Abstract
Aims/Introduction
To investigate the related risk factors of retinopathy in young and middle-aged diabetic patients in order to improve the prognosis of patients.
Materials and Methods
Using clinical practice data from a cohort study at our two research centers, we developed a bivariate logistic regression model to investigate the frightening risk factors potentially for retinopathy in young and middle-aged patients with diabetes, including diabetes type, physical activity level, treatment-related characteristics and laboratory tests.
Results
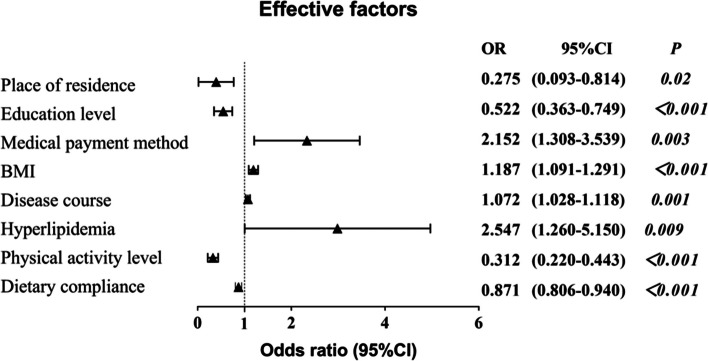
A total of 453 patients with diabetes were investigated, 197 (43.5%) developed retinopathy. The risk of retinopathy was closely related to place of residence (OR: 0.275, 95% CI: 0.093–0.814), education level (OR: 0.522, 95% CI: 0.363–0.749), medical payment method (OR: 2.152, 95% CI: 1.308–3.539), BMI (OR: 1.187, 95% CI: 1.091–1.291), disease course (OR: 1.072, 95% CI: 1.028–1.118), hyperlipidemia (OR: 2.547, 95% CI: 1.260–5.150), physical activity level (OR: 0.312, 95% CI: 0.220–0.443), and dietary compliance (OR 0.871, 95% CI: 0.806–0.940). The area under the receiver operator characteristic curve was 0.915. Goodness of fit (Hosmer–Lemeshow) was 0.658.
Conclusions
The risk of young and middle-aged patients with increased as a result of certain patient characteristics and complications, especially lower dietary compliance and physical activity level.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-024-03821-y.
Keywords: Retinopathy, Diabetes, Risk factors, Prediction model
Introduction
The International Diabetes Federation (IDF) released its 9th edition of the Diabetes Atlas in 2019, which indicates a continuous rise in the number of diabetes patients worldwide, with an average growth rate of 51% [1]. Currently, there are 463 million adults (aged 20–79) with diabetes globally, of which China accounts for 116.4 million, ranking first in the world [2]. Type 2 Diabetes Mellitus (T2DM) is a common chronic disease, accounting for more than 90% of the total number of diabetes patients, posing a serious threat to human health and life [3]. It is estimated that by 2045, there will be 552 million people worldwide with T2DM. Considering the socio-economic conditions over the past 40 years, with the aging population and increasing urbanization in China, coupled with lifestyle changes leading to obesity and overweight, T2DM is showing a trend of becoming younger [4]. The incidence rate of diabetes among young and middle-aged patients is rising year by year, and the harm of chronic complications is becoming more severe.
Diabetes is prone to multiple complications, with an overall prevalence rate of chronic complications of 73.2% [5]. Diabetic retinopathy (DR) is one of the main serious chronic destructive complications of diabetes, a common eye disease that includes macular degeneration, retinal detachment, and retinal vascular diseases, etc. These lesions can affect the structure and function of the retina, thereby affecting vision and visual quality, bringing significant social and economic burdens to patients and the healthcare system [6]. The global prevalence rate of DR is 34.6%, and by 2011, 126.6 million people had DR [7]. If effective and timely measures are not taken, it is expected to reach 191 million by 2030. The prevalence and severity of global DR may be influenced by factors such as racial/ethnic differences, socio-economic conditions, healthcare systems, and lifestyle research. Research results show that there are about 19.5 million diabetic patients in China with DR, of which about 1/5 are at the stage of vision-threatening diabetic retinopathy (VTDR) [8]. Therefore, early identification of risk factors is very important for preventing the occurrence of DR. Currently, the treatment of DR mainly targets the advanced stage, when vision may be affected. Once DR develops to the advanced stage, vision loss may be irreversible. Being the center of the social labor force, the young and middle-aged people are at a point in their life where professional development is critical. The disease’s impact on their lives and the economy is thus significantly greater than that of other age groups. Most existing empirical studies have focused on diabetic patients aged 40 and above. However, the incidence of DR in young and middle-aged diabetic patients is not statistically different from that in older patients. Compared to older diabetic patients, those who develop diabetes at a younger age have a higher risk of DR. High-risk factors for DR, such as obesity, dyslipidemia, and unhealthy lifestyle behaviors, are also prevalent among young and middle-aged individuals. Early screening for diabetic DR patients with poor comorbidity, focusing on modifiable risk factors, can more effectively reduce the risk of DR, especially in middle-aged patients, by achieving early intervention sooner.
Therefore, this study is based on the data of DR patients treated by the Tianjin Eye hospital and the Tianjin Nankai hospital since 2022, and will comprehensively explore the main risk factors for young and middle-aged DR patients, estimate the prevalence rate of young and middle-aged DR and its relationship with the main variable risk factors, especially the relationship with VTDR. This study has certain guiding significance for targeted early individualized intervention and improved prognosis.
Materials and methods
Study design and setting
A double-center retrospective study was performed. All clinical data of young and middle-aged patients (n = 453) with diabetes was collected by researchers from June 2022 to December 2022 in Tianjin Eye hospital and the Tianjin Nankai hospital. The study strictly adhered to the Declaration of Helsinki and was approved by the Ethics Review Committees of Tianjin Eye Hospital, China (Approval number: 2022041). Responses were voluntary and anonymous from all enrolled participants.
Inclusion criteria
Patients who met the following criteria were selected: (1) complete medical records, including general information, admission laboratory examination results, description of the disease’s characteristics, and life style factors; (2) patients diagnosed with DM according to 1999 World Health Organization (WHO) criteria [9] (fasting plasma glucose concentration ≥ 7.0 mmol/L or 2-h post glucose challenge value ≥ 11.1 mmol/L or HbA1c ≥ 6.5%); (3) patients aged ≥ 18 years and ≤ 60 years; (4) patients provided oral informed consent.
Exclusion criteria
Patients who met the following criteria were excluded: (1) pancreatic diabetes, gestational diabetes or other secondary diabetes; (2) Systemic diseases except for diabetes(e.g., infection or previously diagnosed with malignant tumors and other complications); (3) cognitive deficits or mental disorders affect the questionnaire evaluation.
Data collection
We conducted a retrospective study on patients with young and middle-aged diabetes and evaluate every two weeks. The outcome was ‘without DR’ or ‘with DR’. Before carrying out this study, we estimated the sample size. A minimum of 130–195 patients were required on the basis of 10–15 times the possible predictive factors. Finally, a total of 490 patients volunteered for investigation and 454 questionnaires were considered suitable, among which, 36 (7.35%) were excluded for more than 5% missing data. Therefore, the questionnaire had an effective recovery rate of 92.65%.
Measurments
Demographic information sheet
The demographic information sheet was designed by researchers. The potential variables considered in this study comprised gender, age, employment status, education level, medical payment method, marital status, type of diabetes, diabetes treatment, duration of disease, body mass index (BMI), fasting blood glucose (FBG), postprandial blood glucose (PBG), sleep duration in 24 h, smoking, alcohol consumption, dietary compliance, cardiac history, hypertension history, nephropathy history, and family history of DM.
Summary of Diabetes Self-Care Activity Measure(SDSCA)
Diabetes self-care behaviors were assessed using the Arabic version of the Summary of Diabetes Self-Care Activities (SDSCA-Arabic) questionnaire, which is a translated version of the SDSCA originally developed by Toobert et al. SDSCA-Arabic consists of 15 items that assess five domains of diabetes self-care activities, including diet (5 items: 2 items on general diet, 3 items on specific diet), exercise (2 items), blood glucose testing (2 items), medication (2 items), and foot care (4 items). Each item ranged from 0 (none of the days) to 7 days (daily), reflecting the self-reported frequency of performing self-care activities related to diabetic dietary subscale during the past week [10]. Higher scores indicate better self-care behavior. In this study, the dietary subscale was utilized to measure dietary compliance, as it has been validated and demonstrated to have good reliability, with a reported Cronbach's α of 0.718 [11].
International Physical Activity Questionnaire (IPAQ-SF)
The short form of the International Physical Activity Questionnaire (IPAQ-SF) was utilized to assess physical activity levels. In 1998, IPAQ was developed by a group of experts with the aim of providing a simple and cost-effective method for assessing physical activity [12]. This questionnaire has been globally standardized and validated in 12 countries, and it has demonstrated acceptable reliability and validity. The questionnaire showed good value in evaluating three types of physical activity, which based on different MET scores (Metabolic Equivalent of Task, a unit used to estimate the amount of oxygen used by the body during exercise), including walking (3.3 METs), moderate-intensity activities (6.0 METs), and vigorous-intensity activities (8.0 METs). Participants were asked about the types of activities they engaged in, their intensity, and total duration over the past 7 days. Scores were calculated for each category by multiplying the MET score by the minutes and days of participation and then the results were added to arrive at the total IPAQ score (MET-minute/week). According to the recommendations of the International Expert Committee on Physical Activity and the Public Health Guidelines, physical activity was categorized into four levels, including sedentariness, physically inactive, physically active, and highly physically active [13, 14].
Statistical analysis
We used SPSS statistical software v22.0 (IBM Corp., Armonk, NY, USA) to process the data. In the descriptive analysis, the data are represented as frequency and quartile spacing. Differences between the two groups of classified variables were described by the test. Measurement data that did not conform to the normal distribution were described by the Mann–Whitney U test. According to the results of their DR, 453 young and middle-aged population with diabetes were divided into groups without DR (n = 256) and with DR (n = 197) based on fundus photography. In our study, univariate logistic regression analysis was used to test correlations between all selected predictive factors and the occurrence of retinopathy, and bivariate logistic regression analysis (Enter, α = 0.05) was used to analyze significant factors. The risk of developing retinopathy was expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Predictive model discrimination was analyzed by the area under the receiver operating characteristic curve. The calibration of the model was evaluated with a Hosmer–Lemeshow test.
Results
Patients’ characteristics
Of the 453 patients with diabetes in young and middle-aged who met the inclusion criteria, 197 (105 male and 92 female) had DR. In our study, there were more patients aged 45–60 (n = 313, 69.09%) and most participants took medicine regularly (n = 358, 79.03%). In which most patients with comorbidity, including 431 patients with cardiac history, 281 patients with hypertension history, 108 patients with hyperlipidemia, and 46 patients with nephropathy history (Table 1).
Table 1.
Characteristics of 453 diabetic patients regarding DR status
| Variables | None-DR (n = 256) | DR (n = 197) | Z/ | P |
|---|---|---|---|---|
| Sex, n (%) | 0.016 | 0.898 | ||
| Male | 138(53.91) | 105(53.30) | ||
| Female | 118(46.09) | 92(46.70) | ||
| Age (years) | 0.149 | 0.699 | ||
| 18–44 | 81(31.64) | 59(29.95) | ||
| 45–60 | 175(68.36) | 138(70.05) | ||
| Place of residence | 5.149 | 0.023* | ||
| Countryside | 11(4.30) | 19(9.64) | ||
| Urban | 245(95.70) | 178(90.36) | ||
| Working state, n(%) | 75.692 | < 0.001* | ||
| Unemployed | 29(11.33) | 85(43.15) | ||
| Employed | 184(71.88) | 67(34.01) | ||
| Retired | 43(16.80) | 45(22.84) | ||
| Education level, n(%) | 78.355 | < 0.001* | ||
| Junior high school or below | 44(17.19) | 104(52.80) | ||
| Senior high school | 79(30.86) | 58(29.44) | ||
| Bachelor or above | 133(51.95) | 35(17.77) | ||
| Medical payment method, n(%) | 64.325 | < 0.001* | ||
| Urban medical insurance | 215(83.98) | 96(48.73) | ||
| rural medical insurance | 37(14.45) | 90(45.69) | ||
| Self-supported | 4(1.56) | 11(5.58) | ||
| Marital status, n(%) | 4.974 | 0.083 | ||
| Unmarried | 15(5.86) | 22(11.17) | ||
| Married | 230(89.84) | 170(86.29) | ||
| Divorce | 11(4.30) | 5(2.54) | ||
| Smoking, n(%) | 22.025 | < 0.001* | ||
| Non-smokers | 193(75.39) | 114(57.87) | ||
| Quit smoking | 16(6.25) | 38(19.30) | ||
| Smokers | 47(18.36) | 45(22.84) | ||
| Alcohol consumption, n(%) | 15.204 | < 0.001* | ||
| Never | 186(72.66) | 130(65.99) | ||
| Quit drinking | 16(6.25) | 35(17.77) | ||
| Regular | 54(21.09) | 32(16.24) | ||
| Type of diabetes, n(%) | 0.087 | 0.657 | ||
| Type 1 diabetes | 2(0.78) | 3(1.52) | ||
| Type 2 diabetes | 254(99.22) | 194(98.48) | ||
| Treatment of diabetes, n(%) | 19.481 | < 0.001* | ||
| Dietary control | 2(0.78) | 5(2.54) | ||
| Oral hypoglycemic agents | 121(47.27) | 61(30.96) | ||
| Insulin injection | 18(7.03) | 33(16.75) | ||
| Oral medication and insulin injection | 115(44.92) | 98(49.75) | ||
| Sleep duration in 24 h, n(%) | 0.060 | 0.970 | ||
| < 6 h | 15(5.86) | 12(6.09) | ||
| 6 ≤ h < 8 | 78(30.47) | 58(29.44) | ||
| ≥ 8 h | 163(63.67) | 127(64.47) | ||
| Cardiac history, n(%) | 1.150 | 0.283 | ||
| Yes | 246(96.09) | 185(93.91) | ||
| No | 10(3.91) | 12(6.09) | ||
| Hypertension history, n(%) | 3.969 | 0.046* | ||
| Yes | 169(66.02) | 112(56.85) | ||
| No | 87(33.98) | 85(43.15) | ||
| Nephropathy history, n(%) | 6.294 | 0.012* | ||
| Yes | 18(7.03) | 28(14.21) | ||
| No | 238(93.00) | 169(85.79) | ||
| Hyperlipidemia | 26.095 | < 0.001* | ||
| Yes | 84(32.81) | 24(12.18) | ||
| No | 172(67.19) | 173(87.82) | ||
| Family history of DM, n(%) | 5.468 | 0.019* | ||
| Yes | 58(22.67) | 64(32.49) | ||
| No | 198(77.34) | 133(67.51) | ||
| Physical activity level, n(%) | 110.176 | < 0.001* | ||
| Sedentariness | 94(36.72) | 156(79.19) | ||
| Inactive | 43(16.80) | 34(17.26) | ||
| Active | 89(34.77) | 5(2.54) | ||
| Highly Active | 30(11.72) | 2(1.02) | ||
| BMI (kg/m2) | 24.45(22.73,26.42) | 26.22(23.70,28.36) | 19.570 | < 0.001* |
| Systolic blood pressure | 130.00(120.00,140.00) | 120.00(10.7.00,136.00) | 29.342 | < 0.001* |
| Diastolic blood pressure | 80.00(78.00,86.00) | 77.00(70.50,84.50) | 10.822 | 0.001* |
| Disease course (years) | 6.00(3.00,10.00) | 10.00(3.00,16.00) | 11.448 | 0.001* |
| FBG (mmol/L) | 7.00(6.00,7.00) | 7.00(6.00,8.00) | 11.818 | 0.001* |
| PBG (mmol/L) | 9.00(8.00,9.00) | 9.00(8.00,10.00) | 3.586 | 0.058 |
| Dietary compliance(score) | 25.00(23.00,27.00) | 23.00(21.00,26.00) | 25.271 | < 0.001* |
BMI Body mass index, FBG Fasting blood glucose, PBG Postprandial blood glucose
*Statistical significance
Influencing factors associated DR
Results of the tests and Mann–Whitney U showed significant differences between the DR and DR-free groups in characteristics (Tables 1 and 2), such as place of residence (P = 0.023), BMI (P < 0.001), working state (P < 0.001), education level (P < 0.001), medical payment method (P < 0.001), smoking (P < 0.001), alcohol consumption (P < 0.001), diastolic blood pressure (P < 0.001), systolic blood pressure (P = 0.001), treatment of diabetes (P < 0.001), disease course (P = 0.001), FBG (P = 0.001), hypertension history (P = 0.046), nephropathy history (P = 0.012), hyperlipidemia (P < 0.001), family history of DM (P = 0.019), physical activity level (P < 0.001), dietary compliance (P < 0.001) and others had no significant effect on diabetic retinopathy (P > 0.05), as shown in Figs. 1 and 2.
Table 2.
Univariate logistic regression analysis of DR
| Variables | Regression coefficient (β) | OR | 95% CI | P |
|---|---|---|---|---|
| Sex | 0.024 | 1.025 | (0.706,1.487) | 0.898 |
| Age | 0.079 | 1.083 | (0.724,1.620) | 0.699 |
| Place of residence | -0.866 | 0.421 | (0.195,0.906) | 0.027* |
| Working state | -0.312 | 0.732 | (0.559,0.959) | 0.024* |
| Education level | -1.099 | 0.333 | (0.258,0.431) | < 0.001* |
| Medical payment method | 1.261 | 3.530 | (2.396,5.201) | < 0.001* |
| Marital status | -0.627 | 0.534 | (0.303,0.942) | 0.030* |
| Smoking | 0.334 | 1.397 | (1.110,1.758) | 0.004* |
| Alcohol consumption | 0.029 | 1.029 | (0.815,1.300) | 0.809 |
| Type of diabetes | -0.675 | 0.509 | (0.084,3.077) | 0.462 |
| Treatment of diabetes | 0.190 | 1.209 | (0.996,1.468) | 0.050* |
| Sleep duration in 24 h | 0.016 | 1.016 | (0.746,1.383) | 0.921 |
| Cardiac history | 0.467 | 1.596 | (0.675,3.773) | 0.287 |
| Hypertension history | 0.388 | 1.474 | (1.006,2.161) | 0.047* |
| Nephropathy history | 0.784 | 2.191 | (1.174,4.089) | 0.014* |
| Hyperlipidemia | 1.259 | 3.520 | (2.134,5.806) | < 0.001 |
| Family history of DM | -0.496 | 0.609 | (0.401,0.924) | < 0.020* |
| Physical activity level | -1.306 | 0.271 | (0.203,0.361) | < 0.001* |
| BMI | 0.120 | 1.128 | (1.066,1.193) | < 0.001* |
| Systolic blood pressure | -0.032 | 0.968 | (0.957,0.980) | < 0.001* |
| Diastolic blood pressure | -0.032 | 0.969 | (0.950,0.988) | 0.001* |
| Disease course | 0.072 | 1.074 | (1.042,1.107) | < 0.001* |
| FBG (mmol/L) | 0.287 | 1.333 | (1.146,1.549) | < 0.001* |
| PBG (mmol/L) | 0.105 | 1.111 | (0.996,1.240) | 0.059 |
| Dietary compliance | -0.181 | 0.834 | (0.786,0.885) | < 0.001* |
OR Odds ratio, CI Confidence interval, BMI Body mass index
*Statistical significance
Fig. 1.
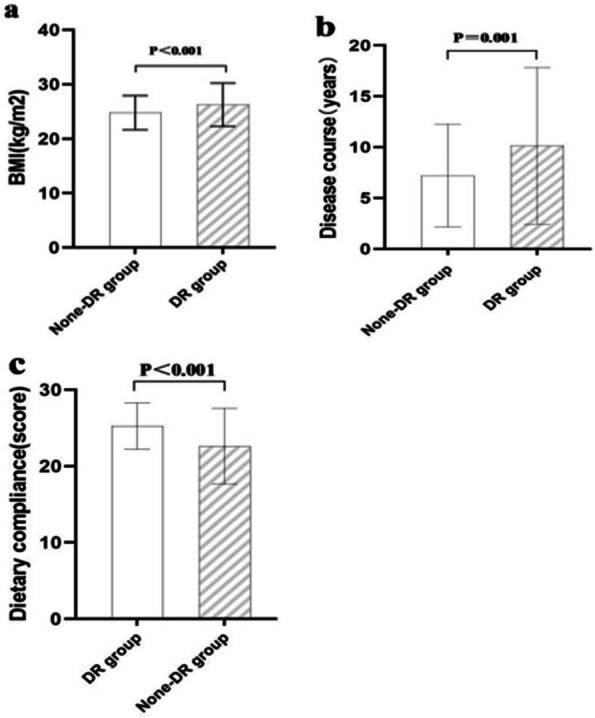
Mann–Whitney U test results of study-related measurement data
Fig. 2.
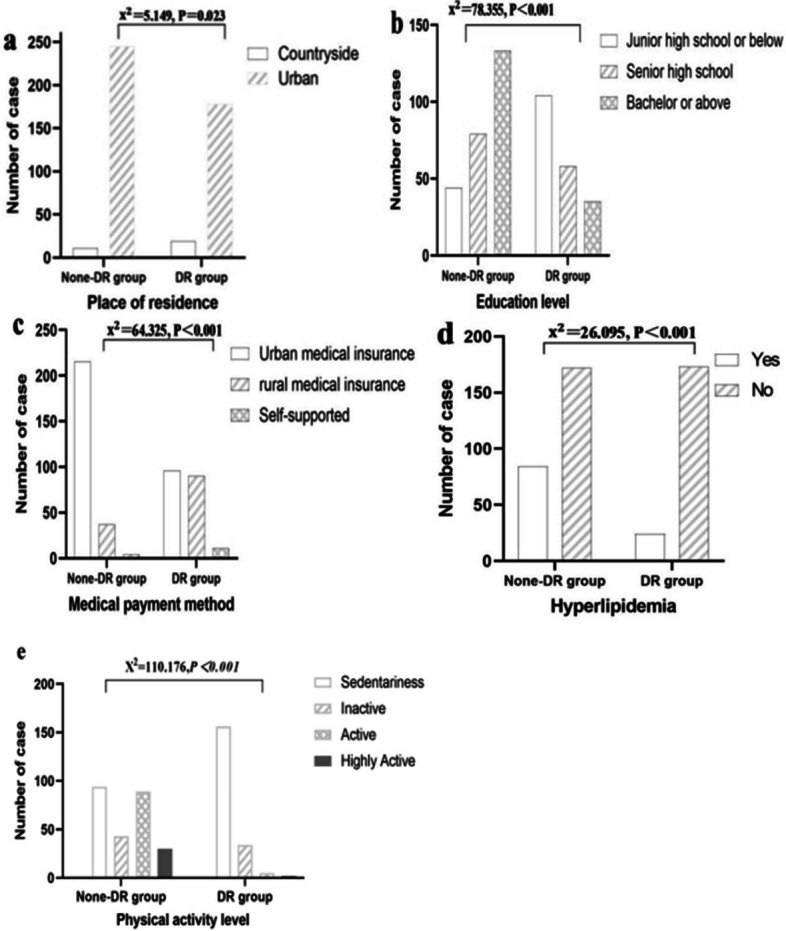
test results of study-related count data
Bivariate logistic regression analysis showed that place of residence, education level, medical payment method, BMI, course of disease, hyperlipidemia, physical activity level, and dietary compliance were independent risk factors of DR in young and middle-aged patients with diabetes. (Table 3; Fig. 3). In addition, the variance inflation factors were all less than 10.0, indicating the absence of multicollinearity, so these variables can be used as predictors to enter the model.
Table 3.
Bivariate logistic regression analysis of DR
| Variables | Regression coefficient (β) | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Place of residence | -1.290 | 0.553 | 5.440 | 0.275 | 0.093–0.814 | 0.020 |
| Education level | -0.651 | 0.185 | 10.314 | 0.522 | 0.363–0.749 | < 0.001 |
| Medical payment method | 0.766 | 0.254 | 10.177 | 2.152 | 1.308–3.539 | 0.003 |
| BMI | 0.171 | 0.043 | 14.644 | 1.187 | 1.091–1.291 | < 0.001 |
| Disease course | 0.070 | 0.021 | 7.526 | 1.072 | 1.028–1.118 | 0.001 |
| Hyperlipidemia | 0.935 | 0.359 | 5.213 | 2.547 | 1.260–5.150 | 0.009 |
| Physical activity level | -1.165 | 0.179 | 42.670 | 0.312 | 0.220–0.443 | < 0.001 |
| Dietary compliance | -0.139 | 0.039 | 11.084 | 0.871 | 0.806–0.940 | < 0.001 |
| Constant | 2.341 | 2.139 | 1.197 | 43.899 | 0.274 |
Fig. 3.
Forest map of bivariate logistic regression analysis
Performance of the prediction model
We generated the ROC curve for the prediction model, which showed an AUC of 0. 915 (95%CI: 0.889–0. 940, P <0.001) (Fig. 4). The sensitivity, specificity, and Youden index of the model were 88.3%, 80.9%, and 35.9%, respectively, and the model has some diagnostic value. The Hosmer–Lemeshow test value was 0.658, indicating the model fits well.
Fig. 4.
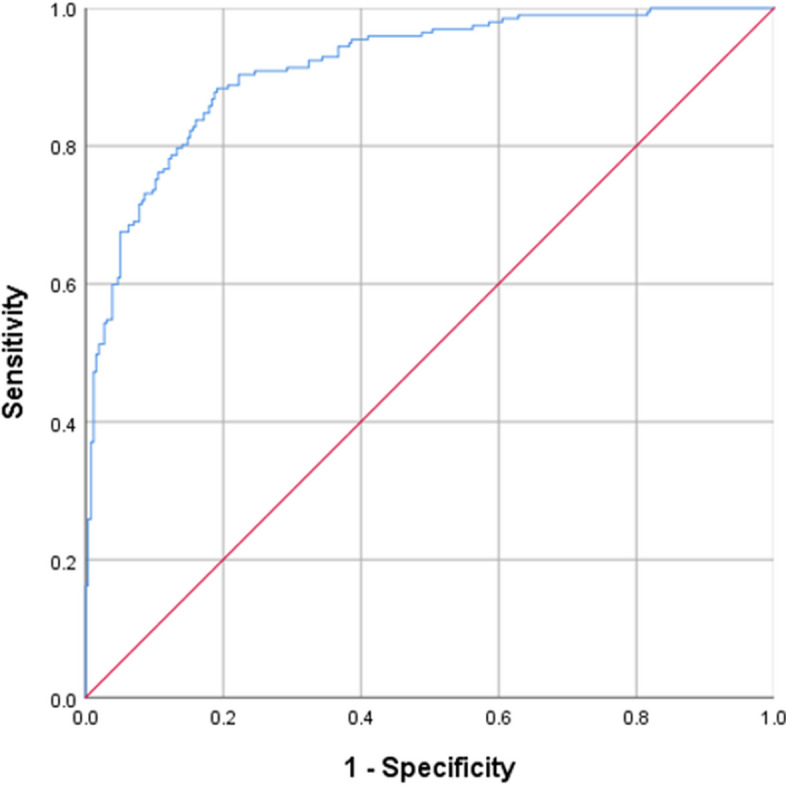
ROC curve
Discussion
DR has profound implications for the daily lives of those affected. The onset of DR can severely impede an individual's capacity to carry out routine self-care activities, thereby diminishing their overall quality of life [15]. Moreover, the condition imposes considerable socioeconomic burdens on both the family units and the broader economy, as it often necessitates extensive medical care and support systems. This has a negative impact on young and middle-aged people with diabetes who have social and family responsibilities [16]. Moreover, the young and middle-aged population has become the fastest growing group in terms of diabetes prevalence and are more likely to face this problem. Prevention of DR is noteworthy. While the majority of existing research, in the form of cohort or case–control studies, has concentrated on identifying risk factors among the elderly population, our study delves into a relatively unexplored demographic. This study has found that the primary risk factors contributing to the development of DR in young and middle-aged diabetic patients encompass a range of sociodemographic and lifestyle elements. Specifically, the place of residence, level of education, method of medical payment, Body Mass Index (BMI), duration of the disease, presence of hyperlipidemia, adherence to dietary recommendations, compliance with medication regimens, and the level of physical activity are the most influential factors in the manifestation of DR in this age group.
The place of residence, level of education, method of medical payment as demographic characteristics of theparticipants are also risk factors for DR. In China, there is still the problem of uneven distribution of medical resources among different residential areas. Urbans have more advanced medical facilities and specialized medical staff, while rural areas may lack these resources. Different insurance payment modalities may affect patients' willingness and ability to access care. Surveys have shown that better health insurance payment models with higher levels of reimbursement promote patients' motivation to be treated and are more inclined to have regular eye exams, whereas uninsured patients may be likely to reduce necessary medical tests and treatments due to financial pressures. Higher educated people are probably better aware of the dangers of diabetes and its consequences, and they're also probably more likely to lead healthy lifestyles or seek out preventive care. The likelihood that a patient's DR will be identified in advance is impacted by each of these variables.
BMI is established as independent risk factors for the development of DR. BMI, recognized as the most widely adopted and earliest indicator for assessing general obesity, has been a cornerstone in our understanding of obesity-related health risks [17]. The research findings underscore a significant association between obesity, as defined by BMI, and the risk of DR among young and middle-aged diabetic patients. Specifically, the result indicate that obesity is linked to a 17% increased risk of developing DR (Odds Ratio = 1.187; 95% Confidence Interval 1.091–1.291). Obesity's impact on ocular health, which is known to induce disruptions in the retinochoroidal microvascular system through a cascade of inflammatory, hormonal, and systemic metabolic alterations [18]. These disruptions are hypothesized to underpin the pathogenesis of DR. The intricate relationship between obesity and ocular health underscores the importance of weight management in the prevention and mitigation of DR. Healthcare providers are encouraged to advocate for weight reduction strategies among overweight or obese patients with diabetes. This proactive approach can significantly lower the risk of DR and other obesity-related complications [19]. Moreover, even for patients with the BMI within the normal range, regular monitoring and proactive lifestyle interventions are essential. This vigilance can help in the early identification of risk factors and the implementation of preventive measures to delay or prevent the onset of ocular complications associated with diabetes.
The manuscript makes a significant contribution by establishing a statistically robust link between hyperlipidemia and DR, with an odds ratio (OR) of 2.547 and a 95% confidence interval (CI) of 1.260 to 5.150. This finding stands out as previous studies have not definitively established a conclusive relationship between hyperlipidemia and DR. Hyperlipidemia is indeed a risk factor for DR, particularly among young and middle-aged individuals. The possible mechanism is that patients with type 2 diabetes mellitus (T2DM) have a disorder of lipid metabolism, leading to abnormal lipid clearance in the retina, which results in an increase in glycation and non-enzymatic oxidation, activating inflammatory responses in the retina and ocular fundus tissues, and increasing the permeability of microvessels [20]. This further disrupts the barrier of the DR in diabetic patients, leading to the occurrence and progression of DR [21]. The discrepancies in findings may be attributed to the use of different measurement techniques and diagnostic criteria for various stages of DR. While earlier studies were based on sub-analyses of clinical trials focused on cardiovascular disease, our data is directly derived from ophthalmic patient outcomes. Recent evidence suggests that lipid-lowering management can effectively mitigate the progression of DR and manage vision loss when it occurs [22]. This underscores the potential of lipid-lowering strategies as a new focus for preventing DR. However, at present, the targets for individualized and precise blood lipid control and lipid-lowering strategies are still being refined and require changes in the patients' own lifestyle habits. Note that further research involving larger-scale trials is needed to clarify the relationship between hyperlipidemia and DR, providing more definitive insights into the role of lipid management in the prevention and treatment of this sight-threatening complication of diabetes.
Furthermore, a stratification analysis was used to explore disease course as an independent risk factor for developing DR in young and middle-aged patients(OR = 1.072; 95%CI1.028–1.118). With the prolongation of the disease, chronic hyperglycemia could damage vascular permeability, and affects retinal homeostasis, which characterize the underlying pathogenesis of DR [23]. Meanwhile, patients with a longer course of diabetes should complete health records by performing fundus examination regularly [24]. Prolonged exposure to the disease not only exacerbates the physical toll on the patient but also casts a significant psychological burden. The financial implications of enduring treatment can lead to heightened anxiety regarding the economic strain on their families, consequently propelling these individuals towards adopting maladaptive coping mechanisms. Such behaviors, in turn, escalate the likelihood of encountering further health complications [8]. Consequently, healthcare providers must extend their vigilance towards those enduring the disease over an extended period. It is imperative to address and alleviate the psychological stress experienced by these patients [25]. By doing so, medical professionals can effectively mitigate the risk of complications, ensuring a more holistic and supportive approach to the management of diabetes and its associated risks.
Dietary compliance and physical activity levels are independent risk factors for DR. Compliance refers to the extent to which patients follow medical advice for treatment. This study was the first to comprehensively assess the relationship between health management compliance in diet for T2DM and the risk of DR. The results showed that the incidence of DR in the group with good overall compliance was significantly lower than in the group with poor compliance, indicating that patients who adhere to health management can significantly improve their risk of developing DR. Optimal control of blood glucose and blood pressure in diabetic patients remains the cornerstone for preventing the development and progression of DR. Previous studies have shown that blood glucose control can be achieved through dietary control [26]. A diet high in salt and sugar increases the risk of hypertension in patients, and hypertension has been confirmed as an important risk factor for DR. In addition, poor dietary habits may lead to dyslipidemia, increasing the risk of atherosclerosis, affecting the blood supply to the patient's eyes, and further exacerbating the risk of disease onset [27]. The "National Guideline for Primary Prevention and Management of Diabetes (2022)" [28] requires primary health care providers to provide comprehensive health management services focused on dietary management to improve blood glucose control and reduce the incidence of complications. Additionally, we found that patients with a sedentary lifestyle have a higher risk of DR than those with an active lifestyle, and increasing physical activity can reduce the risk of DR, consistent with previous literature [23]. Studies have proven that appropriate exercise can improve insulin sensitivity, which helps control blood glucose and thus reduce the damage of diabetes to the retina [28]. At the same time, moderate exercise can improve blood circulation and increase blood supply to the eyes, which is beneficial to the health of the retina. These risk factors suggest that clinical attention should be paid to patients' self-management abilities and lifestyle habits. High-intensity aerobic and resistance exercises should be avoided to reduce the risk of vitreous hemorrhage or retinal detachment. Individualized dietary management should be emphasized to ensure that diabetic patients have a balanced diet.
This study has several limitations: (1)The sample size is relatively small, and the research was conducted at only two hospitals in Tianjin, China. (2)Most of the measurement indicators in this study are derived from clinical records, lacking more precise objective measurements. Additionally, due to limitations, the study did not include confirmed biochemical markers. (3)The study is cross-sectional and lacks long-term measurement of predictive variables.
Conclusions
This study identified eight independent risk factors for the incidence of DR in young and middle-aged patients with diabetes. A reliably simple clinical prediction model constructed, to a certain extent, may aid clinicians in improving patients’ outcomes and decreasing healthcare costs.
Supplementary Information
Acknowledgements
The authors would like to thank the study participants in Tianjin Eye Hospital for their participation. The authors also thank the investigators who assisted in collecting patient information at Tianjin Eye Hospital.
Authors’ contribution
ZHW, XWB, JTL and YXR study conception and design; WJL, ZHX, LW, TS and DS data collection; ZHW, MI, LCand FL data analysis; XWB, MDW and FL manuscript drafting. All authors were involved in the revision of the manuscript and approved the final version of the paper. ZHW and XWB contributed equally to this work and should be considered co-first authors.
Funding
This article was supported by Tianjin Key Medical Discipline(Specialty) Construction Project (No. TJYXZDXK-016A) and grants from National Natural Science Foundation of China (82003092 and 82000949).
Data availability
Data is provided within the supplementary information files.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the ethics committee of Tianjin Eye Hospital, China (Approval number: 2022041). The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhihui Wang and Xiaowen Bai contributed equally to this work and should be considered co-first authors.
Contributor Information
Jinting Li, Email: Snkyyhlb01@tj.gov.cn.
Yongxia Ren, Email: ryx199202@126.com.
References
- 1.Magliano DJ, Boyko EJ and committee IDFDAtes: IDF Diabetes Atlas. In: Idf diabetes atlas. edn. Brussels: International Diabetes Federation © International Diabetes Federation, 2021; 2021.
- 2.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA. Ogurtsova Ket al: Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157: 107843. [DOI] [PubMed] [Google Scholar]
- 3.Westman EC. Type 2 Diabetes Mellitus: A Pathophysiologic Perspective. Front Nutr. 2021;8: 707371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magliano DJ, Sacre JW, Harding JL, Gregg EW and Zimmet PZ: Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. 2020;16(6):321–331. [DOI] [PubMed]
- 5.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. [DOI] [PubMed] [Google Scholar]
- 6.Eggers ED. Visual Dysfunction in Diabetes. Ann Rev Vis Sci. 2023;9:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y. et al: Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–91. [DOI] [PubMed] [Google Scholar]
- 8.Hou X, Wang L, Zhu D, Guo L and Weng J: Prevalence of diabetic retinopathy and vision-threatening diabetic retinopathy in adults with diabetes in China. 2023, 14(1):4296 [DOI] [PMC free article] [PubMed]
- 9.Alberti KG and Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic medicine : a journal of the British Diabetic Association 1998, 15(7):539–553. [DOI] [PubMed]
- 10.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–50. [DOI] [PubMed] [Google Scholar]
- 11.AlJohani KA, Kendall GE, Snider PD. Psychometric Evaluation of the Summary of Diabetes Self-Care Activities-Arabic (SDSCA-Arabic): Translation and Analysis Process. J Transcult Nurs. 2016;27(1):65–72. [DOI] [PubMed] [Google Scholar]
- 12.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A. Sallis JFet al: International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 13.American College of Sports Medicine position statement on the recommended quantity and quality of exercise for developing and maintaining fitness in healthy adults. Med Sci Sports 1978;10(3):vii-x. [PubMed]
- 14.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW. King ACet al: Physical activity and public health. A recommendation from the centers for disease control and prevention and the american college of sports medicine. Jama. 1995;273(5):402–7. [DOI] [PubMed] [Google Scholar]
- 15.Su Z, Wu Z, Liang X, Xie M, Xie J, Li H, Wang X, Jiang F. Diabetic retinopathy risk in patients with unhealthy lifestyle: A Mendelian randomization study. Front Endocrinol. 2022;13:1087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1): 010803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosello F, Vanzo A, Zaffalon C, Polinelli L, Saggin F, Bonacci E, Pedrotti E, Marchini G, Bosello O. Obesity, body fat distribution and eye diseases. Eat Weight Disord. 2024;29(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan H, Wang Y, Xiang Q, Fang S, Chen Y, Chen C, Zhang W, Zhang H, Xia F, Wang Net al: Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. 2020;19(1):118 [DOI] [PMC free article] [PubMed]
- 19.Rong SS and Yu X: Phenotypic and Genetic Links between Body Fat Measurements and Primary Open-Angle Glaucoma. Int J Mole Sci 2023, 24(4). [DOI] [PMC free article] [PubMed]
- 20.Rehman K and Akash MSH: Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? 2017, 118(11):3577–3585. [DOI] [PubMed]
- 21.Hammer SS, Busik JV. The role of dyslipidemia in diabetic retinopathy. Vision Res. 2017;139:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatziralli IP. The role of dyslipidemia control in the progression of diabetic retinopathy in patients with type 2 diabetes mellitus. Diabetes Therapy. 2017;8(2):209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun XJ, Zhang GH, Guo CM, Zhou ZY, Niu YL, Wang L, Dou GR. Associations between psycho-behavioral risk factors and diabetic retinopathy: NHANES (2005–2018). Front Public Health. 2022;10: 966714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W, Wang J, Shen X, Lu W, Wang Y, Li W, Gao Z, Xu J, Li X, Liu Ret al: Establishment and Validation of a Risk Prediction Model for Early Diabetic Kidney Disease Based on a Systematic Review and Meta-Analysis of 20 Cohorts. 2020, 43(4):925–933. [DOI] [PubMed]
- 25.Sun Q, Jing Y, Zhang B, Gu T, Meng R, Sun J and Zhu D: The Risk Factors for Diabetic Retinopathy in a Chinese Population: A Cross-Sectional Study. 2021, 2021:5340453 [DOI] [PMC free article] [PubMed]
- 26.Shah J and Cheong ZY: Dietary Intake and Diabetic Retinopathy: A Systematic Review of the Literature. 2022;14(23). [DOI] [PMC free article] [PubMed]
- 27.Drenjančević-Perić I, Jelaković B, Lombard JH, Kunert MP, Kibel A, Gros M. High-salt diet and hypertension: focus on the renin-angiotensin system. Kidney Blood Press Res. 2011;34(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai C, Jia WP. To promote the integration of prevention and treatment for the whole-course management of diabetes: interpretation of the national guideline for the prevention and control of diabetes in primary care (2022). Zhonghua Nei Ke Za Zhi. 2022;61(7):713–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the supplementary information files.