Abstract
Background:
Serratia has emerged as an important nosocomial opportunistic pathogen, often associated with serious infections. We investigated the antimicrobial resistance trends, predisposing factors, and infection outcomes associated with Serratia species isolated in a secondary-care hospital in Oman.
Materials and Methods:
A retrospective study was conducted at a secondary-care hospital in the northern region of Oman after receiving approval from the research ethics and approval committee of Oman. The relevant data of patients diagnosed with Serratia infection during 2017–2021 was extracted from the Sohar Hospital health records. We statistically analyzed the data using the statistical software STATA version 14.
Results:
A total of 257 non-duplicate Serratia strains were studied. S. marcescens was the predominant (79.4%) isolated species. Serratia strains were more frequently isolated from males (51.4%). The most affected were older people aged > 60 years (29.4%), infants (28%), and patients treated at critical care units. Serratia has demonstrated high resistance to beta-lactams. The susceptibility rates of Serratia strains to tigecycline, ciprofloxacin, trimethoprim-sulfamethoxazole, gentamicin, amikacin, piperacillin-tazobactam, imipenem, and meropenem was high. Septicemia, pneumonia, mechanical ventilation, and hemodialysis were the independent risk factors for increased mortality among studied subjects (P < 0.05).
Conclusions:
Our study results recommend empirical therapy with trimethoprim-sulfamethoxazole, piperacillin-tazobactam, aminoglycosides, and ciprofloxacin as first-line drugs for Serratia infection. The emergence of ESBL producers and carbapenem-resistant strains is worrisome. Regular updating of physicians’ knowledge about antimicrobial profiles, antibiotic prescription policies, and infection control measures is necessary to combat antimicrobial resistance and improve outcomes.
Keywords: Carbapenems, health care-associated infection, multi-drug resistance, Serratia marcescens
INTRODUCTION
Serratia species, once regarded as harmless saprophytes, are now apprehended as important nosocomial opportunistic pathogens. They are gram-negative motile rods belonging to the family Enterobacterales. Serratia are facultative anaerobes capable of surviving in harsh environmental conditions, and these factors account for their ability to thrive in hospital environments, including on the surfaces of inanimate objects such as hospital instruments and equipment.[1,2,3,4] Furthermore, they have a high propensity to acquire antimicrobial resistance and cause a wide range of hospital-acquired infections (HAIs).[1,2,3,4] Within the genus Serratia, researchers have identified over 14 species, with eight of them known to cause human infections. Serratia marcescens accounts for the majority of human infections, while S. odorifera, S. liquefaciens, and S. fonticola are less commonly associated with human infections.[5] Over the years, it has been established that the frequency of infection by Serratia is higher in hospitals in developing countries due to inadequate infection control practices, including insufficient sterilization of instruments and equipment, improper water, and food sanitation.[1,6] S. marcescens is implicated in a wide range of serious infections, such as pneumonia, septicemia, peritonitis, wound infection, urinary tract infection, and meningitis, especially in infants, older people, and persons with a weakened immune system.[7,8,9,10] Additionally, in patients with weakened immune systems who are undergoing prolonged immunosuppressive therapy, underwent previous antimicrobial therapy, mechanical ventilation, repeated hemodialysis, and indwelling catheterization, the risk of severe infection increases.[11] A recent study reported more frequent isolation of S. marcescens from blood (35.4%) and sputum samples (24.6%), suggesting its more frequent association with bloodstream and respiratory infections.[12] Keratitis and endocarditis are less frequently associated infections, especially among contact lens users and intravenous drug users, respectively.[12,13,14]
Treating S. marcescens infection is increasingly becoming difficult because of their exceptional ability to acquire, transfer, and modify the expression of multiple antimicrobial-resistant genes.[2] Recent epidemiologic reports reveal an increase in the rate of antimicrobial resistance among S. marcescens strains.[11] They exhibit resistance to a wide range of antibiotics, such as beta-lactams, macrolides, aminoglycosides, fluoroquinolones, tetracyclines, and carbapenems, and the resistance reflects the pattern of antimicrobial use.[2,15] Plasmid-mediated resistance gene transfer is the major attributing factor for the emergence and dissemination of multiple drug-resistant strains. Like other Enterobacterales, Serratia also acquires resistance genes encoding for extended-spectrum beta-lactamases, carbapenemases, and drug-modifying enzymes.[16,17] The emergence of these multidrug-resistant strains has posed serious challenges, like frequent treatment failures with adverse outcomes.[11]
The rate and pattern of antimicrobial resistance among S. marcescens and other species of Serratia show wide geographic variation. In Oman, studies related to infections caused by S. marcescens and other species of Serratia are limited. Our study explored the risk factors, clinical characteristics, outcome of infection, and antibiotic resistance profile of Serratia spp. recovered from clinical samples of patients who were diagnosed with Serratia infection at a secondary-care hospital in the northern region of Oman during the period from January 2017 to December 2021.
MATERIALS AND METHODS
This retrospective study was conducted at Sohar Hospital, Oman, in collaboration with the College of Medicine and Health Sciences (COMHS), National University of Science and Technology. The study was approved by the institutional review and ethical committee as well as by the Research Ethical Review and Approval Committee (RERAC), Ministry of Health, Oman [approval number: MH/DGHS/NBG/RERAC21/2022]. The study included Serratia isolates recovered from clinical samples of patients during their visit or admission to Sohar Hospital from January 2017 to December 2021. The relevant data of the study subjects, such as demographics, clinical characteristics, risk factors, outcomes of infections, and antibiotic susceptibility patterns of isolated Serratia species, was extracted from the Sohar Hospital's electronic health records. The confidentiality of the patient's details was strictly maintained.
Inclusion criteria: Serratia species isolated from patients with clinical evidence of infection, confirmed by positive bacterial culture and laboratory investigations, were included in the study. Exclusion criteria: Serratia species isolated from patients without clinical evidence of infection were considered probable contamination and excluded. We also excluded patients with incomplete data from the study.
Bacterial identification and antimicrobial susceptibility testing
Clinical samples received at the Sohar microbiology laboratory were inoculated onto MacConkey and blood agar and incubated at 37°C for 18 to 24 hours. The isolated growth was identified by preliminary tests such as the morphological characteristics of the bacterial colony and the Gram stain. Standard guidelines of the Clinical Laboratory Standards Institute (CLSI)[18] were followed to identify the Serratia isolates up to the species level using biochemical tests including indole, methyl red, Voges-Proskauer, and citrate (IMViC), catalase, oxidase, motility, urease, DNAse, gelatinase production, urease, hydrogen sulfide production, arabinose, lysine, and ornithine decarboxylase tests. We further screened the Serratia species for antibiotic susceptibility using the Kirby-Bauer disk diffusion method. The antibiotic disks ampicillin, amoxicillin-clavulanic acid, piperacillin-tazobactam, cefuroxime, cefotaxime, ceftazidime, ciprofloxacin, gentamicin, amikacin, imipenem, and meropenem procured from Oxoid Ltd. in the United Kingdom were used for antibiotic susceptibility testing. The zone of inhibition diameter was interpreted as susceptible (S), intermediate (I), and resistance (R) according to CLSI guidelines.[18] We performed broth microdilution or Epsilometer (E) tests to determine the minimum inhibitory concentrations of colistin and tigecycline. Multidrug-resistance Serratia species were classified by non-susceptibility to at least one agent of three or more antimicrobial classes by standard susceptibility testing methods.[18,19] Serratia strains that have shown non-susceptibility to carbapenems by standard susceptibility testing methods (i.e. a minimum inhibitory concentration of ≥4 mg/mL for imipenem and/or meropenem) were categorized as carbapenem-resistant strains. Initially, we screened the Serratia strains for ESBL production by testing their resistance to ceftazidime. The screen-positive strains were further confirmed for ESBL production by the double-disc synergy test (DDST) and the CLSI confirmatory test.[18]
Statistical analysis
Data was entered in Microsoft Excel and analyzed using the statistical software STATA version 14 [StatCorp LCC, College Station, TX, USA]. We summarized categorical variables as frequencies and proportions. Quantitative variables were summarized as mean with SD (standard deviation) or median with IQR (interquartile range). The statistical significance of the association between the outcome and the categorical risk factors was assessed using the Chi-Square test or Fisher's Exact test as per the assumptions for the test. A P value less than 0.05 is considered significant.
RESULTS
A total of 257 non-duplicate Serratia species isolated from clinical samples of patients who have been treated at Sohar Hospital from January 2017 to December 2021 were studied. Table 1 summarizes the baseline characteristics of the study population. Males (51.4%) had a higher frequency of Serratia species isolation compared to females (48.6%). The frequency of isolation was highest (29.4%) in old people aged > 60 years and in infants (28.0%). The infection was less common among individuals aged 1–20 years (7%), 21–40 years (13.2%), and 41–60 years (21.4%).
Table 1.
Baseline characteristics of the study population (n=257)
| Characteristics | S. marcescens: Number (n) & percentage (%) | Other species*: Number (n) & percentage (%) | Total isolates Number (n) & percentage (%) |
|---|---|---|---|
| Number of isolates | 204 (79.4) | 53 (20.6) | 257 (100) |
| Age distribution (years) | |||
| <1 | 60 (29.4) | 12 (22.6) | 72 (28.0) |
| 1-20 | 18 (8.8) | 1 (1.89) | 19 (7.4) |
| 21-40 | 25 (12.3) | 9 (17.0) | 34 (13.2) |
| 41-60 | 43 (21.1) | 12 (22.6) | 55 (21.4) |
| >60 | 56 (27.5) | 19 (35.8) | 75 (29.4) |
| Gender distribution | |||
| Male | 108 (52.9) | 24 (45.3) | 132 (51.4) |
| Female | 96 (47.1) | 29 (54.7) | 125 (48.6) |
| MDRO (Multidrug-resistant organisms) | |||
| MDR** | 18 (8.8) | 12 (22.6) | 30 (11.7) |
| CR-Serratia*** | 3 (1.5) | 2 (3.8) | 5 (1.9) |
| ESBL**** | 3 (1.5) | 3 (5.7) | 6 (2.3) |
| Non-MDR | 180 (88.2) | 36 (67.9) | 216 (84.0) |
*Other species includes S. liquifaciens (11), S. fonticuli (4), S. odorifera (1), S. ficaria (1), and other unidentified species (36). **MDR: Multidrug-resistant, ***CR-Serratia: Carbapenem-resistant Serratia, ****ESBL: Extended-spectrum beta-lactamases
The majority of the patients had one (36.3%) or multiple (35.3%) comorbidities. Cardiovascular comorbidities, including hypertension, were observed in 28.4% of the studied subjects. Diabetes mellitus (24.7%), renal (10.5%), central nervous system (10.5%), renal (10.5%), and respiratory (4.2%) comorbidities were amongst others. Coinfection with COVID-19 was observed in 8.4% of the studied subjects [Table 2]. S. marcescens was the predominantly isolated species (79.4%), followed by S. liquefaciens (4.3%), S. fonticuli (1.6%), S. ficaria (0.4%), and S. odorifera (0.4%). While 14% were other unidentified Serratia species, Figure 1 depicts the frequency of isolation of Serratia species from different clinical samples. The majority of the strains were isolated from blood (24.2%), pus (23.3%), urine (20.7%), and endotracheal aspirate (12.8%). Less frequently isolated from sputum (8.4%), tissue fragments (3.1%), eye secretions (2.6%), and catheter tips (1.3%). The antimicrobial resistance pattern of Serratia species is shown in Table 3. Overall, high resistance was shown towards ampicillin (99.5%), cefuroxime (99.1%), amoxicillin-clavulanic acid (98.6%), and ceftazidime (48%). By contrast, low resistance was observed against imipenem (8.1%), meropenem (7.6%), trimethoprim-sulfamethoxazole (6.5%), ciprofloxacin (7.6%), gentamicin (9.3%), and amikacin (7.5%). All tested strains showed susceptibility (100%) to tigecycline. A minimum of the strains are multidrug-resistant (11.7%), carbapenem-resistant (2.3%), and extended-spectrum beta-lactamase producers (1.9%). [Table 1]. Table 4 reveals the association between mortality and risk factors. The higher mortality rate (P < 0.05) was observed among patients who had septicemia and/or pneumonia, COVID-19 patients with secondary bacterial infection, patients exposed to mechanical ventilation, and patients who had a history of repeated hemodialysis.
Table 2.
Comorbidities and risk factors (n=190)
| Comorbidities and risk factors* | % (n) |
|---|---|
| One comorbidity | 36.3 (69) |
| More than one comorbidities | 35.3 (67) |
| Type of comorbidity | |
| Diabetes Mellitus | 24.7 (47) |
| Cardiovascular | 28.4 (54) |
| Respiratory | 4.2 (08) |
| Renal | 10.5 (20) |
| Central nervous system | 10.5 (20) |
| COVID-19 | 8.4 (16) |
| Risk factors | |
| Mechanical ventilation | 36.8 (70) |
| Hemodialysis | 5.8 (11) |
| Central venous catheterization | 2.6 (5) |
| Urinary catheterization | 21.6 (41) |
| Prematurity/Low birth weight | 19.5 (37) |
*The data was not found in 67 patients
Figure 1.
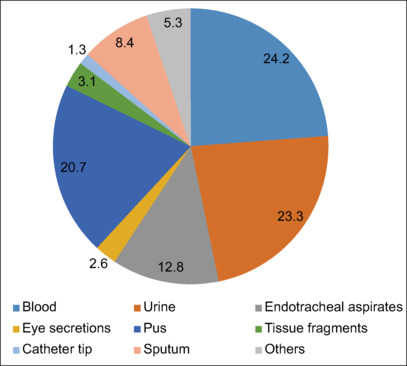
Source/site of Serratia species isolation (n = 257)
Table 3.
Antibiotic susceptibility profile of Serratia species
| Antibiotic | Antibiotic susceptibility percentage (%) and number (n) |
||
|---|---|---|---|
| S. marcescense | Other species | Total | |
| Amikacin | 91.5 (75/82) | 95.8 (23/24) | 92.5 (98/106) |
| Gentamicin | 91.2 (177/194) | 88.5 (46/52) | 90.7 (223/246) |
| Ampicillin | 0.6 (1/160) | 0 (0/48) | 0.5 (1/208) |
| Amoxicillin-clavulanic acid | 1.2 (2/164) | 2.2 (1/45) | 1.4 (3/209) |
| Cefotaxime | 71.6 (101/141) | 70.3 (26/37) | 71.3 (127/178) |
| Cefuroxime | 1.1 (2/176) | 0 (0/49) | 0.9 (2/225) |
| Ceftazidime | 55.4 (31/56) | 42.1 (8/19) | 52.0 (39/75) |
| Piperacillin-tazobactam | 94.2 (98/104) | 93.5 (29/31) | 94.1 (127/135) |
| Ciprofloxacin | 94.8 (184/198) | 90.6 (48/53) | 92.4 (232/251) |
| Nalidixic acid | 76.0 (19/25) | 57.1 (4/7) | 71.9 (23/32) |
| Imipenem | 95.3 (61/64) | 81.8 (18/22) | 91.9 (79/86) |
| Meropenem | 95.6 (65/68) | 83.3 (20/24) | 92.4 (85/92) |
| Trimethoprim-sulfamethoxazole | 94.2 (147/156) | 90.7 (39/43) | 93.5 (186/199) |
| Chloramphenicol | 94.4 (34/36) | 100 (8/8) | 95.5 (42/44) |
| Tetracycline | 62.9 (22/35) | 83.3 (5/6) | 65.9 (27/41) |
| Tigecycline | 100 (8/8) | 100 (2/2) | 100 (10/10) |
Table 4.
Association of risk factors and outcome of infection
| Character | Death | Recovery | P |
|---|---|---|---|
| Septicemia and/or pneumonia | |||
| No septicemia/pneumonia | 12 (10.6) | 101 (89.4) | <0.001 |
| Septicemia/pneumonia | 43 (51.2) | 41 (48.8) | |
| Covid-19 with secondary bacterial infection | |||
| Bacterial infection without COVID-19 | 45 (24.8) | 136 (75.2) | <0.001 |
| Bacterial infection with COVID-19 | 10 (62.5) | 6 (37.5) | |
| Type of pathogens caused infection | |||
| Infection by Non-MDR strains | 46 (27.8) | 119 (72.2) | 0.977 |
| Infection by MDR-strains | 9 (28.1) | 23 (71.9) | |
| Mechanical ventilation | |||
| Patients without mechanical ventilation | 1 (4.0) | 49 (51.0) | <0.001 |
| Patients with mechanical ventilation | 23 (96.0) | 47 (49.0) | |
| Hemodialysis | |||
| No history of repeated hemodialysis | 11 (73.0) | 75 (91.0) | 0.042 |
| History of repeated hemodialysis | 4 (27.0) | 7 (9.0) | |
| Prematurity/preterm birth | |||
| No prematurity/preterm birth | 12 (71.0) | 52 (62.0) | 0.50 |
| History of prematurity/preterm birth | 5 (29.0) | 32 (38.0) |
DISCUSSION
Serratia are relatively low-virulent, often recognized as common opportunistic nosocomial pathogens in the last 3 decades, and have shown an increase in the rate of antimicrobial resistance. They have a propensity for a wide range of healthcare-associated infections (HAIs), especially in immunocompromised or critically ill patients.[1,6] In our study, nearly 80% of the isolated strains were S. marcescens, more frequently isolated from males and in older and younger patients. This finding is consistent with the results of previous studies.[20,21] Serratia are widespread in the environment and can contaminate hospital instruments and equipment. It is relatively less virulent and does not cause primary invasive diseases. However, they cause infection, often serious if they gain entry into an immunocompromised host. The contributing factors to the spread of nosocomial infections include inadequate infection control practices coupled with the increased frequency of modern medical procedures. The risk of transmission of infection is high through the contaminated hands of healthcare workers and exposure to inadequately sterilized mechanical ventilators, hemodialysis units, central venous lines, urinary catheters, injection needles, and surgical interventions.[9,22] Previous reports have revealed the transmission of Serratia infection through contaminated soap dispensers and because of the inadequate hand hygiene practices of healthcare workers.[23,24] Another study by Mcneal et al. demonstrated a reduction in Serratia infection rates of nearly 80% following adherence to standard hand hygiene practices with alcohol-based hand rubs among healthcare workers, indicating the importance of hand hygiene in infection prevention.[25] Approximately 70% of our study participants had one or more underlying comorbidities and were exposed to medical or surgical interventions. Prematurity in newborns, cardiovascular disease, and diabetes mellitus are the most recognized risk factors. Al Maskari et al. and Gastemier et al. have also reported immaturity and low birth weight (<1500 g) in preterm newborns as the major risk factors for Serratia infection and high mortality.[1,26]
Serratia marcescens is a frequent source of hospital outbreaks (HAIs) in both pediatric and adult patients.[27,28] S. marcescens is implicated in a wide range of infections, including serious conditions like septicemia, pneumonia, and meningitis.[29] Furthermore, contact lens users and premature newborns have a high incidence of ocular infections caused by S. marcescens, including conjunctivitis and keratitis.[30] In our study, we predominantly isolated Serratia spp. from the blood, followed by pus, urine, and respiratory secretions. In similar studies, Liou et al. and Simsek et al. found that Serratia spp. were predominantly isolated from respiratory secretions and urine and from blood and sputum cultures, respectively.[9,12] Another study by Madani et al. identified septicemia, purulent conjunctivitis, urinary tract infection, meningitis, and cellulitis among premature newborns.[31] In line with this, in our study, the majority of preterm newborns treated in the special care baby unit (SCBU) had septicemia (35.9%), while conjunctivitis, keratitis, and urinary tract infection were less frequently noticed.
Serratia strains associated with HAIs have shown resistance to several antibiotics. In fact, one key feature of Serratia is their intrinsic resistance to ampicillin, amoxicillin-clavulanic acid, first-generation cephalosporins, macrolides, and colistin.[2,10] Intrinsic resistance is due to the presence of resistance genes on the chromosome. Furthermore, the acquisition of such chromosomal or plasmid-resistant genes by horizontal transfer is considered the most important event that leads to multiple drug resistances, including resistance to aminoglycosides, fluoroquinolones, and third-generation cephalosporins that encompass the mainstay in the therapy of Serratia infections.[32] Worldwide reports indicate varying rates of resistance to these drugs in Serratia isolates, indirectly reflecting patterns of antimicrobial use.[2,33] Aminoglycoside resistance in Serratia is most frequently attributed to the acquisition of plasmid-mediated aminoglycoside-modifying enzymes, including acetyl- and adenyl transferases. While fluoroquinolone resistance in S. marcescens is attributed to mutations in DNA gyrase, altered porin channels, and an increased efflux pump, Simsek et al. reported low resistance of Serratia strains to piperacillin-tazobactam (19.6%), ceftazidime (19.6%), imipenem (13.2%), meropenem (13.2%), amikacin (6.3%), ciprofloxacin (4.4%), trimethoprim-sulfamethoxazole (2.5%), and gentamicin (0.6%).[12] A different study by Ferriera et al. found that Serratia strains were only 18.5% resistant to gentamicin, 14.8% resistant to amikacin, and 18.5% resistant to ciprofloxacin. Furthermore, all strains showed resistance to beta-lactam antibiotics, and 96.3% of strains were resistant to colistin.[16] Our study results were in concordance with these studies, with low resistance ranging from 8 to 11% to gentamicin, amikacin, ciprofloxacin, trimethoprim-sulfamethoxazole, high resistance to ceftazidime (50%), colistin (70%), and near total resistance (>98%) to ampicillin, amoxicillin-clavulanic acid, and cefuroxime.
In the recent past, third-generation cephalosporins such as ceftazidime have been the mainstay of treatment for S. marcescens infection. However, empirical use of these agents has eroded since S. marcescens, like other Enterobacterales, can overproduce AmpC beta-lactamases and extended-spectrum beta-lactamases (ESBL). More often, the drug of choice for AmpC and ESBL-producing S. marcescens is carbapenems that resist inactivation by chromosomal AmpC and plasmid-mediated ESBL beta-lactamases. However, the increased use of carbapenems to treat multidrug-resistant strains has led to the emergence of carbapenem-resistant S. marcescens. Of greater concern is the expression of plasmid-mediated KPC-class carbapenemases among S. marcescens. Dissemination of these strains in many regions is alarming since they can transmit these enzymes to other beta-lactam antibiotics, mediating high-level resistance.[34,35] Ferreira et al. reported 24% of Serratia strains as MDR, and all strains carried KPC-carbapenemase (blaKPC) and extended-spectrum beta-lactamase blaTEM genes.[16] Congruently, in our study, 17% of the strains were MDRs. Out of them, 3% and 1% were ESBL and carbapenemase producers, respectively. Carbapenems are regarded as last-resort antibiotics to treat infections caused by MDR Enterobacterales, and the development of resistance to carbapenems is alarming as it limits the treatment options. Therefore, prompt detection of carbapenemase-producing Serratia and other Enterobacterales is necessary to limit their spread.
We also investigated the outcome of the Serratia infection. Several independent factors, such as pneumonia, septicemia, old age (>65 years), thrombocytopenia (platelet count < 50000/mm3), hyperbilirubinemia (serum total bilirubin >18 mmol/L), prolonged length of ICU stay, mechanical ventilation, and repeated hemodialysis, are associated with increased mortality among patients infected with Serratia.[11,36,37,38] In line with this, in our study we found a significant association (P < 0.001) between mortality and independent risk factors such as bacteremia/pneumonia, COVID-19, and mechanical ventilation.
Limitations
Our study has several limitations. First, because of the retrospective study design, some of the information related to previous antibiotic therapy, length of hospital stays, serum albumin level, platelet count, infection prevention control practices among healthcare workers, etc., is unexploited. Therefore, we could not relate their role in predicting the transmission and outcome of Serratia infection. Second, the exact cause of death and confounding factors determining mortality were not determined. Third, the study did not determine the antimicrobial resistance genes in ESBL and carbapenem-resistant strains. Finally, the study was a single-center study with a small sample size, and hence, to validate our findings, a large multicenter study is recommended.
CONCLUSIONS
Over the last 2–3 decades, Serratia, especially S. marcescens, has emerged as an important nosocomial pathogen, causing a wide range of HAIs. Serratia strains that are resistant to multiple drugs are becoming more common and are linked to high death rates in immunocompromised patients, like babies and the elderly. Clinicians must evaluate the local microbiology data about the antimicrobial resistance pattern of clinical isolates before the selection of antibiotic therapy. Additionally, adhering to effective infection control practices is crucial to reducing the disease burden and limiting the emergence and dissemination of resistant strains.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors express their gratitude to Mr. Jaya Prasad (Information and Technology Personal) as well as all the microbiology laboratory staff of Sohar Hospital for their indispensable assistance in data collection and completing this study.
REFERENCES
- 1.Al-Maskari Z, Al-Hinnai M, Al-Ghabshi L, Panchatcharam SM, Al-Rashdi A, Al-Jardani A. Rapid control of Serratia marcescens outbreak in neonatal intensive care unit, Oman. Infect Dis Diag Treat. 2022;6:191. [Google Scholar]
- 2.Moradigaravand D, Boinett CJ, Martin V, Peacock SJ, Parkhill J. Recent independent emergence of multiple multidrug-resistant Serratia marcescens clones within the United Kingdom and Ireland. Genome Res. 2016;26:1101–9. doi: 10.1101/gr.205245.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali F, Wu J, Kc D. A sixty-nine-year-old female with Serratia marcescens infection. Cureus. 2023;15:e49985. doi: 10.7759/cureus.49985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millán-Lou MI, López C, Bueno J, Pérez-Laguna V, Lapresta C, Fuertes ME, et al. Successful control of Serratia marcescens outbreak in a neonatal unit of a tertiary-care hospital in Spain. Enferm Infecc Microbiol Clin (Engl Ed) 2022;40:248–54. doi: 10.1016/j.eimce.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Williams DJ, Grimont PAD, Cazares A, Grimont F, Ageron E, Pettigrew KA. The genus Serratia revisited by genomics. Nat Commun. 2022;13:5195. doi: 10.1038/s41467-022-32929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prashad Y. A Critical review of the bacteria Serratia marcescens and its impact on human health. Webmedcentral BACT. 2019;10:WMC005573. [Google Scholar]
- 7.Prakash KP, Arora V, Geethanjali PP. Bloodstream Bacterial Pathogens and their Antibiotic Resistance Pattern in Dhahira Region, Oman. Oman Med J. 2011;26:240–7. doi: 10.5001/omj.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghaith DM, Zafer MM, Ismail DK, Al-Agamy MH, Bohol MFF, Al-Qahtani A, et al. First reported nosocomial outbreak of Serratia marcescens harboring bla IMP-4 and bla VIM-2 in a neonatal intensive care unit in Cairo, Egypt. Infect Drug Resist. 2018;11:2211–7. doi: 10.2147/IDR.S174869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q, Zheng B, Li K, Shen P, Xiao Y. A preliminary exploration on the mechanism of the carbapenem-resistance transformation of Serratia marcescens in vivo. BMC Genomics. 2024;25:2. doi: 10.1186/s12864-023-09904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristina ML, Sartini M, Spagnolo AM. Serratia marcescens Infections in Neonatal Intensive Care Units (NICUs) Int J Environ Res Public Health. 2019;16:610. doi: 10.3390/ijerph16040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SB, Jeon YD, Kim JH, Kim JK, Ann HW, Choi H, et al. Risk factors for mortality in patients with Serratia marcescens bacteremia. Yonsei Med J. 2015;56:348–54. doi: 10.3349/ymj.2015.56.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simsek M. Determination of the antibiotic resistance rates of Serratia marcescens isolates obtained from various clinical specimens. Niger J Clin Pract. 2019;22:125–30. doi: 10.4103/njcp.njcp_362_18. [DOI] [PubMed] [Google Scholar]
- 13.Bôas VTV, Almeida Júnior GC, Almeida MTG, Gonçalves MS, Coelho LF. Microbiological analysis of contact lens cases: Impact of the hospital environment. Arq Bras Oftalmol. 2018;81:371–5. doi: 10.5935/0004-2749.20180074. [DOI] [PubMed] [Google Scholar]
- 14.Elkattawy S, Mohammadian M, Williams N, Mowafy A, Ayad S, Noori MAM, et al. Serratia marcescens endocarditis. Cureus. 2021;13:e17346. doi: 10.7759/cureus.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia J, Huang L, Zhang L, Sheng Y, Chu W, Xu H, et al. Genomic characterization of two carbapenem resistant Serratia marcescens isolates causing bacteremia: Emergence of KPC-2- encoding IncR plasmids. Front Cell Infect Microbiol. 2023;13:1075255. doi: 10.3389/fcimb.2023.1075255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira RL, Rezende GS, Damas MSF, Oliveira-Silva M, Pitondo-Silva A, Brito MCA, et al. Characterization of KPC-Producing Serratia marcescens in an Intensive Care Unit of a Brazilian Tertiary Hospital. Front Microbiol. 2020;11:956. doi: 10.3389/fmicb.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omololu-Aso J, Awoderu BO. Extended spectrum β-Lactamase mobile gene encoded variants CTX-M, TEM, SHV, AmPC and FOX among Serratia marcescens, Ile- Ife South Western Nigeria. Int J Immunol Immunother. 2021;7:058. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute . 32nd. Wayne, PA: Clinical and Laboratory Standards for Antimicrobial Susceptibility Testing; 2022. Performance CLSI Supplement M100. Standards Institute. [Google Scholar]
- 19.Alkofide H, Alhammad AM, Alruwaili A, Aldemerdash A, Almangour TA, Alsuwayegh A, et al. Multidrug-resistant and extensively drug-resistant enterobacteriaceae: Prevalence, treatments, and outcomes-A retrospective cohort study. Infect Drug Resist. 2020;13:4653–62. doi: 10.2147/IDR.S283488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daoudi A, Benaoui F, El-Idrissi SN, Soraa N, Rabou-Maoulainine FM. An outbreak of Serratia marcescens in a Moroccan neonatal intensive care unit. Adv Med. 2018;2018:4867134. doi: 10.1155/2018/4867134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zivkovic ZR, Zaric M, Sekulic M, Zornic N, Nesic J, Rosic V, et al. Antimicrobial treatment of Serratia marcescens invasive infections: Systematic review. Antibiotics (Basel) 2023;12:367. doi: 10.3390/antibiotics12020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen DB, Arduino MJ, Patel PR. Hemodialysis-Associated Infections. Chronic Kidney Disease, Dialysis, and Transplantation. 2019:389–410.e8. [Google Scholar]
- 23.World Health Organization (WHO) WHO Guidelines on Hand Hygiene in Health Care. Available from: https://www.who.int/publications/i/item/9789241597906 . [Last accessed on 2024 Jan 30] [Google Scholar]
- 24.Lompo P, Heroes A-S, Agbobli E, Kühne V, Tinto H, Affolabi D, et al. Bacterial contamination of antiseptics, disinfectants and hand hygiene products in healthcare facilities in high-income countries: A scoping review. Hygiene. 2023;3:136–75. [Google Scholar]
- 25.Guel-Gomez M, Angulo-Zamudio UA, Leon-Sicairos N, Flores-Villaseñor H, Mendívil-Zavala E, Plata-Guzmán A, et al. Outbreak of Serratia marcescens in the neonatal intensive care unit of a tertiary care hospital in Mexico. Adv Med. 2023:3281910. doi: 10.1155/2023/3281910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Feng R, Song X, Zhao H. Rare post-operative intracranial abscess due to Serratia marcescens: What we can learn from it? BMC Infect Dis. 2024;24:61. doi: 10.1186/s12879-023-08966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourdin T, Benoit MÈ, Monnier A, Bédard E, Prévost M, Charron D, et al. Serratia marcescens colonization in a neonatal intensive care unit has multiple sources, with sink drains as a major reservoir. Appl Environ Microbiol. 2023;89:e0010523. doi: 10.1128/aem.00105-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakash GK, John G, Hansrivijit P, Divya B, Ellen-Cook BA, Kathryn E, et al. Severe skin infections due to Serratia marcescens: A case associated with cat scratch in a patient with liver disease and review of the literature. Infect Dis Clin Pract. 2021;29:e146–50. [Google Scholar]
- 29.Gupta N, Hocevar SN, Moulton-Meissner HA, Stevens KM, McIntyre MG, Jensen B, et al. Outbreak of Serratia marcescens bloodstream infections in patients receiving parenteral nutrition prepared by a compounding pharmacy. Clin Infect Dis. 2014;59:1–8. doi: 10.1093/cid/ciu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghaith DM, Zafer MM, Ismail DK, Al-Agamy MH, Bohol MFF, Al-Qahtani A, et al. First reported nosocomial outbreak of Serratia marcescens harboring blaIMP-4 and blaVIM-2 in a neonatal intensive care unit in Cairo, Egypt. Infect Drug Resist. 2018;11:2211–7. doi: 10.2147/IDR.S174869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madani TA, Alsaedi S, James L, Eldeek BS, Jiman-Fatani AA, Alawi MM, et al. Serratia marcescens-contaminated baby shampoo causing an outbreak among newborns at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. J Hosp Infect. 2011;8:16–19. doi: 10.1016/j.jhin.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Birk T, Fuentes MAF, Aabo S, Jensen LB. Horizontal transmission of antimicrobial resistance genes from E. coli to Serratia spp. in minced meat using a gfp tagged plasmid. Res Vet Sci. 2020;132:481–4. doi: 10.1016/j.rvsc.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Yang HF, Cheng J, Hu LF, Ye Y, Li JB. Plasmid-mediated quinolone resistance in extended-spectrum-β-lactamase- and AmpC β-lactamase-producing Serratia marcescens in China. Antimicrob Agents Chemother. 2012;56:4529–31. doi: 10.1128/AAC.00493-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tavares-Carreon F, De Anda-Mora K, Rojas-Barrera IC, Andrade A. Serratia marcescens antibiotic resistance mechanisms of an opportunistic pathogen: A literature review. Peer J. 2023;11:e14399. doi: 10.7717/peerj.14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herra C, Falkiner FR. Serratia marcescens. Antimicrobe Microbes. 2018 Available from: http://www.antimicrobe.org/b26.asp . [Last accessed 2023 Oct 02] [Google Scholar]
- 36.Gomes-Cochicho J, Silva JM, Viegas M. Infection of multiple tunneled dialysis catheters resulting from the contamination of the chlorhexidine solution by Serratia marcescens. Cureus. 2023;15:e45693. doi: 10.7759/cureus.45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulu-Kilic A, Alp E, Orhan T, Cevahir F, Ersoy S, Altun D, et al. Clustering of Serratia marcescens infections during six years: Epidemiology and risk factors for mortality. Can J Inf Contr. 2017;32:104–7. [Google Scholar]
- 38.Patton MJ, Orihuela CJ, Harrod KS, Bhuiyan MAN, Dominic P, Kevil CG, et al. COVID-19 bacteremic co-infection is a major risk factor for mortality, ICU admission, and mechanical ventilation. Crit Care. 2023;27:34. doi: 10.1186/s13054-023-04312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


