Abstract
Introduction
In a phase 1 study, bintrafusp alfa was found to have an encouraging clinical activity in patients with previously treated advanced NSCLC. This study evaluated the safety and efficacy of bintrafusp alfa with chemotherapy in patients with stage IV NSCLC regardless of the programmed death-ligand 1 (PD-L1) expression status.
Methods
In this open-label, phase 1b/2 study (NCT03840915), eligible patients were assigned to one of four cohorts. Patients with previously untreated metastatic NSCLC (cohorts A, B, and C) received bintrafusp alfa with chemotherapy as first-line treatment, whereas patients whose disease progressed on previous treatment with programmed cell death protein 1 or PD-L1 inhibitors (cohort D) received bintrafusp alfa with chemotherapy as second-line treatment. The primary objective of this study was to evaluate the safety and tolerability of bintrafusp alfa with chemotherapy.
Results
Four serious and one nonserious treatment-emergent adverse events were considered dose-limiting toxicities, none of which were assessed as related to bintrafusp alfa by the investigator. Any-grade bintrafusp alfa-related adverse events occurred in 20.7% of patients in cohorts A+B+C and in 16.7% of patients in cohort D. Keratoacanthoma was the most common transforming growth factor-β inhibition-mediated skin lesion (cohorts A+B+C: 12.1% and cohort D: 8.3%). In cohorts A+B+C, the overall response rate was 48.3%, and in patients with PD-L1 tumor proportion score of more than or equal to 50.0%, it was 71.4%. On the basis of an interim analysis, the data were considered mature, and no further analysis has been planned.
Conclusion
Bintrafusp alfa with chemotherapy was found to have a manageable safety profile and encouraging clinical activity in patients with stage IV NSCLC.
Keywords: Bintrafusp alfa, TGF-β, Bifunctional, Stage IV, Non–small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death in patients aged above or equal to 50 years,1 and approximately 40.0% of patients with NSCLC are first diagnosed at stage IV.2 Platinum-based combination chemotherapy is the standard of care in patients with stage IV NSCLC,3 resulting in a median overall survival (OS) of 8.0 months and progression-free survival (PFS) of 4.0 months.4 In the last decade, however, the use of targeted therapies and immune checkpoint inhibitors (ICIs) has redefined the treatment options in patients with advanced-stage NSCLC.5, 6, 7
Nevertheless, not all patients can benefit from this treatment, as there remains an unmet need because some patients experience early disease progression and treatment resistance,8,9 and programmed death-ligand 1 (PD-L1)-low or -negative tumors are less likely to respond to therapy.8 This highlights the need for development of new therapeutic strategies, and ICIs are currently being evaluated in combination with chemotherapy and other ICIs as first-line (1L) or second-line (2L) treatment options in patients with advanced and metastatic NSCLC.
The POSEIDON study in patients with stage IV NSCLC found significantly improved PFS with durvalumab plus chemotherapy compared with chemotherapy alone (5.5 versus 4.8 mo; p = 0.0009). This trial also revealed that the addition of tremelimumab, an anticytotoxic T lymphocyte antigen-4, to durvalumab (dual immune checkpoint blockade) and chemotherapy significantly improved OS (14.0 versus 11.7 mo; p = 0.0030) and PFS (6.2 versus 4.8 mo; p = 0.0003) compared with chemotherapy alone. Tremelimumab in combination with durvalumab plus chemotherapy has been recently approved as a 1L treatment option in patients with metastatic NSCLC.2,10 Consistent with the outcome of the CheckMate 9LA phase 3 trial,11 results from the 4-year study update revealed continued long-term, durable efficacy benefit of nivolumab plus ipilimumab with chemotherapy than chemotherapy (4-y OS rate: 21.0% versus 16.0%; median OS: 15.8 versus 11.0 mo, respectively) as a 1L treatment option in patients with stage IV and recurrent NSCLC regardless of PD-L1 expression.12
As the immunosuppressive functions of the PD-L1 pathway within the tumor microenvironment (TME) are well-established, another possible approach is to simultaneously target other immunosuppressive networks within the TME.13 Transforming growth factor-β (TGF-β) signaling is dysregulated in cancers including NSCLC, and it is known to promote invasiveness and metastasis through the induction of epithelial-to-mesenchymal transition.14 Moreover, aberrant TGF-β activity is associated with an immunosuppressive TME and has been implicated in facilitating the emergence of resistance mechanisms to chemotherapy, targeted therapy, and immunotherapy. Furthermore, evidence suggests that aberrant TGF-β signaling serves as a predictive biomarker for the efficacy of anti–PD-L1 therapies15,16; therefore, inhibition of the TGF-β pathway may help overcome anti–PD-L1 resistance.
Bintrafusp alfa (BA) is a first-in-class bifunctional fusion protein composed of the extracellular domain of the TGF-β receptor II (TGF-βRII or TGF-β “trap”) fused to a human IgG1 monoclonal antibody blocking PD-L1. A previous preclinical study revealed that BA elicited stronger antitumor immunity and tumor regression than TGF-β “trap” or anti–PD-L1 alone or in combination.17 BA has been reported to be well-tolerated and efficacious in a phase 1 study of pretreated advanced solid tumors (e.g., bronchopulmonary carcinoid, cervical, pancreatic, colorectal, anal, small bowel tumors).18 Moreover, it has a manageable safety profile and clinical activity in patients with pretreated (but not with ICIs) stage IIIB and IV NSCLC.19 This phase 1b and 2 study explored the safety, tolerability, and preliminary efficacy of BA in combination with chemotherapy in patients with stage IV NSCLC as a 1L and 2L treatment option regardless of their PD-L1 expression status.
Materials and Methods
Study Design and Patients
This global, multicenter, open-label, phase 1b and 2 study (NCT03840915) evaluated BA in combination with chemotherapy in patients with stage IV NSCLC (according to International Association for the Study of Lung Cancer Staging Manual in Thoracic Oncology, version 8) regardless of their PD-L1 expression status. Eligible patients with stage IV NSCLC were assigned to one of the four cohorts (cohorts A, B, C, and D); patients with previously untreated metastatic NSCLC (cohorts A, B, and C) and patients with metastatic NSCLC whose disease progressed on previous PD-(L)1–containing therapy (cohort D included patients who had disease progression after 1L treatment with PD-[L]1 inhibitors in combination with platinum-based chemotherapy or received 1L treatment with platinum-based chemotherapy followed by 2L treatment with PD-[L]1 inhibitors or had received 1L PD-[L]1 inhibitor followed by 2L platinum-based chemotherapy) received BA with chemotherapy (additional details in Supplementary Methods).
All eligible patients received 2400 mg BA as intravenous infusion every 3 weeks20 in combination with chemotherapy for four treatment cycles (the duration of each cycle was 21 d) followed by maintenance therapy for up to 31 cycles or until unacceptable toxicities, confirmed disease progression, or death, whichever occurred first. In cohort A, patients with nonsquamous stage IV with NSCLC were treated with BA plus cisplatin (75 mg/m2) or carboplatin (area under concentration-time curve [AUC] 5) and pemetrexed (500 mg/m2; every 3 weeks) during induction, followed by BA plus pemetrexed every 3 weeks during maintenance. In cohort B, patients with nonsquamous or squamous stage IV NSCLC received BA plus carboplatin (AUC 6) plus paclitaxel (200 mg/m2; every 3 weeks) or nab-paclitaxel (100 mg/m2 on days 1, 8, and 15, in each cycle) during induction, followed by maintenance with BA. In cohort C, patients with nonsquamous or squamous stage IV NSCLC received BA plus gemcitabine (1250 mg/m2 every 3 weeks on days 1 and 8, in each cycle) and cisplatin (75 mg/m2) or carboplatin (AUC 5) every 3 weeks during induction, followed by maintenance with BA. Patients in cohort D received BA plus docetaxel (75 mg/m2; every 3 weeks) during induction, followed by maintenance with BA (Supplementary Fig. 1).
Cohort A also served as a pilot cohort to assess additional safety data and preliminary efficacy. This cohort consisted of an expansion part in which additional patients were enrolled after the safety profile of BA plus chemotherapy was approved by the safety monitoring committee (SMC). Expansion of cohorts B, C, and D was considered based on the emerging safety profile of each combination regimen during the study. Dose-limiting toxicities (DLTs) were observed for 3 weeks starting from week 1 day 1 of the visits. The combinations were considered safe if DLTs were observed in less than or equal to two of eight evaluable patients. If DLTs were observed in more than or equal to three of the eight patients, the SMC recommended approving the corresponding combination based on a review of all relevant parameters, including adverse events (AEs), serious AEs, and benefit–risk assessment, for a particular cohort.
The primary objective of this study was to evaluate the safety and tolerability of BA in combination with chemotherapy measured as occurrence of DLTs during the 3-week observation period, including treatment-emergent AEs (TEAEs) and treatment-related AEs (TRAEs). The secondary objectives of this study included evaluation of the overall response rate (ORR) and PFS according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1), as assessed by the investigator; OS; duration of response (DOR); and pharmacokinetic (PK) profiles. The exploratory or tertiary objective of this study was to evaluate the expression level of PD-L1 and its association with efficacy.
Methodology
Patients aged above or equal to 18 years with a histologically confirmed diagnosis of stage IV NSCLC; adequate renal, hepatic, hematologic, and coagulation functions; an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1; and a life expectancy of more than or equal to 3 months were included in this study. Further eligibility criteria were as follows: availability of fresh biopsies or archived tumor material (<6 mo old, excluding bone biopsies); measurable disease based on RECIST 1.1; and completion of treatment with cytotoxic chemotherapy, biological therapy, or radiation as part of neoadjuvant or adjuvant or unresectable locally advanced therapy at least 6 months before the diagnosis of metastatic disease for cohorts A, B, and C. Toxic effects of previous chemotherapy should have been resolved and confirmed to be grade less than or equal to 1, and patients should have recovered from previous radiation toxicity or previous major surgeries, adverse effects, and complications. Patients enrolled in cohort D who had disease progression on previous 1L treatment were required to have completed therapy at least 28 days before the first study intervention.
The key exclusion criteria were as follows: presence of EGFR mutation, ROS1 rearrangement, BRAF V600E mutation, or ALK translocation in patients with nonsquamous histology (cohorts A, B, C); presence of mixed small cells with a histologic diagnosis of NSCLC; any major surgery 4 weeks before study entry; thoracic radiation therapy with a dose of more than 30 Gy within 6 months of the study; active central nervous system metastases; carcinomatous meningitis; autoimmune disease; organ transplantation; interstitial lung disease; a history of pneumonitis; inability to tolerate computed tomography or magnetic resonance imaging; and concurrent enrollment in another trial (a full list of the inclusion and exclusion criteria is provided in the Supplementary Methods).
After patients received the initial dose, antitumor responses were monitored until unacceptable toxicities, confirmed disease progression, or death, whichever occurred first (additional details in Supplementary Methods). All patients provided written informed consent before enrollment. The study complied with the international standards of Good Clinical Practice (International Conference on Harmonization-GCP) and the Declaration of Helsinki.
Statistical Analysis
A total of 64 eligible patients (safety part, n = 8 in each cohort; expansion part, n = 32) were considered for enrollment, with a total sample size of 40 patients in cohort A. Nevertheless, based on the SMC’s request to fully evaluate the safety of the cohort, 12 patients were enrolled in cohort D instead of the initially planned eight patients. The SMC evaluated the safety and tolerability in each cohort separately after the eighth evaluable patient completed the DLT observation period. The probability of observing DLTs in more than or equal to three of the eight patients was 4.0% when the underlying true DLT rate was 10.0%, but it increased to 85.0% when the underlying true rate was 50.0%. When DLTs were observed in more than or equal to three of the eight patients, the SMC reviewed all relevant parameters for providing recommendations to the cohort.
The expansion part of the study was designed to preliminary assess the efficacy of treatment in patients with nonsquamous tumor types. Under the assumption of a true ORR of 60.0%, the probability of observing more than or equal to 21 responders among 40 treated patients (ORR = 52.5%) was 87.0%. Conversely, when the true response rate was 45.0%, the probability of observing more than or equal to 21 responders was 21.3%. When 21 responders of the 40 treated patients were observed in cohort A, the posterior probability of achieving a true response rate of more than or equal to 55.0% was expected to be 36.9% (using a noninformative beta [1,1] previous distribution).
The objective response and safety end points were presented using descriptive statistics. The Kaplan–Meier method was used to estimate the survival rates and 95% confidence intervals (CIs) of PFS and OS (additional details in the Supplementary Methods). The PK parameters of the first treatment cycle were calculated through noncompartmental analysis. Analyses were performed using statistical software SAS (Statistical Analysis System, SAS Institute, Cary, NC, Windows version 9.4 or higher).
Results
Patients and Treatment
Between April 2019 and November 2020, 70 patients were assigned to one of the four cohorts during the safety and expansion parts of the study (full analysis set: cohort A, n = 40; cohort B, n = 9; cohort C, n = 9; and cohort D, n = 12; Supplementary Fig. 2). The safety and tolerability of the combination treatment were assessed during the 3-week DLT observation period. The study discontinuation rates were 56.9% in cohorts A+B+C and 50.0% in cohort D. Death was the most frequently reported reason for study discontinuation (cohorts A+B+C versus cohort D: 48.3% versus 50.0%). The cutoff date for analysis was May 5, 2021 (defined as 6 mo after the treatment start for the last patient in the expanded cohort A).
The median age was 64 (40–83) years in cohorts A+B+C and 58 (47–75) years in cohort D (Table 1). The proportion of patients with PD-L1 expression of more than or equal to 1.0% to less than 50.0% was similar between cohorts A+B+C (17.2%) and cohort D (16.7%). Of note, the proportion of patients with adenocarcinomas was higher in cohort D (91.7%) than in cohorts A+B+C (75.9%). The median duration of exposure to BA was 19.1 (3.0–90.0) weeks in cohorts A+B+C and 15.5 (3.0–36.0) weeks in cohort D. The proportions of patients who received a relative BA dose intensity of more than 90.0% were 74.1% in cohorts A+B+C and 66.7% in cohort D.
Table 1.
Baseline Patient and Disease Characteristics of the Study Cohorts
| Characteristic | Cohort A, (n = 40) | Cohort B, (n = 9) | Cohort C, (n = 9) | Cohort D, (n = 12) | Cohorts A+B+C, (n = 58) |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Male | 31 (77.5) | 6 (66.7) | 5 (55.6) | 5 (41.7) | 42 (72.4) |
| Female | 9 (22.5) | 3 (33.3) | 4 (44.4) | 7 (58.3) | 16 (27.6) |
| Age | |||||
| Median (range), y | 65 (41.0–83.0) | 61 (56.0–79.0) | 60 (40.0–77.0) | 58 (47.0–75.0) | 64 (40.0–83.0) |
| <65 | 20 (50.0) | 6 (66.7) | 5 (55.6) | 9 (75.0) | 31 (53.4) |
| ≥65 | 20 (50.0) | 3 (33.3) | 4 (44.4) | 3 (25.0) | 27 (46.6) |
| Race | |||||
| White | 14 (35.0) | 6 (66.7) | 2 (22.2) | 3 (25.0) | 22 (37.9) |
| Black | 1 (2.5) | 2 (22.2) | 0 | 0 | 3 (5.2) |
| Others/not collected at the site | 25 (62.5) | 1 (11.1) | 7 (77.8) | 9 (75.0) | 33 (56.9) |
| ECOG performance status | |||||
| 0 | 12 (30.0) | 1 (11.1) | 4 (44.4) | 4 (33.3) | 17 (29.3) |
| 1 | 28 (70.0) | 8 (88.9) | 5 (55.6) | 8 (66.7) | 41 (70.7) |
| PD-L1 TPS,a n (%) | |||||
| 1.0% to <50.0% | 7 (17.5) | 0 | 3 (33.3) | 2 (16.7) | 10 (17.2) |
| ≥50% TPS | 1 (2.5) | 1 (11.1) | 0 | 0 | 2 (3.4) |
| <1.0% TPS | 8 (20.0) | 2 (22.2) | 2 (22.2) | 2 (16.7) | 12 (20.7) |
| Missing | 24 (60.0) | 6 (66.7) | 4 (44.4) | 8 (66.7) | 34 (58.6) |
| No. of previous anticancer therapy regimens, n (%) | |||||
| 1 | 0 | 1 (11.1)b | 3 (33.3)b | 2 (16.7)b | 4 (6.9)b |
| 2 | 0 | 1 (11.1) | 0 | 9 (75.0) | 1 (1.7) |
| 3 | 0 | 0 | 0 | 0 | 0 |
| ≥4 | 0 | 0 | 0 | 1 (8.3) | 0 |
| Tumor histology, n (%) | |||||
| Adenocarcinoma | 38 (95.0) | 2 (22.2) | 4 (44.4) | 11 (91.7) | 44 (75.9) |
| Squamous cell carcinoma | 0 (0.0) | 6 (66.7) | 5 (55.6) | 1 (8.3) | 11 (19.0) |
| Adenosquamous carcinoma | 1 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Other | 1 (2.5) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 2 (3.4) |
| Time since the initial cancer diagnosis | |||||
| Median (range), mo | 1.6 (0.5–123.1) | 2.4 (1.0–141.9) | 2.8 (0.9–32.5) | 18.8 (6.3–40.1) | 1.6 (0.5–141.9) |
| Time since documented, locally advanced, inoperable, or metastatic disease | |||||
| Median (range), mo | 1.3 (0.1–50.1) | 2.2 (0.2–36.1) | 1.6 (0.5–2.6) | 15.7 (4.9–33.7) | 1.4 (0.1–50.1) |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
PD-L1 immunohistochemistry data were generated using clone 22C3.
These patients received adjuvant therapy as part of locally advanced therapy that was allowed only if the therapy was completed at least 6 months before the diagnosis of metastatic disease.
Safety and Tolerability
The DLT analysis set comprised eight patients each in cohorts A, B, and C (n = 24) and 11 patients in cohort D. In total, four serious TEAEs (three cases of serious febrile neutropenia [cohort D, n = 2 and cohort B, n = 1] and one case of serious sepsis [cohort D]) and one nonserious TEAE (nausea in cohort A) were considered DLTs by the investigators (Table 2). All DLTs were resolved with or without the administration of corrective treatment, and none led to discontinuation of BA or other chemotherapy. All DLTs were assessed as related to chemotherapy (and none as related to BA), and the dose of chemotherapy was subsequently reduced for all but one patient.
Table 2.
Summary of DLTs, TEAEs, and TRAEs of Any-Grade and Grade Greater Than or Equal to 3
| Patients, n (%) | Cohort A, (n = 40) | Cohort B, (n = 9) | Cohort C, (n = 9) | Cohort D, (n = 12) | Cohorts A+B+C, (n = 58) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DLTs, n/N (%) | 1/8 (12.5) | 1/8 (12.5) | 0/8 | 2/11 (18.2) | 2/24 (8.3) | |||||
| Any TEAE, n (%) | 40 (100.0) | 9 (100.0) | 9 (100.0) | 12 (100.0) | 58 (100.0) | |||||
| Grade ≥3 TEAE | 32 (80.0) | 8 (88.9) | 7 (77.8) | 12 (100.0) | 47 (81.0) | |||||
| Grade ≥4 TEAE | 16 (40.0) | 3 (33.3) | 5 (55.6) | 6 (50.0) | 24 (41.4) | |||||
| Any serious TEAE | 31 (77.5) | 5 (55.6) | 6 (66.7) | 9 (75.0) | 42 (72.4) | |||||
| Any TEAE leading to permanent discontinuation of at least one study intervention | 19 (47.5) | 2 (22.2) | 7 (77.8) | 5 (41.7) | 28 (48.3) | |||||
| TEAEs leading to death | 6 (15.0) | 1 (11.1) | 0 | 0 | 7 (12.1) | |||||
| Most Common TEAEs (≥50.0%) | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 |
| Anemia | 20 (50.0) | 11 (27.5) | 6 (66.7) | 5 (55.6) | 9 (100.0) | 5 (55.6) | 9 (75.0) | 3 (25.0) | 35 (60.3) | 21 (36.2) |
| Neutropenia | 5 (12.5) | 2 (5.0) | 1 (11.1) | 1 (11.1) | 5 (55.6) | 3 (33.3) | 4 (33.3) | 4 (33.3) | 11 (19.0) | 6 (10.3) |
| Constipation | 7 (17.5) | 0 | 6 (66.7) | 0 | 2 (22.2) | 0 | 2 (16.7) | 0 | 15 (25.9) | 0 |
| Diarrhea | 13 (32.5) | 2 (5.0) | 3 (33.3) | 0 | 2 (22.2) | 0 | 6 (50.0) | 1 (8.3) | 18 (31.0) | 2 (3.4) |
| Nausea | 21 (52.5) | 4 (10.0) | 3 (33.3) | 1 (11.1) | 6 (66.7) | 1 (11.1) | 5 (41.7) | 1 (8.3) | 30 (51.7) | 6 (10.3) |
| Asthenia | 16 (40.0) | 7 (17.5) | 1 (11.1) | 0 | 4 (44.4) | 0 | 7 (58.3) | 0 | 21 (36.2) | 7 (12.1) |
| Decreased appetite | 8 (20.0) | 0 | 3 (33.3) | 0 | 4 (44.4) | 0 | 6 (50.0) | 3 (25.0) | 15 (25.9) | 0 |
| Pruritus | 15 (37.5) | 0 | 3 (33.3) | 0 | 6 (66.7) | 0 | 2 (16.7) | 1 (8.3) | 24 (41.4) | 0 |
| TRAEs, n (%) | ||||||||||
| Any bintrafusp alfa-related AE | 35 (87.5) | 9 (100.0) | 9 (100.0) | 12 (100.0) | 53 (91.4) | |||||
| Any chemotherapy-related AE | 37 (92.5) | 8 (88.9) | 9 (100.0) | 12 (100.0) | 54 (93.1) | |||||
| Any grade ≥3 bintrafusp alfa-related AE | 17 (42.5) | 5 (55.6) | 5 (55.6) | 5 (41.7) | 27 (46.6) | |||||
| Any grade ≥3 chemotherapy-related AE | 23 (57.5) | 6 (66.7) | 7 (77.8) | 9 (75.0) | 36 (62.1) | |||||
| Any serious bintrafusp alfa-related AE | 12 (30.0) | 1 (11.1) | 1 (11.1) | 3 (25.0) | 14 (24.1) | |||||
| Any serious chemotherapy-related AE | 12 (30.0) | 2 (22.2) | 4 (44.4) | 7 (58.3) | 18 (31.0) | |||||
| Any bintrafusp alfa-related AE leading to death | 0 | 0 | 0 | 0 | 0 | |||||
| Any chemotherapy-related AE leading to death | 0 | 0 | 0 | 0 | 0 | |||||
| Bintrafusp Alfa-Related AE (≥40.0%) | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 |
| Anemia | 6 (15.0) | 4 (10.0) | 4 (44.4) | 3 (33.3) | 2 (22.2) | 1 (11.1) | 2 (16.7) | 1 (8.3) | 12 (20.7) | 8 (13.8) |
| Asthenia | 8 (20.0) | 2 (5.0) | 0 | 0 | 3 (33.3) | 0 | 6 (50.0) | 0 | 11 (19.0) | 2 (3.4) |
| Keratoacanthoma | 2 (5.0) | 0 | 1 (11.1) | 0 | 4 (44.4) | 1 (11.1) | 1 (8.3) | 0 | 7 (12.1) | 1 (1.7) |
| Pruritus | 15 (37.5) | 0 | 1 (11.1) | 0 | 6 (66.7) | 0 | 2 (16.7) | 1 (8.3) | 22 (37.9) | 0 |
AE, adverse event; DLT, dose-limiting toxicity; TEAE, treatment-emergent adverse event; TRAE, treatment-related adverse event.
The safety analysis set consisted of 70 patients (cohort A, n = 40; cohort B, n = 9; cohort C, n = 9; and cohort D, n = 12). Any-grade TEAEs were reported in all patients in cohorts A, B, C, and D (Table 2). The most common any-grade TEAEs (≥50.0%) were anemia (60.3% versus 75.0%), asthenia (36.2% versus 58.3%), nausea (51.7% versus 41.7%), diarrhea (31.0% versus 50.0%), and decreased appetite (25.9% versus 50.0%) in cohorts A+B+C and cohort D, respectively. The proportions of patients who experienced grade greater than or equal to 3 TEAEs, grade greater than or equal to 4 TEAEs, and any serious TEAEs are provided in Table 2. The rate of TEAEs leading to permanent discontinuation of BA treatment was 37.9% in cohorts A+B+C versus 3.3% in cohort D.
Seven patients (12.1%) in cohorts A+B+C experienced TEAEs leading to death (one patient each with gastrointestinal hemorrhage, upper gastrointestinal hemorrhage, coronavirus disease 2019 pneumonia, metastases to central nervous system, and acute respiratory distress syndrome and two patients with disease progression). The proportion of patients with grade greater than or equal to 3 TRAEs related to BA was higher in cohorts A+B+C (46.6%) than in cohort D (41.7%). The proportion of patients with grade greater than or equal to 3 TRAEs related to chemotherapy was higher in cohort D (75.0%) than in cohorts A+B+C (62.1%; Table 2).
The proportion of patients with immune-related AEs of special interest (AESIs; 50.0% versus 46.6%) and treatment-emergent anemia (75.0% versus 60.3%) was higher in cohort D than in cohorts A+B+C (Table 3). TGF-β inhibition-mediated skin AEs were reported in 19.0% of patients in cohorts A+B+C and 8.3% of patients in cohort D, with keratoacanthoma being the most common AE (Table 3). The rates of BA-related TGF-β inhibition-mediated skin AEs (17.2% versus 8.3%) and bleeding events (15.5% vs. 8.3%) were higher in cohorts A+B+C than in cohort D.
Table 3.
Summary of AESIs
| Patients With AESI of Any Grade, n (%) | Cohort A (n = 40) | Cohort B (n = 9) | Cohort C (n = 9) | Cohort D (n = 12) | Cohorts A+B+C (n = 58) |
|---|---|---|---|---|---|
| Any AESI | |||||
| Infusion-related reaction | 5 (12.5) | 1 (11.1) | 1 (11.1) | 1 (8.3) | 7 (12.1) |
| Immune-related AE | 19 (47.5) | 3 (33.3) | 5 (55.6) | 6 (50.0) | 27 (46.6) |
| TGF-β inhibition-mediated skin AE | 6 (15.0) | 1 (11.1) | 4 (44.4) | 1 (8.3) | 11 (19.0) |
| Keratoacanthoma | 2 (5.0) | 1 (11.1) | 4 (44.4) | 1 (8.3) | 7 (12.1) |
| Squamous cell carcinoma of skin | 2 (5.0) | 0 | 0 | 0 | 2 (3.4) |
| Hyperkeratosis | 3 (7.5) | 0 | 1 (11.1) | 0 | 4 (6.9) |
| Actinic keratosis | 1 (2.5) | 0 | 0 | 0 | 1 (1.7) |
| Basal cell carcinoma | 1 (2.5) | 0 | 0 | 0 | 1 (1.7) |
| Lip squamous cell carcinoma | 0 | 0 | 0 | 0 | 0 |
| Bowen’s disease | 0 | 0 | 0 | 0 | 0 |
| Treatment-emergent anemia | 20 (50.0) | 6 (66.7) | 9 (100.0) | 9 (75.0) | 35 (60.3) |
| Any bintrafusp alfa-related TEAE of special interest | |||||
| Infusion-related reaction | 4 (10.0) | 0 | 0 | 1 (8.3) | 4 (6.9) |
| Immune-related AE | 18 (45.0) | 1 (11.1) | 4 (44.4) | 6 (50.0) | 23 (39.7) |
| TGF-β inhibition-mediated skin AE | 5 (12.5) | 1 (11.1) | 4 (44.4) | 1 (8.3) | 10 (17.2) |
| Treatment-emergent anemia | 6 (15.0) | 4 (44.4) | 2 (22.2) | 2 (16.7) | 12 (20.7) |
| Any treatment-emergent bleeding event | 19 (47.5) | 5 (55.6) | 8 (88.9) | 8 (66.7) | 32 (55.2) |
| Any treatment-emergent bintrafusp alfa-related bleeding event | 4 (10.0) | 2 (22.2) | 3 (33.3) | 1 (8.3) | 9 (15.5) |
AE, adverse event; AESI, adverse event of special interest; TEAE, treatment-emergent adverse events; TGF-β, transforming growth factor beta.
Overall, 31 patients in cohorts A+B+C and six patients in cohort D died. Most deaths in cohorts A+B+C (44.8%) and cohort D (50.0%) were attributed to PD or disease-related conditions. A higher proportion of deaths were reported within 60 days of study treatment initiation in cohort D than in cohorts A+B+C (8.3% versus 6.9%).
Efficacy
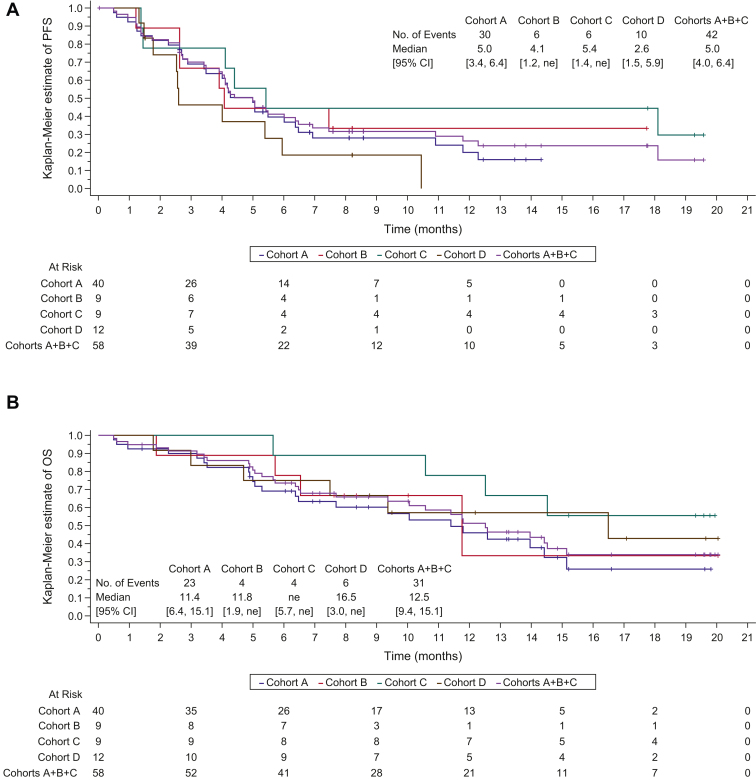
At the time of data cutoff (May 5, 2021), with a median follow-up of 13.8 (95% CI: 8.2–17.8) months in cohorts A+B+C and 8.2 months in cohort D, the median PFS was 5.0 (95% CI: 4.0–6.4) months in cohorts A+B+C and 2.6 (95% CI: 1.5–5.9) months in cohort D (Table 4 and Fig. 1A). After a median follow-up of 15.2 (95% CI: 13.2–19.3) months in cohorts A+B+C and 17.1 (95% CI: 8.8–20.0) months in cohort D, the median OS was 12.5 (95% CI: 9.4–15.1) months and 16.5 (95% CI: 3.0–not estimable [NE]) months, respectively (Table 4 and Fig. 1B).
Table 4.
Investigator-Assessed Efficacy According to RECIST 1.1
| Characteristic, n (%) | Cohort A (n = 40) | Cohort B (n = 9) | Cohort C (n = 9) | Cohort D (n = 12) | Cohorts A+B+C (n = 58) |
|---|---|---|---|---|---|
| Confirmed BOR, n (%) | |||||
| Complete response | 0 | 1 (11.1) | 1 (11.1) | 0 | 2 (3.4) |
| Partial response | 18 (45.0) | 5 (55.6) | 3 (33.3) | 2 (16.7) | 26 (44.8) |
| Stable disease | 9 (22.5) | 0 | 3 (33.3) | 4 (33.3) | 12 (20.7) |
| Progressive disease | 7 (17.5) | 3 (33.3) | 2 (22.2) | 5 (41.7) | 12 (20.7) |
| Not evaluable | 6 (15.0) | 0 | 0 | 1 (8.3) | 6 (10.3) |
| ORR (CR + PR), n (%) | 18 (45.0) | 6 (66.7) | 4 (44.4) | 2 (16.7) | 28 (48.3) |
| DCR,a n (%) | 27 (67.5) | 6 (66.7) | 7 (77.8) | 6 (50.0) | 40 (69.0) |
| Median OSb (95% CI), mo | 11.4 (6.4–15.1) | 11.8 (1.9–NE) | NE (5.7–NE) | 16.5 (3.0–NE) | 12.5 (9.4–15.1) |
| Median PFSb (95% CI), mo | 5.0 (3.4–6.4) | 4.1 (1.2–NE) | 5.4 (1.4–NE) | 2.6 (1.5–5.9) | 5.0 (4.0–6.4) |
| Median DORb (range), mo | 9.6 (3.7–NE) | NE (2.8–NE) | 10.5 (2.8–NE) | 3.4 (3.0–3.8) | 9.6 (4.2–NE) |
BOR, best overall response; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; NE, not estimable; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors.
Confirmed best overall response of complete response, partial response, or stable disease.
Product limit (Kaplan–Meier) estimates.
Figure 1.
Kaplan–Meier curves for (A) investigator-assessed PFS per RECIST 1.1 and (B) OS. CI, confidence interval; NE, not estimable; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors.
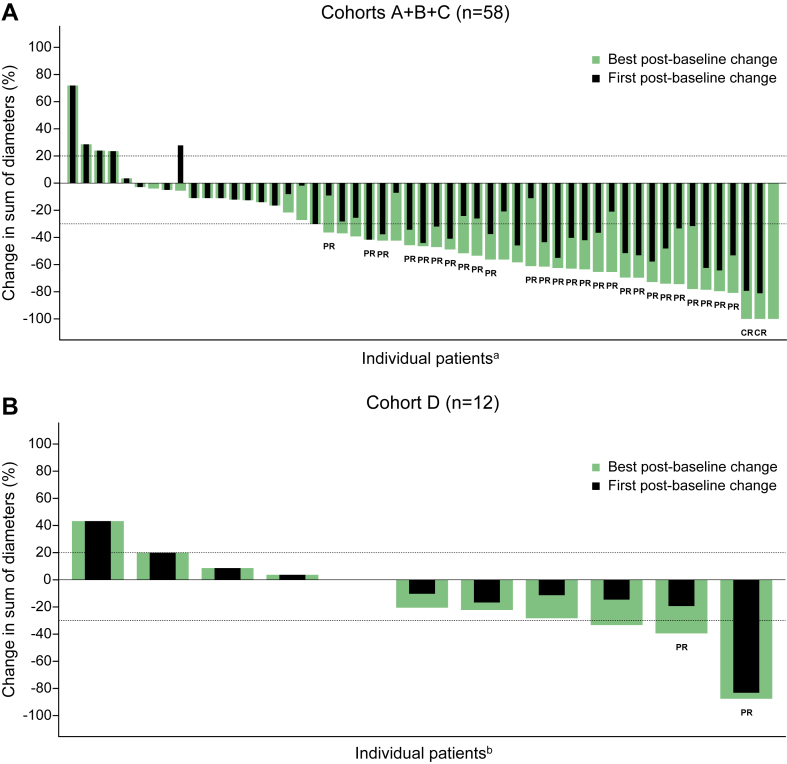
The ORR was 48.3% in cohorts A+B+C (complete response [CR]: n = 2 [3.4%]; partial response [PR]: n = 26 [44.8%]) and 16.7% in cohort D (CR: n = 0; PR: n = 2 [16.7%]) (Table 4 and Fig. 2A and B). The disease control rates were 69.0% in cohorts A+B+C and 50.0% in cohort D. The proportion of not evaluable patients was higher in cohorts A+B+C (10.3%) than in cohort D (8.3%, Table 4). The median DORs were 9.6 (95% CI: 4.2–NE) months in cohorts A+B+C and 3.4 (95% CI: 3.0–3.8) months in cohort D.
Figure 2.
Percent change in the sum of the tumor diameters from baseline at the first and best post-baseline assessments in the (A) cohorts A+B+C and (B) cohort D. CR, complete response; PR, partial response. aThere were five patients with no post-baseline sum of diameters available. bThere was one patient with no post-baseline sum of diameters available.
Level of PD-L1 Expression and Its Association With Efficacy
Overall, 6.3%, 37.5%, and 25.0% of patients in cohorts A+B+C with PD-L1 tumor proportion score (TPS) of less than 1% achieved CR, PR, and stable disease, respectively. Stable disease was achieved in 66.7% of patients in cohort D with PD-L1 TPS of less than 1.0%, but none of them achieved CR or PR (Supplementary Table 1). In patients with PD-L1 TPS of 1.0% to less than 50.0%, PR and stable disease were achieved by 45.8% and 20.8% of patients in cohorts A+B+C, respectively, whereas PR and stable disease each were achieved by 25.0% of patients in cohort D. In patients with PD-L1 TPS of more than or equal to 50%, the ORR was higher in cohorts A+B+C (71.4% [CR: 7.1% and PR: 64.3%]) than in cohort D (25.0% [PR: 25.0%]).
In patients with PD-L1 TPS of more than or equal to 50.0%, the median PFS was higher in cohorts A+B+C (11.8 [95% CI: 2.6–NE] mo) than in cohort D (4.3 [95% CI: 2.5–NE] mo) (Supplementary Figs. 3A and 4A). In cohort D, the median OS was higher in patients with PD-L1 TPS of more than or equal to 50.0% (16.5 [95% CI: 7.5–NE] mo) than in those with PD-L1 TPS of less than 1.0% (NE) and more than or equal to 1.0% to less than 50.0% (7.0 [95% CI: 1.8–NE] mo) (Supplementary Fig. 3B). In cohorts A+B+C, the median OS in patients with PD-L1 TPS of less than 1.0% versus 1.0% to less than 50.0% versus more than or equal to 50.0% was 11.4 (95% CI: 5.3–15.1) months, 14.4 (95% CI: 5.7–NE) months, and 12.6 (95% CI: 6.5–NE), months, respectively (Supplementary Fig. 4B).
Pharmacokinetic Analysis
Pharmacokinetic analyses were performed in 67 of the 70 patients (cohort A, n = 38; cohort B, n = 9; cohort C, n = 8; and cohort D, n = 12) with available samples. Pharmacokinetic profiles with a dose of 2400 mg every 3 weeks were similar across cohorts and between patients who did and did not experience DLTs. In addition, the observed BA first-cycle exposure data (Cmax, AUC, and Ctrough) were consistent with those derived from the published population PK (popPK) model, which was developed using data from BA monotherapy studies with a dose of 1200 mg every 2 weeks (Supplementary Table 2).21,22
Discussion
This study evaluated the role of BA in patients with stage IV NSCLC as 1L or 2L treatment option, regardless of their PD-L1 expression status. On the basis of this interim analysis, the study results were considered mature, and no further analysis has been planned. The safety profile of BA in combination with chemotherapy was manageable, and there were no new safety signals. DLTs were assessed as being unrelated to BA by the investigator. Keratoacanthoma, which is known to occur with TGF-β inhibition, was the most common TGF-β–related skin lesion. Among BA-related AESIs, any-grade infusion-related reaction was the least common event (cohorts A+B+C, 6.9% and cohort D, 8.3%). Any-grade treatment-emergent anemia and bleeding events as AESIs were more common in cohorts A+B+C (20.7% and 15.5%, respectively) than in cohort D (16.7% and 8.3%, respectively).
A total of eight patients experienced grade greater than or equal to 3 bleeding TEAEs (cohorts A+B+C, n = 7 [12.1%] and cohort D, n = 1 [8.3%]), of which gastrointestinal hemorrhage (n = 3; 5.2%) was the most common event that was assessed as unrelated to BA. Of eight patients who experienced grade greater than or equal to 3 bleeding TEAEs, only one patient with hematuria was considered to have a BA-related grade greater than or equal to 3 bleeding TEAE. Two (3.4%) fatal events (grade 5) reported in cohorts A+B+C that were assessed as unrelated to BA treatment were gastrointestinal hemorrhage and upper gastrointestinal hemorrhage (each 1.7%). The proportion of patients with BA-related bleeding events of any grade as AESI in cohorts A+B+C (15.5%) and cohort D (8.3%) in this study was higher than that previously reported for the vascular endothelial growth factor inhibitor bevacizumab (4.0%)23 and lower than that reported for ramucirumab (any-grade bleeding or hemorrhage: 29.0%).24
Overall, the safety outcomes of this study were in line with those of previously published studies on BA.25,26 Most bleeding events observed with BA were grades 1 to 2 epistaxis, which is consistent with the previous finding of a higher frequency of low-grade bleeding events with BA.25,26 At study initiation, both anemia and bleeding events were considered as important potential risks, based on the complex role of TGF-β signaling in the biology of cancer and its angiogenesis.27 On the basis of ongoing safety surveillance that confirmed these risks, these events were reclassified as important identified risks, and appropriate risk mitigation measures were added to the study protocol to ensure timely identification and management of these events. Similarly, the grade greater than or equal to 3 and fatal hemorrhagic events were all assessed as unrelated to BA by the investigators and were mostly manageable by standard of care.
BA was found to have encouraging clinical activity in cohorts A+B+C. The ORR (48.3% versus 16.7%) and disease control rate (69.0% versus 50.0%) were higher in cohorts A+B+C than in cohort D. In cohorts A+B+C, the ORR among patients with PD-L1 expression more than or equal to 50.0% was 71.4%. In previous studies, the confirmed ORRs were 41.5% for durvalumab plus chemotherapy,10 43.3% for cemiplimab plus chemotherapy,28 47.6% for pembrolizumab plus chemotherapy,29 and 48.3% in cohorts A+B+C of this study. The response rates were also high across all three PD-L1 TPS categories in cohorts A+B+C of this study compared with those reported in the EMPOWER-Lung 3 and KEYNOTE-189 trials (PD-L1 TPS <1.0%: 43.8% versus 32.6% versus 32.3%; PD-L1 TPS 1.0%–49.0%: 45.8% versus 43.0% versus 48.4%; and PD-L1 TPS ≥50.0%: 71.4% versus 53.4% versus 61.4%).28,29
The median PFS observed with BA in cohorts A+B+C (5.0 mo) in this study was consistent with that reported for durvalumab in the POSEIDON trial (durvalumab + chemotherapy, 5.5 mo)10 but shorter than that reported in the EMPOWER-Lung 3 (cemiplimab + chemotherapy, 8.2 mo)28 and KEYNOTE-189 (pembrolizumab + chemotherapy, 8.8 mo)29 trials. Similarly, the median OS observed in cohorts A+B+C for BA plus chemotherapy in this study was 12.5 months, whereas that in the POSEIDON (durvalumab + chemotherapy arm),10 EMPOWER-Lung 3,28 and KEYNOTE-18929 trials was 13.3 months, 21.9 months, and NE, respectively. The modest clinical activity observed for BA with chemotherapy in cohort D in this study (ORR, 16.7%) was consistent with the finding of a previously published study on BA in patients with NSCLC resistant or refractory to ICIs (≥3 previous therapies; ORR, 4.8%).30 The median PFS and OS reported in cohort D were 2.6 months and 16.5 months and those in REVEL (ramucirumab + docetaxel) trial were 4.5 months and 10.5 months, respectively.24
Target BA exposures were reached, and the PK profile was consistent with the predicted monotherapy popPK model, suggesting no drug–drug interaction potential for BA with chemotherapy.22 There are several ongoing trials evaluating novel molecular targets, such as lymphocyte activation gene-3, indoleamine 2, 3-dioxygenase 1, and T cell immunoreceptor with IG and ITIM domains in patients with stage IV NSCLC (trials in phase 3: NCT04294810 [tiragolumab + atezolizumab versus atezolizumab]; NCT04738487 [vibostolimab + pembrolizumab versus pembrolizumab]); however, no previous study has evaluated the effects of simultaneous inhibition of TGF-β and PD-L1 pathways.31 Furthermore, a randomized phase 3 trial did not find BA to be superior to pembrolizumab as a 1L treatment option in patients with PD-L1–high advanced NSCLC,32 but we believe the present and published findings of BA18,19,21,22,25,26,30,32 to be relevant for other TGF-β–directed therapies that are currently under investigation in ongoing clinical trials.
The major limitations of the study included the open-label design of the trial and varying chemotherapeutic regimens between cohorts, which may have affected the investigator’s assessment of safety events. Furthermore, the limited duration of the study, its discontinuation rate across cohorts, lack of a direct comparator arm, and small number of patients make it difficult to draw firm conclusions about the efficacy and survival outcomes of the study. It should be noted that the PD-L1 expression data were analyzed in available tissue samples and were not a key inclusion criterion; therefore, the level of PD-L1 expression and association with efficacy were evaluated as an exploratory end point of the study.
In summary, BA in combination with chemotherapy had no new safety signals and had encouraging clinical activity in patients with stage IV NSCLC. Further studies are warranted to understand the role of TGF-β inhibition within the TME.
CRediT Authorship Contribution Statement
Christian Rolfo: Conceptualization, Methodology, Investigation, Resources, Data curation, Formal analysis, Writing - review & editing, Visualization, Project administration.
Laurent Greillier: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Remi Veillon: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Firas Badin: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Francois Ghiringhelli: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Nicolas Isambert: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Astrid Paulus: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Sandrine Hiret: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Surendra Pal Chaudhary: Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Yulia Vugmeyster: Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Masashi Sato: Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Disclosure
Dr. Rolfo received consulting fees from AstraZeneca, Daiichi Sankyo, Regeneron, and Novocure; received payment or honoraria from Bristol Myers Squibb (BMS), Novartis, Invitae, Guardant Health, Novocure, COR2ED, Regeneron, Bayer, Boehringer Ingelheim, AbbVie, and Thermo Fisher; is on data safety monitoring or advisory boards for Invitae, Novartis, Novocure, Janssen, EMD Serono, Bayer, and Regeneron; is a scientific advisory board member of Imagene; is a board member or has leadership roles in International Society of Liquid Biopsy, The European School of Oncology, International Association for Study of Lung Cancer, and Oncology Latin American Association; and received financial or nonfinancial interests from Lung Cancer Research Foundation, National Foundation for Cancer Research, and U54 (National Institute of Health) research grants. Dr. Greillier received consulting fees from BMS, Merck & Co., Kenilworth, NJ, Takeda, Pfizer, Roche, Amgen, Sanofi, Janssen, Eli Lilly, and Novartis; received payment or honoraria from BMS, Merck & Co., Kenilworth, NJ, Takeda, Pfizer, Roche, Amgen, Sanofi, Janssen, Eli Lilly, and Novartis; received support for attending meetings and/or travel from Pfizer, Merck & Co., Kenilworth, NJ, AstraZeneca, Takeda, and Amgen; and is on data safety monitoring or advisory boards for InhaTarget Therapeutics. Dr. Veillon received consulting fees from Merck & Co., Kenilworth, NJ, and Janssen; received payment or honoraria from Roche, AstraZeneca, BMS, Merck & Co., Kenilworth, NJ, Pfizer, Takeda, and Sanofi; and received support for attending meetings and/or travel from Janssen, Takeda, and Sanofi. Dr. Badin received consulting fees from GE Health care; received payment or honoraria from BMS, Jazz Pharmaceuticals, Merck & Co., Kenilworth, NJ, Eli Lilly, Natera, Guardant, Incyte, Amgen, EMD Serono, Regeneron, and Pfizer. Dr. Chaudhary is an employee of EMD Serono. Dr. Vugmeyster is an employee of EMD Serono and has patents for work with EMD Serono. Mr. Sato is an employee of Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA, Darmstadt, Germany. Dr. Hiret received payment or honoraria from Sanofi, AstraZeneca, and BMS; and received support for attending meetings and/or travel from Roche and Novartis. The remaining authors declare no conflict of interest.
Acknowledgments
The authors thank the patients and their families, investigators, co-investigators, and the study teams at each of the participating centers and at the healthcare business of Merck KGaA, Darmstadt, Germany. The authors also thank James L. Gulley, MD, PhD, from the National Cancer Institute, for his valuable comments that improved the manuscript and Xiaoli You, MD, a former employee of EMD Serono, for her many contributions to patient safety during the conduct of the study. Medical writing assistance was provided by Satrupa Das, PhD, CMPP (Merck Specialties Pvt. Ltd., Bengaluru, India, an affiliate of the healthcare business of Merck KGaA, Darmstadt, Germany).
This study was funded by EMD Serono (CrossRefFunder ID: 10.13039/100004755) and was previously part of an alliance between the healthcare business of Merck KGaA, Darmstadt, Germany, and GlaxoSmithKline.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data-sharing portal for the healthcare business of Merck KGaA, Darmstadt, Germany https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been outlicensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA will endeavor to gain agreement to share data in response to requests.
Footnotes
Cite this article as: Rolfo C, Greillier L, Veillon R, et al. Efficacy and Safety of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting Transforming Growth Factor-β and Programmed Death-Ligand 1, Plus Chemotherapy in Patients With Stage IV NSCLC. JTO Clin Res Rep. 2025;6:100748.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2024.100748.
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Non-small cell lung cancer treatment (PDQ®)–health professional version. https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq#_48406
- 3.Azzoli C.G., Baker S., Jr., Temin S., et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller J.H., Harrington D., Belani C.P., et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Chen R., Manochakian R., James L., et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. 2020;13:58. doi: 10.1186/s13045-020-00881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna N.H., Schneider B.J., Temin S., et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2020;38:1608–1632. doi: 10.1200/JCO.19.03022. [DOI] [PubMed] [Google Scholar]
- 7.Singh N., Temin S., Baker S., Jr., et al. Therapy for stage IV non–small-cell lung cancer without driver alterations: ASCO living guideline. J Clin Oncol. 2022;40:3323–3343. doi: 10.1200/JCO.22.00825. [DOI] [PubMed] [Google Scholar]
- 8.Low J.L., Walsh R.J., Ang Y., Chan G., Soo R.A. The evolving immuno-oncology landscape in advanced lung cancer: first-line treatment of non-small cell lung cancer. Ther Adv Med Oncol. 2019;11 doi: 10.1177/1758835919870360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chocarro de Erauso L., Zuazo M., Arasanz H., et al. Resistance to PD-L1/PD-1 blockade immunotherapy. A tumor-intrinsic or tumor-extrinsic phenomenon? Front Pharmacol. 2020;11:441. doi: 10.3389/fphar.2020.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson M.L., Cho B.C., Luft A., et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. 2023;41:1213–1227. doi: 10.1200/JCO.22.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 12.Carbone D.P., Ciuleanu T.E., Schenker M., et al. Four-year clinical update and treatment switching-adjusted outcomes with first-line nivolumab plus ipilimumab with chemotherapy for metastatic non-small cell lung cancer in the CheckMate 9LA randomized trial. J Immunother Cancer. 2024;12 doi: 10.1136/jitc-2023-008189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eser P.Ö., Jänne P.A. TGFβ pathway inhibition in the treatment of non-small cell lung cancer. Pharmacol Ther. 2018;184:112–130. doi: 10.1016/j.pharmthera.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Ramundo V., Palazzo M.L., Aldieri E. TGF-β as predictive marker and pharmacological target in lung cancer approach. Cancers (Basel) 2023;15:2295. doi: 10.3390/cancers15082295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Principe D.R., Doll J.A., Bauer J., et al. TGF-β: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi M., Li T., Niu M., Wu Y., Zhao Z., Wu K. TGF-β: a novel predictor and target for anti-PD-1/PD-L1 therapy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1061394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan Y., Zhang D., Xu C., et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan5488. [DOI] [PubMed] [Google Scholar]
- 18.Strauss J., Heery C.R., Schlom J., et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res. 2018;24:1287–1295. doi: 10.1158/1078-0432.CCR-17-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz-Ares L., Kim T.M., Vicente D., et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J Thorac Oncol. 2020;15:1210–1222. doi: 10.1016/j.jtho.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vugmeyster Y., Wilkins J., Koenig A., et al. Selection of the recommended phase 2 dose for bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1. Clin Pharmacol Ther. 2020;108:566–574. doi: 10.1002/cpt.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkins J.J., Vugmeyster Y., Dussault I., Girard P., Khandelwal A. Population pharmacokinetic analysis of bintrafusp alfa in different cancer types. Adv Ther. 2019;36:2414–2433. doi: 10.1007/s12325-019-01018-0. [DOI] [PubMed] [Google Scholar]
- 22.Vugmeyster Y., Klopp-Schulze L., Rueckert P., et al. Safety and Pharmacokinetics of bintrafusp alfa with Q3W dosing: confirmation of the model-informed dose selection. 11th American Conference on Pharmacometrics. 2020:240. WED-094. [Google Scholar]
- 23.Reck M., von Pawel J., Zatloukal P., et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 24.Garon E.B., Ciuleanu T.E., Arrieta O., et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 25.Vokes E.E., Mornex F., Sezer A., et al. Bintrafusp alfa with CCRT followed by bintrafusp alfa versus placebo with CCRT followed by durvalumab in patients with unresectable stage III NSCLC: a phase 2 randomized study. J Thorac Oncol. 2024;19:285–296. doi: 10.1016/j.jtho.2023.09.1452. [DOI] [PubMed] [Google Scholar]
- 26.Vugmeyster Y., Grisic A.M., Wilkins J.J., et al. Model-informed approach for risk management of bleeding toxicities for bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1. Cancer Chemother Pharmacol. 2022;90:369–379. doi: 10.1007/s00280-022-04468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goumans M.J., Liu Z., ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 28.Gogishvili M., Melkadze T., Makharadze T., et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med. 2022;28:2374–2380. doi: 10.1038/s41591-022-01977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 30.Barlesi F., Isambert N., Felip E., et al. Bintrafusp Alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with non-small cell lung cancer resistant or refractory to immune checkpoint inhibitors. Oncologist. 2023;28:258–267. doi: 10.1093/oncolo/oyac253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivares-Hernández A., González Del Portillo E., Tamayo-Velasco Á., et al. Immune checkpoint inhibitors in non-small cell lung cancer: from current perspectives to future treatments-a systematic review. Ann Transl Med. 2023;11:354. doi: 10.21037/atm-22-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho B.C., Lee J.S., Wu Y.L., et al. Bintrafusp alfa versus pembrolizumab in patients with treatment-naive, programmed death-ligand 1-high advanced NSCLC: a randomized, open-label, phase 3 trial. J Thorac Oncol. 2023;18:1731–1742. doi: 10.1016/j.jtho.2023.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data-sharing portal for the healthcare business of Merck KGaA, Darmstadt, Germany https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When the healthcare business of Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been outlicensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA will endeavor to gain agreement to share data in response to requests.