Abstract
Purpose
Previous studies have linked high-density lipoprotein cholesterol (HDL-C) to gout, but little is known about the dose-effect relationship between serum HDL-C levels and gout flares. This study aimed to quantify the association between the two during urate-lowering therapy initiation and develop a regression equation to predict gout flares.
Patients and Methods
We conducted a prospective, observational, single-center cohort study of men with gout. Patients were identified and grouped according to the level of serum HDL-C (1.16 mmol/L) at baseline and followed-up every four weeks until 12 weeks.
Results
A total of 394 participants completed the study (203 in the low HDL-C group; 191 in the high HDL-C group). The proportion of participants with gout flares in the low HDL-C group was significantly higher than in the high HDL-C group after 12 weeks follow-up (52.2% versus 35.6%, P=0.001). Patients with lower serum HDL-C level had higher risk of gout flares analyzed by restricted cubic spline and when serum HDL-C level = 1.15mmol/L, flareHR = 1. When combined with well-known risk factors, serum HDL-C predicted gout flares with an area under curve (AUC) of 0.75 (95% CI=0.70–0.80). Based on the logistic regression coefficients, we derived the following regression equation: Logit (P)= −2.282+0.05× [disease duration]+1.015× [recurrent flares in the last year]+0.698× [palpable tophus]+0.345× [serum urate]-1.349×[serum HDL-C].
Conclusion
Patients with gout presented a negative linear relationship between serum HDL-C and gout flares. Together with common clinical indicators, the AUC for gout flare prediction increased to 0.75. For patients with gout, remaining serum HDL-C level above 1.15 mmol/L may reduce the risk of gout flares.
Keywords: inflammation, prediction, AUC, life style intervention
Introduction
Gout, a chronic inflammatory disease, is caused by monosodium urate (MSU) crystal deposition, and increased serum urate level is a major risk factor for gout.1 According to the Global Burden of Disease Study 2017, 41 million people worldwide suffered from gout, more than twice as many as those with rheumatoid arthritis.2,3 Gout is characterized by recurrent episodes of acute arthritis called gout flares.4 Gout flares are an important concern for people with gout, the harms of which are not only limited to unbearable pain, actually a case-control study found that gout flares were related to temporary increases in the rates of cardiovascular events.5 The goals of the long-term management of gout are to lower serum urate levels and suppress gout flares.4 Currently, it is relatively clear that the factors inducing gout flares include drinking, a high-purine diet, trauma, obesity, surgery and diuretic.6–9 However, some patients who are unexposed to these factors still suffer from varying frequencies of gouty flares, which prompted us to explore other factors associated with gout flares except for these externalities.
Dyslipidemia is a common complication of gout, the incidence of which is threefold higher in patients with gout and hyperuricemia than general population.10 Studies have shown an association between gout and dyslipidemia, of which triglycerides, total cholesterol and low-density lipoprotein cholesterol (LDL-C) were positively associated with serum urate, while serum HDL-C was contrary.11 Coincidentally, a Mendelian randomization (MR) study showed that an increase in serum HDL-C level led to a reduction in gout risk through direct and indirect pathways.12 These findings suggest that serum HDL-C plays a protective role in the gout population and provide an implication for our study to explore the relationship between serum HDL-C level and gout flares.
Accurate and low-cost screening tools to predict gout flares are critical. Considering the potential involvement of HDL-C in gout, we conducted a follow-up for a period of 12 weeks to determine whether serum HDL-C could be used as a reliable predictive biomarker of gout flares and the dose-effect relationship between the two.
Methods
Study Design
We recruited participants by sending leaflets and promoting in patient groups. All patients who signed the study underwent a two-week washout period, during which they were reminded not to take urate-lowering drugs and to maintain a low-purine diet. Enrolled patients were divided into two groups according to their serum HDL-C levels at baseline. Patients with baseline HDL-C≥1.16 mmol/L were included in the high HDL-C group, and those with baseline HDL-C<1.16mmol/L were assigned to the low HDL-C group.13 All participants attended face-to-face visits every four weeks for 12 weeks.
This study complied with the Declaration of Helsinki, which was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and was registered at the Chinese Clinical Trial Registration Center (#ChiCTR2100043573). All the participants provided written informed consents.
Participant Recruitment
A cohort study was designed to prospectively investigate whether low serum HDL-C levels was an independent factor of gout flares. Inclusion criteria were male sex, age ≥ 18 years old, and meeting the 2015 American College of Rheumatology/European League Against Rheumatism classification criteria for gout.14 Patients were excluded if they met one of the following criteria: presence or history of malignant disease; brain tumor or renal tumor; presence or history of cerebral infarction or myocardial infarction; estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2; serum transaminase level greater than or equal to twice the upper limit of normal range (ULN).
Sample Size
According to our pre-experimental data, the proportion of patients who experienced at least one gout flare was 50% in the low HDL-C group and 35% in the high HDL-C group, which was consistent with a previous report on the proportion of gout flares in general gout patients.15 Based on these data, we estimated that 182 participants in each group would provide >80% power at a significance level of 0.05. Taking an attrition rate of approximately 20% into account, we brought 442 patients of which 221 in each group.16
Treatment
After the two-week wash out period, all participants took febuxostat 20mg once a day or benzbromarone 25 mg once a day according to their usual medicine usage and in our hospital, febuxostat or benzbromarone is the standard treatment for patients with gout. If gout flares occurred during follow-up, colchicine or NSAIDs were prescribed for treatment, whereas anti-inflammatory prophylaxis was not routinely prescribed. In patients with serum transaminase levels more than two-fold that of the ULN, hepatoprotective drugs were administered.
Disease Definition and Data Collection
Hypertension and diabetes were diagnosed on the basis of the doctor’s judgment at the time of study entry or prescribed specific medications. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2); obesity was defined as BMI ≥28 kg/m2.17,18 Metabolic dysfunction-associated steatohepatitis (MASH), kidney stones, and cardiovascular diseases were diagnosed by report in the medical history. Gout flare was defined as more than three of the following criteria: patient-reported flare of resting pain with a visual analog scale score greater than 3 on a scale ranging from 0 to 10, presence of at least one swollen joint and presence of at least one warm joint.19 The estimated glomerular filtration rate (eGFR) was used to assess kidney function and calculated as follows: Cr ≤ 0.9 mg/dL, eGFR = 141 × (Cr/0.9) − 0.411 × (0.993) age; Cr > 0.9 mg/dL, eGFR = 141 × (Cr/0.9) −1.209 × (0.993).age20
At baseline and at every single subsequent visit, patient demographics, including age, height, weight, systolic blood pressure, and diastolic blood pressure were recorded. In addition, comorbidities related to gout such as hypertension, diabetes, MASH, obesity, kidney stones, and cardiovascular disease and corresponding medications were recorded. We collected positive family history, onset age of gout, palpable tophus, and gout flares in the last year as patients’ gout history. Laboratory indicators included HDL-C, low-density lipoprotein cholesterol (LDL-C), serum urate, blood glucose, triglycerides, total cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and C-reactive protein (CRP). Biochemical index were measured using an automatic biochemical analyzer (TBA-40FR, Toshiba Company, Japan). CRP was measured by a latex-enhanced turbidimetric assay using instrumentation provided by Goldsite (Shenzhen, China). We recorded patients with gout flares in each HDL-C group during the follow-up period. Besides, changes in renal function and liver function were the main adverse events (AE) observed in this study.
Statistical Analyses
Statistical analyses were performed using IBM SPSS 26.0, GraphPad Prism V.9 and R 4.3.2. P value < 0.05 was considered statistically significant. Continuous variables were expressed as mean (standard deviation) or median (inter-quartile range), and categorical variables were expressed as numbers (percentages). Independent sample t-test or Wilcoxon signed-rank test was used to compare continuous variables and the Chi-Squared test was used for categorical variables between the two groups. The flare-free and recurrent gout flares-free survival proportions of the participants in the different groups were estimated by Kaplan–Meier curves. Cox regression analysis was used to identify risk factors associated with flares. A restricted cubic spline (RCS) was used to explore the correlation between variables and gout flares within a limited scope. Receiver operating characteristic (ROC) curve analyses were performed to evaluate the predictive efficacy of HDL-C level for gout flares. A sensitivity analysis was performed to test the robustness of the results. Predictive equations were developed using a logistic regression analysis. A mediation analysis was performed using linear and logistic regression analyses.
Results
Study Flow and Baseline Characteristics
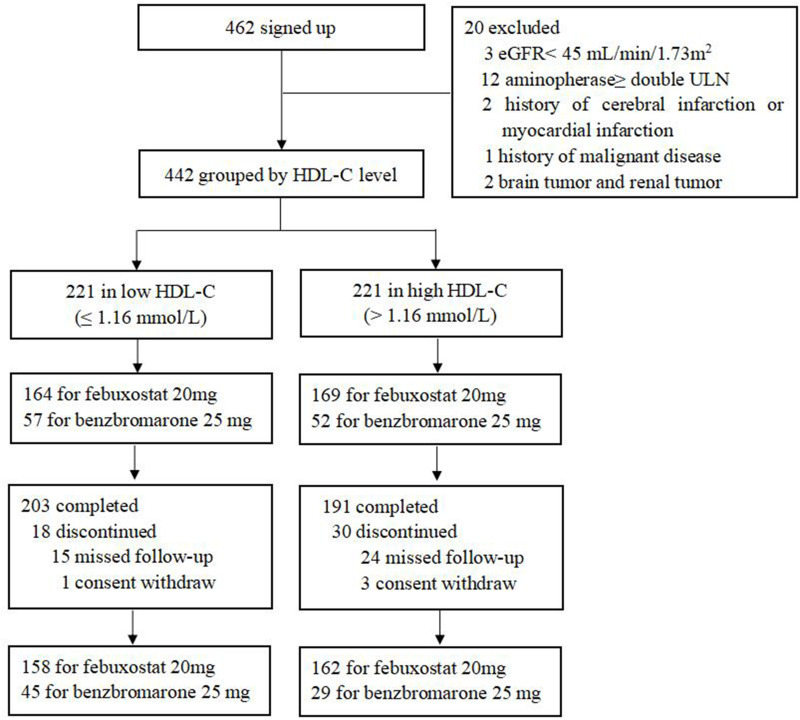
A total of 462 patients signed up our study, 20 patients were excluded for the following reasons: eGFR< 45 mL/min/1.73 m2 (n=3); transaminase > two folds of the upper limits of normal (ULN) (n=12); history of cerebral infarction or myocardial infarction (n=2); history of malignant disease (n=1); brain tumor or renal tumor (n=2). Consistent with the usual ULT drugs, 164 patients were taking febuxostat 20 mg and 57 were taking benzbromarone 25 mg a day in the low HDL-C group; 169 were taking febuxostat 20 mg and 52 were taking benzbromarone 25mg a day in the high HDL-C group. Ultimately, 394 patients completed this follow-up (203 in the low HDL-C group; 191 in the high HDL-C group), and 48 were dropped out due to missed follow-up mainly (Figure 1).
Figure 1.
Flow chart.
Abbreviations: eGFR, estimated glomerular filtration rate; ULN, upper limit of normal range; HDL-C, high-density lipoprotein cholesterol.
Demographic and clinical characteristics of patients are presented in Table 1. Patients in the low HDL-C group had a higher mean BMI (27.2 kg/m2 versus 26.3 kg/m2, P=0.001) than those in the high HDL-C group. Comorbidities such as hypertension, diabetes, MASH, obesity, kidney stones and cardiovascular disease had no differences between the two groups and the medicine application was comparable. While most laboratory indicators were different between the low and high HDL-C group, including higher serum urate (9.3 mg/dL versus 8.8 mg/dL, P<0.001), higher triglyceride (2.1 mmol/L versus 1.4 mmol/L, P<0.001), lower total cholesterol (4.9 mmol/L versus 5.4 mmol/L, P<0.001), lower LDL-C (3.4 mmol/L versus 3.7 mmol/L, P<0.001), higher ALT (26.0 U/L versus 22.0 U/L, P=0.010) and higher CRP (1.76mmol/L versus 1.14mmol/L, P=0.002). In addition, the percentages of participants with flares (85.2% versus 80.1%, P=0.229) and recurrent gout flares (62.1% versus 52.4%, P=0.066) in the last year showed no differences between the two groups.
Table 1.
Baseline Demographic and Clinical Characteristics
| Low HDL-C (N=203) | High HDL-C (N=191) | P value | |
|---|---|---|---|
| Patient demographics | |||
| Age (years) | 39.0 (33.0–49.0) | 45.0 (36.0–55.0) | 0.003 |
| BMI (kg/m2) | 27.2 (25.4–29.2) | 26.3 (24.2–28.4) | 0.001 |
| SBP (mmHg) | 132.0 (122.0–143.0) | 135.0 (124.5–145.0) | 0.149 |
| DBP (mmHg) | 86.0 (80.0–93.0) | 87.0 (78.0–93.5) | 0.903 |
| Comorbidities | |||
| Hypertension | 91 (44.8%) | 89 (46.6%) | 0.762 |
| Diabetes | 21 (10.3%) | 12 (6.3%) | 0.202 |
| MASH | 44 (21.7%) | 32 (16.8%) | 0.251 |
| Obesity | 87 (42.9%) | 69 (36.1%) | 0.182 |
| Kidney stone | 15 (7.4%) | 23 (12.0%) | 0.127 |
| Cardiovascular disease | 2 (1.0%) | 0 (0) | 0.499 |
| Gout history | |||
| Positive family history | 46 (22.7%) | 42 (22.0%) | 0.904 |
| Palpable tophus | 38 (18.7%) | 25 (13.1%) | 0.133 |
| Onset age (years) | 35.0 (29.0–42.0) | 38.0 (30.0–45.3) | 0.014 |
| Disease duration (years) | 4.0 (2.0–9.0) | 6.0 (2.0–10.0) | 0.052 |
| Gout flare frequency | |||
| Less than twice a year | 173 (85.2%) | 153 (80.1%) | 0.229 |
| Twice or more a year | 126 (62.1%) | 100 (52.4%) | 0.066 |
| Concomitant medications | |||
| Febuxostat | 158 (77.8%) | 162 (84.8%) | 0.093 |
| Benzbromarone | 45 (22.2%) | 29 (15.2%) | |
| Losartan | 21 (10.3%) | 23 (12.0%) | 0.633 |
| Fenofibrate | 3 (1.5%) | 9 (4.7%) | 0.080 |
| Metformin | 3 (1.5%) | 1 (0.5%) | 0.624 |
| Hepatoprotectants | 3 (1.5%) | 2 (1.0%) | 1.000 |
| Laboratory indicators | |||
| Serum urate (mg/dL) | 9.3 (8.5–10.0) | 8.8 (8.1–9.4) | <0.001 |
| Blood glucose (mmol/L) | 5.7 (5.4–6.2) | 5.8 (5.5–6.2) | 0.162 |
| Triglyceride (mmol/L) | 2.1 (1.5–3.0) | 1.4 (1.1–2.0) | <0.001 |
| Total cholesterol (mmol/L) | 4.9 (0.9) | 5.4 (1.0) | <0.001 |
| eGFR (mL/min/1.73m²) | 91.7 (17.7) | 91.8 (17.0) | 0.972 |
| ALT (U/L) | 26.0 (18.0–39.0) | 22.0 (17.0–32.0) | 0.010 |
| AST (U/L) | 20.0 (17.0–25.0) | 20.0 (18.0–25.0) | 0.528 |
| HDL-C (mmol/L) | 1.0 (0.9–1.1) | 1.3 (1.2–1.5) | <0.001 |
| CRP (mg/dL) | 1.76 (0.85–3.34) | 1.14 (0.65–1.91) | 0.002 |
| LDL-C (mmol/L) | 3.4 (0.8) | 3.7 (1.0) | <0.001 |
Note: Data are n (%), mean (SD), or median (IQR).
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MASH, metabolic dysfunction-associated steatohepatitis; eGFR, estimated glomerular filtration rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-C, high-density lipoprotein cholesterol; CRP, C-reactive protein; LDL-C, low-density lipoprotein cholesterol.
Independent Predictors of Gout Flares in Gout Patients
As shown in Table 2, disease duration, recurrent flares in the last year, palpable tophus, serum urate level, CRP and serum HDL-C level were risk factors in the univariate analysis. In Model 1, starting with eighteen covariates which might have connection with gout flares, the backward stepwise regression reduced them to three, of which disease duration [HR 1.04 (95% CI 1.01, 1.08)], recurrent flares in the last year [HR 2.43 (95% CI 1.46, 4.04)] and serum HDL-C [HR 0.33 (95% CI 0.14, 0.79)] were independent factors of gout flares. In Model 2, the covariates with P-value<0.1 including disease duration, positive family history, recurrent flares in the last year, palpable tophus, febuxostat, serum urate, triglyceride, eGFR, CRP and serum HDL-C in the univariate analysis were introduced into a multivariate regression model. Finally, recurrent flares in the last year [HR 2.14 (95% CI 1.25, 3.68)] and serum HDL-C [HR 0.40 (95% CI 0.11, 0.87)] were regarded as independent predictors of gout flares in patients who received urate-lowering therapy.
Table 2.
Cox Regression for Factors Associated with Gout Flares
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | 1.01 (0.99,1.02) | 0.445 | ||||
| BMI (kg/m2) | 1.02 (0.98,1.07) | 0.275 | ||||
| Disease duration (years) | 1.03 (1.01,1.06) | 0.009 | 1.04(1.01, 1.08) | 0.022 | 1.04 (1.00,1.08) | 0.075 |
| Positive family history (ref: no) | 1.33 (0.95,1.86) | 0.092 | 1.04 (0.61,1.79) | 0.879 | ||
| Recurrent flares in the last year (≥ 2) | 2.52 (1.79,3.55) | <0.001 | 2.43(1.46, 4.04) | 0.001 | 2.14 (1.25,3.68) | 0.006 |
| Palpable tophus (ref: no) | 1.81 (1.28,2.58) | 0.001 | 1.43 (0.82,2.49) | 0.209 | ||
| At least one comorbidities | 1.29 (0.92,1.82) | 0.140 | ||||
| Febuxostat usage (ref: no) | 1.46 (0.95,2.23) | 0.082 | 1.46 (0.85,2.51) | 0.172 | ||
| Serum urate (mg/dL) | 1.31 (1.17,1.46) | <0.001 | 1.06 (0.89,1.26) | 0.524 | ||
| Blood glucose (mmol/L) | 1.04 (0.83,1.29) | 0.761 | ||||
| Triglyceride (mmol/L) | 1.08 (1.00,1.17) | 0.058 | 1.01 (0.83,1.21) | 0.962 | ||
| Total cholesterol (mmol/L) | 1.08 (0.93,1.29) | 0.318 | ||||
| LDL-C (mmol/L) | 1.08 (0.91,1.27) | 0.380 | ||||
| ALT (U/L) | 1.00 (0.99,1.01) | 0.686 | ||||
| AST (U/L) | 1.00 (0.98,1.02) | 0.922 | ||||
| eGFR (mL/min/1.73m²) | 0.99 (0.98,1.00) | 0.097 | 1.00 (0.99,1.02) | 0.514 | ||
| CRP (mg/dL) | 1.00 (1.03,1.05) | 0.040 | 1.02 (0.99,1.05) | 0.305 | ||
| HDL-C (mmol/L) | 0.35 (0.19,0.63) | 0.001 | 0.33(0.14, 0.79) | 0.014 | 0.40 (0.11,0.87) | 0.026 |
Note: Model 1 followed the backward stepwise selection rule. Model 2 was adjusted for the factors whose P<0.1 in univariate analysis including disease duration, positive family history, recurrent flares in the last year, palpable tophus, febuxostat usage, serum urate, triglyceride, eGFR, CRP and HDL-C.
Abbreviations: BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol. P value in bold means P<0.05 with statistical significance.
Gout Flares of Different HDL-C Groups
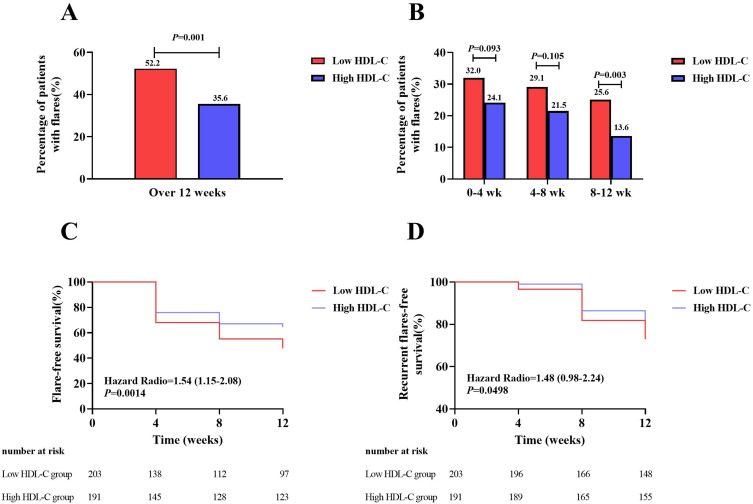
Gout flares were compared in different HDL-C groups. The proportion of participants with gout flares in the low HDL-C group was significantly higher than that in the high HDL-C group after 12 weeks follow-up (52.2% versus 35.6%, P=0.001) (Figure 2A). There was no difference between the two groups at 0–4 weeks and 4–8 weeks, while at 8–12 weeks, the low HDL-C group had a higher incidence of gout flares than the other group (25.6% versus 13.6%, P=0.003) (Figure 2B).
Figure 2.
Gout flares in the low and high HDL-C group over the 12 weeks follow-up. (A) Comparison of percentage of patients with gout flares between the low and high HDL-C group over 12 weeks. (B) Comparison of percentage of patients with gout flares between the low and high HDL-C group every 4 weeks. (C) Comparison of percentage of patients without gout flare between the low and high HDL-C group during the follow-up. (D) Comparison of percentage of patients without recurrent gout flares between the low and high HDL-C group during the follow-up. HDL-C, high-density lipoprotein cholesterol.
The proportion of new cases decreased gradually with every follow-up in the two groups, and survival curves of gout flares [HR 1.54 (95% CI 1.15, 2.08), P=0.0014] (Figure 2C) and recurrent flares (flares≥ 2) [HR 1.48, (95% CI 0.98, 2.24), P=0.0498] (Figure 2D) tested by log-rank analysis showed that there was a significant difference between the low and high groups.
Serum HDL-C Level of Participants with Different Flare Frequencies
According to gout flares frequency, we grouped the participants into 0, 1 and≥ 2 flares groups. Serum HDL-C levels in patients with 1 and ≥ 2 flares were significantly lower than those in the 0 flare group in all participants, while it had no difference between the first two groups. Besides, in low HDL-C group, patients suffering ≥ 2 flares had lower serum HDL-C levels than patients without flares. While in the high serum HDL-C group, patients with different frequencies of gout flares frequency had comparable level of serum HDL-C (Supplementary Figure 1).
The Relationship Between Serum HDL-C and Gout Flares
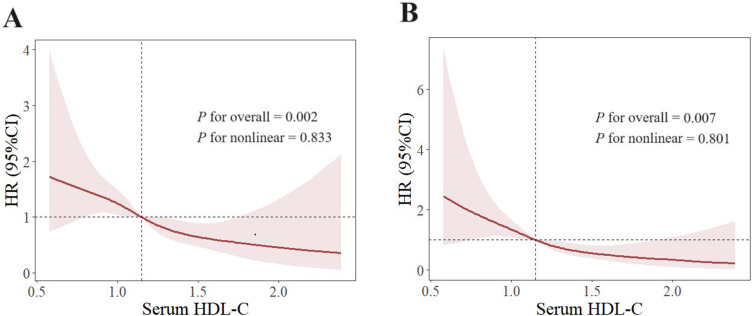
Using restricted cubic splines, a negative linear relationship between serum HDL-C and gout flares was found in the original model (P for nonlinear=0.833) (Figure 3A) and the adjusted model for variables including onset age, disease duration, palpable tophus, BMI, eGFR, serum urate, ALT, AST, blood glucose, triglyceride and LDL-C (P for nonlinear=0.801) (Figure 3B). In addition, when the serum HDL-C level equals to 1.15mmol/L, the HR for gout flares equals to 1 in the two models.
Figure 3.
Restricted cubic spline of the association between serum HDL-C and gout flares. Spline plot of serum HDL-C level and gout flares. (A) Original model to explore relationship between serum HDL-C and gout flares without adjustment. (B) Adjusted for disease duration, palpable tophus, BMI, eGFR, serum urate, ALT, AST, blood glucose, triglyceride, LDL-C. The hazard ratios and 95% confidence intervals (CIs) were calculated with cox regression model. The dashed lines were x–value when HR was 1. BMI, body mass index; eGFR, estimated glomerular filtration rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. P for overall < 0.05 means serum HDL-C and gout flares had correlation. P for nonlinear > 0.05 means correlation between serum HDL-C and gout flares was linear.
Sensitivity Analysis
Sensitivity analyses were performed to test the robustness of the results. These included the exclusion of participants with the extreme 5% of baseline serum HDL-C (< 0.74 or > 1.78 mmol/L) to eliminate their potential influence, the exclusion of patients with baseline serum urate > 10 mg/dL and patients over 60 years old to remove the potential impact of urate and age. The results indicated that even after excluding these factors, the original results were maintained (Supplementary Table 1).
Prediction Efficacy for Gout Flares
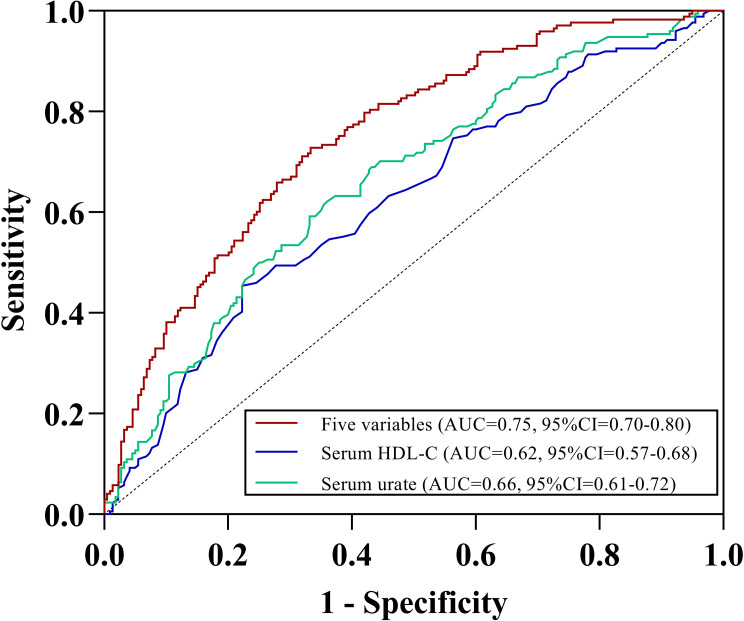
The ROC analyses for gout flares indicated that serum HDL-C could predict gout flares with the AUC of 0.62 (95% CI=0.57–0.68). As a well-known factor associated with gout flares, serum urate could also predict with comparable AUC of 0.66 (95% CI=0.61–0.72). When combined serum HDL-C with serum urate, disease duration, palpable tophus and recurrent flares in the last year (≥ 2), the predictive performance significantly increased with the AUC of 0.75 (95% CI=0.70–0.80) (Figure 4). Multivariate logistic regression analysis was used to develop the prediction equation: Logit (P)= −2.282+0.05× [disease duration]+1.015× [recurrent flares in the last year]+0.698× [palpable tophus]+0.345×[serum urate]-1.349×[serum HDL-C]. Prediction score=elogit(P)/(1+elogit(p)); if the score is greater than 0.5, patients with gout are more likely to experience gout flares. Conversely, if the score was less than 0.5, patients have a lower likelihood of gout flares.
Figure 4.
ROC curve of HDL-C for gout flare over 12 weeks. ROC: receiver operating characteristic. AUC: area under the curve. Five variables including serum HDL-C, serum urate, disease duration, palpable tophus and recurrent flares in the last year (≥ 2).
Safety
Over the 12 weeks study period, liver and renal functions were monitored. Elevated ALT and AST levels were observed during the follow-up. The proportion of participants with increased ALT levels was significantly different between the low and high HDL-C groups (41.9% versus 25.7%, P=0.001), whereas no between-group differences were observed in participants with increased AST levels. Regarding renal function, we did not find any patients with eGFR less than 45 mL/minute/1.73 m2. Besides, there were no gastrointestinal AEs, skin reactions or major adverse cardiac events in either group throughout the trail (Supplementary Table 2).
Discussion
This prospective, observational, single-center cohort study aimed to clarify the relationship between serum HDL-C level and gout flares. Our data present here indicated a negative linear relationship between serum HDL-C and gout flares and when serum HDL-C level=1.15mmol/L, the HR for gout flares=1. By combining serum HDL-C with serum urate, disease duration, palpable tophus and recurrent flares in the last year (≥2), multivariate logistic regression was used to developed a predictive equation. The threshold was set to 0.5 and the predictive performance of serum HDL-C significantly increased with an AUC of 0.75, which may provide a new strategy for gout management.
Earlier studies have shown that serum HDL-C levels were associated with gout, providing evidence that patients with gout had lower serum HDL-C levels than controls,21 while few literature reported a dose-effect association between serum HDL-C and gout flares. Previous studies found that low serum HDL-C could predict gout flares independently,8,22 which is consistent with our conclusion. What’s different was that we grouped different baseline serum HDL-C concentrations according to guidelines and standardized the management of patients taking urate-lowering drugs. Besides, the sample size for each group was determined based on reasonable calculations, thus providing more reliable results. Previous studies have found serum urate,23 palpable tophus and recurrent flares in the last year24 predicted gout flares independently, which corroborated our results. Moreover, compared to these well-known factors associated with gout flares, serum HDL-C level had a more powerful influence on gout flares. Increasing HDL-C level was significantly associated with a decreased HR of 0.40 per 1mmol/L increase, which was much higher than that of serum urate (HR=1.06) and tophus (HR=1.43).
A previous study found that HDL and serum urate had two-way causality and a lower serum HDL-C level was a risk factor of increased serum urate.25 A follow-up study showed that more than 90% of gout flares occurred in patients with a baseline serum urate level of 6 mg/dL or higher.23 In addition, our preliminary research found that higher baseline serum urate levels caused a greater degree of decrease in serum urate levels, both of which led to a higher risk of gout flares.24 These pieces of evidence indicated that low serum HDL-C levels may further lead to a higher risk of gout flares through affecting baseline serum urate levels. To corroborate this conjecture, a mediation analysis was performed using linear and logistic regression, and the results suggested that baseline serum HDL-C could affect subsequent gout flares via the serum urate pathway [ME=−0.49, 95% CI (−0.82, −0.24)], while the direct effect was more dominant [DE=−1.26, 95% CI(−2.10, −0.41)] (Supplementary Figure 2).
Mechanistically, some clinical studies have found serum HDL level decrease during acute inflammatory phase,26,27 which was consistent with our results. The inflammatory response of gout flares is mediated mainly by macrophages and neutrophils.28 Previous studies have shown that HDL exerts anti-inflammatory effects through acting on monocytes and macrophages.29,30 The animal experiment had suggested that injecting monosodium urate crystals into the back airbag of mice induced inflammation while combined injection of HDL could suppress more than half of the leukocyte influx and neutrophils infiltration.31
This study has several strengths. First, our data were obtained from a prospective cohort study, thus endowing our findings with high credibility. In addition, we provided the participants with ULT initiation, making the results more clinically valuable for reference. This study has some limitations. One is its single-center nature, which results in selection bias. Moreover, considering the significant sex disparity in patients with gout, we recruited only male patients with gout in this study. Besides, choosing patients with ULT initiation caused the limitation in generalizability.
In conclusion, this study demonstrated a negative linear relationship between serum HDL-C level and gout flares. Serum HDL-C level was an independent predictor of gout flares, and lower serum HDL-C levels were associated with a higher risk of gout flares in patients with gout. These findings imply that repeated monitoring and maintenance of appropriate serum HDL-C concentrations may help to predict the risk of gout flares. Finally, from a clinical perspective, our findings suggest that in patients with gout and low HDL-C levels, integrating clinical approaches with a healthy lifestyle can maintain a relatively high serum HDL-C level, thereby reducing the risk of gout flares.
Acknowledgments
We thank our patients and the medical staff who assisted in this study.
Funding Statement
This work was sponsored by the National Natural Science Foundation of China (#32470636), Taishan Scholar Programme of Shandong Province (#tsqn202211377) and Natural Science Foundation of Shandong Province (#ZR2024MH031).
Abbreviations
HDL-C, High-density lipoprotein cholesterol; AUC, Area under the curve; MSU, Monosodium urate; LDL-C, Low-density lipoprotein cholesterol; BMI, Body mass index; eGFR, Estimated glomerular filtration; MASH, Metabolic dysfunction-associated steatohepatitis; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; RCS, Restricted cubic spline; ROC, Receiver operating characteristic; HR, Hazard ratios; CI, Confidence interval.
Data Sharing Statement
Data are available from the corresponding authors on request.
Consent for Publication
All authors have agreed to the submission and publication of this study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Article. Nature Revi Disease Pri. 2019;5:17.69. doi: 10.1038/s41572-019-0115-y [DOI] [PubMed] [Google Scholar]
- 2.Global, regional. and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/s0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danve A, Neogi T. Rising Global Burden of Gout: time to Act. Arthritis & rheumatol. 2020;72(11):1786–1788. doi: 10.1002/art.41453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet. 2021;397(10287):1843–1855. doi: 10.1016/s0140-6736(21)00569-9 [DOI] [PubMed] [Google Scholar]
- 5.Cipolletta E, Tata LJ, Nakafero G, Avery AJ, Mamas MA, Abhishek A. Association Between Gout Flare and Subsequent Cardiovascular Events Among Patients With Gout. JAMA. 2022;328(5):440–450. doi: 10.1001/jama.2022.11390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801–808. [PubMed] [Google Scholar]
- 7.Friedman JE, Dallal RM, Lord JL. Gouty attacks occur frequently in postoperative gastric bypass patients. Surg Obes Relat Dis. 2008;4(1):11–13. doi: 10.1016/j.soard.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 8.Mak A, Ho RC, Tan JY, et al. Atherogenic serum lipid profile is an independent predictor for gouty flares in patients with gouty arthropathy. Rheumatol. 2009;48(3):262–265. doi: 10.1093/rheumatology/ken471 [DOI] [PubMed] [Google Scholar]
- 9.DeMarco MA M, Maynard JW, Baer AN, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2012;64(1):121–129. doi: 10.1002/art.33315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatol. 2010;49(7):1229–1238. doi: 10.1093/rheumatology/keq037 [DOI] [PubMed] [Google Scholar]
- 11.Son M, Seo J, Yang S. Association between dyslipidemia and serum uric acid levels in Korean adults: Korea National Health and Nutrition Examination Survey 2016-2017. PLoS One. 2020;15(2):e0228684. doi: 10.1371/journal.pone.0228684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Wang T, Huang S, Zeng P. Evaluation of the causal effects of blood lipid levels on gout with summary level GWAS data: two-sample Mendelian randomization and mediation analysis. J Human Genet. 2021;66(5):465–473. doi: 10.1038/s10038-020-00863-0 [DOI] [PubMed] [Google Scholar]
- 13.Ou W, Jiang T, Zhang N, et al. Role of HDL cholesterol in anthracycline-induced subclinical cardiotoxicity: a prospective observational study in patients with diffuse large B-cell lymphoma treated with R-CHOP. BMJ open. 2024;14(2):e074541. doi: 10.1136/bmjopen-2023-074541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheumatic Dis. 2015;74(10):1789–1798. doi: 10.1136/annrheumdis-2015-208237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK. Serum Uric Acid and the Risk of Incident and Recurrent Gout: a Systematic Review. J Rheumatol. 2017;44(3):388–396. doi: 10.3899/jrheum.160452 [DOI] [PubMed] [Google Scholar]
- 16.Sullivan KM. Open Source Statistics for Public Health. Availablefrom: https://www.openepi.com/SampleSize/SSCohort.htm. Accesed December 12, 2024
- 17.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environm Sci. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 18.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/s0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 19.Gaffo AL, Dalbeth N, Saag KG, et al. Brief Report: validation of a Definition of Flare in Patients With Established Gout. Arthritis rheumatol. 2018;70(3):462–467. doi: 10.1002/art.40381 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Internal Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao S, Kameda K, Matsuzawa Y, Tarui S. Hyperlipoproteinaemia in primary gout: hyperlipoproteinaemic phenotype and influence of alcohol intake and obesity in Japan. Annal rheumatic dise. 1986;45(4):308–313. doi: 10.1136/ard.45.4.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S, Sun M, Li M, et al. Elevated serum CA72-4 predicts gout flares during urate lowering therapy initiation: a prospective cohort study. Rheumatol. 2023;62(7):2435–2443. doi: 10.1093/rheumatology/keac656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick N, Yokose C, Challener GJ, Joshi AD, Tanikella S, Choi HK. Serum Urate and Recurrent Gout. JAMA. 2024;331(5):417–424. doi: 10.1001/jama.2023.26640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang L, Xue X, He Y, et al. The Effect of Decrease in Serum Urate for the Risk of Gout Flares During Urate-Lowering Therapy Initiation Among Chinese Male Gout Patients: a Prospective Cohort Study. J Inflamm Res. 2023;16:3937–3947. doi: 10.2147/jir.S424820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Xian W, Wu D, et al. The role of obesity, type 2 diabetes, and metabolic factors in gout: a Mendelian randomization study. Front Endocrinol. 2022;13:917056. doi: 10.3389/fendo.2022.917056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S, Couret D, Tran-Dinh A, et al. High-density lipoproteins during sepsis: from bench to bedside. Critical Care. 2020;24(1):134. doi: 10.1186/s13054-020-02860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlage S, Gnewuch C, Liebisch G, et al. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009;35(11):1877–1885. doi: 10.1007/s00134-009-1609-y [DOI] [PubMed] [Google Scholar]
- 28.So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol. 2017;13(11):639–647. doi: 10.1038/nrrheum.2017.155 [DOI] [PubMed] [Google Scholar]
- 29.Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arteriosclerosis Thrombosis Vasc Biol. 2008;28(11):2071–2077. doi: 10.1161/atvbaha.108.168690 [DOI] [PubMed] [Google Scholar]
- 30.Burger D, Dayer JM. High-density lipoprotein-associated apolipoprotein A-I: the missing link between infection and chronic inflammation? Autoimmunity Rev. 2002;1(1–2):111–117. doi: 10.1016/s1568-9972(01)00018-0 [DOI] [PubMed] [Google Scholar]
- 31.Scanu A, Luisetto R, Oliviero F, et al. High-density lipoproteins inhibit urate crystal-induced inflammation in mice. Annals of the rheumatic diseases. Mar. 2015;74(3):587–594. doi: 10.1136/annrheumdis-2013-203803 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding authors on request.