Abstract
Background:
Chronic stress can lead to anxiety and depression. Escitalopram is a selective serotonin reuptake inhibitor (SSRI), and crocin is a natural compound derived from saffron. Both of them are used to treat these disorders in clinical and traditional medicine, respectively. This study compared the antidepressant and anxiolytic effects of escitalopram, crocin, and their combination in rats.
Materials and Methods:
Rats were divided into nine groups: control, sham, rest-depression, depression-rest, depression-crocin, depression-escitalopram10, depression-escitalopram20, depression-escitalopram10-crocin, and depression-escitalopram20-crocin. Forced swimming and open field tests (FST and OFT, respectively) were used to evaluate depression, anxiety, and locomotor activity.
Results:
In the FST, the immobility time on day 28 significantly decreased in all depressed groups that received escitalopram, crocin, and their combination compared to the rest-depression group. Whereas, conversely, the time spent at the center in the OFT was significantly higher in similar comparisons. The total distance traveled by the OFT was significantly lower in all depressed groups, except for the depression-escitalopram10 and depression-escitalopram20 groups. The total distance traveled was significantly higher in the depression-escitalopram20 compared to the rest-depression group.
Conclusion:
Crocin, both doses of escitalopram and their combination, reduced depression. A high dose of escitalopram, with and without crocin, was partially more effective than a low dose of escitalopram in reversing depression. There was anxiety-like behavior observed after inducing depression with and without a recovery period. Whereas, crocin alone and both doses of escitalopram, with and without crocin, decreased anxiety-like behaviors in subjects with depression. This effect may be attributed to a modulation of brain neurotransmitter ratios.
Keywords: Crocin, depression, escitalopram, immobilization, stress
INTRODUCTION
Anxiety and depression are two highly prevalent psychological mood disorders in modern society, and they significantly impact people's quality of life.[1,2] Chronic stress has been identified as one of the most significant factors that can trigger these disorders.[3] Moreover, anxiety and depression are associated with dysregulation of the serotonergic system in the brain and mood impairments. Therefore, maintaining serotonin at a normal level can improve mood disorders.[4] Serotonin reuptake inhibitors (SSRIs) increase serotonin levels in various regions of the brain and are commonly prescribed as the initial treatment for chronic stress, anxiety, and depression.[5] Escitalopram is one of the newest SSRI antidepressants indicated for improving anxiety and depression.[6]
On the other hand, certain herbal drugs, such as saffron (Crocus Sativus) have a significant impact on behavior and mood.[7] Crocin is the most biologically active component of saffron[8] that can affect the synthesis of serotonin in the nervous system.[9] It seems that crocin can improve stress, anxiety, and depression.[8,10] Therefore, both escitalopram and crocin affect serotonin secretion through different mechanisms, which have an impact on mood and behavior.[11] Most individuals may need to take medication after experiencing a period of depression. Whereas, some individuals may prefer to avoid medication and believe that they can enhance their well-being by going through a recovery period after reducing stress.[12,13] Furthermore, the effect of the recovery period on reducing anxiety and depressive-like behavior is unclear when compared to natural and chemical medications.
Nowadays, it is crucial to discover new pharmacological approaches that have minimal side effects on the body's physiological system to effectively manage anxiety and depression in individuals. Moreover, despite the various studies conducted on the effects of crocin and escitalopram individually on anxiety and depression, there are no reports comparing the antidepressant and anxiolytic effects of the recovery period, escitalopram, crocin, and their combination in rats with depression. Since natural medications have lower side effects compared to chemical medications, there are specific novel aspects to consider, such as investigating combination therapy. This approach provides an experimental perspective to determine the synergistic potential of natural medications in terms of their antidepressant and anxiolytic effects. This approach might offer a more effective and comprehensive treatment strategy for individuals with anxiety and depression. A second novel aspect of the present study is a well-established rat model of anxiety and depression utilized. This allows for a controlled and reproducible assessment of the effects of escitalopram, crocin, the recovery period, and their combination. The final novel aspect is the comprehensive evaluation that the present study employs, using a range of behavioral measures to assess the antidepressant and anxiolytic effects of the treatments. Overall, the study contributes to the expanding body of research on alternative and complementary treatment approaches for anxiety and depression. Therefore, the present study aimed to investigate the comparative evaluation of the antidepressant and anxiolytic effects of escitalopram, crocin, and their combination in rats.
MATERIALS AND METHODS
Experimental animals
Seventy-two male Wistar adult rats (200–250 g) were obtained from the Isfahan University of Medical Sciences. The rats were housed in an environment with a temperature (23 ± 2°C), light conditions (12 h light from 07:00 to 19:00), and humidity (50% ± 5%). Four rats were housed together in each cage as a colony group. All behavioral studies were conducted in the afternoon from 14:00 to 16:00 and lasted for 28 days. The Ethics Committee for Animal Use at Isfahan University of Medical Sciences approved the study (IR.MUI.MED.REC.1398.606). After a week of adaptation, the animals were randomly assigned to nine groups (n = 8 in each): Control (Con) group, in which the rats received no special treatment. Sham injection (Sh) group, in which the rats received normal saline for the next 14 days. Rest-depression (Rest-Dep) group, in which rats had no special treatment for 14 days, and then chronic restraint stress was applied for 6 h/day for the next 14 days. Depression-rest (Dep-Rest), in which chronic restraint stress for 6 h/day for 14 days, followed by a next 14-day rest (recovery) period. Depression-crocin (Dep-Cr) group, in which chronic restraint stress was applied 6 h/day for 14 days, then the rats received daily injections of crocin for the next 14 days. Depression-Escitalopram10 (Dep-Esc10) and Depression-Escitalopram20 (Dep-Esc20) groups, in which chronic restraint stress was applied 6 h/day for 14 days, then the rats received daily injections of escitalopram (10 and 20 mg/kg, respectively, in each group) for the next 14 days. Depression-Escitalopram10-Crocin (Dep-Esc10-Cr) and Depression-Escitalopram20-Crocin (Dep-Esc20-Cr) groups, in which chronic restraint stress was applied 6 h/day for 14 days, then the rats received daily injections of crocin (30 mg/kg) and escitalopram (10 and 20 mg/kg, respectively, in each group) for the next 14 days [Figure 1].
Figure 1.
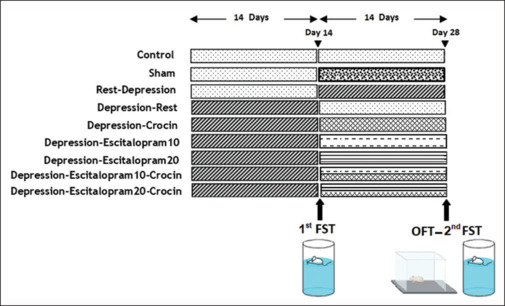
Schematic diagram of the experimental protocol designs. There were two types of behavioral tests, including the forced swimming test (FST) and the open field test (OFT)
Experimental procedures
Depression paradigm
To induce depression, rats were placed in cylindrical restrainers made of Plexiglas for 6 h/day for 14 consecutive days (from 8:00 to 14:00).[11,14]
Chemicals and reagents
The depressed animals received 30 mg/kg of crocin (i.p; Sigma Aldrich Company, America), as well as 10 and 20 mg/kg of escitalopram (Sobhan Daru Co., Iran), dissolved in normal saline for the next 14 consecutive days.[11]
Behavioral paradigms
Forced swimming test
The forced swimming test (FST) is one of the most valid and standardized tests for evaluating depression in rodents.[15] The FST is also used to evaluate the potential antidepressant effects of drugs.[16] A cylindrical glass container with a diameter of 45 cm and a height of 80 cm was used for FST. This cylindrical glass container was filled with water, with a height of 50 cm and a temperature of 23 ± 2°C. Therefore, an animal cannot touch the bottom of the container with its hind legs or tail. Twenty-four hours before the main test, the rat was immersed in this apparatus for 10 minutes to adapt.[17,18] According to a previous study, animals that are subjected to chronic stress and are unable to escape from the situation eventually lose hope of escaping and become immobile over time. Hence, the animal becomes helpless and immobile.
In the FST, immobility time was identified as a significant indicator for measuring the level of depression. An increase in immobility time was considered a reliable indicator of depression induction.[19] On days 14 and 28 of the experiment, the behavior of the rats was recorded by the camera for 300 s. After each session, each rat was carefully placed in a cylindrical container filled with water. Finally, the water in the cylindrical container was changed to prevent it from affecting the next rat. After verifying depression using the FST, the rats were included in the experimental protocol. After testing, the rats were dried with a towel and then transferred back to the storage cage.[20] Notably, in this study, animals conducted to 6 h/day of restraint stress for 14 consecutive days resulted in a success rate of 80-90% in inducing depression through chronic stress prior to the main experiment. In addition, a pilot study was conducted to determine the most effective method of inducing depression with the fewest number of failed animal subjects. However, there are several reasons for the absence of induced depression in certain rats. One of the main reasons is individual differences in stress response due to genetic predispositions and individual coping mechanisms.[21] Some subjects may have a greater innate ability to cope with stressful situations. In addition, the FST is a behavioral test for depression, but it is not a direct measure of depression. Also, rats are prey animals, and they may evolve to cope with stress. This may make them less susceptible to developing depression than each other.[22]
Open field test
The open field test (OFT) was used to assess anxiety-like behavior and locomotor activity. The open field apparatus had dimensions of 90 × 90 × 50 cm. Some grid lines were used to divide each open field into a central part and surrounding regions using a computer. The distance traveled and the time spent in the center during 300 s were measured as indicators of locomotor activity and anxiety levels, respectively.[23] In this study, on days 14 and 28, the OFT was performed for all experimental groups.
Statistical analysis
All behavioral data for days 14 and 28 were separately analyzed using a one-way ANOVA test, followed by Tukey's post-hoc test for comparing multiple groups. Paired sample t-test was used to analyze the immobility times between days 14 and 28 for within-group comparisons only. Furthermore, all data estimated as means ± SEM and P value < 0.05 were considered statistically significant. The computations were executed using SPSS software version 26.0.
RESULTS
In the current study, there was no significant difference between the Con and Sh groups in all behavioral tests. Hence, the Con group was selected as a reference for the following comparisons. In addition, the different experimental groups were also compared to each other.
Assessment of immobility time in the FST
In the FST, the ANOVA assigned different significant levels in immobility time on day 14, F (8,63) = 45.117, P = 0.000; and in immobility time on day 28, F (8,63) = 9.811, P = 0.000. Based on the one-way ANOVA analysis on day 14, the immobility time showed significant enhancements in all experimental groups (P < 0.001), except for the Rest-Dep group compared to the Con group on day 14. This indicates that there were depressive-like behaviors in all depression groups, except for the Rest-Dep group, which had a rest period before the induction of depression [Figure 2a].
Figure 2.
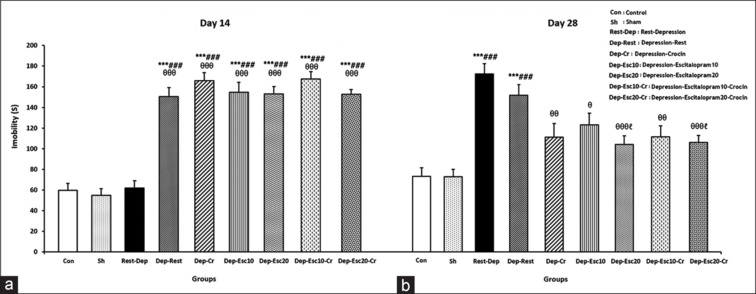
The immobility times of the forced swim test (FST) on days (a) 14 and (b) 28 were measured in all experimental groups (n = 8). Results are expressed as means ± SEM (one-way ANOVA followed by Tukey's post-hoc test). ***P < 0.001 compared to the Con group; ###P < 0.001 compared to the Sh group; ϴP < 0.05, ϴϴP < 0.01 and ϴϴϴP < 0.001 compared to the Rest-Dep group; ℓP < 0.05 compared to the Dep-Rest group
The immobility time showed significant enhancements in the Rest-Dep and Dep-Rest groups (P < 0.001 in both) compared to the Con group on day 28, indicating that there were still depressive-like behaviors in these groups. Also, on day 28, the immobility time significantly decreased in the Dep-Esc10 (P < 0.05), Dep-Cr, and Dep-Esc10-Cr (P < 0.01 in both), Dep-Esc20 and Dep-Esc20-Cr (P < 0.001 in both) compared to the Rest-Dep group [Figure 2b].
The immobility time showed significant decreases (P < 0.05 in both) decreases in the Dep-Esc20 and Dep-Esc20-Cr groups compared to the Dep-Rest group. This indicates that the administration of escitalopram 20 mg/kg, with and without crocin, was more effective in reducing depression than the recovery period after depression [Figure 2b].
The immobility time on the 14th and 28th day was analyzed using a paired sample t-test to evaluate the changes within each group separately. This was done to determine the trend line of depression induction in each group. Significant differences were detected between days 14 and 28 in some experimental groups of the present study (P < 0.01 in the Dep-Cr, Dep-Esc10, Dep-Esc20 groups; also P < 0.001 in the Rest-Dep, Dep-Esc10-Cr, and Dep-Esc20-Cr groups) (in the Con group, t (7) = -1.692 P = 0.134; in the Sh group, t (7) = -4.012 P = 0.005; in the Rest-Dep group, t (7) = -6.948 P = 0.35; in the Dep-Rest, t (7) =1.038 P = 0.334; in the Dep-Cr groups t (7) =5.417 P = 0.001; in Dep-Esc10 t (7) =5.946 P = 0.001; in Dep-Esc20 t (7) =5.076 P = 0.001; in Dep-Esc10-Cr t (7) =7.316 P = 0.000; and in Dep-Esc20-Cr t (7) =7.932 P = 0.000 [Figure 3].
Figure 3.
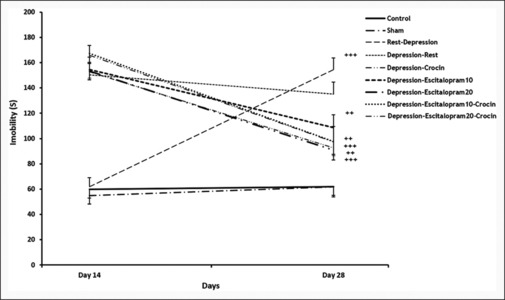
The immobility time on the 14th and 28th day of the forced swim test or FST (within-group) was measured in each group (n = 8). Results are expressed as mean ± SEM (paired sample t-test). ++P < 0.01 and +++P < 0.001 the immobility time of day 14 relative to the immobility time of day 28
Assessment of center spent time in the OFT
In the OFT, the ANOVA assigned different significant levels in center spent time F (8,63) =6.084, P = 0.000). As illustrated in Figure 4, the time spent at the center was significantly lower in the Rest-Dep and Dep-Rest groups (P < 0.01 and P < 0.05, respectively) compared to the Con group. Also, the time spent at the center of those were significantly higher in the Dep-Cr, Dep-Esc10, Dep-Esc20, Dep-Esc10-Cr, and Dep-Esc20-Cr groups (P < 0.05, P < 0.01, P < 0.001, P < 0.01, and P < 0.01, respectively) compared to the Rest-Dep group. In addition, there were significant differences in the center time among the Dep-Esc10, Dep-Esc20, Dep-Esc10-Cr, and Dep-Esc20-Cr groups (P < 0.01 in the Dep-Esc20 group and P < 0.05 in the other groups) compared to the Dep-Rest group [Figure 4].
Figure 4.
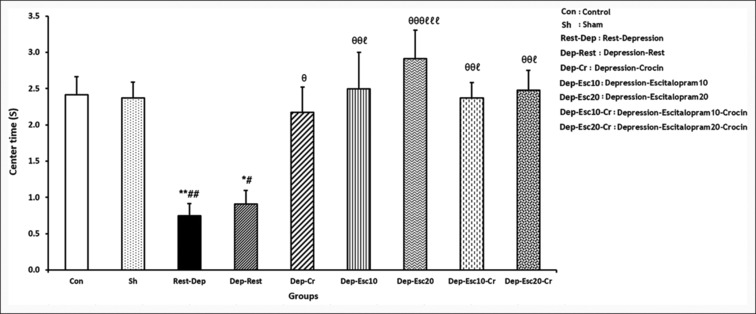
The center time(s) of the open field test (OFT) was measured in all experimental groups. Results are expressed as means ± SEM (one-way ANOVA followed by Tukey's post-hoc test). *P < 0.05 and **P < 0.01 compared to the Con group; #P < 0.05 and ##P < 0.01 compared to the Sh group; ϴP < 0.05, ϴϴP < 0.01 and ϴϴϴP < 0.001 compared to the Rest-Dep group; and ℓP < 0.05 and ℓℓℓP < 0.001 compared to the Dep-Rest group
Assessment of total traveled time in the OFT
In the OFT, the ANOVA assigned different significant levels in the total distance traveled time, F (8,63) = 8.203, P = 0.000. As shown in Figure 5, the total distance traveled was significantly lower in the Rest-Dep, Dep-Rest, Dep-Cr, Dep-Esc10-Cr, and Dep-Esc20-Cr groups (P < 0.001, P < 0.01, P < 0.01, P < 0.05 and P < 0.05, respectively) compared to the Con group. The total distance traveled was significantly higher in the Dep-Esc20 group compared to the Rest-Dep group (P < 0.001). That also significantly increased in the Dep-Esc20 group compared to the Dep-Rest and Dep-Cr groups (P < 0.05 in both) [Figure 5].
Figure 5.
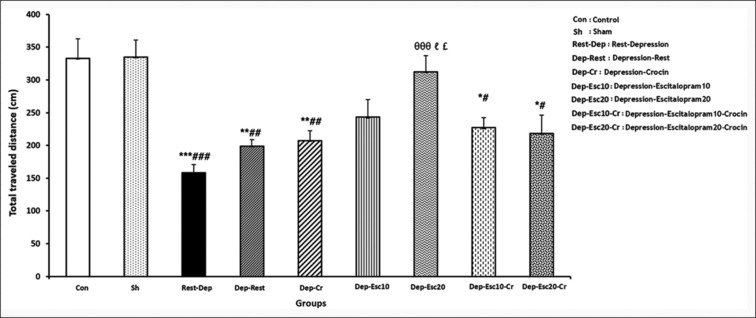
The total distance traveled (in cm) in the open field test (OFT) was measured for all experimental groups (n = 8). Results are expressed as means ± SEM (one-way ANOVA followed by Tukey's post-hoc test). *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the Con group; #P < 0.05, ##P < 0.01, and ###P < 0.001 compared to the Sh group; ϴϴϴP < 0.001 compared to the Rest-Dep group; and ℓP < 0.05 compared to Dep-Rest group; £P < 0.05 compared to Dep-Cr group
DISCUSSION
This study compared the therapeutic effects of different treatments on anxiety, depression, and locomotor activity in male rats. The treatments included a recovery period, two doses of escitalopram (10 and 20 mg/kg), crocin, and the co-administration of both doses of escitalopram with crocin.
The present findings from the FST findings indicated that inducing chronic stress for 14 days resulted in increased despair behavior, suggesting that stress may lead to depression in the experimental subjects. It was demonstrated that depression can be caused by physical, psychological, and neurochemical changes due to chronic stress.[24,25] Some studies reported that 14 days of restraint stress increased depressive-like behavior in rats through changes in hypothalamic-pituitary-adrenal (HPA) axis activity and elevated corticosterone levels.[26,27] On the other hand, a recovery period after depression (without any medication treatment) also showed persistent depressive-like behavior even after a 14-day rest period. In other words, the recovery period alone could not decrease depression. In contrast, Grippo et al. (2003) indicated that chronic stress for four weeks induced depression in rats, and it could be reversed to the baseline levels after a four-week recovery period.[28] These conflicting results may be attributed to a longer recovery period in previous studies compared to the recent study.
Based on other FST findings, it has been observed that various treatments for depression, such as crocin, both doses of escitalopram, and the combination of escitalopram with crocin showed a decrease in depression symptoms. Zhang et al. (2022) demonstrated that crocin reduced depressive-like behavior in the FST.[29] In addition, a dose of 30 mg/day of crocin for eight weeks reversed depressive behaviors.[30] The antidepressant effects of crocin may be attributed to several factors, including changes in the reuptake of neurotransmitters such as dopamine, norepinephrine, and serotonin,[30] the reduction of corticosterone levels,[29,31] and the inhibitory effect of crocin on monoamine neurotransmitters.[29] On the other hand, in the present study, both doses of escitalopram, with and without crocin, were found to reverse depressive-like behavior. However, the dose of 20 mg/kg of escitalopram showed a partial decrease in depression-like behavior that was more effective than the dose of 10 mg/kg. Therefore, crocin had no significant effect on the association with either dose of escitalopram in subjects with depression. In contrast, a study reported that escitalopram (10 mg/kg) did not decrease immobility in subjects with depression.[32] Consistent with these findings, the administration of escitalopram at a dose of 20 mg/kg resulted in a significant decrease in immobility and an increase in swimming behavior in the FST.[32,33] Whereas, Kanekar et al. (2018) reported that escitalopram, at a similar dose, increased immobility in rats under hypoxic stress on the FST.[34] Also, there was a study that reported ineffective or even detrimental effects of SSRI medications.[34] These paradoxes may be related to the type and duration of stress induction and the dosage of SSRIs received. In addition, some studies have also suggested that crocin influences serotonergic pathways by exerting an antagonistic action on the serotonergic receptor site. This may be the reason for the observed effect of crocin in combination with escitalopram.[35,36]
Comparing the immobility time between the 14th and 28th day (within each group and separately) revealed significant differences in the trend of depression-like behavior among the experimental subjects at different levels. There was a proposed beneficial effect of escitalopram, with and without crocin, on reversing the trend of depression. However, this reversing trend was partially better when combining crocin with a dose of 20 mg/kg escitalopram.
Based on the findings of the OFT, anxiety-like behavior was observed both with and without a recovery period following the depression, without receiving any treatment. It seems that a longer duration of the recovery period may be necessary, along with the use of medication, to decrease anxiety-like behavior. Similarly, a study also showed that anxiety-like behaviors persisted after the recovery period in rats.[37] Vyas et al. (2004) suggested that the hypertrophy of the basolateral amygdala did not completely reverse during the recovery period after stress induction. It concluded that certain behavioral changes may persist after the cessation of stress.[37]
Another present finding demonstrated that the administration of crocin, as well as both doses of escitalopram (10 mg/kg and 20 mg/kg), either alone or in combination, resulted in a decrease in anxiety-like behavior in subjects with depression. It should be noted that Ghalandari-Shamami et al. (2019) indicated that crocin decreases anxiety-like behaviors in rats.[38] Another study reported that a sub-chronic regimen of escitalopram at a dose of 5 mg/kg reversed the anxiety and depression induced by single prolonged stress in rats. This effect may be attributed to the impact of escitalopram on serotonin levels in the amygdala.[39] Therefore, the present findings showed that although escitalopram and crocin individually have antidepressant effects, their combination did not demonstrate a significant synergistic effect. Also, those confirmed necessary for natural and specific medication treatment to reduce anxiety-like behaviors. A study reported that treatment with escitalopram prevented anxiety-like behavioral reactivity.[40] Whereas acute administration of escitalopram may increase anxiety.[41] Also, Pinheiro et al. (2008) indicated that the anxiolytic effect of escitalopram appeared at the beginning (acute) and disappeared after 14 days (sub-chronic) of stress, but reappeared after 21 days (chronic).[42] It seems that the initial administration of escitalopram reduces anxiety by decreasing serotonin neurotransmission in the pathway from the raphe nuclei to the amygdala.[42] It is possible that its effect on serotonergic receptors leads to desensitization and finally decreases the anxiolytic impact.[42]
Finally, according to another study by the OFT, locomotor activity was found to be reduced in all subjects with depression who received various treatments, except for those who were administered both doses of escitalopram alone. In support of these findings, some studies showed that depression in the OFT caused a decrease in locomotor activity.[43,44] In the present study, locomotor activity decreased in all subjects receiving crocin, which may have been due to the relaxing effect of crocin. There were different effects of crocin indicated on locomotor activity. For instance, it was reported that crocin can increase, reduce, or have no effect on locomotor activity.[45,46,47,48] In the present study, it appears that the decrease in locomotor activity caused by crocin may be attributed to its relaxing effect on the skeletal muscles in the subjects.[49] In contrast, Hosseinizadeh et al. (2009) did not demonstrate the skeletal muscle relaxant effect of crocin in rodents.[45] Another study showed that crocin, at a dosage of approximately 20 mg/kg, increased locomotor activity due to its stimulating effects on the central nervous system. However, it did not have any significant impact at lower doses.[31] Based on another recent finding, it was discovered that a dose of 20 mg/kg of escitalopram alone increased locomotor activity in individuals with depression. However, a dose of 10 mg/kg of escitalopram had no effect on locomotor activity. The dosage of medication appears to be crucial for locomotor activity responses. A previous study reported that a dose of 10 mg/kg of escitalopram did not affect locomotor activity.[50] In contrast, it was demonstrated that escitalopram, even at a dose of 10 mg/kg, increased locomotor activity.[51] It seems that the type of behavioral tests, the type of rodent, the timing of stress for the induction of depression, and the dose of medication are essential factors for various locomotor activity responses.[43,44,47] However, further investigations are still required to trace and clarify the mechanism(s) involved.
CONCLUSION
To sum up, it is concluded that 14 days of chronic stress-induced depression, and the subsequent recovery period without any medication treatment, could not reverse anxiety and depressive-like behavior. In addition, various treatments for depression, including the administration of crocin, both doses of escitalopram, and their combination, decreased anxiety and depressive behaviors. Although a high dose of escitalopram, with and without crocin, was partially more effective than a low dose in reversing depressive-like behaviors. Crocin also had no significant effect on the association with both doses of escitalopram in subjects with depression. Finally, locomotor activity decreased in all subjects with depression who received different treatments, except for those who were administered only both doses of escitalopram. Locomotor activity decreased in all subjects receiving crocin, which may have been due to the relaxing effect of crocin. Additionally, a dose of 20 mg/kg of escitalopram alone increased locomotor activity in depressed individuals, while a dose of 10 mg/kg had no effect on locomotor activity. The dosage of medication appears to be crucial for locomotor activity responses.
Financial support and sponsorship
The study would not have become possible without the financial support provided by Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This work was supported by grants from the Isfahan University of Medical Sciences in Isfahan, Iran. The present research was conducted with the support received from the Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Shah SMA, Mohammad D, Qureshi MFH, Abbas MZ, Aleem S. Prevalence, psychological responses and associated correlates of depression, anxiety and stress in a global population, during the coronavirus disease (COVID-19) pandemic. Community Ment Health J. 2021;57:101–10. doi: 10.1007/s10597-020-00728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlson F, van Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet. 2019;394:240–8. doi: 10.1016/S0140-6736(19)30934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis MT, Holmes SE, Pietrzak RH, Esterlis I. Neurobiology of chronic stress-related psychiatric disorders: Evidence from molecular imaging studies. Chronic Stress (Thousand Oaks) 2017;1:2470547017710916. doi: 10.1177/2470547017710916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greaney JL, Dillon GA, Saunders EFH, Alexander LM. Peripheral microvascular serotoninergic signaling is dysregulated in young adults with major depressive disorder. J Appl Physiol (1985) 2020;128:100–7. doi: 10.1152/japplphysiol.00603.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, et al. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol Int. 2021;13:387–401. doi: 10.3390/neurolint13030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adjei K, Adunlin G, Ali AA. Impact of sertraline, fluoxetine, and escitalopram on psychological distress among united states adult outpatients with a major depressive disorder. Healthcare (Basel) 2023;11:740. doi: 10.3390/healthcare11050740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monchaux De Oliveira C, Pourtau L, Vancassel S, Pouchieu C, Capuron L, Gaudout D, et al. Saffron extract-induced improvement of depressive-like behavior in mice is associated with modulation of monoaminergic neurotransmission. Nutrients. 2021;13:904. doi: 10.3390/nu13030904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22:16. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui SA, Ali Redha A, Snoeck ER, Singh S, Simal-Gandara J, Ibrahim SA, et al. Anti-depressant properties of crocin molecules in saffron. Molecules. 2022;27:2076. doi: 10.3390/molecules27072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dastgerdi AH, Radahmadi M, Pourshanazari AA, Dastgerdi HH. Effects of crocin on learning and memory in rats under chronic restraint stress with special focus on the hippocampal and frontal cortex corticosterone levels. Adv Biomed Res. 2017;6:157. doi: 10.4103/abr.abr_107_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joodaki M, Radahmadi M, Alaei H. Comparing the therapeutic effects of crocin, escitalopram and co-administration of escitalopram and crocin on learning and memory in rats with stress-induced depression. Malays J Med Sci. 2021;28:50–62. doi: 10.21315/mjms2021.28.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart V. The balance of psychotherapy and pharmacotherapy. Perspect Psychiatr Care. 2000;36:38–58. [PubMed] [Google Scholar]
- 13.Watts M, Dr, Murphy E, Keogh B, Dr, Downes C, Doyle L, Higgins A. Deciding to discontinue prescribed psychotropic medication: A qualitative study of service users’ experiences. Int J Ment Health Nurs. 2021;30(Suppl 1):1395–406. doi: 10.1111/inm.12894. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Xu Y, Yuan X. Validity of chronic restraint stress for modeling anhedonic-like behavior in rodents: A systematic review and meta-analysis. J Int Med Res. 2022;50:3000605221075816. doi: 10.1177/03000605221075816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belovicova K, Bogi E, Csatlosova K, Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip Toxicol. 2017;10:40–3. doi: 10.1515/intox-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker L, Mallien AS, Pfeiffer N, Brandwein C, Talbot SR, Bleich A, et al. Evidence-based severity assessment of the forced swim test in the rat. PLoS One. 2023;18:e0292816. doi: 10.1371/journal.pone.0292816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyko M, Kutz R, Grinshpun J, Zvenigorodsky V, Gruenbaum BF, Gruenbaum SE, et al. The effect of depressive-like behavior and antidepressant therapy on social behavior and hierarchy in rats. Behav Brain Res. 2019;370:111953. doi: 10.1016/j.bbr.2019.111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron LP, Benson CJ, Dunlap LE, Olson DE. Effects of N, N–Dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci. 2018;9:1582–90. doi: 10.1021/acschemneuro.8b00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–57. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015:52587. doi: 10.3791/52587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebner K, Singewald N. Individual differences in stress susceptibility and stress inhibitory mechanisms. Curr Opin Behav Sci. 2017;14:54–64. [Google Scholar]
- 22.Murra D, Hilde KL, Fitzpatrick A, Maras PM, Watson SJ, Akil H. Characterizing the behavioral and neuroendocrine features of susceptibility and resilience to social stress. Neurobiol Stress. 2022;17:100437. doi: 10.1016/j.ynstr.2022.100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimcikova E, Simko J, Karesova I, Kremlacek J, Malakova J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure. 2017;52:35–40. doi: 10.1016/j.seizure.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Lin CC, Yang CP, Cheng PY, Hsiao M, Liu YP. Escitalopram reversibility of the impacts following chronic stress on central 5-HT profiles-Implications to depression and anxiety. Behav Brain Res. 2023;453:114613. doi: 10.1016/j.bbr.2023.114613. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 2017;1:2470547017692328. doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom M, et al. Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. Korean J Physiol Pharmacol. 2013;17:393–403. doi: 10.4196/kjpp.2013.17.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farahbakhsh Z, Radahmadi M. The protective effects of escitalopram on synaptic plasticity in the CA1 region of chronically stressed and non-stressed male rats. Int J Dev Neurosci. 2022;82:748–58. doi: 10.1002/jdn.10224. [DOI] [PubMed] [Google Scholar]
- 28.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–10. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Zhu X, Yu P, Sheng T, Wang Y, Ye Y. Crocin ameliorates depressive-like behaviors induced by chronic restraint stress via the NAMPT-NAD(+)-SIRT1 pathway in mice. Neurochem Int. 2022;157:105343. doi: 10.1016/j.neuint.2022.105343. [DOI] [PubMed] [Google Scholar]
- 30.Jam IN, Sahebkar AH, Eslami S, Mokhber N, Nosrati M, Khademi M, et al. The effects of crocin on the symptoms of depression in subjects with metabolic syndrome. Adv Clin Exp Med. 2017;26:925–30. doi: 10.17219/acem/62891. [DOI] [PubMed] [Google Scholar]
- 31.Alsanie WF, Alamri AS, Abdulaziz O, Salih MM, Alamri A, Asdaq SMB, et al. Antidepressant effect of crocin in mice with chronic mild stress. Molecules. 2022;27:5462. doi: 10.3390/molecules27175462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed AL, Happe HK, Petty F, Bylund DB. Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacology (Berl) 2008;197:433–41. doi: 10.1007/s00213-007-1052-0. [DOI] [PubMed] [Google Scholar]
- 33.Wu R, Xiao D, Shan X, Dong Y, Tao WW. Rapid and prolonged antidepressant-like effect of crocin is associated with GHSR-mediated hippocampal plasticity-related proteins in mice exposed to prenatal stress. ACS Chem Neurosci. 2020;11:1159–70. doi: 10.1021/acschemneuro.0c00022. [DOI] [PubMed] [Google Scholar]
- 34.Kanekar S, Sheth CS, Ombach HJ, Olson PR, Bogdanova OV, Petersen M, et al. Hypobaric hypoxia exposure in rats differentially alters antidepressant efficacy of the selective serotonin reuptake inhibitors fluoxetine, paroxetine, escitalopram and sertraline. Pharmacol Biochem Behav. 2018;170:25–35. doi: 10.1016/j.pbb.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Georgiadou G, Tarantilis PA, Pitsikas N. Effects of the active constituents of Crocus Sativus L., crocins, in an animal model of obsessive-compulsive disorder. Neurosci Lett. 2012;528:27–30. doi: 10.1016/j.neulet.2012.08.081. [DOI] [PubMed] [Google Scholar]
- 36.Lopresti AL, Drummond PD. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum Psychopharmacol. 2014;29:517–27. doi: 10.1002/hup.2434. [DOI] [PubMed] [Google Scholar]
- 37.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Ghalandari-Shamami M, Nourizade S, Yousefi B, Vafaei AA, Pakdel R, Rashidy-Pour A. Beneficial effects of physical activity and crocin against adolescent stress induced anxiety or depressive-like symptoms and dendritic morphology remodeling in prefrontal cortex in adult male rats. Neurochem Res. 2019;44:917–29. doi: 10.1007/s11064-019-02727-2. [DOI] [PubMed] [Google Scholar]
- 39.Lin CC, Tung CS, Liu YP. Escitalopram reversed the traumatic stress-induced depressed and anxiety-like symptoms but not the deficits of fear memory. Psychopharmacology (Berl) 2016;233:1135–46. doi: 10.1007/s00213-015-4194-5. [DOI] [PubMed] [Google Scholar]
- 40.Shalaby A, Kamal S. Effect of escitalopram on GABA level and anti-oxidant markers in prefrontal cortex and nucleus accumbens of chronic mild stress-exposed albino rats. Int J Physiol Pathophysiol Pharmacol. 2009;1:154–61. [PMC free article] [PubMed] [Google Scholar]
- 41.Pettersson R, Näslund J, Nilsson S, Eriksson E, Hagsäter SM. Acute escitalopram but not contextual conditioning exerts a stronger “anxiogenic” effect in rats with high baseline “anxiety” in the acoustic startle paradigm. Psychopharmacology (Berl) 2015;232:1461–9. doi: 10.1007/s00213-014-3783-z. [DOI] [PubMed] [Google Scholar]
- 42.Pinheiro SN, Del-Ben CM, Zangrossi H, Jr, Graeff FG. Anxiolytic and panicolytic effects of escitalopram in the elevated T-maze. J Psychopharmacol. 2008;22:132–7. doi: 10.1177/0269881107079866. [DOI] [PubMed] [Google Scholar]
- 43.Ge JF, Qi CC, Zhou JN. Imbalance of leptin pathway and hypothalamus synaptic plasticity markers are associated with stress-induced depression in rats. Behav Brain Res. 2013;249:38–43. doi: 10.1016/j.bbr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Pei Y, Pan YL, Jia J, Shi C, Yu Y, et al. Enhanced antidepressant-like effects of electroacupuncture combined with citalopram in a rat model of depression. Evid Based Complement Alternat Med. 2013;2013:107380. doi: 10.1155/2013/107380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23:768–74. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 46.Amin B, Nakhsaz A, Hosseinzadeh H. Evaluation of the antidepressant-like effects of acute and sub-acute administration of crocin and crocetin in mice. Avicenna J Phytomed. 2015;5:458–68. [PMC free article] [PubMed] [Google Scholar]
- 47.Jarosik J, Legutko B, Unsicker K, von Bohlen Und Halbach O. Antidepressant-mediated reversal of abnormal behavior and neurodegeneration in mice following olfactory bulbectomy. Exp Neurol. 2007;204:20–8. doi: 10.1016/j.expneurol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Marks W, Fournier NM, Kalynchuk LE. Repeated exposure to corticosterone increases depression-like behavior in two different versions of the forced swim test without altering nonspecific locomotor activity or muscle strength. Physiol Behav. 2009;98:67–72. doi: 10.1016/j.physbeh.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Saeideh S, Yasavoli M, Gholamnezhad Z, Aslani MR, Boskabady MH. The relaxant effect of crocin on rat tracheal smooth muscle and its possible mechanisms. Iran J Pharm Res. 2019;18:1358–70. doi: 10.22037/ijpr.2019.1100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudson R, Zhou Y, Leri F. The combination of escitalopram and aripiprazole: Investigation of psychomotor effects in rats. J Psychopharmacol. 2017;31:1605–14. doi: 10.1177/0269881117732515. [DOI] [PubMed] [Google Scholar]
- 51.Prinssen EP, Ballard TM, Kolb Y, Nicolas LB. The effects of serotonin reuptake inhibitors on locomotor activity in gerbils. Pharmacol Biochem Behav. 2006;85:44–9. doi: 10.1016/j.pbb.2006.07.005. [DOI] [PubMed] [Google Scholar]


