. [DOI] [PubMed] [Google Scholar]
- 9.Bullock J, Rizvi SAA, Saleh AM, et al. Rheumatoid Arthritis: a Brief Overview of the Treatment. Med Princ Pract. 2018;27:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YJ, Anzaghe M, Schulke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. 2020;9. doi: 10.3390/cells9040880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, Di Franco M. Dietary Habits and Nutrition in Rheumatoid Arthritis: can Diet Influence Disease Development and Clinical Manifestations? Nutrients. 2020;12. doi: 10.3390/nu13010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippou E, Nikiphorou E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun Rev. 2018;17:1074–1077. doi: 10.1016/j.autrev.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 13.Petersson S, Philippou E, Rodomar C, Nikiphorou E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: evidence from clinical trials. Autoimmun Rev. 2018;17:1105–1114. doi: 10.1016/j.autrev.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 14.Rondanelli M, Perdoni F, Peroni G, et al. Ideal food pyramid for patients with rheumatoid arthritis: a narrative review. Clin Nutr. 2021;40:661–689. doi: 10.1016/j.clnu.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 15.Skoczynska M, Swierkot J. The role of diet in rheumatoid arthritis. Reumatologia. 2018;56:259–267. doi: 10.5114/reum.2018.77979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen HP, Shin S, Shin KJ, et al. Protective effect of TPP-Niacin on microgravity-induced oxidative stress and mitochondrial dysfunction of retinal epithelial cells. Biochim Biophys Acta Mol Cell Res. 2023;1870:119384. doi: 10.1016/j.bbamcr.2022.119384 [DOI] [PubMed] [Google Scholar]
- 17.Boo YC, Bruno A, Renzi A. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants. 2021;10. doi: 10.3390/antiox11010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044 [DOI] [PubMed] [Google Scholar]
- 19.Wright HL, Lyon M, Chapman EA, Moots RJ, Edwards SW. Rheumatoid Arthritis Synovial Fluid Neutrophils Drive Inflammation Through Production of Chemokines, Reactive Oxygen Species, and Neutrophil Extracellular Traps. Front Immunol. 2020;11:584116. doi: 10.3389/fimmu.2020.584116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo N, Kuroda T, Kobayashi D, Romero MP. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int J Mol Sci. 2021;22. doi: 10.3390/ijms23010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graff EC, Fang H, Wanders D, Judd RL. Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metabolism. 2016;65:102–113. doi: 10.1016/j.metabol.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 22.Kirkland JB, Meyer-Ficca ML. Niacin. Adv Food Nutr Res. 2018;83:83–149. [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Ficca M, Kirkland JB. Niacin. Adv Nutr. 2016;7:556–558. doi: 10.3945/an.115.011239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Sun M, Yu L, Wang Y, Yao Y, Wang D. Niacin Inhibits Apoptosis and Rescues Premature Ovarian Failure. Cell Physiol Biochem. 2018;50:2060–2070. doi: 10.1159/000495051 [DOI] [PubMed] [Google Scholar]
- 25.Mirzaaghasi S, Froushani SMA. Immunomodulatory Effects of Combined Nicotinic Acid and Prednisolone in Adjuvant-induced Arthritis. Antiinflamm Antiallergy Agents Med Chem. 2023;22:104–112. doi: 10.2174/0118715230264101230925060355 [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Eisenberg R, Mowrey WB, et al. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant. 2020;35:1171–1178. doi: 10.1093/ndt/gfz134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying H, Gao L, Liao N, Xu X, Yu W, Hong W. Association between niacin and mortality among patients with cancer in the NHANES retrospective cohort. BMC Cancer. 2022;22:1173. doi: 10.1186/s12885-022-10265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang G, Ren Z, Wang K. Association between human papillomavirus infection or immunization and risk for rheumatoid arthritis. Front Immunol. 2023;14:1130217. doi: 10.3389/fimmu.2023.1130217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Xie S. Dietary fiber intake associated with risk of rheumatoid arthritis among U.S. adults: NHANES 2010-2020. Medicine. 2023; 102:e33357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999-2018. Arthritis Res Ther. 2023;25:34. doi: 10.1186/s13075-023-03018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, Ma X, Liang H, et al. Dietary Magnesium Intake Affects the Association Between Serum Vitamin D and Type 2 Diabetes: a Cross-Sectional Study. Front Nutr. 2021;8:763076. doi: 10.3389/fnut.2021.763076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stebbins RC, Noppert GA, Aiello AE, Cordoba E, Ward JB, Feinstein L. Persistent socioeconomic and racial and ethnic disparities in pathogen burden in the United States, 1999-2014. Epidemiol Infect. 2019; 147:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo W, Song Y, Sun Y, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: evidence from NHANES 2011-2018. Front Endocrinol. 2022;13:1071465. doi: 10.3389/fendo.2022.1071465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong X, Li S, Sun J, Li Y, Zhang D. Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: national Health and Nutrition Examination Survey (NHANES) 2011-2014. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu W, Zhang W, et al. Association between blood lead levels and hyperlipidemiais: results from the NHANES (1999-2018). Front Public Health. 2022;10:981749. doi: 10.3389/fpubh.2022.981749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Shang S, Lee K-H. Relationship between Sleep and Hypertension: findings from the NHANES (2007-2014). Int J Environ Res Public Health. 2021;18. doi: 10.3390/ijerph19010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao H, Liu Y, Tsai TC, Schwartz J, Ji JS. Association Between Blood Lead Level and Uncontrolled Hypertension in the US Population (NHANES 1999-2016). J Am Heart Assoc. 2020; 9:e015533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Li H. Association of serum vitamin C with liver fibrosis in adults with nonalcoholic fatty liver disease. Scand J Gastroenterol. 2022;57:872–877. doi: 10.1080/00365521.2022.2041085 [DOI] [PubMed] [Google Scholar]
- 39.Kadura S, Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev. 2021;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia D, Bai P, Wan N, et al. Niacin Attenuates Pulmonary Hypertension Through H-PGDS in Macrophages. Circ Res. 2020;127:1323–1336. doi: 10.1161/CIRCRESAHA.120.316784 [DOI] [PubMed] [Google Scholar]
- 41.Lohani M, Dhasmana A, Haque S, et al. Niacin deficiency modulates genes involved in cancer: are smokers at higher risk? J Cell Biochem. 2019;120:232–242. doi: 10.1002/jcb.27324 [DOI] [PubMed] [Google Scholar]
- 42.Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the Central Nervous System: an Update of Biological Aspects and Clinical Applications. Int J Mol Sci. 2019;21:20. doi: 10.3390/ijms21010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietary Reference Intakes for Thiamin. Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline. Washington (DC); 1998. [PubMed] [Google Scholar]
- 44.Minihane AM, Vinoy S, Russell WR, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin B, Yang M, Fu H, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17:86. doi: 10.1186/s13075-015-0601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber M, Hoffman R, Cao Y, Zhu -Y-Y, Guan K, Chen Y-M. The Mediterranean diet: health, science and society. Br J Nutr. 2015;113. doi: 10.1017/S0007114515004134 [DOI] [PubMed] [Google Scholar]
- 47.Dhuguru J, Dellinger RW. Defining NAD(P)(H). Catabolism Nutrients. 2023;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huscher D, Sengler C, Gromnica-Ihle E, et al. Clinical presentation, burden of disease and treatment in young-onset and late-onset rheumatoid arthritis: a matched-pairs analysis taking age and disease duration into account. Clin Exp Rheumatol. 2013;31:256–262. [PubMed] [Google Scholar]
- 50.Carbone LD, Buzkova P, Fink HA, et al. Association of Dietary Niacin Intake With Incident Hip Fracture, BMD, and Body Composition: the Cardiovascular Health Study. J Bone Miner Res. 2019;34:643–652. doi: 10.1002/jbmr.3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minto C, Vecchio MG, Lamprecht M, Gregori D. Definition of a tolerable upper intake level of niacin: a systematic review and meta-analysis of the dose-dependent effects of nicotinamide and nicotinic acid supplementation. Nutr Rev. 2017;75:471–490. doi: 10.1093/nutrit/nux011 [DOI] [PubMed] [Google Scholar]
- 52.Meyers CD, Kamanna VS, Kashyap ML. Niacin therapy in atherosclerosis. Curr Opin Lipidol. 2004;15:659–665. doi: 10.1097/00041433-200412000-00006 [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa Y, Terao C. The Impact of Cigarette Smoking on Risk of Rheumatoid Arthritis: a Narrative Review. Cells. 2020;9:475. doi: 10.3390/cells9020475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myasoedova E, Davis J, Matteson EL, Crowson CS. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann Rheum Dis. 2020;79:440–444. doi: 10.1136/annrheumdis-2019-216694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju L, Wei X, Yu D, et al. Dietary Micronutrient Status and Relation between Micronutrient Intakes and Overweight and Obesity among Non-Pregnant and Non-Lactating Women Aged 18 to 49 in China. Nutrients. 2022;15:14. doi: 10.3390/nu15010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radu AF, Bungau SG, Tian R. Management of Rheumatoid Arthritis: an Overview. Cells. 2021;11:10. doi: 10.3390/cells11010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol. 2009;21:279–283. doi: 10.1097/BOR.0b013e32832a2e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullie P, Clarys P, Hulens M, Vansant G. Dietary patterns and socioeconomic position. Eur J Clin Nutr. 2010;64:231–238. doi: 10.1038/ejcn.2009.145 [DOI] [PubMed] [Google Scholar]
- 59.Li L, Zhang B, Wang HJ, et al. Sociodemographic Factors Associated with Dietary Intake of Thiamine, Riboflavin, and Niacin among Chinese Adults in 2015. Biomed Environ Sci. 2020;33:660–669. doi: 10.3967/bes2020.087 [DOI] [PubMed] [Google Scholar]
- 60.Spence JD, Comina M, Monti E. Nutrition and Risk of Stroke. Nutrients. 2019;11. doi: 10.3390/nu12010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J, Liu J, Tian R, et al. A Next Generation Sequencing-Based Protocol for Screening of Variants of Concern in Autism Spectrum Disorder. Cells. 2021;11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15:9–17. doi: 10.1038/s41584-018-0109-2 [DOI] [PubMed] [Google Scholar]
- 63.Jang S, Kwon EJ, Lee JJ. Rheumatoid Arthritis: pathogenic Roles of Diverse Immune Cells. Int J Mol Sci. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ouboussad L, Burska AN, Melville A, Buch MH. Synovial Tissue Heterogeneity in Rheumatoid Arthritis and Changes With Biologic and Targeted Synthetic Therapies to Inform Stratified Therapy. Front Med Lausanne. 2019;6:45. doi: 10.3389/fmed.2019.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Z, Ma D, Yang H, et al. Fibroblast-like synoviocytes in rheumatoid arthritis: surface markers and phenotypes. Int Immunopharmacol. 2021;93:107392. doi: 10.1016/j.intimp.2021.107392 [DOI] [PubMed] [Google Scholar]
- 66.Yoshitomi H. Regulation of Immune Responses and Chronic Inflammation by Fibroblast-Like Synoviocytes. Front Immunol. 2019;10:1395. doi: 10.3389/fimmu.2019.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.La Paz S M-D, Carmen Naranjo M D, Lopez S, et al. Immediate-release niacin and a monounsaturated fatty acid-rich meal on postprandial inflammation and monocyte characteristics in men with metabolic syndrome. Clin Nutr. 2023;42:2138–2150. doi: 10.1016/j.clnu.2023.08.017 [DOI] [PubMed] [Google Scholar]
- 68.Ferreira RG, Matsui TC, Gomides LF, et al. Niacin inhibits carrageenan-induced neutrophil migration in mice. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:533–540. doi: 10.1007/s00210-013-0854-3 [DOI] [PubMed] [Google Scholar]
- 69.Horimatsu T, Blomkalns AL, Ogbi M, et al. Niacin protects against abdominal aortic aneurysm formation via GPR109A independent mechanisms: role of NAD+/nicotinamide. Cardiovasc Res. 2020;116:2226–2238. doi: 10.1093/cvr/cvz303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karunaratne TB, Okereke C, Seamon M, Purohit S, Wakade C, Sharma A. Niacin and Butyrate: nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients. 2020;13:13. doi: 10.3390/nu13010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pope JE. Management of Fatigue in Rheumatoid Arthritis. RMD Open. 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zielinski MR, Systrom DM, Fatigue RNR. Sleep, and Autoimmune and Related Disorders. Front Immunol. 2019;10:1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellocchi C, Carandina A, Montinaro B, et al. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int J Mol Sci. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wuerch E, Urgoiti GR, Yong VW. The Promise of Niacin in Neurology. Neurotherapeutics. 2023;20:1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials in the current study are available from the corresponding author on reasonable request.