Abstract
The global population growth is driving up the demand for agricultural products, while traditional farming methods like those from the Green Revolution are becoming unsustainable due to climate change. To address these challenges and ensure agricultural sustainability, innovative techniques, such as nanotechnology, are essential to meet rising food demands and enhance agricultural sustainability. Nanotechnology, which promotes a more sustainable and resilient agricultural system while enhancing food security, is a key catalyst for the Agri-tech revolution. This review offers a progressive analysis of nanotechnology's role in managing plant stress. It explores how precision agriculture, particularly via nanosensors, is enhancing our comprehension of plant stress conditions. The integration of nanotechnology with genetic engineering methods, notably CRISPR-Cas technology, is also examined. Furthermore, the review considers the potential toxicological effects of nanoparticles (NPs) on both the environment and plants. Our review has the potential to make a significant impact on human food security by enhancing food production and availability while promoting sustainable agricultural practices. By tackling these challenges, we can contribute to a more reliable and sustainable food supply for the global population.
Keywords: CRISPR, Crop protection, Nanosensor, Plant stress, Precision agriculture
1. Introduction
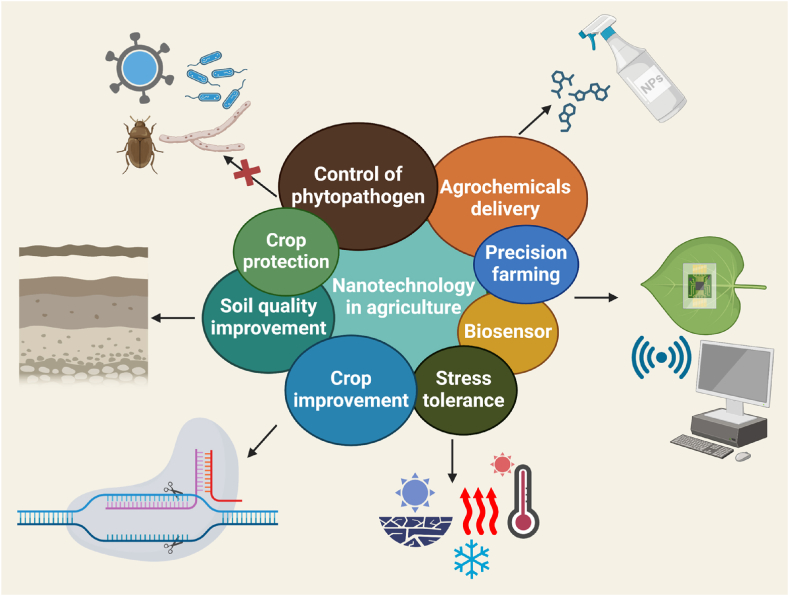
Sustainable agriculture provides a holistic solution to the growing challenges in the agricultural sector, including environmental degradation, social inequality, and economic instability. The current agricultural practices, while sufficient to meet the current global food demand, are unsustainable due to inefficient resource utilization, environmental degradation, and the flattening of yield curves caused by soil degradation and climate change. The pressing challenges posed by the overuse of agrochemicals, including pesticides and fertilizers, lead to soil and water contamination, the persistence of these chemicals in the environment, resistance in pests and weeds, and the significant contribution of agriculture to greenhouse gas emissions [1]. The increasing global demand for agricultural products, driven by rising per capita income and population growth, necessitates a 50–80 % increase in yield production by 2050 [2]. Climate change exacerbates these challenges by increasing the frequency and severity of extreme weather events, such as droughts, floods, and heatwaves, which directly impact crop yields and soil health. For instance, according to recent statistics, climate change could reduce global wheat yields by up to 6 % by 2050, while maize yields might drop by 4 %–20 % in tropical regions [3]. Moreover, studies predict that rising temperatures could lead to a decline in the yields of staple crops, which in turn threatens food security on a global scale [2]. These adverse effects highlight the urgent need for innovative solutions These challenges are exacerbated by biological stresses from pests and pathogens, leading to significant global yield losses, particularly in food-insecure regions. The USDA has announced an investment exceeding $46 million in research and programs dedicated to sustainable agriculture [4]. This investment is aimed at supporting efforts to promote sustainable farming practices and technologies. Addressing these challenges requires a shift towards more sustainable agricultural practices, including the adoption of precision agriculture, integrated pest management, and the development of more resilient crop varieties. The integration of nanotechnology into agriculture is spearheading a revolutionary transformation in the sector, fundamentally altering how we approach food production. Nanotechnology offers many opportunities in agriculture field (Fig. 1) for example; by developing nanopesticides for control plant pathogens, nanofungicides, nanofertilizers for plant growth, targeted delivery of fertilizers, plant disease management, increase in plant productivity, promotes low consumption of nutrients and water [5]. The application of nanoparticles (NPs) in agriculture offers significant potential to enhance plant stress tolerance and improve agricultural productivity [6]. NPs possess unique physicochemical properties, such as a high surface-to-volume ratio, which can be leveraged to alter plant physiology and protect against various biotic and abiotic stress conditions. This technological advancement represents a paradigm shift in agriculture, moving towards highly efficient, sustainable, and resilient farming practices, thereby addressing the growing global food demand in an environmentally responsible manner. The implementation of NPs in agriculture necessitates thorough research and controlled delivery systems to ensure their safety and efficacy. The use of NPs in agriculture represents a new frontier that can significantly improve plant stress management and overall agricultural productivity. Despite the promising applications of NPs, challenges such as tissue toxicity, low transformation efficiencies, and environmental impact must be addressed. Research has highlighted the need for standardized leaching methods to quantify NP exposure and ensure environmental safety. Moreover, advancements in nanobiotechnology and the integration of artificial intelligence (AI) in agricultural practices can lead to the development of novel nano-biosensors, nano-fertilizers, and biodegradable nanofibers, enhancing the precision and effectiveness of NP applications. As a result, nanotechnology stands poised to revolutionize agriculture, offering sustainable solutions to meet global food demands.
Fig. 1.
Applications of nanotechnology in different aspects.
Nanobiosensors, with their non-invasive and high-sensitivity characteristics, are revolutionizing crop health monitoring by improving resource utilization and managing crop loss due to diseases [7]. Various NPs, such as metal oxides and magnetic NPs, enhance the capabilities of these sensors, making them invaluable in precision agriculture. The adoption of nanosensors not only aids in precise monitoring of plant physiological and soil conditions but also promotes efficient resource use and reduces environmental pollution. By providing real-time data on plant health, soil moisture levels, nutrient availability, and pest presence, these sensors enable farmers to make informed decisions [8]. Moreover, Nanosensors, particularly those utilizing GPS technology, offer significant advantages in monitoring crop growth and soil conditions, leading to more efficient resource utilization and reduced environmental impact. Consequently, the application of nanosensors in precision agriculture stands as a sustainable and eco-friendly approach to tackle agricultural challenges such as abiotic and biotic stresses and ensure food security for the growing population. Ongoing research, coupled with supportive government policies, is crucial for maximizing the benefits of nanotechnology in agriculture, thereby promoting sustainable and scientifically advanced farming practices. This review presents a forward-thinking examination of nanotechnology and its role in plant stress management. It delves into how precision agriculture, particularly through the use of nanosensors, is advancing our understanding of plant stress conditions. The integration of nanotechnology with genetic engineering techniques, especially CRISPR-Cas technology, is also discussed. Additionally, the review addresses the potential toxicological impacts of nanoparticles (NPs) on both the environment and plants.
The proposed review emphasizes the critical need for sustainable agriculture to address the growing challenges of environmental degradation, social inequality, and economic instability in the agricultural sector. It highlights the unsustainability of current practices due to inefficient resource utilization and climate change, which exacerbate soil degradation and yield declines. Moreover, it underscores the potential of nanotechnology to revolutionize agriculture through innovations like nanofertilizers, nanopesticides, and nanosensors, enhancing productivity and resilience. It calls for continued research, regulatory frameworks, and supportive policies to ensure the safe and effective implementation of nanotechnology, ultimately promoting sustainable and resilient farming practices to meet global food demands. By providing a forward-thinking examination of these issues, the review sets the foundation for a detailed discussion on how sustainable agricultural practices, coupled with nanotechnology, can address the global food demand in an environmentally responsible manner.
2. Nanotechnological approach and stress management
Plant stress refers to any condition or factor that adversely affects the growth, development, productivity, or survival of plants. These stress factors can be biotic or abiotic. Plant stress can threaten global food security in several ways for example; reduced crop yields, increased vulnerability to pest and diseases, soil degradation, water scarcity and climate change impacts. In the context of the agricultural revolution, nanotechnology emerges as a game-changer, offering innovative solutions to mitigate these stressors and enhance crop resilience. Nanotechnology offers promising solutions to mitigate plant stress and enhanced crop resilience by playing role in NPs based delivery system, nano sensors for early detection, NPs for soil improvement, nanotechnology for genetic engineering. By integrating nanotechnology into agricultural practices, scientists and professionals can develop more effective and environmentally sustainable techniques to combat plant stress, thereby securing and boosting global food production in the face of mounting challenges.
2.1. Nanotechnological approaches for biotic stress mitigation
Nano-biotechnology offers innovative strategies to manage biotic stress in plants, which includes diseases caused by pathogens like fungi, bacteria, viruses, and pests. By integrating nanotechnology into biotic stress management strategies, researchers and agricultural practitioners can develop more effective, targeted, and sustainable solutions for protecting crops against diseases and pests, thereby ensuring global food security.
2.1.1. Nanoparticles in plant pathogen management and disease resilience
Over a million chemical pesticides undergo testing every year in order to develop efficient and specific pesticides [9]. However, a mere 0.1 % of the pesticides that are added as supplements are actually successful in combating the intended pest. The rest of these chemicals end up polluting the environment, harming species that were not the intended target, and contributing to the development of insects, weeds, and infections that are resistant to pesticides [10]. In addition, they possess the capacity to penetrate food chains, therefore facilitating the accumulation and amplification of their presence leading to human and animal health risk [11]. Nanotechnology can play role in disease management through the development and controlled release of nanopesticides, nanoinsecticides, nanobactericides, and nanofungicides in agricultural areas [12]. Nanomaterials (NMs) exhibit several characteristics that make them potential candidates for use as pesticides in agriculture applications. The greater stability, long shelf life and improved water solubility of NMs are two characteristics that augment the pesticides potential and bioefficacy. Nano-pesticides are demonstrating a promising ability in circumventing the limitations of traditional pesticides because of the properties they exhibit, which include regulated release of active components over an extended period of time and delayed disintegration.
In addition, they are less often required in smaller amount to achieve desired results than conventional pesticides. By leveraging these characteristics, nanomaterials offer promising opportunities to develop next-generation pesticides that are more effective, environmentally friendly, and sustainable for crop protection. For example; carbofuran formulations utilizing PEG-900 as a hydrophilic segment showed superior bioefficacy against Meloidogyne incognita nematodes in tomato plants, with CP2 (PEG-900, 20 ppm) being the most effective in reducing nematode penetration and gall formation both in pot and field conditions [13]. PEG-900-based carbofuran formulations for effective management of root-knot nematodes in tomato crops, emphasizing the importance of polymer choice in pesticide formulation design for enhanced pest control efficacy. Nanopermethrin, prepared using a microemulsion-based method, exhibits higher efficacy against Aedes aegypti compared to regular microparticular permethrin [14]. Furthermore, nanopermethrin shows no antibacterial activity against common bacteria, no phytotoxic effects on selected plant seeds, and significantly lower cytotoxicity in Allium cepa cells compared to microparticular permethrin, suggesting its potential as a safer and effective alternative for pest control in agricultural areas [14]. Moreover, this also fulfil the demand for safer pesticide alternatives that maintain high efficacy against target pests while minimizing adverse effects on non-target organisms and the environment, aligning with integrated pest management principles.
Recent study is showing the potential shift towards NPs-based insectprotectants for grain storage due their improved safety profile. For example; silica oxide (SiO2) and aluminium oxide (Al2O3) NPs show promising efficacy as alternatives to malathion for protecting stored grains against Sitophilus oryzae [15]. In addition, these NPs showed significant insecticidal effects, reducing weight loss, and showing relatively low adverse effects compared to malathion. In addition, the development of a nanoemulsion containing Pimpinella anisum essential oil, with enhanced stability and efficacy, offers a promising alternative for managing the red flour beetle (Tribolium castaneum) in stored products [16]. The nanoemulsion demonstrated significant toxicity to adult beetles and their progeny, supported by morphological and histological evidence. This approach addresses the limitations of traditional essential oils, contributes to eco-friendly pest management, and reduces reliance on synthetic insecticides, aligning with sustainable agriculture practices. However, further research is needed to assess their practical effectiveness and safety in protecting stored products.
Porous hollow silica nanoparticles (PHSNPs) as a controlled delivery system for the water-soluble pesticide validamycin showing a high loading capacity achieved through a supercritical fluid loading method [17]. It exhibits a multi-staged release profile of validamycin, making them a promising candidate for precise and prolonged pesticide delivery in agriculture, addressing the need for both immediate and sustained release. The successful encapsulation of IMI microcrystals using chitosan and sodium alginate, resulting in prolonged release kinetics. Photocatalysts, particularly SDS/Ag/TiO2, showed high activity in degrading IMI under UV and natural light, with the novel nano-SDS/Ag/TiO2-IMI formulation exhibiting higher toxicity against Martianus dermestoides adults compared to pure IMI, highlighting its potential as a photodegradable insecticide with reduced environmental impact [18]. Thus, the findings providing a sustainable and efficient approach to control insect pests using photodegradable pesticides encapsulated in biocompatible materials, aiming to enhance efficacy while minimizing environmental harm.
Fungal infections can cause a range of detrimental effects on crop productivity, including reduced photosynthesis, nutrient and water uptake limitations, structural damage, reproductive impairments, toxin production, and post-harvest losses. Exploring nanomaterials as promising antifungal agents to address concerns about the environmental and health impacts of conventional synthetic fungicides, aiming for sustainable and effective plant disease control methods is a need now a days. For example; Ag-NPs were mainly employed to combat fungal infestation in several crop groups, such as Graminae, Crucifereae, and Solanaceae. The main purpose of this NPs was to hinder the growth and spread of fungal mycelia and/or conidia. Cracked cell walls [19] and deformed and ruptured mycelium [20] are documented negative effects of using Ag-NP. Cu and Ag-NPs inhibit the spread of mycelia in the powdery mildew-infected leaves of Quercus robur [21]. Ag-NPs were also reported to act against Fusarium avenaceum, Bacillus megaterium, Pseudomonas needle, F. graminearum, F. color and Erwinia sp. [22]. Beside they also show fungicidal activity against the Penicillium digitatum, A. citri, and Alternaria alternate [23]. However, in a comparative study, Cu-NPs was found to be most effective among the tested NPs in inhibiting fungal growth, followed by ZnO-NPs and Ag-NPs in preventing fungal gray mold disease caused by Botrytis cinerea in pulm and tomatoes [24]. The results suggest that Cu-NPs and ZnO-NPs could serve as eco-friendly alternatives to conventional synthetic fungicides, with potential applications in plant disease management due to their superior fungitoxic effects and ability to suppress fungal diseases on fruit.
Synthesis and characterization of chitosan nanoparticles (ChNP) with enhanced antibacterial and antibiofilm properties compared to chitosan and chitin was recorded and supported by physiochemical analyses and evaluation of minimum inhibitory concentrations (MIC) against common pathogens [25]. In another study, they were reported to exhibit fungicidal activity for Fusarium solani and Aspergillus niger [26]. The presence of primary amine groups in chitosan, coupled with the ionic gelation method using tripolyphosphate, contributes to the superior antimicrobial and antifungal activity of ChNP, making them promising candidates for combating medical pathogens and biofilm formation. Other metal-oxide NPs also have antifungal potential, including MgO-NPs against Phomopsis vexans fungus [27], silica-NPs against Alternaria solani [28], TiO2 -NPs against Cercospora beticola [29], CuS NPs against Gibberella fujikuroi [30] and SiO2−NPs against Radicisi lycopersici and Fusarium oxysporum [31]. With projected population growth and increasing challenges from plant pathogens like Fusarium wilt, there's a pressing need for innovative agricultural technologies. For example; silica-NPs with tenable dissolution rates effectively enhanced watermelon growth and suppressed Fusarium wilt disease, resulting in significant increases in fruit yield [32]. The controlled hydrolysis of NPs facilitated efficient silicic acid delivery to plants, highlighting the potential of nano-enabled agriculture in addressing pathogen pressure and meeting future food demands sustainably.
NPs have also show promising potential to inhibit the growth and spread of bacterial and viral disease. For example; Ag-NPs were reported to act against Escherichia coli [33,34], Bacillus subtilis [34], Pseudomonas aeruginosa [34], Pseudomonas syringae [35]. Similarly, Au-NPs exhibited antibactericidal activity against E. coli [36], and MgO-NPs for Ralstonia solanacearum [37]. Several studies have also been reported for bactericidal potential of TiO2-NPs against P. syringae (sugar beet disease) [29], Venturia inaequalis (apple scab disease), F. solani (Fusarium wilt disease) [38], and Dickeya dadantii (stem and root rot) [39]. In a recent study ZnO NPs found to be effective in inhibiting the growth of Pantonea ananatis at lower concentration and reduced maize white spot [40]. MoS2-CuNPs exhibit significantly higher antibacterial activity against Xanthomonas oryzae pv. oryzae (Xoo) compared to commercial copper bactericides, leading to a substantial reduction in disease severity in rice leaves [41]. The protective film formed by MoS2-CuNPs, along with increased trichome density and enhanced antioxidant enzyme activities, highlights their potential as multifunctional agents for plant protection and growth promotion. By leveraging the synergistic effect of MoS2-CuNPs, this research offers a novel approach to combat phytopathogens like Xoo while enhancing plant resilience and growth, thus addressing key challenges in modern plant protection and agricultural sustainability. Moreover, CuO NPs, effectively reduce tomato bacterial wilt (TBW) incidence by altering rhizosphere bacterial community composition [42]. CuO NPs enhanced beneficial bacterial populations like Streptomyces and Sphingomonadaceae, while suppressing pathogenic bacteria, offering a promising strategy for TBW management and plant health improvement. The study's findings are bridging the gap in addressing the urgent need for sustainable and efficient approaches to control soil-borne plant diseases, such as Ralstonia solanacearum, which causes bacterial wilt [42].
Metal NPs are also playing systemic resistance against the virus. For example; ZnO-NPs effectively induce resistance in tomato plants against Tomato mosaic virus (ToMV) by enhancing growth indices, photosynthetic attributes, and antioxidant defence systems [43]. This suggests ZnO-NPs as a safe and cost-effective antiviral agent to mitigate ToMV-induced damage in tomatoes, highlighting their potential for sustainable disease management in agriculture. SiO2 NPs effectively manage the Meloidogyne incognita, Pectobacterium betavasculorum, and Rhizoctonia solani disease complex in beetroot [44]. Seed priming with SiO2 NPs, particularly at 200 mg L−1, significantly enhances plant growth, chlorophyll content, defense enzyme activities, and reduces disease severity, making it a promising strategy for sustainable disease management in beetroot cultivation. The above findings underscore the importance of NPs-based approaches in enhancing plant health and resilience against multiple pathogens, highlighting the potential of nanotechnology in agricultural disease management and emphasizing the importance of sustainable and eco-friendly strategies for crop protection and food security.
Agricultural weeds are plants that grow in crop fields or agricultural areas where they are not desired. These plants compete with cultivated crops for resources such as nutrients, water, and sunlight, which can reduce crop yields and quality. Weeds can also harbor pests and diseases, interfere with farm machinery operations, and impede harvest efficiency, therefore NPs are being employed to generate nanoherbicides. For example; poly (epsilon-caprolactone) NPs containing atrazine effectively control target plant species while reducing herbicide mobility in soil and mitigating genotoxicity, thus offering a dual benefit of weed control and environmental safety [45]. In another study, nanoencapsulation of paraquat using chitosan and sodium tripolyphosphate (TPP) resulted in a less toxic herbicidal formulation with preserved effectiveness against weeds [46]. Similarly, nanoencapsulation of atrazine using poly(ε-caprolactone) (NC + ATZ) enhances herbicidal efficacy by facilitating foliar uptake, targeted delivery, and chloroplast degradation in Brassica juncea plants [47]. In another studyt, atrazine-nanocapsules outperformed commercial atrazine in their ability to suppress Bidens pilosa L. (hairy beggarticks) and Amaranthus viridis L. (slender amaranth) [48]. The above findings addressing the challenges posed by atrazine's persistent contamination and environmental impact by leveraging nanotechnology to enhance herbicidal activity while minimizing adverse effects. This innovative nanotechnology-based formulation offers a promising solution for reducing herbicide usage, maintaining efficacy at lower concentrations, and mitigating environmental contamination associated with traditional herbicides like atrazine. This approach addresses concerns of toxicity to nontarget organisms and soil sorption while maintaining herbicidal activity. Table 1 list the role of NPs in crop disease management.
Table 1.
Nanoparticles in crop disease management via variable Mechanisms.
| NP | Target Pathogens | Host Plant | Application/Mechanism | Reference |
|---|---|---|---|---|
| Copper Phosphate trihydrate Cu3(PO4)2.3H2O, Copper sulfate (CuSO4) | Rhizoctonia solani | Solanum lycopersicum | Inhibited the spread of the disease by triggering the defensive mechanism. | [49] |
| Copper (Cu) | Acidovorax citrulli | Citrullus lanatus L. | Inhibited the spread of disease by activating stomatal immunity. | [50] |
| Colletotrichum capsici | Capsicum annum | Inhibited the spread of the disease by preventing the growth of the pathogen. | [51] | |
| Clavibacter michiganensis | Solanum lycopersicum | Increased immunity to the disease by stimulating the activity of APX, SOD, GPX, and phenylalanine ammonia-lyase (PAL). | [52] | |
| Copper Oxide (CuO) | Helicoverpa armigera | Gossypium sp. | Provide tolerance to insect in Bt cotton by inducing expression of Bt toxin proteins related genes in plant. | [53] |
| Spodoptera littoralis | Gossypium sp. | Provide tolerance to cotton leafworm by impairing vital enzymes. | [54] | |
| Meloidogyne incognita | Vigna unguiculata | Inhibited root gall formation by impeding the nematode production and reduced egg mass by amending mitochondrial activity, and triggering ROS generation and apoptotic paths. | [55] | |
| Fusarium oxysporum f. sp. chrysanthemi | Chrysanthemum | Inhibited the severity of disease and enhanced plant biomass. | [56] | |
| Sitophilus granarius and Rhyzopertha dominica | Triticum aestivum | Enhanced motility of insects, thereby causing enhanced morphological characteristics, pigment content, and antioxidant enzymes (SOD, POD, APX) activity of plant. | [57] | |
| Copper sulfide (CuS) | Gibberella fujikuroi | Oryza sativa | Inhibited the spread of disease by hampering microbial growth because of interaction among cell and copper ions of NP. | [30] |
| Iron (II, III) oxide (Fe3O4) | Tobacco mosaic virus | Nicotiana benthamiana | Inhibited the spread of the TMV disease by promoting antioxidants and salicylic acid genes expression. | [58] |
| Iron (II) oxide (FeO) | Ralstonia Solanacearum | Solanum lycopersicum | Inhibited the spread of the bacterial wilt disease by modifying the bacterial flora in the rhizosphere and triggering antioxidant enzymes. | [42] |
| Iron (III) oxide (Fe2O3) | Ralstonia solanacearum | Solanum lycopersicum | Inhibited the severity of disease by reducing the growth of bacteria by disintegrating its cell wall, thereby, enhancing plant growth and biomass. | [59] |
| Turnip mosaic virus (TuMV) | Nicotiana benthamiana | Inhibited occurrence and severity of disease by hampering viral proliferation through impeding its replication, synthesis of viral coat protein and triggering phytohormone content in plants. | [60] | |
| Magnesium oxide (MgO) | Colletotrichum gloeosporioides | Persea americana and Carica papaya | Inhibited the spread of the fungi by hampering conidial germination and inflicting structural damage. | [61] |
| Ralstonia solanacearum | Solanum lycopersicum | Inhibited bacterial wilt by triggering systemic resistance through enhanced production of Pathogen resistance proteins, and β-1,3- glucanase and tyloses in the apoplastic pathway and xylem of pith tissues. | [37] | |
| Phytophthora nicotianae and Thielaviopsis basicola | Nicotiana tabacum L. | Inhibited the onset of black shank and black root rot by impeding spore germination, hyphal development and sporangium formation and by enhanced ROS production to disintegrate membrane integrity of pathogen. | [62] | |
| Manganese (Mn) | Fusarium oxysporum f. sp. Niveum | Citrullus lanatus L. | Inhibited the spread of the Fusarium wilt by activating antioxidative and defence mechanism in plants. | [63] |
| Manganese oxide (MnO) | Fusarium oxysporum f. sp. Niveum | Citrullus lanatus L. | Inhibited the spread of the disease by activating the transcription of genes linked to defence. | [64] |
| Silver (Ag) | Pectobacterium carotovorum | Beta vulgaris L | Inhibited soft rot by activating antioxidative defence mechanism. | [65] |
| Xanthomonas oryzae pv. oryzae | Oryza sativa | Inhibited disease spread by triggering antioxidative defence mechanism in plant. | [66] | |
| Acidovorax oryzae | Oryza sativa | Impeded the development of biofilms, the motility of swarms, and pathogen survival. | [67] | |
| Alternaria solani | Solanum lycopersicum | Enhanced antibacterial activity of the particles due to surface coating with Trichoderma viridae secondary metabolites, confers resistance against fungal spores. | [68] | |
| Aspergillus flavus | Oryza sativa | Promoted plant growth by minimizing aflatoxin synthesis. | [69] | |
| Fusarium oxysporum f. sp. Cicero | Cicer arietinum | Inhibited wilt disease by impeding the growth of mycelia. | [70] | |
| Alternaria brassicicola | Arabidopsis thaliana | Induce systemic resistance against fungal spores by enhanced phenolic compound production through promoting PAL activity. | [71] | |
| Alternaria alternate | Solanum lycopersicum | Inhibited disease spread by activating antioxidant and defence mechanism by promoting efficacy of hydrolytic enzymes | [72] | |
| Pseudomonas syringae pv. syringae | Prunus persica | Inhibited bacterial canker spread by enhanced antibacterial activity | [35] | |
| Meloidogyne incognita | Trachyspermum ammi. | Enhanced plant growth, biochemical parameters, and activities of antioxidant (SOD, POD, CAT, APX) and thus inhibited the production of galls, root knot and egg masses. | [73] | |
| Aspergillus flavus | Oryza sativa | Inhibited the proliferation of fungus and reduces the synthesis of osmolytes, enzymatic and non-enzymatic antioxidants. | [74] | |
| Xanthomonas oryzae pv. oryzae | Oryza sativa | Enhances growth, antioxidant enzyme activity (CAT and POD), and nutrient uptake by plant thereby inhibiting the bacterial growth and the lesion size, induced by bacterial leaf blight. | [66] | |
| Alternaria solani | Solanum lycopersicum | Inhibited the formation of fungal spores and improved fresh weight and pigment content, whereas decreases proline, polyphenol oxidase ad SOD activity in plant | [68] | |
| Tomato spotted wilt virus (TSWV) | Solanum tuberosum | Supressed onset, and spread by reducing the number of local lesions. | [75] | |
| Tomato mosaic virus (ToMV) and Potato virus Y (PVY) | Solanum lycopersicum | Induce systemic acquired resistance and thus reduces onset and severity of disease by enhancing the activities of peroxidase and polyphenol oxidase, total soluble protein and pigment content in plant. | [76] | |
| Calcium phosphate (Ca3(PO4)2) | Rhizoctonia solani | Solanum tuberosum | Inhibited the spread and severity of black scurf infection by enhanced catalase and peroxidase activity. | [77] |
| Silicon (Si) | Botrytis fabae | Vicia faba | Inhibited the occurrence and severity of chocolate spot disease by enhancing the activities of polyphenol oxidase, protective enzymes, and peroxidase. | [78] |
| Meloidogyne incognita | Solanum melongena | Inhibited root gall formation by impeding the nematode production and reduced egg mass by impeding egg hatching potential and inducing malfunction in the cellular system. | [79] | |
| Silica (SiO2) | Xanthomonas campestris pv. carotae, Pectobacterium carotovorum, Rhizoctonia solani, Fusarium solani, Alternaria dauci | Daucus carota | Provide tolerance to microbes and enhances plant growth by restricting pathogen entry through enriching the accretion of silica in double layer beneath the cell cuticle. | [80] |
| Fusarium fujikuroi | Oryza sativa | Inhibited the occurrence and severity of bakanae disease through reduced fungus sporulation, and enhanced electrolyte leakage, silica content and peroxidase activity. | [81] | |
| Fusarium oxysporum f. sp. niveum | Citrullus lanatus | Inhibited the occurrence of Fusarium wilt and promoted plant growth by efficiently delivering silicic acid. | [32] | |
| Pseudomonas syringae | Arabidopsis thaliana | Induce systemic and local resistance against bacterial disease by triggering the salicylic acid mediated defence pathway. | [82] | |
| Dickeya dadantii | Phalaenopsis pulcherrima | Induced resistance against the spread of soft rot disease by triggering the synthesis of resistance related proteins and hard leaves layer. | [83] | |
| Magnaporthe oryzae | Oryza sativa | Induced resistance against the blast by upregulation of genes related to salicylic acid pathway. | [84] | |
| Tomato Yellow Leaf Curl Virus (TYLCV) | Solanum lycopersicum | Diminished severity of the disease, viral titer, coat protein genes and enhances growth of tomato. | [85] | |
| Titanium dioxide (TiO2) | Puccinia striiformis f. sp. Tritici | Triticum aestivum L. | Inhibited yellow stripe rust disease by impeding the proliferation and growth of fungus, and by enhancing photosynthesis, and proteins associated with defence responses in plants. | [86] |
| Bipolaris sorokiniana | Triticum aestivum L. | Minimized the spread of spot blotch disease by enhancing the antimicrobial activity, physiological and metabolic profile of plant. | [87] | |
| Dickeya dadantii | Ipomoea batatas | Impeded the development of biofilms, swimming motility, and pathogen survival. | [39] | |
| Cercospora beticola | Beta vulgaris | Inhibited the severity and spread of leaf spot infection by inducing sporangial membrane deformation and disruption that results in mortality. | [29] | |
| Broad Bean Stain Virus (BBSV) | Vicia faba | Minimized the severity of disease and enhanced growth of Vicia faba by reducing viral titer and by inducing the expression of pathogenesis-related genes and defence genes of salicylic acid signaling pathway. | [88] | |
| Cerium dioxide (CeO2) | Fusarium oxysporum f. sp. Lycopersici | Solanum lycopersicum | Inhibited the spread of disease by enhanced photosynthetic and antioxidant capability | [89] |
| Chitosan | Fusarium andiyazi | Solanum lycopersicum | Promote resistance against wilt by inhibiting radial growth of mycelia through enhanced defence system of plant | [90] |
| Puccinia triticina | Triticum aestivum | Inhibited rust disease by impeding pustule size, ROS production, and enhancing the activity of antioxidant enzymes and expression of PR1-PR5 and PR10 genes. | [91] | |
| Zinc oxide (ZnO) | F. oxysporum f. sp. Melongenae | Solanum melongena | Inhibited disease spread by triggering physiological and biochemical mechanism in plant. | [92] |
| F. oxysporum f. sp. Lycopersici | Solanum lycopersicum | Inhibited disease spread by triggering defence response in plant. | [93] | |
| Pectobacterium betavasculorum, Meloidogyne incognita, and Rhizoctonia solani | Beta vulgaris subsp. Vulgaris | Inhibited the severity and spread of root rot, root knot, and soft rot facilitated disease complex by inducing fungal hyphae death by impeding the growth of conidia and conidiospores. | [44] | |
| Tomato mosaic tobamovirus | Solanum lycopersicum | Induces systemic resistance against virus by mediating cellular integrity. | [43] | |
| Xanthomonas oryzae pv. oryzae | Oryza sativa | Impeded the development of biofilms, and bacterial growth by disintegrating its cell wall and promoting ROS production. Thereby, increasing plant biomass and reducing the infected area. | [94] | |
| Fusarium solani | Solanum lycopersicum | Inhibited the germination of spores and growth of mycelia and had no considerable effect on plant biomass. | [95] | |
| Tobacco mosaic virus (TMV) | Nicotiana benthamiana | Inhibits viral replication and accretion by enhancing plant growth and modulating plant immunity that includes ROS accumulation, enhanced activity of antioxidant enzymes (POD, CAT), SA and abscisic acid content and expression of systemic resistance-related genes (PR1 and PR2). | [96] | |
| Fusarium oxysporum | Cicer arietinum L. | Inhibits the onset and severity of Fusarium wilt by enhancing plant antioxidant enzyme activity (SOD, POD, CAT), photosynthetic rate, growth attributes, and enriching the sugar, phenol, and protein content. | [97] | |
| Polyhydroxyalkanoates with metribuzin | ---- | Elsholtzia ciliate | Encapsulation improves stability of herbicide that cause sustained release of herbicide against weed. | [98] |
2.2. Nanotechnological approaches for abiotic stress mitigation
Abiotic stress refers to adverse environmental conditions that can negatively impact plant growth, development, and productivity. These stresses are non-living factors such as drought, salinity, extreme temperatures (cold or heat stress), heavy metal toxicity, and nutrient deficiencies or imbalances. Abiotic stress can lead to physiological disorders, reduced crop yields, and economic losses in agriculture. Nanotechnology plays significant role in mitigating abiotic stress through various innovative approaches for example; enhanced nutrient uptake and water management, stress signalling modulation, soil improvement, metal remediation, temperature regulation, precision agriculture and genetic engineering.
2.2.1. Drought, salinity and temperature stress mitigation
Plants encounter many environmental challenges during their life cycles, which prompts the evolution of defense systems at several stages. Drought and salinity stress are significant threats to global food security due to their adverse effects on crop production and yield. These conditions result in the loss of vigor, impair several biochemical processes associated with plant defense systems, and consequently lead to significant crop damage [99]. One primary abiotic factor affecting agricultural productivity is drought. In plants, another prevailing form of abiotic stress is soil salinity, which has a fundamental impact on plant productivity [100]. Moreover, the primary cause of osmotic and specific ionic effects is the presence of excessive salts, resulting in the dilapidation of explicit metabolic processes in crop plants. Subsequently, at every phase of plant growth and development, salt stress asserts a disruptive influence, diminishing crop yield [101]. In recent years, nanotechnology in agriculture has earned significant attention because of its capacity to grant swift and promising solutions necessary for sustainable farming [102]. Different forms of NPs are being used and have proven to favourably impact plant growth and yield by amending their physiological mechanisms. For example; a novel fertilization strategy shows promise in mitigating water scarcity and salinity challenges for sustainable crop production, highlighting the potential of nanotechnology in agricultural resilience and food security [103]. Wherein, the foliar application of ZnO NP alleviates drought stress effects on eggplant, enhancing plant growth, yield, and water productivity. This nano-scale fertilization approaches like ZnO NP addressing critical agricultural challenges, such as water scarcity and salinity, contributes to improved crop performance and resource use efficiency [103]. In another study, foliar application of ZnO-NPs on cucumber seedlings increased plant biomass under drought stress by enhancing both enzymatic and nonenzymatic antioxidative systems, reducing ROS production and malondialdehyde levels [104]. ZnO-NPs significantly improved plant growth, yield, and photosynthetic pigments in salinity-stressed in wheat during vegetative and maturity stages [105]. AgNPs from Capparis spinosa extract can alleviate salt-induced toxicity in germinating Triticum aestivum L. grains [106]. AgNPs as a priming agent mitigate salinity stress effects on crop germination and growth, contributing to crop resilience and food security amidst changing environmental conditions. In another study, iron, copper, cobalt, and zinc oxide NPs effectively enhance drought tolerance in soybean plants, as evidenced by improved physiological traits and upregulated expression of drought-responsive genes [107]. Additionally, Se-NPs, SiO2-NPs, and Se/SiO2-NPs was found in combating the adverse effects of drought in strawberry plant growth and yield [108]. Exogenous spraying of Se/SiO2 increased drought tolerance by enhancing the efficiency of antioxidant enzymes such as APX, CAT, SOD, and GPX, as well as minimizing lipid peroxidation and hydrogen peroxide (H2O2). Similarly, various dosages of SiNPs displayed anti-stress effects against drought stress in Crataegus sp. seedlings and promoting a rise in chlorophylls, carotenoids, proline, and MDA content [109].
Si-NPs have shown immense potential as a silicon resource that could be used to improve plant tolerance to abiotic stress [110]. By exploring the synergistic effects of nano silicon and gypsum on mitigating salinity stress, a practical and sustainable solutions for improving plant performance and resilience under challenging environmental conditions was reported in Jatropha integerrima [111]. The study concludes that combining foliar application of nano silicon and soil addition of gypsum effectively mitigates the harmful effects of salinity stress on J. integerrima plants irrigated with saline water, especially at higher concentrations. This combined treatment improves vegetative growth, flowering traits, and reduces the accumulation of harmful ions and secondary metabolites, making it a promising and economical strategy for salt-stressed ornamental plants [111]. In tomato plants; Si-NPs application, particularly through foliar spray, effectively mitigates salt-induced stress [112]. It was reported that Si-NPs enhanced growth parameters, mineral uptake, photosynthesis rates, and antioxidant enzyme activity, counteracting the negative effects of salinity stress.
Rapeseed seedling growth was found to be improved under saline condition upon applying ZnO-NPs [113], while rice seeds loaded with ZnO-NPs caused increased growth and yield under drought stress compared with un-primed seeds [114]. A recent study demonstrated that Se-NPs and ZnO-NPs improved ABA and gibberellin gene expression in germinating rapeseed under salt stress, which caused enhanced germination of primed seeds over unprimed and hydro-primed seeds [115]. By enhancing germination parameters, nutrient absorption, osmotic protection, antioxidant defense and gene expression related to stress tolerance; the application of ZnOPs priming highlights the potential of nanotechnology in mitigating salinity stress effects on crop productivity. The approach is offering sustainable solutions for agricultural resilience and economic yield enhancement along with addressing the urgent need for effective strategies to overcome salinity-induced micronutrient imbalances and oxidative stress, crucial factors impacting plant growth and food production in saline-affected areas. The application of TiO2 NPs effectively mitigates the negative effects of salinity on Moldavian balm plants, improving agronomic traits, antioxidant enzyme activity, and essential oil content while reducing H2O2 concentration [116]. Similarly, nano-priming TiO2 enhanced germination rate and seedling vigour under salt stress in Zea mays L [117]. The findings could be promising strategy to enhance plant tolerance against abiotic stressors like salinity, highlighting their role in sustainable agriculture and crop resilience.
Carbon nanotubes (CNTs) have wide range of application due their unique properties which include high strength, flexibility, thermal conductivity and electrical conductivity. Moreover, CNT play role in nanotechnology in mitigating abiotic stress for example; multi-walled CNT effectively enhanced salt resistance in grape seeds, enhancing germination rate and seedling development [118]. In addition, the combined treatment of nano-silica and CNTs significantly enhanced salt tolerance in grape seeds and seedlings [119]. Similarly, foliar treatment with CNTs mitigates water stress effects on peanut plants, enhancing growth, productivity, physiological parameters, and nutrient contents [120]. Higher CNT concentrations (40 mg L−1) show greater efficacy in improving plant responses to drought stress, highlighting the potential of nanotechnology in enhancing crop resilience. The application of graphene (Nano-C) enhance alfalfa growth and stress tolerance at lower dose (5 g kg−1), while higher doses (10–20 g kg−1) lead to phytotoxic effects [121]. A synergistic coupling effect of Nano-C and pH within specific ranges optimizes plant physiological responses, highlighting the importance of precise Nano-C application for plant health and productivity. In another study, graphene oxide-glycine betaine NPs effectively alleviate salinity stress Ocimum basilicum enhancing agronomic trait, photosynthetic components, membrane integrity, and oxidant and non-oxidant accumulation [122]. This research highlights the potential of nanotechnology, as a promising approach for sustainable agriculture by mitigating salinity-induced damage and promoting plant growth under stress conditions. The above nanotechnological approach provides sustainable solutions for improving plant resilience and performance under challenging environmental conditions, highlighting its potential for agricultural resilience and economic yield enhancement. By enhancing plant tolerance to abiotic stresses, nanotechnology improves crop productivity and stability in saline-affected and drought-prone areas. This increases the availability of food resources and supports sustainable agriculture, addressing urgent food security challenges posed by climate change and soil degradation. Table 2 is showing effect of different nanoparticles impact on some plant species with their actions.
Table 2.
Effect of different nanoparticles impact on some plant species with their actions.
| Plant species | Common name | Family | Nanoparticle | Size | Concentration | Action | References |
|---|---|---|---|---|---|---|---|
| Abelmoschus esculentus L. | Okra | Malvaceae | Zinc oxide (ZnO) | 16–35 nm | 10 mgL−1 | Enhancement of the contents of the photosynthetic pigments, activity of both SOD and CAT, lowered accumulation of proline and total soluble sugar | [123] |
| Arundinaria pygmaea | Pygmy Bamboo | Poaceae | Silicon dioxide (SiO2) | 20 nm | 100 μM | Increased protective enzymes, chlorophyll content and fluorescence, as well as plant biomass and shoot length | [124] |
| Capsicum annuum L. | Cayenne Pepper | Solanaceae | Manganese (Mn) | – | 0.1, 0.5, 1.0 mgL−1 | Controlled salinity-modulated molecular responses | [125] |
| Dracocephalum moldavica | Moldavian dragonhead | Lamiaceae | Titanium oxide (TiO2) | 20–30 nm | 0, 50, 100 and 200 mgL−1 | Improved agronomic traits and increased antioxidant enzyme activity, increased essential oil content under 100 mg L−1 TiO2 | [126] |
| Glycine max | Soybean | Fabaceae | Silver (Ag) | 15 nm | 5 ppm | Enhancement of root length/weight and hypocotyl length/weight of soybean | [127] |
| Hordeum vulgare | Barley | Poaceae | Silicon (Si) | – | 125, 250 mgL−1 | Modified the plant morpho -physiological and antioxidative attributes and synthesis of specific metabolites | [128] |
| Lycopersicum esculentum | Tomato | Solanaceae | Silicon dioxide (SiO2) | – | 1–2 mM | Increased root growth, weight, seed germination | [129] |
| Mangifera indica | Mango | Anacardiaceae | Zinc oxide (ZnO) and Silicon (Si) | nZnO <100 nm Si = 5–15 nm |
ZnO (50, 100, and 150 mgL−1) Si (150 and 300 mgL−1) |
Improved resistance mechanism and annual productivity | [130] |
| Musa acuminata | Cavendish Banana | Musaceae | Silicon (Si) | – | 0, 200, 400 and 600 mgL−1 | Mitigated oxidative stress of in vitro derived plant | [131] |
| Ocimum basilicum L. | Basil | Lamiaceae | Titanium oxide (TiO2) | – | – | Modulated toxic effects, improved biomass accumulation and RWC | [132] |
| Oryza sativa L. | Rice | Poaceae | Zinc oxide (ZnO) | 30 nm | 50 mgL−1 | Regulated the antioxidative system and chilling response transcription factors | [133] |
| Solanum melongena L. | Eggplant | Solanaceae | Zinc oxide (ZnO) | – | 50 and 100 ppm | Improved growth characteristics and increased fruit yield | [134] |
| Trigonella foenum-graecum | Fenugreek | Fabaceae | Zinc oxide (ZnO) | 10–30 nm | 0, 1000, and 3000 ppm | Upregulation of protein and proline levels, enhancement of the antioxidant's activities, reduction in H2O2 and MDA levels | [135] |
| Triticum aestivum L. | Bread Wheat | Poaceae | Titanium oxide (TiO2) | – | 500, 1000, and 2000 mg kg−1 | Improved growth, antioxidant system and photosynthetic performance | [136] |
| Zea mays L. | Corn | Poaceae | Copper (Cu) | 30–40 nm | 3.33, 4.44 and 5.55 mgL−1 | Higher biomass grain yield | [137] |
| Olea Europaea | Olive | Oleaceae | Silicon (Si) | – | 0, 150, 200 mgL−1 | improvement of the mechanical resistance, growth, and productivity | [138] |
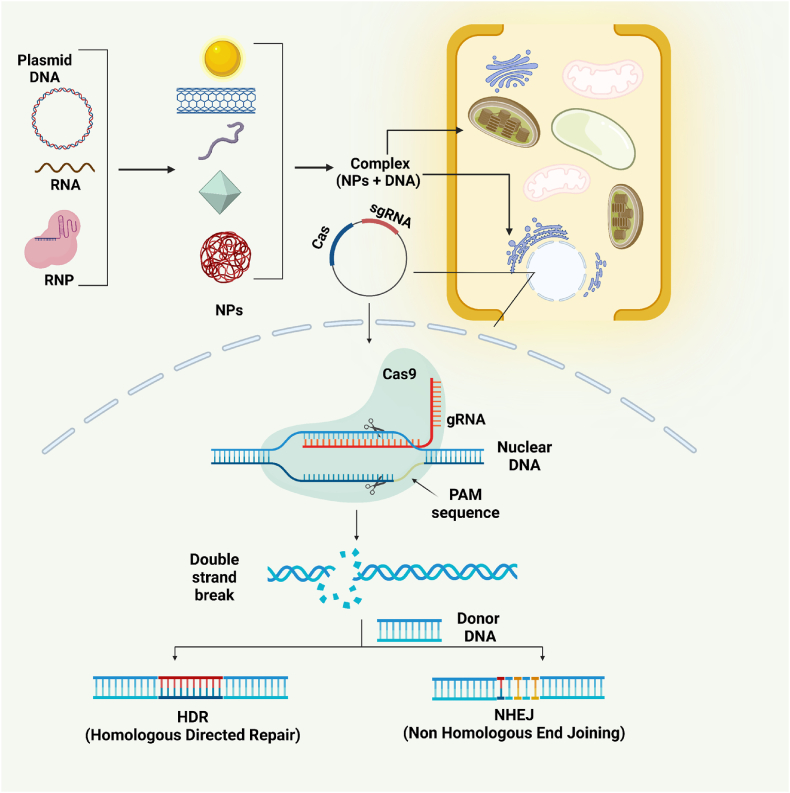
3. Revolutionizing agriculture: nanosensors at the forefront
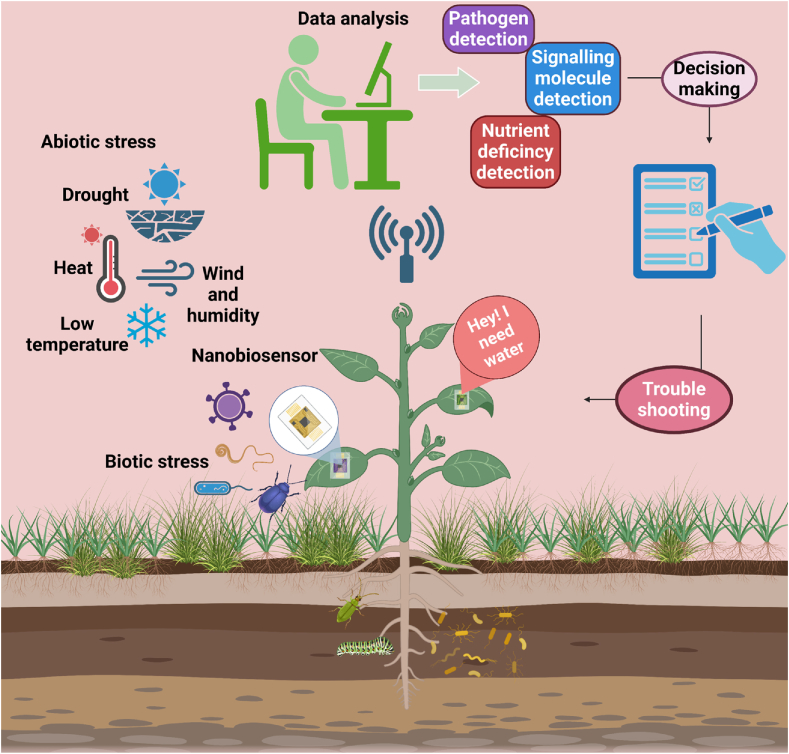
In traditional farming practices, the use of chemically synthesised fertilizers has paved the way for several problems, such as soil and water pollution, drought, salinity, pest resistance in various crops, and more [139,140]. The extensive and challenging task of determining pollutants, heavy metals, and hazardous substances derived from domestic and industrial waste sources, as well as monitoring soil and other dynamic invigorating structures, necessitates rapid, long-lasting, and productive detection methods [141]. Plant nanosensors can facilitate interaction between plants and farmers while also providing protection and sustainability to the plants [142]. Nanosensors are cost effective and real time powerful sensing devices with a dimension of ≤100 nm [143], and have the capacity to offer widespread sensing possibilities for monitoring the health of crops when they are under environmental stress [8]. A nanobiosensor is a device that merges nanotechnology with biological sensing elements to detect specific biological or chemical molecules at the molecular level. It incorporates a variety of nanomaterials, such as NPs, nanowires, carbon nanotubes (CNT), quantum dots etc., to enhance their sensing abilities [8,144]. Nanosensors in agriculture, showcasing their versatility in detecting and monitoring various targets such as pathogens, pesticides, fertilizers, soil conditions, and environmental factors like temperature and humidity. The rapid, accurate, and real-time data provided by nanosensors offer significant advancements in precision farming, enabling timely interventions and improved crop management practices [145]. Nano-biosensors are employed for the spontaneous evaluation of nutrient balance, stress levels, and plant health conditions, along with global navigation satellite systems and global positioning systems [146]. By incorporating nanotechnology with biological sensing elements, nanobiosensors enhance the detection of specific molecules at the molecular level, improving the accuracy and efficiency of crop management. This real-time data enables farmers to make informed decisions, ensuring optimal nutrient balance, stress management, and overall plant health. The use of nanosensors in agriculture not only boosts productivity and sustainability but also increases resilience against biotic and abiotic stresses, marking a significant advancement in precision farming and driving the Agri-tech revolution forward. The importance of integrating nanotechnology into agricultural systems for enhanced sustainability, productivity, and resilience against biotic and abiotic stresses has emphasized in the following sections. Fig. 2 depicts the role of nanosensor in stress management.
Fig. 2.
Role of Nano sensor in stress management.
3.1. Nano-enabled sensing solutions for mitigating biotic and abiotic stress in agriculture
Nano-enabled sensing systems show great potential in reducing both living organism-related and non-living factor-related pressures in agriculture. These cutting-edge technologies, utilizing sensors based on nanomaterials, provide accurate and swift identification of plant diseases, pests, nutritional deficiencies, and environmental factors including drought and salinity [144,147]. Nanosensors enhance plant resilience by detecting and responding to stress conditions through intricate molecular and cellular mechanisms. These sensors, often composed of nanoparticles, carbon nanotubes, or quantum dots, are designed to interact with specific plant metabolites, hormones, or signaling molecules that are indicative of stress conditions such as drought, salinity, or pathogen attack [144]. When a plant experiences stress, it produces ROS, stress-related hormones like ABA, and other biochemical markers [139]. Nanosensors embedded in plant tissues can detect these changes due to their high sensitivity and specificity. For instance, upon detecting elevated ROS levels or changes in ABA concentration, nanosensors can trigger a response by releasing encapsulated nutrients, antioxidants, or protective agents directly to the stressed cells. This targeted delivery helps mitigate the adverse effects of stress by stabilizing cell membranes, maintaining osmotic balance, and activating stress-responsive genes. Additionally, nanosensors can provide real-time data on plant health, allowing for timely interventions to enhance crop resilience and productivity. By understanding and leveraging these mechanistic interactions, nanotechnology offers a powerful tool for improving plant stress tolerance and ensuring sustainable agricultural practices.
Nano-enabled sensors offer farmers the ability to access up-to-date information on the health of their plants, the condition of the soil, and the presence of pests. This enables farmers to make well-informed decisions, efficiently utilize resources, and perform precise treatments. This approach has potential to fundamentally transform agricultural methods, bolster crop resilience, minimize yield losses, and make substantial contributions to global food security by ensuring sustainable and efficient agricultural production in the midst of progressively intricate problems. The extensive use of pesticides, insecticides, and fungicides to manage plant biological stresses poses significant challenges to sustainable agricultural productivity and global food security. While these chemicals aim to reduce yield, loss caused by pests and diseases, their overuse can lead to negative environmental consequences and the development of resistance in pests and pathogens [148]. Therefore, there is a critical need for innovative, efficient, and eco-friendly technologies to manage biological stresses in agriculture. Nanotechnology offers promising solutions through the development of nanomaterial-based engineered sensors for precision agriculture. These sensors enable swift recognition and monitoring of plant health under various environmental and pathogen-related stresses. Researchers have demonstrated the effectiveness of nanomaterial-based sensors in detecting stress signaling molecules, causal organisms of plant diseases, and soil-borne pathogens responsible for crop losses. Examples include sensors for detecting H2O2 stress signaling, bacterial and fungal pathogens, viruses, and nematodes. Moreover, the health of rhizosphere soil, including different gases, trace heavy metals, micronutrients, and compounds, is identified using these nano sensors [149]. Furthermore, the high sensitivity and minimum detectable threshold of nano sensors make them competent for use in smart agriculture applications [150]. A range of nano sensors is necessary, which are designed to aim at distinct entities such as weeds, crops, pest organisms, fungi and viruses [151]. For example; a robust toolkit for dynamic monitoring of H2O2 in the cytosol and mitochondria of Arabidopsis using the fusion protein roGFP2-Orp1 was developed [152]. This give a deeper understanding of intracellular H2O2 dynamics and its regulatory role in plant redox signaling, paving the way for dissecting complex redox mechanisms and NADPH oxidase-mediated ROS signaling in plant. HeAptDNA-SWCNT sensors were implemented to detect H2O2 stress signalling molecules, enabling the monitoring of plant health when exposed to both environmental and pathogen-induced stresses [153]. Moreover, effective methods of monitoring signalling molecules in non-model plant species are also been developed for example; ratiometric quantum dot sensor for glucose [154]. Hemin-complexed DNA aptamer-coated single-walled carbon nanotubes (SWCNT) for H2O2 [153], AT15-coated carbon nanotubes for NO and nanoneedle transistor-based sensors for Ca2+ [155].
Moreover, a lateral flow immunoassay nano biosensor platform comprised of GNPs can detect the soil-borne pathogen Ralstonia solanacearum in potato plants [156]. Furthermore, a "lateral flow biosensor" (LFBS) utilizing GNP technology has been implemented for identifying Phytophthora inoculum, which triggers blight disease in tomato and potato plants. Quantum dot-based biosensors have been implemented for the identification of Citrus tristeza virus and Graphene virus A [157]. The researchers employed Au-NPs to detect the soil pathogen Ralstonia solanacearum, known to cause bacterial wilt in potatoes [158]. An interdigitated microelectrode array biosensor coupled with Ag detects Escherichia coli, with a recognition threshold of 1 CFU mL−1 [159]. Similarly, gas sensors (GSs) utilizing micro-electro-mechanical systems (MEMs) to detect the insect sex pheromone (C17H34O2) of stink bugs (Euschistus heros) at concentrations ranging from 0.005 to 0.03 μg mL⁻1 [160]. Impedimetric biosensors containing gold NPs can detect viruses, including citrus tristeza virus (CTV), in real-time with a detectable level ranging between 0.1 and 10 μM [161]. For the detection of nematodes, phytoplasmas, and viroids, the researchers also developed nano-tuned biosensors [162]. Leveraging nano sensors in remote sensing is known for its extremely effective methods which include detecting, predicting and monitoring insect pests and plant diseases in a wide range of fruit orchards and crops [163]. In pest control activities, insect pheromones and semio-chemicals are commonly used for pest detection and monitoring. They also play an important role in selecting suitable circumstances for pesticide application programmes [164]. An efficient and highly precise nano biosensor was fabricated based on SERS implementing ssDNA and SWCNT conjugation to detect the presence of Hg2+ [165]. To detect Pb2, an optical nano biosensor was created using DNAzyme graphene QDs and AuNPs [166]. Applying in situ synthesised fluorescent MOFs in plants, a study was aimed to determine and report the level of acetone detected in the environment by monitoring changes in fluorescence following contact with these volatile substances. These observations facilitate real-time monitoring and handling of plant problems. These findings demonstrate the wide-ranging capabilities of biosensor technologies, from detecting plant pathogens and viruses to monitoring bacterial contaminants and insect pheromones, all of which are critical for agricultural sustainability and environmental monitoring.
NPs with genetically programmed nanoscales are being used as the latest approach for building smart biosensors. This approach employs genetically encoded sensors capable of providing real-time information on any transformations in the level or activity of the targeted biomolecules of interest [167]. Applying genetically encoded nanoscale to screen stress-responsive biomolecules is an entirely novel approach to designing smart biosensors. These sensors are capable of recording real-time shifts in the level or activity of the biomolecules of demand [168]. Moreover, the evolution of genetically encoded biosensors, particularly calcium indicators, has greatly enhanced our understanding of plant physiology and development by enabling real-time monitoring of key molecules [169]. This study highlights the importance of biosensors as invaluable tools for unravelling the intricacies of plant molecular dynamics, paving the way for innovative strategies in agricultural and environmental sciences. Plant nanosensors play crucial role in monitoring abiotic stressors by providing real time, sensitive and specific detection of key environmental parameters. For example, signals from oxygen and nitric oxide participate in response to both biotic and abiotic stress [155]. Identification of nitric oxide has been made possible by a fluorescent ratio metric sensor utilizing carbon nanotube. These sensors hold the potential to respond to the complexity present in the environment [168]. Visual imaging incorporates the Fluorescence Resonance Energy Transfer (FRET) technique, which makes use of fluorescent markers such as GCamp and YC3.6, along with genetically encoded variants, which allow quick and highly precise transient calcium molecules [169]. Moreover, two different sensors have been discovered for the rapid detection of auxins such as 1-naphthalene acetic acid (NAA) and 2,4-dichloro phenoxy acetic acid (2,4-D) [170]. Under biotic and abiotic stress conditions, volatile compounds such as extracellular adenosine triphosphate (ATP), glutathione, salicylic acid, and phytoalexin levels in Hordeum vulgare, Triticum aestivum L, and Nasturtium officinale can be detected using optical sensors. These aspects play a key role in the emergence of plant diseases.
Nanobiosensors are highly responsive and prudent and are capable of identifying the existence of herbicides if a specific biomolecule interacts with the target herbicide [171]. Nanobiosensors can be used to determine the antibacterial activity of different species of plants. Therefore, ethanol serves as a solvent in the fabrication of several biosensors and to investigate the characteristics of sixteen different plants. Using E. coli RFM 443, two bacterial strains (E. coli) were transformed and later tested for antibacterial activity. Luminescence biosensors are frequently employed to determine the antibacterial activity of all plants [172]. Potentiostate biosensors with field-effect transistors and choline esterase can be used to detect glycol alkaloids. Cadmium eradication from water has been examined and reported employing alumina silica NPs and anatase nanomaterials as water-purifying agents [173]. Nanotechnology innovation additionally established a more advanced method of treating wastewater to remove the presence of heavy metals [174]. Furthermore, TiO2-embedded ultrafiltration membranes facilitate rapid water splitting over the membrane surface, promoting pollutant deterioration via photocatalytic processes [175]. By enabling precise and real-time monitoring of plant health, stress conditions, and contaminants, these advanced technologies help optimize crop yields, reduce losses, and ensure safer food production, thereby addressing global food security challenges. The use of nanosensors and genetically encoded biosensors provides unprecedented sensitivity and specificity in detecting key biomolecules and environmental parameters. This allows for early intervention and precise management of plant health, leading to improved crop resilience, reduced dependency on harmful chemicals, and sustainable agricultural practices.
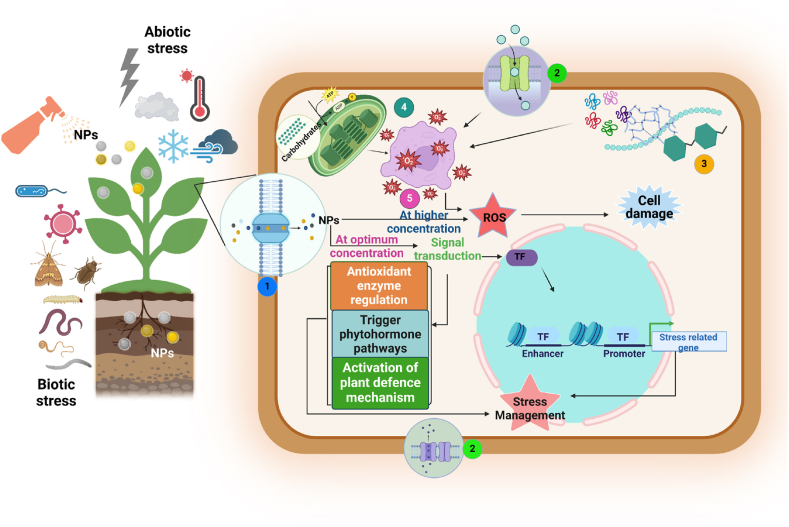
4. Mechanism underlying nano-particle based plant protection
In the field of disease management, NPs have become highly effective tool, providing innovative strategies to counteract phytopathogens and lessen the severe consequences of plant diseases [176]. They can act as strong antibacterial agents by preventing pathogen development, multiplication, and ultimately infection initiation; additionally, they can modulate plant immunity by controlling important defence-related processes, which enhances resistance against pathogens [177]. NPs possess antimicrobial properties due to their minute size and huge surface area, that enable them to engage with microorganisms and impede their viability and capacity to function. They do so by rupturing microbial cell membranes and organelles, impairing cellular functions, and persuading oxidative stress that eventually end up in the disintegration and death of microbial cells, therefore halting the development and spread of disease [63]. Beside this they also impede the key physiological processes in pathogens thereby impairing their nutritional uptake, cell multiplication and enzymatic activities that ultimately reduces pathogen population and disease onset (Fig. 3). For example; Ag NPs disturbs the permeability of plasma membrane of F. oxysporum spores and penetrate through it, to impair respiratory system of cell that can lead to fungal death [178]. Iron and copper nanocomposites distort cellular structure and impede metabolic pathways of Xanthomonas oryzae pv. oryzae to prevent their invasion in the plant cell. Beside this these NPs also form a protective layer around leaves to prevent the invasion of microbes into plant [179]. MgO prevent the proliferation of Phytophthora nicotianae and Tielaviopsis basicola by inhibiting their spore germination and multiplication. Besides, NPs (Cu, Mn) impair the structural integrity of biomolecules (nucleic acid, proteins) to hamper pathogen growth. Carbon NMs were reported to cause spore plasmolysis of F. graminearum fungus by restricting water uptake through clinging on cell membranes of spores [180].
Fig. 3.
Mechanism of interaction of nanomaterials in mitigating plant stress. 1. NPs uptake in plant cell. 2. Membrane leakage. 3. Degradation of macromolecules. 4. Loss of photosystem.
NPs can obstruct the pathogen's ability to adhere to host tissues, grow chemotropically or invasively, or build infection structures, thereby hampering pathogen colonization and disease induction to host plant [181]. They achieve this by altering the surface characteristics (physical barriers), fleeing off pathogens [182], and hindering biofilm formation [183]. In order to survive adverse environments and the host immune system, bacteria synthesize biofilms [184]. Li et al. [185] reported the formation of biofilms as an important step for the establishment of chronic infection. Ag NPs, ZnO NPs, Fe2O3 NPs among others can impede the synthesis of biofilms by bacterial cell during plant infection to several diseases [94]. Zinc oxide and manganese oxide NPs prevent the formation of biofilms by devastating rice bacterial pathogens Xanthomonas oryzae pv. oryzae, and Acidovorax oryzae, thereby preventing the onset of infection on plant surface [94]. Similarly, chitosan coated iron and magnesium nanocomposites also inhibit the formation of biofilm by these two pathogens in rice by enriching antimicrobial ability of microbes via triggering influx of nano magnesium and iron into their cells [182]. Hossain et al. [39], reported that TiO2 inhibited biofilm formation by Dickeya dadantii in Ipomoea batatas. Beside this, Ag NPs were reported to reduces the formation of aflatoxin by the Aspergillus flavus thus reducing its toxicity on the Oryza sativa plant, causing improved rice plant growth [69].
Furthermore, plant surfaces are modified by NPs of copper, sulfur, and manganese, which reduces the ability of pathogens to bind to and colonize them [186]. NPs bolster the physical barriers that function as the primary barrier against the attachment, invasion and colonization of pathogens on plant surface such as root surfaces, leaf surfaces, cuticles, cell walls, and trichomes. Manganese-NPs modify the root surface of watermelon by forming a nanotextured layer to restrict the invasion of Fusarium oxysporum f. sp. Niveum into plant cell [50]. Besides, NPs also enriches the structural integrity of cell walls and cuticles in plants to restrict the entry of pathogen [187]. According to Siddiqui et al. [80] to stop pathogen invasion, silica particles got embedded on the cell's cuticle layer. Likewise, silica NPs alter the structure of cell wall in Arabidopsis thaliana to restrict the invasion of Pseudomonas syringae pv. tomato thereby preventing spread and severity of disease [188]. NPs can disrupt cell wall, preventing pathogenic growth by leaking essential nutrients and nuclear substances, leading to bacterial death and impaired biological functions. Besides, cell membranes function as barriers and offer a stable environment for all of the important biological functions that occur inside cell and NPs were reported to disrupt the cellular membrane [62] (Fig. 3). In addition, NPS alter the structure of trichomes that play an essential role in fleeing off pathogens [63]. Trichomes of soybean and watermelon have been reported to be well maintained by Cu and silica NPs [63]. NPs also trigger the immune system of plants to initiate defence responses to defend against pathogens [175]. Along with being a ground-breaking strategy, NPs causes plants to develop systemic acquired resistance (SAR)- an extensive and robust shield, which protects them from diverse pathogens [189]. SAR induction by NPs in plants can be due to multiple reasons one being is its interaction and activation of other signalling pathways [190]. Salicylic acid (SA) and jasmonic acid (JA) level and signalling pathways were reported to get modified by NPs so as to activate immune responses in plants to resist the pathogens attack [167]. Studies related to the possible crosstalk between NPs and phtohormones to resist pathogen attack is untraversed, however fewer studies have reported their synergetic action [191]. Cu NPs were reported to resist bakanae disease caused by Gibberella fujikuroi in Oryza sativa through regulating salicylic acid and jasmonic acid interceded defence pathway [30]. Likewise, silica-NPs inhibited the occurrence of bacterial disease caused by Pseudomonas syringae in Arabidopsis thaliana by inducing systemic and local resistance through salicylic acid responsive genes mediated defence pathway. NPs also play an important role in the regulation of oxidative stress by the plants. Plants' oxidative stress markers include malondialdehyde (MDA) and hydrogen peroxide (H2O2), and their capacity to detoxify them in response to stress is shown by the amount of both enzymatic and non-enzymatic antioxidants. According to Ma et al. [192] hydroxyapatite NP supplementation impeded the development of Fusarium disease in tomato by enriching Peroxidase (POD) activity. Therefore, inhibiting pathogenic growth by enhanced ROS production with the use of NPs can be efficiently used for pathogen management in plants.
Using NPs in plant protection is their ability to effectively inhibit the growth and spread of pathogens through multiple mechanisms, such as disrupting microbial cell membranes, inducing oxidative stress, impairing pathogen metabolism, and forming physical barriers. Additionally, NPs can trigger plant immune responses, enhance antioxidant activity, and stimulate the production of antimicrobial compounds, providing a comprehensive defense against various plant diseases. These findings are crucial for addressing the problem of food security, as they offer a sustainable and efficient alternative to conventional chemical pesticides, reducing crop losses due to diseases and increasing agricultural productivity. By improving crop resilience and yield, NPs can help ensure a stable food supply for a growing global population.
However, challenges remain that need to be addressed for the widespread application of NPs in agriculture. These include understanding the long-term environmental and health impacts of NPs, optimizing their formulations for specific crops and pathogens, and ensuring cost-effective and scalable production methods. Additionally, regulatory frameworks must be established to ensure the safe use of NPs in agricultural practices. Further research is needed to refine the interaction mechanisms between NPs and plant hormones, minimize potential adverse effects, and achieve precise control over desired responses to ensure the safety and efficacy of NPs in crop protection.
5. Nanoparticles toxicity to the environment and the crops
While nanotechnology holds significant promise for advancing agricultural practices, it is crucial to address the potential environmental and health risks associated with nanoparticles [193]. Because of their small size and special characteristics, NPs can reach the environment through a variety of channels, including soil absorption, atmospheric precipitation, and human activity [193]. Their environmental accumulation is influenced by factors like soil pH, ionic content, and organic matter, which affect NPs transformation and dissolution. These particles can inhibit essential microbial populations, particularly in wastewater treatment plants, and pose risks to aquatic and terrestrial ecosystems. Toxicological studies reveal that NPs can impact plant health adversely by inducing oxidative stress, altering enzyme activity, and modifying gene expression. Unfortunately, there are worries over the potentially dangerous characteristics of synthetic NMs due to their widespread manufacture and introduction into natural systems. Nanotoxicology is a fast-growing subject that looks into the possible effects of NMs on living things. Numerous variables, including the dispersion medium, surface features, size and concentration of NMs, plant species, and kind of cell lines, influence the effect and the toxicity mechanisms. An array of plant-based experimental systems has been used up to this point to evaluate the effects of NMs [194]. For example; It should be taken into consideration to use metrics for NMs' detection, measurement, and characterization. Examples of critical metrics for characterizing nanoparticles are nano tracking analysis (NTA) and transmission electron microscopy (TEM) for environmental sample acquisition. Furthermore, for the mass to size ratio in a suspension, field flow fractionation with inductively coupled plasma mass spectrometry (FFF-ICP-MS) is used [195]. Utilizing metrics based on biological dose-response is a crucial aspect of assessing engineered nanomaterials (ENMs) and their possible toxicity in the environment. Several barriers stand in the way of accurately characterizing nanomedicines (NMs). These include limited concentrations of aqueous exposure, high background levels of naturally occurring colloidal components, and inadequate techniques for isolating NMs from the matrix without artifacts. In additions; a variety of techniques are used in in vivo toxicological investigations to determine the effects of nanomaterials on living things in order to determine their nanotoxicity [196]. In order to monitor any potential negative consequences, these investigations frequently include giving nanoparticles to model creatures like fish, insects, or rats. Acute and chronic toxicity testing are important techniques that evaluate both short- and long-term exposure to nanoparticles in order to determine the health consequences that occur right away and over time. These studies evaluate a range of endpoints, such as behavioural modifications, growth rates, and survival. Furthermore, histological analyses are carried out to detect cellular and tissue injury, and biochemical tests assess changes in metabolic and enzymatic processes. Organ-specific toxicity is also evaluated by means of focused investigations on important organs such the kidneys, liver, and lungs [197].
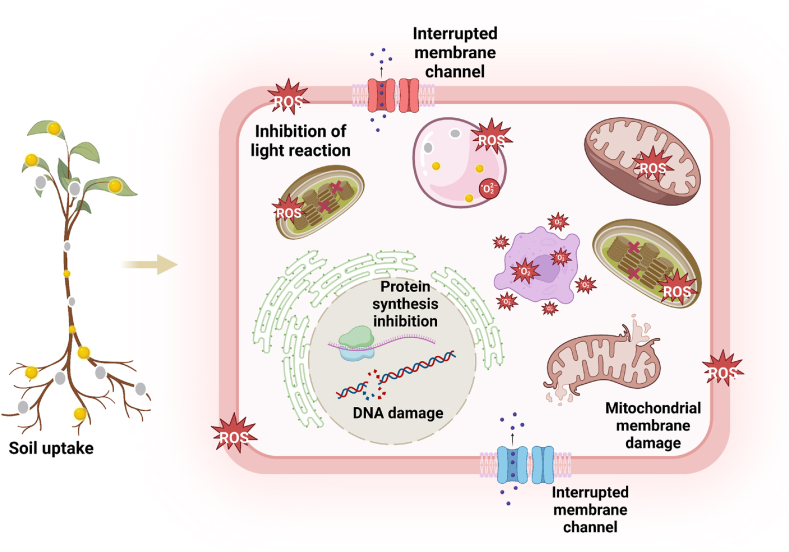
Additionally, bioaccumulation in aquatic organisms can expose higher organisms, including humans, to NPs through the food chain [197]. NPs can be absorbed by plant roots and transported to aerial parts, accumulating in cellular structures, which can lead to a cascade of harmful events, such as cell membrane destabilization, changes in the osmotic balance of the cells, enzymes deactivation and production of ROS which consequently, changed metabolism and cause damage to the target cells (Fig. 4). The rate of bioaccumulation varies based on NPs properties and environmental factors, such as soil organic matter, which can reduce uptake. Commonly bioaccumulated nanoparticles, like copper oxide and cerium oxide, can impair photosynthesis, transpiration, and overall plant health. Their introduction into the food chain poses potential health risks to humans due to their persistence and accumulation in organs.
Fig. 4.
Uptake of NPs and possible toxicological effect.
Microbial flora of soil plays an essential role in defining the quality of soil as it causes organic matter decomposition, help in the uptake of water and nutrient and nitrogen fixation in legumes and cereals. Hence these rhizospheric microbes enhance growth, productivity and make the plant more resistant to disease [198]. Therefore, any kind of harmful effect produced by NPS to this ecological service is a matter of growing concern [198]. It is well known that major NPs released in the environment go to the soils [199]. As per the report the engineered inorganic NPs are more harmful to soil microbes than organic NPs. In addition, Ag NPs are present in over 100 pesticides [199] and its poisonous effect on the ecosystem can't be ignored. A phototoxic and genotoxic effects of citrate coated Ag NPs were reported previously. This might be due to the transition of spherical silver into powder of silver oxides or ions. In Brassica sp that AgNPs are more toxic than AgNO3 and decrease the efficiency of photosystem II, reduce assimilation of CO2, change in stomatal conductance and produce numerous ROS [200]. In other hand the phototoxicity of Ag NPs can be reduced by coating them with polyvinyl pyrrole. Hence, the biocompatible coating can enhance the possibility of the utilization of Ag NPs in the agricultural field. AgNPs cause phototoxic and genotoxic effects, disrupt microbial communities, and impair plant growth and photosynthetic efficiency by generating ROS and altering physiological processes. These findings highlight the significant ecological risks posed by AgNPs compared to organic NPs.
The effect of ZnO NPs on the growth of Solanum melongena seedling in ex vitro as well as in vitro conditions was examined [201]. During in vitro germination seeds were cultured on a medium augmented with 5, 10,15, and 20 mg L−1 ZnO NPs while in greenhouse conditions 0, 5, 10, 15, 20, and 100 mg ZnO NPs kg− 1 were added in cocopeat used for seeds sowing. After analysing the data, it was determined that ZnO NPs produce a phytotoxic effect at higher concentrations during tissue culture. Furthermore, ZnO NPs also produce a negative effect on eggplant growing in greenhouse conditions. Scanning electron microscope (SEM) proved the presence of ZnONPs in various parts of in vitro, as well as ex vitro, grew eggplants seedlings.
While NPs offer significant potential for enhancing agricultural practices, their environmental and health risks must be carefully managed. NPs can enter the environment through various pathways and accumulate in soil and water, potentially disrupting microbial populations, aquatic and terrestrial ecosystems, and plant health. Bioaccumulation in the food chain poses additional risks to human health. Specific nanoparticles, such as silver, zinc oxide, and copper oxide, have been shown to have phytotoxic and genotoxic effects, impairing plant growth and photosynthesis, and disrupting soil microbial communities. These challenges underscore the need for rigorous assessment and regulation of NPs to mitigate their adverse impacts. Developing a comprehensive framework for monitoring, assessing, and governing NP use, alongside advancements in technology and interdisciplinary research, is crucial to ensuring that the benefits of nanotechnology are realized while minimizing environmental and health risks. Responsible application and regulation will be key to leveraging NPs effectively in agriculture, safeguarding ecosystem health and public safety.
6. CRISPR-Cas and nanotechnology synergy: solutions for agricultural challenges
In addition to their critical role in food security, plants are pivotal in the synthesis of biomaterials, biofuels, and medicines. Enhancing plant capabilities beyond their natural limits is a viable strategy to meet the growing global demands sustainably. Historically, crop breeding marked the first significant step in plant improvement, followed by the Green Revolution, which introduced high-yielding semi-dwarf wheat and rice cultivars [202]. Traditional breeding techniques, on the other hand, are labour-intensive, time-consuming, genetically non-targeted, and prone to unintentionally introducing undesired traits. As a result, their capacity to introduce unique traits that are absent from the crossed species is limited. Genetic engineering techniques, such as particle bombardment and Agrobacterium tumefaciens transformation, have significantly broadened the range of traits that can be introduced and enhanced in plants compared to traditional breeding methods. However, random gene insertion, genetic instability, limited species range and unintended phenotypic effects are some potential drawback of these genetic engineering methods. Modern nuclease-based genome-editing techniques, like CRISPR (clustered regulatory interspaced short palindromic repeats)-Cas and TALEN (transcription activator-like effector nucleases), are quick, accurate, genetically targeted, and capable of introducing novel traits into particular location of the genome [203]. Nanotechnology and CRISPR-Cas technology complement each other significantly in advancing plant biotechnology. NPs serve as efficient carriers for CRISPR-Cas components, ensuring their stable and efficient delivery into plant cells, addressing a major challenge of delivering large molecular complexes into plant tissues [204] (Fig. 5). For example, DNA nanostructures have been successfully used to deliver CRISPR-Cas9 components into Arabidopsis thaliana, achieving high efficiency and low off-target effects. Nanomaterials also enable the delivery of CRISPR components into a wide range of plant species, including those resistant to traditional genetic transformation methods. Gold nanoparticles have demonstrated successful gene editing in wheat cells, a traditionally difficult crop to transform [205]. In tomatoes, recent studies have shown that the CRISPR/Cas9 system has been used to enhance fruit quality, disease resistance, and shelf life. For instance, CRISPR/Cas9-induced gene replacement via homology-directed repair has generated tomato lines with extended shelf life [206]. Furthermore, optimizing nanomaterial properties enhances the efficiency of homology-directed repair (HDR), critical for precise genome editing, by improving the uptake and stability of donor DNA templates. High-throughput screening of single-guide RNAs (sgRNAs) and alternative nucleases is also facilitated by nanotechnology, accelerating the identification of the most effective CRISPR components [204]. Specific successful applications include the use of carbon nanotubes for delivering CRISPR-Cas9 components into rice cells, leading to targeted mutations for disease resistance, silica nanoparticles in tomatoes to enhance fruit quality and shelf life, and magnetic nanoparticles in maize to overcome delivery challenges in monocot plants, demonstrating the transformative potential of nanotechnology in genetic improvements of major crops [205].
Fig. 5.
Nanoparticle mediated delivery of CRISPR–Cas reagents or CRISPR–Cas–sgRNA ribonucleoprotein (RNP) into plant cell [202,205]. Cas endonuclease for site specific cleavage of double strand DNA and single guide RNAs (sgRNAs) that hybridizes with target sequences. PAM (protospacer adjacent motif), a few nucleotide long CRISPR RNA binding region in the genome, required for Cas9 recognition of the target sequence. One of two plant DNA repair pathways is activated upon the creation of a double-stranded break (DSB). Point mutations or gene replacement result from homology-directed repair (HDR), which uses a DNA donor template that is similar to the target region. Much more often than HDR, NHEJ, which is prone to errors and produces little insertions or deletions (indels), is demonstrated. Transfection of protoplasts or particle bombardment are two ways that RNPs can enter a plant cell. As an alternative, plasmids with the genes encoding Cas and the sgRNA can be transfected into cells using protoplasts, particles, or Agrobacterium-mediated transformation.
CRISPR-Cas technology holds immense promise in plant biology and agriculture, especially for improving crop resilience and incorporating new traits [206]. However, there are significant challenges that hinder its widespread application. These include difficulties in delivering CRISPR components, limitations in plant tissue culture techniques, and methods that are specific to certain species. Furthermore, regulatory issues and societal acceptance create additional barriers to the adoption of CRISPR-engineered plants. To fully harness the potential of CRISPR in agriculture, ongoing research and innovation are crucial to overcoming these obstacles. As per the reports, different nanomaterials have been used for the delivery of agrochemicals as discussed above. Moreover, DNA and proteins have been inserted into plant cells via nanomaterials for genetic engineering purposes [203,[207], [208], [209], [210]]. The integration of CRISPR-Cas genetic engineering with nanotechnology represents a transformative approach to overcoming significant barriers in plant biotechnology. By enhancing cargo delivery, species independence, germline transformation, and gene editing efficiency, nanomaterials have the potential to significantly improve the applicability and success of CRISPR-mediated genome editing in plants [211,212]. Nanotechnology shows promise in addressing key challenges of CRISPR-Cas technology in plants. It enables efficient delivery of CRISPR components, potentially overcoming delivery limitations and expanding the range of plant species for genome editing. Nanomaterials also offer opportunities to improve homology-directed repair (HDR) efficiency and provide a platform for high-throughput screening of sgRNAs and alternative nucleases. The optimization of nanomaterial chemistries and delivery mechanisms presents a promising pathway to unlock the full potential of CRISPR-Cas technology in plant genetic engineering, paving the way for sustainable agricultural innovations. However, further optimization and research are needed to fully exploit nanotechnology's potential in plant genetic engineering. Plant genome editing using CRISPR-Cas has proven successful in a number of plant species, however some challenges are with adapting the technology. For example; optimization of cargo size; delivery to cell organelles and evaluating how well they work with tissue culture protocols. Moreover, globally varied and complex regulations around genetically modified crops have an effect on market access and innovation.
To sum up, the amalgamation of nanotechnology with CRISPR-Cas genetic engineering signifies a noteworthy progression in the field of plant biotechnology, providing resolutions to several constraints associated with conventional breeding and genetic engineering techniques. This synergistic strategy can increase crop resilience and productivity, facilitate the introduction of novel traits, and improve the efficiency and precision of gene editing. Key issues including enhanced homology-directed repair, species independence, and effective distribution of CRISPR components can all be addressed by nanomaterials. However, more work needs to be done to optimize the chemistries of nanomaterials, delivery systems, and tissue culture procedures before these technologies can be successfully applied. Furthermore, thorough safety evaluations and efficient regulatory frameworks are necessary to guarantee the moral and secure application of nanotechnology in agriculture. In order to overcome current obstacles and fully utilize CRISPR-Cas and nanotechnology to advance sustainable agriculture practices, improve global food security, and support environmental sustainability, more research and innovation in this area are necessary.
7. Negative impacts of nanotechnology in agriculture
The release of nanomaterials into the environment raises concerns about long-term ecological impact because nanoparticles can accumulate in soil and water, disrupting microbial communities, affecting soil health, and entering the food chain, posing risks to human and animal health. Agricultural workers also face health risks from direct exposure to nanoparticles during their production, handling, and application, underscoring the need for strict safety protocols. A details discussion of nanotoxicity has been carried out in heading 5.0. Furthermore, the rapid advancement of nanotechnology frequently outpaces regulatory frameworks, creating gaps in guidelines for safe usage, quality control, and environmental protection [5]. Due to ethical concerns about unequal access that favor major agribusinesses over small-scale farmers, this regulatory uncertainty may prevent promising nanotechnologies from being widely adopted and may create hurdles to market entrance [213]. Small and medium-sized farms may find it difficult to afford the initial investment necessary for nanotechnology research, development, and deployment, which could result in differences in benefits and access. In addition, a careful assessment of the cost-effectiveness of nano-enabled solutions is necessary to guarantee that farmers will see a real return on their investment. Although they provide more effective nutrient delivery and need less fertilizer, nanofertilizers give rise to worries about the buildup of nanoparticles and their long-term effects on soil health [214].
Nanopesticides are more effective at controlling certain pests in smaller amounts, however they come with serious environmental hazards and have an effect on creatures that are not their intended targets [215]. Through real-time monitoring and resource optimization, nanosensors make precision agriculture possible. However, they come with a high cost and demand a strong digital infrastructure, which raises privacy concerns regarding data. While controlled agrochemical release nanocarriers increase efficacy and decrease application frequency, they also run the danger of releasing toxic compounds or breaking down into hazardous by-products. A responsible application of nanotechnology in agriculture requires a well-balanced strategy that includes strict safety evaluations, strong legal protections, and fair access plans. To fully utilize these cutting-edge technologies while minimizing their negative effects, scientists, politicians, and stakeholders must engage in ongoing research and communication [214].
8. Regulatory and ethical consideration
To achieve a safe and responsible application, the use of nanotechnology in agriculture requires thorough consideration of ethical and regulatory considerations. The guidelines that are now in place for the applications of nanotechnology in agriculture are dispersed and fluctuate greatly between geographical areas. The FDA (Food and Drug Administration, USA), EPA (Environmental Protection Agency, USA), and regulatory authorities in the EU (European Union) are just a few of the agencies whose safety guidelines largely govern the use of nanotechnology in agriculture (https://www.fda.gov/science-research/nanotechnology-programs-fda/fdas-approach-regulation-nanotechnology-products; https://www.epa.gov/sciencematters/advancing-epas-understanding-next-generation-pesticides; https://euon.echa.europa.eu/bg/nanodata/sectors/agriculture/overview/regulation).
Under its statutory authority, the FDA monitors products utilizing nanotechnology, evaluating their efficacy and safety in light of the special qualities of nanomaterials. In the meantime, to guarantee that pesticide products fulfill safety requirements for both the environment and human health, the EPA mandates that all pesticide products including those incorporating nanomaterials; go through extensive safety reviews prior to registration.
Due to the gaps in the regulatory framework caused by this lack of standardization, there are possible dangers related to the use of NPs, including environmental contamination, unintentional impacts on non-target creatures, and concerns about human health [216]. This absence of a cohesive regulatory approach leaves consumers and producers vulnerable to the unidentified risks of novel agricultural technologies. Standardized laws and quality control procedures that can offer precise and uniform rules for the creation, testing, and application of nanotechnology in agriculture are desperately needed to address these issues. This entails creating safety evaluation procedures, keeping an eye on long-term environmental effects, and guaranteeing transparency in the application of nanomaterials. Regular monitoring and assessment of nano-enabled products is necessary to support these practices. The focus should be on the products' composition and potential effects at every stage of their lifespan, from production to consumption [217]. Strict quality control procedures can reduce hazards and improve the effectiveness of agricultural nanotechnology applications. Additionally, ethical issues are crucial, especially when it comes to guaranteeing fair access to these cutting-edge technologies and defending the rights and welfare of regional communities and smallholder farmers. We can exploit the benefits of nanotechnology in agriculture while limiting hazards and increasing public trust in these creative solutions by creating a strong regulatory framework and addressing ethical concerns.
Incorporating regulatory and ethical considerations into the use of nanotechnology in agriculture requires a holistic approach that intersects with dimensions such as scale, finance, technology, institutions, governance, and skills [218]. Regulatory frameworks must be scaled to address the diverse contexts in which nanotechnology is applied, ensuring that guidelines are adaptable and comprehensive for both large-scale and smallholder farming. Financial mechanisms are essential for supporting the development and implementation of these regulations, as well as for funding research into safety and efficacy. Technology advancement necessitates ongoing updates to regulatory standards to keep pace with innovations, while institutions and governance structures must be equipped to enforce these standards and address emerging ethical concerns. Skill development is critical, as educating stakeholders on regulatory compliance and ethical practices ensures that technologies are used responsibly and safely. By integrating these dimensions, a robust regulatory framework can be established that not only supports the advancement of nanotechnology but also addresses the challenges and ethical considerations inherent in its application, fostering sustainable and equitable growth in agriculture.
8.1. Conclusion and future prospects
Meeting the increasing food demands and overcoming challenges posed by climate change necessitates the adoption of innovative agricultural techniques, with a particular emphasis on leveraging nanotechnology. This review highlights nanotechnology's role in promoting sustainable agriculture and revolutionizing the agri-tech sector, focusing on enhancing food security and managing plant stress through precision agriculture and genetic engineering like CRISPR-Cas technology. The review aims to contribute significantly to human food security by advocating for sustainable practices and leveraging nanotechnology's potential for a more resilient food supply. In order to increase plant resistance to biotic and abiotic factors, nanotechnology has produced a number of useful tools. These tools include nanofertilizers, which increase plant growth and resilience by improving nutrient delivery and uptake efficiency; nanopesticides, which provide targeted pest control with minimal environmental impact, reducing chemical residues and resistance issues; and nanosensors, which allow for precise monitoring of plant health and soil conditions and real-time adjustments to optimize plant stress management. Nanopesticides and nanosensors are particularly effective for plant resistance and sustainable agriculture among these technologies. Nanopesticides are a better option for environmentally friendly pest management because of their exceptional efficacy in targeting certain pests while having a minimal impact on the environment. Because of their ability to monitor in real time and make decisions based on data, nanosensors greatly improve the accuracy and efficiency of resource management, which improves crop resilience and management.
Integrating nanotechnology with CRISPR-Cas genome editing presents both significant challenges and benefits for global food security. One major challenge is ensuring the safe and efficient delivery of CRISPR reagents to target cells and organelles in plants without causing unintended off-target effects or environmental harm. This synergy of nanotechnology and genetic engineering stands to revolutionize agriculture, creating robust, productive crops and significantly contributing to global food security amidst climate change and growing population demands. However, the benefits are substantial for example this integration could enable precise and rapid genetic modifications to enhance crop resistance to pests, diseases, and abiotic stresses like drought and salinity, thus increasing yield and resilience. Future prospects include developing more sophisticated nanoscale delivery systems to improve the specificity and efficiency of gene editing, as well as comprehensive regulatory frameworks to address safety concerns. This combined technological approach has the potential to revolutionize agriculture by creating more robust and productive crops, thereby contributing to global food security in an era of climate change and growing population demands.
Despite advances in combining biochemical, physiological, and genetic approaches, our understanding of plant responses to environmental stresses remains incomplete, particularly in early signaling and stress sensing. The major challenge humanity can face by integrating nanotechnology into agriculture is managing and mitigating the potential toxicological effects of NPs on the environment and human health. While nanotechnology offers significant benefits in enhancing agricultural sustainability and food security, the introduction of NPs into ecosystems raises concerns about their long-term environmental impact, bioaccumulation, and potential toxicity to plants, animals, and humans. Ensuring that the benefits outweigh the risks requires comprehensive, multidisciplinary research to understand the interactions between NPs and biological systems, establish safe usage guidelines, and develop robust regulatory frameworks to monitor and control nanoparticle application in agriculture.
Based on the review's findings, future proposals should focus on creating sophisticated nanoscale delivery systems that guarantee the safe and precise application of nanomaterials in plants, reducing the risk of off-target effects and environmental damage. To address safety issues and create standards for the responsible use of nanotechnology in agriculture, regulatory structures must be strengthened. Encouraging interdisciplinary research will improve our knowledge of how nanoparticles interact with biological systems, which is essential for creating applications that are secure and efficient. Furthermore, combining genetic engineering methods like CRISPR-Cas with nanotechnology will result in resilient and fruitful crops that can tolerate both biotic and abiotic challenges, greatly enhancing global food security in the face of population growth and climate change.
Funding
This work was financially supported by The National Natural Science Foundation for Scholars of China (31870595) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The authors are also grateful for Metasequoia Faculty Research Start-up Funding (163100036 and 163100028) at the Bamboo Research Institute, Nanjing Forestry University.
CRediT authorship contribution statement
Zishan Ahmad: Writing – review & editing, Writing – original draft, Conceptualization. Shareen Niyazi: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Assima Firdoos: Writing – review & editing. Chunye Wang: Formal analysis. Muhammad Aamir Manzoor: Writing – review & editing, Visualization. Muthusamy Ramakrishnan: Writing – review & editing. Anamica Upadhyay: Writing – review & editing. Yulong Ding: Writing – review & editing, Project administration, Funding acquisition, Conceptualization.
Data availability statement
The data associated with our study has not been deposited anywhere.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All the figures were created using https://www.biorender.com/, accessed on 3 March 2024.
References
- 1.Willett W., Rockström J., Loken B., Springmann M., Lang T., Vermeulen S., Garnett T., et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–492. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- 2.The National Academies Press. Science Breakthroughs to Advance Food and Agricultural Research by 2023 (2018). The National Academies Press.
- 3.Gomiero T. Soil degradation, land scarcity and food security: reviewing a complex challenge. Sustainability. 2016;3:281. [Google Scholar]
- 4.U. S. Department of Agriculture Press Release . 2023. Press release No. 0086.23.https://www.usda.gov/media/press-releases [Google Scholar]
- 5.Ahmad Z., Tahseen S., Wasi A., Ganie I.B., Shahzad A., Emamverdian A., Ramakrishnan M., Ding Y. Nanotechnological interventions in agriculture. Nanomaterials. 2022;12:2667. doi: 10.3390/nano12152667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pramanik B., Sar P., Bharti R., Gupta R.K., Purkayastha S., Sinha S., Chattaraj S., Mitra D. Multifactorial role of nanoparticles in alleviating environmental stresses for sustainable crop production and protection. Plant Physiol. Biochem. 2023;201 doi: 10.1016/j.plaphy.2023.107831. [DOI] [PubMed] [Google Scholar]
- 7.Usman M., Farooq M., Wakeel A., Nawaz A., Cheema S.A., Rehman H.U., Ashraf I., Sanaullah M. Nanotechnology in agriculture: current status, challenges and future opportunities. Sci. Total Environ. 2020;15(721) doi: 10.1016/j.scitotenv.2020.137778. Epub 2020 Mar 6. PMID: 32179352. [DOI] [PubMed] [Google Scholar]
- 8.Zain M., Ma H., Nuruzzaman M., Chaudhary S., Nadeem M., Shakoor N., Azeem I., Aiwang D., Sun C., Ahamad T. Nanotechnology based precision agriculture for alleviating biotic and abiotic stress in plants. Plant Stress. 2023 [Google Scholar]
- 9.Resh, V. H.; & Cardé, R. T. (Eds.). Encyclopedia of Insects. Academic press2009.
- 10.Rai M., Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- 11.Tahir H.M., Basheer T., Ali S., Yaqoob R., Naseem S., Khan S.Y. Effect of pesticides on biological control potential of neoscona theisi (araneae: araneidae). J ins. Sci. 2019;2:17. doi: 10.1093/jisesa/iez024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazarika A., Yadav M., Yadav D.K., Yadav H.S. An overview of the role of nanoparticles in sustainable agriculture. Agric. Biotechnol. 2022;43 [Google Scholar]
- 13.Pradhan S., Roy I., Lodh G., Patra P., Choudhury S.R., Samanta A., Goswami A. Entomotoxicity and biosafety assessment of PEGylated acephate nanoparticles: a biologically safe alternative to neurotoxic pesticides. J Env. Sci. Health. Part B. 2013;7:559–569. doi: 10.1080/03601234.2013.774891. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S., Shiny R.S., Anjali P.J., Jerobin C.H., et al. Distinctive effects of nano-sized permethrin in the environment. Environ. Sci. Pollut. Res. 2013;20:2593–2602. doi: 10.1007/s11356-012-1161-0. [DOI] [PubMed] [Google Scholar]
- 15.Gamal M.M.Z. Conference Proceedings. Vol. 6. 2018. Nano-Particles: A Recent Approach for Controlling Stored Grain Insect Pests; pp. 88–94. No. 5. [Google Scholar]
- 16.Hashem A.S., Awadalla S.S., Zayed G.M., Maggi F., Benelli G. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum—insecticidal activity and mode of action. Environ. Sci. Pollut. Control Ser. 2018;25:18802–18812. doi: 10.1007/s11356-018-2068-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu F., Wen L.-X., Li Z.-Z., Yu W., Sun H.-Y., Chen J.-F. Porous hollow silica nanoparticles as controlled delivery system for water-soluble pesticide. Mater. Res. Bull. 2006;12:2268–2275. [Google Scholar]
- 18.Guan Y.F., Pearce R.C., Melechko A.V., Hensley D.K., Simpson M.L., Rack P.D. Pulsed laser dewetting of nickel catalyst for carbon nanofiber growth. Nanotechnology. 2008;19(23):235604. doi: 10.1088/0957-4484/19/23/235604. Nanotechnology. [DOI] [PubMed] [Google Scholar]
- 19.Ouda S.M. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014;1:34. [Google Scholar]
- 20.Narayanan K.B., Park H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014;140:185–192. [Google Scholar]
- 21.Olchowik J., Bzdyk R.M., Studnicki M., Bederska-Błaszczyk M., Urban A., Aleksandrowicz-Trzcińska M. The effect of silver and copper nanoparticles on the condition of English oak (Quercus robur L.) seedlings in a container nursery experiment. Forests. 2017;8(9):310. [Google Scholar]
- 22.Khan M., Khan A.U., Hasan M.A., Yadav K.K., Pinto M.M., Malik N., Yadav V.K., Khan A.H., Islam S., Sharma G.K. Agro-nanotechnology as an emerging field: a novel sustainable approach for improving plant growth by reducing biotic stress. Appl. Sci. 2021;5:2282. [Google Scholar]
- 23.Abdelmalek G.A.M., Salaheldin T.A. Silver nanoparticles as a potent fungicide for citrus phytopathogenic fungi. J. Nano Res. 2016;3(5) [Google Scholar]
- 24.Malandrakis A.A., Kavroulakis N., Chrysikopoulos C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. TotalEnviron. 2019;670:292–299. doi: 10.1016/j.scitotenv.2019.03.210. [DOI] [PubMed] [Google Scholar]
- 25.Divya K., Vijayan S., George T.K., Jisha M.S. Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers Polym. 2017;2:221–230. doi: 10.1007/s12221-017-6690-1. [DOI] [Google Scholar]
- 26.Xing K., Shen X., Zhu X., Ju X., Miao X., Tian J., Feng Z., Peng X., Jiang J., Qin S. Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int. J. Biol. Macromol. 2016;82:830–836. doi: 10.1016/j.ijbiomac.2015.09.074. [DOI] [PubMed] [Google Scholar]
- 27.Sharma N., Jandaik S., Kumar S., Chitkara M., Sandhu I. S. Synthesis. Characterisation and antimicrobial activity of manganese- and iron-doped zinc oxide nanoparticles. J. Exp. Nanosci. 2016;1:54–71. doi: 10.1080/17458080.2015.1025302. [DOI] [Google Scholar]
- 28.Derbalah A., Shenashen M., Hamza A., Mohamed A., El Safty S. Antifungal activity of fabricated mesoporous silica nanoparticles against early blight of tomato. EJBAS. 2018;2:145–150. doi: 10.1002/ps.4420. [DOI] [PubMed] [Google Scholar]
- 29.Hamza A., El-Mogazy S., Derbalah A. Fenton reagent and titanium dioxide nanoparticles as antifungal agents to control leaf spot of sugar beet under field conditions. J. Plant Protect. Res. 2016;56:270–278. [Google Scholar]
- 30.Shang H., Ma C., Li C., White J.C., Polubesova T., Chefetz B., Xing B. Copper sulfide nanoparticles suppress Gibberella fujikuroi infection in rice (Oryza sativa L.) by multiple mechanisms: contact-mortality, nutritional modulation and phytohormone regulation. Environ. Sci.: Nano. 2020;9:2632–2643. [Google Scholar]
- 31.Akpinar I., Sar T., Unal M. International Workshop Plant Health: Challenges and Solutions. 2017. Antifungal effects of silicon dioxide nanoparticles (SiO2 NPs) against various plant pathogenic fungi. [Google Scholar]
- 32.Kang H., Elmer W., Shen Y., Zuverza-Mena N., Ma C., Botella P.…Haynes C.L. Silica nanoparticle dissolution rate controls the suppression of fusarium wilt of watermelon (Citrullus lanatus) Environ. Sci. Tech. 2021;20:13513–13522. doi: 10.1021/acs.est.0c07126. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Serrano C., Guzmán-Moreno J., Ángeles-Chávez C., Rodríguez-González V., Ortega-Sigala J.J., Ramírez-Santoyo R.M., Vidales-Rodríguez L.E. Biosynthesis of silver nanoparticles by Fusarium scirpi and its potential as antimicrobial agent against uropathogenic Escherichia coli biofilms. PLoS One. 2020;3 doi: 10.1371/journal.pone.0230275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohanta Y.K., Panda S.K., Bastia A.K., Mohanta T.K. Biosynthesis of silver nanoparticles from Protium serratum and investigation of their potential impacts on food safety and control. Front. Micrbiol. 2017;8 doi: 10.3389/fmicb.2017.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahryari F., Rabiei Z., Sadighian S. Antibacterial activity of synthesized silver nanoparticles by sumac aqueous extract and silver-chitosan nanocomposite against Pseudomonas syringae pv. Syringae. J. Plant Pathol. 2020;2:469–475. doi: 10.1007/s42161-019-00478-1. [DOI] [Google Scholar]
- 36.Dang H., Fawcett D., Poinern G.E.J. Green synthesis of gold nanoparticles from waste macadamia nut shells and their antimicrobial activity against Escherichia coli and Staphylococcus epidermis. IJMRS. 2019;4 [Google Scholar]
- 37.Imada K., Sakai S., Kajihara H., Tanaka S., Ito S. Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol. 2016;4:551–560. doi: 10.1111/ppa.12443. [DOI] [Google Scholar]
- 38.Boxi S.S., Mukherjee K., Paria S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology. 2016;8 doi: 10.1088/0957-4484/27/8/085103. [DOI] [PubMed] [Google Scholar]
- 39.Hossain A., Abdallah Y., Ali M.A., Masum M.M.I., Li B., Sun G., Meng Y., Wang Y., An Q. Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen Dickeya dadantii. Biomolecules. 2019;12:863. doi: 10.3390/biom9120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mamede M.C., Mota R.P., Silva A.C.A., Tebaldi N.D. Nanoparticles in inhibiting pantoea ananatis and to control maize white spot. Ciência Rural. 2021;52 [Google Scholar]
- 41.Li Y., Liu Y., Yang D., Jin Q., Wu C., Cui J. Multifunctional molybdenum disulfide-copper nanocomposite that enhances the antibacterial activity, promotes rice growth and induces rice resistance. J. Hzard. Mater. 2020;394 doi: 10.1016/j.jhazmat.2020.122551. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H., Lv L., Ahmed T., Jin S., Shahid M., Noman M., Osman H.-E.H., Wang Y., Sun G., Li X. Effect of the nanoparticle exposures on the tomato bacterial wilt disease control by modulating the rhizosphere bacterial community. Int. J. Mol. Sci. 2021;1:414. doi: 10.3390/ijms23010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sofy A.R., Sofy M.R., Hmed A.A., Dawoud R.A., Alnaggar A.E.-A.M., Soliman A.M., El-Dougdoug N.K. Ameliorating the adverse effects of tomato mosaic tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules. 2021;5:1337. doi: 10.3390/molecules26051337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan M.R., Siddiqui Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta Vulgaris L.) Sci. Hortic. 2020;265 [Google Scholar]
- 45.Pereira A.E., Grillo R., Mello N.F., Rosa A.H., Fraceto L.F. Application of poly (epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hzard. Mater. 2014;268:207–215. doi: 10.1016/j.jhazmat.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Grillo R., Pereira A.E., Nishisaka C.S., De Lima R., Oehlke K., Greiner R., Fraceto L.F. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: an environmentally safer alternative for weed control. J. Hzard. Mater. 2014;278:163–171. doi: 10.1016/j.jhazmat.2014.05.079. [DOI] [PubMed] [Google Scholar]
- 47.Bombo A.B., Pereira A.E.S., Lusa M.G., De Medeiros Oliveira E., De Oliveira J.L., Campos E.V.R., De Jesus M.B., Oliveira H.C., Fraceto L.F., Mayer J.L.S. A mechanistic view of interactions of a nanoherbicide with target organism. J. Agric. Food Chem. 2019;16:4453–4462. doi: 10.1021/acs.jafc.9b00806. [DOI] [PubMed] [Google Scholar]
- 48.Sousa G.F., Gomes D.G., Campos E.V., Oliveira J.L., Fraceto L.F., Stolf-Moreira R., Oliveira H.C. Post-emergence herbicidal activity of nanoatrazine against susceptible weeds. Front. Environ. Sci. 2018;6:12. [Google Scholar]
- 49.Shen Y., Borgatta J., Ma C., Elmer W., Hamers R.J., White J.C. Copper nanomaterial morphology and composition control foliar transfer through the cuticle and mediate resistance to root fungal disease in tomato (Solanum lycopersicum) J. Agric. Food Chem. 2020;68(41):11327–11338. doi: 10.1021/acs.jafc.0c04546. [DOI] [PubMed] [Google Scholar]
- 50.Noman M., Ahmed T., Ijaz U., Shahid M., Nazir M.M., Azizullah, Song F. Bio‐functionalized manganese nanoparticles suppress Fusarium wilt in watermelon (Citrullus lanatus L.) by infection disruption, host defense response potentiation, and soil microbial community modulation. Small. 2023;2 doi: 10.1002/smll.202205687. [DOI] [PubMed] [Google Scholar]
- 51.Iliger K.S., Sofi T.A., Bhat N.A., Ahanger F.A., Sekhar J.C., Elhendi A.Z., Al-Huqail A.A., Khan F. Copper nanoparticles: green synthesis and managing fruit rot disease of chilli caused by colletotrichum capsici. Saudi J. Biol. Sci. 2021;2:1477–1486. doi: 10.1016/j.sjbs.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cumplido-Nájera C.F., González-Morales S., Ortega-Ortíz H., Cadenas-Pliego G., Benavides-Mendoza A., Juárez-Maldonado A. The application of copper nanoparticles and potassium silicate stimulate the tolerance to clavibacter michiganensis in tomato plants. Sci. Hortic. 2019;245:82–89. [Google Scholar]
- 53.Le Van N., Ma C., Shang J., Rui Y., Liu S., Xing B. Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere. 2016;144:661–670. doi: 10.1016/j.chemosphere.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 54.Ayoub H.A., Khairy M., Elsaid S., Rashwan F.A., Abdel-Hafez H.F. Pesticidal activity of nanostructured metal oxides for generation of alternative pesticide formulations. J. Agric. Food Chem. 2018;22:5491–5498. doi: 10.1021/acs.jafc.8b01600. [DOI] [PubMed] [Google Scholar]
- 55.Tauseef A., Gupta J., Rehman A., Uddin I. Differential response of cowpea towards the CuO nanoparticles under Meloidogyne incognita stress. South Afr. J. Bot. 2021;139:175–182. [Google Scholar]
- 56.Elmer W.H., Zuverza-Mena N., Triplett L.R., Roberts E.L., Silady R.A., White J.C. Foliar application of copper oxide nanoparticles suppresses Fusarium wilt development on Chrysanthemum. Environ. Sci. Technol. 2021;15:10805–10810. doi: 10.1021/acs.est.1c02323. [DOI] [PubMed] [Google Scholar]
- 57.Badawy A.A., Abdelfattah N.A., Salem S.S., Awad M.F., Fouda A. Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology. 2021;3:233. doi: 10.3390/biology10030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai L., Cai L., Jia H., Liu C., Wang D., Sun X. Foliar exposure of Fe3O4 nanoparticles on nicotiana benthamiana: evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. J. Hzard. Mater. 2020;393:122415. doi: 10.1016/j.jhazmat.2020.122415. [DOI] [PubMed] [Google Scholar]
- 59.Alam T., Khan R.A.A., Ali A., Sher H., Ullah Z., Ali M. Biogenic synthesis of iron oxide nanoparticles via skimmia laureola and their antibacterial efficacy against bacterial wilt pathogen Ralstonia solanacearum. Mater. Sci. Eng. 2019;98:101–108. doi: 10.1016/j.msec.2018.12.117. [DOI] [PubMed] [Google Scholar]
- 60.Hao Y., Yuan W., Ma C., White J.C., Zhang Z., Adeel M., Zhou T., Rui Y., Xing B. Engineered nanomaterials suppress turnip mosaic virus infection in tobacco (Nicotiana benthamiana) Environ. Sci.: Nano. 2018;7:1685–1693. [Google Scholar]
- 61.De la Rosa-García S.C., Martínez-Torres P., Gómez-Cornelio S., Corral-Aguado M.A., Quintana P., Gómez-Ortíz N.M. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018;1 [Google Scholar]
- 62.Chen J., Wu L., Lu M., Lu S., Li Z., Ding W. Comparative study on the fungicidal activity of metallic MgO nanoparticles and macroscale MgO against soilborne fungal phytopathogens. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noman M., Ahmed T., White J.C., Nazir M.M., Azizullah, Li D., Song F. Bacillus altitudinis-stabilized multifarious copper nanoparticles prevent bacterial fruit blotch in watermelon (Citrullus lanatus L.): direct pathogen inhibition, in planta particles accumulation, and host stomatal immunity modulation. Small. 2023;19(15):2207136. doi: 10.1002/smll.202207136. [DOI] [PubMed] [Google Scholar]
- 64.Elmer W., De La Torre-Roche R., Pagano L., Majumdar S., Zuverza-Mena N., Dimkpa C., Gardea-Torresdey J., White J.C. Effect of metalloid and metal oxide nanoparticles on Fusarium wilt of watermelon. Plant Dis. 2018;7:1394–1401. doi: 10.1094/PDIS-10-17-1621-RE. [DOI] [PubMed] [Google Scholar]
- 65.Ghazy N.A., Abd El-Hafez O.A., El-Bakery A.M., El-Geddawy D.I.H. Impact of silver nanoparticles and two biological treatments to control soft rot disease in sugar beet (Beta vulgaris L) Egypt J. Biol. Pest Control. 2021;1:3. doi: 10.1186/s41938-020-00347-5. [DOI] [Google Scholar]
- 66.Ahmed T., Shahid M., Noman M., Niazi M.B.K., Mahmood F., Manzoor I., Zhang Y., Li B., Yang Y., Yan C. Silver nanoparticles synthesized by using Bacillus cereus SZT1 ameliorated the damage of bacterial leaf blight pathogen in rice. Pathogens. 2020;3:160. doi: 10.3390/pathogens9030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masum M.M.I., Siddiqa M.M., Ali K.A., Zhang Y., Abdallah Y., Ibrahim E., Qiu W., Yan C., Li B. Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial Brown stripe. Front. Microbiol. 2019;10:820. doi: 10.3389/fmicb.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumari M., Pandey S., Bhattacharya A., Mishra A., Nautiyal C.S. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol. Biochem. 2017;121:216–225. doi: 10.1016/j.plaphy.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Ejaz M., Raja N.I., Mashwani Z.-U.-R., Ahmad M.S., Hussain M., Iqbal M. Effect of silver nanoparticles and silver nitrate on growth of rice under biotic stress. IET nanobiotech. 2018;7:927–932. doi: 10.1049/iet-nbt.2018.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaur P., Thakur R., Duhan J.S., Chaudhury A. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicer Arietinum) JCTB. 2018;11:3233–3243. [Google Scholar]
- 71.Kumari M., Pandey S., Mishra S.K., Giri V.P., Agarwal L., Dwivedi S., Pandey A.K., Nautiyal C.S., Mishra A. Omics-based mechanistic insight into the role of bioengineered nanoparticles for biotic stress amelioration by modulating plant metabolic pathways. Front. Bioeng. Biotechnol. 2020;8:242. doi: 10.3389/fbioe.2020.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahawar H., Prasanna R., Gogoi R., Singh S.B., Chawla G., Kumar A. Synergistic effects of silver nanoparticles augmented calothrix elenkinii for enhanced biocontrol efficacy against Alternaria blight challenged tomato plants. 3 Biotech. 2020;10:1–10. doi: 10.1007/s13205-020-2074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Danish M., Altaf M., Robab M.I., Shahid M., Manoharadas S., Hussain S.A., Shaikh H. Green synthesized silver nanoparticles mitigate biotic stress induced by Meloidogyne incognita in trachyspermum Ammi (L.) by improving growth, biochemical, and antioxidant enzyme activities. ACS Omega. 2021;17:11389–11403. doi: 10.1021/acsomega.1c00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sultana T., Javed B., Raja N.I., Mashwani Z.-R. Silver nanoparticles Elicited physiological, biochemical, and antioxidant modifications in rice plants to control Aspergillus flavus. Green Process. Synth. 2021;1:314–324. [Google Scholar]
- 75.Shafie R.M., Salama A.M., Farroh K.Y. Silver nanoparticles activity against tomato spotted wilt virus. Middle East J. Agric. Res. 2018;7:1251–1267. [Google Scholar]
- 76.Noha K., Bondok A.M., El-Dougdoug K.A. Evaluation of silver nanoparticles as antiviral agent against ToMV and PVY in tomato plants. Sciences. 2018;1:100–111. [Google Scholar]
- 77.El-Shewy E.S. The efficacy of copper oxide, tri-calcium phosphate and silicon dioxide nanoparticles in controlling black scurf disease of potato. Ann. Agri. Sci. Moshtohor. 2019;1:129–138. [Google Scholar]
- 78.Hasan K.A., Soliman H., Baka Z., Shabana Y.M. Efficacy of nano-silicon in the control of chocolate spot disease of Vicia faba L. Caused by Botrytis Fabae. EJBAS. 2020;1:53–66. doi: 10.1080/2314808X.2020.1727627. [DOI] [Google Scholar]
- 79.El-Ashry R.M., El-Saadony M.T., El-Sobki A.E., El-Tahan A.M., Al-Otaibi S., El-Shehawi A.M., Saad A.M., Elshaer N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022;2:920–932. doi: 10.1016/j.sjbs.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siddiqui Z.A., Hashmi A., Khan M.R., Parveen A. Management of bacteria Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, and fungi Rhizoctonia solani, Fusarium solani and Alternaria dauci with silicon dioxide nanoparticles on carrot. Int. J. Veg. Sci. 2020;6:547–557. [Google Scholar]
- 81.Elamawi R.M., Tahoon A.M., Elsharnoby D.E., El-Shafey R.A. Bio-production of silica nanoparticles from rice husk and their impact on rice bakanae disease and grain yield. Arch. Phytopathol. Pflanzenschutz. 2020;9–10:459–478. doi: 10.1080/03235408.2020.1750824. [DOI] [Google Scholar]
- 82.El-Shetehy M., Moradi A., Maceroni M., Reinhardt D., Petri-Fink A., Rothen-Rutishauser B., Mauch F., Schwab F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021;3:344–353. doi: 10.1038/s41565-020-00812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nisaq G.J., Sudarsono S., Sukma D. vol. 694. IOP Publishing; 2021. Nano silica spray increase Phalaenopsis pulcherrima growth and resistance against Dickeya dadantii infection. (IOP Conference Series: Earth and Environmental Science). [Google Scholar]
- 84.Du J., Liu B., Zhao T., Xu X., Lin H., Ji Y., Li Y., Li Z., Lu C., Li P., Zhao H., Li Y., Yin Z., Ding X. Silica nanoparticles protect rice against biotic and abiotic stresses. J. Nanobiotechnol. 2022;1:197. doi: 10.1186/s12951-022-01420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Sawy M.M., Elsharkawy M.M., Abass J.M., Hagag E.S. Inhibition of tomato yellow leaf curl virus by zingiber off cinaleand mentha longifolia. JAA. 2017;1:1–6. [Google Scholar]
- 86.Satti S.H., Raja N.I., Ikram M., Oraby H.F., Mashwani Z.-U.-R., Mohamed A.H., Singh A., Omar A.A. Plant-based titanium dioxide nanoparticles trigger biochemical and proteome modifications in Triticum aestivum L. Under biotic stress of puccinia striiformis. Molecules. 2022;13:4274. doi: 10.3390/molecules27134274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Satti S.H., Raja N.I., Javed B., Akram A., Mashwani Z.-R., Ahmad M.S., Ikram M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris Sorokiniana. PLoS One. 2021;2 doi: 10.1371/journal.pone.0246880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elsharkawy M.M., Derbalah A. Antiviral activity of titanium dioxide nanostructures as a control strategy for broad bean strain virus in faba bean. Pest Manag. Sci. 2019;3:828–834. doi: 10.1002/ps.5185. [DOI] [PubMed] [Google Scholar]
- 89.Adisa I.O., Reddy Pullagurala V.L., Rawat S., Hernandez-Viezcas J.A., Dimkpa C.O., Elmer W.H., White J.C., Peralta-Videa J.R., Gardea-Torresdey J.L. Role of cerium compounds in Fusarium wilt suppression and growth enhancement in tomato (Solanum lycopersicum) J. Agric. Food Chem. 2018;24:5959–5970. doi: 10.1021/acs.jafc.8b01345. [DOI] [PubMed] [Google Scholar]
- 90.Chun S.-C., Chandrasekaran M. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Int. J. Biol. Macromol. 2019;125:948–954. doi: 10.1016/j.ijbiomac.2018.12.167. [DOI] [PubMed] [Google Scholar]
- 91.Elsharkawy M.M., Omara R.I., Mostafa Y.S., Alamri S.A., Hashem M., Alrumman S.A., Ahmad A.A. Mechanism of wheat leaf rust control using chitosan nanoparticles and salicylic acid. J. Fungi. 2022;3:304. doi: 10.3390/jof8030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abdelaziz A.M., Salem S.S., Khalil A.M.A., El-Wakil D.A., Fouda H.M., Hashem A.H. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. Biometals. 2022;3:601–616. doi: 10.1007/s10534-022-00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bouqellah N.A., El-Sayyad G.S., Attia M.S. Induction of tomato plant biochemical immune responses by the synthesized zinc oxide nanoparticles against wilt-induced Fusarium oxysporum. Int. Microbiol. 2023;2:435–448. doi: 10.1007/s10123-023-00404-7. [DOI] [PubMed] [Google Scholar]
- 94.Ogunyemi S.O., Zhang M., Abdallah Y., Ahmed T., Qiu W., Ali M.A., Yan C., Yang Y., Chen J., Li B. The bio-synthesis of three metal oxide nanoparticles (ZnO, MnO2, and MgO) and their antibacterial activity against the bacterial leaf blight pathogen. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.588326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malandrakis A.A., Kavroulakis N., Avramidou M., Papadopoulou K.K., Tsaniklidis G., Chrysikopoulos C.V. Metal nanoparticles: phytotoxicity on tomato and effect on symbiosis with the Fusarium solani FsK strain. Sci. Total Environ. 2021;787 doi: 10.1016/j.scitotenv.2021.147606. [DOI] [PubMed] [Google Scholar]
- 96.Cai L., Liu C., Fan G., Liu C., Sun X. Preventing viral disease by ZnONPs through directly deactivating TMV and activating plant immunity in Nicotiana benthamiana. Environ. Sci.: Nano. 2019;12:3653–3669. [Google Scholar]
- 97.Farhana, Munis M.F.H., Alamer K.H., Althobaiti A.T., Kamal A., Liaquat F., Haroon U., Ahmed J., Chaudhary H.J., Attia H. ZnO nanoparticle-mediated seed priming induces biochemical and antioxidant changes in chickpea to alleviate Fusarium wilt. J. Fungi. 2022;7:753. doi: 10.3390/jof8070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vijayamma R., Maria H.J., Thomas S., Shishatskaya E.I., Kiselev E.G., Nemtsev I.V., Sukhanova A.A., Volova T.G. A study of the properties and efficacy of microparticles based on P(3HB) and P(3HB/3HV) loaded with herbicides. J. App. Polym. Sci. 2022;10 doi: 10.1002/app.51756. [DOI] [Google Scholar]
- 99.Kamran M., Parveen A., Ahmar S., Malik Z., Hussain S., Chattha M.S., Saleem M.H., Adil M., Heidari P., Chen J.-T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Inter. J. Mol. Sci. 2019;1:148. doi: 10.3390/ijms21010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alharbi B.M., Elhakem A.H., Alnusairi G.S., Soliman M.H., Hakeem K.R., Hasan M.M., Abdelhamid M.T. Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ. 2021;4:208–220. [Google Scholar]
- 101.Evelin H., Devi T.S., Gupta S., Kapoor R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front. Plant Sci. 2019;10 doi: 10.3389/fpls.2019.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rastogi A., Tripathi D.K., Yadav S., Chauhan D.K., Živčák M., Ghorbanpour M., El-Sheery N.I., Brestic M. Application of silicon nanoparticles in agriculture. 3 Biotech. 2019;9:1–11. doi: 10.1007/s13205-019-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Semida W.M., Abdelkhalik A., Mohamed G.F., Abd El-Mageed T.A., Abd El-Mageed S.A., Rady M.M., Ali E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.) Plants. 2021;2:421. doi: 10.3390/plants10020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghani M.I., Saleem S., Rather S.A., Rehmani M.S., Alamri S., Rajput V.D., Kalaji H.M., Saleem N., Sial T.A., Liu M. Foliar application of zinc oxide nanoparticles: an effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere. 2022;289 doi: 10.1016/j.chemosphere.2021.133202. [DOI] [PubMed] [Google Scholar]
- 105.Adil M., Bashir S., Bashir S., Aslam Z., Ahmad N., Younas T., Asghar R.M.A., Alkahtani J., Dwiningsih Y., Elshikh M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.932861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abou-Zeid H., Ismail G. The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L to salt stress. Egypt. J. Bot. 2018;1:73–85. [Google Scholar]
- 107.Linh T.M., Mai N.C., Hoe P.T., Lien L.Q., Ban N.K., Hien L.T.T., Chau N.H., Van N.T. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomater. 2020;2020:1–13. [Google Scholar]
- 108.Zahedi S.M., Moharrami F., Sarikhani S., Padervand M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-74273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ashkavand P., Tabari M., Zarafshar M., Tomásková I., Struve D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leśne Prace Badawcze. 2015;76(4) [Google Scholar]
- 110.Rajput V.D., Minkina T., Feizi M., Kumari A., Khan M., Mandzhieva S., Sushkova S., El-Ramady H., Verma K.K., Singh A. Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology. 2021;8:791. doi: 10.3390/biology10080791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashour H.A., Mahmoud A.W.M. Response of Jatropha integrrima plants irrigated with different levels of saline water to nano silicon and Gypsum. J Agr. Stud. 2017;5:1–26. [Google Scholar]
- 112.Alam P., Arshad M., Al-Kheraif A.A., Azzam M.A., Al Balawi T. Silicon nanoparticle-induced regulation of carbohydrate metabolism, photosynthesis, and ROS homeostasis in Solanum lycopersicum subjected to salinity stress. ACS Omega. 2022;36:31834–31844. doi: 10.1021/acsomega.2c02586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El-Badri A.M., Batool M., Mohamed I.A., Khatab A., Sherif A., Wang Z., Salah A., Nishawy E., Ayaad M., Kuai J. Modulation of salinity impact on early seedling stage via nano-priming application of zinc oxide on rapeseed (Brassica Napus L.) Plant Physiol. Biochem. 2021;166:376–392. doi: 10.1016/j.plaphy.2021.05.040. [DOI] [PubMed] [Google Scholar]
- 114.Waqas Mazhar M., Ishtiaq M., Hussain I., Parveen A., Hayat Bhatti K., Azeem M., Thind S., Ajaib M., Maqbool M., Sardar T. Seed nano-priming with zinc oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS One. 2022;3 doi: 10.1371/journal.pone.0264967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Badri A.M., Batool M., Wang C., Hashem A.M., Tabl K.M., Nishawy E., Kuai J., Zhou G., Wang B. Selenium and zinc oxide nanoparticles modulate the molecular and Morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021;225:112695. doi: 10.1016/j.ecoenv.2021.112695. [DOI] [PubMed] [Google Scholar]
- 116.Gohari G., Mohammadi A., Akbari A., Panahirad S., Dadpour M.R., Fotopoulos V., Kimura S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of dracocephalum moldavica. Sci. Rep. 2020;1:912. doi: 10.1038/s41598-020-57794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shah T., Latif S., Saeed F., Ali I., Ullah S., Alsahli A.A., Jan S., Ahmad P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. Sci. 2021;1 [Google Scholar]
- 118.Li Y., Liu M., Yang X., Zhang Y., Hui H., Zhang D., Shu J. Multi-walled carbon nanotubes enhanced the antioxidative system and alleviated salt stress in grape seedlings. Sci. Hort. 2022;293 doi: 10.1016/j.scienta.2021.110698. [DOI] [Google Scholar]
- 119.Li Y., Sheng Y., Shu J., Hao S., Wang J., Huang Q., He K., Qi J., Liu J. Effects of nano-silica and multi-walled carbon nanotubes on grape seedlings under salt stress. Agronomy. 2024;14:622. doi: 10.3390/agronomy14030622. [DOI] [Google Scholar]
- 120.Bakry B.A., Sadak M.S., Al Ashkar N.M., Ibrahim O.M., Okla M.K., El-Tahan A.M. The role of carbon nanotubes in improving drought tolerance via upregulation of the physiological processes of peanut plants grown in sandy soils. Agronomy. 2024;3:611. [Google Scholar]
- 121.Chen Z., Wang Q. Graphene ameliorates saline-alkaline stress-induced damage and improves growth and tolerance in alfalfa (medicago sativa L.) Plant Physiol. Biochem. 2021;163:128–138. doi: 10.1016/j.plaphy.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 122.Ganjavi A.S., Oraei M., Gohari G., Akbari A., Faramarzi A. Glycine betaine functionalized graphene oxide as a new engineering nanoparticle lessens salt stress impacts in sweet basil (Ocimum basilicum L.) Plant Psysiol. Biochem. 2021;162:14–26. doi: 10.1016/j.plaphy.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 123.Alabdallah N.M., Alzahrani H.S. The potential mitigation effect of ZnO nanoparticles on (Abelmoschus esculentus L. Moench) metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020;27:3132–3137. doi: 10.1016/j.sjbs.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Emamverdian A., Ding Y., Mokhberdoran F., Ahmad Z., Xie Y. Determination of heavy metal tolerance threshold in a bamboo species (Arundinaria pygmaea) as treated with silicon dioxide nanoparticles. Glob.Ecol. Conserv. 2020;24(1306):1–14. [Google Scholar]
- 125.Ye Y., Cota-Ruiz K., Hernández-Viezcas J.A., Valdés C., Medina-Velo I.A., Turley R.S., Peralta-Videa J.R., Gardea-Torresdey J.L. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: a sustainable approach for agriculture. ACS Sustain Chem Eng. 2020;8:1427–1436. [Google Scholar]
- 126.Gohari G., Mohammadi A., Akbari A., Panahirad S., Dadpour M.R., Fotopoulos V., Kimura S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020;10:912. doi: 10.1038/s41598-020-57794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hashimoto T., Mustafa G., Nishiuchi T., Komatsu S. Comparative analysis of the effect of inorganic and organic chemicals with silver nanoparticles on soybean under flooding stress. Int. J. Mol. Sci. 2020;21(4) doi: 10.3390/ijms21041300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghorbanpour M., Mohammadi H., Kariman K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci.: Nano. 2020;7:443–461. [Google Scholar]
- 129.Haghighi M., Afifipour Z., Mozafarian M. The effect of N-Si on tomato seed germination under salinity levels. J. Biol. Environ. Sci. 2012;6:87–90. [Google Scholar]
- 130.Elsheery N.I., Helaly M.N., El-Hoseiny H.M., Alam-Eldein S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy. 2020;10:558. [Google Scholar]
- 131.Mahmoud L.M., Dutt M., Shalan A.M., El-Kady M.E., El-Boray M.S., Shabana Y., Grosser J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. South Afr. J. Bot. 2020;132:155–163. [Google Scholar]
- 132.Kiapour H., Moaveni P., Habibi D., Sani B. Evaluation of the application of gibbrellic acid and titanium dioxide nanoparticles under drought stress on some traits of basil (Ocimum basilicum L.) International Journal of Agronomy and Agricultural Research. 2015;6:138–150. [Google Scholar]
- 133.Song Y., Jiang M., Zhang H., Li R. Zinc oxide nanoparticles alleviate chilling stress in rice (Oryza sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules. 2021;26:2196. doi: 10.3390/molecules26082196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Semida W.M., Abdelkhalik A., Mohamed G.F., Abd El-Mageed T.A., Abd El-Mageed S.A., Rady M.M., Ali E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.) Plants. 2021;10:421. doi: 10.3390/plants10020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Noohpisheh Z., Amiri H., Mohammadi A., Farhadi S. Effect of the foliar application of zinc oxide nanoparticles on some biochemical and physiological parameters of Trigonella foenum-graecum under salinity stress. Plant Biosyst. International Journal of Plant Biology. 2021;155:267–280. [Google Scholar]
- 136.Faraji J., Sepehri A. Exogenous nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system and photosynthetic performance of wheat seedlings under drought stress. J Soil Sci. Plant Nut. 2020;20:703–714. [Google Scholar]
- 137.Van Nguyen D., Nguyen H.M., Le N.T., Nguyen K.H., Nguyen H.T., Le H.M., Nguyen A.T., Dinh N.T.T., Hoang S.A., Van Ha C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Reg. 2021;40:1–12. [Google Scholar]
- 138.Hassan I.F., Ajaj R., Gaballah M.S., Ogbaga C.C., Kalaji H.M.M.H. M., S. Foliar application of nano-silicon improves the physiological and biochemical characteristics of ‘Kalamata’ Olive subjected to deficit irrigation in a semi-Arid climate. Plants. 2021;11(12):1561. doi: 10.3390/plants11121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chung J., Sepunaru L., Plaxco K.W. On the disinfection of electrochemical aptamer-based sensors. ECS Sensors Plus. 2022;1 doi: 10.1149/2754-2726/ac60b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stefan-van Staden R.I. Perspective—challenges in biomedical analysis: from classical sensors to stochastic sensors. ECS Sensors Plus. 2022;1 [Google Scholar]
- 141.Shahbazi R., Salouti M., Amini B., Jalilvand A., Naderlou E., Amini A., Shams A. Highly selective and sensitive detection of Staphylococcus aureus with gold nanoparticle-based core-shell nano biosensor. Mol. Cell. Probes. 2018;41:8–13. doi: 10.1016/j.mcp.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 142.Khandelwal A., Joshi R., Mukherjee P., Singh S.D., Shrivastava M. Use of bio-based nanoparticles in agriculture. Int. J. Appl. Agric. Res. 2019:89–100. [Google Scholar]
- 143.Bhagat R., Ingle A.P., Chen H. Nanotechnology in Agriculture and Agroecosystems. Elsevier; 2023. Nanosensors and nanobiosensors for sustainable agriculture; pp. 93–112. [Google Scholar]
- 144.Mariyam S., Upadhyay S.K., Chakraborty K., Verma K.K., Duhan J.S., Muneer S., Meena M., Sharma R.K., Ghodake G., Seth C.S. Nanotechnology, a frontier in agricultural science, a novel approach in abiotic stress management and convergence with new age medicine-A review. Sci. Total Environ. 2023 doi: 10.1016/j.scitotenv.2023.169097. [DOI] [PubMed] [Google Scholar]
- 145.Gupta S., Seth C.S. Salicylic acid alleviates chromium (VI) toxicity by restricting its uptake, improving photosynthesis and augmenting antioxidant defense in Solanum lycopersicum L. Physiol. Mol. Biol. Plants. 2021;27:2651–2664. doi: 10.1007/s12298-021-01088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tshabalala Z.P., Mokoena T.P., Motaung D.E. Nanosensors for Smart Agriculture. Elsevier; 2022. Current commercial nanosensors and devices/products used in agriculture; pp. 165–181. [Google Scholar]
- 147.Dong B.R., Jiang R., Chen J.F., Xiao Y., Lv Z.Y., Chen W.S. Strategic nanoparticle-mediated plant disease resistance. Crit. Rev. Biotechnol. 2023;1:22–37. doi: 10.1080/07388551.2021.2007842. [DOI] [PubMed] [Google Scholar]
- 148.Ijaz M., Khan F., Ahmed T., Noman M., Zulfiqar F., Rizwan M., Chen J., Siddique K.H., Li B. Nanobiotechnology to advance stress resilience in plants: current opportunities and challenges. Materials Today Bio. 2023 doi: 10.1016/j.mtbio.2023.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peng C., Xia Y., Zhang W., Wu Y., Shi J. Proteomic analysis unravels response and antioxidation defense mechanism of rice plants to copper oxide nanoparticles: comparison with bulk particles and dissolved Cu ions. ACS Agr. Sci. Tech. 2022;3:671–683. [Google Scholar]
- 150.Gauba A., Hari S.K., Ramamoorthy V., Vellasamy S., Govindan G., Arasu M.V. The versatility of green synthesized zinc oxide nanoparticles in sustainable agriculture: a review on metal-microbe interaction that rewards agriculture. Physiol. Mol. Plant Path. 2023 [Google Scholar]
- 151.Saritha G.N.G., Anju T., Kumar A. Nanotechnology-big impact: how nanotechnology is changing the future of agriculture? J Agr. Food Res. 2022;10 [Google Scholar]
- 152.Nietzel T., Elsässer M., Ruberti C., Steinbeck J., Ugalde J.M., Fuchs P.…Schwarzländer M. The fluorescent protein sensor ro GFP 2‐Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor‐induced oxidative burst in Arabidopsis. New Phytol. 2019;3:1649–1664. doi: 10.1111/nph.15550. [DOI] [PubMed] [Google Scholar]
- 153.Wu H., Nißler R., Morris V., Herrmann N., Hu P., Jeon S.-J., Kruss S., Giraldo J.P. Monitoring plant health with near-infrared fluorescent H 2 O 2 nanosensors. Nano Lett. 2020;4:2432–2442. doi: 10.1021/acs.nanolett.9b05159. [DOI] [PubMed] [Google Scholar]
- 154.Li J., Wu H., Santana I., Fahlgren M., Giraldo J.P. Standoff optical glucose sensing in photosynthetic organisms by a quantum dot fluorescent probe. ACS Appl. Mater. Interfaces. 2018 Aug 29;10(34):28279–28289. doi: 10.1021/acsami.8b07179. Epub 2018 Aug 14. PMID: 30058800. [DOI] [PubMed] [Google Scholar]
- 155.Giraldo J.P., Landry M.P., Kwak S.Y., Jain R.M., Wong M.H., Iverson N.M.…Strano M.S. A ratiometric sensor using single chirality near‐infrared fluorescent carbon nanotubes: application to in vivo monitoring. Small. 2015;32:3973–3984. doi: 10.1002/smll.201403276. [DOI] [PubMed] [Google Scholar]
- 156.Borse V., Srivastava R. Process parameter optimization for lateral flow immunosensing. Mater. Sci. Energy Technol. 2019;3:434–441. [Google Scholar]
- 157.Tereshchenko A., Fedorenko V., Smyntyna V., Konup I., Konup A., Eriksson M., Yakimova R., Ramanavicius A., Balme S., Bechelany M. ZnO films formed by atomic layer deposition as an optical biosensor platform for the detection of grapevine virus A-type proteins. Biosens. Bioelectron. 2017;92:763–769. doi: 10.1016/j.bios.2016.09.071. [DOI] [PubMed] [Google Scholar]
- 158.Khaledian S., Nikkhah M., Shams-bakhsh M., Hoseinzadeh S. A sensitive biosensor based on gold nanoparticles to detect Ralstonia solanacearum in soil. J. Gen. Plant Pathol. 2017;83:231–239. [Google Scholar]
- 159.Wilson D., Materón E.M., Ibáñez-Redín G., Faria R.C., Correa D.S., Oliveira Jr O.N. Electrical detection of pathogenic bacteria in food samples using information visualization methods with a sensor based on magnetic nanoparticles functionalized with antimicrobial peptides. Talanta. 2019;194:611–618. doi: 10.1016/j.talanta.2018.10.089. [DOI] [PubMed] [Google Scholar]
- 160.Brezolin A.N., Martinazzo J., Steffens J., Steffens C. Nanostructured cantilever sensor using with pani/MWCNT-COOH nanocomposites applied in the detection of pheromone. J. Mater. Sci. Mater. Electron. 2020;8:6008–6016. doi: 10.1007/s10854-020-03152-w. [DOI] [Google Scholar]
- 161.Khater M., De La Escosura-Muñiz A., Quesada-González D., Merkoçi A. Electrochemical detection of plant virus using gold nanoparticle-modified electrodes. Anal. Chim. Acta. 2019;1046:123–131. doi: 10.1016/j.aca.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 162.Bind S., Bind S., Chandra D. Unravelling Plant-Microbe Synergy. Elsevier; 2023. Microbial nanotechnology: a green approach towards sustainable agriculture; pp. 195–211. [Google Scholar]
- 163.Abd El-Ghany N.M., Abd El-Aziz S.E., Marei S.S. A review: application of remote sensing as a promising strategy for insect pests and diseases management. Environ. Sci. Poll. Res. 2020;27:33503–33515. doi: 10.1007/s11356-020-09517-2. [DOI] [PubMed] [Google Scholar]
- 164.Martinazzo J., Ballen S.C., Steffens J., Steffens C. Sensing of pheromones from euschistus heros (F.) stink bugs by nanosensors. Sens. Actuators. Rep. 2022;4 [Google Scholar]
- 165.Yang X., He Y., Wang X., Yuan R. A SERS biosensor with magnetic substrate CoFe2O4@ Ag for sensitive detection of Hg2+ Appl. Surf. Sci. 2017;416:581–586. [Google Scholar]
- 166.Niu X., Zhong Y., Chen R., Wang F., Liu Y., Luo D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Acuators B Chem. 2018;255:1577–1581. [Google Scholar]
- 167.Walia A., Waadt R., Jones A.M. Genetically encoded biosensors in plants: pathways to discovery. Annu. Rev. Plant Biol. 2018;1:497–524. doi: 10.1146/annurev-arplant-042817-040104. [DOI] [PubMed] [Google Scholar]
- 168.Mittal D., Kaur G., Singh P., Yadav K., Ali S.A. Nanoparticle-based sustainable agriculture and food science: recent advances and future outlook. Front. Nanotechnol. 2020;2 [Google Scholar]
- 169.Wu J., Prole D.L., Shen Y., Lin Z., Gnanasekaran A., Liu Y., Chen L., Zhou H., Chen S.W., Usachev Y.M. Red fluorescent genetically encoded Ca2+ indicators for use in mitochondria and endoplasmic reticulum. Biochem. J. 2014;1 doi: 10.1042/BJ20140931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tripathi D., Singh M., Pandey-Rai S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress. 2022;6 [Google Scholar]
- 171.Choudhary M.K., Singh M., Saharan V. Application of nanobiosensors in agriculture. Popular Kheti. 2015;3:130–135. [Google Scholar]
- 172.Litescu S.C., Eremia S.A., Diaconu M., Tache A., Radu G.-L. Biosensors applications on assessment of reactive oxygen species and antioxidants. Environ. Biosen. 2011;1:35–40. [Google Scholar]
- 173.Pacheco S., Tapia J., Medina M., Rodriguez R. Cadmium ions adsorption in simulated wastewater using structured alumina–silica nanoparticles. J. Non-Cryst. Solids. 2006;52–54:5475–5481. [Google Scholar]
- 174.Mohammed A.S., Kapri A., Goel R. Heavy metal pollution: source, impact, and remedies. Biomanagement of metal-contaminated soils. 2011:1–28. [Google Scholar]
- 175.Zhao L., Bai T., Wei H., Gardea-Torresdey J.L., Keller A., White J.C. Nanobiotechnology-based strategies for enhanced crop stress resilience. Nature Food. 2022;10:829–836. doi: 10.1038/s43016-022-00596-7. [DOI] [PubMed] [Google Scholar]
- 176.Elmer W., Ma C., White J. Nanoparticles for plant disease management. Current Opinion in Environmental Science & Health. 2018;6:66–70. [Google Scholar]
- 177.Kumar A., Choudhary A., Kaur H., Guha S., Mehta S., Husen A. Potential applications of engineered nanoparticles in plant disease management: a critical update. Chemosphere. 2022;295 doi: 10.1016/j.chemosphere.2022.133798. [DOI] [PubMed] [Google Scholar]
- 178.Abkhoo J., Panjehkeh N. Evaluation of antifungal activity of silver nanoparticles on Fusarium oxysporum. Int. J. Infec. 2017;4:2. [Google Scholar]
- 179.Ahmed T., Lv L., Noman M., Masood H.A., Rizwan M., Ijaz M., Hatamleh A.A., Al-Dosary M.A., Ali H.M., Chen J. Transcriptomic and proteomic profiling reveals toxicity and molecular action mechanisms of bioengineered chitosan-iron nanocomposites against Xanthomonas oryzae pv. Oryzae. Pest. Biochem. Physiol. 2023;193 doi: 10.1016/j.pestbp.2023.105447. [DOI] [PubMed] [Google Scholar]
- 180.Wang X., Liu X., Chen J., Han H., Yuan Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon. 2014;68:798–806. [Google Scholar]
- 181.Ma C., Borgatta J., Hudson B.G., Tamijani A.A., De La Torre-Roche R., Zuverza-Mena N., Shen Y., Elmer W., Xing B., Mason S.E. Advanced material modulation of nutritional and phytohormone status alleviates damage from soybean sudden death syndrome. Nature nanotechnol. 2020;12:1033–1042. doi: 10.1038/s41565-020-00776-1. [DOI] [PubMed] [Google Scholar]
- 182.Ahmed T., Noman M., Jiang H., Shahid M., Ma C., Wu Z., Nazir M.M., Ali M.A., White J.C., Chen J. Bioengineered chitosan-iron nanocomposite controls bacterial leaf blight disease by modulating plant defense response and nutritional status of rice (Oryza sativa L.) Nano Today. 2022;(45):101547. [Google Scholar]
- 183.Chen J., Mao S., Xu Z., Ding W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv. 2019;7:3788–3799. doi: 10.1039/c8ra09186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vestby L.K., Grønseth T., Simm R., Nesse L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics. 2020;2:59. doi: 10.3390/antibiotics9020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li Y., Zhang P., Li M., Shakoor N., Adeel M., Zhou P.…Rui Y. Application and mechanisms of metal‐based nanoparticles in the control of bacterial and fungal crop diseases. Pest Manag. Sci. 2023;1:21–36. doi: 10.1002/ps.7218. [DOI] [PubMed] [Google Scholar]
- 186.Cao X., Wang C., Luo X., Yue L., White J.C., Elmer W., Dhankher O.P., Wang Z., Xing B. Elemental sulfur nanoparticles enhance disease resistance in tomatoes. ACS Nano. 2021;7:11817–11827. doi: 10.1021/acsnano.1c02917. [DOI] [PubMed] [Google Scholar]
- 187.Kim S.G., Kim K.W., Park E.W., Choi D. Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathology. 2002;10:1095–1103. doi: 10.1094/PHYTO.2002.92.10.1095. [DOI] [PubMed] [Google Scholar]
- 188.Ross A., Somssich I.E. A DNA-based real-time PCR assay for robust growth quantification of the bacterial pathogen Pseudomonas syringae on Arabidopsis thaliana. Plant Methods. 2016;1:48. doi: 10.1186/s13007-016-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hussain M., Shakoor N., Adeel M., Ahmad M.A., Zhou H., Zhang Z., Xu M., Rui Y., White J.C. Nano-enabled plant microbiome engineering for disease resistance. Nano Today. 2023;48:101752. [Google Scholar]
- 190.Kandhol N., Singh V.P., Peralta-Videa J., Corpas F.J., Tripathi D.K. Silica nanoparticles: the rising star in plant disease protection. Trends Plant Sci. 2022;1:7–9. doi: 10.1016/j.tplants.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 191.Vinković T., Novák O., Strnad M., Goessler W., Jurašin D.D., Para\djiković N., Vrček I.V. Cytokinin response in pepper plants (capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017;156:10–18. doi: 10.1016/j.envres.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 192.Ma C., Li Q., Jia W., Shang H., Zhao J., Hao Y., Li C., Tomko M., Zuverza-Mena N., Elmer W., White J.C., Xing B. Role of nanoscale hydroxyapatite in disease suppression of Fusarium -infected tomato. Environ. Sci. Technol. 2021;20:13465–13476. doi: 10.1021/acs.est.1c00901. [DOI] [PubMed] [Google Scholar]
- 193.Murali M., Gowtham H.G., Singh S.B., Shilpa N., Aiyaz M., Alomary M.N., Alshamrani M., Salawi A., Almoshari Y., Ansari M.A. Fate, bioaccumulation and toxicity of engineered nanomaterials in plants: current challenges and future prospects. Sci. Total Environ. 2022;811 doi: 10.1016/j.scitotenv.2021.152249. [DOI] [PubMed] [Google Scholar]
- 194.Mahjouri S., Movafeghi A., Divband B., Kosari-Nasab M. Toxicity impacts of chemically and biologically synthesized CuO nanoparticles on cell suspension cultures of Nicotiana tabacum. Plant Cell Tissue Organ Cult. PCTOC) 2018;135:223–234. [Google Scholar]
- 195.Von der Kammer F., Ferguson P.L., Holden P.A., Masion A., Rogers K.R., Klaine S.J., Koelmans A.A., Horne N., Unrine J.M. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ. Toxicol. Chem. 2012;31:32–49. doi: 10.1002/etc.723. [DOI] [PubMed] [Google Scholar]
- 196.Tarrahi R., Mahjouri S., Khataee A. A review on in vivo and in vitro nanotoxicological studies in plants: a headlight for future targets. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111697. [DOI] [PubMed] [Google Scholar]
- 197.Bundschuh M., Filser J., Lüderwald S., McKee M.S., Metreveli G., Schaumann G.E., Schulz R., Wagner S. Nanoparticles in the environment: where do we come from, where do we go to? Environ. Sci. Eur. 2018;1:6. doi: 10.1186/s12302-018-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dinesh R., Srinivasan V., Hamza S. In: Zingiberaceae Crops—Present and Future. Singh H.P., Parthasarathy V.A., Kandiannan K., Krishnamurthy K.S., editors. Westville Publishing House; New Delhi: 2012. Nutrition; pp. 255–287. [Google Scholar]
- 199.Mishra S., Singh B.R., Singh A., Keswani C., Naqvi A.H., Singh H.B. Biofabricated silver nanoparticles act as a strong fungicide against bipolaris sorokiniana causing spot blotch disease in wheat. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097881. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Satya Sai M.V., Revati G.D., Ramya R., Swaroop A.M., Maheswari E., Kumar M.M. Knowledge and perception of farmers regarding pesticide usage in a rural farming village, southern India. Indian J. Occup. Environ. Med. 2019;23:32–36. doi: 10.4103/ijoem.IJOEM_121_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vishwakarma K., Singh V.P., Prasad S.M., Chauhan D.K., Tripathi D.K., Sharma S. Silicon and plant growth promoting rhizobacteria differentially regulate AgNP-induced toxicity in Brassica juncea: implication of nitric oxide. J. Hazard Mater. 2020;390 doi: 10.1016/j.jhazmat.2019.121806. [DOI] [PubMed] [Google Scholar]
- 202.Thunugunta T., Channa Reddy A., Kodthalu Seetharamaiah S., Ramanna Hunashikatti L., Gowdra Chandrappa S., Cherukatu Kalathil N., Dhoranapalli Chinnappa Reddy L.R. Impact of Zinc oxide nanoparticles on eggplant (S. melongena): studies on growth and the accumulation of nanoparticles. IET Nanobiotechnol. 2018;12(6):706–713. doi: 10.1049/iet-nbt.2017.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Khush G.S. IR varieties and their impact. Int. Rice Res. Inst. 2005 https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Khush+G.S.%2C+IR+varieties+and+their+impact%2C+Int.+Rice+Res.+Inst.+%282005%29.&btnG=#d=gs_cit&t=1733108392358&u=%2Fscholar%3Fq%3Dinfo%3AFzQ51r1ujGAJ%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Den [Not available] [Google Scholar]
- 204.Wang P., Lombi E., Zhao F.J., Kopittke P.M. Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. 2016 Aug 1;21(8):699–712. doi: 10.1016/j.tplants.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 205.Demirer G.S., Zhang H., Matos J.L., Goh N.S., Cunningham F.J., Sung Y., Chang R., Aditham A.J., Chio L., Cho M.J., Staskawicz B. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019 May;14(5):456–464. doi: 10.1038/s41565-019-0382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xia X., Cheng X., Li R., et al. Advances in application of genome editing in tomato and recent development of genome editing technology. Theor. Appl. Genet. 2021;134:2727–2747. doi: 10.1007/s00122-021-03874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mitter N., Worrall E.A., Robinson K.E., Li P., Jain R.G., Taochy C., Fletcher S.J., Carroll B.J., Lu G.Q., Xu Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants. 2017 Jan 9;3(2) doi: 10.1038/nplants.2016.207. [DOI] [PubMed] [Google Scholar]
- 208.Mahfouz M.M., Piatek A., Stewart C.N. Jr Genome engineering via TALENs and CRISPR/Cas9 systems: challenges and perspectives. Plant Biotechnol. J. 2014;12:1006–1014. doi: 10.1111/pbi.12256. [DOI] [PubMed] [Google Scholar]
- 209.Demirer G.S., Silva T.N., Jackson C.T., Thomas J.B., W. Ehrhardt D., Rhee S.Y., Mortimer J.C., Landry M.P. Nanotechnology to advance CRISPR–cas genetic engineering of plants. Nat. Nanotechnol. 2021;3:243–250. doi: 10.1038/s41565-021-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Martin-Ortigosa S., Peterson D.J., Valenstein J.S., Lin V.S.-Y., Trewyn B.G., Lyznik L.A., Wang K. Mesoporous silica nanoparticle-mediated intracellular cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 2014;2:537–547. doi: 10.1104/pp.113.233650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kwak S.-Y., Lew T.T.S., Sweeney C.J., Koman V.B., Wong M.H., Bohmert-Tatarev K., Snell K.D., Seo J.S., Chua N.-H., Strano M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nature nanotechnol. 2019;5:447–455. doi: 10.1038/s41565-019-0375-4. [DOI] [PubMed] [Google Scholar]
- 212.Zhu H., Li C., Gao C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020;21:661–677. doi: 10.1038/s41580-020-00288-9. [DOI] [PubMed] [Google Scholar]
- 213.Kah M., Hofmann T. Nanopesticide research: current trends and future priorities. Environ. Int. 2014 Feb 1;63:224–235. doi: 10.1016/j.envint.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 214.Pánková L., Aulová R., Jarolímek J. Economic aspects of precision agriculture systems. AGRIS on-line Papers in Economics and Informatics. 2020 Sep 30;12(3):59–67. [Google Scholar]
- 215.Kah M., Beulke S., Tiede K., Hofmann T. Nanopesticides: state of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013 Jan 1;43(16):1823–1867. [Google Scholar]
- 216.Mwaanga P. Risks, uncertainties, and ethics of nanotechnology in agriculture. New Visions in Plant Science. 2018 Sep 19;22(3) [Google Scholar]
- 217.Amenta V., et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015;73:463–476. doi: 10.1016/j.yrtph.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 218.Jellason N.P., Robinson E.J., Ogbaga C.C. Agriculture 4.0: is sub-saharan Africa ready? Appl. Sci. 2020;11(12):5750. doi: 10.3390/app11125750. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with our study has not been deposited anywhere.