Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) poses a significant clinical challenge, especially in older patients with HT. This study aimed to identify the factors influencing HFpEF occurrence in elderly patients with HT.
Methods
Elderly patients with HT were categorized into two groups: no HFpEF group and HFpEF group based on HFpEF diagnosis. Demographic, clinical, laboratory and echocardiographic data was conducted. Logistic regression analysis and joint prediction modeling were used to identify predictive factors for HFpEF.
Results
Several factors were associated with HFpEF, including age, body mass index, duration of HT, atrial fibrillation (AF), chronic kidney disease (CKD), stroke, systolic blood pressure (SBP), serum creatinine (SCr), N-terminal pro brain natriuretic peptide (NT-proBNP), heart rate, serum sodium, low density lipoprotein cholesterol (LDL-c), triglyceride, left ventricular ejection fraction (LVEF), E/e’ ratio, left atrial diameter, tricuspid regurgitation velocity, mitral regurgitation and C-reactive protein (CRP) levels. The joint prediction model shown high accuracy, with an area under the curve (AUC) of 0.840.
Conclusions
This study provided insights into the incidence rate and risk factors of HFpEF in elderly patients with HT. Key determinants included age, blood pressure, biomarkers, and echocardiographic parameters.
Keywords: Incidence rate, Risk factors, HFpEF, Elderly, Hypertension
Introduction
Heart failure with preserved ejection fraction (HFpEF) has become a complex and common clinical condition, particularly among older individuals with hypertension (HT) [1, 2]. As the population continues to age, the burden of HFpEF is expected to increase, necessitating a comprehensive understanding of its incidence rate and associated risk factors [3, 4]. HFpEF, characterized by diastolic dysfunction and maintained left ventricular ejection fraction (LVEF), accounts for a substantial portion of heart failure cases, especially in the elderly population [5–7]. With a complex pathophysiology involving comorbidities, aging-related changes, and inflammatory processes, HFpEF presents unique diagnostic and therapeutic challenges [8, 9]. HT, as a prevalent cardiovascular risk factor, is intricately linked to the development and progression of HFpEF, further emphasizing the need for detailed investigation into the incidence rate and specific risk factors of HFpEF in elderly patients with HT [10–12]. Recent studies have highlighted the importance of statistical models in predicting survival time and identifying risk factors for patients with heart failure. For instance, Ashine et al. [13] used Bayesian methods to evaluate the survival time of heart failure patients, demonstrating the potential of advanced statistical approaches in this field. Similarly, Ashine et al. [14] applied a Bayesian accelerated failure time (AFT) shared frailty model combined with integrated nested Laplace approximation to estimate time-to-death and identify risk predictors for heart failure patients. These studies highlight the value of complex statistical techniques in understanding the heterogeneity of heart failure outcomes and identifying high-risk patients. However, despite these advancements, there remains a need for comprehensive risk prediction models specifically targeting elderly HT patients with HFpEF. Current research has largely focused on individual risk factors and their associations with HFpEF, but there is a lack of integrated approaches that consider the interplay between demographic, clinical, laboratory, and echocardiographic factors [15]. To address this gap, our study aimed to conduct a thorough analysis of these factors to identify predictive elements linked to HFpEF in elderly patients with HT.
Methods
Study design and population
This study employed a retrospective case-control design. Elderly patients with HT admitted to our hospital between January 2020 and May 2023 were stratified into two groups based on the development of HFpEF: the no HFpEF group and HFpEF group. Patients in the HFpEF group met the diagnostic criteria for HFpEF outlined by the European Society of Cardiology [16]. The no HFpEF group consisted of patients without heart failure. Inclusion Criteria: (1) patients exhibited signs of left atrial or right atrial enlargement or left ventricular hypertrophy on echocardiography, and were diagnosed with primary HT based on the diagnostic criteria in the “Chinese Guidelines for the Prevention and Treatment of Hypertension” (systolic blood pressure > 140mmHg and diastolic blood pressure > 90mmHg) [17]; (2) patients had a disease duration of > 5 years, were receiving treatment with one of the following medications: diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor-neprilysin inhibitors, calcium channel blockers or beta-blockers, and had a LVEF of ≥ 50% on echocardiography; (3) age ≥ 65 years; (4) patients were conscious and in a stable condition. Exclusion Criteria: (1) patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with mid-range ejection fraction (HFmrEF) (LVEF < 50%); (2) patients with various secondary HT or severe infectious diseases; (3) patients with coronary artery stenosis exceeding 50%; (4) patients who had a history of myocardial infarction, heart valve disease, chronic kidney insufficiency, dilated cardiomyopathy, hypertrophic cardiomyopathy, or rheumatic heart valve disease within the previous three months; (5) patients with malignant tumors.
Date collection
Data was systematically obtained from medical records. Moreover, 5 mL of fasting venous blood was drawn from the patient’s elbow, and serum separation was performed. Serum levels of hemoglobin, serum creatinine (SCr), N-terminal pro-brain natriuretic peptide (NT-proBNP), serum sodium and serum potassium were measured using a fully automatic biochemical analyzer (BS-280, Mindray, China). C-reactive protein (CRP) levels were quantified using the enzyme-linked immunosorbent assay (ELISA). Blood plasma, obtained after heparin anticoagulation and centrifugation, was used to assess total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c) using enzymatic colorimetric techniques.
Echocardiographic examination
Echocardiography was performed using an ultrasound diagnostic instrument equipped with an M5Sc 2D probe (2-4.5 MHz) and a 4Vc 4D probe (2.5-4 MHz). Patients were positioned in the left lateral decubitus position. During image acquisition, patients were instructed to breathe calmly or, if necessary, to hold their breath to ensure clear and stable images. The following measurements were taken: LVEF, left atrial diameter, E/e’ ratio and heart rate. Pulsed-wave Doppler sampling at the mitral valve orifice was used to assess of mitral regurgitation. Continuous-wave Doppler measured tricuspid regurgitation velocity.
Statistical analysis
Using G*Power 3.1.9.7, the “Means: Difference between two independent means (two groups)” option under t tests was selected for Post hoc analysis. The settings were as follows: Two tails mode, Effect size d = 0.6, α err prob = 0.05. Subsequently, the sample sizes of the two groups were entered, and the Power (1-β err prob) was calculated, resulting in a Power of 0.867. SPSS 29.0 statistical software (SPSS Inc., Chicago, IL, USA) was utilized for data analysis. Normally distributed continuous data were presented in the format (mean ± SD). Categorical data were represented using [n (%)] and the chi-square test was employed. Normal distribution of continuous variables was evaluated using the Shapiro-Wilk method. A two-sided P < 0.05 was considered statistically significant. Covariates demonstrating significant differences in both the difference analysis were included in the logistic regression analysis. Variables with differences in logistic regression analysis were included receiver operating characteristic (ROC) analysis. The predictive value of each index for the occurrence of HFpEF in elderly patients with HT was evaluated using the area under the curve (AUC).
Results
Demographic characteristics
Based on the table, elderly patients with HT with HFpEF had a significantly higher mean age (70.24 ± 3.78 vs. 68.45 ± 4.12 years, P = 0.020), higher body mass index (30.25 ± 6.89 vs. 26.91 ± 6.56, P = 0.014), longer duration of hypertension (11.97 ± 2.96 vs. 10.58 ± 3.57 years, P = 0.025), higher proportion of atrial fibrillation (31.43% vs. 13.64%, P = 0.033), higher proportion of chronic kidney disease (25.71% vs. 9.09%, P = 0.024) and higher proportion of stroke(17.14% vs. 4.55%, P = 0.037) compared to those without HFpEF (Table 1). No significant differences were found in gender distribution, smoking habits, drinking habits, diabetes prevalence, coronary artery disease, and peripheral arterial disease between the two groups (P > 0.05).
Table 1.
Demographic characteristics
| Parameter | No HFpEF group (n = 110) |
HFpEF group (n = 35) |
t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 68.45 ± 4.12 | 70.24 ± 3.78 | 2.392 | 0.020 |
| Gender | ||||
| Male | 55 (50.00%) | 16 (45.71%) | 0.061 | 0.804 |
| Female | 55 (50.00%) | 19 (54.29%) | ||
| Body mass index (kg/m2) | 26.91 ± 6.56 | 30.25 ± 6.89 | 2.533 | 0.014 |
| Smoking | 40 (36.36%) | 14 (40.00%) | 0.035 | 0.852 |
| Drinking | 35 (31.82%) | 12 (34.29%) | 0.004 | 0.949 |
| Duration of hypertension (years) | 10.58 ± 3.57 | 11.97 ± 2.96 | 2.299 | 0.025 |
| Diabetes | 30 (27.27%) | 15 (42.86%) | 2.329 | 0.127 |
| CAD | 20 (18.18%) | 10 (28.57%) | 1.171 | 0.279 |
| AF | 15 (13.64%) | 11 (31.43%) | 4.567 | 0.033 |
| CKD | 10 (9.09%) | 9 (25.71%) | 5.067 | 0.024 |
| Stroke | 5 (4.55%) | 6 (17.14%) | 4.348 | 0.037 |
| PAD | 25 (22.73%) | 9 (25.71%) | 0.018 | 0.893 |
Note: CAD = Coronary artery disease; AF = Atrial fibrillation; CKD = Chronic kidney disease; PAD = Peripheral arterial disease
Clinical characteristics
The HFpEF group showed significantly higher systolic blood pressure (145.23 vs. 140.23 mmHg, P = 0.033) and diastolic blood pressure (96.26 vs. 94.46 mmHg, P = 0.009) compared to the non-HFpEF group (Table 2). SCr and NT-proBNP levels were also significantly higher in the HFpEF group. However, no differences were found in heart rate and hemoglobin levels between the two groups.
Table 2.
Clinical characteristics of study participants
| Parameter | No HFpEF group (n = 110) |
HFpEF group (n = 35) |
t | P |
|---|---|---|---|---|
| SBP (mm/Hg) | 140.23 ± 10.26 | 145.23 ± 12.15 | 2.195 | 0.033 |
| DBP (mm/Hg) | 94.46 ± 3.48 | 96.26 ± 3.59 | 2.645 | 0.009 |
| SCr (mg/dL) | 1.12 ± 0.27 | 1.27 ± 0.33 | 2.468 | 0.017 |
| NT-proBNP (pg/mL) | 472.14 ± 50.17 | 520.46 ± 100.28 | 2.743 | 0.009 |
| Heart rate (bpm) | 78.23 ± 5.49 | 80.47 ± 6.47 | 1.851 | 0.070 |
| Hemoglobin (g/dL) | 14.13 ± 1.03 | 13.85 ± 1.25 | 1.175 | 0.245 |
Note: SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; SCr = Serum Creatinine; NT-proBNP = N-terminal pro-brain natriuretic peptide
Laboratory tests
In the elderly hypertensive patient population, the HFpEF group showed significantly lower serum sodium levels (P = 0.005), HDL cholesterol levels (P = 0.026) and higher LDL cholesterol levels (P = 0.005), triglycerides (P = 0.012) and CRP (P = 0.028) compared to the non-HFpEF group (Table 3). However, no significant variations were found in the serum potassium and total cholesterol levels.
Table 3.
Laboratory tests of study participants
| Parameter | No HFpEF group | HFpEF group | t | P |
|---|---|---|---|---|
| (n = 110) | (n = 35) | |||
| Serum sodium (mmol/L) | 140.25 ± 2.48 | 138.52 ± 3.14 | 2.978 | 0.005 |
| Serum potassium (mmol/L) | 4.18 ± 0.36 | 4.28 ± 0.47 | 1.19 | 0.24 |
| TC (mmol/L) | 5.23 ± 0.85 | 5.57 ± 0.96 | 1.828 | 0.073 |
| LDL-c (mmol/L) | 3.16 ± 0.67 | 3.58 ± 0.75 | 2.947 | 0.005 |
| HDL-c (mmol/L) | 1.15 ± 0.31 | 1.04 ± 0.23 | 2.268 | 0.026 |
| Triglycerides (mmol/L) | 1.48 ± 0.57 | 1.83 ± 0.71 | 2.626 | 0.012 |
| CRP (mg/L) | 3.09 ± 0.58 | 3.51 ± 1.05 | 2.275 | 0.028 |
Note: TC = Total Cholesterol; LDL-c = low-density lipoprotein cholesterol; HDL-c = high-density lipoprotein cholesterol; CRP = C-reactive protein
Echocardiographic parameters
The HFpEF group showed a significantly reduced LVEF (P = 0.040) and elevated E/e’ ratio (P = 0.006), left atrial diameter (P = 0.037) and tricuspid regurgitation velocity (P = 0.026) compared to the non-HFpEF group (Table 4). In addition, mitral regurgitation in the no HFpEF group was mainly mild, while in the HFpEF group, it was predominantly moderate (P = 0.044).
Table 4.
Echocardiographic parameters of study participants
| Parameter | No HFpEF group | HFpEF group | t/χ2 | P |
|---|---|---|---|---|
| (n = 110) | (n = 35) | |||
| LVEF (%) | 53.36 ± 5.14 | 50.26 ± 8.13 | 2.12 | 0.040 |
| E/e’ Ratio | 10.65 ± 2.69 | 12.38 ± 3.24 | 2.851 | 0.006 |
| Left Atrial Diameter (mm) | 38.26 ± 3.51 | 40.27 ± 5.16 | 2.152 | 0.037 |
| Tricuspid Regurgitation Velocity (m/s) | 2.08 ± 0.53 | 2.38 ± 0.72 | 2.293 | 0.026 |
| Mitral Regurgitation | ||||
| Mild | 58 (52.73%) | 10 (28.57%) | 6.229 | 0.044 |
| Moderate | 42 (38.18%) | 20 (57.14%) | ||
| Severe | 10 (9.09%) | 5 (14.29%) |
Note: LVEF = Left Ventricular Ejection Fraction
Logistic regression analysis
Age, body mass index, duration of hypertension, atrial fibrillation, chronic kidney disease, stroke, systolic blood pressure, SCr levels, NT-proBNP, heart rate, serum sodium, LDL cholesterol, triglyceride levels, LVEF, E/e’ ratio, left atrial diameter, tricuspid regurgitation velocity, mitral regurgitation and CRP levels all demonstrated significant correlations with HFpEF in elderly patients with HT (Table 5).
Table 5.
Logistic regression analysis between risk factors and HFpEF in elderly patients with HT
| Parameter | Odds ratio | 95%CI | P |
|---|---|---|---|
| Age | 1.120 | 1.016–1.244 | 0.026 |
| Body mass index | 1.076 | 1.017–1.143 | 0.013 |
| Duration of Hypertension | 1.129 | 1.008–1.274 | 0.041 |
| AF | 2.903 | 1.166–7.124 | 0.02 |
| CKD | 3.462 | 1.257–9.48 | 0.015 |
| Stroke | 4.345 | 1.227–16.064 | 0.022 |
| SBP | 1.044 | 1.007–1.084 | 0.021 |
| DBP | 1.119 | 1.046–1.204 | 0.002 |
| SCr | 6.745 | 1.709–29.331 | 0.008 |
| NT-proBNP | 1.010 | 1.005–1.017 | < 0.001 |
| Heart Rate | 1.070 | 1.001–1.146 | 0.049 |
| Serum Sodium | 0.783 | 0.666–0.908 | 0.002 |
| LDL-c | 2.385 | 1.36–4.375 | 0.003 |
| Triglycerides | 2.591 | 1.356–5.214 | 0.005 |
| LVEF | 0.920 | 0.86–0.98 | 0.011 |
| E/e’ Ratio | 1.237 | 1.079–1.435 | 0.003 |
| Left Atrial Diameter | 1.136 | 1.031–1.264 | 0.013 |
| Tricuspid Regurgitation Velocity | 2.463 | 1.261–5.087 | 0.011 |
| Mitral Regurgitation | 1.918 | 1.089–3.436 | 0.025 |
| CRP | 2.197 | 1.296–3.899 | 0.005 |
Note: AF = Atrial Fibrillation; CKD = Chronic Kidney Disease; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; SCr = Serum Creatinine; NT-proBNP = N-terminal pro-brain natriuretic peptide; LDL-c = low-density lipoprotein cholesterol; LVEF = Left Ventricular Ejection Fraction; CRP = C-reactive protein
ROC analysis
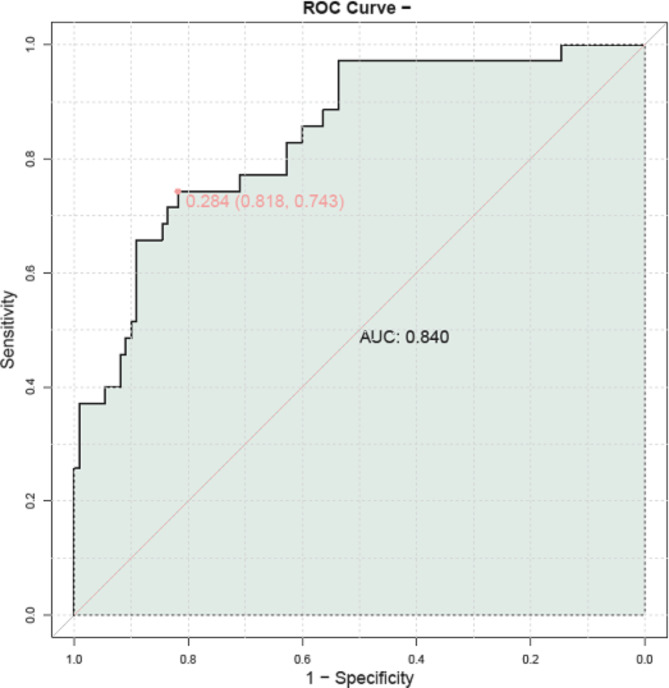
The predictive value of each risk factor for the occurrence of HFpEF in elderly patients with HT was comprehensively evaluated. Sensitivities and specificities were computed for each parameter, resulting in specific values for the AUC and the Youden index (Table 6). Among the parameters assessed, the AUC ranged from 0.563 to 0.678, demonstrating varying degrees of predictive power, with diastolic blood pressure and NT-proBNP showing relatively higher AUC values. These results offer valuable perspective on how these risk factors could be utilized to predict the onset of HFpEF in elderly individuals with HT. Finally, this study combined significant predictive risk factors to construct a joint model for predicting the occurrence of HFpEF in elderly patients with HT. The results demonstrated an AUC value of 0.840 for the joint model (Fig. 1), suggesting that the combined factors model holds a significantly high predictive ability for the occurrence of HFpEF in elderly patients with HT.
Table 6.
The predictive value of each risk factor for the occurrence of HFpEF in elderly patients with HT
| Parameter | Sensitivities | Specificities | AUC | Youden index |
|---|---|---|---|---|
| Age | 0.486 | 0.755 | 0.619 | 0.241 |
| Body mass index | 0.771 | 0.509 | 0.644 | 0.28 |
| Duration of Hypertension | 0.914 | 0.327 | 0.61 | 0.241 |
| AF | 0.314 | 0.864 | 0.589 | 0.178 |
| CKD | 0.257 | 0.909 | 0.583 | 0.166 |
| Stroke | 0.171 | 0.955 | 0.563 | 0.126 |
| SBP | 0.771 | 0.464 | 0.629 | 0.235 |
| DBP | 0.743 | 0.609 | 0.678 | 0.352 |
| SCr | 0.314 | 0.964 | 0.622 | 0.278 |
| NT-proBNP | 0.571 | 0.836 | 0.655 | 0.407 |
| Serum Sodium | 0.429 | 0.891 | 0.663 | 0.32 |
| LDL-c | 0.629 | 0.673 | 0.659 | 0.302 |
| Triglycerides | 0.429 | 0.891 | 0.646 | 0.320 |
| LVEF | 0.543 | 0.727 | 0.621 | 0.270 |
| E/e’ Ratio | 0.629 | 0.655 | 0.651 | 0.284 |
| Left Atrial Diameter | 0.657 | 0.618 | 0.631 | 0.275 |
| Tricuspid Regurgitation Velocity | 0.743 | 0.536 | 0.635 | 0.279 |
| Mitral Regurgitation | 0.714 | 0.527 | 0.622 | 0.241 |
| CRP | 0.314 | 1.000 | 0.63 | 0.314 |
Note: AF = Atrial Fibrillation; CKD = Chronic Kidney Disease; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; SCr = Serum Creatinine; NT-proBNP = N-terminal pro-brain natriuretic peptide; LDL-c = low-density lipoprotein cholesterol; LVEF = Left Ventricular Ejection Fraction; CRP = C-reactive protein
Fig. 1.
ROC curve analysis of joint prediction model for HFpEF in elderly patients with HT
Discussion
The frequency and determining factors of HFpEF in elderly individuals with HT have gained increasing attention in cardiovascular medicine. This retrospective case-control study aimed to clarify the frequency and risk factors for HFpEF in elderly patients with HT. The demographic characteristics of the study participants revealed several notable associations between age, body mass index, duration of hypertension, atrial fibrillation, chronic kidney disease, stroke, and the occurrence of HFpEF. The logistic regression analysis underscored the significant predictive value of these demographic factors in relation to HFpEF, with age, body mass index, duration of hypertension, atrial fibrillation, chronic kidney disease, and stroke emerging as independent risk factors for the development of HFpEF. These findings align with existing literature [18–20], emphasizing the cumulative impact of aging, comorbidities, and cardiovascular risk factors on HFpEF pathogenesis. Notably, the observed correlations and predictive associations emphasize the importance of comprehensive geriatric assessment and targeted management strategies for elderly patients with HT at risk of HFpEF.
Elevated blood pressure has been widely recognized as a major factor in the development and progression of HFpEF [21–23]. This investigation revealed that the clinical features of the HFpEF group exhibited notably elevated levels of both systolic and diastolic blood pressure in comparison to the non-HFpEF group. Moreover, the logistic regression analysis validated the predictive significance of systolic and diastolic blood pressure concerning the occurrence of HFpEF, underscoring the pivotal role of HT in the pathophysiology of HFpEF. These findings highlight the importance of aggressive blood pressure control and targeted antihypertensive therapies in mitigating the risk of HFpEF in elderly patients with HT.
The laboratory examinations in this research offered valuable perspectives on the correlation between particular biomarkers and HFpEF in elderly individuals with HT. Notably, serum Cr and NT-proBNP emerged as strong predictors of HFpEF occurrence, consistent with their established roles as markers of renal function and cardiac stress, respectively. Additionally, the correlation between serum sodium levels and HFpEF implies a possible connection between electrolyte imbalances and cardiac dysfunction in these patients. These results underscore the potential utility of renal and cardiac biomarkers in risk stratification and prognostication in elderly patients with HT at risk of HFpEF.
Echocardiographic parameters are crucial in diagnosing and characterizing HFpEF [24–26], and the findings from this study align with established echocardiographic features of HFpEF. Specifically, the HFpEF group demonstrated significantly lower LVEF, higher E/e’ ratio, left atrial diameter and tricuspid regurgitation velocity compared to the non-HFpEF group. Significantly, these echocardiographic parameters were recognized as important indicators of HFpEF occurrence in the logistic regression analysis, emphasizing their diagnostic and prognostic significance in elderly individuals with HT who are suspected of having HFpEF. These findings emphasize the central role of echocardiography in the comprehensive assessment of elderly patients with HT at risk of HFpEF, supporting the incorporation of these parameters into risk prediction models and clinical decision-making algorithms.
Inflammatory markers have garnered increasing attention in the context of HFpEF, reflecting the intertwined relationships between inflammation, endothelial dysfunction, and cardiovascular disease [27–29]. In this investigation, higher CRP levels were observed in the HFpEF group, suggesting a possible pro-inflammatory environment associated with HFpEF in elderly individuals with HT. The logistic regression analysis further confirmed the predictive value of CRP, underscoring their potential as novel targets for risk stratification and therapeutic interventions in HFpEF. These findings support the rationale for exploring anti-inflammatory strategies in the management of HFpEF and highlight the need for further research into the mechanistic links between inflammation, HT, and HFpEF pathophysiology.
The logistic regression equation facilitated the identification of a constellation of risk factors and biomarkers associated with HFpEF in elderly patients with HT. Notably, these findings reflect the multifaceted nature of HFpEF pathogenesis and underscore the importance of a multidimensional approach to risk assessment and management in this patient population. The joint prediction model constructed in this study further demonstrated a significantly high predictive value, with an AUC of 0.873. This underscores the potential utility of integrating demographic, clinical, laboratory and echocardiographic into a comprehensive risk prediction model for HFpEF, offering a practical tool for risk stratification and prognostication in real-world clinical settings.
The study has several limitations that should be recognized. First, its retrospective nature could have introduced inherent biases and limitations in the collection and analysis of data. Moreover, the study cohort was derived from a single-center setting, potentially limiting the generalizability of the findings to wider patient populations. Currently, the combined prediction model in this study has not been validated in an independent external dataset. Although internal validation results show that the model has high predictive performance, external validation is a critical step to assess the model’s generalizability. Future studies should use independent cohort data to externally validate the model to ensure its stability and reliability across different populations. Despite including multiple important risk factors in the model, there may still be unconsidered confounding factors. For example, lifestyle factors (such as diet and physical activity), socioeconomic status, and medication adherence can significantly influence the development of HFpEF. Due to data limitations, these factors were not included in our analysis. Future research should further explore these potential confounders and incorporate them into the model to enhance predictive accuracy. Additionally, the observational design of the study precludes causal inferences, and further prospective studies were warranted to confirm the predictive value of the identified risk factors and biomarkers in relation to HFpEF occurrence in elderly patients with HT.
Conclusions
In conclusion, this study provides valuable insights into the frequency and risk factors of HFpEF in elderly patients with HT. Key determinants include age, blood pressure, biomarkers and echocardiographic parameters. These findings have implications for risk assessment, early identification, and targeted interventions. Future research should validate these findings in diverse population and explore additional confounders to improve clinical outcomes for HFpEF patients.
Acknowledgements
Not applicable.
Abbreviations
- HFpEF
Heart failure with preserved ejection fraction
- HT
Hypertension
- LVEF
Left ventricular ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- HFmrEF
Heart failure with mid-range ejection fraction
- SCr
Serum creatinine
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- CRP
C-reactive protein
- ELISA
Enzyme-linked immunosorbent assay
- TC
Total cholesterol
- TG
Triglycerides
- HDL-c
High-density lipoprotein cholesterol
- LDL-c
Low-density lipoprotein cholesterol
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- CAD
Coronary artery disease
- AF
Atrial fibrillation
- CKD
Chronic kidney disease
- PAD
Peripheral arterial disease
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis was performed by MC and QR. The first draft of the manuscript was written by MC and WTL. QR commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was not supported by any sponsor or funder.
Data availability
The data involved in the present study can be provided under reasonable request.
Declarations
Ethics approval and consent to participate
This study received approval from the Institutional Review Board and Ethics Committee of Chengdu Seventh People’s Hospital. Informed consent was waived for this retrospective study because it solely utilized de-identified patient data, which had no potential harm or impact on patient care. The Institutional Review Board and Ethics Committee of Chengdu Seventh People’s Hospital granted approval for this waiver in accordance with the regulatory and ethical guidelines governing retrospective research studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sathyanarayanan SP, Oberoi M, Saad Shaukat MH, Stys T, Stys A. Heart Failure with Preserved Ejection Fraction: Concise Review. South Dak medicine: J South Dak State Med Association. 2022;75(11):513–7. [PubMed] [Google Scholar]
- 2.Upadhya B, Kitzman DW. Heart failure with preserved ejection fraction: New approaches to diagnosis and management. Clin Cardiol. 2020;43(2):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(1):16–37. [DOI] [PubMed] [Google Scholar]
- 4.Wintrich J, Abdin A, Böhm M. Management strategies in heart failure with preserved ejection fraction. Herz. 2022;47(4):332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat reviews Cardiol. 2017;14(10):591–602. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat reviews Cardiol. 2020;17(9):559–73. [DOI] [PubMed] [Google Scholar]
- 7.Pagel PS, Tawil JN, Boettcher BT, Izquierdo DA, Lazicki TJ, Crystal GJ, et al. Heart Failure With Preserved Ejection Fraction: A Comprehensive Review and Update of Diagnosis, Pathophysiology, Treatment, and Perioperative Implications. J Cardiothorac Vasc Anesth. 2021;35(6):1839–59. [DOI] [PubMed] [Google Scholar]
- 8.Deichl A, Wachter R, Edelmann F. Comorbidities in heart failure with preserved ejection fraction. Herz. 2022;47(4):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz-Salvador P, Adão R, Vasconcelos I, Leite-Moreira AF, Brás-Silva C. Heart Failure with Preserved Ejection Fraction: a Pharmacotherapeutic Update. Cardiovasc Drugs Ther. 2023;37(4):815–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosiano MF, Tobin R, Mentz RJ, Greene SJ. Physical Functioning in Heart Failure With Preserved Ejection Fraction. J Card Fail. 2021;27(9):1002–16. [DOI] [PubMed] [Google Scholar]
- 11.Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2016;375(19):1868–77. [DOI] [PubMed] [Google Scholar]
- 12.Omote K, Verbrugge FH, Borlaug BA. Heart Failure with Preserved Ejection Fraction: Mechanisms and Treatment Strategies. Annu Rev Med. 2022;73:321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashine T, Muleta G, Tadesse K. Assessing survival time of heart failure patients: using Bayesian approach[J]. J Big Data. 2021;8(1):156. [Google Scholar]
- 14.Ashine T, Tadesse Likassa H, Chen DG. Estimating Time-to-Death and Determining Risk Predictors for Heart Failure Patients: Bayesian AFT Shared Frailty Models with the INLA Method[J]. Stats. 2024;7(3):1066–83. [Google Scholar]
- 15.Redfield MM, Borlaug BA. Heart Failure With Preserved Ejection Fraction: A Review. JAMA. 2023;329(10):827–38. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J Am Coll Cardiol. 2023;81(18):1810–34. [DOI] [PubMed] [Google Scholar]
- 17.2018 Chinese Guidelines for Prevention and Treatment of Hypertension-A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. Journal of geriatric cardiology: JGC. 2019;16(3):182–241. [DOI] [PMC free article] [PubMed]
- 18.Shim CY. Stress Testing in Heart Failure with Preserved Ejection Fraction. Heart Fail Clin. 2021;17(3):435–45. [DOI] [PubMed] [Google Scholar]
- 19.Jhund PS. SGLT2 Inhibitors and Heart Failure with Preserved Ejection Fraction. Heart Fail Clin. 2022;18(4):579–86. [DOI] [PubMed] [Google Scholar]
- 20.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70(20):2476–86. [DOI] [PubMed] [Google Scholar]
- 21.Desai AS, Lam CSP, McMurray JJV, Redfield MM. How to Manage Heart Failure With Preserved Ejection Fraction: Practical Guidance for Clinicians. JACC Heart Fail. 2023;11(6):619–36. [DOI] [PubMed] [Google Scholar]
- 22.Campbell P, Rutten FH, Lee MM, Hawkins NM, Petrie MC. Heart failure with preserved ejection fraction: everything the clinician needs to know. Lancet (London England). 2024;403(10431):1083–92. [DOI] [PubMed] [Google Scholar]
- 23.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat reviews Cardiol. 2014;11(9):507–15. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. [DOI] [PubMed] [Google Scholar]
- 25.Guazzi M, Naeije R. Right Heart Phenotype in Heart Failure With Preserved Ejection Fraction. Circulation Heart Fail. 2021;14(4):e007840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peikert A, Martinez FA, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, et al. Efficacy and Safety of Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction According to Age: The DELIVER Trial. Circulation Heart Fail. 2022;15(10):e010080. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circul Res. 2019;124(11):1598–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy YNV, Borlaug BA, Gersh BJ. Management of Atrial Fibrillation Across the Spectrum of Heart Failure With Preserved and Reduced Ejection Fraction. Circulation. 2022;146(4):339–57. [DOI] [PubMed] [Google Scholar]
- 29.Samson R, Le Jemtel TH. Therapeutic Stalemate in Heart Failure With Preserved Ejection Fraction. J Am Heart Association. 2021;10(12):e021120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data involved in the present study can be provided under reasonable request.