Abstract
Flammulina velutipes (F. velutipes) residues polysaccharide (FVRP) is a high molecular weight polysaccharide with diverse bioactivities extracted from F. velutipes residues (FVR). However, high molecular weight polysaccharides have been shown to face significant challenges in crossing the cell membrane barrier, thereby limiting their absorption and application in the body. Therefore, an ultrasonic-assisted H2O2-Fe3+ method was employed for the first time to degrade FVRP, resulting in the production of a new polysaccharide, FVRPF. Compared with FVRP, there was no significant difference in the main chemical structure of FVRPF, but the monosaccharide composition ratio varied. and FVRPF had lower molecular weight and stronger antioxidant capacity. Moreover, FVRPF could be degraded by human microbiota, modulate gut microbiota composition, and increase the production of total short-chain fatty acids (SCFAs). These findings suggest that FVRPF holds potential as a promising prebiotic for applications in the food and pharmaceutical industries.
Keywords: Degradation, Flammulina velutipes residues, Polysaccharide, In vitro fermentation
Graphical abstract
Highlights
-
•
FVRPF was degraded by ultrasonic assistant H2O2-Fe3+ method for the first time.
-
•
FVRPF had lower molecular weight and higher antioxidant abilities.
-
•
FVRPF exhibited excellent prebiotic properties based on human fecal fermentation.
1. Introduction
F. velutipes, also known as the golden needle mushroom or enokitake, is a globally popular edible and medicinal fungus (Hao et al., 2023; Ye et al., 2024; Zhang et al., 2024). With the extensive cultivation of F. velutipes, the production of FVR, the byproduct derived from the edible fruiting bodies of F. velutipes, has significantly increased in China (Li, Zhang, Sheng, & Qing, 2018; Yang et al., 2024). FVR serves as a solid medium for the cultivation of F. velutipes and retains substantial amounts of fruiting bodies and mycelium after harvest. It has the advantage of abundant availability and low cost. Furthermore, FVR contains various effective components of F. velutipes, such as polysaccharides, amino acids, Fe, Ca, Zn, and Mg, along with certain metabolites from the fruiting bodies (Leong, Ma, Chang, & Yang, 2022; Liu et al., 2016; Wang, Yang, Song, Zhang, & Jia, 2021; Wei et al., 2023). Meanwhile, FVRP has gained considerable attention, focusing primarily on extraction, purification, and activity analysis. FVRP can regulate gut microbiota, promote human immunity, modulate the immune system, regulate lipid metabolism, improve prebiotic activity, and mitigate heavy metal toxicity (Hao et al., 2023; Hao, Liao, Wang, Lao, & Liao, 2021). In our previous study, ultrasonic-assisted extraction identified FVRP as a novel polysaccharide with a molecular weight (Mw) of 335.29 kDa, exhibiting significant antioxidant and prebiotic activities (Liu et al., 2022; Liu et al., 2023; Zhang et al., 2024). However, its high Mw may limit its biological activity and restrict its development and application in the pharmaceutical and nutraceutical industries (Lin et al., 2019; Yao et al., 2022; Yao et al., 2024). In contrast, low-Mw polysaccharides exhibit superior bioavailability and enhanced probiotic potential (Fan et al., 2024). Hence, reducing the Mw of FVRP is crucial to improving its biological activity and establishing a foundation for its application in food and health products.
Degradation is the most direct method for reducing the Mw of polysaccharides (Hao et al., 2021). Currently, polysaccharide degradation methods can be primarily categorized into physical, chemical, and biological approaches. In general, biodegradation is defined as the conversion of a substrate into smaller molecules, or CO2 and H2O, through the enzymatic activity of microorganisms such as fungi and bacteria (Da Rocha, Da Costa, Da Costa, & de Mello Ferreira, 2024). However, biodegradation methods, despite their high specificity, are costly (Manheim, Lin, Kong, Biba, & Zhuang, 2022). Chemical degradation methods include acid-base degradation, degradation using organic reagents, and free radical degradation. The use of acid-base and organic reagents in degradation processes might result in reagent residues, chemical contamination, and other risks. In contrast, free radical degradation has been widely used for polysaccharide degradation due to its advantages, including mild reaction conditions, cost-effectiveness, and environmental protection (Wang et al., 2024), including the H2O2-Vc (Ma et al., 2021; Wang et al., 2024), H2O2-Fe2+ (Xu et al., 2018), and H2O2-Fe3+ systems (Arantes & Milagres, 2006). In addition, combining chemical methods with physical methods can achieve a more effective degradation outcome (Yan et al., 2021). While the primary structure of polyspermous polysaccharide PCP remains unchanged after ultrasonic treatment, its trihelical and surface structures are disrupted. Compared with intact PCP, the degradation products exhibit reduced thermal stability and apparent viscosity but enhanced antioxidant and enzyme inhibition activities (Liu et al., 2024). Ultrasonic-assisted H2O2-Vc and ultrasonic-assisted H2O2-Fe2+ have been used to degrade polysaccharides. The results showed that the degradation treatment alters the chemical composition and monosaccharide ratios of the two degraded products, reduces their Mw, and enhances their antioxidant activity. Moreover, a characteristic analysis indicated that ultrasonic-assisted H2O2-Vc and ultrasonic-assisted H2O2-Fe2+ merely disrupt the glycosidic linkages between sugar units, thereby reducing the Mw and altering the chemical composition and monosaccharide ratio. However, these methods have no significant effect on the primary structure of polysaccharides extracted from green seaweed C. cylindricum (Yan et al., 2021). Wang et al. employed ultrasonic-assisted H2O2 in the degradation of Longan polysaccharide, and their findings showed that the Mw, particle size, and viscosity of Longan polysaccharide decreased following ultrasonic/H2O2 degradation, while its solubility and prebiotic activity were improved. Notably, ultrasonic/H2O2 degradation did not modify the chemical composition or monosaccharide profile of LP but did alter their ratio (Wang, Zhang, et al., 2024). Recent studies have indicated that H2O2-Fe3+ not only significantly degrades cellulose and hemicellulose substrates but also enhances the effectiveness of the degradation reaction, especially for hemicellulose substrates (Arantes & Milagres, 2006). However, the ultrasonic-assisted H2O2-Fe3+ method has not yet been applied to the degradation of naturally occurring active polysaccharides, and its effects on their physicochemical properties remains unclear. Therefore, we hypothesize that the ultrasonic-assisted H2O2-Fe3+ degradation method could improve the bioactivity of polysaccharides by reducing Mw, breaking glucosidic bonds, and altering the chemical composition and monosaccharide ratio.
In this study, FVRPF was degraded from FVRP for the first time using an ultrasonic-assisted H₂O₂-Fe3+ approach. The physicochemical and structural characteristics, in vitro antioxidant activity, and the effect of FVRPF on alleviating copper-induced damage in yeast were evaluated. Additionally, the prebiotic properties of FVRPF were investigated, focusing on its influence on gut microbiota (Arantes & Milagres, 2006) and short-chain fatty acids (SCFAs) using an in vitro human fecal fermentation model. This study aimed to provide a theoretical foundation for the development and application of FVRPF as an antioxidant and prebiotic, offering novel insights into its bioactivities, especially in promoting gut health.
2. Materials and experimental methods
2.1. Materials and reagents
FVRP and a wild-type yeast strain (BY4742) were previously extracted and preserved in our laboratory (Liu et al., 2021; Liu et al., 2022). Fructooligosaccharides (FOS) were provided by Beijing Solarbio Life Sciences Co., Ltd. (Beijing, China). Standards for SCFAs, including Acetic acid, Propanoic acid, Isobutyric acid, N-Butyric acid, Isovaleric acid, N-Valeric acid, and N-Caproic acid, were supplied by Aladdin Biotechnology Co., Ltd. (Shanghai, China). All other chemicals and reagents used were of analytical grade.
2.2. Preparation of FVRPF
The ultrasonic-assisted H2O2-Fe3+ method was employed to degrade FVRP, following protocols described in previous studies (Arantes & Milagres, 2006; Arantes & Milagres, 2007; Yan et al., 2021). A solution of FVRP (5 mg/mL) was adjusted to pH 3.0 using 1.0 mol/L HCl, then combined with a 0.035 % H2O2 solution and FeCl3 (0.2 g/L). The resulting mixture was degraded at 80 °C and 600 W (SCIENTZ, China) for 30 min and subsequently neutralized using 1.0 M NaOH. FVRPF was obtained through dialysis (48 h, Mw: 500 Da), followed by concentration and lyophilization.
2.3. Analysis of physicochemical characteristics
The levels of polysaccharides, reducing sugars, proteins, and total phenolics were determined using the following methods: the phenol‑sulfuric acid method (Dou, Chen, Huang, & Fu, 2021), the DNS method (Liu et al., 2022), the m-hydroxybiphenyl method (Bai et al., 2022), the Coomassie Brilliant Blue method (Dou et al., 2021), and the Folin–Ciocalteu method (Kerdsomboon, Chumsawat, & Auesukaree, 2021).
The monosaccharide composition of FVRPF was analyzed using a high-performance liquid chromatography (HPLC) method with slight modifications (Dai et al., 2010). FVRPF was hydrolyzed with 2.0 M trifluoroacetic acid (TFA) at 120 °C for 4 h and subsequently derivatized with 1-phenyl-3-methyl-5-pyrazolone (PMP) under alkaline conditions. The PMP-derivatized FVRPF and monosaccharide standards (5 μL each) were separately loaded onto a Dionex CarboPac PA10 liquid chromatography column. The mobile phase consisted of 0.1 M NaOH and a mixture of 0.1 M NaOH and 0.2 M NaAc at a volume ratio of 60:40.
FVRP and FVRPF solutions at a concentration of 0.1 mg/mL were prepared and added to a sample dish for particle size and Zeta potential analysis using a nanoparticle potential analyzer (NANO ZS90, Malvern, UK) (Zhang, Chen, et al., 2024).
The Mw of FVRP and FVRPF was determined using previously described methods with a gel chromatography system equipped with a multi-angle laser light scattering (GPC-MALLS) detector (Li et al., 2021; Liu et al., 2016). Astra software was used for data processing.
The dried samples of FVRP and FVRPF were individually mixed with potassium bromide and pressed into tablets for Fourier Transform Infrared (FT-IR) spectroscopy analysis. Measurements were conducted using a Nexus 470 FT-IR spectrometer (Thermo Nicolet, USA) within the range of 4000–400 cm−1.
2.4. Evaluation of antioxidant activity of FVRPF
2.4.1. In vitro antioxidant activity analysis
The reducing power and scavenging activities of FVRP and FVRPF on ABTS, DPPH, and hydroxyl radicals were evaluated based on methods described in previous studies (Cui et al., 2018; Fleita, El-Sayed, & Rifaat, 2015; Liu et al., 2016; Liu et al., 2022). Ascorbic acid (Vc), a natural antioxidant, was used as a positive control.
2.4.2. Antioxidant ability based on CuSO4-induced oxidative damage in a yeast model
The wild-type yeast strain BY4742 was cultured in YPD medium to the logarithmic growth phase (OD600 = 1.0 ± 0.2) (Kerdsomboon et al., 2021; Rattanawong, Kerdsomboon, & Auesukaree, 2015). Eight experimental groups were prepared: the blank group (CK), the CuSO4 group, the CuSO4 + FVRP group, the CuSO4 + FVRPF group, the CuSO4 + Vc group, the FVRP group, the FVRPF group, and the Vc group. A total of 107 yeast cells were inoculated into YPD medium containing 8 mM CuSO4, with or without FVRPs (1 mg/mL) or Vc (1 mg/mL), and incubated at 30 °C with shaking at 150 rpm for 16 h, respectively. OD600 values were measured using a multifunctional enzyme-labeling instrument (TECAN-Spark, Switzerland). Simultaneously, 2.5 μL of each cell suspension was serially diluted (10−1, 10−2, 10−3, 10−4, and 10−5) and spotted onto YPD solid medium. Plates were incubated at 30 °C for 16 h, and images were captured.
Each group of yeast cells was centrifuged at 3000 rpm for 6 min, washed three times with PBS, and then adjusted to an OD600 value of 0.6–0.8. Enzyme solution was extracted from the prepared yeast cells using an ultrasonic-assisted method. The sample was subjected to ultrasound at 300 W for 3 s with a 9 s interval, repeated continuously for 10 min in an ice-water bath. Subsequently, the enzyme solution was obtained by centrifugation at 12000 rpm and 4 °C for 10 min. A BCA assay kit (Abbkine, China) was used to quantify the protein concentration. The levels of nitric oxide (NO) and reactive oxygen species (ROS), as well as the activities of SOD and GSH-PX, were measured and normalized to protein content (U/mg protein).
2.5. In vitro fermentation characteristics of FVRPF
2.5.1. In vitro fermentation
Fecal samples were collected from six healthy individuals (three males and three females, aged 23–27 years, with a BMI of 18.5–24) who met specific inclusion criteria. These individuals had no history of intestinal diseases and had not taken antibiotics within the preceding 90 days. Equal amounts of pre-meal fecal samples were combined in a sterile tube, and a 10 % (w/v) fecal suspension was prepared by promptly adding sterile physiological saline. Following homogenization for 1 min, the supernatant was obtained by centrifugation at 500 ×g and 4 °C for 5 min, yielding a fecal microbial suspension for subsequent testing (Li et al., 2023).
The fermentation medium was prepared following the method described in a previous study. Pure water, 10 mg/mL of FVRPF, and FOS were added to the fermentation liquid, which was then autoclaved and designated as the CK group, FVRPF group, and FOS group, respectively. Then, 10 % of a fecal microbial suspension was inoculated into the medium, followed by anaerobic fermentation at 37 °C for 0, 6, 12, 24, and 48 h, respectively (Guo et al., 2024).
2.5.2. Detection of physicochemical characteristics and in vitro antioxidant abilities of fermentation broth
After simulating fermentation, the fermentation broth was collected by centrifugation at 2300 rpm at 4 °C for 10 min. The content of polysaccharides, reducing sugars, and uronic acid in the four fermentation supernatants were analyzed following the method described in Section 2.3. Additionally, the reducing power, radical scavenging activity against ABTS and DPPH, and total reducing capacity were evaluated according to the method outlined in Section 2.4.1.
2.5.3. FT-IR analysis
Ten milliliters of the fermentation broth, as described in Section 2.5.1, were subjected to alcohol precipitation, dialysis, and subsequent concentration via lyophilization to obtain polysaccharide powders, which were then characterized using FT-IR analysis (Wang, Zhang, et al., 2024).
2.5.4. Detection of pH and SCFAs
The pH of the supernatant from the fermentation liquid at various time points was analyzed using a pH meter (INESA, Shanghai, China). For the determination of SCFAs content, 1 mL of the fermentation supernatant was thoroughly mixed with 0.2 mL of a 25 % metaphosphate solution and centrifuged at 10000 rpm for 15 min. The SCFAs content was then measured using gas chromatography (Agilent Technologies, USA) equipped with a Nukol capillary column (30 m × 0.32 mm × 0.25 μm) (Ding et al., 2019).
2.5.5. 16S rDNA sequencing analysis
Total bacterial DNA was extracted from the fermentation broth using a DNA extraction kit (Laboratories, USA). The V3-V4 region of 16S rDNA was amplified and sequenced using the Illumina MiSeq system (GENE DENOVO, Guangzhou, China) (Li et al., 2023). Alpha and beta diversity analyses of the samples were performed using Motur and QIIME software. LEfSe analysis was conducted to identify statistically significant biomarkers between groups and to analyze differential microbial communities. Spearman correlation analysis was performed to explore the relationships between microbial species, biochemical indicators, and functional genes. Heatmaps of functional genes were generated using the RStudio “pheatmap” package (version 4.2.0).
2.6. Statistical analysis
Data are presented as the mean ± standard deviation (SD) from three independent measurements. Differences were analyzed using one-way analysis of variance (ANOVA) and independent sample t-tests, conducted with Origin 2021 software (OriginLab, Northampton, MA, USA) and IBM SPSS 26.0 software (International Business Machines Corporation, USA), respectively. A p-value of <0.05 was considered statistically significant.
3. Results and discussion
3.1. Physicochemical properties of FVRPF
FVRPF was prepared from FVRP, a polysaccharide derived from FVR, using an ultrasonic-assisted H₂O₂-Fe3+ method, achieving a recovery rate of 40.39 % ± 0.43 %. The physicochemical properties of FVRP (Liu et al., 2022) and FVRPF are presented in Table 1. The polysaccharide content of FVRPF was equivalent to that of FVRP. However, the uronic acid and total phenolic content of FVRPF were significantly lower than those of FVRP, while the protein and reducing sugar (CR) content remained unchanged. This is likely due to the ultrasonic-assisted H₂O₂-Fe3+ treatment, which may induce polysaccharide chain breakage, leading to the exposure of active groups and subsequent decline in the content of uronic acid and total phenolics (Gong et al., 2021).
Table 1.
Chemical composition, particle size, potential, molecular weight and monosaccharide composition of FVRP and FVRPF.
| Physicochemical properties | FVRP | FVRPF | |
|---|---|---|---|
| Polysaccharides (%) | 84.20 ± 1.00a | 84.53 ± 0.41a | |
| Protein (%) | 0.50 ± 0.06a | 0.53 ± 0.05a | |
| Uronic acid (%) | 8.80 ± 0.10a | 6.05 ± 0.01b | |
| Total phenol (%) | 0.60 ± 0.03a | 0.27 ± 0.02b | |
| Reducing sugar (%) | 0.20 ± 0.01a | 0.18 ± 0.02a | |
| Mw (kDa) | 335.29a | 256.06b | |
| Particle size (nm) | 465.43 ± 37.57a | 226.73 ± 6.05b | |
| PDI | 0.71 ± 0.04a | 0.54 ± 0.02b | |
| ζ-potential (mV) | −8.50 ± 0.50b | −9.61 ± 0.60a | |
| Monosaccharides (%) | Fuc | 9.30 | 7.51 |
| Rha | 7.35 | 6.86 | |
| Ara | 5.65 | 8.78 | |
| Gal | 16.99 | 16.29 | |
| Glc | 8.32 | 9.91 | |
| Xyl | 17.18 | 17.15 | |
| Man | 15.76 | 26.71 | |
| Gal-UA | 16.19 | 4.36 | |
| Glc-UA | 3.25 | 2.44 | |
Note: Different letters indicate significant differences between samples (P < 0.05).
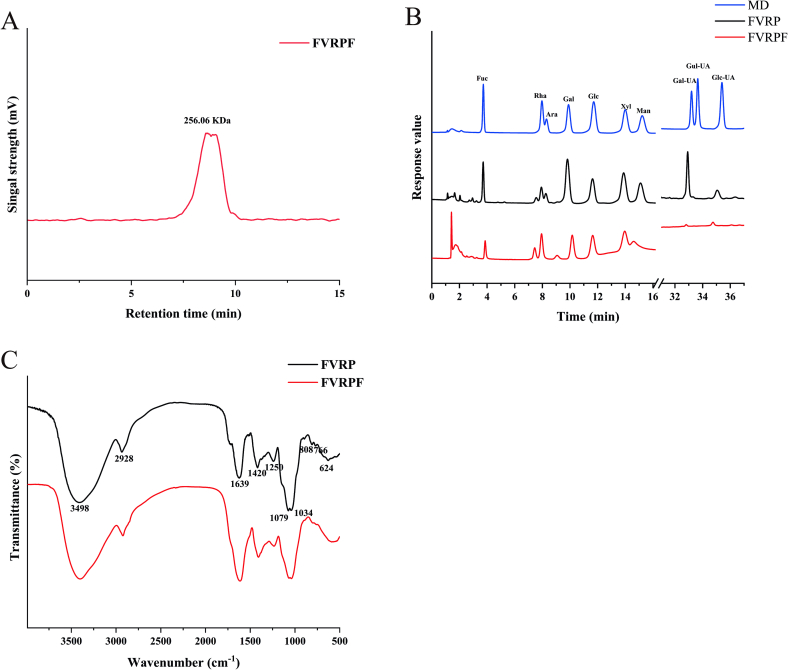
Table 1 According to Fig. 1A, the Mw of FVRPF was 256.06 kDa (Table 1), which is smaller than that of FVRP (335.29 kDa) (Liu et al., 2022). This suggests that the molecular chain of FVRP was partially degraded during the process, resulting in a reduction in Mw (Liu, Liu, et al., 2024; Ma et al., 2022). Particle size distribution, polymer dispersion index (PDI), and zeta potential are critical factors influencing the stability of polysaccharides (Yang et al., 2024). Generally, reducing particle size can enhance the biological activity of polysaccharides, while a lower PDI indicates a uniform distribution of polysaccharides and improved stability (Chen, You, Ma, Zhao, & Kulikouskaya, 2021). The particle size of FVRPF was 226.73 ± 6.05 nm, significantly smaller than that of FVRP (465.43 ± 37.57 nm). Similarly, the PDI value of FVRPF was significantly lower than that of FVRP. These findings align with results from degraded polysaccharides in strawberry fruits (Liu, Liu, et al., 2024), indicating that the degradation process was effective, resulting in a comparatively uniform and stable FVRPF. Zeta potential is commonly used to characterize the surface charge properties of polysaccharides, with higher absolute zeta potential values indicating better dispersion of polysaccharides during the dissolution process (Yang, Tao, et al., 2024). The absolute zeta potential of FVRPF (9.61 ± 0.60) was significantly higher than that of FVRP (8.50 ± 0.50) (Table 1). FVRP exhibited negative surface charges, indicating that degradation promoted molecular ionization and reduced aggregation in FVRPF. Furthermore, the reduction in PDI, accompanied by an increase in zeta potential (Zhang et al., 2024), supports the conclusion that the stability of FVRPF is higher than that of FVRP. These results were similar to that the Mw, particle size, and PDI values of degraded polysaccharides from strawberries processed using the Vc-H2O2 method were smaller than those of PSP (Liu et al., 2024).
Fig. 1.
Molecular weight (A), Monosaccharide composition (B) and FT-IR spectrum (C) of FVRPF.
As shown in Table 1 and Fig. 1B, FVRP and FVRPF primarily comprised fucose (Fuc), rhamnose (Rha), arabinose (Ara), galactose (Gal), glucose (Glc), xylose (Xyl), mannose (Man), galacturonic acid (Gal-UA), and glucuronic acid (Glc-UA), consistent with FVRP (Liu et al., 2021). The monosaccharide composition of FVRP remained unchanged after ultrasonic-assisted H2O2-Fe3+ treatment, but the molar ratios differed, aligning with previous studies (Chen et al., 2020; Chen et al., 2021; Wang et al., 2024). For example, degraded Holothuria mexicana glycosaminoglycans treated with the H2O2-Vc method exhibit the same monosaccharide composition as the native polysaccharide (Mou, Wang, Li, Qi, & Yang, 2018). Except for the Xyl content, the levels of most other monosaccharides were affected. Notably, the content of Man increased significantly, likely due to the complex composition of polysaccharides and the non-disproportionate attack of uncontrolled free radicals (Yang, Zhang, et al., 2024; Zhu, Chen, Chang, Qiu, & You, 2023). Additionally, FVRPF showed a higher Glc content than FVRP. Since gut microbiota are better able to utilize glucose, FVRPF is hypothesized to have superior prebiotic activity compared to FVRP. Conversely, the Gal-UA content decreased considerably, which may indicate that glycosides linked to Gal-UA are more susceptible to targeted free radical attacks, leading to the loss of some small polysaccharide molecules containing Gal-UA during dialysis (Yao et al., 2022). These findings suggest that while FVRPF prepared via ultrasonic-assisted H2O2-Fe3+ degradation retained the same chemical and monosaccharide composition as FVRP, the proportions of monosaccharides were significantly altered. Compared with FVRP, FVRPF showed lower Mw, particle size, and PDI, but higher zeta potential. In general, the ultrasonic-assisted H2O2-Fe3+ method had a notable impact on the physicochemical properties of polysaccharides.
3.2. FT-IR assay of FVRPF
The absorption peaks of FVRPF were observed at approximately 3498 cm−1, 2928 cm−1, 1639 cm−1, 1420 cm−1, 1250 cm−1, 1079 cm−1, 1034 cm−1, 808 cm−1, 766 cm−1, and 624 cm−1 (Fig. 1C), which align with the previously reported results for FVRP (Liu et al., 2022). FVRPF exhibited infrared characteristic peaks similar to those of FVRP (Yao et al., 2024), indicating that ultrasonic-assisted H2O2-Fe3+ degradation did not alter the primary functional groups of FVRP but, disrupted some glycosidic bonds (Yao et al., 2024). Similarly, Wang et al. reported no differences in the main functional groups and chemical bonds between LP and DLP when employing ultrasonic-assisted H2O2 degradation of Longan polysaccharide (Wang, Xue, et al., 2024), which is consistent with our results. Hence, the ultrasonic-assisted H2O2 method was shown to have a certain effect on the physicochemical characteristics of polysaccharides without altering the main structure of polysaccharide.
3.3. Antioxidant ability assay
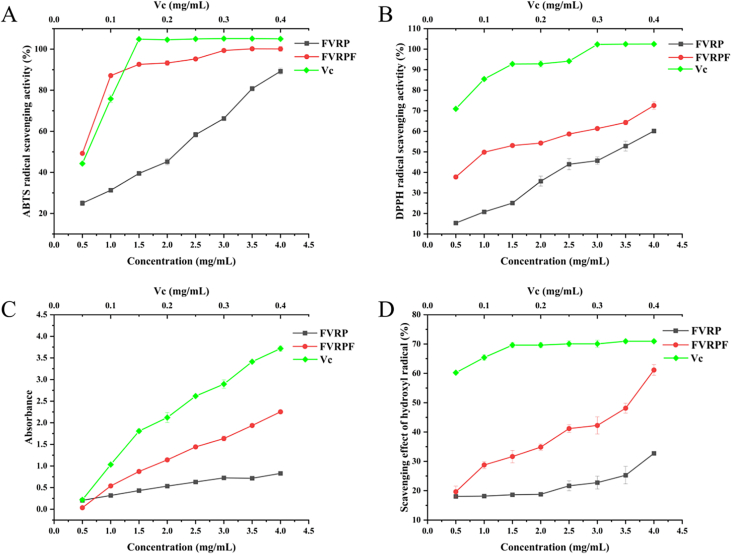
The in vitro antioxidant activities of FVRP and FVRPF were evaluated. The ABTS free radical scavenging assay is a commonly employed method to assess total antioxidant capacity (Yuan et al., 2023). DPPH, a stable lipophilic free radical, is widely used for assessing the antioxidant activity of various substances. Hydroxyl radicals, which are among the most prevalent free radicals, are considered to be destructive to nearly all biomolecules in living cells (Qi et al., 2024; Zhai et al., 2024). The results demonstrated that the in vitro antioxidant activity of FVRPF was notably higher than that of FVRP, with both exhibiting a dose-dependent increase (Fig. 2). The antioxidant activities of polysaccharides are closely associated with the Mw, monosaccharides, and chemical composition. Polysaccharides with lower Mw are thought to offer a larger surface area and greater opportunities for interactions with free radicals, thereby enhancing their antioxidant activity (Yan et al., 2021). Consistent with previous findings, FVRPF exhibited stronger antioxidant activity compared to FVRP (Liu et al., 2022; Yao et al., 2024). Therefore, the ultrasonic-assisted H2O2-Fe3+ technique enhanced the antioxidant properties of FVRP.
Fig. 2.
Antioxidant activity of FVRPs. ABTS radical scavenging activity (A), DPPH radical scavenging activity (B), Reducing power (C), Hydroxyl radical scavenging activity (D).
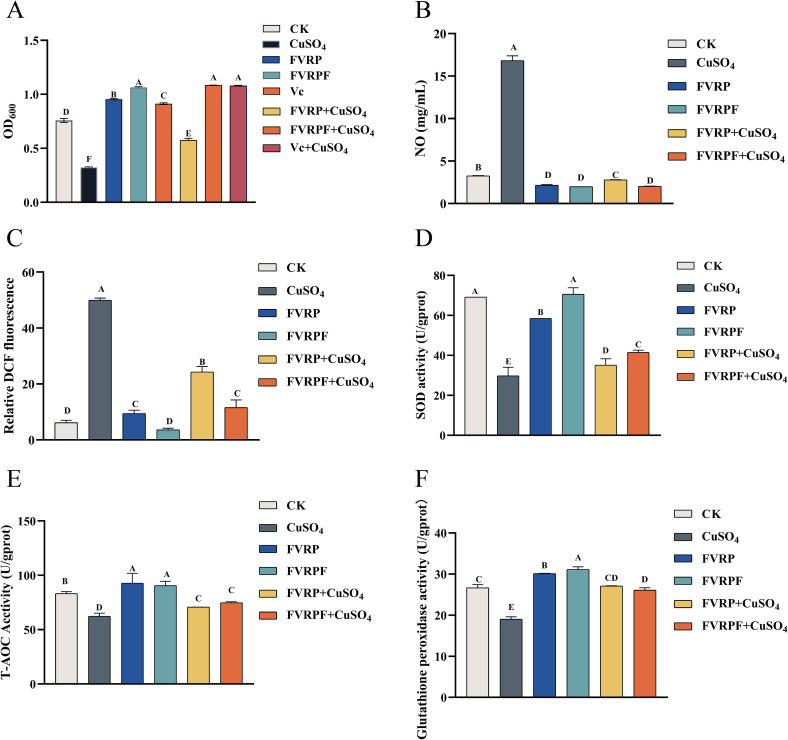
The cytotoxicity of heavy metals is primarily attributed to an increase in endogenous oxidative stress (Shi, Zou, Chen, Zheng, & Ying, 2016). A yeast model for oxidative damage induced by heavy metals can be employed to evaluate the antioxidant capabilities of active compounds (Henkler, Brinkmann, & Luch, 2010). In this study, the viability of yeast was tested to assess the protective effects of FVRPs against oxidative damage induced by CuSO4. To further evaluate the antioxidant potential of FVRPF, we measured levels of NO and ROS, total antioxidant capacity, and the activities of SOD and GSH-Px. The OD600 results illustrated that FVRPF notably alleviated the toxicity of CuSO₄, exhibiting similar efficacy to the positive drug Vc. Additionally, FVRP significantly promoted the growth of yeast cells, though the improvement effect was considerably lower than that of FVRPF (Fig. 3A). The growth patterns observed on PDA plates were consistent with the OD600 results (Fig. S1).
Fig. 3.
In vivo activity of FVRPF. Effects of FVRPF on cell density of yeast cells at OD600 under CuSO4 stress (A), NO content (B), ROS content (C), SOD activity (D), T-AOC activity (E), GSH-Px activity (F).
Note: Different letters indicate significant differences between samples (P < 0.05).
Compared with the CK group, the NO content in the CuSO4 group was markedly elevated, further indicating that the damage was induced by CuSO4. After treatment with FVRP and FVRPF, the NO content was more significantly reduced by FVRPF than by FVRP (Fig. 3B). The overproduction of ROS can result in oxidative stress and cellular damage and is commonly used as an indicator of oxidative stress in living cells (Wu et al., 2022). Both FVRP and FVRPF demonstrated a significant ability to reduce ROS levels in yeast cells damaged by CuSO4; however, FVRP exhibited a significantly greater effect than FVRPF (Fig. 3C). SOD is a critical antioxidant enzyme that plays an essential role in protecting cells from oxidative stress (Chai et al., 2018). This study showed that both FVRP and FVRPF significantly enhanced SOD activity in yeast cells compared with the model group, with FVRPF demonstrating a significantly stronger effect (Fig. 3D). Furthermore, FVRPF significantly improved total antioxidant capacity and GSH-Px activity in yeast cells damaged by CuSO₄ (Fig. 3E, F), consistent with its effects on SOD activity. These findings indicate that, compared to FVRP, FVRPF exhibited a stronger capacity to reduce the production of NO and ROS,improve SOD activity, GSH-Px, and total antioxidant capacity in CuSO4-induced yeast cells.
Polysaccharides have been extensively studied as dietary antioxidants due to their potential to improve health by neutralizing free radicals (Guo, Deng, et al., 2024). Some studies have reported a negative correlation between the antioxidant capacity of polysaccharides and their Mw (Lee et al., 2024; Tao et al., 2024). Low-Mw polysaccharides are believed to possess more reduced hydroxyl groups, a larger surface area, and greater opportunities for interaction with free radicals (Li, Lei, et al., 2024). Additionally, the antioxidant activity of polysaccharides is influenced by their functional groups, chemical composition, and spatial configuration (Chen et al., 2023; Yang, Tao, et al., 2024; Zhang, Wang, et al., 2024). Degradation may also lead to the release of electrophilic groups and generating polysaccharides with more reduced hydroxyl groups capable of targeting and reacting with active free radicals (Yang, Tao, et al., 2024). In the present study, compared to FVRP, FVRPF exhibited a lower Mw, particle size, and PDI, as well as stronger antioxidant activity. In summary, ultrasonic-assisted H2O2-Fe3+ degradation improved the antioxidant activity of polysaccharides derived from FVR. This method represents a novel approach for developing polysaccharides with improved antioxidant properties in the future.
3.4. Fermentation characteristics of FVRPF
3.4.1. Changes in physicochemical characteristics and in vitro antioxidant ability of fermentation broth
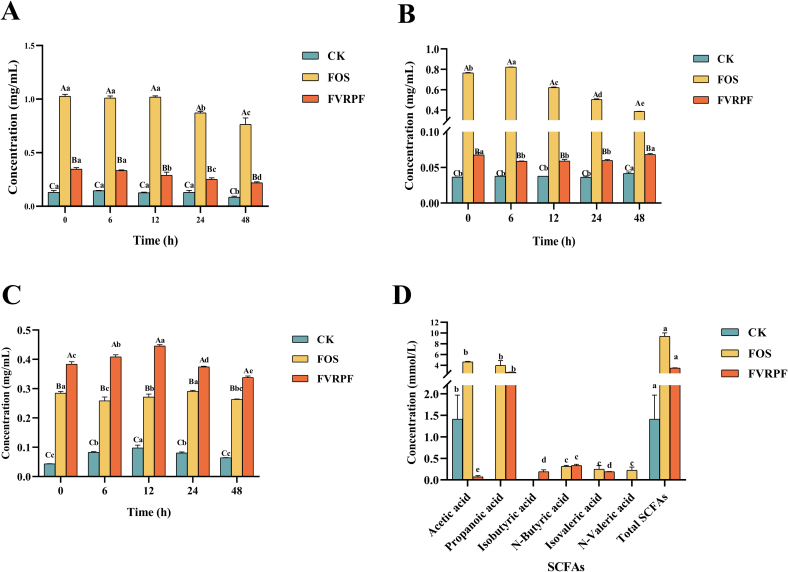
The gut microbiota plays a crucial role in human health, particularly in immune system function and energy metabolism (Zhang et al., 2024). Generally, the gut microbiota can degrade indigestible polysaccharides to produce energy (Wang, Zhang, et al., 2024; Xu et al., 2019). The prebiotic activity of polysaccharides is closely associated with their utilization by probiotics and depends on various physicochemical properties, chemical composition, and structural characteristics of polysaccharides, such as the Mw and chemical composition of polysaccharides (Fan et al., 2024; Wang, Xu, et al., 2024). To investigate the utilization of FVRPF by human gut microbiota (Li et al., 2022), an in vitro fecal fermentation model was employed. The physicochemical properties and structural changes of FVRPF were also examined during in vitro fermentation. Fig. 2 illustrates the changes in the chemical composition of FVRPF during human fecal fermentation. The polysaccharide content in the fermentation broth of the CK group, FOS group, and FVRPF group decreased markedly with prolonged fermentation time (Fig. 4A). Among them, the polysaccharide content in the fermentation broth of the FVRPF group exhibited a significant decline after 48 h of fermentation, implying that FVRPF is readily degradable by human gut microbiota. The gut microbiota may hydrolyze FVRPF through glucoside hydrolase activity, producing reducing sugars that provide a continuous supply of carbon source for the gut microbiota. Moreover, the utilization of polysaccharides by the gut microbiota resulted in changes in the concentrations of CR and carbohydrates (Wei et al., 2024). In the FVRPF group, CR content decreased significantly at 6 h and increased significantly at 48 h (Fig. 4B). Therefore, this pattern in the reducing sugar content during fermentation aligns with previous research findings (Zhang, Zhu, et al., 2024). Furthermore, the uronic acid content in the 24 h fermentation broth across all groups showed a significant decrease. In the FVRPF group, the uronic acid content in fermentation broth increased significantly between 0 and 12 h, but then exhibited a downward trend, ultimately decreasing by 23 % at 48 h (Fig. 4C). Herein, we speculate that FVRPF is degraded and utilized by human gut microbiota, with its utilization rate increasing as fermentation time progresses.
Fig. 4.
Total polysaccharide content (A), reducing sugar content (B), uronic acid content (C), and SCFAs content (H) of FVRPF after in vitro fermentation.
Note: Different lowercase letters indicate significant differences between samples at different times (P < 0.05), and different uppercase letters indicate significant differences between different samples at the same time (P < 0.05).
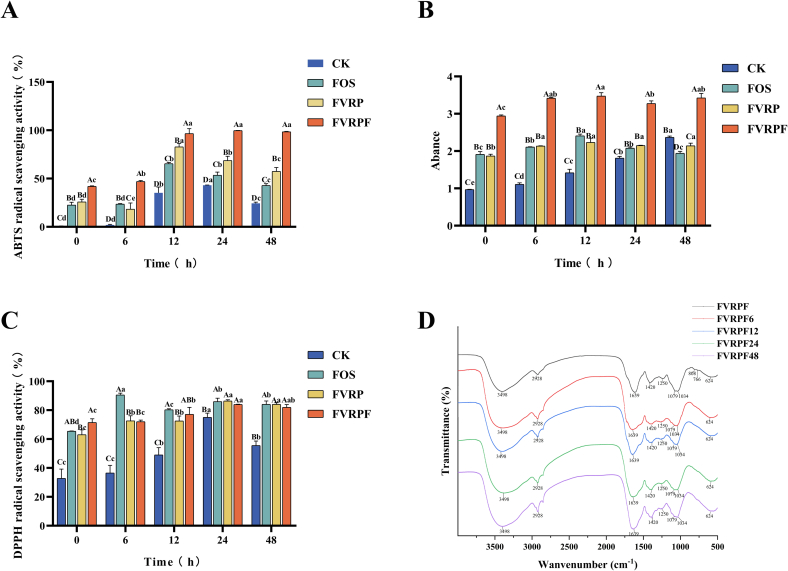
The in vitro antioxidant capacities of FVRPF in the fermentation broth were evaluated based on reducing ability, DPPH scavenging rate and ABTS scavenging rate. As shown in Fig. 5, the ABTS scavenging activity, reducing ability, and DPPH scavenging activity of the CK group, FOS group, FVRP group, and FVRPF group all increased notably with prolonged fermentation time (Fig. 5A–C). After 48 h of in vitro fermentation, the ABTS scavenging activity and reducing power of the FVRPF group were considerably stronger than those of the other three groups. Notably, the reducing power of FVRPF exhibited an upward trend throughout the fermentation process, surpassing the levels observed in the other groups. Additionally, the DPPH scavenging activity of FVRPF was comparable to that of the FOS group as fermentation time progressed. These results indicate that the in vitro antioxidant capacities of FVRPF improved significantly during the fermentation process and were significantly higher than those of FVRP (Zhang, Chen, et al., 2024). This suggests that the degradation process enhances the in vitro antioxidant properties of polysaccharides, aligning with findings from previous research (Yan et al., 2021).
Fig. 5.
ABTS radical scavenging ability (A), reduction ability (B), DPPH radical scavenging ability (C), and FT-IR spectrum (D) of FVRPF after in vitro fermentation.
Note: Different lowercase letters indicate significant differences between samples at different times (P < 0.05), and different uppercase letters indicate significant differences between different samples at the same time (P < 0.05).
3.4.2. FT-IR assay
Polysaccharides were extracted from the fermentation broth of FVRPF at different fermentation time, and their FT-IR spectral changes were analyzed to elucidate their degradation properties by in vitro gut microbiota. The FT-IR results demonstrated that the FT-IR spectra of FVRPF after in vitro fermentation remained largely consistent (Fig. 5D). This finding suggests that the main chemical structure of FVRPF was stable and not degraded by human gut microbiota during simulated in vitro fermentation, aligning with results from previous studies (Sun et al., 2024; Zhang, Zhu, et al., 2024). Furthermore, the FT-IR analysis of the in vitro fecal fermentation of polyspermous polysaccharides exhibited similar spectral absorption bands throughout the fermentation process (0–48 h). This indicates that in vitro fermentation did not affect any other structure, apart from the disruption of glycosidic bonds (Chen et al., 2024).
3.4.3. pH change of fermentation broth
The variation in pH is a critical indicator in the polysaccharide fermentation process (Gao et al., 2024a). The pH changes in the fermentation broth over time are displayed in Fig. S2. The pH values of the CK, FOS, and FVRPF groups all exhibited a downward trend as the fermentation process progressed. Compared with that of the CK group, the pH value of the FOS group decreased significantly. Similarly, the pH value of the FVRPF group was lower than that of the CK group after 24 h of fermentation. The in vitro fecal fermentation of oolong tea polysaccharides also showed a marked decrease in pH after fermentation (Wu, Huang, et al., 2022). This outcome suggests that treatment with FVRPF promotes the generation of substantial amounts of acidic metabolites. Previous research has proved that pH changes during simulated in vitro fermentation are closely associated with the production of SCFAs, thereby reducing the pH of the surrounding environment (Guo et al., 2022). Furthermore, a mildly acidic environment can improve the proliferation of beneficial gut microbiota while inhibiting the growth of harmful gut microbiota (Wu et al., 2022). Therefore, FVRPF might exhibit physiological activities that support intestinal health.
3.4.4. Changes in SCFAs after in vitro human fecal fermentation
Most polysaccharides are resistant to digestion by the human digestive tract and reach the cecum and colon (Gao et al., 2024b). There, they can be degraded and utilized by specific gut microbiota to produce SCFAs, which play a crucial role in regulating intestinal pH and supporting the function of intestinal epithelial cells (Guo et al., 2022). SCFAs, the primary metabolites generated from gut bacterial fermentation of prebiotic polysaccharides, are essential for maintaining intestinal health. Fig. 4D presents the SCFA content in the CK, FOS, and FVRPF groups after 48 h of fecal fermentation. The results indicate that the total SCFA levels across the groups followed the order: FOS > FVRPF > CK, consistent with the observed pH values in each group. The CK group only produced one type of Acetic acid. FOS group primarily produced Propanoic acid and Acetic acid. In contrast, the FVRPF group was predominantly enriched in Propanoic acid, Acetic acid, Isobutyric acid, N-Butyric acid, and Isovaleric acid. Among these, Acetic acid, one of the most common SCFAs in peripheral circulation, is absorbed by colonic epithelial cells to provide energy for multiple organs and tissues (Ferreira-Lazarte, Moreno, Cueva, Gil-Sánchez, & Villamiel, 2019). Additionally, Acetic acid is the primary end product of the fermentation processes carried out by bifidobacteria and lactic acid bacteria (Hu et al., 2023). Propanoic acid, a major energy substrate for intestinal epithelial cells, not only influences liver and cholesterol metabolism, appetite suppression, immune regulation, and energy homeostasis but may also enhance systemic insulin sensitivity (Fu et al., 2019; Wang et al., 2019). Similarly, N-Butyric acid serves as a predominant energy source for intestinal epithelial cells and plays a critical role in mitigating diabetes, regulating host gene expression, and influencing cell differentiation and apoptosis (Guan et al., 2020; Ziegler, Kerimi, Poquet, & Williamson, 2016). FVRPF exhibited a similar trend in SCFA changes to FVRP, which has been demonstrated to possess prebiotic activity (Zhang, Wang, et al., 2024). In addition, the production of Propionic acid is primarily driven by the fermentation of Glc and Man, whereas the fermentation of Gal and Man notably enhances N-Butyric acid levels (Fu et al., 2018). Elevated levels of Propionic and Butyric acids have been shown to be associated with the utilization of Man and Gal (Li, Lei, et al., 2024). Considering the monosaccharide composition of FVRPF (Table 1), these findings suggest that the increased SCFA production may be attributed to the high consumption of Gal and Man in FVRPF. Overall, these results indicate that FVRPF holds promising potential for enhancing the production of SCFAs, possibly by promoting Propionic acid synthesis and acidifying the intestinal environment, thereby contributing to the regulation of intestinal immunity.
3.4.5. Influence of FVRPF on the diversity of human gut microbiota
The gut microbiota plays a role in energy collection and storage, various metabolic functions, and immunomodulation, thereby significantly influencing human health and disease (Clemente, Ursell, Parfrey, & Knight, 2012; Li, Wang, Wang, Hu, & Chen, 2016). As dietary components, polysaccharides can modulate gut microbiota and SCFA levels, thereby promoting the growth of microbiota, regulating the human circulatory system, and contributing to overall health (Duque et al., 2021). Therefore, to investigate the effects of FVRPF, high-throughput sequencing was conducted to examine the influence of FVRPF on gut microbiota after 48 h of fermentation.
Alpha diversity was evaluated using the Shannon, ACE, Simpson, Chao1, and Sobs indices (Fig. S3A–E). The Chao1, ACE, and Sobs indices are typically influenced by species abundance, while the Shannon and Simpson indices reflect community diversity (Grice et al., 2009). Fig. S3A–E illustrate changes in these indices for evaluating alpha diversity. With increasing sequencing depth and sample size, the Shannon, Simpson, and Sobs-based curves tended to plateau, indicating that the gut microbiota data were reliable and sufficient. Notably, the FVRPF group showed slightly higher Shannon, Simpson, and Sobs indices compared with the other groups. Thus, FVRPF had a more favorable effect on maintaining the richness and diversity of gut microbiota, aligning with previous research (Zhang, Wang, et al., 2024). To assess beta diversity in the gut microbiota, principal coordinate analysis (PCoA) was performed to examine correlations among gut microbiota structures across different groups at the OTU level (Luo et al., 2023). PCoA1 and PCoA2 accounted for 61.53 % and 35.21 % of the variance in sample composition, respectively (Fig. S3F). Distances between the CK, FOS, and FVRPF groups were greater, while distances within the same group were closer, indicating marked differences in the composition of gut microbiota among groups. This reflects the response of gut microbiota to different carbon sources, consistent with findings from previous studies (Peng et al., 2024). In summary, these results indicate that FVRPF plays a significant role in modulating the structure of gut microbiota.
3.4.6. Impact of FVRPF on the composition of gut microbiota
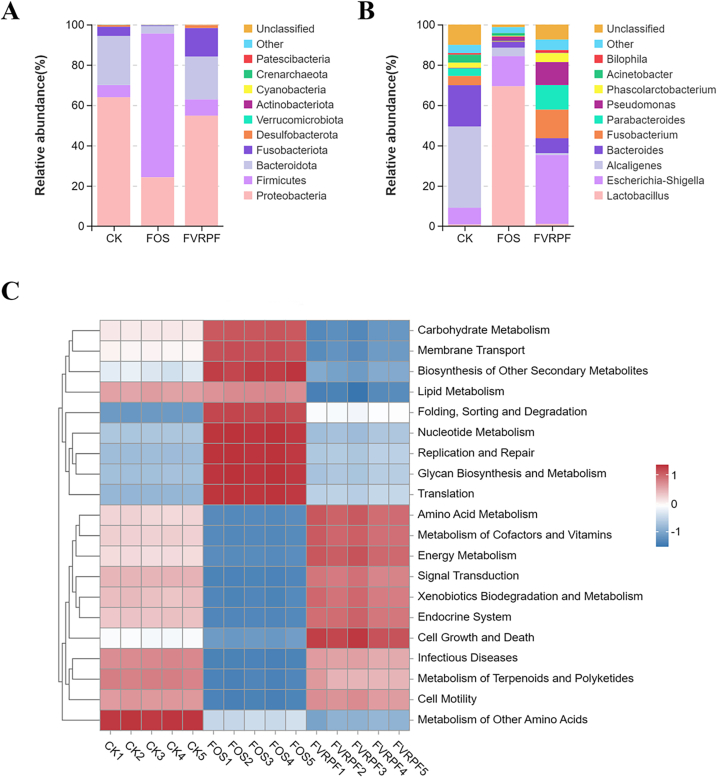
The effect of FVRPF on gut microbiota composition was investigated using 16S rDNA sequencing analysis. The distribution of microbial composition at the phylum level after 48 h of fermentation is shown in Fig. 6A. The predominant microorganisms in the CK group were Proteobacteria, Firmicutes, Bacteroidota, and Fusobacteria, consistent with previous findings (Hu et al., 2023). In the FOS group, the dominant microbiota were Proteobacteria, Firmicutes, and Bacteroidota compared with the CK group. Specifically, the relative abundance of Firmicutes in the FOS group was markedly increased, while the relative abundance of Proteobacteria and Bacteroidota was significantly decreased. Notably, the relative abundance of Bacteroidota in the FOS group was lower than in the CK group, potentially due to the lower pH after 48 h of fermentation. For example, the relative abundance of Bacteroidetes was higher in the FOS group (Wu et al., 2021). Meanwhile, the relative abundances of Firmicutes and Fusobacteria in the FVRPF group exhibited a slight increase compared with the CK group, while the levels of Proteobacteria and Bacteroidota were slightly lower than those observed in the CK group. Firmicutes have been shown to produce SCFAs through the fermentation of carbohydrates (Tawfick, Xie, Zhao, Shao, & Farag, 2022), which may explain the higher SCFA content observed in the FVRPF group compared to the CK group (Fig. 4D). Proteobacteria is the most distinct bacterial phylum and is common in healthy human fecal microbiota. An excessive presence of Proteobacteria in the gut can lead to microbiota imbalances, leading to low-grade inflammation (Huang et al., 2020). Fusobacteria, known as pathogenic bacteria, tend to increase in response to nutritional deficiencies and are closely associated with the development of gastric cancer (Xu, Chen, Liu, & Cheong, 2020). Bacteroidota, a major beneficial gut microbiota, plays a key role in SCFA production through the fermentation and utilization of polysaccharides (Liu et al., 2021). According to reports, Bacteroidota degrade and utilize polysaccharides via a series of carbohydrate-active enzymes, including glycoside hydrolases and polysaccharide hydrolases. The metabolites derived from polysaccharide fermentation can, in turn, be utilized by other gut microbiota (Guo et al., 2024). Additionally, Bacteroidota can act as an anti-inflammatory agent. Contributing to the maintenance of gut microbiota, improved metabolism in obese individuals, and enhanced immune function. Moreover, variations in microbiota composition at the genus level were also evaluated (Fig. 6B). The abundances of Fusobacterium, Escherichia/Shigella, Parabacteroides, and Pseudomonas were higher in the FVRPF group compared with both the CK and FOS groups. On the contrary, the abundance of Alcaligenes in the FVRPF group was notably lower than that in the CK and FOS groups, while the abundance of Bacteroides was remarkably lower in the CK group but higher than in the FOS group. Alcaligenes has been implicated in peritonitis (Wang et al., 2018). Nevertheless, the higher abundance of Escherichia-Shigella in the FVRPF group may result from the utilization and absorption of carbon substances with low Mw by Escherichia-Shigella, which promotes its growth (Guan et al., 2022). This phenomenon suggests that Escherichia-Shigella becomes the dominant bacterial colony following FVRPF fermentation. These results demonstrate that FVRPF has the potential to modulate the composition and abundance of gut microbiota in in vitro fermentation systems.
Fig. 6.
The relative abundance of the gut microbiota at the phylum level (A), the relative abundance of bacterial community at the genus level (B), functional predictions for gut microbiota (C).
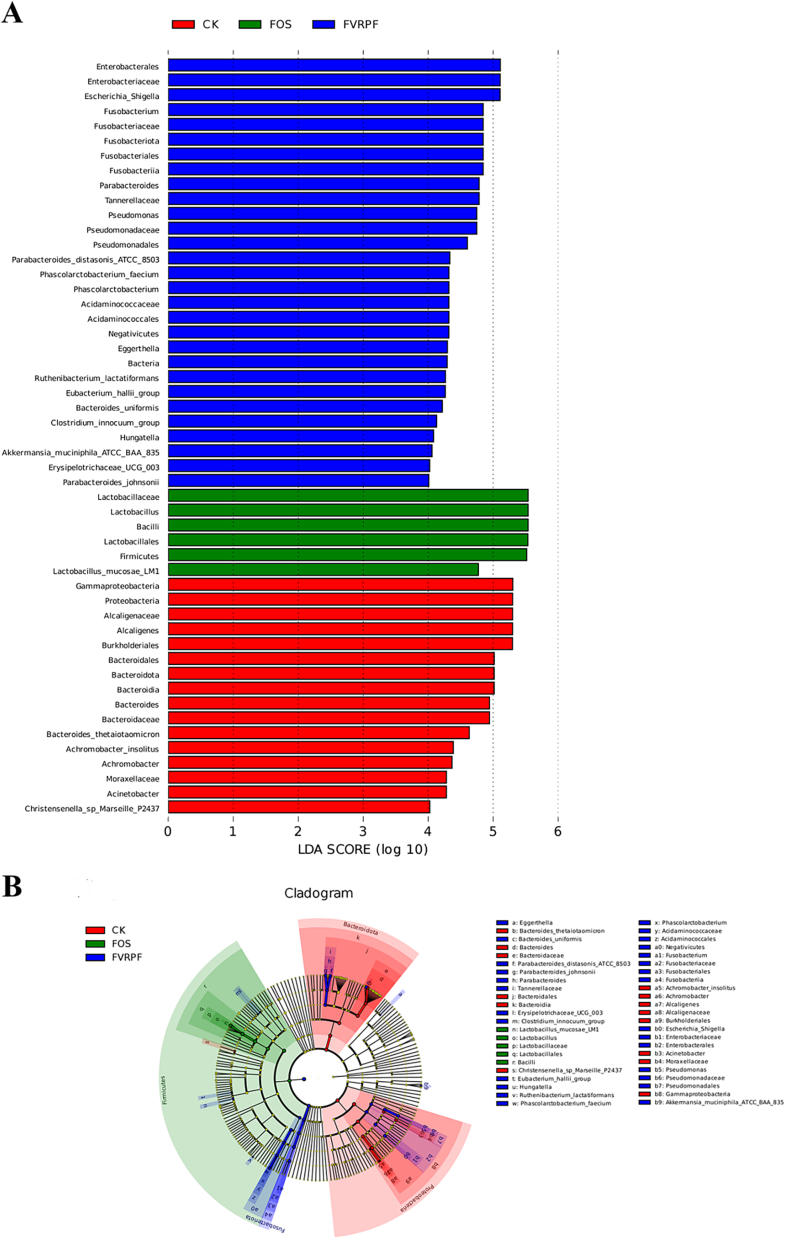
3.4.7. LEfSe analysis
Different microbial communities generated during in vitro fermentation were examined using LEfSe and LDA analyses (Fig. 7A, B). A total of 48 genera had LDA scores above 4.0, with 15, 6, and 27 species identified in the CK, FOS, and FVRPF groups, respectively, showing significant differences among the groups. In addition, Bacteroides and Parabacteroides were the predominant bacteria in the FVRPF group, while Firmicutes dominated the FOS group. Both Bacteroidota and Parabacteroides are beneficial bacteria with a strong capacity to degrade polysaccharides (Zhang et al., 2021). They are also major producers of SCFAs, same as Firmicutes (Liu et al., 2021). These microbial communities likely contribute to the overproduction of SCFAs in the FOS and FVRPF groups (Fig. 4D).
Fig. 7.
Histogram showing LDA-based distribution (A), and Cladogram (B).
Parabacteroides mitigates obesity and metabolic dysfunction by producing secondary bile acids and succinic acid (Li et al., 2022). Bacteroidota can utilize the succinate pathway to produce Propanoic acid (Chen et al., 2018). Hence, the increased abundance of Bacteroidota and Parabacteroides may be attributed to the accumulation of Propanoic acid in the FVRPF group, consistent with FVRP (Zhang, Chen, et al., 2024). Mori Folium polysaccharides (MP) have been shown to regulate gut microbiota composition and promote the proliferation of beneficial bacteria in an in vitro fermentation model (Zhang, Zhu, et al., 2024). These results suggest that FVRPF has the potential to modulate gut microbiota balance and promote the growth of specific probiotics.
3.4.8. Prediction of metabolic function of gut microbiota
KEGG is an integrated database that connects genomes, biological pathways, diseases, and drugs. It is commonly used for annotating the functions of gut microbiota (Hu et al., 2021; Zhou, Chen, Sun, Li, & Huang, 2019). Compared with the CK and FOS groups, the FVRPF group demonstrated significant upregulation of metabolic pathways, such as amino acid metabolism, metabolism of cofactors and vitamins, energy metabolism, signal transmission, xenobiotics biodegradation and metabolism, endocrine system, and cell growth and death. In contrast, it exhibited significantly downregulated carbohydrate metabolism, membrane transport, biosynthesis of other secondary metabolism, lipid metabolism, folding, sorting, and degradation, and metabolism of other amino acids (Fig. 7B). The KEGG analysis indicated that polysaccharides utilized by human gut microbiota increase metabolic enzyme activity, primarily those involved in amino acid metabolism, factors and vitamins, and energy metabolism (Li et al., 2020). Additionally, Bacteroidota, a key genus involved in host amino acid metabolism, reduces host cardiovascular injury by promoting the accumulation of branched-chain amino acids (Qiao et al., 2022). Alterations in lipid metabolism within the intestinal microbiota are closely associated with host metabolic syndrome (Li et al., 2020). These metabolic processes may accelerate energy production and conversion while enhancing the host's defense mechanisms. The functional prediction results suggest that FVRPF enhances biosynthesis and metabolic processes, accelerates energy production and conversion in the body, and helps stabilize host defense mechanisms.
4. Conclusion
In this study, the ultrasonic-assisted H2O2-Fe3+ method was employed to investigate the chemical composition, antioxidant capacity, and prebiotic properties of FVRPF, a product derived from the degradation of FVRP. Compared with FVRP, the main functional groups of FVRPF remained unchanged, although the proportions of its chemical and monosaccharide compositions were altered. Moreover, the Mw, particle size, and PDI of FVRPF decreased, while the zeta potential increased compared with FVRP. Notably, FVRPF exhibited significantly higher antioxidant capacity than FVRP. In addition, FVRPF demonstrated a degree of stability and exhibited a significant improvement in antioxidant activity during in vitro human fecal fermentation. Simultaneously, FVRPF influenced the composition and abundance of gut microbiota, promoted the growth of specific probiotics, and increased the production of SCFAs during in vitro fermentation. It might enhance intestinal defense mechanisms, as indicated by KEGG analysis. Therefore, FVRPF has potential as a natural antioxidant or novel prebiotic dietary ingredient for regulating gut health by improving the gut microbiota environment, offering promising applications in the food, cosmetic, and pharmaceutical industries. However, further systematic research is needed to elucidate the specific changes in human gut microbiota and the metabolic processes influenced by FVRPF.
CRediT authorship contribution statement
Liping Wang: Writing – original draft, Visualization, Formal analysis, Data curation, Conceptualization. Yao Zhang: Writing – original draft, Visualization, Formal analysis, Data curation, Conceptualization. Xinyuan Zang: Writing – review & editing, Validation. Yiting Yang: Validation, Supervision. Wanting Wang: Writing – review & editing. Jingbo Zhang: Software. Yunxiang Que: Investigation. Fengxiang Liang: Validation, Supervision. Tiezhu Wang: Writing – review & editing, Formal analysis. Jian Zhang: Writing – review & editing, Validation, Supervision, Resources. Hongxia Ma: Writing – review & editing, Validation, Supervision, Resources. Lili Guan: Writing – review & editing, Validation, Supervision, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Jilin Provincial Scientific and Technological Development Program (20230101247JC and 20240303085NC).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.102049.
Contributor Information
Jian Zhang, Email: as1220@163.com.
Hongxia Ma, Email: mahongxia@jlau.edu.cn.
Lili Guan, Email: llguan@jlau.edu.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Arantes V., Milagres A.M.F. Degradation of cellulosic and hemicellulosic substrates using a chelator-mediated Fenton reaction. Journal of Chemical Technology & Biotechnology. 2006;81(3):413–419. [Google Scholar]
- Arantes V., Milagres A.M.F. The effect of a catecholate chelator as a redox agent in Fenton-based reactions on degradation of lignin-model substrates and on COD removal from effluent of an ECF kraft pulp mill. Journal of Hazardous Materials. 2007;141(1):273–279. doi: 10.1016/j.jhazmat.2006.06.134. [DOI] [PubMed] [Google Scholar]
- Bai C., Chen R., Tan L., Bai H., Tian L., Lu J.…Chi Y. Effects of multi-frequency ultrasonic on the physicochemical properties and bioactivities of polysaccharides from different parts of ginseng. International Journal of Biological Macromolecules. 2022;206:896–910. doi: 10.1016/j.ijbiomac.2022.03.098. [DOI] [PubMed] [Google Scholar]
- Chai Z., Huang W., Zhao X., Wu H., Zeng X., Li C. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of polysaccharide from Cynanchum auriculatum Royle ex Wight. International Journal of Biological Macromolecules. 2018;119:1068–1076. doi: 10.1016/j.ijbiomac.2018.08.024. [DOI] [PubMed] [Google Scholar]
- Chen G., Xie M., Wan P., Chen D., Ye H., Chen L.…Liu Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chemistry. 2018;244:331–339. doi: 10.1016/j.foodchem.2017.10.074. [DOI] [PubMed] [Google Scholar]
- Chen S.K., Wang X., Guo Y.Q., Song X.X., Yin J.Y., Nie S.P. Exploring the partial degradation of polysaccharides: Structure, mechanism, bioactivities, and perspectives. Comprehensive Reviews in Food Science and Food Safety. 2023;22(6):4831–4870. doi: 10.1111/1541-4337.13244. [DOI] [PubMed] [Google Scholar]
- Chen W., Dong M., Wang L., Wu J., Cong M., Yang R., Yu N., Zhou A., Liang J. In vitro digestive and fermentation characterization of Polygonatum cyrtonema polysaccharide and its effects on human gut microbiota. LWT. 2024;203 [Google Scholar]
- Chen X., You L., Ma Y., Zhao Z., Kulikouskaya V. Influence of UV/H2O2 treatment on polysaccharides from Sargassum fusiforme: Physicochemical properties and RAW 264.7 cells responses. Food and Chemical Toxicology. 2021;153 doi: 10.1016/j.fct.2021.112246. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang R., Li Y., Li X., You L., Kulikouskaya V., Hileuskaya K. Degradation of polysaccharides from Sargassum fusiforme using UV/H2O2 and its effects on structural characteristics. Carbohydrate Polymers. 2020;230 doi: 10.1016/j.carbpol.2019.115647. [DOI] [PubMed] [Google Scholar]
- Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: An integrative view. CELL. 2012;148(6):1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Liu X., Li S., Hao L., Du J., Gao D.…Lu J. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. International Journal of Biological Macromolecules. 2018;117:256–263. doi: 10.1016/j.ijbiomac.2018.05.134. [DOI] [PubMed] [Google Scholar]
- Da Rocha R.F.P., Da Costa M.P.M., Da Costa A.C.A., de Mello Ferreira I.L. Study of the degradation in an ultisol of alginate-chitosan complex and its stability and applicability as a soil conditioner. International Journal of Biological Macromolecules. 2024;264 doi: 10.1016/j.ijbiomac.2024.130384. [DOI] [PubMed] [Google Scholar]
- Dai J., Wu Y., Chen S., Zhu S., Yin H., Wang M., Tang J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydrate Polymers. 2010;82(3):629–635. [Google Scholar]
- Ding Y., Yan Y., Peng Y., Chen D., Mi J., Lu L.…Cao Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. International Journal of Biological Macromolecules. 2019;125:751–760. doi: 10.1016/j.ijbiomac.2018.12.081. [DOI] [PubMed] [Google Scholar]
- Dou Z., Chen C., Huang Q., Fu X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. International Journal of Biological Macromolecules. 2021;183:1548–1559. doi: 10.1016/j.ijbiomac.2021.05.131. [DOI] [PubMed] [Google Scholar]
- Duque A.L.R.F., Demarqui F.M., Santoni M.M., Zanelli C.F., Adorno M.A.T., Milenkovic D.…Sivieri K. Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110657. [DOI] [PubMed] [Google Scholar]
- Fan H., Li R., Zhang Y., Xu X., Pan S., Liu F. Effect of H2O2/ascorbic acid degradation and gradient ethanol precipitation on the physicochemical properties and biological activities of pectin polysaccharides from Satsuma mandarin. International Journal of Biological Macromolecules. 2024;280 doi: 10.1016/j.ijbiomac.2024.135843. [DOI] [PubMed] [Google Scholar]
- Ferreira-Lazarte A., Moreno F.J., Cueva C., Gil-Sánchez I., Villamiel M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®) Carbohydrate Polymers. 2019;207:382–390. doi: 10.1016/j.carbpol.2018.11.088. [DOI] [PubMed] [Google Scholar]
- Fleita D., El-Sayed M., Rifaat D. Evaluation of the antioxidant activity of enzymatically-hydrolyzed sulfated polysaccharides extracted from red algae; Pterocladia capillacea. LWT - Food Science and Technology. 2015;63(2):1236–1244. [Google Scholar]
- Fu X., Cao C., Ren B., Zhang B., Huang Q., Li C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydrate Polymers. 2018;183:230–239. doi: 10.1016/j.carbpol.2017.12.048. [DOI] [PubMed] [Google Scholar]
- Fu Y., Zhang J., Chen K., Xiao C., Fan L., Zhang B., Ren J., Fang B. An in vitro fermentation study on the effects of Dendrobium officinale polysaccharides on human intestinal microbiota from fecal microbiota transplantation donors. Journal of Functional Foods. 2019;53:44–53. [Google Scholar]
- Gao K., Peng X., Wang J., Wang Y., Pei K., Meng X.…Liu Y. In vivo absorption, in vitro simulated digestion and fecal fermentation properties of polysaccharides from Pinelliae Rhizoma Praeparatum cum Alumine and their effects on human gut microbiota. International Journal of Biological Macromolecules. 2024;266 doi: 10.1016/j.ijbiomac.2024.131391. [DOI] [PubMed] [Google Scholar]
- Gao K.X., Peng X., Wang J.Y., Wang Y., Pei K., Meng X.L.…Liu Y.J. In vivo absorption, in vitro simulated digestion and fecal fermentation properties of polysaccharides from Pinelliae Rhizoma Praeparatum cum Alumine and their effects on human gut microbiota. International Journal of Biological Macromolecules. 2024;266(Pt 2) doi: 10.1016/j.ijbiomac.2024.131391. [DOI] [PubMed] [Google Scholar]
- Gong Y., Ma Y., Cheung P.C., You L., Liao L., Pedisić S., Kulikouskaya V. Structural characteristics and anti-inflammatory activity of UV/H2O2-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food and Chemical Toxicology. 2021;152 doi: 10.1016/j.fct.2021.112157. [DOI] [PubMed] [Google Scholar]
- Grice E.A., Kong H.H., Conlan S., Deming C.B., Davis J., Young A.C.…NISC, C. S. P. Topographical and temporal diversity of the human skin microbiome. Science (American Association for the Advancement of Science) 2009;324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan N., He X., Wang S., Liu F., Huang Q., Fu X.…Zhang B. Cell Wall integrity of pulse modulates the in vitro fecal fermentation rate and microbiota composition. Journal of Agricultural and Food Chemistry. 2020;68(4):1091–1100. doi: 10.1021/acs.jafc.9b06094. [DOI] [PubMed] [Google Scholar]
- Guan X., Feng Y., Jiang Y., Hu Y., Zhang J., Li Z.…Hu W. Simulated digestion and in vitro fermentation of a polysaccharide from lotus (Nelumbo nucifera Gaertn.) root residue by the human gut microbiota. Food Research International. 2022;155 doi: 10.1016/j.foodres.2022.111074. [DOI] [PubMed] [Google Scholar]
- Guo D., Lei J., He C., Peng Z., Liu R., Pan X.…Geng X. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from Clitocybe squamulose. International Journal of Biological Macromolecules. 2022;208:343–355. doi: 10.1016/j.ijbiomac.2022.03.126. [DOI] [PubMed] [Google Scholar]
- Guo W., Wang X., Wang B., Zhang Y., Zhao F., Qu Y., Yao L., Yun J. In vitro digestion and fecal fermentation behaviors of exopolysaccharide from Morchella esculenta and its impacts on hypoglycemic activity via PI3K/Akt signaling and gut microbiota modulation. Food Chemistry: X. 2024;24 doi: 10.1016/j.fochx.2024.101870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Deng R., Wang Y., Qu M., Liu P., Gao J. Extraction, separation, and antioxidant activity of polysaccharides from peony seed shell. Industrial Crops and Products. 2024;222 [Google Scholar]
- Hao R., Zhou X., Zhao X., Lv X., Zhu X., Gao N.…Li D. Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Science of the Total Environment. 2023;877 doi: 10.1016/j.scitotenv.2023.162910. [DOI] [PubMed] [Google Scholar]
- Hao Y., Liao X., Wang X., Lao S., Liao W. The biological regulatory activities of Flammulina velutipes polysaccharide in mice intestinal microbiota, immune repertoire and heart transcriptome. International Journal of Biological Macromolecules. 2021;185:582–591. doi: 10.1016/j.ijbiomac.2021.06.175. [DOI] [PubMed] [Google Scholar]
- Henkler F., Brinkmann J., Luch A. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers. 2010;2(2):376–396. doi: 10.3390/cancers2020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Liu C., Jiang W., Zhu H., Zhang H., Qian H., Zhang W. Chronic in vitro fermentation and in vivo metabolism: Extracellular polysaccharides from Sporidiobolus pararoseus regulate the intestinal microbiome of humans and mice. International Journal of Biological Macromolecules. 2021;192:398–406. doi: 10.1016/j.ijbiomac.2021.09.127. [DOI] [PubMed] [Google Scholar]
- Hu W., Di Q., Liang T., Zhou N., Chen H., Zeng Z.…Shaker M. Effects of in vitro simulated digestion and fecal fermentation of polysaccharides from straw mushroom (Volvariella volvacea) on its physicochemical properties and human gut microbiota. International Journal of Biological Macromolecules. 2023;239 doi: 10.1016/j.ijbiomac.2023.124188. [DOI] [PubMed] [Google Scholar]
- Huang F., Hong R., Yi Y., Bai Y., Dong L., Jia X.…Wu J. In vitro digestion and human gut microbiota fermentation of longan pulp polysaccharides as affected by Lactobacillus fermentum fermentation. International Journal of Biological Macromolecules. 2020;147:363–368. doi: 10.1016/j.ijbiomac.2020.01.059. [DOI] [PubMed] [Google Scholar]
- Kerdsomboon K., Chumsawat W., Auesukaree C. Effects of Moringa oleifera leaf extracts and its bioactive compound gallic acid on reducing toxicities of heavy metals and metalloid in Saccharomyces cerevisiae. CHEMOSPHERE. 2021;270 doi: 10.1016/j.chemosphere.2020.128659. [DOI] [PubMed] [Google Scholar]
- Lee Q., Xue Z., Luo Y., Lin Y., Lai M., Xu H.…Zeng F. Low molecular weight polysaccharide of Tremella fuciformis exhibits stronger antioxidant and immunomodulatory activities than high molecular weight polysaccharide. International Journal of Biological Macromolecules. 2024;281 doi: 10.1016/j.ijbiomac.2024.136097. [DOI] [PubMed] [Google Scholar]
- Leong Y.K., Ma T., Chang J., Yang F. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresource Technology. 2022;344(Pt A) doi: 10.1016/j.biortech.2021.126157. [DOI] [PubMed] [Google Scholar]
- Li D., Wang P., Wang P., Hu X., Chen F. The gut microbiota: A treasure for human health. Biotechnology Advances. 2016;34(7):1210–1224. doi: 10.1016/j.biotechadv.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Li H., Liu S., Liu Y., Li W., Niu A., Ren P.…Guan L. Effects of in vitro digestion and fermentation of Nostoc commune Vauch. Polysaccharides on properties and gut microbiota. Carbohydrate Polymers. 2022;281 doi: 10.1016/j.carbpol.2021.119055. [DOI] [PubMed] [Google Scholar]
- Li H., Liu Y., Zhou J., Liu S., Liu Y., Yang Y.…Guan L. The protective mechanism of a novel polysaccharide from Lactobacillus-fermented Nostoc commune Vauch. On attenuating cadmium-induced kidney injury in mice. International Journal of Biological Macromolecules. 2023;226:1444–1454. doi: 10.1016/j.ijbiomac.2022.11.256. [DOI] [PubMed] [Google Scholar]
- Li H., Xie Z., Zhang Y., Liu Y., Niu A., Liu Y., Zhang L., Guan L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Bioscience. 2021;44 [Google Scholar]
- Li J., Pang B., Yan X., Shang X., Hu X., Shi J. Prebiotic properties of different polysaccharide fractions from Artemisia sphaerocephala Krasch seeds evaluated by simulated digestion and in vitro fermentation by human fecal microbiota. International Journal of Biological Macromolecules. 2020;162:414–424. doi: 10.1016/j.ijbiomac.2020.06.174. [DOI] [PubMed] [Google Scholar]
- Li W., Lei J., Qu Mo M., Li J., Wei J., Liu Y.…Wu D. Impacts of ultrasound-assisted Fenton degradation and alkaline de-esterification on structural properties and biological effects of pectic polysaccharides from Tartary buckwheat leaves. Ultrasonics Sonochemistry. 2024;106 doi: 10.1016/j.ultsonch.2024.106895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang D., Sheng F., Qing H. Adsorption characteristics of copper (II), zinc (II) and mercury (II) by four kinds of immobilized fungi residues. Ecotoxicology and Environmental Safety. 2018;147:357–366. doi: 10.1016/j.ecoenv.2017.08.058. [DOI] [PubMed] [Google Scholar]
- Li Y., Wu B., Chen J., Veeraperumal S., Wei J., Tan K., Zhong S., Cheong K. Prebiotic characteristics of added-value polysaccharides from jackfruit peel waste during in vitro digestion and fecal fermentation. LWT. 2023;187 [Google Scholar]
- Lin S., AL-Wraikat M., Niu L., Zhou F., Zhang Y., Wang M.…Wang L. Degradation enhances the anticoagulant and antiplatelet activities of polysaccharides from Lycium barbarum L. leaves. International Journal of Biological Macromolecules. 2019;133:674–682. doi: 10.1016/j.ijbiomac.2019.04.147. [DOI] [PubMed] [Google Scholar]
- Liu M., Liu J., Li G., Zhang D., Qin D., Wang L., Xu Y. Functional properties, structural characteristics, and anti-complementary activities of two degraded polysaccharides from strawberry fruits. International Journal of Biological Macromolecules. 2024;269 doi: 10.1016/j.ijbiomac.2024.132263. [DOI] [PubMed] [Google Scholar]
- Liu W., Qin Y., Shi J., Wu D., Liu C., Liang J., Xie S. Effect of ultrasonic degradation on the physicochemical characteristics, GLP-1 secretion, and antioxidant capacity of Polygonatum cyrtonema polysaccharide. International Journal of Biological Macromolecules. 2024;274 doi: 10.1016/j.ijbiomac.2024.133434. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li H., Ren P., Che Y., Zhou J., Wang W.…Guan L. Polysaccharide from Flammulina velutipes residues protects mice from Pb poisoning by activating Akt/GSK3β/Nrf-2/HO-1 signaling pathway and modulating gut microbiota. International Journal of Biological Macromolecules. 2023;230 doi: 10.1016/j.ijbiomac.2023.123154. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li Y., Ke Y., Li C., Zhang Z., Wu Y.…Wu W. In vitro saliva-gastrointestinal digestion and fecal fermentation of Oudemansiella radicata polysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohydrate Polymers. 2021;251 doi: 10.1016/j.carbpol.2020.117041. [DOI] [PubMed] [Google Scholar]
- Liu Y., Sun Y., Li H., Ren P., Inam M., Liu S.…Guan L. Optimization of ultrasonic extraction of polysaccharides from Flammulina velutipes residue and its protective effect against heavy metal toxicity. Industrial Crops and Products. 2022;187 [Google Scholar]
- Liu Y., Zhang B., Ibrahim S.A., Gao S., Yang H., Huang W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydrate Polymers. 2016;145:71–77. doi: 10.1016/j.carbpol.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Luo Q., Li X., Li H., Kong K., Li C., Fang Z.…Zeng Z. Effect of in vitro simulated digestion and fecal fermentation on boletus auripes polysaccharide characteristics and intestinal flora. International Journal of Biological Macromolecules. 2023;249 doi: 10.1016/j.ijbiomac.2023.126461. [DOI] [PubMed] [Google Scholar]
- Ma C., Bai J., Shao C., Liu J., Zhang Y., Li X.…Wang L. Degradation of blue honeysuckle polysaccharides, structural characteristics and antiglycation and hypoglycemic activities of degraded products. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110281. [DOI] [PubMed] [Google Scholar]
- Ma Y., Xiu W., Wang X., Yu S., Luo Y., Gu X. Structural characterization and in vitro antioxidant and hypoglycemic activities of degraded polysaccharides from sweet corncob. Journal of Cereal Science. 2022;108 [Google Scholar]
- Manheim J., Lin M., Kong J., Biba M., Zhuang P. Identification and quantitation of isomeric pneumococcal polysaccharides by partial chemical degradation followed by mass spectrometry. Carbohydrate Polymers. 2022;289 doi: 10.1016/j.carbpol.2022.119465. [DOI] [PubMed] [Google Scholar]
- Mou J., Wang C., Li Q., Qi X., Yang J. Preparation and antioxidant properties of low molecular holothurian glycosaminoglycans by H2O2/ascorbic acid degradation. International Journal of Biological Macromolecules. 2018;107:1339–1347. doi: 10.1016/j.ijbiomac.2017.10.161. [DOI] [PubMed] [Google Scholar]
- Peng F., Yu Z., Niu K., Du B., Wang S., Yang Y. In vivo absorption, in vitro digestion, and fecal fermentation properties of non-starch polysaccharides from Chinese chestnut kernels and their effects on human gut microbiota. Food Chemistry: X. 2024;24 doi: 10.1016/j.fochx.2024.101829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Zhang J., Wang K., Cheng Y., Sheng Q., Kurtovic I.…Yue T. Tibetan kefir grains fermentation alters physicochemical properties and improves antioxidant activities of Lycium barbarum pulp polysaccharides. Food Chemistry. 2024;453 doi: 10.1016/j.foodchem.2024.139659. [DOI] [PubMed] [Google Scholar]
- Qiao S., Liu C., Sun L., Wang T., Dai H., Wang K.…Liu H. Gut Parabacteroides merdae protects against cardiovascular damage by enhancing branched-chain amino acid catabolism. Nature Metabolism. 2022;4(10):1271–1286. doi: 10.1038/s42255-022-00649-y. [DOI] [PubMed] [Google Scholar]
- Rattanawong K., Kerdsomboon K., Auesukaree C. Cu/Zn-superoxide dismutase and glutathione are involved in response to oxidative stress induced by protein denaturing effect of alachlor in Saccharomyces cerevisiae. Free Radical Biology and Medicine. 2015;89:963–971. doi: 10.1016/j.freeradbiomed.2015.10.421. [DOI] [PubMed] [Google Scholar]
- Shi X., Zou Y., Chen Y., Zheng C., Ying H. Overexpression of a water-forming NADH oxidase improves the metabolism and stress tolerance of Saccharomyces cerevisiae in aerobic fermentation. Frontiers in Microbiology. 2016;7:1427. doi: 10.3389/fmicb.2016.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Liang X., Xu X., Wang L., Xiao W., Ma Y.…Lei P. In vitro digestion and fecal fermentation of basidiospore-derived exopolysaccharides from Naematelia aurantialba. International Journal of Biological Macromolecules. 2024;261 doi: 10.1016/j.ijbiomac.2024.129756. [DOI] [PubMed] [Google Scholar]
- Tao L., Wu Q., Liu H., Bi Y., Song S., Wang H.…Xiong B. Improved the physicochemical properties and bioactivities of oligosaccharides by degrading self-extracting/commercial ginseng polysaccharides. International Journal of Biological Macromolecules. 2024;279(Pt 4) doi: 10.1016/j.ijbiomac.2024.135522. [DOI] [PubMed] [Google Scholar]
- Tawfick M.M., Xie H., Zhao C., Shao P., Farag M.A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. International Journal of Biological Macromolecules. 2022;208:948–961. doi: 10.1016/j.ijbiomac.2022.03.218. [DOI] [PubMed] [Google Scholar]
- Wang J., Xu X., Zou X., Zhang R., Jia X., Dong L.…Huang F. Effect of ultrasound assisted H2O2 degradation on longan polysaccharide: Degradation kinetics, physicochemical properties and prebiotic activity. International Journal of Biological Macromolecules. 2024;282 doi: 10.1016/j.ijbiomac.2024.136902. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang A., Hu Y., Yuan X., Qiu Y., Dong C. Polysaccharides from fructus corni: Extraction, purification, structural features, and biological activities. Carbohydrate Research. 2024;538 doi: 10.1016/j.carres.2024.109072. [DOI] [PubMed] [Google Scholar]
- Wang M., Chen G., Chen D., Ye H., Sun Y., Zeng X., Liu Z. Purified fraction of polysaccharides from Fuzhuan brick tea modulates the composition and metabolism of gut microbiota in anaerobic fermentation in vitro. International Journal of Biological Macromolecules. 2019;140:858–870. doi: 10.1016/j.ijbiomac.2019.08.187. [DOI] [PubMed] [Google Scholar]
- Wang W., Yang S., Song S., Zhang J., Jia F. Flammulina velutipes mycorrhizae dietary fiber improves lipid metabolism disorders in obese mice through activating AMPK signaling pathway mediated by gut microbiota. Food Bioscience. 2021;43 [Google Scholar]
- Wang X., Wang W., Gao Q., Wang X., Lei C., Zhu F. Chrysomya megacephala larvae feeding favourably influences manure microbiome, heavy metal stability and greenhouse gas emissions. Microbial Biotechnology. 2018;11(3):498–509. doi: 10.1111/1751-7915.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xue J., Zhang R., Li Y., Li X., Ding Y.…Chu X. Prebiotic characteristics of degraded polysaccharides from Acanthopanax senticosus polysaccharide on broilers gut microbiota based on in vitro digestion and fecal fermentation. Poultry Science. 2024;103(7) doi: 10.1016/j.psj.2024.103807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Xiao H., Wei Y., Nguepi Tsopmejio I.S., Sun C., Wu H.…Song H. Longitudinal study of the effects of Flammulina velutipes stipe wastes on the Cecal microbiota of laying hens. mSystems. 2023;Vol. 8(1) doi: 10.1128/msystems.00835-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Wang L., Chen X., Wang Y., Tong L., Han Q.…Guo D. Anti-inflammatory activity of boletus aereus polysaccharides: Involvement of digestion and gut microbiota fermentation. Food Chemistry: X. 2024;21 doi: 10.1016/j.fochx.2023.101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Liu W., Yuan Q., Gan R., Hu Y., Wang S., Zou L. Dynamic variations in physicochemical characteristics of oolong tea polysaccharides during simulated digestion and fecal fermentation in vitro. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yuan Q., Guo H., Fu Y., Li F., Wang S., Gan R. Dynamic changes of structural characteristics of snow chrysanthemum polysaccharides during in vitro digestion and fecal fermentation and related impacts on gut microbiota. Food Research International. 2021;141 doi: 10.1016/j.foodres.2020.109888. [DOI] [PubMed] [Google Scholar]
- Wu J., Huang R., Jiao D., Liu S., Liu H., Liu H. Protection by Hosta ventricosa polysaccharides against oxidative damage induced by t-BHP in HepG2 cells via the JNK/Nrf2 pathway. International Journal of Biological Macromolecules. 2022;208:453–462. doi: 10.1016/j.ijbiomac.2022.03.134. [DOI] [PubMed] [Google Scholar]
- Xu S., Aweya J.J., Li N., Deng R., Chen W., Tang J., Cheong K. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chemistry. 2019;289:177–186. doi: 10.1016/j.foodchem.2019.03.050. [DOI] [PubMed] [Google Scholar]
- Xu S., Chen X., Liu Y., Cheong K. Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. International Journal of Biological Macromolecules. 2020;152:748–756. doi: 10.1016/j.ijbiomac.2020.02.305. [DOI] [PubMed] [Google Scholar]
- Xu Y., Niu X., Liu N., Gao Y., Wang L., Xu G.…Yang Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chemistry. 2018;243:26–35. doi: 10.1016/j.foodchem.2017.09.107. [DOI] [PubMed] [Google Scholar]
- Yan S., Pan C., Yang X., Chen S., Qi B., Huang H. Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe2+−ultrasonic treatment: Structural characterization and antioxidant activity. International Journal of Biological Macromolecules. 2021;182:129–135. doi: 10.1016/j.ijbiomac.2021.03.193. [DOI] [PubMed] [Google Scholar]
- Yang M., Tao L., Wang Z., Li L., Zhao C., Shi C.…Tian Y. Effects of UV/H2O2 degradation on Moringa oleifera lam. Leaves polysaccharides: Composition, in vitro fermentation and prebiotic properties on gut microorganisms. Food Chemistry: X. 2024;22 doi: 10.1016/j.fochx.2024.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang X., Zhang J., Wang T., Liu S., Ma H., Inam M., Guan L. Green ultrasonic-assisted enzymatic extraction of polysaccharides from Flammulina velutipes residues by response surface methodology. Sustainable Chemistry and Pharmacy. 2024;41 [Google Scholar]
- Yao Q., Pu L., Dong B., Zhu D., Wu W., Yang Q. Effects of ultrasonic degradation on physicochemical and antioxidant properties of Gleditsia sinensis seed polysaccharides. Carbohydrate Research. 2024;545 doi: 10.1016/j.carres.2024.109272. [DOI] [PubMed] [Google Scholar]
- Yao W., Liu M., Chen X., You L., Ma Y., Hileuskaya K. Effects of UV/H2O2 degradation and step gradient ethanol precipitation on Sargassum fusiforme polysaccharides: Physicochemical characterization and protective effects against intestinal epithelial injury. Food Research International. 2022;155 doi: 10.1016/j.foodres.2022.111093. [DOI] [PubMed] [Google Scholar]
- Ye Z., Yu L., Zhang C., Gao Y., Zhao J., Narbad A.…Tian F. Modulation of gut microbiota and metabolites by Flammulina velutipes polysaccharides during in vitro human fecal fermentation: Unveiling Bacteroides as a potential primary degrader. Food Chemistry. 2024;450 doi: 10.1016/j.foodchem.2024.139309. [DOI] [PubMed] [Google Scholar]
- Yuan S., Wang J., Li X., Zhu X., Zhang Z., Li D. Study on the structure, antioxidant activity and degradation pattern of polysaccharides isolated from lotus seedpod. Carbohydrate Polymers. 2023;316 doi: 10.1016/j.carbpol.2023.121065. [DOI] [PubMed] [Google Scholar]
- Zhai C., Lin Y., Mao C., Li X., Zhang R., Liu J., Zhang L. Construction, characterization, antioxidant activity and effects on properties in vitro digestion of selenium nanoparticles decorated with Cyperus esculentus polysaccharides. Food Bioscience. 2024;59 [Google Scholar]
- Zhang J., Chen X., Wang Y., Zhan Q., Hu Q., Zhao L. Study on the physicochemical properties and antioxidant activities of Flammulina velutipes polysaccharide under controllable ultrasonic degradation based on artificial neural network. International Journal of Biological Macromolecules. 2024;261 doi: 10.1016/j.ijbiomac.2024.129382. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhu T., Wang Y., Zhang B., Zhang H., Han L.…Fu Z. Effects of in vitro simulated digestion and fecal fermentation on the structure and regulating the glucose and lipid activity of a polysaccharide from Mori folium. International Journal of Biological Macromolecules. 2024;280 doi: 10.1016/j.ijbiomac.2024.135595. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wu P., Tian Y., Liu B., Huang L., Liu Z.…Sun J. Gut microbiota serves a predictable outcome of short-term low-carbohydrate diet (LCD) intervention for patients with obesity. Microbiology Spectrum. 2021;9(2) doi: 10.1128/Spectrum.00223-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Huang S., Guo Y., Xie X., Chen G., Cao C.…Cheng S. Chitosan from the base of Flammulina velutipes stipe alleviates oral Candida albicans infection via modulating Th-17 cell differentiation and Streptococcus mutans. International Journal of Biological Macromolecules. 2024;274 doi: 10.1016/j.ijbiomac.2024.132879. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang L., Qiu Z., Yang Y., Wang T., Inam M.…Guan L. Comprehensive evaluation of Flammulina velutipes residues polysaccharide based on in vitro digestion and human fecal fermentation. International Journal of Biological Macromolecules. 2024;281 doi: 10.1016/j.ijbiomac.2024.136487. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen L., Sun G., Li Y., Huang R. Alterations in the gut microbiota of patients with silica-induced pulmonary fibrosis. Journal of Occupational Medicine and Toxicology. 2019;14:5. doi: 10.1186/s12995-019-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Chen Y., Chang S., Qiu H., You L. Degradation kinetic models and mechanism of isomaltooligosaccharides by hydroxyl radicals in UV/H2O2 system. Carbohydrate Polymers. 2023;300 doi: 10.1016/j.carbpol.2022.120240. [DOI] [PubMed] [Google Scholar]
- Ziegler K., Kerimi A., Poquet L., Williamson G. Butyric acid increases transepithelial transport of ferulic acid through upregulation of the monocarboxylate transporters SLC16A1 (MCT1) and SLC16A3 (MCT4) Archives of Biochemistry and Biophysics. 2016;599:3–12. doi: 10.1016/j.abb.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.