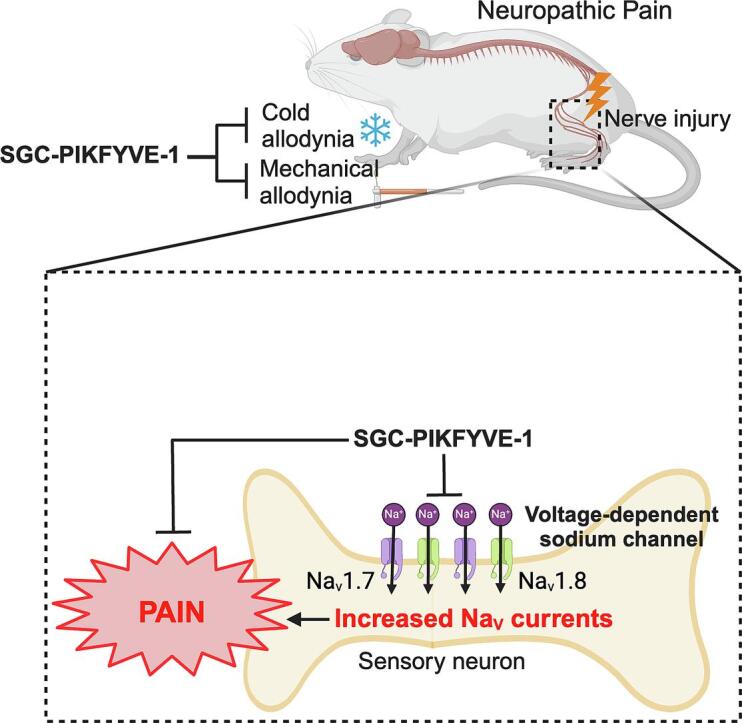
Graphical abstract
Keywords: PIKfyve inhibitor, Pharmacokinetics, Sodium channels, Sensory neurons, Neuropathic pain
Highlights
-
•
The lipid kinase PIKfyve generates phosphatidylinositol (3,5)-bisphosphate (PI(3,5)P2).
-
•
SGC-PIKFYVE-1, a potent and selective inhibitor of PIKfyve, has been used as a chemical probe for this kinase.
-
•
PIKfyve inhibition reportedly changes membrane dynamics and ion transport.
-
•
SGC-PIKFYVE-1 inhibited voltage-gated sodium currents in rat sensory neurons.
-
•
SGC-PIKFYVE-1 reversed spared nerve injury-induced mechanical and cold nociception.
Abstract
PIKfyve (1-phosphatidylinositol 3-phosphate 5-kinase), a lipid kinase, plays an important role in generating phosphatidylinositol (3,5)-bisphosphate (PI(3,5)P2). SGC-PIKFYVE-1, a potent and selective inhibitor of PIKfyve, has been used as a chemical probe to explore pathways dependent on PIKfyve activity. Based on reported changes in membrane dynamics and ion transport in response to PIKfyve inhibition, we hypothesized that pharmacological inhibition of PIKfyve could modulate pain. Acute treatment with SGC-PIKFYVE-1 (10 µM) inhibited voltage-gated sodium currents through the inhibition of Nav1.7 and Nav1.8 channels, without affecting voltage-gated calcium or potassium currents in sensory neurons. Additionally, systemic administration of SGC-PIKFYVE-1 (30 mg/kg) alleviated mechanical and cold sensitivity induced by neuropathic or inflammatory pain in both male and female mice, without causing motor impairments. Although other functions of PIKfyve are well characterized, its role in inhibiting chronic pain has not been fully elucidated. Our study provides proof-of-concept for this alternative approach to pain management. Collectively, these results highlight the inhibitory effects of PIKfyve as a promising avenue for further exploration in chronic pain treatment.
1. Introduction
PIKfyve (1-phosphatidylinositol 3-phosphate 5-kinase), a phosphoinositide kinase, plays crucial roles in neurons by regulating intracellular membrane trafficking and signaling pathways (Rivero-Ríos & Weisman, 2022). It is involved in the synthesis of phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2), a key lipid second messenger that regulates various cellular processes (Sbrissa et al., 2012). In neurons, PIKfyve is essential for maintaining endolysosomal homeostasis, synaptic vesicle trafficking, and neurite outgrowth. Additionally, it modulates neurotransmitter release and receptor recycling, significantly impacting synaptic transmission and neuronal plasticity (Rivero-Ríos & Weisman, 2022). Dysregulation of PIKfyve has been implicated in neurodegenerative diseases such as amyotrophic lateral sclerosis, Alzheimer's, and Parkinson's diseases, highlighting its importance in neuronal function and disease pathogenesis (Barlow-Busch et al., 2023, Hung et al., 2023). Overall, PIKfyve serves as a critical regulator of neuronal physiology and represents a potential therapeutic target for neurological disorders.
While the specific role of PIKfyve in regulating nociceptive sensitization in peripheral sensory neurons remains unknown, several lines of evidence suggest its potential involvement. For instance, PIKfyve induces the internalization and degradation of Cav1.2 channels, a type of voltage-gated calcium channel, in response to N-methyl-d-aspartic acid receptor activation in cortical neurons (Tsuruta et al., 2009). Conversely, knockdown of PIKfyve prevents Cav1.2 degradation and increases neuronal susceptibility to excitotoxicity, suggesting a protective role for PIKfyve-mediated signaling in neuronal health (Tsuruta et al., 2009). Additionally, the pharmacological inhibition or genetic deletion of PIP5K and PI4K, lipid kinases that regulate levels of phosphatidylinositol 4,5-bisphosphate (PIP2), have been associated with attenuated sensitization of TRPV1 channels (Loo et al., 2015). Furthermore, the analysis of tissues from the Pikfyve gene-trap mouse, a hypomorph with ∼10 % of the normal level of PIKfyve protein mutants, revealed that PIKfyve is critical in sensory neurons of dorsal root ganglia (DRG), which contains several ion channels involved in pain signaling (Zolov et al., 2012). Voltage-gated sodium (Na+) channels are essential for membrane excitation in neurons and play a key role in the development and maintenance of several pain syndromes, including inflammatory pain and neuropathic pain. While some current pain treatments targets sodium channels directly, their therapeutic use is often limited by adverse side effects (Dib-Hajj et al., 2009). As a result, an emerging approach in chronic pain management focuses on indirectly targeting these channels by regulating their interactions with cytosolic proteins, offering a potentially safer therapeutic alternative. However, the role of PIKfyve in regulating sodium ion channels, which are major contributors to pain signaling in sensory neurons, remains unclear.
Highly potent and selective small molecule inhibitors of PIKfyve have been characterized, including the recently described PIKfyve chemical probe, SGC-PIKFYVE-1 (Drewry et al., 2022). This compound is proposed to bind non-covalently to the orthosteric site of PIKfyve, resulting in the inhibition of its enzymatic function in vitro. When profiled against the screenable human kinome (403 wild-type human kinases), it was found to be highly selective for PIKfyve. When used at 1 µM, SGC-PIKFYVE-1 inhibited viral entry in multiple cell types and performed comparably to the clinically used PIKfyve inhibitor, apilimod. SGC-PIKFYVE-1 and apilimod also impacted lysosomal homeostasis when SH-SY5Y cells were treated at 5 µM (Drewry et al., 2022).
In this study, we demonstrate that SGC-PIKFYVE-1 is stable in vivo, exhibits excellent oral bioavailability, and is highly brain penetrant. Using electrophysiological recordings, we show that acute treatment with SGC-PIKFYVE-1 inhibits voltage-gated sodium channels but does not affect the activity of voltage-gated calcium or potassium channels expressed in sensory neurons. Additionally, systemic administration of SGC-PIKFYVE-1 produces an antinociceptive effect in a neuropathic pain model. Collectively, our findings provide evidence that PIKfyve modulates the nociceptive signaling and inhibits sodium with no effect on voltage-gated calcium and potassium channels.
2. Material and methods
2.1. Animals
6–8 weeks old male CD-1 and Balb/C mice were used in the rodent pharmacokinetic and brain penetration studies and maintained by Pharmaron, Inc. 10–12 weeks old male C57Bl/6J mice from The Jackson Laboratory, and 4–5 weeks old female Sprague-Dawley rats (∼75 g) from Envigo were housed in acrylic cages under controlled conditions (temperature 23 °C ± 1 °C and 50 % humidity) with 12/12 light–dark cycles and tap water and food ad libitum. All animal procedures and protocols were performed with the approval of University of Florida (IACUC202400000002), following the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985) and the Guidelines on Ethical Standards for Investigation of Experimental Pain in Animals (Zimmermann, 1983).
2.2. General Information for chemical synthesis
Reagents were purchased from reputable commercial suppliers and used as delivered, without purification. The reported 1H spectrum was collected in methanol‑d4 and recorded on a Varian INOVA 400 MHz (MHz) spectrometer. Peak positions are given in parts per million (ppm) and coupling constants (J values) are expressed in hertz (Hz). Purity was assessed via 1H NMR spectrometry and LC–MS. The same protocol as was previously published was used to remake the reported compound (Drewry et al., 2022).
SGC-PIKFYVE-1
The analytical data for SGC-PIKFYVE-1 matches that previously reported (Drewry et al., 2022): 1H NMR (400 MHz, methanol‑d4) δ 8.97 (s, 1H), 7.84 (s, 1H), 7.43 – 7.37 (m, 2H), 4.32 (s, 2H), 3.35 (t, J = 6.5 Hz, 2H), 3.05 (s, 6H), 2.85 – 2.82 (m, 2H), 2.16 – 2.10 (m, 2H). Purity (LC–MS): 100 %. Appearance: light yellow solid.
2.3. Dorsal root ganglion neuron culture
6-week-old female Sprague Dawley rats were euthanized following the guidelines of the American Veterinary Medical Association for the euthanasia of animals. In brief, lumbar and thoracic DRG were dissected and enzymatically dissociated in DMEM media (Cat. No. 11965, Thermo Fisher Scientific, Waltham, MA) containing collagenase type I (1.66 mg/mL; Cat. No. LS004194, Worthington Biochemical, Lakewood, NJ) and neural protease (1.04 mg/mL; Cat. No. LS02104, Worthington Biochemical, Lakewood, NJ) for 50 min at 37 °C with gentle agitation. Subsequently, the sensory neurons were centrifugated at 800 rpm for 5 min and resuspended in complete DRG media (DMEM s upplemented with 1 % penicillin/streptomycin sulfate (Cat. No. 15140, Life Technologies, Carlsbad, CA), 10 % fetal bovine serum, and 15 ng/ml nerve growth factor (Cat. No. N2513, Millipore Sigma, St. Louis, MO). The dissociated cells were then seeded on poly-D-lysine-and laminin-coated coverslips and incubated at 37 °C and 5 % CO2. Cultures were utilized within 24 h after seeding.
2.4. Whole cell patch-clamp recordings of Na+, Ca2+ and K+ in dissociated neurons
The DRG neurons were transferred from the culture medium to an external solution to record voltage-gated sodium channels, as we previously described (Gomez et al., 2023). All the currents were recorded in the presence of either 0.1 % DMSO (control), 1 µM or 10 µM of SGC-PIKFYVE-1, ProTxII 5 nM (Schmalhofer et al., 2008) or VX-548 5 nM (Jones et al., 2023) in the external solution. Each coverslip was kept in the recording chamber for ∼ 50 min.
For sodium currents the external solution contained (in mM): 130 NaCl, 30 tetraethylammonium chloride, 3 KCl, 1 CaCl2, 0.5 CdCl2, 1 MgCl2, 10 D-glucose, and 10 HEPES (pH 7.3, mOsm/L = 310–315). The patch electrodes were elaborated from borosilicate glass capillaries and displayed electrical resistances of ∼ 2–4 mΩ. The internal solution contained (in mM): 140 CsF, 1.1 Cs-EGTA, 10 NaCl, and 15 HEPES (pH 7.3 adjusted with CsOH, and mOsm/L = 300). Current-voltage (I-V) curves were generated by applying 150 ms voltage steps ranging from −70 to + 60 mV with increments of + 5 mV. The holding potential was set at −110 mV. To estimate current density (pA/pF), the current amplitude was normalized to cell size (pF). The voltage-dependence of inactivation protocol was assessed by application of 1-s prepulses from −120 to 0 mV in increments of + 10 mV from a holding potential of −110 mV, followed by a test pulse at + 10 mV. The Nav channels voltage-dependence of inactivation curves were obtained by normalizing the current amplitude at the + 10 mV test pulse to the maximum current (Imax).
For total calcium current recordings, the external solution consisted of the following (in mM): 110 N-methyl-D-glucamine, 10 BaCl2, 30 TEA-Cl, 10 HEPES, 10 glucose, 0.001 TTX (pH 7.29 adjusted with TEA-OH, and mOsm/L = 310). Patch pipettes were filled with an internal solution containing (in mM): 150 CsCl2, 10 HEPES, 5 Mg-ATP, and 5 BAPTA, (pH 7.24 adjusted with CsOH, and mOsm/L = 305). Peak Ca2+ current was acquired by applying 200-millisecond voltage steps from − 70 to + 60 mV in 10-mV increments from a holding potential of − 90 mV to obtain the current–voltage (I-V) relation. Steady-state inactivation (SSI) curves were obtained by applying an H-infinity protocol that consisted of 1.5-seconds conditioning pre-pulses from − 100 to + 30 mV in 10-mV increments followed by a 20-millisecond test pulse to + 10 mV.
To isolate potassium currents (IK+), DRG neurons were bathed in external solution composed of (in mM): 140 N-methyl-glucamine chloride, 5 KCl, 1 MgCl2, 2 CaCl2, 10 D-glucose and 10 HEPES (pH adjusted to 7.3 with KOH and mOsm/L = 313). Recording pipettes were filled with internal solution containing (in mM): 140 KCl, 2.5 MgCl2, 4 Mg-ATP, 0.3Na-GTP, 2.5 CaCl2, 5 EGTA, and 10 HEPES (pH adjusted to 7.3 with KOH and mOsm/L = 320). From a holding potential of −60 mV, total IK+ activation was determined by applying 300-millisecond voltage steps from − 80 to + 60 mV in 10-mV increments. To estimate current density (pA/pF), the current amplitude was normalized to cell size (pF). Voltage-dependence of activation and inactivation curves were fitted with the Boltzmann equation. The voltage-dependence of activation curves were obtained from the I-V protocol using the equation: G = I/(Vmem-Erev), where I represents the current amplitude, Vm is the test potential, and Erev is the extrapolated reversal potential of each neuron. The conductance (G) was normalized to the maximum conductance (Gmax). Activation curves were then fitted with the Boltzmann equation:
Here, G is the conductance, Gmax is the maximal channel conductance obtained from the Boltzmann fit, V0.5 is the voltage corresponding to half maximal activation, Vm is the potential and κ is a slope factor.
Inactivation curves were obtained by dividing the peak current recorded at the test pulse by the maximal current (Imax) steady-state inactivation (SSI) was fitted with the equation:
Where I is the current, Imax represents the maximal current obtained from the Boltzmann fit, V0.5 is the potential for half-maximal inactivation of Imax, Vm is the pre-pulse membrane potential, and κ is a slope factor.
Capacitive artifacts and series resistance were compensated. Recordings made from cells with greater than a 20 % shift in series resistance compensation error were excluded from the analysis. All experiments were performed at room temperature (∼23 °C). The experimenters were blinded to the conditions tested. We recorded from unstained small-diameter DRGs (ranging between 7─20 picoFarads (pF)), which correspond to C-fibers, characterized by having unmyelinated axons and known be involved in pain transmission.(Fauci et al., 2008) The cell sizes (in pF) of DRGs recorded in this set of experiments (DMSO 13.42 ± 1.43, SGC-PIKFYVE-1 12.98 ± 1.095; p = 0.7664).
2.5. Rodent pharmacokinetic studies
Snapshot or standard pharmacokinetic (PK) was performed as previously described (Potjewyd et al., 2022, Yang et al., 2023) at Pharmaron Inc using male CD-1 mice (6–8 weeks, 20–30 g) or male Balb/C mice (6–8 weeks, 20–30 g) dosed either by intraperitoneally (IP) or orally (PO). Two animals were dosed per route of administration for snapshot PK experiments and three animals were dosed per route of administration for standard PK experiments. Since SGC-PIKFYVE-1 was prepared as a TFA salt, the actual dose level was calculated using a calibration factor (1.34) to account for the salt. For IP and PO administration at 10 mg/kg, a single dose of the compound was administered as a 0.74 mg/mL solution in NMP/Solutol/PEG-400/normal saline (v/v/v/v, 10:5:30:55). For IP and PO administration at 30 mg/kg, a single dose of the compound was administered as a 2.23 mg/mL solution in NMP/Solutol/PEG-400/normal saline (v/v/v/v, 10:5:30:55). Mice were given free access to food and water. Approximately 0.3 mL of blood was collected via the dorsal metatarsal vein at 0.5, 1, 3, and 5 h post-dose for snapshot PK or at 0.8, 0.25, 0.5, 1, 2, 4, 8, and 24 h post-dose for standard PK. Blood from each sample was transferred into a plastic microcentrifuge tube containing EDTA-K2 (anticoagulant), and then centrifuged at 4000g for 5 min at 4 °C to separate and collect plasma. Samples were stored at −75 °C until analysis. Analyses of samples were carried out using a Prominence LC-30AD, AB Sciex Triple Quan 5500 LC–MS/MS instrument fitted with one of the following columns: HALO 9A C18 (2.7 μm, 2.1 × 50 mm), YMC-Triart C18 (3 µm, 2.1 x 33 mm), or YMC-Triart C18 (3 µm, 3.0 x 50 mm). The same mobile phase of 5–95 % acetonitrile in water with 0.1 % formic acid was used with each column.
2.6. Brain penetration studies
Brain penetration studies was conducted as previously described (Potjewyd et al., 2022) at Pharmaron Inc using three male CD-1 mice (6–8 weeks, 20–30 g) dosed by IP administration. Since SGC-PIKFYVE-1 was prepared as a TFA salt, the actual dose level was calculated using a calibration factor (1.34) to account for the salt. To achieve a 30 mg/kg concentration, a single dose of the compound was administered as a 2.23 mg/mL solution in NMP/Solutol/PEG-400/normal saline (v/v/v/v, 10:5:30:55). Mice were given free access to food and water. To analyze plasma concentrations of SGC-PIKFYVE-1, approximately 0.3 mL of blood was collected via the dorsal metatarsal vein at 1-hour post-dose. Blood from this sample was transferred into a plastic microcentrifuge tube containing EDTA-K2 (anticoagulant), and then centrifuged at 4000g for 5 min at 4 °C to separate and collect plasma. Samples were stored at −75 °C until analysis. At 1-hour post-dose, mice were euthanized and fully exsanguinated prior to brain collection. The brain samples were collected and stored in weighted tubes at −75 °C until analysis. Brain samples were weighed and homogenized with PBS at a ratio of 1 g of brain tissue to 3 mL of PBS volume (W/V, 1:3) prior to analysis. The final concentration of SGC-PIKFYVE-1 was the detected value corrected by a multiplication factor of four. Analyses of samples were carried out using a Prominence LC-30AD, AB Sciex Triple Quan 5500 LC–MS/MS instrument fitted with a YMC-Triart C18 (3 µm, 2.1 x 33 mm) column. A mobile phase of 5–95 % acetonitrile in water with 0.1 % formic acid was used for elution.
2.7. Spared nerve injury (SNI)
All mice surgeries were conducted following a previously published (Decosterd & Woolf, 2000). In brief, mice were anesthetized with isoflurane (5 % for induction and 2 % for maintenance). The branches of sciatic nerve were surgically exposed, and common peroneal and tibial branches were ligated with 7–0 silk suture and axotomized at 2 mm distal to the ligation, leaving the sural branch intact.
2.8. Capsaicin pain model
Male and female mice were injected with either 12 µg in 10 µL capsaicin or vehicle (80 % saline, 10 % ethanol, 10 % Tween80) in the left footpad using a 30G syringe, while under anesthesia with isoflurane (5 % for induction and 2 % for maintenance). One hour after the capsaicin injection, mechanical allodynia was assessed using calibrated von Frey filaments.
2.9. Nociceptive behaviors
Animals were habituated in transparent acrylic cages on a mesh grid floor for 60 min before the experiment. Immediately after the habituation period, von Frey filaments were used to determine the 50 % paw withdrawal threshold using the up-down method as previously described (Chaplan et al., 1994, Dixon, 1980). The 50 % withdrawal threshold was determined according to the following equation:
50 % Threshold (g) = (10^[Xf + κδ]/10000).
Where Xf is the value of the last von Frey filament used (in logarithmic units), κ is the correction factor based on the response patterns of a calibration table and the tabulated value based on the pattern of positive and negative responses, and δ indicates the average differences between stimuli logarithmic units (Chaplan et al., 1994). The presence of mechanical allodynia was considered when the paw mechanical withdrawal threshold was < 0.2 g.
Cold allodynia was determined as previously described (Gomez et al., 2023). After the von Frey test, 20 µL of acetone (Sigma-Aldrich, MO, USA) were applied onto the lateral side of the plantar surface of each hind paw using a syringe connected to PE-90 tubing. The cumulative time spent licking, slapping, flinching, or shaking the hind paw for 60 s was used to determine the aversion time to a cold stimulus.
2.10. Evaluation of motor coordination
Motor performance was assessed using the Rotarod test. Male and female were placed on a rotating cylinder set at a constant speed of 14 rpm. Training sessions were conducted over two consecutive days before the test, with one session per day (10 and 5 min, respectively). On the day of the experiment, the effect of intraperitoneal injection of SGC-PIKFYVE-1 (30 mg/kg) on motor function was evaluated by recording the latency of fall over a 5-minute period. Each time a mice fell from the Rotarod, it was placed back on the cylinder, and the session continued.
2.11. Compound administration
To mitigate the stress induced by injecting compounds into mice, SGC-PIKFYVE-1 was dissolved in 30 % DMSO and administrated at dose of 30 mg/kg via intraperitoneal injection and then a 1-hour waiting period was allowed before assessing the mechanical and cold allodynia.
2.12. Statistical analysis
PK parameters were calculated from the mean plasma concentration versus time and a non-compartmental model using WinNonlin 8.3 (Phoenix). Brain penetration was calculated using the plasma concentration and brain concentration data for each mouse to calculate a ratio in each animal, and then these ratios were averaged. PK and brain penetration studies are presented as standard deviation (SD) and coefficient of variation (CV). The differences for mechanical allodynia were calculated by 2-way analysis of variance (ANOVA) followed by the Tukey test because independent groups were used. Statistical analyze was performed using SigmaPlot v12.0 (Systat Software Inc., Chicago, IL). The plots were generated using GraphPad Prism 10 (GraphPad Software Inc., La Jolla, CA). The sample size for behavioral assays was calculated with 80 % power and an expected effect size of d = 2.2 in behavioral experiments, with a significance level set to 0.05 using G*Power (version 3.1.9.2), as previously described (Barragán-Iglesias et al., 2019). Electrophysiology data were assessed for Gaussian distribution using a D’Agostino-Pearson test using GraphPad Prism 10 (GraphPad Software Inc., La Jolla, CA). Statistical significance of differences between means was determined using Student’s t-test or one-way ANOVA test, followed by a Tukey’s multiple comparations test for peak current density and voltage-dependence of activation and inactivation parameters, unless otherwise specified. All values are presented as the mean ± SEM. Statistical significance was accepted at P < 0.05.
3.0. Results
3.1 SGC-PIKFYVE-1 is stable and well tolerated in vivo.
Previously, we introduced SGC-PIKFYVE-1 (Supplementary Fig. 1A, analog 17 in original publication (Drewry et al., 2022)) as a cell-active chemical probe that potently and selectively inhibits PIKfyve. However, previous studies using this compound have been limited to in vitro experiments. To determine whether this compound is suitable for in vivo applications, we first administered SGC-PIKFYVE-1 to young, non-diseased CD-1 mice. Initial snapshot PK studies monitored the stability of SGC-PIKFYVE-1 over a five-hour time course when dosed at 10 mg/kg either via IP or PO administration. These data showed that SGC-PIKFYVE-1 exhibited oral bioavailability and remained relatively stable in vivo (Drewry et al., 2022) (Supplementary Fig. S1B). Subsequently, a longer time course of twenty-four hours was assessed in CD-1 mice. At the same dose (10 mg/kg), SGC-PIKFYVE-1 was no longer detectable after eight hours (note: no time points were sampled between eight and twenty-four hours) (Supplementary Fig. S1C). These initial PK studies demonstrated that SGC-PIKFYVE-1 exhibits promising stability and bioavailability in vivo, supporting its potential suitability for further in vivo applications.
A higher dose of 30 mg/kg was next administered either IP or PO, and the plasma concentration of SGC-PIKFYVE-1 was monitored for twenty-four hours. Despite the higher dose, SGC-PIKFYVE-1 was still not detectable after eight hours (Supplementary Fig. S1D). However, SGC-PIKFYVE-1 exhibited similar stability in vivo with a 3.5-hour half-life when administered orally and did not induce any adverse effects in the mice. To sustain the in vivo concentration of SGC-PIKFYVE-1, we next dosed twice daily (BID) at 30 mg/kg IP or PO. This dosing regimen enabled maintenance of the plasma concentration of SGC-PIKFYVE-1 well above its cellular affinity for PIKfyve (Supplementary Fig. S1E). Finally, to determine whether the PK results observed in CD-1 mice were consistent in other strains of mice, we dosed Balb/C mice with 10 or 30 mg/kg SGC-PIKFYVE-1 IP or PO. The plasma concentration of SGC-PIKFYVE-1 was evaluated up to five hours after dosing and found to be maintained well above the cellular affinity for PIKfyve in this second strain of mice (Supplementary Fig. S2). These additional PK studies helped refine an optimal dosing regimen for SGC-PIKFYVE-1 and confirmed that it exhibits promising stability and bioavailability in mice.
3.2. SGC-PIKFYVE-1 is highly brain penetrant
The well-tolerated 30 mg/kg dose was employed to ascertain the brain penetration of SGC-PIKFYVE-1 in CD-1 mice one hour after dosing. Analysis of multiple mice treated with this dose and sampling of brain and plasma concentrations revealed a mean brain/plasma ratio of 0.6, indicating significant brain penetrance by SGC-PIKFYVE-1 (Table S1 and S2). This result confirmed that an appreciable concentration of SGC-PIKFYVE-1 reaches the mouse brain.
3.3. SGC-PIKFYVE-1 inhibits Nav channels in sensory neurons
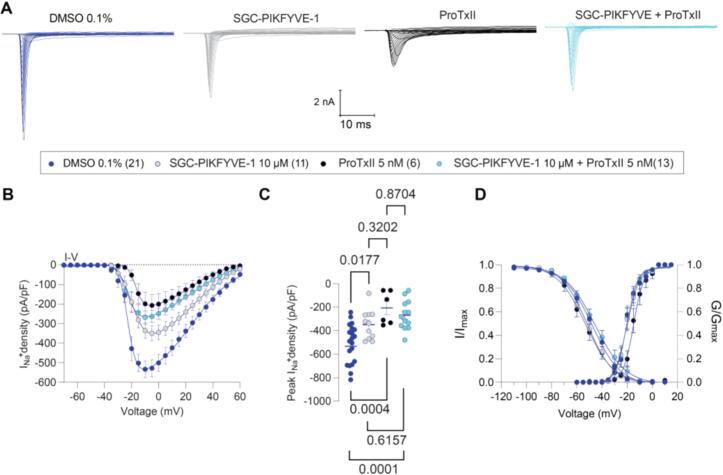
Voltage-gated sodium channels (VGSCs) are important mediators of neuronal excitability, essential for initiating and propagating action potentials to the central nervous system (CNS) (Bennett et al., 2019). Notably, Nav1.7, Nav1.8 and Nav1.9 are abundantly expressed in the peripheral terminals along the axons and within the cell bodies of sensory neurons in both DRG and trigeminal ganglia (TG) (Bennett et al., 2019). Dysregulation or aberrant expression of these sodium channel subtypes has been implicated in neuropathic pain conditions (Aromolaran and Goldstein, 2017, Emery et al., 2016). To assess the effect of SGC-PIKFYVE-1 on total Nav currents, whole-cell voltage-clamp recordings were conducted to obtain current–voltage (I-V) relationships from cultured small (cell sizes 7–20 picoFarads) rat DRG neurons in the presence of the compound in the bath solution. Acute application of SGC-PIKFYVE-1 at 10 µM, but not at 1 µM, resulted in a decrease in the density of sodium currents (Table 1; Fig.1, Fig.2). To determine which subtypes of sodium channels are involved in this effect, we used blocked Nav1.7 channels with the selective blocker ProToxin-II (ProTxII) (Schmalhofer et al., 2008) and Nav.18 with its selective blocker VX-548 (Jones et al., 2023). The acute application of ProTxII alone led to a reduction in sodium currents comparable to the reduction observed when ProTxII was coapplied with 10 µM SGC-PIKFYVE-1 (Fig. 1A-C). The voltage-dependence of activation remained unchanged in the presence of SGC-PIKFYVE-1 compared to the vehicle (Table 1 and Fig. 1D). In contrast, SGC-PIKFYVE-1 induced a 4-mV hyperpolarizing shift in the voltage-dependence of inactivation (Table 1 and Fig. 1D). Examination of the maximal slope conductance and the I-V data at the more depolarized potentials shows that SGC-PIKFYVE-1 causes a small reduction on whole cell sodium current that is augmented further by protoxin and is also quite a bit less pronounced than the effect of protoxin itself, suggesting that there is a substantial Nav1.7 component left that is unaffected by the inhibitor.
Table 1.
Biophysical properties of the assessed voltage gated channels from rat DRG neurons in the absence and presence of SGC-PIKFYVE-1.
| Condition | IV curve | Activation | Fast inactivation | |||||
|---|---|---|---|---|---|---|---|---|
|
Peak/density (pA/pF) |
n |
V0.5 (mV) |
k | n |
V0.5 (mV) |
k | n | |
| Total sodium currents + 0.1 % DMSO | −281.2 ± 32.24 | 15 | −21.776 ± 0.50 | 4.93 ± 0.44 | 15 | −53.6 ± 1.56 | −13.64 ± 1.53 | 15 |
| Total sodium currents + SGC-PIKFYVE-1 (1 µM) | −370.4 ± 46.81 | 20 | −21.09 ± 0.74 | 6.54 ± 0.66 | 20 | −51.69 ± 1.67 | −14.49 ± 1.69 | 20 |
| Total sodium currents + 0.1 % DMSO | −533.2 ± 42.99 | 21 | −20.48 ± 0.48 | 4.25 ± 0.42 | 21 | −47.42 ± 0.79 | −11.11 ± 0.74 | 21 |
| Total sodium currents + SGC-PIKFYVE-1 (10 µM) | −348.7 ± 38.42* | 11 | −20.11 ± 0.63 | 4.89 ± 0.56 | 11 | −51.51 ± 0.79* | −10.32 ± 0.73 | 11 |
| Total sodium currents + ProTxII (5 nM) | −207.3 ± 57.12* | 6 | −14.89 ± 0.57* | 4.17 ± 0.49 | 6 | −53.17 ± 1.24* | −11.11 ± 1.15 | 6 |
| Total sodium currents + SGC-PIKFYVE-1 (10 µM) + ProTxII (5 nM) | −268.8 ± 34.33 | 13 | −20.23 ± 0.48 | 4.00 ± 0.42 | 13 | −45.41 ± 1.28 | −12.06 ± 1.21 | 13 |
| Total sodium currents + 0.1 % DMSO | −358.2 ± 32.87 | 16 | −24.61 ± 0.44 | 3.70 ± 0.39 | 16 | −52.39 ± 1.14 | −12.25 ± 1.08 | 16 |
| Total sodium currents + SGC-PIKFYVE-1 (10 µM) | −189.7 ± 47.11* | 6 | −20.18 ± 0.55 | 3.73 ± 0.47 | 6 | −58.85 ± 1.79* | −10.25 ± 1.63 | 6 |
| Total sodium currents + VX-548 (5 nM) | −200.5 ± 35.37* | 11 | −19.51 ± 0.98* | 6.18 ± 0.88* | 11 | −59.85 ± 1.10* | −9.98 ± 0.99 | 11 |
| Total sodium currents + SGC-PIKFYVE-1 (10 µM) + VX-548 (5 nM) | −186.1 ± 26.63 | 14 | −21.43 ± 0.61* | 4.85 ± 0.53 | 14 | −59.37 ± 1.23* | −10.62 ± 1.12 | 14 |
| Total calcium currents + 0.1 % DMSO | −107.71 ± 14.20 | 20 | −4.68 ± 0.73 | 5.99 ± 0.64 | 20 | −36.91 ± 0.73 | 0.99 ± 0.01 | 20 |
| Total calcium currents + SGC-PIKFYVE-1 (10 µM) | −113.06 ± 13.24 | 20 | −4.31 ± 0.67 | 5.73 ± 0.58 | 20 | −34.76 ± 1.79 | 0.98 ± 0.02 | 20 |
| Total potassium currents + 0.1 % DMSO | 184.90 ± 17.17 | 15 | − | − | − | − | − | − |
| Total potassium currents + SGC-PIKFYVE-1 (10 µM) | 213.92 ± 21.52 | 12 | − | − | − | − | − | − |
Values are presented as means ± SEM. Gating properties were calculated from fits of the data obtained from the indicated number of individual cells to the Boltzmann equation. The values include V0.5, which represents the midpoint potential (mV) for voltage-dependent of activation or inactivation, and k, the slope factor. These values pertain to Fig.2, Fig.3, Fig.4. * p < 0.05 with respect to 0.1 % DMSO.
Fig.1.
SGC-PIKFYVE-1 decreases total Nav currents recorded in DRG neurons. (A) Representative traces of total Na+ currents recorded from a DRG neuron under control conditions (dark blue), in the presence of 10 µM of SGC-PIKFYVE-1 (gray), 5 nM ProTxII (black) or SGC-PIKFYVE-1 + ProTxII (light blue) in the external solution. (B) Summary of current density versus voltage (I-V) curves obtained from the previously described conditions. (C) Peak current density plot as in (B). (D) Voltage dependence of activation obtained from current traces as in (B), both current density and channel conductance were normalized to their corresponding maximal values (i.e., Imax and Gmax) that were calculated from each DRG neuron. The average maximal values (Gmax and Imax), and the kinetic parameters describing the corresponding voltage dependences of activation and inactivation are given in Table 1. Data are presented as the mean ± SEM.
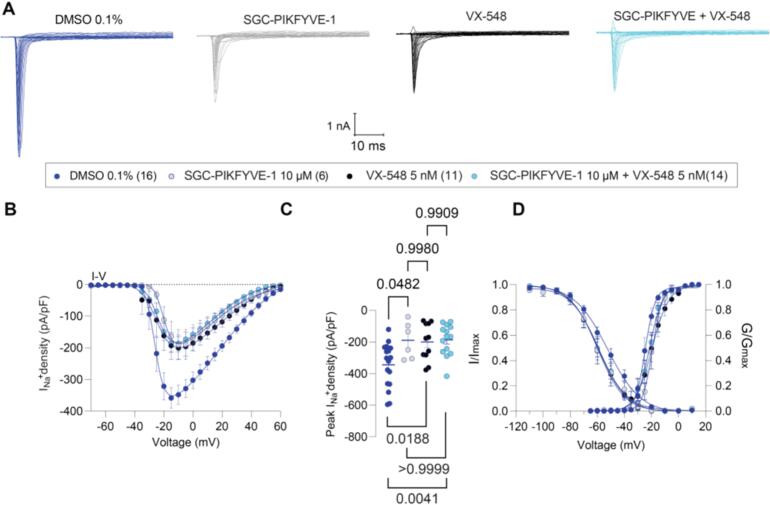
Fig.2.
SGC-PIKFYVE-1 decreases total Nav currents recorded in DRG neurons via NaV1.8 channels. (A) Representative traces of total Na+ currents recorded from a DRG neuron under control conditions (dark blue), in the presence of 10 µM of SGC-PIKFYVE-1 (gray), 5 nM VX-548 (black) or SGC-PIKFYVE-1 + VX-548 (light blue) in the external solution. (B) Summary of current density versus voltage (I-V) curves obtained from the previously described conditions. (C) Peak current density plot as in (B). (D) Voltage dependence of activation obtained from current traces as in (B), both current density and channel conductance were normalized to their corresponding maximal values (i.e., Imax and Gmax) that were calculated from each DRG neuron. The average maximal values (Gmax and Imax), and the kinetic parameters describing the corresponding voltage dependences of activation and inactivation are given in Table 1. Data are presented as the mean ± SEM.
Furthermore, we also assessed the contribution of Nav1.8 channels to the effect of 10 µM SGC-PIKFYVE-1. Our results showed that the inhibition caused by the Nav1.8-selective blocker VX-548 (Jones et al., 2023) did not increase when coapplied with 10 µM SGC-PIKFYVE-1 in the external solution (Fig. 2A-C). The I-V data with SGC-PIKFYVE-1 shows that the inhibition completely overlays that of VX-548 and the combination of VX-548 plus SGC-PIKFYVE-1 − this suggests that Nav1.8 inhibition fully precludes the effects of PIKfyve modulation and indicates that it is mostly Nav1.8 that is the target. Collectively, these results show that SGC-PIKFYVE-1 reduces total voltage-gated sodium currents in DRG neurons by inhibiting both Nav1.7 and Nav1.8 (preferentially) channels.
3.4. SGC-PIKFYVE-1 does not inhibit Cav channels in sensory neurons
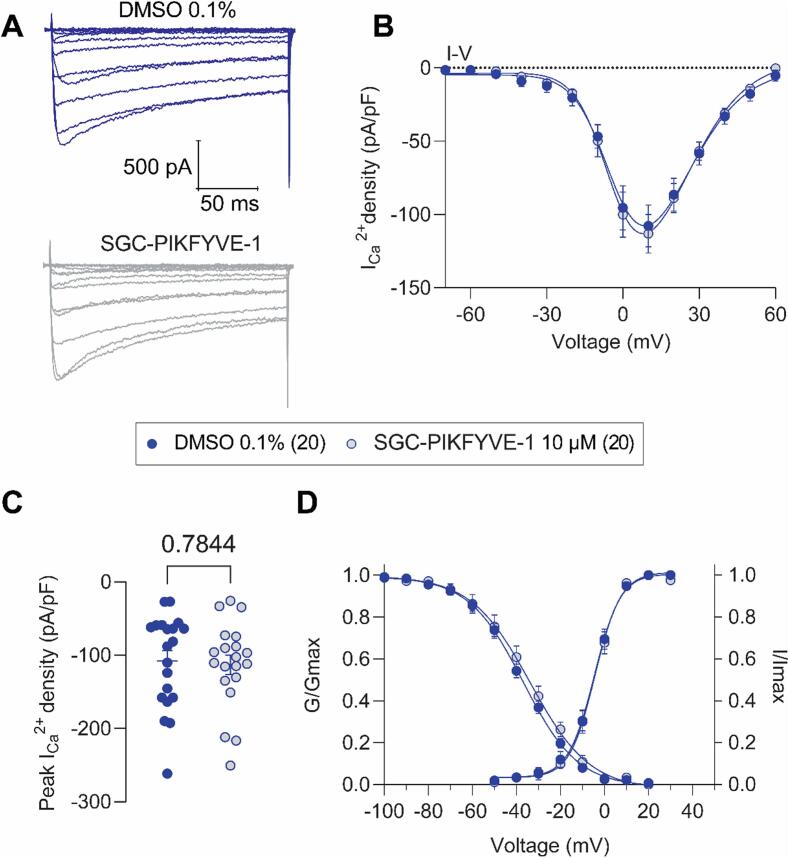
Voltage-gated calcium channels (VGCCs) are functionally expressed in DRG neurons where they play an important role in the transmission and modulation of pain signals (Aromolaran & Goldstein, 2017). To investigate whether the VGCCs currents are modulated by SGC-PIKFYVE-1, we performed whole-cell patch-clamp recordings on isolated rat DRG neurons. Fig. 3A shows representative family of traces, where the amplitude of total calcium currents in DRG neurons pre-treated with SGC-PIKFYVE-1 appeared similar compared with the control condition (0.1 % DMSO). The current amplitude was normalized to the cell size and the subsequent current density was plotted versus the voltage applied (Fig. 3B). Both the current density (Fig. 3B) and the peak current (Fig. 3C) were unaffected by SGC-PIKFYVE-1. Moreover, SGC-PIKFYVE-1 did not affect the biophysical properties of steady-state inactivation and activation (Fig. 3C and Table 1). Taken together, our results show that PIKfyve inhibition does not affect calcium channels.
Fig.3.
SGC-PIKFYVE-1 does not affect total Ca2+ currents recorded in DRG neurons. (A) Representative traces of total Ca2+ currents recorded from a DRG neuron under control conditions (top) or a neuron in presence of 10 µM of SGC-PIKFYVE-1 in the external solution (bottom). (B) Summary of current density versus voltage (I-V) curves obtained from a total of 20 control (0.1 % DMSO; blue circles) and 20 SGC-PIKFYVE-1-treated DRG neurons (circles filled in light grey). (C) Peak current density plot as in (B). (D) Voltage dependence of activation obtained from current traces as in (B), both current density and channel conductance were normalized to their corresponding maximal values (i.e., Imax and Gmax) that were calculated from each DRG neuron. The average maximal values (Gmax and Imax), and the kinetic parameters describing the corresponding voltage dependences of activation and inactivation are given in Table 1. Data are presented as the mean ± SEM.
3.5. SGC-PIKFYVE-1 does not inhibit potassium channels in sensory neurons
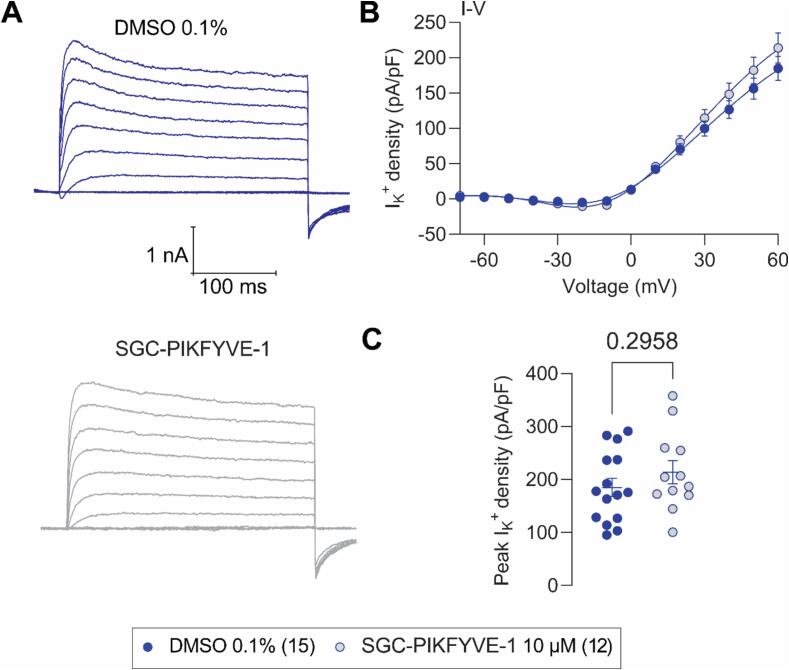
As potassium ions are critical components in generating action potentials and modulating neuronal excitability and by extension, propagating nociceptive signaling, we used whole cell patch-clamp electrophysiology to assess the effects of SGC-PIKFYVE-1 on potassium currents (IK+) in rat DRG neurons. Fig. 4A shows representative family of current traces of total IK+ following acute treatment with 10 μM SGC-PIKFYVE-1, or control (0.1 % DMSO), as indicated. Treatment with SGC-PIKFYVE-1 did not alter IK+ current or peak densities (Fig. 4A-C).
Fig.4.
SGC-PIKFYVE-1 does not affect total K+ currents recorded in DRG neurons. (A) Representative traces of total K+ currents recorded from a DRG neuron under control conditions (top) or a neuron in presence of 10 µM of SGC-PIKFYVE-1 in the external solution (bottom). (B) Summary of current density versus voltage (I-V) curves obtained from a total of 15 control (0.1 % DMSO; blue circles) and 12 SGC-PIKFYVE-1-treated DRG neurons (circles filled in light grey). (C) Peak current density plot as in (B).
3.5. SGC-PIKFYVE-1 exerts an antinociceptive effect in both neuropathic male and female mice
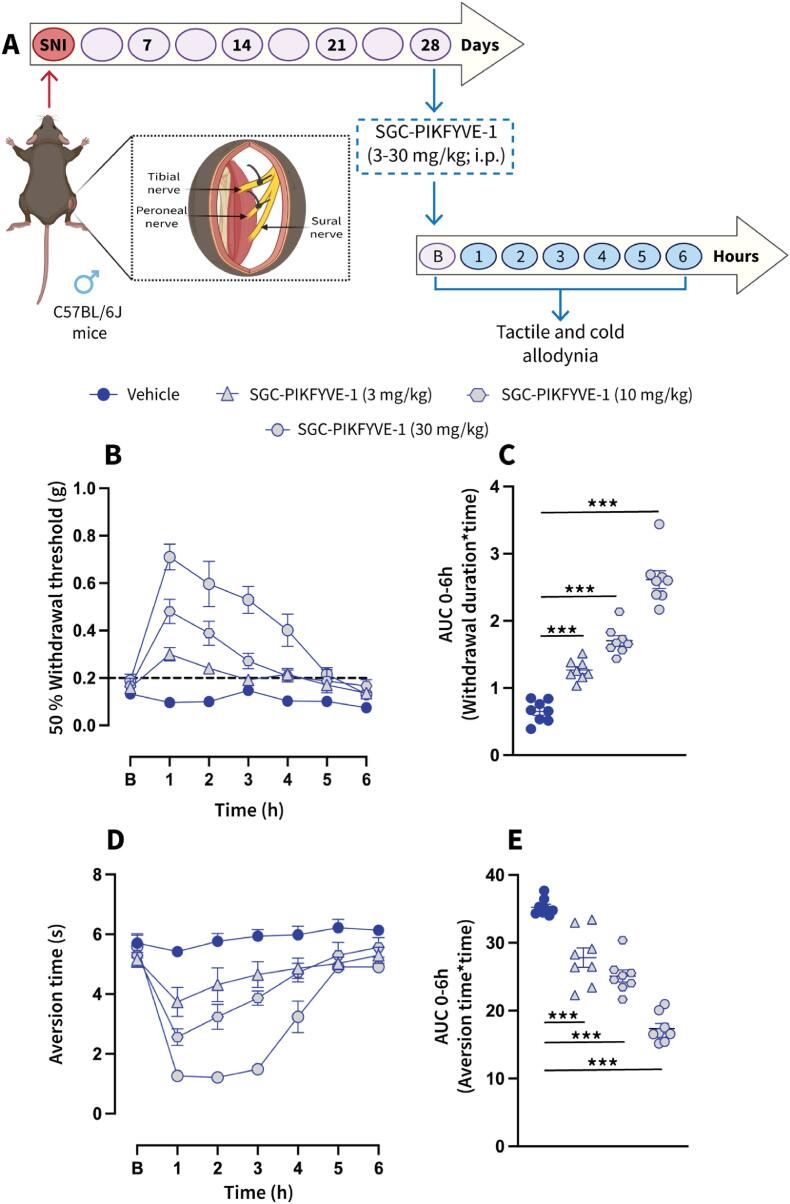
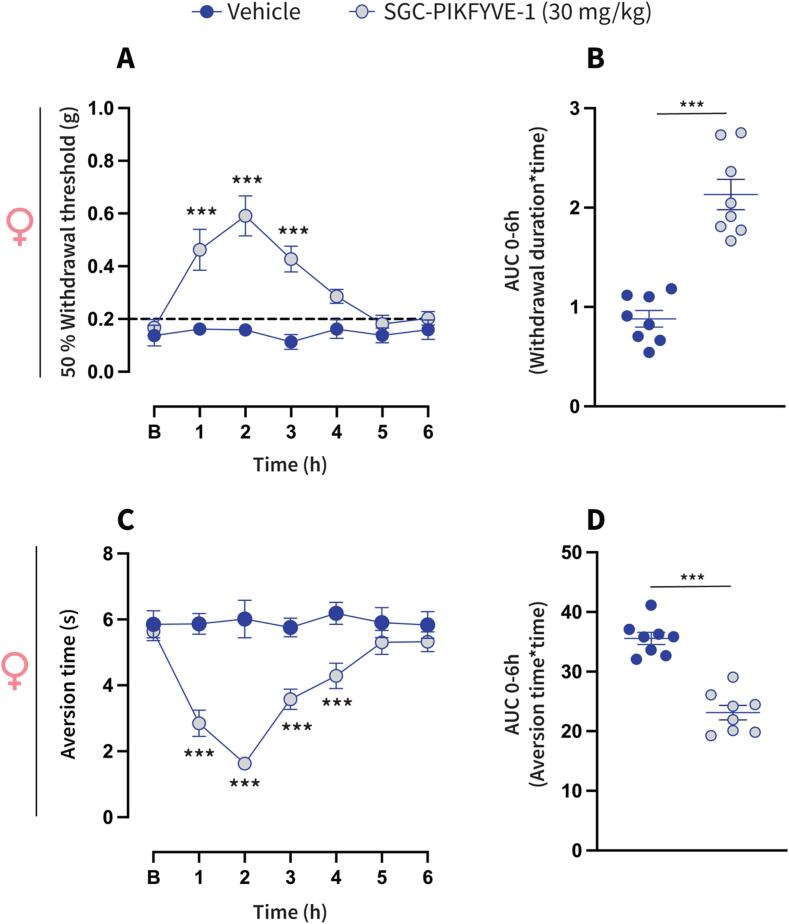
Neuropathic pain is characterized by enhanced excitability of sensory neurons, resulting in exaggerated or spontaneous firing of action potentials (Liu et al., 2000, Wu et al., 2001). Our electrophysiological recordings indicate that SGC-PIKFYVE-1 reduces neuronal excitability in DRG neurons. Therefore, we investigated the potential antinociceptive properties of SGC-PIKFYVE-1 in vivo. For that purpose, we used the spared nerve injury (SNI) model, a well-establish peripheral neuropathic pain model that mimics symptoms such as the mechanical and cold allodynia observed in patients with neuropathic pain (Decosterd & Woolf, 2000). We assessed the effect of intraperitoneal injection of SGC-PIKFYVE-1 on mechanical allodynia twenty-eight days post-injury, a time at which neuropathic pain-like behaviors have been firmly established (Fig. 5A). Our results show that a single injection of SGC-PIKFYVE-1 (30 mg/kg) induced an increase of the paw mechanical withdrawal threshold for four hours in male neuropathic mice (Fig. 5B). The antiallodynic effect of SGC-PIKFYVE-1 in male neuropathic mice was dose-dependent (3, 10 and 30 mg/kg) (Fig. 5C). As cold allodynia is a significant symptom experienced by many neuropathic pain patients (MacDonald et al., 2021), we explored the effect of SGC-PIKFYVE-1 administration (3, 10 and 30 mg/kg) on this behavior in the neuropathic mice. Intraperitoneal injection of SGC-PIKFYVE-1 reduced SNI-induced cold allodynia for six hours in a dose-dependent manner in male mice (3, 10 and 30 mg/kg) (Fig. 5D-E). Since sex plays an important role in the analgesic effect of different drugs (Mogil et al., 2024), we determine the effect of the highest dose of SGC-PIKFYVE-1 (30 mg/kg) in female neuropathic mice. Intraperitoneal administration of SGC-PIKFYVE-1 reduced SNI-induced tactile (Fig. 6A-B) and cold (Fig. 6C-D) allodynia in female mice. No differences were observed in mechanical (F=(1,1,6) = 1.607, p = 0.146) or cold allodynia (F=(1,1,6) = 1.437, p = 0.202). Collectively, these results suggest that SGC-PIKFYVE-1 induces an antinociceptive effect in neuropathic male and female mice likely mediated by the blockade of sodium channels.
Fig.5.
Intraperitoneal administration of SGC-PIKFYVE-1 reduces spared nerve injury (SNI)-induced nociceptive behaviors in male mice. (A) SNI model schematic and timeline of the experimental approach used to determine the antinociceptive effects of SGC-PIKFYVE-1 on tactile and cold allodynia in male mice. (B) Baseline paw withdrawal threshold measurements were conducted before intraperitoneal administration of SGC-PIKFYVE-1 in male mice with neuropathic pain. The time course of a single intraperitoneal (IP) injection of SGC-PIKFYVE-1 (3–30 mg/kg) or vehicle (DMSO 30 %) on mechanical allodynia were measured from 0 to 6 h post injection on day 28 post-SNI (n = 8 mice). Von Frey filaments were used to determine the 50 % paw withdrawal threshold using the up-down method. Results were compared using two-way ANOVA with factors: time * treatment, followed by the Tukey post hoc test. **P < 0.01 and ***P < 0.001 vs vehicle group. (C) Quantification of area under the curve (AUC) of the time course of the effect induced by SGC-PIKFYVE-1 from 0 to 6 h (n = 8 mice). Results were analyzed using one-way ANOVA, followed by the Dunnett post hoc test. ***P < 0.001 vs vehicle group. (D) Baseline aversion time responses were performed before SGC-PIKFYVE-1 in male neuropathic mice. The time course of a single intraperitoneal (IP) injection of SGC-PIKFYVE-1 (30 mg/kg) or vehicle (DMSO 30 %) on cold allodynia were measured from 0 to 6 h post injection on day 28 post-SNI (n = 8 mice). Results were compared using two-way ANOVA, with factors: time * treatment, followed by the Tukey post hoc test. (E) Quantification of AUC in panel D between 0 and 6 h. SGC-PIKFYVE-1 (30 mg/kg) reversed SNI-induced cold allodynia. ***P < 0.001 vs vehicle group; one-way ANOVA followed by the Dunnett post hoc test. n = 8 mice. For all panels, error bars indicate mean ± SEM. B, Baseline.
Fig.6.
SGC-PIKFYVE-1 reduces nociceptive behaviors inducedby nerve injury in female mice (A) Baseline paw withdrawal threshold measurements were conducted before intraperitoneal administration of SGC-PIKFYVE-1 in female mice with neuropathic pain. The time course of a single intraperitoneal (IP) injection of SGC-PIKFYVE-1 (30 mg/kg) or vehicle (DMSO 30 %) on mechanical allodynia were measured from 0 to 6 h post injection on day 28 post-SNI (n = 8 mice). Von Frey filaments were used to determine the 50 % paw withdrawal threshold using the up-down method. Results were compared using two-way ANOVA with factors: time * treatment, followed by the Tukey post hoc test. **P < 0.01 and ***P < 0.001 vs vehicle group. (B) Quantification of area under the curve (AUC) of the time course of the effect induced by SGC-PIKFYVE-1 from 0 to 6 h (n = 8 mice). Results were analyzed using one-way ANOVA, followed by the Dunnett post hoc test. ***P < 0.001 vs vehicle group. (C) Baseline aversion time responses were performed before SGC-PIKFYVE-1 in female neuropathic mice. The time course of a single intraperitoneal (IP) injection of SGC-PIKFYVE-1 (30 mg/kg) or vehicle (DMSO 30 %) on cold allodynia were measured from 0 to 6 h post injection on day 28 post-SNI (n = 8 mice). Results were compared using two-way ANOVA, with factors: time * treatment, followed by the Tukey post hoc test. (D) Quantification of AUC in panel C between 0 and 6 h. SGC-PIKFYVE-1 (30 mg/kg) reversed SNI-induced cold allodynia. ***P < 0.001 vs vehicle group; one-way ANOVA followed by the Dunnett post hoc test. n = 8 mice. For all panels, error bars indicate mean ± SEM. B, Baseline.
3.6. SGC-PIKFYVE-1 reduces the mechanical sensitivity in an inflammatory pain model in male and female mice
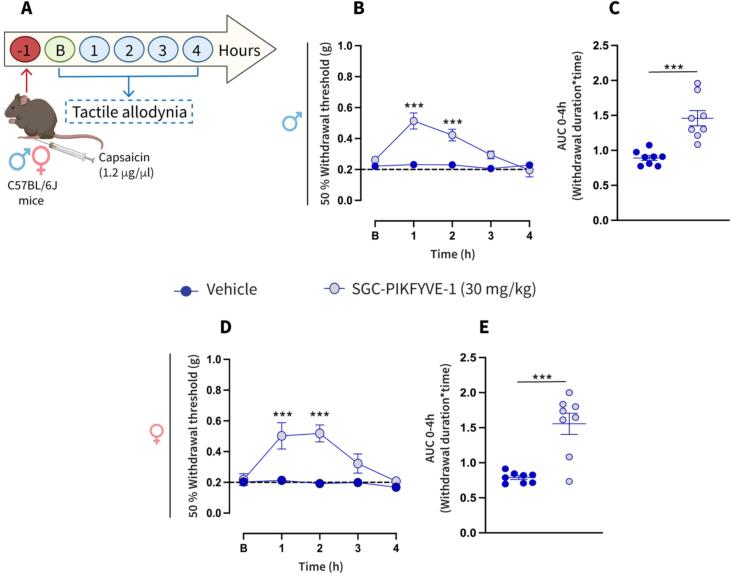
To further profile the antinociceptive efficacy of SGC-PIKFYVE-1, we tested if PIKfyve inhibition can reduce the inflammatory pain induced by intraplantar administration of capsaicin. Male and female mice received an intraplantar injection of capsaicin and 1 h later the effect of SGC-PIKFYVE-1 was determined on mechanical allodynia (Fig. 7A). SGC-PIKFYVE-1 injection (30 mg/kg) reduced the tactile and cold allodynia induced by capsaicin for 2 h in male neuropathic mice (Fig. 7B-C). Additionally, we observed that SGC-PIKFYVE-1 was also able to reduce the nociceptive behaviors in female mice (Fig. 7D-E). SGC-PIKFYVE-1 (30 mg/kg) showed a similar effect in male and female mice (F=(1,1,4) = 0.703, p = 0.591). Taken together, our findings suggests that the SGC-PIKFYVE-1 exerts an antinociceptive effect in the capsaicin-induce tactile allodynia in both male and female mice, likely mediated by the blockade of sodium channels.
Fig.7.
Intraperitoneal administration of SGC-PIKFYVE-1 reduces capsaicin-induced mechanical allodynia in both female and male mice (A) Timeline of the experimental approach used to determine the antinociceptive effects of SGC-PIKFYVE-1 on tactile allodynia in male and female mice with inflammatory pain. (B) Baseline paw withdrawal threshold measurements were conducted before intraperitoneal administration of SGC-PIKFYVE-1 in male mice previously injected with capsaicin. The time course of a single intraperitoneal (IP) injection of SGC-PIKFYVE-1 (30 mg/kg) or vehicle (DMSO 30 %) on mechanical allodynia were measured from 0 to 4 h post injection, starting 1 h after the capsaicin injection (n = 8 mice). Von Frey filaments were used to determine the 50 % paw withdrawal threshold using the up-down method. Results were compared using two-way ANOVA with factors: time * treatment, followed by the Tukey post hoc test. **P < 0.01 and ***P < 0.001 vs vehicle group. (C) Quantification of area under the curve (AUC) of the time course of the effect induced by SGC-PIKFYVE-1 from 0 to 4 h (n = 8 mice). Results were analyzed using one-way ANOVA, followed by the Dunnett post hoc test. ***P < 0.001 vs vehicle group. (D) Baseline paw withdrawal threshold measurements were conducted before intraperitoneal administration of SGC-PIKFYVE-1 in female mice previously injected with capsaicin. The time course of a single intraperitoneal (IP) injection of SGC-PIKFYVE-1 (30 mg/kg) or vehicle (DMSO 30 %) on mechanical allodynia were measured from 0 to 4 h post injection, starting 1 h after the capsaicin injection (n = 8 mice). Von Frey filaments were used to determine the 50 % paw withdrawal threshold using the up-down method. Results were compared using two-way ANOVA with factors: time * treatment, followed by the Tukey post hoc test. **P < 0.01 and ***P < 0.001 vs vehicle group. (E) Quantification of area under the curve (AUC) of the time course of the effect induced by SGC-PIKFYVE-1 from 0 to 4 h (n = 8 mice). Results were analyzed using one-way ANOVA, followed by the Dunnett post hoc test. ***P < 0.001 vs vehicle group. B, Baseline.
3.7. SGC-PIKFYVE-1 treatment does not affect somatosensation or motor coordination in naïve mice
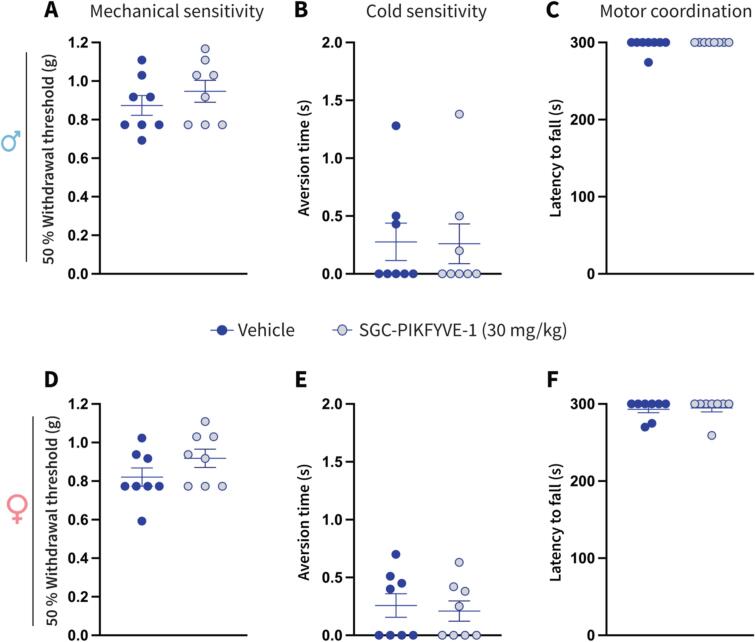
Our pharmacological approach demonstrated that SGC-PIKFYVE-1 exerts an antinociceptive effect in both male and female mice. To ensure that this PIKfyve inhibitor does not interfere with somatosensation or motor function, we injected the higher dose of SGC-PIKFYVE-1 (30 mg/kg) in both male and female naïve mice. Our findings show that SGC-PIKFYVE-1 did not induce changes on the mechanical and cold sensitivity, as well as motor coordination in both male (Fig. 8A-C) and female (Fig. 8D-F) naïve mice. Taken together, these results demonstrate that SGC-PIKFYVE-1 does not affect somatosensation or motor function in naïve male and female mice.
Fig.8.
SGC-PIKFYVE-1 does not induce adverse effects in female and male naïve mice. Effect of a single intraperitoneal (IP) injection of SGC-PIKFYVE-1 (30 mg/kg) or vehicle (DMSO 30 %) on (A) mechanical, (B) cold sensitivity and (C) motor coordination in male naïve mice, was measured 1 h post injection (n = 8 mice). Mechanical sensitivity was assessed using Von Frey filaments with the up-down method. Cold sensitivity was measured by applying an acetone drop to the hind paw, and motor coordination was evaluated using the rotarod test. Administration of SGC-PIKFYVE-1 or vehicle did not induce changes on (D) mechanical, (E) cold sensitivity and (F) motor coordination in female naïve mice motor impairments in male mice. Effect of SGC-PIKFYVE-1 injection (30 mg/kg) was measured 2 h post injection (n = 8 mice). All panels were compared using a Student’s t-test.
4. Discussion
PIKfyve is an enzyme with a significant role in cellular processes. This lipid kinase phosphorylates phosphatidylinositol-3-phosphate (PI(3)P) to produce phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2), which is crucial for regulating membrane homeostasis, endosomal trafficking, and autophagy (Drewry et al., 2022). PIKfyve contains a FYVE finger domain, which is characteristic of proteins involved in various signaling pathways related to membrane trafficking and dynamics (Rivero-Ríos & Weisman, 2022).
PIKfyve has been implicated in several diseases, including cancer, viral infections like COVID-19, and neurodegenerative disorders. Its inhibitor, apilimod, has shown potential in suppressing viral replication and improving the survival of motor neurons in amyotrophic lateral sclerosis (ALS) models (Drewry et al., 2022, Hung et al., 2023). These links to disease make PIKfyve a promising target for therapeutic interventions in these conditions. Despite the well-recognized roles of PIKfyve in physiology and pathophysiology, there are not many tools to study this kinase. Development of a selective chemical probe would represent an important first step in enabling target validation and unearthing novel biological functions of PIKfyve. In this study, we took advantage of a previously published PIKfyve chemical probe, SGC-PIKFYVE-1, which binds PIKfyve in cells with high affinity, is extremely selective for PIKfyve, and performs comparably to the clinically used PIKfyve inhibitor, apilimod (Drewry et al., 2022). At concentrations of 1–5 µM, SGC-PIKFYVE-1 inhibited viral entry and impacted lysosomal homeostasis with a similar efficacy as apilimod, which was dosed at the same concentrations.
Our study demonstrates that inhibition of PIKfyve using SGC-PIKFYVE-1 reduced the sodium currents mediated by Nav1.7 and Nav1.8 channels in rat sensory neurons. This dual-targeting approach provides a significant advantage over current sodium channel inhibitors, which often lack selectivity or target only a single channel subtype. Targeting both Nav1.7 and Nav1.8 sodium channels may be a promising strategy for curbing chronic pain because these channels play distinct but complementary roles in regulating the excitability of nociceptors (Bennett et al., 2019). Nav1.7 amplifies subthreshold depolarizations, bringing the membrane potential closer to the firing threshold, while Nav1.8 drives action potential generation and shapes the action potential waveform. This interplay is crucial in pain conditions like inherited erythromelalgia (IEM), where mutant Nav1.7 enhances Nav1.8′s function, increasing hyperexcitability. Targeting both channels with a single drug could offer more effective pain relief by addressing both the initiation and propagation of neuronal excitation. Inhibiting Nav1.8, even partially, may reduce neuronal excitability and spontaneous activity in DRG neurons. Therefore, our findings suggest that pharmacological inhibition of PIKfyve might modulate pain by reducing the activity of Nav1.7 and Nav1.8 channels in sensory neurons. A limitation of our study is that without demonstrating that knockdown of PIKfyve precludes the effects of the inhibitor, we cannot rule out a direct effect on the channels. Furthermore, we acknowledge that PIKfyve has also been implicated in the modulation of other ion channels relevant to pain signaling. For instance, PIKfyve induces the internalization and degradation of Cav1.2 channels in cortical neurons (Tsuruta et al., 2009). In contrast, we found that PIKfyve inhibition did not alter the activity of voltage-gated calcium channels. A possible explanation to this discrepancy with our results could be attributed to differences in the types of neurons used, suggesting that PIKfyve does not regulate calcium channels in the peripheral nervous system. Additionally, it has been reported that PI4K-mediated PIP2 production is important for the desensitization of inward-rectifier potassium channels and voltage-gated potassium channels in rat taste receptor cells (Zhao & Herness, 2009). Conversely, our findings show that SGC-PIKFYVE-1 application does not affect the total potassium current in sensory neurons. Nevertheless, further research is necessary to fully elucidate the role of PIKfyve and other lipid kinases (PI4K) in the modulation of ion channels implicated in pain signaling. Our findings provide preliminary insights into the effects of SGC-PIKFYVE-1 on the activity of voltage-gated sodium channels. Notably, the pharmacological blockade of these channels is associated with analgesic effects during pain (Emery et al., 2016). Based on this, we hypothesized that the inhibition of sodium channels by SGC-PIKFYVE-1 in vitro might translate to antinociceptive effects when this compound is administered in vivo. In this sense, our data show that systemic administration of SGC-PIKFYVE-1 reversed nociceptive behaviors induced by a nerve injury or inflammation in both male and female mice, suggesting that SGC-PIKFYVE-1 may exert its antinociceptive effect, at least in part, through the blockade of Nav1.7 and Nav1.8 channels. This promising antinociceptive efficacy of SGC-PIKFYVE-1, lasting upwards of four hours following a single dose, is well supported by its pharmacokinetic stability. SGC-PIKFYVE-1 maintained concentrations well above those required to engage PIKfyve in cells. A modest half-life of ∼ 3.5 h was observed following intraperitoneal administration, and oral bioavailability was appreciable. Dose-escalation studies demonstrated that a 30 mg/kg dose is also well tolerated and that twice daily dosing would be optimal for SGC-PIKFYVE-1 in CD-1 mice. Furthermore, most currently available sodium channel blockers used for pain treatment are non-specific across sodium channel subtypes, leading to undesirable side effects that limit their clinical utility. In contrast, our findings demonstrate that SGC-PIKFYVE-1 exerts its effect through the inhibition of Nav1.7 and Nav1.8 channels without affecting somatosensation or motor function in naïve mice, supporting its potential as a more targeted and safer therapeutic option.
Brain penetration is critical for the effectiveness of drugs targeting the central nervous system (CNS), including those used to treat pain (Shi & Mader, 2018). While achieving optimal brain penetration is desirable, it presents challenges due to the blood–brain barrier (BBB), which restricts the entry of many substances into the brain. Various strategies such as using brain-penetrating peptides (Eiselt et al., 2020), monoclonal antibodies, nanoparticles, and prodrugs have been explored to enhance drug delivery to the brain (Di et al., 2013). While progress has been made, the balance between achieving sufficient brain exposure for therapeutic effects and minimizing potential side effects remains a formidable challenge. Systemic administration of an analgesic capable of crossing the BBB can modulate pain by interacting with targets across the CNS, potentially impacting sensory and affective aspects of pain through supraspinal mechanisms (Bannister & Dickenson, 2020). The favorable physicochemical properties of SGC-PIKFYVE-1 resulted in a significant fraction of the compound reaching the brain within one hour following intraperitoneal administration, indicating its excellent blood–brain barrier penetration and making it an ideal candidate for evaluation in animal models of chronic pain. We acknowledge that a limitation of our brain penetration assays is that we only evaluated the intraperitoneal route based on its reliability for ensuring consistent and rapid drug absorption. This route allows us to achieve predictable systemic exposure, which is crucial for understanding the compound's pharmacokinetic properties and its direct effects on the central nervous system. We did not include the oral route in this study because of concerns about the compound's bioavailability and first-pass metabolism, which might introduce variability in the results. However, we acknowledge the importance of evaluating multiple administration routes and will incorporate the oral route in future studies to assess the broader translational potential of the compound. Furthermore, another limitation is that our electrophysiology data cannot exclude direct inhibition of sodium channels independent of its original reported effect on the PIKfyve enzyme. However, previous reports indicate that turnover effects on Nav can be observed on a time scale similar consistent with the experiments reported here (Fréal et al., 2023). Therefore, activity-dependent function of Nav channels could be further studied by combining electrophysiological recordings with or without SGC-PIKFYVE-1 to test whether our compound may have a role on Nav channels turnover or through alternative mechanisms in DRG neurons. Additionally, a significant limitation of our study arises from the multifaceted role of PIKfyve in cellular signaling. PIKfyve inhibition leads to decreased levels of phosphatidylinositol 4,5-bisphosphate (PIP2), a lipid crucial for signaling downstream of various G-protein-coupled receptors, receptor tyrosine kinases, and ion channels implicated in nociceptive pathways. Recent findings suggest that reducing PIP2 levels in neurons can alleviate nociceptive behaviors, presenting a novel avenue for pain management (Loo et al., 2015). Specifically, PIKfyve is known to modulate the function of ion channels such as Cav1.2, TRPV1, KCNQ, and Kv7, which play critical roles in the pathophysiology of chronic pain. Given the diverse signaling pathways influenced by PIKfyve, the mechanisms underlying the observed antinociceptive effects of SGC-PIKFYVE-1 likely involve complex interactions beyond sodium channel modulation alone. Thus, while our study provides valuable insights into the functional and pharmacological effects of PIKfyve inhibition in sensory neurons, further research is necessary to fully elucidate the mechanisms underlying its therapeutic potential in pain management. Consequently, our findings highlight the need for additional in vitro electrophysiological experiments using DRG neurons or heterologous systems. These experiments would enable assessment of the contribution of various ion channels implicated in pain signaling, as well as different compound concentrations, to clarify the precise mechanisms through which SGC-PIKFYVE-1 exerts its therapeutic effects.
5. Conclusion
Our data offers proof-of-concept that acute pharmacological inhibition of PIKfyve could be a novel strategy for pain management. Furthermore, our findings provide a framework for follow-up studies to further explore the full mechanism of action of SGC-PIKFYVE-1 and to assess its efficacy in other types of acute and inflammatory pain.
CRediT authorship contribution statement
Erick J. Rodríguez-Palma: Writing – review & editing, Visualization, Validation, Resources, Investigation, Formal analysis. Santiago Loya-Lopez: Writing – review & editing, Visualization, Resources, Investigation, Formal analysis. Sophia M. Min: Writing – review & editing, Visualization, Validation, Resources, Investigation, Formal analysis. Aida Calderon-Rivera: Writing – review & editing, Visualization, Resources, Investigation, Formal analysis. Kimberly Gomez: Formal analysis, Investigation. Rajesh Khanna: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Funding acquisition, Conceptualization. Alison D. Axtman: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Structural Genomics Consortium (SGC) is a registered charity (number 1097737) that receives funds from Bayer AG, Boehringer Ingelheim, the Canada Foundation for Innovation, Eshelman Institute for Innovation, Genentech, Genome Canada through Ontario Genomics Institute, EU/EFPIA/OICR/McGill/KTH/Diamond, Innovative Medicines Initiative 2 Joint Undertaking, Janssen, Merck KGaA (aka EMD in Canada and USA), Pfizer, the São Paulo Research Foundation-FAPESP, and Takeda. Research reported in this publication was supported in part by the NC Policy Collaboratory and National Institutes of Health awards (NINDS (NS098772 and NS120663) and NIDA (DA042852) to R.K. The graphical abstract was created with Biorender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynpai.2024.100174.
Contributor Information
Rajesh Khanna, Email: r.khanna@ufl.edu.
Alison D. Axtman, Email: alison.axtman@unc.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aromolaran K.A., Goldstein P.A. Jan-Dec). Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy; cause and effect? Mol Pain. 2017;13 doi: 10.1177/1744806917714693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister K., Dickenson A.H. Jul). Central Nervous System Targets: Supraspinal Mechanisms of Analgesia. Neurotherapeutics. 2020;17(3):839–845. doi: 10.1007/s13311-020-00887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow-Busch I., Shaw A.L., Burke J.E. Aug). PI4KA and PIKfyve: Essential phosphoinositide signaling enzymes involved in myriad human diseases. Curr Opin Cell Biol. 2023;83 doi: 10.1016/j.ceb.2023.102207. [DOI] [PubMed] [Google Scholar]
- Barragán-Iglesias P., Kuhn J., Vidal-Cantú G.C., Salinas-Abarca A.B., Granados-Soto V., Dussor G.O., Campbell Z.T., Price T.J. Jan). Activation of the integrated stress response in nociceptors drives methylglyoxal-induced pain. Pain. 2019;160(1):160–171. doi: 10.1097/j.pain.0000000000001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D.L., Clark A.J., Huang J., Waxman S.G., Dib-Hajj S.D. Apr 1). The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol Rev. 2019;99(2):1079–1151. doi: 10.1152/physrev.00052.2017. [DOI] [PubMed] [Google Scholar]
- Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Jul). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Decosterd I., Woolf C.J. Aug). Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–158. doi: 10.1016/s0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Di, L., Rong, H., & Feng, B. (2013, 2013/01/10). Demystifying Brain Penetration in Central Nervous System Drug Discovery. Journal of medicinal chemistry, 56(1), 2-12. https://doi.org/10.1021/jm301297f. [DOI] [PubMed]
- Dib-Hajj S.D., Black J.A., Waxman S.G. Voltage-Gated Sodium Channels: Therapeutic Targets for Pain. Pain Medicine. 2009;10(7):1260–1269. doi: 10.1111/j.1526-4637.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- Dixon W.J. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Drewry D.H., Potjewyd F.M., Bayati A., Smith J.L., Dickmander R.J., Howell S., Taft-Benz S., Min S.M., Hossain M.A., Heise M., McPherson P.S., Moorman N.J., Axtman A.D. Oct 13). Identification and Utilization of a Chemical Probe to Interrogate the Roles of PIKfyve in the Lifecycle of β-Coronaviruses. J Med Chem. 2022;65(19):12860–12882. doi: 10.1021/acs.jmedchem.2c00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiselt É., Otis V., Belleville K., Yang G., Larocque A., Régina A., Demeule M., Sarret P., Gendron L. Jul). Use of a Noninvasive Brain-Penetrating Peptide-Drug Conjugate Strategy to Improve the Delivery of Opioid Pain Relief Medications to the Brain. J Pharmacol Exp Ther. 2020;374(1):52–61. doi: 10.1124/jpet.119.263566. [DOI] [PubMed] [Google Scholar]
- Emery E.C., Luiz A.P., Wood J.N. Aug). Nav1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin Ther Targets. 2016;20(8):975–983. doi: 10.1517/14728222.2016.1162295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci, A., Kasper, D., Braunwald, E., Hauser, E., Longo, D., Jameson, J., & Loscalzo, J. (2008). Harrison’s principle of internal medicine (Vol. 17). McGraw-Hill.
- Fréal A., Jamann N., Ten Bos J., Jansen J., Petersen N., Ligthart T., Hoogenraad C.C., Kole M.H.P. Sep 15). Sodium channel endocytosis drives axon initial segment plasticity. Sci Adv. 2023;9(37):eadf3885. doi: 10.1126/sciadv.adf3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, K., Santiago, U., Nelson, T. S., Allen, H. N., Calderon-Rivera, A., Hestehave, S., Rodríguez Palma, E. J., Zhou, Y., Duran, P., Loya-Lopez, S., Zhu, E., Kumar, U., Shields, R., Koseli, E., McKiver, B., Giuvelis, D., Zuo, W., Inyang, K. E., Dorame, A., Chefdeville, A., Ran, D., Perez-Miller, S., Lu, Y., Liu, X., Handoko, Arora, P. S., Patek, M., Moutal, A., Khanna, M., Hu, H., Laumet, G., King, T., Wang, J., Damaj, M. I., Korczeniewska, O. A., Camacho, C. J., & Khanna, R. (2023, Nov 21). A peptidomimetic modulator of the Ca(V)2.2 N-type calcium channel for chronic pain. Proc Natl Acad Sci U S A, 120(47), e2305215120. https://doi.org/10.1073/pnas.2305215120. [DOI] [PMC free article] [PubMed]
- Hung S.-T., Linares G.R., Chang W.-H., Eoh Y., Krishnan G., Mendonca S., Hong S., Shi Y., Santana M., Kueth C., Macklin-Isquierdo S., Perry S., Duhaime S., Maios C., Chang J., Perez J., Couto A., Lai J., Li Y., Alworth S.V., Hendricks E., Wang Y., Zlokovic B.V., Dickman D.K., Parker J.A., Zarnescu D.C., Gao F.-B., Ichida J.K. PIKFYVE inhibition mitigates disease in models of diverse forms of ALS. Cell. 2023;186(4):786–802.e728. doi: 10.1016/j.cell.2023.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J., Correll, D. J., Lechner, S. M., Jazic, I., Miao, X., Shaw, D., Simard, C., Osteen, J. D., Hare, B., Beaton, A., Bertoch, T., Buvanendran, A., Habib, A. S., Pizzi, L. J., Pollak, R. A., Weiner, S. G., Bozic, C., Negulescu, P., & White, P. F. (2023, Aug 3). Selective Inhibition of Na(V)1.8 with VX-548 for Acute Pain. N Engl J Med, 389(5), 393-405. https://doi.org/10.1056/NEJMoa2209870. [DOI] [PubMed]
- Liu C.N., Wall P.D., Ben-Dor E., Michaelis M., Amir R., Devor M. Apr). Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85(3):503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Loo L., Wright B.D., Zylka M.J. Apr). Lipid kinases as therapeutic targets for chronic pain. Pain. 2015;156 Suppl 1(0 1):S2–s10. doi: 10.1097/01.j.pain.0000460345.92588.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, D. I., Luiz, A. P., Iseppon, F., Millet, Q., Emery, E. C., & Wood, J. N. (2021, Jul 28). Silent cold-sensing neurons contribute to cold allodynia in neuropathic pain. Brain, 144(6), 1711-1726. https://doi.org/10.1093/brain/awab086. [DOI] [PMC free article] [PubMed]
- Mogil J.S., Parisien M., Esfahani S.J., Diatchenko L. Aug). Sex differences in mechanisms of pain hypersensitivity. Neurosci Biobehav Rev. 2024;163 doi: 10.1016/j.neubiorev.2024.105749. [DOI] [PubMed] [Google Scholar]
- Potjewyd F.M., Annor-Gyamfi J.K., Aubé J., Chu S., Conlon I.L., Frankowski K.J., Guduru S.K.R., Hardy B.P., Hopkins M.D., Kinoshita C., Kireev D.B., Mason E.R., Moerk C.T., Nwogbo F., Pearce K.H., Jr., Richardson T.I., Rogers D.A., Soni D.M., Stashko M., Wang X., Wells C., Willson T.M., Frye S.V., Young J.E., Axtman A.D. Use of AD Informer Set compounds to explore validity of novel targets in Alzheimer's disease pathology. Alzheimers Dement. 2022;8(1):e12253. doi: 10.1002/trc2.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Ríos P., Weisman L.S. Jun). Roles of PIKfyve in multiple cellular pathways. Curr Opin Cell Biol. 2022;76 doi: 10.1016/j.ceb.2022.102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrissa, D., Ikonomov, O. C., Filios, C., Delvecchio, K., & Shisheva, A. (2012, Aug). Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P by means of the PIKfyve inhibitor YM201636. American Journal of Physiology-Cell Physiology, 303(4), C436-C446. https://doi.org/10.1152/ajpcell.00105.2012 [DOI] [PMC free article] [PubMed]
- Schmalhofer W.A., Calhoun J., Burrows R., Bailey T., Kohler M.G., Weinglass A.B., Kaczorowski G.J., Garcia M.L., Koltzenburg M., Priest B.T. Nov). ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008;74(5):1476–1484. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- Shi, Y., & Mader, M. (2018, 2018/06/15/). Brain penetrant kinase inhibitors: Learning from kinase neuroscience discovery. Bioorganic & medicinal chemistry letters, 28(11), 1981-1991. https://doi.org/https://doi.org/10.1016/j.bmcl.2018.05.007. [DOI] [PubMed]
- Tsuruta F., Green E.M., Rousset M., Dolmetsch R.E. PIKfyve regulates CaV1.2 degradation and prevents excitotoxic cell death. Journal of Cell Biology. 2009;187(2):279–294. doi: 10.1083/jcb.200903028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Ringkamp M., Hartke T.V., Murinson B.B., Campbell J.N., Griffin J.W., Meyer R.A. Apr 15). Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21(8):RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Ong H.W., Dickmander R.J., Smith J.L., Brown J.R., Tao W., Chang E.F., Moorman N.J., Axtman A.D., Willson T.M. Optimization of 3-Cyano-7-cyclopropylamino-pyrazolo[1,5-a]pyrimidines Toward the Development of an In Vivo Chemical Probe for CSNK2A. ACS Omega. 2023 doi: 10.1021/acsomega.3c05377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.L., Herness S. Jan 15). Resynthesis of phosphatidylinositol 4,5-bisphosphate mediates adaptation of the caffeine response in rat taste receptor cells. J Physiol. 2009;587(2):363–377. doi: 10.1113/jphysiol.2008.165167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Jun). Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zolov S.N., Bridges D., Zhang Y., Lee W.W., Riehle E., Verma R., Lenk G.M., Converso-Baran K., Weide T., Albin R.L., Saltiel A.R., Meisler M.H., Russell M.W., Weisman L.S. Oct 23). In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci U S A. 2012;109(43):17472–17477. doi: 10.1073/pnas.1203106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.