Abstract
Tuta absoluta (Lepidoptera: Gelechiidae) is one of the most significant invasive and destructive pests worldwide, causing serious economic losses to the tomato industry. Rhizosphere microorganism, such as arbuscular mycorrhizal fungi (AMF) and Pseudomonas bacteria, can interact with plants individually or collectively to improve plant growth and resistance to pests and disease. However, the effects of AMF, Pseudomonas, and their interactions on plant responses to insect herbivores remain unclear. A pot experiment was conducted to investigate the effects of single/dual inoculation with AMF (Funneliformis mosseae, M) and Pseudomonas putida (P) on the growth and defense of tomato variety Dafen (Solanum lycopersicum L.) in response to infestation by T. absoluta, as well as the growth, development, and enzyme activity of insect. The results showed that M, P, and MP promoted tomato growth by increasing nutrient concentrations, with the growth-promoting effect of dual-inoculation significantly surpassing that of single inoculation. M, P, and MP still improved tomato growth in T. absoluta infestation, with biomass increases of 57.34%, 54.46%, and 255.49%. M, P, and MP significantly increased the defense ability of tomato, with jasmonic acid concentrations increasing by 42.15%, 60.87% and 90.02%, and phenylalanine ammonia-lyase activity increasing by 47.40%, 47.68%, and 59.97%. The inoculation treatments inhibited the growth and development of T. absoluta, reduced its feeding, prolonged its growth and development, decreased egg weight, and increased the activity of protective and detoxifying enzymes. Overall, our results indicated that AMF and bacteria can stimulate each other, positively influence tomato growth and enhance resistance to T. absoluta. These findings indicate the feasibility of AMF and bacteria in combinations as potential biocontrol agents for the management of T. absoluta.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05952-2.
Keywords: Arbuscular mycorrhizal fungi, Pseudomonas, Microbe-plant-herbivore interactions, Bottom-up effects, Tuta absoluta
Background
Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), which originated in South America and was first described by Meyrick in the highlands of Peru in 1917, is among the most important invasive and destructive pest globally [1]. The pest has occurred and harmed more than 110 countries and regions across South and Central America, Europe, Africa and Asia [2]. T. absoluta can harm 41 species of plants across nine families, primarily feeding on Solanaceous species, but also impacting Amaranthaceae, Leguminosae, and Malvaceae families, with a particular preference for tomatoes [3]. Since its first invaded Xinjiang Uygur Autonomous Region of China in 2017, it has successfully colonized the southwest and northwest regions of China, affecting a total of 33 cities across seven provinces [4]. Furthermore, the pest can cause a loss of 80–100% of tomato yield when it occurs seriously, resulting in significant economic losses for the tomato industry (RMB 80–400 billion) in China [5]. Currently, the pest shows a trend of continuous spread in China, and effective control of this pest is urgently needed.
In recent decades, bottom-up effects have been recognized as an important link for optimizing integrated pest management (IPM) [6]. Rhizosphere microorganisms, such as arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR), indirectly induce bottom-up effects on pest control through their interactions with plants [7, 8]. AMF can form mutualistic relationships with over 80% of terrestrial plants and develop structural arbuscules that expand the root absorption area, thereby enhancing the host plant’s ability to absorb and utilize nutrients and water from the soil [9–11]. Additionally, AMF can also enhance resistance against pathogens and herbivores [12]. Studies focusing on AMF and tomato have demonstrated that AMF not only promotes tomato growth but also induces defense mechanisms, consequently inhibiting the growth and development of Helicoverpa armigera and Spodoptera exigua [13–15]. PGPR, a group of plant rhizosphere microorganisms, promotes growth and induces systemic resistance by activating nutrient uptake and producing plant hormones, antibiotics and other compounds [16, 17]. Pseudomonas spp., a significant species of PGPR, has been shown to enhance tomato growth and yield by facilitating nutrient activation and hormone production [18, 19], while also increasing tomato resistance to biological stresses such as pathogens and nematodes [20–22]. However, AMF and Pseudomonas can have either positive or negative effects on tomato-insect interactions, depending on the specific species of fungi, bacteria, and insects involved.
Both AMF and PGPR are closely associated with plant roots, leading to potential interactions within the rhizosphere. The mycelium of AMF can serve as attachment sites for PGPR and secrete hyphal exudates that facilitate bacterial growth. In return, PGPR can assist AMF in colonizing plant roots, and their secretions may enhance the growth and development of AMF hyphae and spores [23]. Several studies have demonstrated that the dual inoculation of AMF and Pseudomonas can significantly enhance plant phosphorus absorption, promote plant growth, and improve resistance to drought, salinity, as well as pathogenic microbes and nematodes [24–27]. However, few studies regarding the effects of dual inoculation of AMF and Pseudomonas on plant and insect feeding. Additionally, the feedback effects of AMF and Pseudomonas on plants are influenced by species specificity and environmental conditions. Behn [28] found that in the absence of pathogenic bacteria, the growth-promoting effects of AMF and Pseudomonas were superior to those of single inoculation; however, single inoculation exhibited greater control over pathogenic bacteria than combined inoculation. The impact of insect herbivory on the feedback effects of dual inoculation of AMF and PGPR on plants remains unknown.
Previous studies have demonstrated that Funneliformis mosseae and Pseudomonas putidis exist in the rhizosphere of tomato plants and have positive feedback effect on tomato [29–31]. However, the effects of single or dual inoculation of these microorganisms on tomato growth in the infestation of T. absoluta are unclear. This study investigated the effects of single or dual inoculation with F. mosseae, P. putidis on tomato growth parameters, defense parameters of tomato when infested with T. absoluta, development duration and detoxifying enzymes activities of T. absoluta. Additionally, the study compared the AMF colonization rate and the density of P. putidis under different inoculation in both infested with or without T. absoluta. The objective was to elucidate the roles of F. mosseae and P. putida in the response of tomato to T. absoluta infestation and to determine the interaction between these two microorganisms. The findings of this research provide both theoretical and technical insights for utilizing beneficial microorganisms to mitigate damage caused by T. absoluta and enhance ecosystem benefits.
Methods
Plants, insect and soil
Tomato variety Dafen was selected as the host plant due to its suitability for both greenhouse and open field cultivation, as well as high yield and good resistance to various plant diseases. Seeds were obtained from Huaifang Four Seasons Spring Seed Industry Co., Ltd. Before planting, the seeds were surface-sterilized in 1.5% sodium hypochlorite solution, rinsed five times with sterile distilled water, submerged in 70% ethanol for 1 min, and then submerged in sterile distilled water for 2 h.
Tuta absoluta were collected from tomato fields in Xinhua Township, Xinping Yi and Dai Autonomous County, Yuxi City, Yunnan Province (24°06′32″N, 101°51′22″E), and reared indoors in artificial climate chambers (27 ± 2℃ photoperiod 16 h L: 8 h D, RH 70% ± 5%) with artificial diets over ten generations.
The soil was collected from the tomato growing soil at the Kedihua Experimental Station in Yiliang County, Kunming City, Yunnan Province (25°17′02"N, 103°28′75"E). The soil was sandy loam, was passed through a 10-mesh sieve and mixed with perlite in a ratio 2:1 (v/v) as plant culture substrate. The properties of the plant culture substrate were as follows: pH = 6.59, 2.84 g·kg−1 of total nitrogen, 3.20 g·kg−1 of total phosphorus, 506.70 g·kg−1 of total potassium, 31.54 mg·kg−1 of available phosphorus, 106.38 mg·kg−1 of available potassium, 23.55 mg·kg−1 of ammonium nitrogen, 9.95 mg·kg−1 of nitrate nitrogen. The culture substrate was sterilized in an autoclave at 121℃ for 2 h. Each planting pot (h = 12 cm, Φ = 13.5 cm) was filled with 600 g of mixture.
Microbial inoculant
The Funneliformis mosseae fungus was obtained from Qingdao Agricultural University. The fungal inoculant was propagated with maize (Zea mays) in a soil-sand mixture in a greenhouse for three months. The inoculant was a mixture of rhizosphere soil containing fragments of colonized roots, spores and hyphae, with a spore density of 300 spore g−1 inoculant. The Pseudomonas putida (bio-53094) inoculant was obtained from Zhili Zhongte (Wuhan) Biological Technology Co., Ltd., and was cultured in LB medium at 28 °C for 24 h, diluted and adjusted to obtain a concentration of 1 × 108 colony forming units CFU/mL.
Experimental design
The experiment was laid out in a factorial with complete randomized design with two factors: (1) inoculant treatments: control (CK, no AM fungi or PGPR added), inoculation with F. mosseae (M), P. putida (P), F. mosseae and P. putida (MP), and (2) infestation treatment: with T. absoluta infestation and without T. absoluta infestation. For the M and MP treatments, fifty grams of AM fungal inoculum, containing about 300 spores per gram, were mixed with the soil. In the non-AMF treatments, autoclaved inoculant was added in addition to the fifty grams of soil. For the P and MP treatments, 20 mL of P. putida suspension (1 × 108 CFU) were added to the soil. In the non-bacterial treatments, 20 mL autoclaved bacterial suspension was used. After 50 days, nine first instar larvae of T. absoluta were introduced to each plant, with one larva on each branch of the tomato [32]. The experiment included six replicates (pots) for each treatment. Irrigation was conducted with sterile water daily and Hoagland nutrient solution was provided every 14 days during the cultivation period.
Measurement
Plant biomass
After T. absoluta pupated, the pupa was removed from the tomato plant, and harvesting of the plant started. The tomato plants were extracted from the soil, and their roots were washed to remove any soil residue. To determine the dry weight of the aerial parts and roots, these organs were placed in an oven at 80℃ for 72 h. After the samples got dried, they were weighed using a digital scale [33].
Nutritional quality
Tomato leaves were ground with distilled water (1:10, g:mL), incubated in a water bath at 95℃ for 10 min, and centrifuged at 4000 rpm for 10 min, the soluble sugar concentration was determined using anthrone colorimetry [34]. Tomato leaves were immersed in a 0.86% normal saline solution (1:9, g:mL) and centrifuged at 3500 rpm for 10 min at 4℃, the protein concentration was determined using the BCA protein quantification method [35]. Starch and chlorophyll concentrations were determined according to the kit instructions of Beijing Box Shenggong Technology Co., LTD.
Antioxidant defense enzymes activities, secondary metabolite and defense hormones concentrations
The protective enzyme activity and secondary metabolite concentrations of leaves were measured following the feeding of T. absoluta. Tomato leaves (0.1 g) were ground in 0.9 mL of 0.86% normal saline solution and then centrifuged at 3500 rpm for 10 min at 4℃. Enzyme activity was determined using kits from the Nanjing Jiancheng Institute of Bioengineering for peroxidases (POD), superoxide dismutase (SOD), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL). The leaves were dried to a constant weight, crushed through a 40-mesh sieve, weighed to 100 mg and added to the extraction solution, extracted at 60℃ for 30 min, centrifuged for 10 min at 12000 rpm, and the supernatant was taken as the sample to be measured. The total phenols and flavonoid concentrations were determined using kits from Beijing Box Shenggong Technology Co., LTD.
The tomato leaves (0.1 g) were ground in Phosphate-buffered saline (1:9, g:mL), and then centrifuged at 3000 rpm for 10 min. The concentrations of jasmonic acid (JA) and salicylic acid (SA) were determined according to the instructions provided by the ELISA kit from Shanghai Enzyme-Linked Biotechnology Co., Ltd.
Leaf loss rate
The leaf loss rate of T. absoluta feeding is described by Fateme et al. [32]. Briefly, when T. absoluta was in its 3rd instar (two weeks after infestation), the infested leaves were removed. The feeding leaf area and the remaining leaf area were subsequently measured using a scanner (Epson Expression 10000XL; Epson, Long Beach, CA, USA) and analyzed with WinRhizo Software (Regent Instruments Inc., Québec City, QC, Canada) to calculate the total leaf area. The biomass of the remaining leaf area was measured, allowing for the calculation of weight per unit leaf area [36].
Development duration, pupa weight and enzyme activity of T. absoluta
The inoculated T. absoluta were divided into two groups. One group was used to examine the effects of feeding on tomato treated with different inoculant on the growth development duration and pupal weight of T. absoluta. The other group was used to detect the effects of feeding on tomato treated with different inoculant on the protective and detoxifying enzyme activities of T. absoluta. The infested leaves that were surrounded were examined daily under binocular microscope from the one-day old larvae until the pupae appeared to determine the developmental stages of larvae. The 3rd instar larvae of T. absoluta were collected for enzyme activity determination. The larvae of the 3rd instar of T. absoluta were collected under different treatments and weighed accurately. Subsequently, the samples were immersed in a 0.86% normal saline solution (1:9, g:mL) and homogenized, centrifuged at 12,000 rpm for 15 min at 4℃. Enzyme activity was determined using kits from Nanjing Jiancheng Institute of Bioengineering for SOD, CAT, glutathione S-transferase (GST), and carboxylesterase (CarE), as well as Shanghai Yuancheng Biotechnology Co., Ltd. for cytochrome P450 (CYP450) activity. The activities of protective enzymes (SOD, CAT) and detoxifying enzymes (GST, CarE, CYP450) were measured and calculated following the manufacturer’ s instructions. Enzyme activity was expressed as enzyme mg/protein [37]. The absorbance was measured using a microplate reader (DR-3518L).
Pseudomonas density and AMF colonization rate
For P. putida density measurement, 1 g of rhizosphere soil was combined with 9 mL of LB liquid medium to prepare a soil solution. The density of P. putida in the rhizosphere soil was counted with dilution plate method 14 h after incubation at 28℃ [38]. Soil suspensions were diluted with sterile water, evenly spread onto LB medium plates using a coating rod, and incubated at 37℃ for 24 h. Soil suspensions with a dilution of 10–4 were selected for colony counting, allowing for the determination of the number of Pseudomonas per gram of soil.
To measure the AMF colonization rate, roots selected from each replicate were cut into 100 segments of 1 cm. Then, the segments were washed 3 to 4 times with water and immersed in 10% potassium hydroxide (KOH) at 90℃ for 10 min. The samples were washed with water and then immersed in 1% hydrochloric acid for 10 min. In the next step, the samples were immersed in acid fuchsin solution (consisting of lactic acid, glycerol, and distilled water in equal proportions and acid fuchsin by 0.01% weight/volume) at 90℃ for 30 min. The roots were removed from acid fuchsin solution and then transferred to vials containing lactic acid. The processed root segments were then examined under a microscope at 40 × magnification. The colonization rate of AMF was calculated by combining the percentage of AM fungal structures (hyphae, vesicles, arbuscules and spores) from 100 root segments of each treatment [39, 40], with three replicates for each treatment.
Statistical analysis
The data obtained from the measurements of plant biomass, nutrient concentrations, antioxidant defense enzyme activity, secondary metabolite and defense hormone concentration, leaf loss rate, AMF colonization rate, bacterial density, development duration, pupa weight and enzyme activity of T. absoluta were statistically analyzed using the Statistical Package for Social Sciences (SPSS) program, version 27.0 for Windows (SPSS Inc. Chicago, IL, USA). The significance of differences between the mean values of different inoculation treatments was determined through one-way analysis of variance. Duncan’s multiple range test was used to compare the means. The significance probability levels of the results were fixed at P < 0.05. Pearson’s rank correlation coefficient was used to analyze the correlation between AMF colonization or the density of P. putida and plant growth parameters. All visual analyses were performed using Origin 2022 software.
Results
Effect of microbial inoculants and T. absoluta infestation on the tomato growth
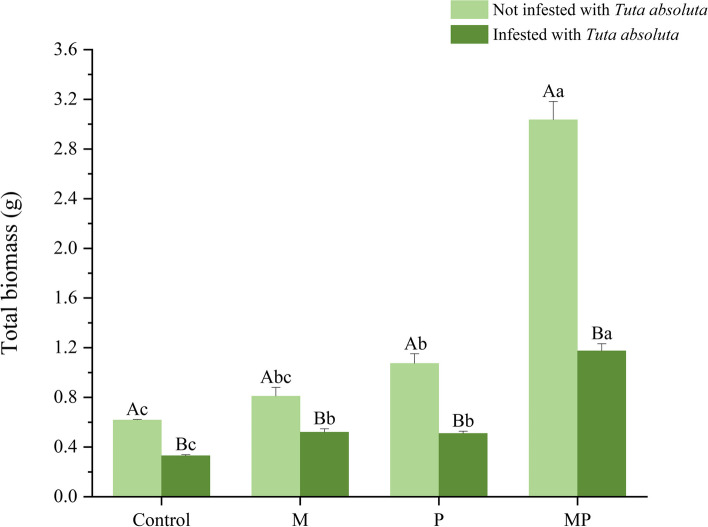
The effect of different microbial inoculants (F. mosseae (M), P. putida (P), their combination (MP)) on tomato plant biomass were different in the absence of T. absoluta infestation (Fig. 1). Specifically, inoculation with P. putida and dual-inoculation with F. mosseae and P. putida significantly increased total biomass by 57.58% and 310.61%, respectively. In contrast, while inoculation with F. mosseae increased total biomass, this increase was not statistically significant compared to the control treatment. T. absoluta infestation negatively affected tomato growth; however, the inoculant treatments partially offset the growth inhibition caused by this infestation. Specifically, the total biomass of tomato infested with T. absoluta in inoculation of F. mosseae, P. putida, and F. mosseae and P. putida significantly increased by 57.34%, 54.46%, and 255.49%, respectively, compared to the uninoculated treatment (Fig. 1). The total biomass of the dual-inoculated MP treatment was significantly greater than of the inoculated M and P treatments.
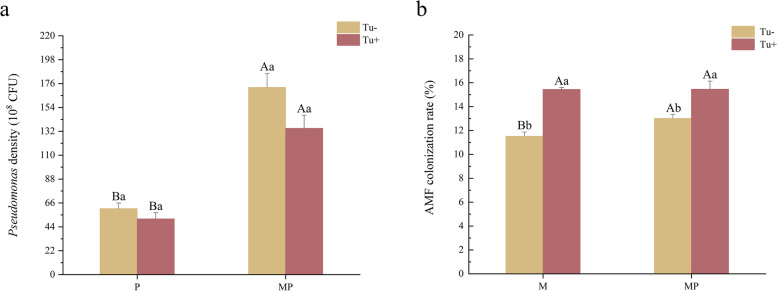
Fig. 1.
Effect on total biomass (g) of tomato inoculated with different treatments involving different inoculants and T. absoluta. M, inoculated with Funneliformis mosseae. P, inoculated with Pseudomonas putida. MP, dual inoculated with F. mosseae and P. putida. Different uppercase letters are indicating significant differences (P < 0.05) between treatments with or without Tuta absoluta infestation. Different lowercase letters are indicating significant difference of P < 0.05 among the four inoculation treatments. Error bars represent ± SD of the mean
In the absence of T. absoluta infestation, the concentrations of soluble sugar, starch, and chlorophyll in the M treatments showed no significant difference when compared to the control; however, the protein concentration significantly increased (P < 0.05). The nutrient concentrations (soluble sugar, protein, starch, and chlorophyll) in the P and MP treatments were significantly higher (P < 0.05), with the nutrient concentrations in the MP treatment being significantly higher than that in the P treatment (P < 0.05, Table 1). Infestation by T. absoluta significantly reduced the nutrient concentration in the tomato plants, while the inoculation treatment partially mitigated this negative impact. Specifically, the soluble sugar concentration in the M treatment was significantly higher than in the other two inoculation treatments, and the chlorophyll concentration in the MP treatment was significantly increased compared to the other inoculation treatments. The protein and starch concentration in the P and MP treatments were significantly higher than those in the M treatment.
Table 1.
Growth parameters (soluble sugar, protein, starch, chlorophyll) of tomato inoculated with different inoculants and T. absoluta treatment
| Treatments | Soluble sugar (μg/g) | Protein (mg/mL) | Starch (mg/g) | Chlorophyll (mg/g) |
|---|---|---|---|---|
| Control | 2.83 ± 0.25 Ab | 6.77 ± 0.53 Ac | 8.02 ± 0.60 Ac | 33.10 ± 3.36 Ab |
| M | 4.21 ± 0.11 Bb | 7.65 ± 0.43 Ab | 8.72 ± 0.33 Ac | 30.83 ± 1.21 Ab |
| P | 5.40 ± 0.48 Ab | 8.98 ± 0.39 Aa | 14.57 ± 2.93 Ab | 36.18 ± 0.33 Ab |
| MP | 19.35 ± 1.87 Aa | 9.17 ± 0.28 Aa | 20.47 ± 1.93 Aa | 51.078 ± 5.35 Aa |
| Control-T | 3.87 ± 0.54 Ac | 6.75 ± 0.56 Ac | 6.85 ± 2.17 Ab | 15.94 ± 1.45 Bd |
| M-T | 8.00 ± 0.67 Aa | 7.65 ± 0.43 Ab | 7.51 ± 1.07 Ab | 33.67 ± 3.19 Ab |
| P–T | 5.53 ± 0.19 Ab | 8.98 ± 0.39 Aa | 14.30 ± 0.85 Aa | 23.21 ± 2.78 Bc |
| MP-T | 5.67 ± 0.22 Bb | 9.17 ± 0.28 Aa | 13.33 ± 2.52 Ba | 42.29 ± 3.72 Aa |
M, inoculated with Funneliformis mosseae. P, inoculated with Pseudomonas putida. MP, inoculated with F. mosseae and P. putida. Control-T, infested with T. absoluta. M-T, inoculated with F. mosseae and infested with T. absoluta. P–T, inoculated with P. putida and infested with T. absoluta. MP-T, dual inoculated with F. mosseae and P. putida and infested with T. absoluta. The data are mean ± SD. Different uppercase letters are indicating significant differences (P < 0.05) between treatments with or without T. absoluta infestation. Different lowercase letters are indicating significant difference of P < 0.05 among the four treatments
Effect of microbial inoculants and T. absoluta infestation on the tomato defense
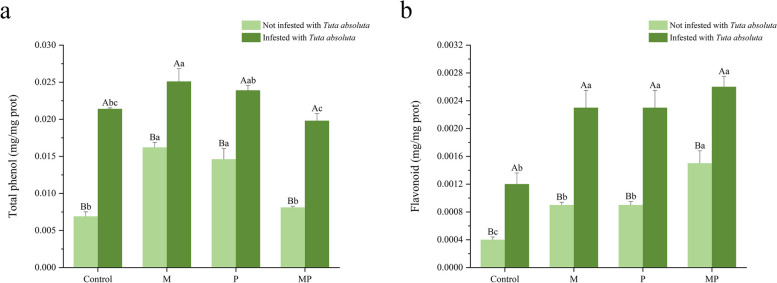
In terms of total phenol in plants, the M and P treatments significantly increased the total phenol concentration in tomato without T. absoluta infestation, by 134.78% and 111.59%, respectively, compared to the uninoculated treatment. Infestation by T. absoluta significantly increased the total phenol concentration in tomato. Among the plants infected by T. absoluta, only the M treatment significantly increased the total phenol concentration of tomato, by 17.29% (Fig. 2a). Regarding flavonoids, the concentration in plants infested with T. absoluta was significantly higher than in those without infestation. Both in the presence and absence of T. absoluta infestation, the M, P, and MP treatments significantly increased flavonoid concentrations compared to the uninoculated treatment (P < 0.05), with the co-inoculated treatment was higher flavonoid concentration than the other inoculated treatments (P < 0.05, Fig. 2b).
Fig. 2.
Effect on secondary metabolites of tomato inoculated with different treatments involving different inoculants and T. absoluta. a Total phenol concentration. b Flavonoid concentration. Different uppercase letters are indicating significant differences (P < 0.05) between treatments with or without T. absoluta infestation. Different lowercase letters are indicating significant difference of P < 0.05 among the four inoculation treatments. Error bars represent ± SD of the mean
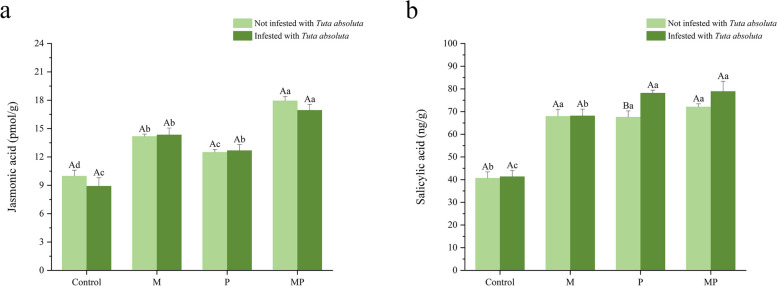
Compared to non-infested T. absoluta, there was no significant difference in jasmonic acid concentration among all inoculated treatments of tomato infested with T. absoluta (Fig. 3a). In the absence of T. absoluta infestation, jasmonic acid concentrations in tomato inoculated with M, P, and MP increased by 42.08%, 25.35%, and 79.86%, respectively. In the infestation of T. absoluta, salicylic acid concentrations in tomato inoculated with M, P, MP increased by 42.15%, 60.87%, and 90.02%, respectively. In comparison to non-infested T. absoluta, there was no significant difference in the salicylic acid concentration in tomato infested with T. absoluta, except for the P treatment (Fig. 3b). In the absence of T. absoluta infestation, salicylic acid concentrations in tomato inoculated with M, P, and MP increased by 66.99%, 66.16%, and 77.24%, respectively. In the infestation of T. absoluta, salicylic acid concentrations in tomato inoculated with M, P, and MP increased by 64.90%, 89.11%, and 90.90%, respectively.
Fig. 3.
Effect on endogenous hormone in tomato inoculated with different treatments involving different inoculants and T. absoluta. a JA (Jasmonic acid) of tomato. b SA (Salicylic acid) of tomato. Different uppercase letters are indicating significant differences (P < 0.05) between treatments with or without T. absoluta. Different lowercase letters are indicating significant difference of P < 0.05 among the four inoculations treatments. Error bars represent ± SD of the mean
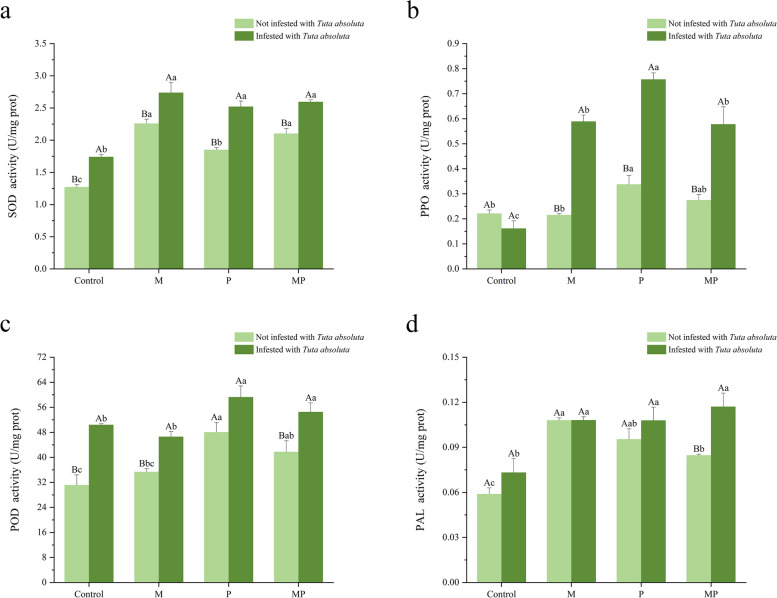
In comparison to tomato non-infested by T. absoluta, the activity of SOD was significantly increased in all inoculation treatments infested by T. absoluta; the activity of PPO was significantly increased in the M, P, and MP treatments; the activity of POD was significantly increased in the C, M, and MP treatments; the activity of PAL was significantly increased in the MP treatment (Fig. 4). The effects of different inoculation treatments on the antioxidant enzyme activity of tomato were different. Compared to the uninoculated treatment without T. absoluta infestation, the SOD activity in tomato treated with M, P, and MP increased by 77.59%, 45.26%, and 65.25%; the PPO activity in tomato treated with P increased by 52.59%; the POD activity in tomato treated with P and MP increased by 54.28% and 34.12%; the PAL activity of tomato treated with P, M, and MP increased by 86.21%, 61.97%, and 43.80%. In contrast to the control treatment infested by T. absoluta, the SOD activity in tomato treated with M, P, the MP increased by 44.91%, 49.16%, and 75.38%; the PPO activity in tomato treated with MP, M, and P increased by 258.15%, 265.16%, and 269.31%; the POD activity in tomato treated with P and MP increased by 17.02% and 27.20%; the PAL activity in tomato treated with P, M, and MP increased by 47.40%, 47.68%, and 59.97%.
Fig. 4.
Effect on antioxidant defense enzymes activity in tomato inoculated with different treatments involving different inoculants and T. absoluta. a SOD activity. b PPO activity. c POD activity. d PAL activity. Different uppercase letters are indicating significant differences (P < 0.05) between treatments with or without T. absoluta infestation. Different lowercase letters are indicating significant difference of P < 0.05 among the four inoculation treatments. Error bars represent ± SD of the mean
AMF colonization rate and Pseudomonas density
No bacterial colonies in the rhizosphere soil of the no P. putida inoculation treatments. The population density of Pseudomonas in tomato rhizosphere soil treated with dual inoculation was significantly higher than that treated with single inoculation (Fig. 5a). Compared to the P treatment, the population density of P. putida in the rhizosphere soil of the MP treatment increased by 183.73%. The population density of P. putida of the MP treatment increased by 162.55% when infested by T. absoluta. While T. absoluta infestation resulted in a decrease in population density of Pseudomonas in both the P and MP treatments, no significant difference was observed when compared to the non-infestation treatment.
Fig. 5.
Density of Pseudomonas (a) and mycorrhizal colonization of tomato roots (b) infected or not with T. absoluta under different inoculations. Tu-, infested without T. absoluta; Tu + , infested with T. absoluta. Different lowercase letters in the figure indicate a significant difference of P < 0.05 between treatments
No AMF colonization in tomato roots in the no F. mosseae inoculation treatments. In the treatment without T. absoluta infestation, the AMF colonization rate in the MP treatment was significantly higher than that in the M treatment, with an increase of 13.08% (Fig. 5b). However, in the treatment with T. absoluta infestation, no significant difference was observed between the AMF colonization rate in the MP treatment and M treatment. Compared to the treatment without T. absoluta infestation, the M and MP treatments with T. absoluta infestation significantly increased by 34.16% and 18.75%, respectively, indicating that T. absoluta infestation increased the AMF colonization rate.
Correlation of root colonization rate of F. mosseae, the population density of P. putida with plant parameters
The correlation between root colonization rates and tomato growth and defense parameters, under different inoculation treatments during T. absoluta infestation was examined (Table S1). In both the M and MP treatments, the colonization rate of F. mosseae was positively correlated with biomass, soluble sugar, chlorophyll, flavonoids, concentrations of soluble sugar, chlorophyll, flavonoids, JA and SA, as well as the enzyme activities of SOD, PPO, and PAL. In the MP treatment, the mycorrhizal colonization rate demonstrated a significant positive correlation with starch and protein.
The correlation between population density of Pseudomonas and the growth and defense parameters of tomato infested by T. absoluta revealed that both P and MP treatments were positively correlated with biomass, concentrations of soluble sugars, protein, starch, chlorophyll, flavonoids, JA, and SA, the enzyme activities of SOD and PPO (Table S2).
Effects of different inoculation treatments on the loss rates of tomato leaves, development duration, protective and detoxifying enzymes of T. absoluta
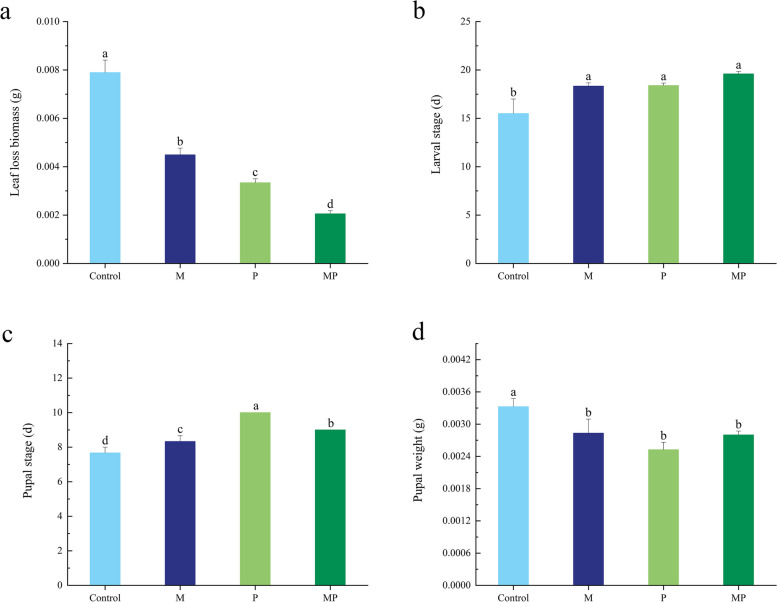
The inoculation treatment significantly inhibited the feeding on tomato leaf by T. absoluta. The loss rates of tomato leaves treated with M, P, MP inoculation decreased by 43.09%, 57.67%, and 74.02%, respectively, compared to the uninoculated treatment (Fig. 6a). The larval period of the T. absoluta feeding on uninoculated tomato was 15.50 ± 1.50 d, while the larval period for those feeding on the inoculated tomato (M, P, MP) was significantly longer, measuring 18.33 ± 0.33 d, 18.40 ± 0.24 d, and 19.60 ± 0.24 d, respectively (Fig. 6b). Compared to the uninoculated treatment, the inoculation treatment significantly prolonged the pupal stage of the T. absoluta, which was arranged in the following order: P > MP > M > C (Fig. 6c). Additionally, the pupal weight of T. absoluta feeding on tomatoes treated with inoculation M, P, MP were reduced, but no significant differences were observed among the three inoculation treatments (Fig. 6d). These results indicate that inoculation with arbuscular mycorrhizal fungi (AMF) and Pseudomonas influences the growth and development of the T. absoluta by affecting the tomatoes.
Fig. 6.
The effects of different inoculation treatments on growth and development of T. absoluta. a Leaf loss biomass. b Larval stage of T. absoluta. c Pupal stage of T. absoluta. d Pupal weight of T. absoluta. Different lowercase letters in the figure indicate a significant difference of P < 0.05 between treatments
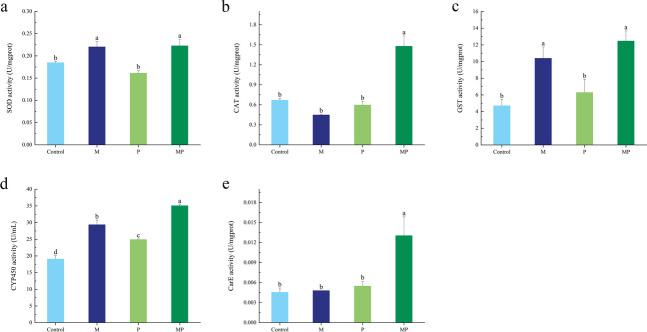
Different inoculation treatments have different effects on the activities of protective and detoxifying enzymes in the T. absoluta (Fig. 7). The M, P, and MP treatments significantly enhanced the activity of the CYP450 enzyme, with the following order: MP > M > P > C. Both the M and MP treatments significantly increased the activities of SOD and GST enzymes in the T. absoluta (P < 0.05), while the enzyme activities of the P treatment were not significantly different from those in the uninoculated treatment. For CAT and CarE enzyme activities, only the MP treatment increased compared to the uninoculated treatment, while the other two inoculation treatments showed no significant difference.
Fig. 7.
The effects of different inoculation treatments on protective detoxification enzyme activities of T. absoluta. a SOD activity. b CAT activity. c GST activity. d CYP450 activity. e CarE activity. Different lowercase letters in the figure indicate a significant difference of P < 0.05 between treatments
Discussion
Beneficial microorganisms provide essential support to plants in response to herbivorous growth and survival through various direct and indirect mechanisms [41, 42]. Arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria have been widely recognized for their roles in improving plant growth and nutritional status [43, 44]. Our study found that the biomass of tomato treated with microbial inoculation was significantly higher than that of the non-inoculated treatment, both in non-infested and infested with T. absoluta (Fig. 1). AMF and PGPR enhance the plant’s ability to absorb and transport nutrients, thereby promoting growth through the synthesis of essential substances such as sugars, proteins, starch, and chlorophyll. Our results are consistent with those of Minchev et al. and He et al., which found that F. mosseae and P. putida can enhance tomato growth [19, 45]. The T. absoluta infests tomato and damages their plant tissues, leading to a decrease in various nutrient concentrations and causing tomato wilt or even death [46]. AMF and PGPR can improve plant tolerance to biotic stress by enhancing their growth following herbivory [47, 48]. Our results showed that inoculation with F. mosseae, P. putida and their dual-inoculation can mitigate the inhibitory effect of T. absoluta infestation on tomato by increasing the concentrations of soluble sugar, protein, starch, and chlorophyll (Table 1). Previous studies have also shown that F. mosseae and P. putida significantly enhance tomato tolerance to both biotic and abiotic stresses by improving nutrient concentration and photosynthesis [13, 49, 50]. Additionally, our study found that co-inoculation with F. mosseae and P. putida resulted in the highest biomass of tomato infested by T. absoluta, indicating that the dual-inoculation treatment was most effective in improving the tomato tolerance to this pest. Previous studies have demonstrated that AMF and PGPR not only enhance plant tolerance but also improve plant resistance to herbivores, thereby reducing herbivore performance [12]. Further research is needed to identify the defense mechanisms of beneficial microorganisms in tomato and their effectiveness against T. absoluta.
An increasing number of studies have demonstrated that microorganisms can facilitate plant in allocating resources to both tolerance and resistance traits simultaneously [51]. Plant resistance to herbivores may be closely related to the presence of microbial communities in the soil [52]. The physiological process of plant defense against insect feeding primarily involves the synthesis of secondary metabolites, phytohormone regulation, and the enhancement of antioxidant defense enzymes activity [53, 54]. Our results showed that the total phenol and flavonoid concentration in tomato infested with T. absoluta was significantly increased, which is consistent with previous studies that reported an increase in total phenol and flavonoid accumulation in plants following insect feeding [12]. The inoculation of F. mosseae, P. putida, and their dual-inoculation resulted in the production of substantial amounts of total phenols and flavonoids, with further increase in their concentration observed upon infestation by T. absoluta. In the infestation of T. absoluta, the total phenol concentration of tomato was significantly higher in inoculation with F. mosseae compared to the uninoculated treatment, conformity with the suggestions of Fateme et al. [12] that the total phenol concentration in plants treated with increased significantly following T. absoluta infestation, thereby initiating a defense response. In the infestation of T. absoluta, the flavonoid concentration was significantly enhanced by inoculation of F. mosseae, P. putida, and their dual-inoculation. Flavonoids serve to protect plants from herbivores by influencing the behavior, growth, and development of insects [55]. Additionally, our results showed that inoculation of F. mosseae, P. putida, and their dual-inoculation significantly increased jasmonic acid (JA) and salicylic acid (SA) concentrations in tomato. Numerous studies have demonstrated that JA and SA pathways play crucial roles in AMF and PGPR mediated defense initiation, thereby triggering systemic resistance against pathogen or insect infestation [54, 56]. However, the infestation by T. absoluta did not significantly increase in the levels of these two hormones. Chen et al. [57] compared the biochemical responses of tomatoes and eggplants to T. absoluta feeding and found that the concentrations of JA and SA in tomato affected by the T. absoluta did not increase and were significantly lower than tose in eggplants, rendering tomato more susceptible to feeding. Thereby, inoculation with F. mosseae, P. putida, and their dual-inoculation can enhance tomato resistance by increasing JA and SA concentrations and modulating the associated signaling pathways. Biological stress often leads to the production of reactive oxygen species (ROS), which can be toxic to plant cells [58]. AMF and Pseudomonas have been shown to stimulate the production of antioxidant defense enzymes, thereby promoting plant growth [59, 60]. Our results found that the inoculation of F. mosseae and P. putida, whether alone or in combination, can enhance the activities of antioxidant defense enzymes in tomato infested by T. absoluta. Furthermore, the infestation of tomato by T. absoluta significantly increases the activities of SOD, POD, and PPO in tomato leaves, which is plant response to insect feeding. These enzymes also play a role in mediating the synthesis of certain secondary metabolites, which can inhibit insect feeding and performance [60, 61]. Our results are consistent with those of Fateme et al. [12] and Senthilraja et al. [41] which showed that the antioxidant defense enzymes in tomato inoculated with AMF or PGPR significantly increases during insect infestation, rendering the plants less suitable for the insects. Overall, our results indicated that F. mosseae and/or P. putida can indirectly enhance tomato resistance to T. absoluta by increasing secondary metabolite concentrations, phytohormone concentrations, and antioxidant defense enzyme activities.
Several studies have demonstrated that AMF and Pseudomonas spp. can mutually promote the growth and development of both microorganisms [62, 63]. Our results showed that F. mosseae and P. putida mutually promoted their growth; the dual-inoculation treatment significantly increased both the AMF colonization rate and Pseudomonas density. Insect feeding can have positive, neutral, or negative effects on AMF colonization rates and the bacterial density in rhizosphere soil [64, 65]. Our findings revealed that infestation with T. absoluta did not significantly affect the density of P. putida in the rhizosphere soil, while it did significantly enhance the AMF colonization rate in tomato roots. This result is similar to those documented in Fateme et al. [32], which also reported that T. absoluta infestation increased the AMF colonization rate and mitigated the impacts of insect feeding by enhancing nutrient absorption. This phenomenon is attributed to alterations in root exudates induced by AMF due to herbivory, as well as the potential increase in photosynthetic rates or root carbon exudation following plant tissue damage, which may facilitate AMF colonization [66]. The AMF colonization rate of root and bacterial density in rhizosphere soil are positively correlated with the feedback of these two microorganisms to plants [49, 67]. Our correlation analysis found that both the AMF colonization rate and P. putida density were positively correlated with most of the growth and defense traits in tomatoes infested by T. absoluta. This positive correlation was further enhanced by dual-inoculation treatment (Table S1, S2). Our results found that dual-inoculation with F. mosseae and P. putida significantly alleviated the inhibitory effects of T. absoluta infestation on tomato biomass, and significantly increased the flavonoids and jasmonic acid concentrations in the infestation of T. absoluta. These findings are consistent with previous studies that demonstrate dual-inoculation treatments with AMF and Pseudomonas significantly enhance plant defense against both biotic and abiotic stresses [49, 68], while also promoting plant growth and yield [44, 69]. Further studies on the growth and development of T. absoluta are necessary to confirm the alterations in these plant indicators.
This study also investigated the effects of different microbial treatments on leaf loss rate, development duration, and enzyme activities of T. absoluta. We found that larvae consumed fewer leaves in inoculated plants, with minimal leaf consumption in the dual-inoculated treatment. Fateme et al. [12] also found that inoculation with AMF inhibited leafminer feeding by enhancing nutrient absorption and stimulating the production of phenolic compounds. PGPR can also regulate the volatiles, metabolites, and defense structures of plant leaves, thereby inhibiting insect herbivory [59, 70]. Our findings indicate that the larval and pupal stages of the T. absoluta were significantly prolonged, and the pupal weight was significantly reduced in feeding on tomato leaves treated with inoculants. Our results are consistent with the findings of Fateme et al. [12] and Senthilraja et al. [41], which indicated that inoculation with AMF and PGPR prolongs the generation time of T. absoluta. This extended developmental period may result in prolonged exposure to natural enemies, potentially enhancing the effectiveness of biological control. When T. absoluta fed on tomato leaves treated with different inoculants, the protective and detoxifying enzyme activities increased, suggesting that the tomato treated with inoculation had enhanced defense against the T. absoluta compared to the non-inoculated treatment. Tomato inoculated with F. mosseae and/or P. putida presence compounds that inhibit feeding, reduce development, and decrease egg laying, thereby impeding the growth and development of the T. absoluta. This experiment utilizes pot experiments to investigate the effect of F. mosseae and/or P. putida on the responses of tomato to T. absoluta herbivory. Given that the function of microorganisms is highly dependent on environmental conditions, their influence on plant growth and defense mechanisms may be significantly affected by various environmental factors [71]. Therefore, our experimental methods and results require further validation in field conditions.
Conclusion
Our results indicated that F. mosseae and/or P. putida can enhance both the growth and defense abilities of tomato, and these two microorganisms can form a synergistic effect to have stronger positive feedback on the tomato. The T. absoluta exhibits reduced adaptability when feeding on tomato leaf inoculated with F. mosseae and/or P. putida, manifested as a decrease in leaf loss rate, inhibition of growth period and egg weight, and increased activity of protective and detoxifying enzymes. In the future, F. mosseae and P. putida may be utilized to resist pests and enhance yields in tomato production, which is an important way to achieve green, healthy, and sustainable agricultural development.
Supplementary Information
Acknowledgements
The authors acknowledge the facility support of the State Key Laboratory for Conservation and Utilization of Bioresources in Yunnan, and also grateful to the editors and the reviewers for their valuable comments and help.
Clinical trial number
Not applicable.
Abbreviations
- AMF
Arbuscular mycorrhizal fungal
- IPM
Integrated pest management
- PGPR
Plant growth-promoting rhizobacteria
- C
Uninoculated treatment used as the control
- M
Inoculated with Funneliformis mosseae
- P
Inoculated with Pseudomonas putida
- MP
Dual inoculated with F. mosseae and P. putida
- JA
Jasmonic acid
- SA
Salicylic acid
Authors’ contributions
W.Y.Z.: conducted the investigation, performed analyses, and wrote the main manuscript text. E.W.D.: conceived and designed the research, data curation, revised the manuscript. R.C.L.: sampling and carried out the comparative analysis. Y.P.C.: conceptualized the study, assisted with the writing, and reviewed and edited the manuscript. Z.X.S.: revised the manuscript. F.R.G.: conceptualized and revised the manuscript, supervised, provided funding, and coordinated the work related to this manuscript.
Funding
This study was funded by the National Key R&D Program of China (grant No. 2021YFD1400200).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenyuan Zhao and Ewei Du should be considered joint first author.
References
- 1.Jabamo T, Ayalew G, Goftishu M, Wakgari M. Integrated effect of insecticide and sex pheromone on the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Crop Prot. 2023;171:106285. [Google Scholar]
- 2.Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narváez-Vasquez CA, et al. Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci. 2010;83(3):197–215. [Google Scholar]
- 3.Gharekhani G, Salek-Ebrahimi H. Evaluating the damage of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on some cultivars of tomato under greenhouse condition. Arch Phytopath Plant. 2013;47(4):429–36. [Google Scholar]
- 4.Zhang GF, Xian XQ, Zhang YB, Zhang R, Ma DY, Liu WX, et al. Warning of the dispersal of a newly invaded alien species, tomato leaf miner Tuta absoluta (Meyrick), in China. Plant Prot. 2020;46:281–6. [Google Scholar]
- 5.Zhang GF, Xian XQ, Zhang YB, Liu WX, Liu H, Feng XD, et al. Outbreak of the south American tomato leafminer, Tuta absoluta, in the Chinese mainland: Geographic and potential host range expansion. Pest Manag Sci. 2021;77:5475–88. [DOI] [PubMed] [Google Scholar]
- 6.Han P, Desneux N, Becker C, Larbat R, Le Bot J, Adamowicz S, et al. Bottom-up effects of irrigation, fertilization and plant resistance on Tuta absoluta: Implications for Integrated Pest Management. J Pest Sci. 2019;92(4):1359–70. [Google Scholar]
- 7.Bani K, Asghari B, Naeem K. PGPR modulation of secondary metabolites in tomato infested with Spodoptera litura. Agronomy. 2020;10(6):778. [Google Scholar]
- 8.Frew A, Antunes PM, Cameron DD, Hartley SE, Johnson SN, Rillig MC, et al. Plant herbivore protection by arbuscular mycorrhizas: A role for fungal diversity? New phytol. 2021;3:1022–31. [DOI] [PubMed] [Google Scholar]
- 9.Smith SE. 2008. Mycorrhizal symbiosis. 3rd ed. Academic Press. 2021:273-81
- 10.Brundrett MC, Tedersoo L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018;4:1108–15. [DOI] [PubMed] [Google Scholar]
- 11.Jiang YN, Wang WX, Xie QJ, Liu N, Liu LX, Wang DP, et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356:1172–5. [DOI] [PubMed] [Google Scholar]
- 12.Fateme S, Shahnaz S-N, Guy S. Effect of arbuscular mycorrhizal colonization on tomato defense metabolites and population parameters of Tuta absoluta (Meyrick). Arthropod-Plant Inte. 2024;18(2):339–51. [Google Scholar]
- 13.Camila A, Laura FB, Josefina B, María SV. Arbuscular mycorrhizal fungi in tomato tolerance to pathogens and nematodes: A comprehensive review. Sci Hortic-Amsterdam. 2024;329(1): 112969. [Google Scholar]
- 14.Song YY, Ye M, Li CY, Wang RL, Wei XC, Luo SM, et al. Priming of anti-herbivore defense in tomato by arbuscular mycorrhizal fungus and involvement of the jasmonate pathway. J Chem Ecol. 2013;39(7):1036–44. [DOI] [PubMed] [Google Scholar]
- 15.Shrivastava G, Ownley HB, Augé MR, Toler H, Dee M, Vu A, et al. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis. 2015;65(2):65–74. [Google Scholar]
- 16.Kehri HK, Akhtar O, Zoomi I, Pandey D. Arbuscular mycorrhizal fungi: taxonomy and its systematics. Inter J Life Sci Res. 2018;6(4):58–71. [Google Scholar]
- 17.Fasusi OA, Babalola OO, Adejumo TO. Harnessing of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi in agroecosystem sustainability. CABI Agric Biosci. 2023;4:26. [Google Scholar]
- 18.Hernández-Montiel LG, Chiquito CCJ, Murillo Amador B, Vidal H, Librado QA, Evanjelina E, et al. Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. J Soil Sci Plant Nut. 2017;17(4):1003–12. [Google Scholar]
- 19.He YH, Pantigoso HA, Wu ZS, Vivanco JM. Co-inoculation of Bacillus spp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J Appl Microbiol. 2019;1:196–207. [DOI] [PubMed] [Google Scholar]
- 20.Nicolás P, Oscar M, Sonia F, Virginia L, Marisa R. Potential of Pseudomonas putida PCI2 for the protection of tomato plants against fungal pathogens. Curr Microbiol. 2016;73(3):346–53. [DOI] [PubMed] [Google Scholar]
- 21.Zhai YL, Shao ZZ, Cai MM, Zheng LY, Li GY, Yu ZN, et al. Cyclo(l-Pro-l-Leu) of Pseudomonas putida MCCC 1A00316 isolated from antarctic soil: identification and characterization of activity against Meloidogyne incognita. Molecules. 2019;24(4):768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang F, Ding L, Zhao D, Fan HY, Zhu XF, Duan YX, et al. Identification and functional analysis of tomato microRNAs in the biocontrol bacterium Pseudomonas putida induced plant resistance to Meloidogyne incognita. Phytopathology. 2022;112(11):2373–82. [DOI] [PubMed] [Google Scholar]
- 23.Sharma M, Saini I, Kaushik P, Aldawsari MM, Al Balawi T, Alam P. Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi J Biol Sci. 2021;28(7):3685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elisa B, Simone C, Nadia M, Paola M, Francesco M, Andrea C, et al. Arbuscular mycorrhizal fungi and plant growth-promoting Pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza. 2017;27(1):1–11. [DOI] [PubMed] [Google Scholar]
- 25.Hidri R, Mahmoud OMB, Farhat N, Cordero I, Pueyo JJ, Debez A, et al. Arbuscular mycorrhizal fungus and rhizobacteria affect the physiology and performance of Sulla coronaria plants subjected to salt stress by mitigation of ionic imbalance. J Plant Nutr Soil Sci. 2019;182(3):451–62. [Google Scholar]
- 26.Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, et al. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 2015;17:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicola I, Xavier C, Klaus S, Marie F, Daniela V, Geoffrey J, et al. Combined field inoculations of Pseudomonas bacteria, arbuscular mycorrhizal fungi, and entomopathogenic nematodes and their effects on wheat performance. Front Plant Sci. 2017;8:1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behn O. Influence of Pseudomonas fluorescens and arbuscular mycorrhiza on the growth, yield, quality and resistance of wheat infected with Gaeumannomyces graminis. J Plant Dis Protect. 2008;115(1):4–8. [Google Scholar]
- 29.Marro N, Caccia M, Doucet ME, Marta C, Alejandra B, Paola L. Mycorrhizas reduce tomato root penetration by false root-knot nematode Nacobbus aberrans. Appl Soil Ecol. 2018;124:262–5. [Google Scholar]
- 30.Dong CJ, Wang LL, Li Q, Shang QM. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE. 2019;14(11):e0223847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SW, Ahn IP, Lim JW, Lee YH. Pseudomonas putida strain 17 isolated from replant soil promotes tomato growth and inhibits conidial germination of soilborne plant pathogens. Plant Patholog J. 2005;21(3):244–51. [Google Scholar]
- 32.Fateme S, Shahnaz S, Ebrahim S. The impact of arbuscular mycorrhizal fungi on tomato plant resistance against Tuta absoluta (Meyrick) in greenhouse conditions. J Asia-Pacific Entomology. 2022;25(3):11971. [Google Scholar]
- 33.Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 2008;18:287–96. [DOI] [PubMed] [Google Scholar]
- 34.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6. [Google Scholar]
- 35.Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 1987;148:350–82. [Google Scholar]
- 36.Sohrabi F, Nooryazdan HR, Gharati B, Saeidi Z. Plant Resistance to the Moth Tuta absoluta (Meyrick) (Lepidoptera:Gelechiidae) in Tomato Cultivars. Neotrop Entomol. 2017;46:203–9. [DOI] [PubMed] [Google Scholar]
- 37.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 38.Liu RJ, Dai M, Wu X, Li M, Liu XZ. Suppression of the root-knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] on tomato by dual inoculation with arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria. Mycorrhiza. 2012;22:289–96. [DOI] [PubMed] [Google Scholar]
- 39.Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55(1):158–61. [Google Scholar]
- 40.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. [DOI] [PubMed] [Google Scholar]
- 41.Senthilraja G, Anand T, Kennedy JS, Raguchander T, Samiyappan R. Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leafminer insect and collar rot pathogen. Physiol Mol Plant P. 2013;82:10–9. [Google Scholar]
- 42.Vincze ÉB, Becze A, Laslo É, Mara G. Beneficial soil microbiomes and their potential role in plant growth and soil fertility. Agriculture. 2024;14:152. [Google Scholar]
- 43.Begum N, Wang L, Ahmad H, Akhtar K, Roya R, Khan MI, et al. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb Ecol. 2022;83:971–88. [DOI] [PubMed] [Google Scholar]
- 44.Zhang LL, Zuluaga MYA, Pii Y, Barone A, Amaducci S, Miras-Moreno B, et al. A Pseudomonas plant growth promoting rhizobacterium and arbuscular mycorrhiza differentially modulate the growth, photosynthetic performance, nutrients allocation, and stress response mechanisms triggered by a mild Zinc and Cadmium stress in tomato. Plant Sci. 2023;337:111873. [DOI] [PubMed] [Google Scholar]
- 45.Minchev Z, Ramírez-Serrano B, Dejana L, Lee DAS, Zitlalpopoca-Hernandez G, Orine D, et al. Beneficial soil fungi enhance tomato crop productivity and resistance to the leaf-mining pest Tuta absoluta in agronomic conditions. Agron Sustain Dev. 2024;44(6):1–16. [Google Scholar]
- 46.Santana P, Kumar L, Silva RD, Picanço M. Global geographic distribution of Tuta absoluta as affected by climate change. J Pest Sci. 2019;92:1373–85. [Google Scholar]
- 47.Hosseini A, Hosseini M, Schausberger P. Plant Growth-Promoting Rhizobacteria enhance defense of strawberry plants against spider mites. Front Plant Sci. 2022;12:783578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lina B, Michael JS. The effect of mycorrhizal seed treatments on rice growth, yield, and tolerance to insect herbivores. J Pest Sci. 2021;94:375–92. [Google Scholar]
- 49.Papadopoulou A, Matsi T, Kamou N, Avdouli D, Mellidou I, Karamanoli K. Decoding the potential of a new Pseudomonas putida strain for inducing drought tolerance of tomato (Solanum lycopersicum) plants through seed biopriming. J Plant Physiol. 2022;271:153658. [DOI] [PubMed] [Google Scholar]
- 50.Litsa A, Poulaki EG, Vasilis D, Mavrommati M, Amourgis GG, Tjamos SE. Enhancing botrytis disease management in tomato plants: insights from a Pseudomonas putida strain with biocontrol activity. J Appl Microbiol. 2024;135(4):4. [DOI] [PubMed] [Google Scholar]
- 51.Frew A, Powell JR, Johnson SN. Aboveground resource allocation in response to root herbivory as affected by the arbuscular mycorrhizal symbiosis. Plant Soil. 2019;447(1–2):463–73. [Google Scholar]
- 52.Pang ZQ, Chen J, Wang TH, Gao CS, Li ZM, Guo LE, et al. Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci. 2021;1(12): 621276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song YY, Cui HY, Guo WX, Sindhu L, Lv SH, Li LL, et al. Endophytic fungi improved wheat resistance to Rhopalosiphum padi by decreasing its feeding efficiency and population fitness. Ecotox Environ Safe. 2023;270: 115865. [DOI] [PubMed] [Google Scholar]
- 54.Aljbory Z, Chen MS. Indirect plant defense against insect herbivores: a review. Insect Sci. 2018;25(1):2–23. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Xia X, Zhang Z, Nong B, Zeng Y, Wu Y, et al. Identification of anthocyanin biosynthesis genes in rice pericarp using PCAMP. Plant Biotechnol J. 2019;17(9):1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shu P, Zhang SJ, Li YJ, Wang XY, Yao L, Sheng JP, et al. Over-expression of SlWRKY46 in tomato plants increases susceptibility to Botrytis cinerea by modulating ROS homeostasis and SA and JA signaling pathways. Plant Physiol Bioch. 2021;166:1–9. [DOI] [PubMed] [Google Scholar]
- 57.Chen LM, Li XW, He TJ, Li PJ, Liu Y, Zhou SX, et al. Comparative biochemical and transcriptome analyses in tomato and eggplant reveal their differential responses to Tuta absoluta infestation. Genomics. 2021;113(4):2108–21. [DOI] [PubMed] [Google Scholar]
- 58.Mylona PV, Polidoros AN, Scandalios JG. Modulation of antioxidant responses by arsenic in maize. Free Radical Bio Med. 1998;25(4–5):576–85. [DOI] [PubMed] [Google Scholar]
- 59.Bano A, Muqarab R. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.). Plant Biology. 2017;19(3):406–12. [DOI] [PubMed] [Google Scholar]
- 60.Yu L, Zhang WT, Geng YY, Liu KS, Shao XQ. Cooperation with arbuscular mycorrhizal fungi increases plant nutrient uptake and improves defenses against insects. Front Ecol Evol. 2022;10:833389. [Google Scholar]
- 61.Kumar PA, Bhasker K, Nikhil KSB, Srinivas P. Role of phenylpropanoids and flavonoids in plant defense mechanism. Int J Environ Clim Change. 2023;13(9):2951–60. [Google Scholar]
- 62.Yu L, Zhang H, Zhang WT, Liu KS, Liu M, Shao XQ. Cooperation between arbuscular mycorrhizal fungi and plant growth-promoting bacteria and their effects on plant growth and soil quality. PeerJ. 2022;10:e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pivato B, Offre P, Marchelli S, Barbonaglia B, Mougel C, Lemanceau P, et al. Bacterial effects on arbuscular mycorrhizal fungi and mycorrhiza development as influenced by the bacteria, fungi, and host plant. Mycorrhiza. 2009;19(2):81–90. [DOI] [PubMed] [Google Scholar]
- 64.Van der HM, Bennett JA, Pither J, Hart M. Longterm effects of grazing on arbuscular mycorrhizal fungi. Agr Ecosyst Environ. 2017;243:27–33. [Google Scholar]
- 65.Khaitov B, Patino-Ruiz JD, Pina T, Schausberger P. Interrelated effects of mycorrhiza and free-living nitrogen fixers cascade up to aboveground herbivores. Ecol Evol. 2015;5(17):3756–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narula N, Kothe E, Behl RK. Role of root exudates in plant-microbe interactions. J Appl Bot Food Qual. 2009;82(2):122–30. [Google Scholar]
- 67.Jian QH, Zhang TR, Wang YY, Guan L, Li LL, Wu LN, et al. Biocontrol potential of plant growth-promoting rhizobacteria against plant disease and insect pest. Antonie Van Leeuwenhoek. 2024;117:92. [DOI] [PubMed] [Google Scholar]
- 68.Sharma IP, Sharma AK. Physiological and biochemical changes in tomato cultivar PT-3 with dual inoculation of mycorrhiza and PGPR against root-knot nematode. Symbiosis. 2017;71:175–83. [Google Scholar]
- 69.Baniyaghob AM, Hamid M, Khalil K, Faezeh R, Raziyeh A. Arbuscular mycorrhizal fungi (AMF) and Plant Growth-promoting Rhizobacteria (PGPR) as an alternative to mineral fertilizers to improve the growth, essential oil profile, and phenolic content of Satureja Macrantha L. J Crop Health. 2021;76:347–56. [Google Scholar]
- 70.Rinkee K, Ekta P, Sayyada B, Shahla F, Saurabh P. Plant growth promoting rhizobacteria (PGPR) induced protection: A plant immunity perspective. Physiol Plantarum. 2024;176(5):e14495. [DOI] [PubMed] [Google Scholar]
- 71.Lee DAS, Macheda D, Saha H, Ploll U, Orine D, Biere A. Tackling the context-dependency of microbial-induced resistance. Agron. 2021;11:1–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].