Abstract
Background
In observational studies, frailty has been strongly associated with mental disorders. However, the mechanisms underlying the association between frailty and mental disorders remain unclear.
Methods
We conducted a two-sample Mendelian randomization (MR) study to assess the causal relationship between frailty, as measured by the frailty index (FI), and ten common mental disorders. The datasets involved European ancestry individuals and included measurements of the FI (N = 175,226), schizophrenia (SCZ; N = 320,404), major depressive disorder (MDD; N = 143,265), bipolar disorder (N = 337,199), insomnia (N = 462,341), obsessive–compulsive disorder (N = 33,925), anxiety disorders (N = 463,010), autism spectrum disorder (N = 46,351), anorexia nervosa (N = 14,477), opioid-related mental and behavioral disorders (N = 215,650), and mental and behavioral disorders due to use of other stimulants including caffeine (N = 215,570).
Results
Two-sample MR analyses were performed using inverse variance weighting followed by various sensitivity and validation analyses. Genetically predicted SCZ (odds ratio [OR] = 1.019, 95% confidence interval [CI] 1.005–1.033) and MDD (OR = 1.211, 95% CI 1.092–1.343) had significant causal effects on FI. In the reverse MR analysis, we discovered that MDD was significantly and causally affected by FI (OR = 1.290, 95% CI 1.133–1.469). No causal links were identified between the FI and the other eight common mental disorders. In the Multivariable MR, the estimated MDD effect on FI is comparable to the univariate IVW estimate (OR = 1.298; 95% CI, 1.175 to 1.435), while the estimated SCZ effect on FI fails to be significant compared to the univariate estimate. The results of the sensitivity and validation analyses confirmed stabilization.
Conclusions
Our study found evidence of a causal relationship between SCZ, MDD, and frailty and explored the underlying mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06409-4.
Keywords: Mental disorders, Schizophrenia, Major depressive disorder, Frailty, Mendelian randomization, Causality
Introduction
As awareness of health has increased in recent years, frailty, which may have a variety of adverse outcomes, has received considerable attention [1]. The definition of frailty was first proposed by Fried et al.(2001) [2]. It is considered as a group of syndromes characterized by impaired stress tolerance due to decreased muscle, nutritional deficiencies, hormonal changes, and increased inflammation leading to decreased function of different organs. Frailty dimensions can represent an assessment of biological rather than physiological age, which in turn allows for a valid estimation of an individual's health status [3]. As the global population ages, the prevalence of frailty is likely to increase [4]. Ma, L et al. [5] recently selected 5,844 elderly participants from seven cities based on well-established clustering, stratification, and random selection statistical sampling techniques and found a 9.9% prevalence of frailty. According to a 21-cohort survey involving 61,500 participants, 10.7% of community-dwelling older adults were frail [6]. In addition to being associated with different study population characteristics, however, the prevalence of frailty varies widely across studies, ranging from 4.0% to 59.1% [7], which may also be due to differences in how it is measured. To our knowledge, a number of frailty assessments are available, although the two most commonly used are the frailty phenotype (FP) [2] and the frailty index (FI) [8]. FP refers to a clinical syndrome. Specifically, at least three of the following five manifestations need to be present at the same time: fatigue, weakness, slow walking speed, unintentional weight loss, and low physical activity. Investigations into the frailty phenotype have been extensive and have involved numerous disciplines such as dentistry [9], infectious diseases [10], and cardiovascular [11]. Nonetheless, the presentation of FP may overlap with clinical symptoms of other chronic diseases such as depression, which may lead to misinterpretation of the respondent's condition. The FI is based on the deficit accumulation model and operationalized as the proportion of deficits present in an individual out of the total number of age-related health variables considered. In addition, throughout a person's life expectancy, deficit accumulation is thought to be a stochastic process. Thus, FI lessens the impact of the previously mentioned overlapping symptoms. With the widespread use of stable and reliable FI, further research on frailty has been conducted.
Evidence from epidemiological studies suggests a strong correlation between mental disorders and physical frailty [12–14]. Borges et al. [15] investigated 315 elderly outpatients (mean age 72.1 years, 68.3% female) participating in a cohort study and found that the prevalence of frailty was 14.5%, 46.5%, and 65.1% in those who were nondepressed, subthreshold depressed, and severely depressed, respectively. When the results of the study were validated using the FI-36 index, the prevalence of frailty was found to be 10.2%, 20.9%, and 30.2%, respectively. Although the data obtained varied, the overall trend was consistent. Studies confirming insomnia [16], schizophrenia (SCZ) [17], bipolar disorder [BD] [18], and frailty have produced conclusions similar to those above. In addition, a systematic review that included 20 cross-sectional studies and one longitudinal study from 1,272 references retrieved also found that frail older adults were more likely to exhibit symptoms of anxiety [19]. When the Belgian Bone Club investigated the epidemiology of osteoporosis in frail individuals, patients with anorexia nervosa (AN) were included in the study [20]. Frailty-related analyses have also been addressed in substance abuse research [21]. However, the relationship between autism spectrum disorders (ASD), obsessive–compulsive disorder (OCD), and frailty has been scarcely explored. Interestingly, emerging evidence shows that the conclusions of some previous studies are partial, and mental disorders and frailty could have a bidirectional association [2, 22]. Other researchers have raised concerns about frailty for current young and middle-aged adults [23]. Nevertheless, given that most reviews are cross-sectional epidemiological studies or short-term follow-up studies, it is difficult to conclude the causal relationship between frailty and mental disorders.
With the rapid development of genomics, Mendelian randomization (MR) analysis is widely used in various medical fields [24–26]. The instrumental variables obtained by the MR method are single nucleotides that are closely associated with clinical phenotypes. The influence of confounding and reverse causal associations on study conclusions in observational studies is effectively avoided by using the simulation of random assignment in human genetic processes [27]. Therefore, it is plausible that MR analysis was used to verify the causal association between mental disorders and frailty. Recently, Ni Sang et al. [28] designed and analyzed the causal association between depression and frailty risk using the MR method. However, considering the evident association of frailty with multiple psychiatric disorders and the effective reduction of clinical symptom overlap at assessment by FI, an MR study of the causal association between various common mental disorders and frailty is still needed.
In the present study, instrumental variables obtained from large genetic data significantly associated with ten common mental disorders (SCZ [29], major depressive disorder [MDD] [30], BD (https://gwas.mrcieu.ac.uk/datasets/ukb-a-525/), insomnia [ISN] (https://gwas.mrcieu.ac.uk/datasets/ukb-b-3957/), anxiety disorder [AD] (https://gwas.mrcieu.ac.uk/datasets/ukb-b-11311/), ASD (https://gwas.mrcieu.ac.uk/datasets/ieu-a-1185/), OCD (https://gwas.mrcieu.ac.uk/datasets/ieu-a-1189/), AN [31], opioid-related mental and behavioral disorders [MBDO] (https://gwas.mrcieu.ac.uk/datasets/finn-b-F5_OPIOIDS/), and mental and behavioral disorders due to use of other stimulants [MBDS] (https://gwas.mrcieu.ac.uk/datasets/finn-b-F5_STIMUL/)) were used to analyze the underlying association with frailty in a two-sample MR. Then, the inverse MR was used to detect the association between the two at the genetic level. Finally, multivariate MR was used to explore the direct effect of mental disorders on FI [32]. The purpose of this study was to provide an accurate and comprehensive assessment of the causal association between mental disorders and frailty from a genetic perspective.
Methods
Study design
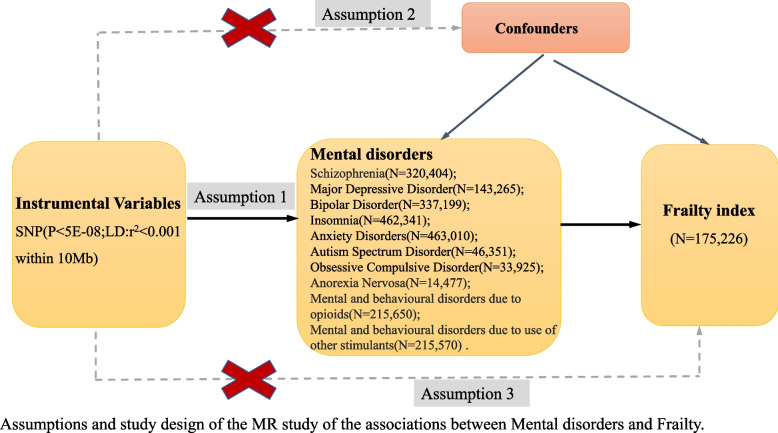
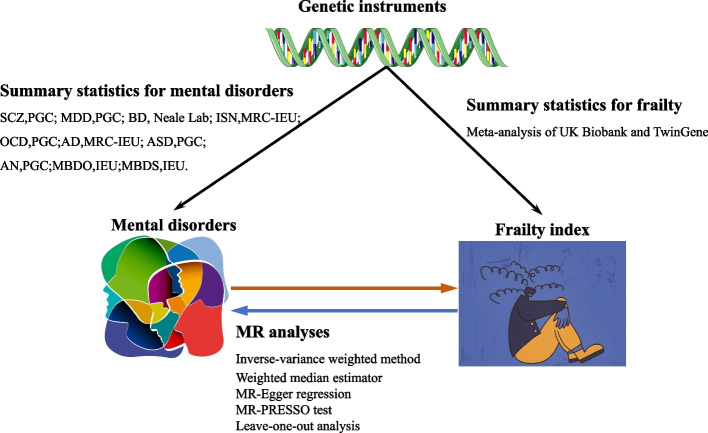
An overview of the MR framework is shown in Fig. 1. To comprehensively assess the causal association between the ten common psychiatric disorders and frailty, a two-sample MR analysis was first performed (e.g., MR analysis of the causal association from the ten psychiatric disorders to frailty), and the reverse analysis was then performed (from frailty to the ten mental disorders) (Fig. 2). All publicly available summary statistics of the GWAS data used for the analysis were downloaded and obtained from the Psychiatric Genomics Consortium (PGC), Neale lab, UK biobank, and Integrative Epidemiology Unit (IEU). Therefore, no additional ethical approval or informed consent was required. This study followed the STROBE-MR guidelines [33].
Fig. 1.
Diagram for Mendelian randomization (MR). MR was developed on the premise of three assumptions. First, SNPs designated as instrumental variables (IVs) should be extremely connected to exposure (Assumption 1). Second, SNPs selected as IVs are required to be independent of confounders (Assumption 2). Third, rather than being directly correlated, IVs and FI (outcome) only have a relationship through mental disorders (exposure) (Assumption 3)
Fig. 2.
Study design of the associations between mental disorders and FI. First, we genetically assessed the causal associations between frailty and ten common mental disorders by 2-sample MR; we then assessed the causal associations between the two aforementioned by reverse MR. Multiple sensitivity analyses confirmed the robustness of the findings
Data source for frailty
Summary statistics for the frailty phenotypic measure of frailty were obtained from a recent meta-analysis of a genome-wide association study (GWAS) in the UK Biobank and TwinGene, Sweden, which included 175,226 participants of European ancestry [34] (Table 1). FI is a continuous measure, expressed as the proportion of the combined total of all age-related health deficits with more than 40 components, covering a wide range of physical and mental health domains [8]. FI, as a proxy for overall health, has been validated as a strong predictor of many adverse health outcomes and has been demonstrated to be more appropriate than other measures for assessing frailty at younger ages [35–37].
Table 1.
The GWAS summary data used in the MR study
| Phenotype | Consortium/ author |
Year | Participants | SNP(N) | Age | N_IVs | URL/PMID |
|---|---|---|---|---|---|---|---|
| Exposure/Outcome | |||||||
| SCZ |
PGC/ Trubetskoy V |
2022 |
320,404 (Ncase:76,755) |
7,585,078 | - | 206 | https://gwas.mrcieu.ac.uk/datasets/ieu-b-5099/; 35,396,580 |
| MDD | PGC/Wray | 2018 |
143,265 (Ncase:45,591) |
1,048,575 | - | 19* | https://doi.org/10.6084/m9.figshare.21655784; 29,700,475 |
| BD |
Neale lab/ UK-B |
2017 |
337,199 (Ncase:303) |
10,894,596 | 44–69 | 27* | https://gwas.mrcieu.ac.uk/datasets/ukb-a-525/ |
| ISN |
MRC-IEU/ UK-B/ Ben Elsworth |
2018 | 462,341 | 9,851,867 | 44–69 | 42 | https://gwas.mrcieu.ac.uk/datasets/ukb-b-3957/ |
| AD |
MRC-IEU/ UK-B/ Ben Elsworth |
2018 |
463,010 (Ncase:1,523) |
9,851,867 | 44–69 | 0 | https://gwas.mrcieu.ac.uk/datasets/ukb-b-11311/ |
| ASD | PGC | 2017 |
46,351 (Ncase:18,382) |
9,112,386 | - | 16* | https://gwas.mrcieu.ac.uk/datasets/ieu-a-1185/ |
| OCD | PGC | 2017 |
33,925 (Ncase:26,888) |
8,409,517 | - | 2* | https://gwas.mrcieu.ac.uk/datasets/ieu-a-1189/ |
| AN | PGC/Duncan | 2017 |
14,477 (Ncase:3,495) |
10,641,224 | 5* | https://gwas.mrcieu.ac.uk/datasets/ieu-a-1186/; 28,494,655 | |
| MBDO | IEU | 2021 |
215,650 (Ncase:651) |
16,380,458 | 20–80 | 1* | https://gwas.mrcieu.ac.uk/datasets/finn-b-F5_OPIOIDS/ |
| MBDS | IEU | 2021 |
215,570 (Ncase:571) |
16,380,435 | 20–80 | 1* | https://gwas.mrcieu.ac.uk/datasets/finn-b-F5_STIMUL/ |
| Outcome/Exposure | |||||||
| FI | Atkins JL | 2021 |
175,226 (Female:90,396) |
7,589,717 | 41–87 | 15 | https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90020053/; 34,431,594 |
Abbreviations: SCZ schizophrenia, MDD major depressive disorder, BD bipolar disorder, ISN insomnia, OCD obsessive compulsive disorder, AD anxiety disorders, ASD autism spectrum disorder, AN anorexia nervosa, MBDO opioid-related mental and behavioral disorders, MBDS mental and behavioral disorders due to use of other stimulants, including caffeine, FI frailty index, PGC Psychiatric Genomics Consortium, IEU integrative epidemiology unit, N_IVs number of instrumental variables,
*the P threshold is 1e-6
Selection of genetic instruments
We selected genetic instruments for ten common psychiatric disorders from the largest GWAS meta-analysis involving European ancestry individuals conducted by PGC, Neale lab, UK biobank, and IEU (Table 1). Independent IVs were obtained after aggregation based on the 1000 Genomes Project linkage disequilibrium (LD) structure (r2 < 0.001 within 10 Mb). Overlapping proxy SNPs in LD (r2 = 0.8) were used when SNPs did not appear in the summary statistics of the corresponding psychiatric phenotype. SNPs that were strongly linked to the current study outcome (p < 5e-08) were also excluded from the IVs before MR analysis was performed. To obtain sufficient IVs for analysis, the P threshold is adjustable to 1e-06.
IVs for the FI were obtained from large GWAS data for 175,226 participants from the UK and Sweden. In addition, we screened IVs for FI at a strict genome-wide statistical significance (p < 5e-08) (Table S10).
Statistical analysis
To better adjust for significant heterogeneity among SNP effects, the multiplicative random effects model in the inverse variance weighting (IVW) approach was applied in this study as the main analysis in a bidirectional MR study [38]. In the multivariate setting, the covariance between the SNP effects for each exposure was fixed at zero. The weighted mean of SNP effects is provided in the IVW approach, where the intercept is constrained to zero [38]. However, assuming that instrumental SNPs show horizontal pleiotropy, the results may be biased, which is a major source of bias in MR settings. Accordingly, univariate IVW estimates were compared with a range of other well-established MR methods to strengthen the robustness of the findings, including weighted median [39], simple model [40], weighted model [41], and MR-Egger regression [39]. To avoid the effect of reverse causal association, we also performed a two-sample reverse directional MR analysis. Based on evidence that SCZ is genetically significantly linked to MDD [30], we analyzed estimates of the immediate effect of the other mental disorder on FI by adjusting one mental disorder in multivariable MR (MVMR). Selecting random effects or fixed effects based on heterogeneity in the univariable MR (UVMR), we performed the IVW MR method in MVMR.
Complementary analysis
To further check the robustness of the results, the Cochran Q statistic was used to check for evidence of heterogeneity (a potential indicator of pleiotropy) in the IVW estimator. The MR Egger intercept test was conducted to detect the presence of directional pleiotropy [39]. Leave-one-out analyses were performed to assess whether the overall estimate was driven by a single SNP. MR-pleiotropy residual sum and outlier (MR-PRESSO) were employed to detect outliers and all MR analyses were repeated after deleting these outlier SNPs. The R2 values explaining the exposure variance and the F-statistic for each SNP were calculated by linear regression analysis of the IVs. F-statistics greater than 10 indicate that the IV is strong and that there is only a marginal amount of bias is attributable to sample overlap [42]. In the sensitivity analyses of MVMR, MVMR-Egger, MVMR-Lasso, and MVMR-Median were applied.
The TwoSampleMR (version 0.5.7), MendelianRandomization (version 0.7.0), and MRPRESSO (version 1.0.0) packages in R software (version 4.2.3) were used for the analysis [40]. Significant correlations were required to meet the following requirements: the MR estimates exceeded standard statistical significance (p < 0.05), the direction of the effect in the sensitivity analyses was generally consistent, and the MR-Egger intercept showed a limited effect of horizontal pleiotropy.
Results
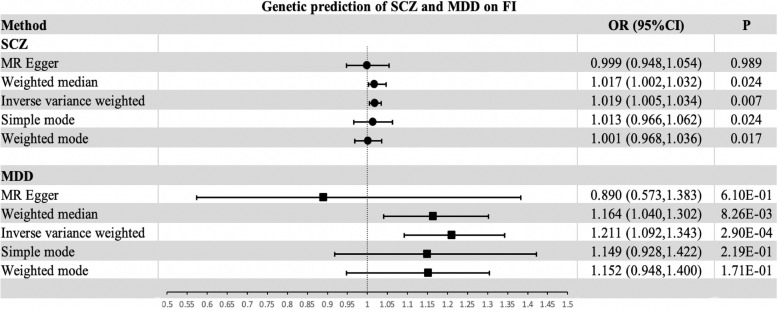
Details of the GWAS database for the ten common psychiatric disorders used in this study and publicly available GWAS data from UK Biobank and TwinGene are presented in Table 1. Based on the screening criteria for IVs, we separately identified key SNPs significantly associated with each of the GWAS phenotypes for the ten psychiatric disorders and frailty, which were subsequently used as independent IVs (TableS1-10). In the two-sample MR study, a statistically significant bidirectional causal relationship was found between the genetic prediction of MDD and FI (MR: odds ratio [OR] = 1.211, 95% confidence interval [CI] 1.092–1.343, p = 2.90e–04; reverse MR: OR = 1.290, 95% CI 1.133–1.469, p = 1.17e–04). Genetically predicted SCZ had a significant causal effect on FI (OR = 1.019, 95% CI 1.005–1.033, p = 0.007), while no significant causal effect of FI on SCZ was found. Nevertheless, no statistically significant causal association was found between BD, ISN, AD, ASD, OCD, AN, MBDO, MBDS, and FI.
Genetically predicted SCZ on FI in univariate MR
According to the selection criteria of genetic instruments in this study, 206 SNPs significantly linked to SCZ were identified (Table S1). The IVW approach shows the causal effect of genetically predicted SCZ on FI (OR = 1.019, 95% CI 1.005–1.033, p = 0.007) (Table S11, Figure S1, and Fig. 3). The sensitivity analysis showed heterogeneity in the estimated effect of SCZ on FI (p < 0.05), but not horizontal pleiotropy (p > 0.05) (Table 2). To control for the effect of heterogeneity on the study, we used a random effects model for the MR analysis. The F-statistics, which were calculated by obtaining the IV of SCZ, were 35.34–135.13, indicating that the intensity of the obtained IVs was good (Table S12). The leave-one-out analysis showed that the estimated effects were relatively stable after excluding any single SNP (Figure S2). The scatterplot and funnel plot demonstrated that the results of the IVW method are robust (Figures S3 and S4). In assessing the causal association between SCZ and FI, the MR-PRESSO test identified ten outliers (Table 2). The causal estimates generated from the MR-PRESSO and MR-PRESSO correction approaches, which eliminate outliers, produced consistent findings (Table S13). Nevertheless, the IVW approach detected no causal association between FI and genetic liability to SCZ in the inverse MR analysis (p = 0.370) (Table S13). Heterogeneity was also present in the estimated effect of FI on SCZ (p < 0.05), but no horizontal pleiotropy was detected (p > 0.05) (Table 2). The F-statistics calculated from the IVs of FI were 39.71–85.38 (Table S14). The MR-PRESSO test found three outliers in the evaluation of FI and genetic liability to SCZ (Table 2).
Fig. 3.
Effect of genetic risk of SCZ and MDD on FI using various methods. Abbreviations: SCZ, schizophrenia; MDD, major depressive disorder; FI, frailty index; MR, mendelian randomization
Table 2.
Association of genetically predicted psychiatric disorders and FI in the sensitivity analysis
| Exposure | Outcome | N_IVs | IVW Q statistic | Q_P value | MR-Egger intercept | Egger_P value | No. of outlier detected by MR-PRESSO |
|---|---|---|---|---|---|---|---|
| SCZ | FI | 206 | 460.34 | 6.91e-24 | 0.0012 | 0.458 | 10 |
| MDD | FI | 19 | 33.29 | 1.53e-02 | 0.0072 | 0.177 | 1 |
| FI | SCZ | 15 | 63.82 | 2.48e-08 | -0.0353 | 0.118 | 3 |
| FI | MDD | 15 | 17.78 | 1.23e-01 | 0.0067 | 0.648 | - |
Abbreviations: IVW Inverse variance weighting, MR-PRESSO Mendelian Randomization-Pleiotropy RESidual Sum and Outlier, SCZ schizophrenia, MDD major depressive disorder, FI frailty index, N_IVs number of instrumental variables
Genetically predicted MDD and FI in univariate MR
A total of 19 SNPs were eligible for IVs and were substantially associated with MDD (Table S2). Using the IVW approach, genetically determined MDD had a significant effect on FI (OR = 1.211, 95% CI 1.092–1.343, p = 2.90e–04) (Table S11, Figure S5, and Fig. 3). Similarly, heterogeneity was present in the genetic estimation of the effect of MDD on FI (p < 0.05), but no horizontal pleiotropy was detected (p > 0.05) (Table 2). Meanwhile, the random effects model was chosen to reduce the effect of heterogeneity. The F-statistics of the IVs obtained, which were closely related to MDD and had good intensity, were 24.03–37.36 (Table S15). From the leave-one-out analysis, it was evident that the estimated overall effect was relatively robust after removing any one SNP (Figure S6). Both scatter plot and funnel plot show that the results of the IVW method are reliable (Figures S7 and S8). In terms of genetically predicting the effect of MDD on FI, no outliers were found by MR-PRESSO (Table 2). In addition, in the reverse MR analysis, we found a causal effect of FI on MDD (OR = 1.290, 95% CI 1.133–1.469, p = 1.17e–04) (Table S11 and Figure S9). Heterogeneity in the causal effects of FI on MDD was similarly observed (p < 0.05), while no horizontal pleiotropy was identified (p > 0.05). The leave-one-out method further confirms the robustness of the results (Figure S10). The scatter plot and funnel plot both confirmed the robustness of the results derived from the IVW method (Figures S11 and S12). No outliers were found in the MR-PRESSO analysis (Table S13).
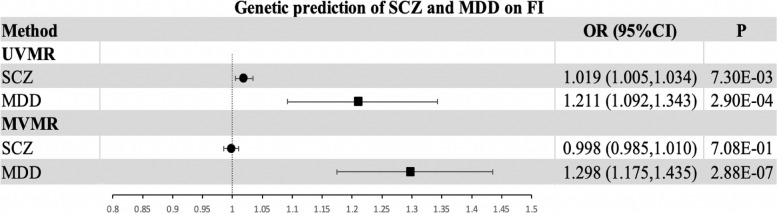
Genetically predicted MDD and SCZ on FI in Multivariable MR
Given that MDD and SCZ are genetically associated with each other, we conducted MVMR to estimate the direct effect of the other mental disorder (e.g., MDD) on FI in the context of controlling one of these mental disorders (e.g., SCZ). In the MVMR analysis, we extracted 19 SNPs significantly associated with MDD and 303 SNPs associated with SCZ that met the criteria for IVs (p = 1e-06). Information on instrumental variables for MDD and SCZ used for MVMR is presented separately in Tables S16 and S17. In MVMR, the estimated effect of MDD on FI is equivalent to the univariate IVW estimate (Multivariable IVW OR = 1.298; 95% CI, 1.175 to 1.435; p = 2.88e-07) (Fig. 4). However, comparing the univariate IVW estimates, the estimated effect of SCZ on FI was not statistically significant (p > 0.05) (Fig. 4). MVMR-Egger intercept analyses showed no horizontal pleiotropy (p = 0.800) (Table S18). In sensitivity analyses, MVMR-Lasso and MVMR-Median analyses provide evidence that the study findings are reliable and robust (Table S18).
Fig. 4.
Effect of genetic risk of SCZ and MDD on FI in UVMR and MVMR. Abbreviations: SCZ, schizophrenia; MDD, major depressive disorder; FI, frailty index; UVMR, univariable mendelian randomization; MVMR, multivariable mendelian randomization
Discussion
Although several observational studies have explored frailty and mental disorders (e.g., INS, AD, MDD, SCZ), conclusions have been mixed and it is difficult to determine a causal association between the two. Therefore, the present MR study aimed to assess the causal relationship between ten common mental disorders and frailty. The findings demonstrated that genetically predicted SCZ and MDD are causally associated with increased FI, whereas no causal association was found between the other eight mental disorders and the FI. In contrast, no causal associations between the FI and ten common mental disorders were found in the reverse MR analysis.
Our findings suggested that the genetic liability to SCZ may causally increase the FI. SCZ is a common, chronic, and severe mental disorder. The majority of SCZ patients struggle to achieve full remission, which places an intense financial strain on families and communities, and intensifies care-related challenges [43]. It is not surprising that a proportion of people with SCZ are hospitalized for long periods of time [44]. As a result, the health status of these patients is all the more alarming. According to a systematic review, the life expectancy of schizophrenia patients is 10–20 years less than the average [45]. A sufficient and plausible explanation is that patients with SCZ are more susceptible to aging compared to the general population [46]. Accumulating evidence of ineffective self-care, chronic unhealthy lifestyle habits (e.g., inadequate diet, physical inactivity, and excessive smoking), and cardiotoxicity (e.g., clozapine) and metabolic disturbances (e.g., olanzapine) associated with continued use of second-generation antipsychotics tend to plague SCZ patients [47–51]. In fact, these problems contribute dramatically to the premature frailty and mortality of people with SCZ. Ming-Tsun Tsai et al. [52] investigated 561 individuals with chronic SCZ at baseline and carried out a follow-up at 18 months. The findings revealed that frailty was remarkably frequent in chronic SCZ patients and was substantially linked with the likelihood of adverse clinical events. A retrospective cohort study of treatment‑resistant SCZ also provides novel evidence for these observations [13]. In addition, the accelerated aging hypothesis in SCZ was proposed in 2008 [53] and is based on the assumption that, compared to the general population, SCZ patients tend to experience the physiological changes of aging at a younger age. In a human plasma multi-omics investigation, Campeau et al. [54] discovered that both the diagnosis of SCZ and age had a substantial impact on the plasma proteome of the participants studied. Premature aging in SCZ is widely associated with marked dysregulation of inflammatory and metabolic system components. In further research, individuals with SCZ have been demonstrated to frequently have substantial reductions in brain capacity, cognitive performance, bone density, and leukocyte telomere length [55–58]. In brief, the susceptibility of SCZ patients to frailty and aging may be explained by a combination of external variables and premature internal functioning. Emerging genetic evidence further substantiates this theory [59]. However, a recent study has not established a causal link between schizophrenia and frailty [60]. Even a comprehensive analysis utilizing UK Biobank data yielded no positive findings [61]. Therefore, emerging evidence regarding the relationship between SCZ and FI needs to be supplemented by preclinical and clinical studies.
In addition to SCZ, the genetic liability to MDD distinctly increased the risk of frailty in the current study. According to the World Health Organization, MDD is the primary cause of mental and physical impairment worldwide [62]. Globally, more than 264 million people currently suffer from MDD [63]. Depression is also commonplace among the elderly, with a frequency of 10%–20% [64]. Epidemiological evidence demonstrates that frailty is also frequently observed in the elderly population, with a prevalence similar to that of depression [5]. Both frailty and depression are also characterized by similar clinical symptoms, such as deficiency in daily activities, poor self-care, and loss of interest [65]. In addition, adverse medical outcomes including unintentional weight loss, falls, disability, hospitalization, and even death are intimately associated with depression and frailty [66–68]. In this regard, it is not difficult to recognize that the criteria for determining depression and frailty are highly overlapping as well as disparate and that the relationship between depression and frailty is essential since they both have pronounced health consequences for the elderly population, with widely differing treatment strategies [69–71]. A recent distinctive study further elaborated on the fact that frailty occurs more frequently in people with depression from a gender perspective [71]. Furthermore, a systematic review and meta-analysis of a cohort of 84,351 older adults aged 65 years and older showed that older adults with depression were more susceptible to frailty than those without depression [72]. Yan Liu, et al. [73], on the other hand, also validated the previously mentioned assertion by using data model fitting to explore the association between depression and frailty. Notably, both emerging and accumulating evidence provide further support for our findings [14, 66, 74–76]. The causal association between depression and frailty may be due to several explanations. First, in contrast to frailty, depression usually appears at an earlier age. Second, mood swings, low motivation, reduced sleep, a sedentary lifestyle, and social isolation associated with late-life depression often contribute to frailty [77]. Third, more severe late-life depression is related to a higher prevalence of frailty [78]. Moreover, bereavement, cognitive impairment, and multiple illnesses that characterize later life inevitably exacerbate frailty [79]. Furthermore, the somatic burden of chronic antidepressants combined with sleeping medications further intensifies this issue [80]. Finally, a portion of common pathophysiological pathways may also exist. The above facts can be explained, at least in part, by overlapping mechanisms, such as inflammatory events, oxidative stress, mitochondrial dysfunction, and variability in the response of the hypothalamic–pituitary–adrenal axis to elevated cortisol levels [81, 82]. Obviously, future research will further elucidate the pathological mechanisms underlying the association between depression and frailty.
While a proportion of observational studies provide evidence for a relationship between common mental disorders and frailty, genetically predicting nine other common mental disorders failed to detect a causal association with frailty, as did reverse analyses of MR [12, 14, 18]. On the other hand, the results of MR inverse analysis found a significant and causal relationship between FI and MDD. It was pointed out in a clinical study (N = 5,303) that a bidirectional association exists between depression and frailty [2]. A systematic review and meta-analysis of 24 studies also indicated an interaction between depression and frailty [83]. The evidence for a bidirectional association between depression and frailty was strengthened by the multivariate MR analysis of Ni Sang et al. [28]. Both the frailty phenotype and the frailty index have been genetically found to be bi-directionally associated with depression [84]. These findings, initially based on GWAS data for depression from the UK Biobank, were further corroborated by GWAS data from the FinnGen database [59]. The pathologic mechanisms underlying the interaction between frailty and depression may also be similar. However, a systematic review and meta-analysis involving 84,531 older adults found no evidence of a significant effect of frailty on depression [72]. Notably, Morin RT et al. have indicated that the severity of depression in older adults is unrelated to frailty [85]. Hence, given that frailty is a syndrome of aging that involves multi-system impairment, further research involving the pathomechanisms of mental disorders and frailty is needed.
Accumulating evidence demonstrates that MDD and SCZ, although two common mental disorders are inextricably connected [86]. Symptomatologically, there are many similarities between the lack of motivation and reduced activity exhibited by depression and the negative symptoms of SCZ [87]. In terms of clinical diagnosis, evidence that the relationship is not simply a dichotomy is the listing of schizoaffective disorder and post-schizophrenic depression [88, 89]. With regard to health hazards, they cause serious consequences such as impairment of social functioning, disruption of interpersonal relationships, and even impact on personal or public safety [90–92]. In pathological mechanisms, both are closely associated with neurotransmitters [93]. Likewise, emerging Meta-analyses have shown that genetic prediction of MDD is remarkably associated with SCZ [30]. Various indications show that the two mental disorders are associated to some extent and may involve co-morbidities. MVMR provides a better understanding of the direct impact of the two mental disorders on FI. In the MVMR, independent of SCZ, no significant variation in the effect of MDD on FI was observed. However, by adjusting MDD, the impact of SCZ on FI is greatly diminished. Our findings challenge Kraepelin's dichotomous model of major mental disorders [94]. On the basis of this belief in the existence of a "natural" disease entity, Kraepelin divided the major mental disorders according to early-onset dementia or SCZ versus manic-depressive psychosis or bipolar disorder and mood disorders. The syndrome of the Kraepelin dichotomy is characterized by its mutual exclusivity and stability over time. In clinical work, however, it is not difficult to find a large cluster of intermediate symptoms between the dichotomies, such as depressive episodes with psychotic symptoms [95]. A systematic review of 39 clinical studies spanning nearly 40 years showed a trend towards greater diagnostic stability in SCZ over time [96], whereas the results of another review on the overall stability of mood disorder diagnoses across the lifespan hints at a high degree of variability in the diagnostic stability of affective disorders [97]. In adjusted MDD, evidence of a non-significant effect of SCZ on FI at the genetic level strengthens the basis for the overlap of some pathologic mechanisms between the two psychiatric disorders. In terms of phenotypic analysis, a single symptom such as depressed mood, delusions, or irritability is combined in different permutations to give an anxiety-depression syndrome or a hallucinatory-delusional syndrome. Not surprisingly, the composition of the syndromes is characterized by symptom intersection. It is also not difficult to explain the overlap of symptoms between MDD and SCZ. From the point of view of pathological mechanisms, both may involve dysregulation of the dopamine system [98]. The theory is that interneuron dysfunction in SCZ leads to dysregulation of the dopamine system and occurs in other mental disorders as well. Once the interneurons are abnormal, a disruption of rhythmic activity and coherence in exaggerated brain regions may develop. There is evidence that interneurons are especially susceptible to oxidative stress-induced damage during early postnatal development, prior to the formation of the protective perineuronal network [99]. Considering the shared susceptibility of both disorders to stress sensitivity, it is plausible that exacerbated stress responses and exposure during adolescence, resulting in damage to the parvalbumin neurons, may play a role in the development of SCZ. If an individual is protected from mental stress during the peri-adolescent period of parvalbumin susceptibility, they may become prone to developing depression later in life when exposed to stronger stress responses [98].
The association between frailty and psychiatric disorders extends beyond SCZ and MDD to include other conditions, such as insomnia and remains a focal point of ongoing clinical research. The majority of previous discussions between insomnia and frailty have focused on the elderly population. A meta-analysis of insomnia and frailty in older adults showed that the two were independently associated [100]. Previous studies show that older adults who sleep for longer or shorter periods of time, insufficient daytime sleep, and poor sleep quality are more likely to be frail [101, 102]. On the other hand, frail older adults are also prone to insomnia [103]. Recent genetic analyses have found that the two are related and share genes [104]. However, our study did not find positive results, which may be due to different selection criteria for IVs. The relationship between insomnia and frailty needs to be corroborated by more high-level research evidence.
Our study did not identify a genetic association between anxiety disorders and frailty. However, a systematic review of 25 studies involving 2,499 patients reported a high prevalence of frailty in individuals with severe mental illnesses, including anxiety disorders [105]. Furthermore, anxiety has been independently associated with frailty in studies of postmenopausal women [106]. Longitudinal data from the UK Biobank, encompassing up to 500,000 participants, confirmed a significant association between anxiety disorders and frailty [18]. This finding was confirmed by research on genetic analysis [60, 107]. Additionally, bidirectional associations between genetically determined anxiety and frailty have been observed [59]. Mixed results may stem from factors such as confounding variables in observational studies and variations in genetic data sources used in MR studies. The application of the larger GWAS data on anxiety disorders enables us to remain confident in our findings.
Frailty is notably prevalent in patients with bipolar disorder, a major psychiatric condition [105]. Analysis of UK Biobank data further supports the association between bipolar disorder and frailty [18]. The association between genetic susceptibility to bipolar disorder and frailty has also been established [108]. A bidirectional association between affective disorders and frailty has also been reported [59]. In contrast, some studies have found no evidence of a genetic predisposition to bipolar disorder and frailty [60]. The same is true for our study. These conflicting findings highlight the need to consider confounding factors and the potential impact of differing genetic susceptibility across populations.
The remaining five psychiatric disorders (ASD, OCD, AN, MBDO, and MBDS) have limited research related to frailty, and our study did not find enough IVs for these conditions. Even after adjusting the selection criteria for IVs (P = 1*10–6), only ASD received a sufficiently large number of IVs, whereas still no positive results were found. With deeper research, their association may be able to be dialed in.
Our research has several important implications and strengths. We obtained IVs for each of the ten common mental disorders from a large GWAS database and analyzed bidirectional causality between mental disorders and frailty using MR methods to reduce the risk of reverse causality bias and the effect of confounders. In addition, frailty, as a reversible variable, gives health and community workers more space for prevention and improvement [109]. Nevertheless, our research also has some limitations. First, given that the GWAS of the IVs obtained in this study were of European ancestry, the findings should be generalized to other races with caution. Second, due to the failure to obtain a sufficient number of IVs, some mental disorders (e.g., OCD) could not be analyzed in the MR analysis. An updated and larger GWAS is needed to polish the study. Third, although our study employed bidirectional MR analyses, the mechanisms underlying the association between mental disorders and frailty are complex. The interference of confounding factors still cannot be completely avoided [110], and research findings should be interpreted with caution. Fourth, overlapping samples of common psychiatric disorders and FI potentially biased MR estimates. Since overlapping samples may reduce the effective sample size, the efficacy of the statistical test is reduced. Finally, the FI, in comparison with the frailty phenotype, is comprehensive and robust. However, its application is limited at present, especially in low-income countries [111].
Conclusion
In conclusion, from a genetic perspective, the current MR analyses provide further evidence for a causal relationship between SCZ, MDD, and frailty and explore the underlying pathologic mechanisms for the aforementioned associations.
Supplementary Information
Supplementary Material 1: Supplementary material for this paper is Tables S1 – 15.
Acknowledgements
We sincerely thank all the participants for the data material and the researchers who freely shared the data.
Abbreviations
- AD
Anxiety disorder
- AN
Anorexia nervosa
- ASD
Autism spectrum disorder
- BD
Bipolar disorder
- FI
Frailty index
- FP
Frailty phenotype
- GWAS
Genome-wide association study
- IEU
Integrative Epidemiology Unit
- ISN
Insomnia
- IVs
Instrumental variables
- IVW
Inverse variance weighting
- MBDS
Mental and behavioral disorders due to use of other stimulants
- MBDO
Opioid-related mental and behavioral disorders
- MDD
Major depressive disorder
- MR
Mendelian randomization
- MR-PRESSO
MR-pleiotropy residual sum and outlier
- MVMR
Multivariable Mendelian randomization
- OCD
Obsessive compulsive disorder
- PGC
Psychiatric Genomics Consortium
- SCZ
Schizophrenia
- SNPs
Single nucleotide polymorphisms
- UVMR
Univariable mendelian randomization
Authors’ contributions
Y.R. F., W.X. S., and X.B. Z. contributed to the conception and design of the study. S.J. T. and X.H. W. organized the database and carried out the statistical analyses. W.X. S. wrote the first draft of the manuscript. P. S. and J. C. wrote parts of the manuscript. All authors participated in revising the manuscript, and read and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China(81930033); Suzhou Key Laboratory (SZS2024016); Suzhou Clinical Key disciplines for Geriatric Psychiatry (SZXK202116); Suzhou clinical Medical Center for mood disorders (Szlcyxzx202109); Shanghai Municipal Science and Technology Major Project (2018SHZDZX05); Suzhou Science and Technology Development Programme Youth Project(SKYD2023160) and National Mentorship Training Program for Young Health Professionals in Suzhou (Qngg2022027).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This MR study was conducted based on publicly available summary statistics from a large genome-wide association study (GWAS) and all participants provided written informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm–issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao L, Zhou Y, Liu H, Shi M, Wei Y, Xia Y. Bidirectional Longitudinal Study of Frailty and Depressive Symptoms Among Older Chinese Adults. Front Aging Neurosci. 2022;14:791971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Lepeleire J, Iliffe S, Mann E, Degryse JM. Frailty: an emerging concept for general practice. Br J Gen Pract. 2009;59:e177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience. 2017;39:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L, Tang Z, Zhang L, Sun F, Li Y, Chan P. Prevalence of Frailty and Associated Factors in the Community-Dwelling Population of China. J Am Geriatr Soc. 2018;66:559–64. [DOI] [PubMed] [Google Scholar]
- 6.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92. 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed]
- 7.Rohrmann S. Epidemiology of Frailty in Older People. In: Veronese N, editor. Frailty and Cardiovascular Diseases. Cham: Springer International Publishing; 2020. p. 21–7. [Google Scholar]
- 8.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of Deficits as a Proxy Measure of Aging. Scientific World Journal. 2001;1:323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiltunen K, Saarela RKT, Kautiainen H, Roitto H-M, Pitkälä KH, Mäntylä P. Relationship between Fried’s frailty phenotype and oral frailty in long-term care residents. Age Ageing. 2021;50:2133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felker G, Enel P, Petit N, Retornaz F, Darque A, Ravaux I. Frailty phenotype is associated with antiretroviral exposure among older persons living with HIV. Curr Opin HIV AIDS. 2021;16:271–7. [DOI] [PubMed] [Google Scholar]
- 11.Laddu DR, Ozemek C, Sabbahi A, Severin R, Phillips SA, Arena R. Prioritizing movement to address the frailty phenotype in heart failure. Prog Cardiovasc Dis. 2021;67:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernal-López C, Potvin O, Avila-Funes JA. Frailty is Associated with Anxiety in Community-Dwelling Elderly Adults. J Am Geriatr Soc. 2012;60:2373–4. [DOI] [PubMed] [Google Scholar]
- 13.Pearson E, Siskind D, Hubbard R, Gordon E, Coulson E, Arnautovska U, et al. Frailty and Treatment-Resistant Schizophrenia: A Retrospective Cohort Study. Community Ment Health J. 2023;59:105–9. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Hou T, Nkimbeng M, Li Y, Taylor JL, Sun X, et al. Associations between symptoms of pain, insomnia and depression, and frailty in older adults: A cross-sectional analysis of a cohort study. Int J Nurs Stud. 2021;117:103873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borges MK, Aprahamian I, Romanini CV, Oliveira FM, Mingardi SVB, Lima NA, et al. Depression as a determinant of frailty in late life. Aging Ment Health. 2021;25:2279–85. [DOI] [PubMed] [Google Scholar]
- 16.Tang JY, Luo H, Tse M, Lum TY, Wong GH, Li SX. The relationship between insomnia symptoms and frailty in community-dwelling older persons: a path analysis. Sleep Med. 2021;84:237–43. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Hou X, Ma X, Wu D. Frailty among inpatients with Schizophrenia: Status, influencing factors, and their correlation with quality of life. Front Psychiatry. 2023;13:1067260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutz J, Choudhury U, Zhao J, Dregan A. Frailty in individuals with depression, bipolar disorder and anxiety disorders: longitudinal analyses of all-cause mortality. BMC Med. 2022;20:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan M, Bhanu C, Frost R. The association between frailty and anxiety: A systematic review. Int J Geriat Psychiatry. 2023;38:e5918. [DOI] [PubMed] [Google Scholar]
- 20.Gielen E, Bergmann P, Bruyère O, Cavalier E, Delanaye P, Goemaere S, et al. Osteoporosis in Frail Patients: A Consensus Paper of the Belgian Bone Club. Calcif Tissue Int. 2017;101:111–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lake S, Buxton J, Walsh Z, Cooper ZD, Socías ME, Fairbairn N, et al. Methadone Dose, Cannabis Use, and Treatment Retention: Findings From a Community-based Sample of People Who Use Unregulated Drugs. J Addict Med. 2023;17:e18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemoto Y, Sato S, Kitabatake Y, Nakamura M, Takeda N, Maruo K, et al. Bidirectional relationship between insomnia and frailty in older adults: A 2-year longitudinal study. Arch Gerontol Geriatr. 2021;97:104519. [DOI] [PubMed] [Google Scholar]
- 23.Borges MK, Jeuring HW, Marijnissen RM, Van Munster BC, Aprahamian I, Van Den Brink RHS, et al. Frailty and affective disorders throughout adult life: A 5-year follow-up of the Lifelines Cohort Study. J American Geriatrics Society. 2022;70:3424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allman PH, Aban IB, Tiwari HK, Cutter GR. An introduction to Mendelian randomization with applications in neurology. Multiple Sclerosis and Related Disorders. 2018;24:72–8. [DOI] [PubMed] [Google Scholar]
- 25.Khasawneh LQ, Al-Mahayri ZN, Ali BR. Mendelian randomization in pharmacogenomics: The unforeseen potentials. Biomed Pharmacother. 2022;150:112952. [DOI] [PubMed] [Google Scholar]
- 26.Sleiman PM, Grant SF. Mendelian Randomization in the Era of Genomewide Association Studies. Clin Chem. 2010;56:723–8. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization Nat Rev Methods Primers. 2022;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang N, Li B-H, Zhang M-Y, Wei M, Fang R-X, Liu W-J, et al. Bidirectional causal relationship between depression and frailty: a univariate and multivariate Mendelian randomisation study. Age and Ageing. 2023;52:afad113. [DOI] [PubMed]
- 29.Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.eQTLGen, 23andMe, the Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, Wray NR, Ripke S, Mattheisen M, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81. [DOI] [PMC free article] [PubMed]
- 31.Duncan L, Yilmaz Z, Gaspar H, Walters R, Goldstein J, Anttila V, et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am J Psychiatry. 2017;174:850–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkins JL, Jylhävä J, Pedersen NL, Magnusson PK, Lu Y, Wang Y, et al. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell. 2021;20:e13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326:1614. [DOI] [PubMed] [Google Scholar]
- 34.Wightman DP, Jansen IE, Savage JE, Shadrin AA, Bahrami S, Holland D, et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat Genet. 2021;53:1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–70. [DOI] [PubMed] [Google Scholar]
- 36.Theou O, Tan ECK, Bell JS, Emery T, Robson L, Morley JE, et al. Frailty Levels in Residential Aged Care Facilities Measured Using the Frailty Index and FRAIL-NH Scale. J Am Geriatr Soc. 2016;64:e207–12. [DOI] [PubMed] [Google Scholar]
- 37.Williams DM, Jylhävä J, Pedersen NL, Hägg S. A Frailty Index for UK Biobank Participants. J Gerontol A Biol Sci Med Sci. 2019;74:582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7: e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stock J, Yogo, M. In Identification and Inference for Econometric Models. 2005th edition. Cambridge: Cambridge University Press. p. 80–108.
- 43.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. The Lancet. 2022;399:473–86. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Jiang F, Needleman J, Guo M, Chen Y, Zhou H, et al. A sequence analysis of hospitalization patterns and service utilization in patients with major psychiatric disorders in China. BMC Psychiatry. 2021;21:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4:295–301. [DOI] [PubMed] [Google Scholar]
- 46.Taube C, Mentzel C, Glue P, Barak Y. Aging of persons with schizophrenia: analysis of a national dataset. Int Psychogeriatr. 2023;:1–8. [DOI] [PubMed]
- 47.Sagud M, Mihaljevic Peles A, Pivac N. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32:402–8. [DOI] [PubMed] [Google Scholar]
- 48.Van Zonneveld SM, Haarman BCM, Van Den Oever EJ, Nuninga JO, Sommer IEC. Unhealthy diet in schizophrenia spectrum disorders. Curr Opin Psychiatry. 2022;35:177–85. [DOI] [PubMed] [Google Scholar]
- 49.Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry. 2017;16:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanniah G, Kumar S. Clozapine associated cardiotoxicity: Issues, challenges and way forward. Asian J Psychiatr. 2020;50:101950. [DOI] [PubMed] [Google Scholar]
- 51.Malhotra N, Grover S, Chakrabarti S, Kulhara P. Metabolic Syndrome in Schizophrenia. Indian J Psychol Med. 2013;35:227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai M-T, Lee S-M, Chen H-K, Wu B-J. Association between frailty and its individual components with the risk of falls in patients with schizophrenia spectrum disorders. Schizophr Res. 2018;197:138–43. [DOI] [PubMed] [Google Scholar]
- 53.Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campeau A, Mills RH, Stevens T, Rossitto L-A, Meehan M, Dorrestein P, et al. Multi-omics of human plasma reveals molecular features of dysregulated inflammation and accelerated aging in schizophrenia. Mol Psychiatry. 2022;27:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayora M, Fraguas D, Abregú-Crespo R, Recio S, Blasco MA, Moises A, et al. Leukocyte telomere length in patients with schizophrenia and related disorders: a meta-analysis of case-control studies. Mol Psychiatry. 2022;27:2968–75. [DOI] [PubMed] [Google Scholar]
- 56.Jung D-U, Kelly DL, Oh M-K, Kong B-G, Kang J-W, Lee S-J, et al. Bone Mineral Density and Osteoporosis Risk in Older Patients With Schizophrenia. J Clin Psychopharmacol. 2011;31:406–10. [DOI] [PubMed] [Google Scholar]
- 57.Loewenstein DA, Czaja SJ, Bowie CR, Harvey PD. Age-Associated Differences in Cognitive Performance in Older Patients With Schizophrenia: A Comparison With Healthy Older Adults. Am J Geriatr Psychiatry. 2012;20:29–40. [DOI] [PubMed] [Google Scholar]
- 58.Veijola J, Guo JY, Moilanen JS, Jääskeläinen E, Miettunen J, Kyllönen M, et al. Longitudinal Changes in Total Brain Volume in Schizophrenia: Relation to Symptom Severity. Cognition and Antipsychotic Medication PLoS ONE. 2014;9:e101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma L, Liu Z, Fu L, Fan J, Kong C, Wang T, et al. Bidirectional causal relational between frailty and mental illness: a two-sample Mendelian randomization study. Front Psychiatry. 2024;15:1397813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma T, Chen M, Cheng X, Bai Y. Assessment of Bidirectional Relationships between Frailty and Mental Disorders: A Bidirectional Mendelian Randomization Study. J Am Med Dir Assoc. 2024;25:506-513.e29. [DOI] [PubMed] [Google Scholar]
- 61.Huang W, Lin C, Liu M. Bidirectional causal associations between aging and major mental disorders: A population-based study using the two-sample mendelian randomization method from the UK biobank (AM-SRNMA 002). Arch Gerontol Geriatr. 2024;127:105578. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organ. Depression. 2021.
- 63.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–40. [DOI] [PubMed] [Google Scholar]
- 64.Rodda J, Walker Z, Carter J. Depression in older adults. BMJ. 2011;343 sep28 1:d5219–d5219. [DOI] [PubMed]
- 65.Maștaleru A, Abdulan IM, Ștefăniu R, Lefter N, Sandu IA, Pîslaru AI, et al. Relationship between Frailty and Depression in a Population from North-Eastern Romania. IJERPH. 2022;19:5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almeida OP, Hankey GJ, Yeap BB, Golledge J, Norman PE, Flicker L. Depression, Frailty, and All-Cause Mortality: A Cohort Study of Men Older than 75 Years. J Am Med Dir Assoc. 2015;16:296–300. [DOI] [PubMed] [Google Scholar]
- 67.Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47:193–200. [DOI] [PubMed] [Google Scholar]
- 68.Martín-Fernández J, Del Nido-Varo LP, Vázquez-de-la-Torre-Escalera P, Candela-Ramírez R, Ariza-Cardiel G, García-Pérez L, et al. Health Related Quality of Life in Major Depressive Disorder: evolution in time and factors associated. Actas Esp Psiquiatr. 2022;50:15–26. [PMC free article] [PubMed]
- 69.Mezuk B, Lohman M, Dumenci L, Lapane KL. Are Depression and Frailty Overlapping Syndromes in Mid- and Late-life? A Latent Variable Analysis. Am J Geriatr Psychiatry. 2013;21:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho IY, Kang J, Ko H, Sung E, Chung PW, Kim C. Association Between Frailty-Related Factors and Depression among Older Adults. Clin Gerontol. 2022;45:366–75. [DOI] [PubMed] [Google Scholar]
- 72.Chu W, Chang S, Ho H, Lin H. The Relationship Between Depression and Frailty in Community-Dwelling Older People: A Systematic Review and Meta-Analysis of 84,351 Older Adults. J Nurs Scholarsh. 2019;51:547–59. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Meng H, Tu N, Liu D. The Relationship Between Health Literacy, Social Support, Depression, and Frailty Among Community-Dwelling Older Patients With Hypertension and Diabetes in China. Front Public Health. 2020;8:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ribeiro O, Duarte N, Teixeira L, Paúl C. Frailty and depression in centenarians. Int Psychogeriatr. 2018;30:115–24. [DOI] [PubMed] [Google Scholar]
- 75.Oyon J, Serra-Prat M, Limon E, Ferrer M, Pastor N, Palomera E, et al. Depressive symptom severity is a major risk factor for frailty in community-dwelling older adults with depression. A prospective study Family Practice. 2022;39:875–82. [DOI] [PubMed] [Google Scholar]
- 76.Borges MK, Romanini CV, Lima NA, Petrella M, Da Costa DL, An VN, et al. Longitudinal Association between Late-Life Depression (LLD) and Frailty: Findings from a Prospective Cohort Study (MiMiCS-FRAIL). J Nutr Health Aging. 2021;25:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.The Agecode Study Group, Hajek A, Brettschneider C, Posselt T, Lange C, Mamone S, et al. Predictors of frailty in old age–results of a longitudinal study. J Nutr Health Aging. 2016;20:952–7. [DOI] [PubMed]
- 78.Oyon J, Serra-Prat M, Ferrer M, Llinares A, Pastor N, Limón E, et al. Psychosocial factors associated with frailty in the community-dwelling aged population with depression. A cross-sectional study Atención Primaria. 2021;53:102048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weyerer S, Eifflaender-Gorfer S, Wiese B, Luppa M, Pentzek M, Bickel H, et al. Incidence and predictors of depression in non-demented primary care attenders aged 75 years and older: results from a 3-year follow-up study. Age Ageing. 2013;42:173–80. [DOI] [PubMed] [Google Scholar]
- 80.Brown PJ, Ciarleglio A, Roose SP, Garcia CM, Chung S, Alvarez J, et al. Frailty Worsens Antidepressant Treatment Outcomes in Late Life Depression. Am J Geriatr Psychiatry. 2021;29:944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belvederi Murri M, Pariante C, Mondelli V, Masotti M, Atti AR, Mellacqua Z, et al. HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology. 2014;41:46–62. [DOI] [PubMed] [Google Scholar]
- 82.Brown PJ, Rutherford BR, Yaffe K, Tandler JM, Ray JL, Pott E, et al. The Depressed Frail Phenotype: The Clinical Manifestation of Increased Biological Aging. Am J Geriatr Psychiatry. 2016;24:1084–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. [DOI] [PubMed] [Google Scholar]
- 84.Zhu J, Zhou D, Nie Y, Wang J, Yang Y, Chen D, et al. Assessment of the bidirectional causal association between frailty and depression: A Mendelian randomization study. J Cachexia Sarcopenia Muscle. 2023;14:2327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morin RT, Insel P, Bickford D, Nelson C, Mackin RS. Depression Severity, but Not Cognitive Impairment or Frailty, is Associated with Disability in Late-Life Depression. Clin Gerontol. 2020;43:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Upthegrove R, Marwaha S, Birchwood M. Depression and Schizophrenia: Cause, Consequence or Trans-diagnostic Issue? SCHBUL. 2016;:sbw097. [DOI] [PMC free article] [PubMed]
- 87.Krynicki CR, Upthegrove R, Deakin JFW, Barnes TRE. The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr Scand. 2018;137:380–90. [DOI] [PubMed] [Google Scholar]
- 88.Jeczmien P, Levkovitz Y, Weizman A, Carmel Z. Post-psychotic depression in schizophrenia. Isr Med Assoc J. 2001;3:589–92. [PubMed] [Google Scholar]
- 89.Malaspina D, Owen MJ, Heckers S, Tandon R, Bustillo J, Schultz S, et al. Schizoaffective Disorder in the DSM-5. Schizophr Res. 2013;150:21–5. [DOI] [PubMed] [Google Scholar]
- 90.Porcelli S, Van Der Wee N, Van Der Werff S, Aghajani M, Glennon JC, Van Heukelum S, et al. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. 2019;97:10–33. [DOI] [PubMed] [Google Scholar]
- 91.Hor K, Taylor M. Review: Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24 4_suppl:81–90. [DOI] [PMC free article] [PubMed]
- 92.Inoue K, Otsuka K, Onishi H, Cho Y, Shiraishi M, Narita K, et al. Multi-institutional survey of suicide death among inpatients with schizophrenia in comparison with depression. Asian J Psychiatr. 2020;48:101908. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura S. Integrated pathophysiology of schizophrenia, major depression, and bipolar disorder as monoamine axon disorder. Front Biosci (Schol Ed). 2022;14:4. [DOI] [PubMed] [Google Scholar]
- 94.Kraepelin E. Klinische Psychiatrie. 7th edition. Germany: Leipzig; 1904.
- 95.Häfner H, An Der Heiden W, Maurer K. Evidence for separate diseases?: Stages of one disease or different combinations of symptom dimensions?. Eur Arch Psychiatry Clin Neurosc. 2008;258:85–96. [DOI] [PubMed]
- 96.Palomar-Ciria N, Cegla-Schvartzman F, Lopez-Morinigo J-D, Bello HJ, Ovejero S, Baca-García E. Diagnostic stability of schizophrenia: A systematic review. Psychiatry Res. 2019;279:306–14. [DOI] [PubMed] [Google Scholar]
- 97.De La Vega D, Piña A, Peralta FJ, Kelly SA, Giner L. A Review on the General Stability of Mood Disorder Diagnoses Along the Lifetime. Curr Psychiatry Rep. 2018;20:29. [DOI] [PubMed] [Google Scholar]
- 98.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steullet P, Cabungcal JH, Monin A, Dwir D, O’Donnell P, Cuenod M, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr Res. 2016;176:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wen Q, Yan X, Ren Z, Wang B, Liu Y, Jin X. Association between insomnia and frailty in older population: A meta-analytic evaluation of the observational studies. Brain and Behavior. 2023;13:e2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balomenos V, Ntanasi E, Anastasiou CA, Charisis S, Velonakis G, Karavasilis E, et al. Association Between Sleep Disturbances and Frailty: Evidence From a Population-Based Study. J Am Med Dir Assoc. 2021;22:551-558.e1. [DOI] [PubMed] [Google Scholar]
- 102.Nakakubo S, Makizako H, Doi T, Tsutsumimoto K, Hotta R, Lee S, et al. Long and Short Sleep Duration and Physical Frailty in Community-Dwelling Older Adults. J Nutr Health Aging. 2018;22:1066–71. [DOI] [PubMed] [Google Scholar]
- 103.Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R, et al. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74:659–66. [DOI] [PubMed] [Google Scholar]
- 104.Song Z, Li W, Han Y, Xu Y, Wang Y. Investigating the shared genetic architecture between frailty and insomnia. Front Aging Neurosci. 2024;16:1358996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pearson E, Siskind D, Hubbard RE, Gordon EH, Coulson EJ, Warren N. Frailty and severe mental illness: A systematic review and narrative synthesis. J Psychiatr Res. 2022;147:166–75. [DOI] [PubMed] [Google Scholar]
- 106.García-Vigara A, Fernandez-Garrido J, Chedraui P, Monllor-Tormos A, García-Pérez MÁ, Tarín JJ, et al. Association between anxiety and frailty in postmenopausal women. Gynecol Endocrinol. 2024;40:2329714. [DOI] [PubMed] [Google Scholar]
- 107.Chen J-H, Lei H, Wan Y-F, Zhu X-C, Zeng L-Y, Tang H-X, et al. Frailty and psychiatric disorders: A bidirectional Mendelian randomization study. J Affect Disord. 2024;356:346–55. [DOI] [PubMed] [Google Scholar]
- 108.Xiao H, Zhu W, Jing D. Association between frailty and common psychiatric disorders: A bidirectional Mendelian randomization study. J Affect Disord. 2025;371:1–5. [DOI] [PubMed] [Google Scholar]
- 109.Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res Rev. 2019;50:81–8. [DOI] [PubMed] [Google Scholar]
- 110.Park C, Ko FC. The Science of Frailty: Sex Differences. Clin Geriatr Med. 2021;37:625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary material for this paper is Tables S1 – 15.
Data Availability Statement
No datasets were generated or analysed during the current study.