Abstract
Exosomes have emerged as a crucial focus in advancing the diagnosis and treatment of osteoarthritis (OA). However, there are limited bibliometric studies on this topic. This study aimed to delineate the literature landscape on exosomes in OA, identifying global research trends and key areas. We utilised the Web of Science Core Collection to retrieve articles published from 2004 to 2023. Our analysis included 456 publications across 671 institutions from 40 countries/regions. Publication volume, citations, and emerging research foci and trends were examined. Our results reveal a consistently increased interest in exosomes related to OA over the past two decades. Prominent institutions contributing to this research include Shanghai Jiao Tong University and Shenzhen University. The leading journal for these publications is the International Journal of Molecular Sciences, with Stem Cell Research & Therapy being the most frequently co-cited journal. Notable scholars in this field are Li Duan, Yujie Liang, Xiao Xu, and Wei Seong Toh, with Shipin Zhang emerging as the most co-cited author. The principal research themes were elucidating how exosomes contribute to OA pathology and developing novel therapeutic approaches. Research hotspots and new trends are linked to terms such as “cartilage,” “mesenchymal stem cell,” “miRNA,” “treatment,” and “biomarkers.” This comprehensive analysis offers valuable insights into the prevailing scientific discourse, pivotal topics, and potential future directions. It could serve as a foundational reference for researchers exploring exosomes and their utility in OA diagnostics and therapeutics.
Keywords: Bibliometrics, Osteoarthritis, Exosomes, CiteSpace, VOSviewers
1. Introduction
Osteoarthritis (OA) is a prevalent chronic degenerative joint disorder, primarily affecting weight-bearing joints, leading to significant structural damage and symptoms, such as pain, stiffness, and disability [1]. OA is characterised by progressive cartilage degradation, synovitis, subchondral bone sclerosis, and osteophyte formation [[2], [3], [4]]. OA is commonly classified into primary and secondary types; the etiology of primary OA however remains unknown [5]. The risk factors for OA include age, obesity, sex, genetics, and joint injuries [6].
The aging global population, coupled with rising global obesity rates and increased frequency of joint injuries, is expected to cause the prevalence of OA to escalate. Currently, an estimated 250 million people worldwide suffer from OA [7]. A longitudinal study in China highlighted that symptomatic knee OA affects 10.3 % of women and 5.7 % of men aged 60 and above, contributing to approximately 15 million new cases of OA globally [8,9]. The economic burden imposed by OA on families and society is substantial.
Treatment options for OA range from non-pharmacological interventions and analgesics to non-steroidal anti-inflammatory drugs, intra-articular corticosteroid injections, and surgical procedures, such as joint replacement [10]. However, no targeted and effective disease-modifying drug is currently available for OA. Thus, understanding the pathogenesis of OA is crucial for developing new, effective therapeutic strategies. Accumulating evidence has indicated a significant role of exosomes in the pathogenesis of OA [11], representing a promising area for investigation.
Exosomes are vital mediators of intercellular communication, involved in numerous physio-pathological processes. OA-related exosomes are derived from various cell types, such as chondrocytes, subchondral bone cells, synovial fluid, synoviocytes, and osteoclasts, and contain lipids, nucleic acids (mRNA, miRNA, and small non-coding RNA), metabolites, and proteins. These components enable exosomes to regulate gene expression and subsequent activities in target cells, thereby influencing OA progression. Furthermore, their ability to function as natural carriers offers opportunities for targeted drug development, utilising their cargo of diverse miRNAs and proteins for precise OA treatment. Recent advances have shown promising outcomes in leveraging exosomes for OA therapy [[12], [13], [14], [15], [16]].
Bibliometric analysis employs philological, mathematical, and statistical methods to analyse literature and its bibliometric characteristics, such as authorship, institutional affiliations, publication outlets, geographic distribution, and citation metrics [[17], [18], [19]]. Börner K et al. presented the use of visualisation techniques in analyzing knowledge domains, highlighting bibliometrics' role in pinpointing research themes and trends [20]. Hood W W and Wilson C S examined the evolution and fundamental concepts of bibliometrics, delving into its uses in scientific research and information science [21]. Collectively, these studies offer an expansive view of bibliometric methodologies, encompassing citation analysis, visualisation techniques, and evaluative metrics, all of which are extensively applied within the field of bibliometrics. While similar studies have been conducted for diseases like cardiovascular disease [22], cancer [23], autoimmune diseases [24], and diabetes [25], bibliometric analyses specifically on OA and exosomes are lacking. The present study aimed to fill this gap by reviewing publications on exosomes in OA from 2004 to 2023, aiming to uncover global research trends and forecast future development.
2. Materials and methods
A search was conducted in the Web of Science Core Collection (WoSCC) database for studies conducted between 1 January 2004 and 31 December 2023.
2.1. Data collection
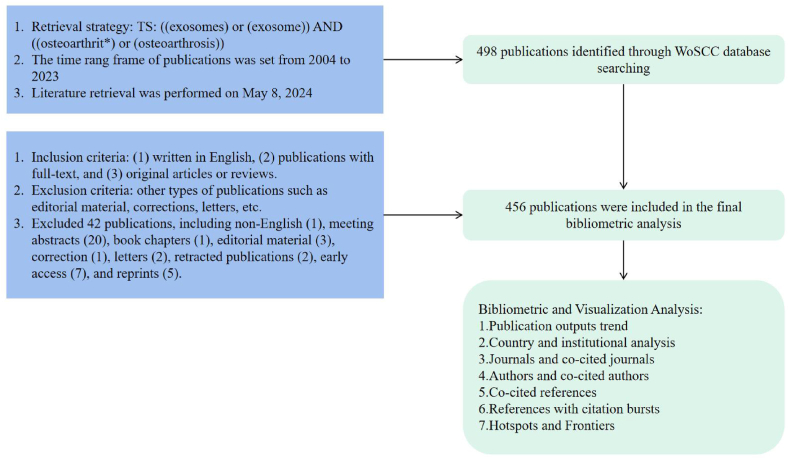
Our data collection strategy employed the specific search key words “exosomes or exosome” and “osteoarthrit∗ or osteoarthrosis,” targeting the expansive literature on the field. The article types were restricted to “article” and “review” (Fig. 1). This approach ensured the inclusion of relevant and impactful research publications.
Fig. 1.
Publication screening flowchart.
2.2. Data analysis
For bibliometric analysis, we utilised VOSviewer (version 1.6.18) [26], a specialised software known for its robust capabilities in generating network visualisations, such as collaboration, cooccurrence, and co-citation networks [27,28]. This analysis covered various bibliometric aspects, including country/region, institution, journal, co-cited journal, author, co-cited author, and keyword co-occurrence. In this study, we only used author keywords. VOSviewer generates network maps where nodes represent authors, countries/regions, institutions, or journals; the size of each node correlates with the entity's prominence, while colours denote different classifications. The thickness of the lines between nodes illustrates the strength of collaboration or co-citation relationships [29,30].
Additionally, we employed CiteSpace (version 6.1.R1), developed by Professor Chaomei Chen, for more nuanced visual analysis [31]. This software is pivotal for generating dual-map overlays of journals and detecting references characterised by strong citation bursts, indicative of emerging trends or shifts in the field. We obtained data on journal quartiles and impact factors from the Journal Citation Reports 2022.
For quantitative analysis, we used Microsoft Office Excel 2022, which provided the necessary tools for data manipulation and trend analysis. Furthermore, Scimago Graphica was utilised to create visual representations of the global distribution of national publications, highlighting the international scope and collaboration in the research of exosomes in OA.
3. Results
3.1. Trend in publication outputs
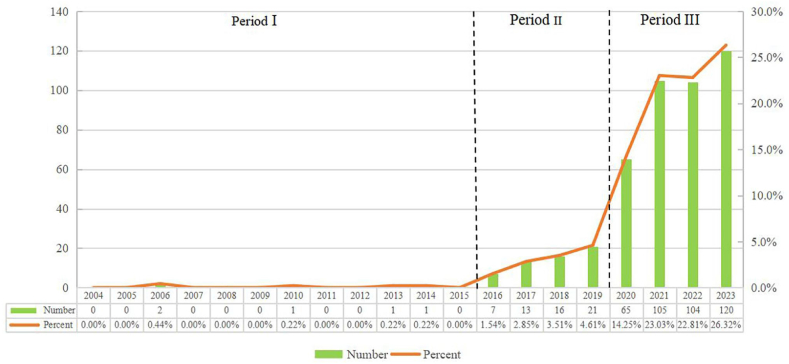
The search yielded a total of 456 studies on exosomes in OA from 2004 to 2023. Based on the annual publication volume, this period can be divided into three distinct phases (Fig. 2). The first phase, from January 2004 to December 2015, represents the nascent stage of exosome research in OA, with a total of 5 publications. The second phase, from January 2016 to December 2019, marks the developmental stage, during which publication numbers gradually increased to an average of 14 annually. The third phase, from January 2020 to December 2023, showed a significant surge in research activity, with an average of 98 publications per year. Notably, the year 2020 saw 65 publications, tripling the number from 2019. By 2023, annual publications had reached 120, indicating a robust and growing interest in the role of exosomes in OA. Overall, the volume of research publications during the third phase markedly outpaced that of previous phases, reflecting an accelerating advancement in the field.
Fig. 2.
Annual output of exosome research in OA.
3.2. Country and institutional contributions
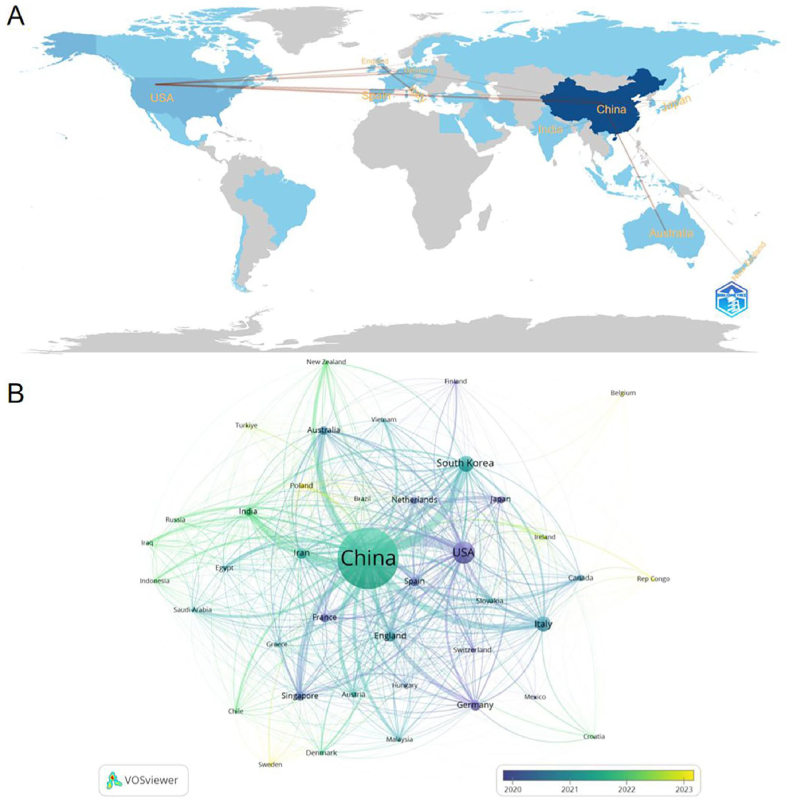
A comprehensive analysis revealed contributions from 42 countries/regions and 671 institutions on exosome research related to OA. The top ten countries/regions included Italy, United Kingdom, Germany, Spain, China, South Korea, India, Iran, the United States, and Australia, demonstrating a dominant presence of Europe and Asia, each contributing four countries/regions (Table 1). Notably, China was the foremost contributor with 287 publications, accounting for 51.90 % of the total, followed by the United States with 51 publications (9.22 %), South Korea with 28 (5.06 %), and Italy with 26 (4.70 %). Collectively, these countries/regions represented 61.12 % of the total output. For a more detailed analysis, we selected 26 countries/regions with at least two publications for a national collaboration network visualisation. The result demonstrated robust international cooperation (Fig. 3). For instance, China had significant collaborations with South Korea, the United States, Japan, and Australia, while the United States maintained strong collaborative ties with Singapore, Italy, China, and Canada.
Table 1.
Top 10 countries/regions and institutions on research of exosomes in OA.
| Rank | Country | Counts | Institution | Counts |
|---|---|---|---|---|
| 1 | China (Asia) | 287(51.90 %) | Shanghai Jiao Tong University (China) | 16(2.23 %) |
| 2 | USA (North America) | 51(9.22 %) | Shenzhen University(China) | 15(1.24 %) |
| 3 | South Korea (Asia) | 28(5.06 %) | Central South University(China) | 14(1.16 %) |
| 4 | Italy (Europe) | 26(4.70 %) | Chinese University of Hong Kong(China) | 14(1.16 %) |
| 5 | England (Europe) | 15(2.71 %) | Sichuan University(China) | 14(1.16 %) |
| 6 | Germany(Europe) | 13(2.35 %) | Zhejiang University(China) | 14(1.16 %) |
| 7 | Iran (Asia) | 13(2.35 %) | Sun Yat-sen University(China) | 12(0.99 %) |
| 8 | Singapore(Asia) | 11(1.99 %) | Anhui Medical University(China) | 11(0.91 %) |
| 9 | Spain(Europe) | 11(1.99 %) | Nanjing Medical University(China) | 11(0.91 %) |
| 10 | Australia(Oceania) | 10(1.81 %) | National University of Singapore(Singapore) | 11(0.91 %) |
Fig. 3.
The geographical distribution (A) and visualisation of countries (B) based on exosome research in OA.
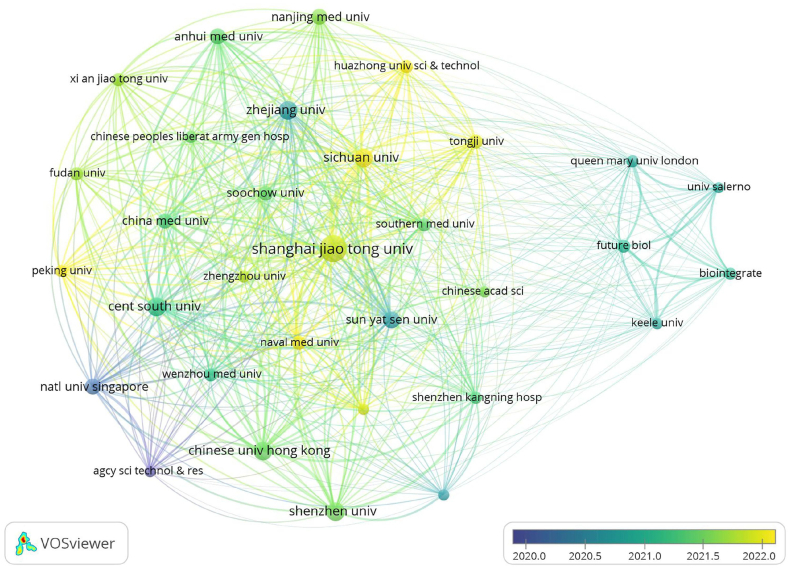
Regarding institutional contributions, the top 10 prolific institutions were all based in China. Leading this group were Shanghai Jiao Tong University with 16 publications (2.23 %); Shenzhen University with 15 (1.24 %); and Central South University, Chinese University of Hong Kong, Sichuan University, and Zhejiang University, each contributing 14 publications (1.16 %). For further analysis, 32 institutions with six or more publications were selected for visualisation of the institutional collaboration network. Notable close collaborations were observed among Shenzhen University, Southern University of Science & Technology, and Shenzhen Kangning Hospital, as well as among Zhejiang University, Sun Yat Sen University, and China Medical University (Fig. 4).
Fig. 4.
The visualisation of institutions based on exosome research in OA.
3.3. Journal publication and Co-cited analysis
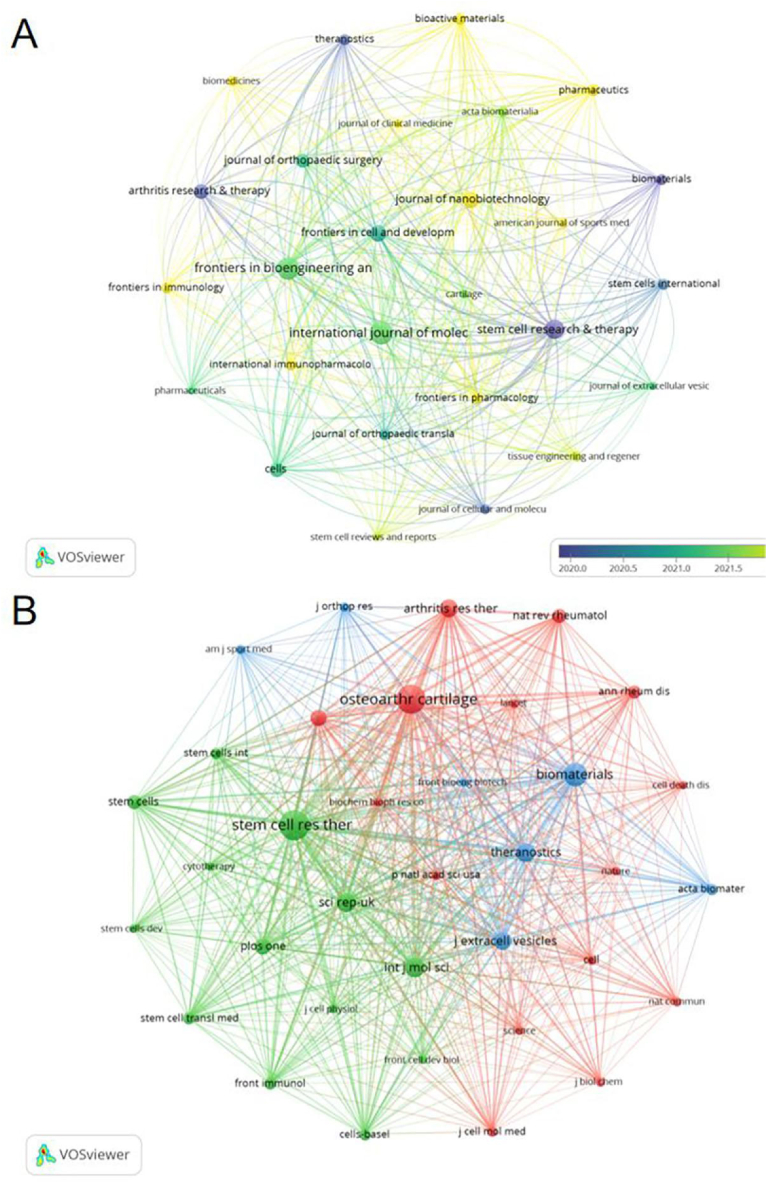
Research on exosomes in OA was disseminated through 202 journals. The International Journal of Molecular Sciences led with 20 publications (4.39 %), followed by Frontiers in Bioengineering and Biotechnology with 19 publications (4.17 %), Stem Cell Research & Therapy with 15 (3.29 %), and Frontiers in Cell and Developmental Biology with 12 (2.63 %). Among the top 10 journals, the Journal of Nanobiotechnology boasted the highest impact factor at 10.2, with Stem Cell Research & Therapy following at an impact factor of 7.5. For further analysis, 27 journals with 4 or more publications were selected for journal network mapping (Fig. 5A). This mapping illustrated active citation relationships, notably between Frontiers in Bioengineering and Biotechnology and other leading journals, such as International Journal of Molecular Sciences, Frontiers in Cell and Developmental Biology, and Stem Cell Research & Therapy.
Fig. 5.
The visualisation of journals (A) and co-cited journals (B) based on exosome research in OA.
Table 2 highlights that among the top 10 co-cited journals, three were cited over 600 times. Stem Cell Research & Therapy was the most cited journal, with 1187 citations, followed by Osteoarthritis and Cartilage with 1124 citations, and Biomaterials with 840 citations. Biomaterials featured the highest impact factor at 14, with Arthritis & Rheumatology close behind at 13.3. A co-citation network was created for journals cited at least 180 times, illustrating prominent co-citation dynamics (Fig. 5B). This network revealed strong co-citation links between Stem Cell Research & Therapy and journals like the Journal of Extracellular Vesicles and Biomaterials.
Table 2.
Top 10 journals and co-cited journals for research of exosomes in OA.
| Rank | Journal | Count | IF | Q | Co-cited Journal | Co-citation | IF | Q |
|---|---|---|---|---|---|---|---|---|
| 1 | International Journal of Molecular Sciences | 20(4.39 %) | 5.6 | Q1 | Stem Cell Research & Therapy | 1187 | 7.5 | Q1 |
| 2 | Frontiers in Bioengineering and Biotechnology | 19(4.17 %) | 5.7 | Q1 | Osteoarthr and Cartilage | 1124 | 7 | Q1 |
| 3 | Stem Cell Research & Therapy | 15(3.29 %) | 7.5 | Q1 | Biomaterials | 840 | 14 | Q1 |
| 4 | Frontiers in Cell and Developmental Biology | 12(2.63 %) | 5.5 | Q2 | International Journal of Molecular Sciences | 658 | 5.6 | Q1 |
| 5 | Journal of Nanobiotechnology | 12(2.63 %) | 10.2 | Q1 | Scientific Reports | 622 | 4.6 | Q2 |
| 6 | Arthritis Research & Therapy | 10(2.19 %) | 4.9 | Q2 | Theranostics | 603 | 12.4 | Q1 |
| 7 | Cells | 10(2.19 %) | 6 | Q2 | Journal of Extracellular Vesicles | 579 | 0.1 | Q1 |
| 8 | Journal of Orthopaedic Surgery and Research | 9(1.97 %) | 2.6 | Q2 | Arthritis Research & Therapy | 565 | 4.9 | Q2 |
| 9 | Bioactive Materials | 7(1.54 %) | 0.1 | Q1 | PLoS One | 480 | 3.7 | Q2 |
| 10 | Pharmaceutics | 7(1.54 %) | 5.4 | Q1 | Arthritis & Rheumatology | 468 | 13.3 | Q1 |
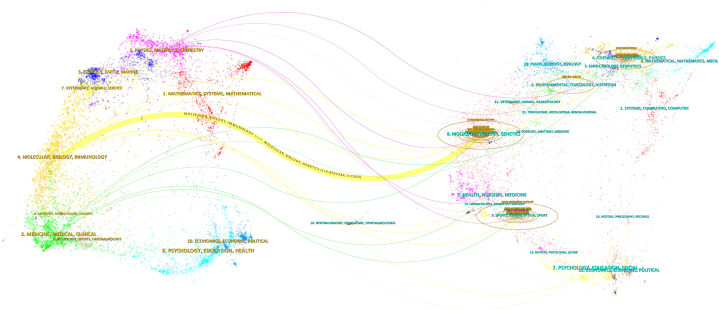
The dual-map overlay of journals, depicted in Fig. 6, visually represents the interrelationships between citing and cited journals [32]. The major citation pathway (colored orange) indicates that articles from journals categorized under molecular biology and immunology are predominantly cited by molecular biology and genetics fields.As shown in Fig. 6, the major citation path in orange indicates that studies published in “molecular, biology, immunology” journals were mainly cited in “molecular, biology, genetics” journals.
Fig. 6.
The dual-map overlay of journals on exosome research in OA.
3.4. Author contributions and Co-citation networks
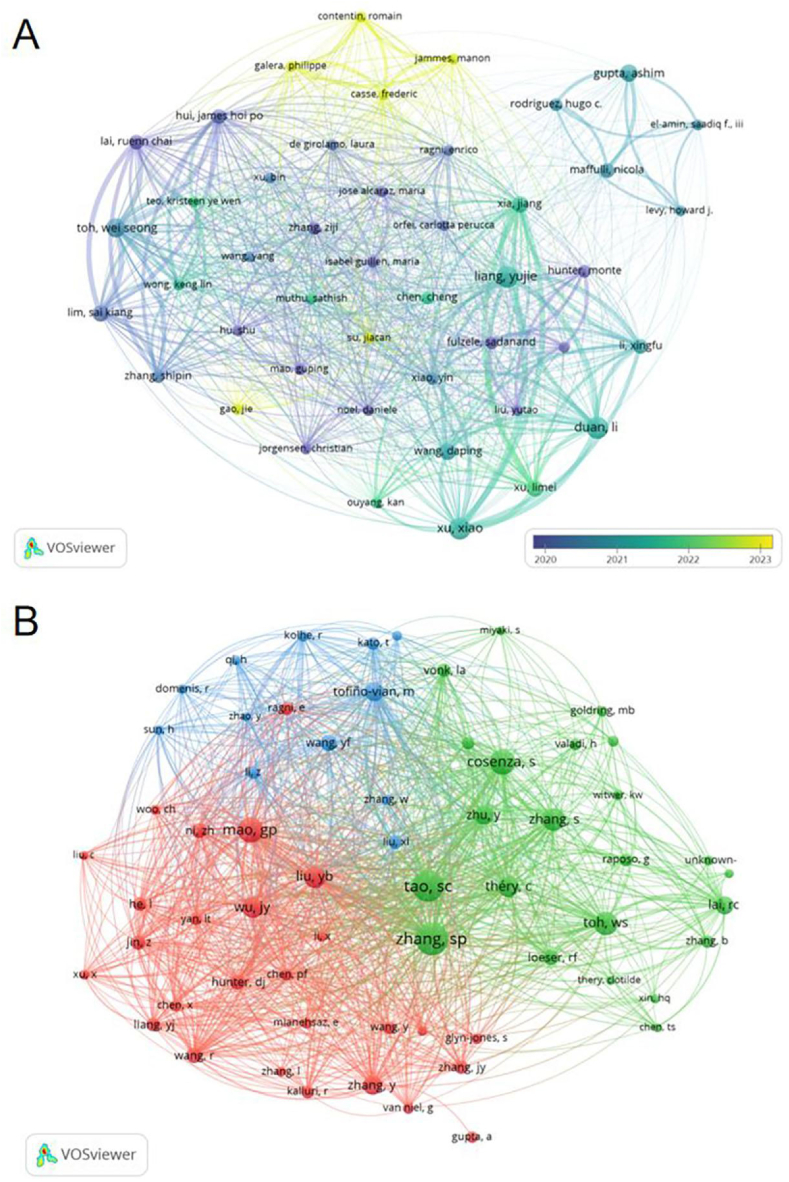
A total of 2694 authors contributed to the research on exosomes in OA. The top 10 authors, each having published four or more papers, are highlighted in Table 3. We developed a collaborative network (Fig. 7A) for authors who met this publication threshold. Prominent among these are Li Duan, Yujie Liang, Xiao Xu, Ashim Gupta, Daping Wang, Sadanand Fulzele, Xingfu Li, Nicola Maffulli, Wei Seong Toh, and Limei Xu, each represented by large nodes in the network. This network also revealed substantial collaboration among authors; for example, Yujie Liang closely collaborated with Xiao Xu, Li Duan, and Daping Wang, while Sadanand Fulzele was notably linked with Monte Hunter.
Table 3.
Top 10 authors and co-cited authors on research of exosomes in OA.
| Rank | Authors | Count | Co-Cited Authors | Citations |
|---|---|---|---|---|
| 1 | Li Duan | 11 | Shipin Zhang | 247 |
| 2 | Yujie Liang | 11 | Shi-Cong Tao | 235 |
| 3 | Xiao Xu | 11 | Stella Cosenza | 173 |
| 4 | Wei Seong Toh | 9 | Guping Mao | 171 |
| 5 | Ashim Gupta | 8 | Wei Seong Toh | 156 |
| 6 | Sai Kiang Lim | 7 | Shuai Zhang | 142 |
| 7 | Daping Wang | 7 | Yubao Liu | 136 |
| 8 | Jiang Xia | 7 | Clotilde Théry | 132 |
| 9 | Limei Xu | 7 | Jiangyi Wu | 128 |
| 10 | James Hoi Po Hui | 6 | Yan Zhang | 116 |
Fig. 7.
The visualisation of authors (A) and co-cited authors (B) based on exosome research in OA.
In terms of co-citations, 15,055 authors were recognised, with four authors being co-cited over 170 times (Table 3). Shipin Zhang led with 247 co-citations, followed by Shicong Tao with 235, and Stella Cosenza with 173. A co-citation network was constructed for authors with at least 40 co-citations, visualised in Fig. 7B. This network not only mapped out the frequency of co-citations but also highlighted active collaborations, such as the partnership between Shipin Zhang and Shicong Tao as well as between Guping Mao and Rui Wang.
3.5. Analysis of Co-cited references
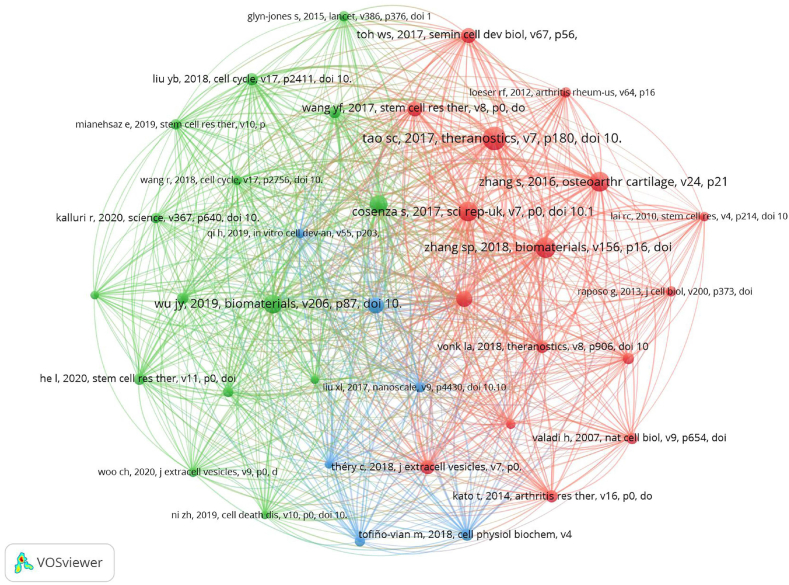
Over the past two decades, there have been 20,405 co-cited references related to exosomes in OA. Table 4 lists the top 10 co-cited references, with each being cited at least 89 times; seven of these were cited 100 times or more. To provide a comprehensive view of the research connections within this field, we constructed a co-citation network map from references that were co-cited at least 40 times, as depicted in Fig. 8. This map highlights several key papers with strong co-citation links. Notably, “Tao S C, 2017, Theranostics” shows significant co-citation relationships with “Zhang S P, 2018, Biomaterials,” “Zhu Y, 2017, Stem cell research & therapy,” and “Zhang S, 2016, Osteoarthritis and cartilage,” among others.
Table 4.
Top 10 co-cited references on research of exosomes in OA.
| Rank | Co-cited reference | Ref | Citations |
|---|---|---|---|
| 1 | Tao S C, 2017, Theranostics, V7, P180 | [33] | 170 |
| 2 | Zhang S P, 2018, Biomaterials, V156, P16 | [34] | 138 |
| 3 | Cosenza S, 2017, Scientific reports, V7, P0 | [35] | 129 |
| 4 | Zhang S, 2016, Osteoarthritis and cartilage, V24, P2135 | [36] | 129 |
| 5 | Wu J Y, 2019, Biomaterials, V206, P87 | [37] | 120 |
| 6 | Mao G P, 2018, Stem cell research & therapy, V9, P0 | [38] | 116 |
| 7 | Zhang S P, 2019, Biomaterials, V200, P35 | [39] | 100 |
| 8 | Zhu Y, 2017, Stem cell research & therapy, V8, P0 | [40] | 99 |
| 9 | Toh Wei Seong, 2017, Seminars in cell & developmental biology, V67, P56 | [41] | 90 |
| 10 | Wang Y F, 2017, Stem cell research & therapy, V8, P0 | [42] | 89 |
Fig. 8.
The visualisation of authors (A) and co-cited authors (B) based on exosome research in OA.
3.6. Identification of references with citation bursts
References with citation bursts are those that experience a sharp increase in citations over a specific period, indicating significant impact or relevance in the field. As illustrated in Fig. 9, we identified eight references with strong citation burst using Citespace analysis. The red bars in Fig. 9 indicate periods of intense citation activity, with each bar representing a year. These citation bursts were first noted in 2014 and extended to as late as 2017.
Fig. 9.
Top 10 references with strong citation bursts.
The reference with the highest citation burst strength is “Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes” by Tomohiro Kato et al. which experienced bursts from 2017 to 2021. The reference with the second highest burst strength (10.91) is “Exosomes derived from human embryonic mesenchymal stem cells (MSCs) promote osteochondral regeneration.” by S Zhang et al. showing citation bursts from 2016 to 2019. The bursts strength of these eight references ranged from 6.43 to 18.34, and their duration of influence lasted from 2 to 4 years. Table 5 provides a summary of the main content from the publications of these eight references, ordered as they appear in Fig. 9.
Table 5.
The main research contents of the 8 references with strong citations bursts.
| Rank | Strength | Main research contents | Ref |
|---|---|---|---|
| 1 | 10.91 | Exosomes from IL-1β stimulated synovial fibroblasts induce OA-like changes both in vitro and in ex vivo models. | [43] |
| 2 | 18.34 | The efficacy of human embryonic mesenchymal stem cell exosomes in cartilage repair, and the utility of mesenchymal stem cell exosomes as a therapeutic alternative to cell-based mesenchymal stem cell therapy. | [36] |
| 3 | 9.66 | Osteoarthritis pathogenesis recognition and detection methods. | [44] |
| 4 | 8.63 | The potential of SMSC-140-Exos in the prevention of OA was confirmed. | [33] |
| 5 | 6.75 | Mesenchymal stem cell exosome is immunologically active. | [45] |
| 6 | 6.75 | The current state of extracellular vesicle cell biology and focus on endosome-derived exosomes. | [46] |
| 7 | 6.43 | Pluripotent stem cell-derived mesenchymal stem cells can be induced to secrete exosomes, thus providing a potential novel therapeutic approach for OA. | [40] |
| 8 | 8.24 | The therapeutic potential of exosomes from human embryonic stem cell-induced mesenchymal stem cells in alleviating osteoarthritis. | [42] |
3.7. Research hotspots and emerging frontiers
Keyword co-occurrence analysis is an effective method to quickly identify research hotspots within a specific field. Table 6 summarises the top 20 high-frequency keywords in the study of exosomes in OA. Notably, “MSCs” and “extracellular vesicles” appeared more than 50 times, highlighting them as primary research directions.
Table 6.
Top 20 keywords on research of exosomes in OA.
| Rank | Keywords | Counts | Rank | Keywords | Counts |
|---|---|---|---|---|---|
| 1 | exosomes | 366 | 11 | knee osteoarthritis | 66 |
| 2 | osteoarthritis | 337 | 12 | microRNAs | 60 |
| 3 | mesenchymal stem cells | 189 | 13 | apoptosis | 59 |
| 4 | extracellular vesicles | 186 | 14 | stromal cells | 59 |
| 5 | inflammation | 98 | 15 | chondrocytes | 53 |
| 6 | cartilage | 84 | 16 | articular cartilage | 52 |
| 7 | knee | 76 | 17 | Stem cells | 51 |
| 8 | expression | 74 | 18 | repair | 50 |
| 9 | proliferation | 70 | 19 | in-vitro | 42 |
| 10 | regeneration | 69 | 20 | cartilage repair | 40 |
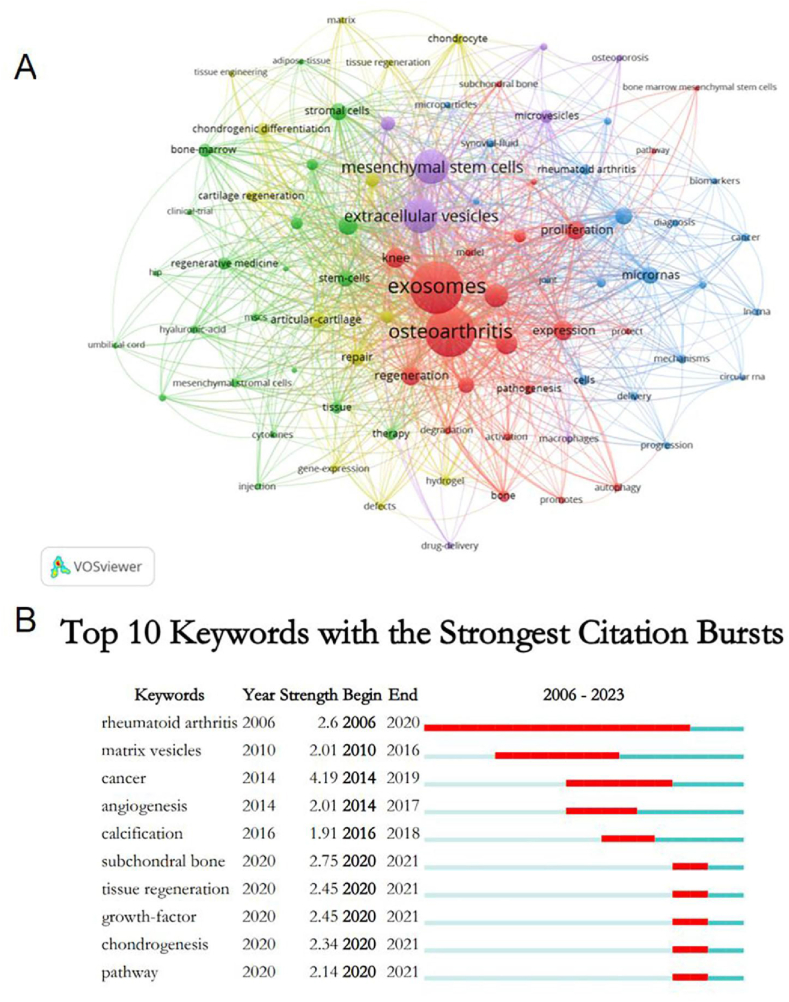
Fig. 10A illustrates an analysis of keywords with occurrences of five or more using VOSviewer. The visualisation delineated the strength of research connections through the thickness of the edges between nodes. Five distinct clusters were identified, each representing different research directions: 1) Red Cluster: includes keywords such as “activation”, “autophagy”, “bone”, “bone marrow mesenchyme”, “cartilage”, etc. 2) Green Cluster: comprises “adipose-derived stem cell”, “adipose-tissue”, “bone-marrow”, “clinical-trial”, etc. 3) Blue Cluster: contains “angiogenesis”, “apoptosis”, “biogenesis”, “biomarkers”, etc. 4) Yellow Cluster: focuses on “articular-cartilage”, “cartilage regeneration”, “cartilage repair”, “chondrocyte”, etc. 5) Purple Cluster: encompasses “drug-delivery”, “extracellular vesicles”, “in-vitro”, “macrophages”, etc.
Fig. 10.
Keyword cluster analysis (A) and keyword citation bursts analysis (B).
Additionally, keyword citation burst analysis helps to reflect the temporal dynamics of research interests. Fig. 10B shows the timeline of keyword citation bursts, where the blue line indicates the duration, and the red line marks the peak period of citation activity. Keywords began showing bursts in 2006, continuing for 15 years. “Cancer” experienced the strongest burst (strength = 4.19), followed by “subchondral bone” (strength = 2.75). The most recent keywords with significant citation bursts, appearing in 2020, include “subchondral bone”, “tissue regeneration”, “growth factor”, “chondrogenesis”, and “pathway”, indicating current and evolving focal points in the field.
4. Discussion
From 2004 to 2015, exosome research in OA was in its infancy, averaging only 0.42 publications per year, indicating a nascent stage with limited foundational research. This period saw a gradual development, with total publication reaching 57 from 2016 to 2019. However, a significant surge occurred from 2020 to 2023, with 394 papers published, averaging 98 annually and peaking at 120 in 2023. This dramatic increase underscores a burgeoning interest in exosome research, especially during the last three years. China was the predominant contributor to global publications, followed by the United States, highlighting their pivotal roles in this research area.
Notable collaborations were observed among virous countries/regions, with China and the United States engaging extensively, albeit with potential for deeper cooperative efforts. In particular, institutions like Shenzhen University and the Chinese University of Hong Kong demonstrated extensive collaboration networks. Conversely, Shanghai Jiao Tong University, despite its high output, showed limited collaborative engagements, which could impact long-term research progress.
The primary publication journal in this specific field was the International Journal of Molecular Sciences, favoured by researchers due to its accessibility and impact. High-impact journals, such as the Journal of Nanobiotechnology and Stem Cell Research & Therapy, also featured prominently, underscoring their relevance and influence on exosome research. The spectrum of research covered diverse disciplines, including medicine, biology, and engineering, yet there was a notable lack of research reports of clinical trials, pointing to ongoing experimental efforts rather than clinical application.
Among authors, Li Duan, Yujie Liang, Xiao Xu, Wei Seong Toh, and Ashim Gupta were the most prolific, collectively authoring 50 articles. Their works focused on innovative exosome-based treatments for OA. Notably, their research on hybrid and engineered exosomes, as well as the use of digoxin for joint inflammation, has opened new avenues for therapeutic interventions [[33], [34], [35]]. They also explored the targeted delivery of Kartogenin using engineered exosomes, showing promise for enhancing chondrogenesis both in vitro and in vivo [36]. The study on miR-140-encapsulated exosomes further highlighted potential strategies for inducing MSCs chondrogenesis and reducing cartilage matrix degradation [37].
In terms of citation impact, Shipin Zhang emerged as a leading figure, significantly influencing the field through his work on MSC exosome-mediated cartilage repair and joint healing [38]. His works, particularly those combining MSC exosomes with hyaluronic acid, have not only elucidated key biological mechanisms but also set the stage for upcoming clinical trials targeting osteochondral lesions [[39], [40], [41]]. These studies underscore the potential of exosomes as therapeutic agents in OA, paving the way for future clinical applications.
The foundational literature in the field of exosomes in OA is formed by frequently co-cited references and articles [42]. Among these, the top 10 co-cited references are pivotal for understanding the role of exosomes in OA. Notable among these is the work by Shicong Tao et al., in 2017, which explored the effects of exosomes from miR-140-5p-overexpressing human synovial MSCs on articular chondrocyte proliferation and migration, significantly decreasing extracellular matrix formation [43]. S. Zhang et al., in 2016 demonstrated that intra-articular injections of exosomes derived from human embryonic MSCs could promote cartilage repair and regeneration in a rat OA model [44]. Further contributions include those by Shipin Zhang, who in 2018 illustrated how MSCs exosomes can repair osteochondral defects and alleviate temporomandibular joint pain and degeneration [38,39]. Stella Cosenza et al., in 2017 showed the chondroprotective and anti-inflammatory effects of exosomes in vitro and in vivo [45]. The significant research by Guping Mao et al., in 2018 identified the role of exosomal miR-92a-3p in cartilage development via the WNT5A pathway, offering potential new avenues for OA treatment [46]. These key references underscore the critical role of exosome components, biological functions, and their potential clinical applications in OA research.
Emerging topics and research frontiers in the field of exosomes in OA were highlighted by the references experiencing citation bursts, indicating a rising interest and significance in recent years [47]. The focus on the biological role of endogenous exosomes in cartilage repair and the innovative engineering of exogenous exosomes to alleviate OA symptoms mark pivotal areas of exploration. These exogenous exosomes, often derived from cells with immunomodulatory functions, such as MSCs, illustrate a progressive shift towards targeted therapeutic strategies.
Excluding common terms, such as ‘osteoarthritis’ and ‘exosome’, keyword analysis revealed an emphasis on ‘cartilage’, ‘MSCs’, ‘microRNA’, ‘treatment’, and ‘biomarkers’. This selection indicates that current research predominantly concentrates on specific areas. For instance, exosomes modulate chondrocyte functions and cartilage integrity through the delivery of bioactive molecules like proteins, mRNAs, and miRNAs. Different sources of exosomes play distinct roles in OA progression, some promoting chondrocyte proliferation, while others inhibit apoptosis [48,49].
In the area of MSCs, MSC-derived exosomes are at the forefront of OA treatment due to their capacity to modulate inflammation and promote tissue regeneration [[50], [51], [52], [53]]. The efficacy of these exosomes in accelerating cartilage repair suggests significant potential for clinical applications [[54], [55], [56]]. Regarding miRNAs, the therapeutic potential is considerable, with various studies demonstrating that miRNA-containing exosomes can regulate gene expression essential for chondrocyte survival and cartilage repair [12,[57], [58], [59]].
The role of exosomes as treatment vectors highlights a move towards less invasive and more targeted therapeutic strategies in OA management, emphasising their importance in cartilage regeneration and inflammation modulation. Additionally, the diagnostic potential of exosomes is being increasingly recognised, with research identifying specific miRNAs and other molecular contents as promising biomarkers for early OA detection. This dynamic area of study suggests that exosomes hold the key to revolutionary approaches in both the treatment and diagnosis of OA.
It should be noted that this study has several limitations. Notably, its reliance solely on WoSCC data excludes potentially relevant research published in other databases. However, the WoSCC comprises multiple databases, including the Science Citation Index Expanded (SCI-E), Social Science Citation Index (SSCI), Arts and Humanities Citation Index (AHCI), Conference Proceedings Citation Index (CPCI), BIOSIS Citation Index (BIOSIS), and Current Chemical Reactions (CINS). Addressing the limitations of relying solely on Web of Science, Harzing AW and Alakangas S conducted a comparison of the accuracy of Google Scholar, Scopus, and Web of Science in terms of literature indexing and classification [60]. Their findings revealed that Web of Science exhibits advantages in certain fields. Furthermore, Yeung AWK found that Web of Science can allocate document types more accurately than Scopus [61].
5. Conclusion
In recent years, exosomes have demonstrated significant research value and clinical potential in the field of OA. The growing volume of publications underscores a global recognition of their importance, with numerous significant findings already reported. China and the United States are at the forefront of this research, yet there is a clear need for enhanced collaboration and communication across countries/regions and institutions to further advance the field. Studies have shown that the composition of exosomes changes with the progression of OA, including specific increases or decreases in cytokines or miRNAs during the early, middle, and late stages of the disease. This suggests that exosomes could serve as vital biological markers for diagnosing OA in the future. Moreover, exosomes play a crucial role in mediating intercellular communication within joint tissues, activating various signalling pathways that may help to prevent the progression of OA. There is also promising evidence that exosomes derived from MSCs can repair damaged cartilage and bone. As natural carriers, exosomes contain various biologically active substances including proteins, miRNAs, nucleic acids, and lipids. This allows for targeted drug research based on different contents, providing insights for targets and precise treatments of OA. However, future studies will be necessary to fully unleash the potential of exosomes in the prevention and treatment of OA.
CRediT authorship contribution statement
Hui Xu: Writing – review & editing, Writing – original draft, Funding acquisition, Data curation. Zhen Wang: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Conceptualization. Zheng Wang: Writing – review & editing, Writing – original draft, Software, Project administration, Formal analysis. Juntao Chen: Writing – original draft, Project administration, Investigation, Formal analysis, Data curation. Chi Zhao: Writing – review & editing, Visualization, Validation, Resources, Methodology, Formal analysis. Bingxin Kang: Writing – original draft, Supervision, Methodology, Investigation, Data curation. Xirui Xu: Writing – original draft, Methodology, Data curation, Conceptualization. Jun Shen: Writing – review & editing, Software, Project administration, Investigation. Mengmeng Li: Writing – original draft, Visualization, Supervision, Methodology, Investigation. Jieyao Diao: Writing – original draft, Supervision, Software, Resources, Data curation. Jun Xie: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Data curation. Lianbo Xiao: Writing – review & editing, Writing – original draft, Validation, Supervision, Funding acquisition, Data curation.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the key specialty construction project of Changning District Health Commission in 2023 (No. 20231003); the project supported by Shanghai Science and Technology Commission (No. 21Y11921500); National Advantage Specialty of Traditional Chinese Medicine; Shanghai 2023 ′Science and Technology Innovation Action Plan' Medical Innovation Research Special Project (No. 23Y11921900); Shanghai Health Care Commission Project (No. 202140169); Changning District Science and Technology Commission Project (No. CNKW2020Y23); the 2022 Central Plains Talent Plan (Talent Education Series); the Central Plains Youth Top Talent Project (No. Yu Talent Office [2022] No. 5); and 2024 Henan Provincial Special Project for Scientific Research of Traditional Chinese Medicine (No. 2024ZY3060).
Contributor Information
Jun Xie, Email: leoxie199@126.com.
Lianbo Xiao, Email: xiao_lianbo@163.com.
References
- 1.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J. Osteoarthritis. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Walsh D.A., Sofat N., Guermazi A., Hunter D.J. Osteoarthritis bone marrow lesions. Osteoarthritis Cartilage. 2023;31(1):11–17. doi: 10.1016/j.joca.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Kalamegam G., Memic A., Budd E., Abbas M., Mobasheri A. A comprehensive review of stem cells for cartilage regeneration in osteoarthritis. Adv. Exp. Med. Biol. 2018;1089:23–36. doi: 10.1007/5584_2018_205. [DOI] [PubMed] [Google Scholar]
- 4.Arias C., Salazar L.A. Autophagy and polyphenols in osteoarthritis: a focus on epigenetic regulation. Int. J. Mol. Sci. 2022;23(1):421. doi: 10.3390/ijms23010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z., Huang C., Jiang Q., Zheng Y., Liu Y., Liu S., Chen Y., Mei Y., Ding C., Chen M., Gu X., Xing D., Gao M., He L., Ye Z., Wu L., Xu J., Yang P., Zhang X., Zhang Y., Chen J., Lin J., Zhao L., Li M., Yang W., Zhou Y., Jiang Q., Chu C., Chen Y., Zhang W., Tsai W., Lei G., He D., Liu W., Fang Y., Wu D., Lin J., Wei C., Lin H., Zeng X. Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition) Ann. Transl. Med. 2020;8(19):1–19. doi: 10.21037/atm-20-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glyn-Jones S., Palmer A.J.R., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 7.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 8.Tang X., Wang S., Zhan S., Niu J., Tao K., Zhang Y., Lin J. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol. 2016;68(3):648–653. doi: 10.1002/art.39465. [DOI] [PubMed] [Google Scholar]
- 9.Jacob L.K.K. Osteoarthritis and the incidence of fracture in the United Kingdom: a retrospective cohort study of 258,696 patients. Osteoarthritis Cartilage. 2021;29(2):215–221. doi: 10.1016/j.joca.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Da Costa B.R., Pereira T.V., Saadat P., Rudnicki M., Iskander S.M., Bodmer N.S., Bobos P., Gao L., Kiyomoto H.D., Montezuma T., Almeida M., Cheng P., Hincapie C.A., Hari R., Sutton A.J., Tugwell P., Hawker G.A., Juni P. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. 2021;375 doi: 10.1136/bmj.n2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosenza S., Ruiz M., Maumus M., Jorgensen C., Noel D. Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem Cell-Derived vesicles. Int. J. Mol. Sci. 2017;18(4):889. doi: 10.3390/ijms18040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Fan A., Lu L., Pan Z., Ma M., Luo S., Liu Z., Yang L., Cai J., Yin F. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem. Pharmacol. 2022;206 doi: 10.1016/j.bcp.2022.115343. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y., Wu K., Ding D. Chondrogenic potential of human umbilical cord mesenchymal stem cells cultured with Exosome-Depleted fetal bovine serum in an osteoarthritis mouse model. Biomedicines. 2022;10(11):2773. doi: 10.3390/biomedicines10112773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R., Xu B. TGFβ1-modified MSC-derived exosome attenuates osteoarthritis by inhibiting PDGF-BB secretion and H-type vessel activity in the subchondral bone. Acta Histochem. 2022;124(7) doi: 10.1016/j.acthis.2022.151933. [DOI] [PubMed] [Google Scholar]
- 15.Dong J., Li L., Fang X., Zang M. Exosome-Encapsulated microRNA-127-3p released from bone Marrow-Derived mesenchymal stem cells alleviates osteoarthritis through regulating CDH11-Mediated Wnt/beta-Catenin pathway. J. Pain Res. 2021;14:297–310. doi: 10.2147/JPR.S291472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen C., Lin L., Zou R., Lin F., Liu Y. Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle. 2022;21(3):289–303. doi: 10.1080/15384101.2021.2019411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B., Xing D., Zhu Y., Dong S., Zhao B. The state of exosomes research: a global visualized analysis. BioMed Res. Int. 2019;(1) doi: 10.1155/2019/1495130. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke L., Lu C., Shen R., Lu T., Ma B., Hua Y. Knowledge mapping of drug-induced liver injury: a scientometric investigation (2010-2019) Front. Pharmacol. 2020;11:842. doi: 10.3389/fphar.2020.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Huang L., Liu M., Gao H., Li W. Scientific knowledge graph of acupuncture for migraine: a bibliometric analysis from 2000 to 2019. J. Pain Res. 2021;14:1985–2000. doi: 10.2147/JPR.S314174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.B Rner K., Chen C., Boyack K.W. Visualizing knowledge domains. Annu. Rev. Inf. Sci. Technol. 2003;37:179–255. [Google Scholar]
- 21.William W., HoodConcepción S. Wilson, the literature of bibliometrics, scientometrics, and informetrics. Scientometrics. 2001;52:291–314. [Google Scholar]
- 22.Ma D., Guan B., Song L., Liu Q., Fan Y., Zhao L., Wang T., Zhang Z., Gao Z., Li S., Xu H. A bibliometric analysis of exosomes in cardiovascular diseases from 2001 to 2021. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.734514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S., Gao Y., Liu M., Bu Y., Zhang J. Top 100 most-cited articles on exosomes in the field of cancer: a bibliometric analysis and evidence mapping. Clin. Exp. Med. 2021;21(19):181–194. doi: 10.1007/s10238-020-00624-5. [DOI] [PubMed] [Google Scholar]
- 24.Wu F., Gao J., Kang J., Wang X., Niu Q., Liu J., Zhang L. Knowledge mapping of exosomes in autoimmune diseases: a bibliometric analysis (2002-2021) Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.939433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehghanbanadaki H., Aazami H., Razi F., Nasli-Esfahani E., Norouzi P., Hashemi E. The global trend of exosome in diabetes research: a bibliometric approach. Diabetes Metabol. Syndr. 2022;16(4) doi: 10.1016/j.dsx.2022.102450. [DOI] [PubMed] [Google Scholar]
- 26.Eck N.J.V., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung A.W.K., Mozos I. The innovative and sustainable use of dental panoramic radiographs for the detection of osteoporosis. Int. J. Environ. Res. Publ. Health. 2020;17(7):2449. doi: 10.3390/ijerph17072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X., Yan E., Cui M., Hua W. Examining the usage, citation, and diffusion patterns of bibliometric mapping software: a comparative study of three tools. J Informetr. 2018;12(2):481–493. [Google Scholar]
- 29.Wu H., Cheng K., Guo Q., Yang W., Tong L., Wang Y., Sun Z. Mapping knowledge structure and themes trends of osteoporosis in rheumatoid arthritis: a bibliometric analysis. Front. Med. 2021;8 doi: 10.3389/fmed.2021.787228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Zheng Y., Xia M., Wu Y., Liu X., Xie S., Wu Y., Wang M. Knowledge domain and emerging trends in vinegar research: a bibliometric review of the literature from WoSCC. Foods. 2020;9(2):166. doi: 10.3390/foods9020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Synnestvedt M.B., Chen C., Holmes J.H. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA annual symposium proceedings. AMIA Annu Symp Proc. 2005;2005:724–728. [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C. Science mapping: a systematic review of the literature. J Data Inf Sci. 2017;2(2):1–40. [Google Scholar]
- 33.Tao S., Yuan T., Zhang Y., Yin W., Guo S., Zhang C. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Cosenza S., Ruiz M., Toupet K., Jorgensen C., Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H.P., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Wu J., Kuang L., Chen C., Yang J., Zeng W., Li T., Chen H., Huang S., Fu Z., Li J., Liu R., Ni Z. L, MiR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. doi: 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Mao G., Zhang Z., Hu S., Zhang Z., Chang Z., Huang Z., Liao W., Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018;9:1–13. doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S., Teo K.Y.W., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y., Wang Y., Zhao B., Niu X., Hu B., Li Q., Zhang J., Ding J., Chen Y., Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017;8:1–11. doi: 10.1186/s13287-017-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017;67:56–64. doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Yu D., Liu Z., Zhou F., Dai J., Wu B., Zhou J., Heng B.C., Zou X.H., Ouyang H., Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017;8:1–13. doi: 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato T., Miyaki S., Ishitobi H., Nakamura Y., Nakasa T., Lotz M.K., Ochi M. Exosomes from IL-1 beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014;16(4):1–11. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 45.Zhang B., Yin Y., Lai R.C., Tan S.S., Choo A.B., Lim S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cell. Dev. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 46.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y., Xu X., Xu L., Iqbal Z., Ouyang K., Zhang H., Wen C., Duan L., Xia J. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12(11):4866–4878. doi: 10.7150/thno.69368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., Wang D., Xia J. Chondrocyte-Targeted MicroRNA delivery by engineered exosomes toward a Cell-Free osteoarthritis therapy. Acs Appl Mater Inter. 2020;12(33):36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 49.Jia H., Duan L., Yu P., Zhou Y., Liu R., Wang H. Digoxin ameliorates joint inflammatory microenvironment by downregulating synovial macrophage M1-like-polarization and its-derived exosomal miR-146b-5p/Usp3&Sox5 axis. Int Immunopharmacol. 2022;111 doi: 10.1016/j.intimp.2022.109135. [DOI] [PubMed] [Google Scholar]
- 50.Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., Li W., Liu J., Xiong J., Li B., Xia J., Wang D., Duan L. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- 51.Duan L., Liang Y., Xu X., Xiao Y., Wang D. Recent progress on the role of miR-140 in cartilage matrix remodelling and its implications for osteoarthritis treatment. Arthritis Res. Ther. 2020;22(1):194. doi: 10.1186/s13075-020-02290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong K.L., Zhang S., Wang M., Ren X., Afizah H., Lai R.C., Lim S.K., Lee E.H., Hui J.H.P., Toh W.S. Intra-Articular injections of mesenchymal stem cell exosomes and hyaluronic acid improve structural and mechanical properties of repaired cartilage in a rabbit model. Arthroscopy. 2020;36(8):2215. doi: 10.1016/j.arthro.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S., Wong K.L., Ren X., Teo K.Y.W., Afizah H., Choo A.B.H., Lai R.C., Lim S.K., Hui J.H.P., Toh W.S. Mesenchymal stem cell exosomes promote functional osteochondral repair in a clinically relevant porcine model. Am. J. Sports Med. 2022;50(3):788–800. doi: 10.1177/03635465211068129. [DOI] [PubMed] [Google Scholar]
- 54.Chen C.M. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006;57(3):359–377. [Google Scholar]
- 55.Miao Y., Zhang Y., Yin L. Trends in hepatocellular carcinoma research from 2008 to 2017: a bibliometric analysis. PeerJ. 2018;6 doi: 10.7717/peerj.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rui Wang, Bin Xu. Honggang, TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle. 2018;17(24):2756–2765. doi: 10.1080/15384101.2018.1556063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thoma D.S., Lim H.C., Sapata V.M., Yoon S.R., Jung R.E., Jung U.W. Recombinant bone morphogenetic protein-2 and platelet-derived growth factor-BB for localized bone regeneration. Histologic and radiographic outcomes of a rabbit study. Clin Oral Implan Res. 2017;28(11):e236–e243. doi: 10.1111/clr.13002. [DOI] [PubMed] [Google Scholar]
- 58.Hosseinzadeh M., Kamali A., Baghaban Eslaminejad M., Hosseini S. Higher ratios of chondrocyte to mesenchymal stem cells elevate the therapeutic effects of extracellular vesicles harvested from chondrocyte/mesenchymal stem cell co-culture on osteoarthritis in a rat model. Cell Tissue Res. 2023;394(1):145–162. doi: 10.1007/s00441-023-03819-w. [DOI] [PubMed] [Google Scholar]
- 59.Rizzo M.G., Best T.M., Huard J., Philippon M., Hornicek F., Duan Z., Griswold A.J., Kaplan L.D., Hare J.M., Kouroupis D. Therapeutic perspectives for inflammation and senescence in osteoarthritis using mesenchymal stem cells, mesenchymal stem Cell-Derived extracellular vesicles and senolytic agents. Cells. 2023;12(10):1421. doi: 10.3390/cells12101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venugopal C.C.S.J. Dosage and passage dependent neuroprotective effects of exosomes derived from rat bone marrow mesenchymal stem cells: an in vitro analysis. Curr. Gene Ther. 2017;17(5):379–390. doi: 10.2174/1566523218666180125091952. [DOI] [PubMed] [Google Scholar]
- 61.Li P., Lv S., Jiang W., Si L., Liao B., Zhao G., Xu Z., Wang L., Zhang J., Wu H., Peng Q., Li Z., Qi L., Chi G., Li Y. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann. Transl. Med. 2022;10(18):976. doi: 10.21037/atm-22-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]