Abstract
Background
Hypoxic hepatitis (HH) is commonly seen in critically ill patients, such as those with cardiac shock, sepsis, and respiratory failure. However, data are limited regarding its impact on the prognosis of patients with cardiac arrest (CA).
Methods
We conducted a systematic review and meta-analysis of studies from PubMed, EMBASE, and the Cochrane Library from inception to July 30, 2024. Studies were included if they focused on adult CA patients with HH compared to controls and had a clear definition of HH (defined as a rapid elevation in liver enzyme levels > 20 times the upper limit of normal after CA). The primary outcome was all-cause mortality.
Subgroup analyses, sensitivity analyses, and generic inverse variance analyses were conducted.
Results
Six studies with 3,005 adults were included. The median prevalence of HH was 16.3 % (ranging from 7.2 to 24.7 %). Overall, patients with HH had a significantly higher risk of all-cause mortality than those without (odds ratio [OR] = 3.49; 95 % CI, 2.19–5.57; P < 0.00001). This finding was confirmed in subgroups, sensitivity analyses, and regression analyses. HH patients were more likely to have a poor neurological outcome (OR = 2.73; 95 % CI, 1.37–5.42; P = 0.004), post-CA shock (OR = 5.77; 95 % CI, 1.76–18.94; P = 0.004), cardiac failure (OR = 35.84; 95 % CI, 6.02–213.31; P < 0.0001), and higher lactate levels (mean difference [MD] = 4.10 mmol/L; 95 % CI, 2.89–5.31; P < 0.00001). In addition, HH required more continuous renal replacement therapy (OR = 4.19; 95 % CI, 3.02–5.82; P < 0.00001), vasopressor therapy, time to return of spontaneous circulation (MD = 5.0 min; 95 % CI, 3.02–6.97; P < 0.00001) but not mechanical ventilation (OR = 1.40; 95 % CI, 1.00–1.97; P = 0.05).
Conclusions
Hypoxic hepatitis is not a rare complication after CA, and was independently associated with all-cause mortality. Further prospective, well-designed studies are needed to validate our findings.
Keywords: Hypoxic hepatitis, Cardiac arrest, Mortality, Neurological outcome, Meta-analysis
Introduction
Despite significant advances in post-cardiac arrest (CA) therapy[1], the hospital mortality of patients with CA remains high. This is associated with the post-CA syndrome that develops in patients after ROSC. The syndrome includes both CA and cardiopulmonary resuscitation-induced ischemia/reperfusion injury[2]. Clinicians have long been more concerned about the impact of this injury on the brain and have taken various measures to avoid neurologic deterioration[3]. Recently, multi-organ damage occurring after CA has attracted clinical attention. In a previous meta-analysis, Sandroni et al. reported that acute kidney injury occurs early in more than 50 % of CA patients and is associated with increased mortality[4]. Another study has shown that nearly 70 % of cases may worsen with post-CA shock[5]. However, there is less literature on liver dysfunction in this setting.
Hypoxic hepatitis (HH), also known as “hypoxic liver injury”, “ischemic hepatitis” or “shock liver,” is thought to be the result of decreased systemic blood flow and is a life-threatening complication commonly reported in ICU patients[6]. As evidenced by a previous meta-analysis, 78.2 % and 23.4 % of patients with ischemic hepatitis experienced induced acute cardiac events, or sepsis, respectively. The proportion of patients with a documented history of hypotensive events of any duration was 52.9 %. The overall survival rate of HH patients at discharge was 51 % (ranging from 23.1 to 85.7 %)[7]. However, this meta-analysis did not mention CA patients in analyzing etiology, morbidity, and prognosis. On the other hand, Henrion believed that one of the diagnostic criteria of HH is a sharp increase in serum aminotransferase levels to at least 20 times the upper limit of normal (ULN) [8]. It is uncertain whether this criterion is appropriate for CA patients.
Recently, several studies have been published on this topic9, 10, 11. Therefore, we aimed to conduct a systematic review and meta-analysis to explore the incidence of HH in survivors after CA and the impact on the prognosis. We also performed subgroup analyses and sensitivity analyses to examine potential confounders and test the robustness of our findings.
Methods
This study protocol has been registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols database (INPLASY 202480051). We conducted this study following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) checklist[12] (Additional File 1).
Search strategy and data sources
Two authors (J-HS and Y-BG) independently performed a comprehensive literature search to identify studies that explored the effect of HH in patients after CA following ROSC. The search databases included PubMed, Embase, and the Cochrane Library from their inception dates until July 30, 2024. We used search strategies by combining medical subject headings (MeSH) and free text terms: “cardiac arrest,” “heart arrest,” and “hypoxic hepatitis,” with no language restrictions. Details of the complete search strategy are summarized in Additional File 2 In addition, we screened the reference lists of the included studies to avoid omitting potentially relevant studies.
Inclusion criteria and study selection
Eligible studies were included if they met the following PICOS criteria: (1) participants: adult patients after ROSC with HH; (2) exposure: HH (defined as a rapid elevation in liver enzyme levels > 20 times the ULN); (3) comparisons: non-HH patients; (4) outcomes: mortality and other predefined important outcomes; and (5) study design: cohort, case-control or randomized controlled design.
We excluded the studies that met the following criteria: (1) studies that enrolled patients < 18 years old, women of pregnant or breastfeeding; (2) publications only in the abstract, letters to the editor without sufficient data, and review article; and (3) studies defined HH as liver enzyme levels < 20 times the ULN.
Data extraction
The two authors independently extracted relevant data from eligible articles. The extracted data included the study characteristics (the first author’s name, publication year), patient characteristics (age, gender, patient population, disease severity, body mass index), details of the acupuncture technique procedure, control protocols, and predefined outcomes.
Outcomes
The primary outcome was all-cause short-term mortality (ie., ICU, 28-day, or hospital mortality, the longest follow-up period was preferred). Secondary outcomes included important clinical outcomes (i.e., neurological outcome, ICU or hospital length of stay, duration of mechanical ventilation [MV]), severe complications (i.e., shock, heart failure, infection, time to return of spontaneous circulation [ROSC], lactate levels, and rearrest) post-CA; and patients with advanced organ supportive techniques (i.e., renal replacement therapy [RRT], MV, and vasopressor therapy). Meta-analyses of these predefined outcomes were implemented only when at least 2 studies included were available.
Quality assessment
The two authors independently assessed the study quality of each included study using the Newcastle-Ottawa Scale (NOS)[13]. We evaluated publication bias by visual inspection funnel plots when at least 10 studies were included in the current meta-analysis. Disagreements were resolved by thorough discussion and consensus.
Statistical analysis
We combined the results from all relevant studies to estimate the pooled risk ratios (RRs) with 95 % confidence intervals (CIs) for categorical variables and mean differences (MDs) with 95 % CIs for continuous variables as. For studies that reported the median with an interquartile range (IQR) as the measure of treatment effect, we presented the mean of the median and the standard deviations (SD) of the IQR, as described in a previous study[14]. We used the I2 statistic to test for heterogeneity, with values of I2 < 50 % and I2 > 50 % indicating low and high heterogeneity, respectively. A fixed-effect model was used when I2 < 50 %, and a random-effect model was used when I2 > 50 %, using the Mantel-Haenszel method[15].
For the analysis of each predefined outcome, we conducted meta-analyses of relevant studies. To investigate the potential influencing factors for the primary outcome, we conducted subgroup analyses by pooling studies based on (1) Out of hospital CA (OHCA)%: 100 % or < 100 %; (2) shockable rhythm (SR)%: ≥50 % or < 50; and (3) Design: single-center study vs. multi-center study, (4) Mortality: short-term (30-day, ICU stay, or hospital stay) or long-term (≥90-day) mortality; and (5) location: Europe or non-Europe. To assess the robustness of the results, sensitivity analyses were performed by sequentially excluding each study at a time to explore whether an individual study's particular result drove the results. For studies using regression analyses to adjust for the effect of confounders on mortality, we merged mortality estimates with corresponding standard errors by using the generic inverse variance method in the analysis. The significance level for P values was set at 0.05. Review Manager software (version 5.4) was used for all statistical analyses.
Results
Searching results
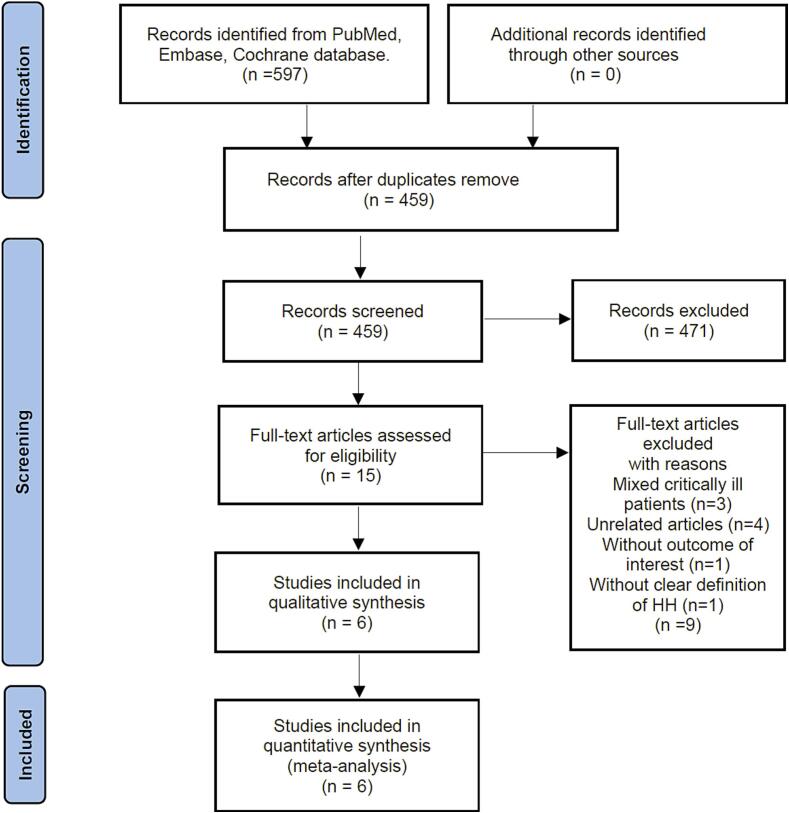
Following de-duplication, we eliminated 459 records from the 597 found when searching the predefined databases. After screening titles and abstracts, 444 records were deemed ineligible. Finally, we conducted a full-text review and excluded 6 articles, resulting in 6 retrospective studies with 3,005 patients being included in the final analysis9, 10, 11, 16, 17, 18. (Fig. 1).
Fig. 1.
Flow chart of literature selection.
Study characteristics and quality assessment
Table 1 summarizes the main characteristics of the included studies. These studies were performed between 2015 and 2024, with sample sizes ranging from 148 to 1,068. A total of 489 and 2,516 survivals were analyzed in the HH group and the non-HH groups, respectively. These studies were conducted in France (n = 2), Korea (n = 2), Belgium (n = 1), and Austria (n = 1). Of these studies, three recruited OHCA patients9, 16, 18, and the remaining three focused on both in-hospital CA (IHCA) and OHCA patients10, 11, 17. The average incidence of HH in all included studies was 16.3 % (ranging from 7.2 %-24.7 %). Regarding the definition of HH, all studies used a rapid elevation in liver enzyme levels > 20 times the ULN9, 10, 11, 16, 17, 18.
Table 1.
Characteristics of the included studies.
| Study | Country | Design | N | Age, year | Gender, % | HH definition | HH, N (%) | Short-term mortality | OHCA, % | SR, % | TTM, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Champigneulle 2016 [16] | France | R, SC | 632 | 60.8 | 68.8 | >20 ULN | 72 (11.4) | ICU | 100 | 55.6 | 94.1 |
| Delignette 2024 [9] | France | R, MC | 418 | 64.0 | 59.1 | >20 ULN | 61 (14.6) | 28-day | 100 | 73.4 | 100 |
| Lee 2020 [10] | Korea | R, SC | 365 | 61.5 | 69.0 | >20 ULN | 90 (24.7) | Hospital, 30-day | 13.2 | 32.1 | NR |
| Lesu 2018 [17] | Belgium | R, SC | 374 | 62.0 | 64.7 | >20 ULN | 27 (7.2) | ICU, hospital | 59.4 | 35.3 | 88.5 |
| Oh 2015 [18] | Korea | R, SC | 148 | 54.8 | 72.0 | >20 ULN | 20 (13.5) | Hospital | 100 | 30.4 | 79.7 |
| Roedl 2019 [11] | Austria | P, SC | 1068 | 61.0 | 72.0 | >20 ULN | 219 (20.5) | 28-day | 75 | 51 | 62 |
ALT = alanine transaminase; AST = aspartate transaminase; HH = hypoxic hepatitis; ICU = intensive care unit; N = sample size; NR = not report; OHCA = out of hospital cardiac arrest; SR = shockable rhythm; TTM = target temperature management.
We evaluated the quality of each included study using the NOS tool for cohort studies (Additional File 3). Overall, the assessment shows the study quality ranged from moderate to high quality, with scores ranging from 7 to 9 on the NOS scale. Five studies were considered high quality16, 17, 9, 10, 11, and the remaining one was moderate[18].
Primary outcome
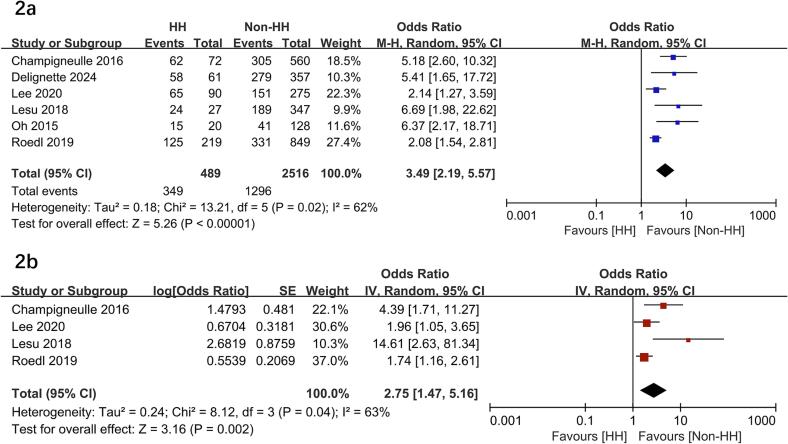
All included studies compared mortality between patients with or without HH9, 10, 11, 16, 17, 18. Among these patients, 489 had HH, and 349 died (71.2 %) compared to 2,516 non-HH patients with 1,296 deaths (51.5 %). Patients with HH had a significantly higher risk of mortality than those without (OR = 3.49; 95 % CI, 2.19–5.57; P < 0.00001) with the heterogeneity of 62 % observed (Fig. 2a).
Fig. 2.
Forest plots of the hypoxia hepatitis on mortality rate in patients following cardiac arrest. HH, hypoxia hepatitis.
Subsequently, we performed subgroup analyses to investigate the potential heterogeneity sources. As to between-groups mortality analyses, HH had significantly higher mortality rates in all the subgroups, including OHCA%, geographic location, study design, and SR% (P values ranging from 0.01 to < 0.00001 with I2 ranging from 0 % to 74 %) (Table 2). In the sensitivity analysis, excluding any single study did not significantly change the overall combined OR (all P values < 0.00001 with I2 ranging from 47 % to 68 %). In addition, four studies using multivariate logistic regression analyses were pooled to assess the risk for mortality. The pooled data showed HH was associated with an increased risk of mortality (adjusted OR = 2.75, 95 % CI 1.47–5.16, P = 0.002) (Fig. 2b).
Table 2.
Subgroup analyses of the effect of hypoxic hepatitis on mortality.
| Study characteristics | Studies number | Patient number |
Events in hypoxic hepatitis group | Events in non-hypoxic hepatitis group | Odds ratio (95 % CI) |
I2 | p |
|---|---|---|---|---|---|---|---|
| All included studies | 6 | 3005 | 349 of 489 (15.3 %) | 1296 of 2516 (30.0 %) | 3.49 (2.19, 5.57) | 62 % | <0.00001 |
| Out of hospital cardiac arrest % =100 % | 3 | 1098 | 135 of 153 (15.5 %) | 625 of 1045 (30.5 %) | 5.48 (3.26, 9.23) | 0 % | <0.00001 |
| Out of hospital cardiac arrest % <100 % | 3 | 1907 | 214 of 336 (14.6 %) | 671 of 1471 (27.3 %) | 2.35 (1.57, 3.54) | 41 % | <0.0001 |
| Shockable rhythm % ≤50 % | 3 | 887 | 104 of 137 (9.8 %) | 381 of 750 (27.8 %) | 3.94 (1.68, 09.25) | 62 % | 0.002 |
| Shockable rhythm % >50 % | 3 | 2118 | 245 of 352 (20.6 %) | 915 of 1766 (32.0 %) | 3.49 (1.65, 7.41) | 74 % | 0.001 |
| 28-day mortality | 3 | 1851 | 243 of 3770 (20.6 %) | 750 of 3481 (32.0 %) | 2.20 (1.71, 2.82) | 23 % | <0.00001 |
| ICU mortality | 2 | 1006 | 86 of 99 (20.6 %) | 484 of 907 (32.0 %) | 5.72 (3.14, 10.40) | 0 % | <0.00001 |
| Hospital mortality | 3 | 887 | 104 of 137 (20.6 %) | 381 of 750 (32.0 %) | 3.94 (1.68, 9.25) | 62 % | 0.002 |
| Single-center study | 5 | 2587 | 291 of 428 (15.1 %) | 1017 of 2159 (29.8 %) | 3.33 (2.03, 5.45) | 66 % | <0.00001 |
| Multi-center study | 1 | 418 | 58 of 61 (14.6 %) | 279 of 357 (27.3 %) | 5.41 (1.65, 17.72) | − | 0.005 |
| Europe | 3 | 1424 | 144 of 160 (7.5 %) | 773 of 1264 (23.3 %) | 5.49 (3.22, 9.38) | 0 % | <0.00001 |
| Non-Europe | 3 | 1581 | 205 of 329 (18.1 %) | 523 of 1252 (32.5 %) | 2.43 (1.58, 3.75) | 48 % | <0.0001 |
Secondary outcomes
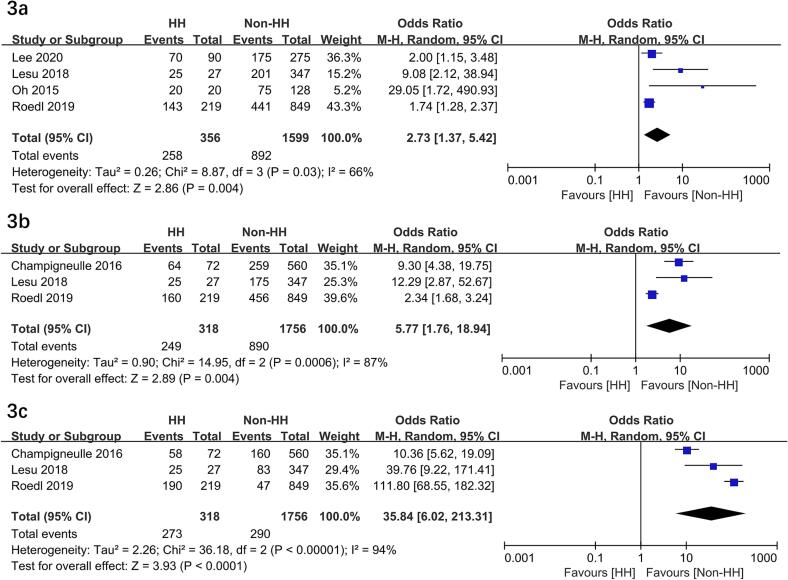
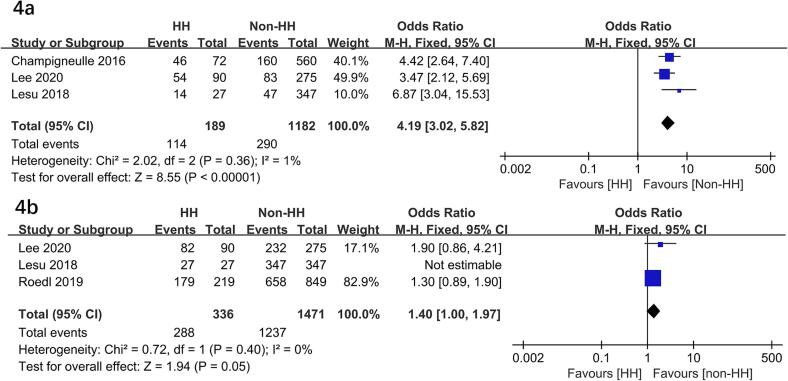
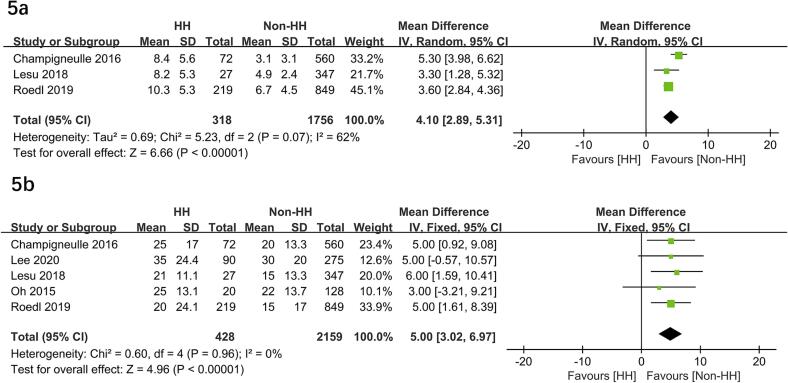
HH significantly higher risks of poor neurological outcome (OR = 2.73; 95 % CI, 1.37–5.42; P = 0.004)10, 11, 17, 18, post-CA shock (OR = 5.77; 95 % CI, 1.76–18.94; P = 0.004)11, 16, 17, cardiac failure (OR = 35.84; 95 % CI, 6.02-213.31; P < 0.0001)11, 16, 17, and lactate levels (MD = 4.10 mmol/L; 95 % CI, 2.89-5.31; P < 0.00001)11, 16, 17. In addition, HH required more CRRT (OR = 4.19; 95 % CI, 3.02–5.82; P < 0.00001)10, 16, 17, time to ROSC (MD = 5.0 min; 95 % CI, 3.02–6.97; P < 0.00001)10, 11, 16, 17, 18 but a nearly significant difference in mechanical ventilation (OR = 1.40; 95 % CI, 1.00–1.97; P = 0.05)10, 11, 17 (Fig. 3, Fig. 4, Fig. 5). In addition, two studies compared the outcome of vasopressor therapy between the HH and control groups (HH vs. control: P = 0.081 and P < 0.01, respectively) 10, 17.
Fig. 3.
Forest plots of the hypoxia hepatitis on poor neurological outcome (3a), post-CA shock (3b), and cardiac failure (3c) in patients following cardiac arrest. HH, hypoxia hepatitis.
Fig. 4.
Forest plots of the effects of hypoxia hepatitis on CRRT (4a) and mechanical ventilation (4b) in patients following cardiac arrest. HH, hypoxia hepatitis.
Fig. 5.
Forest plots of the effects of hypoxia hepatitis on lactate levels (5a) and time to ROSC (5b) in patients following cardiac arrest. HH, hypoxia hepatitis.
Discussion
We focused on patients after ROSC who developed HH during post-CA treatment. The pooled results from six studies of 3,005 patients showed that patients with HH tended to have a higher risk of all-cause mortality, with mortality rates ranging from 7.2 % to 24.7 %9, 10, 11, 16, 17, 18. Specifically, HH significantly increased the risk of mortality in CA survival (OR = 3.49, P < 0.00001). Further subgroup, sensitivity analyses, and generic inverse variance analyses confirmed this finding. HH was associated with a significantly higher risk of poor neurological outcome, post-CA shock, cardiac failure, and higher lactate levels and required more CRRT and vasopressor therapy.
Prevalence
Our results show that the mean prevalence of HH in post-CA patients is 16.3 %, with considerable variation between the included studies. To understand the prevalence of HH, we need to pay attention to the following points. First, the included studies followed the Henrion criteria for HH[8]. That is, a clinical diagnosis of HH is made if a person’s serum aminotransferases are at least 20 times the ULN and they have signs of heart, respiratory, or circulatory failure, and other etiologies of acute hepatocellular necrosis have been ruled out. Thus, the differences in HH prevalence may be due to the threshold of AST/ALT used to define it. In a previous study of 94 patients with OHCA, Nam et al. found that HH occurred in 35 patients (37.2 %)[19]. This is in line with this author's definition of HH as an increase in serum aminotransferase levels reaching at least eight times the upper limit of normal.
Second, more people post-CA have HH than in other critical populations. Under the same Henrion criteria definition, HH was observed more frequently in post-CA patients than those with severe COVID-19[20], respiratory failure[21], cardiac shock[22], and acute myocardial infarction[23]. In another study comprising 1,066 critically ill patients, 118 patients developed HH (defined as > 10 times the ULN), of whom 35 (30 %) were CA patients[24]. The high incidence of HH in CA may be related to the hepatic supply mechanism[25]. Specifically, although the liver is protected against ischemia by a dual blood supply through the hepatic artery and portal venous system[25], this unique dual blood supply does not work during CA. Thus, CA may represent the greatest risk factor for HH among many critical diseases.
Third, the six studies included in this study were retrospective in design9, 10, 11, 16, 17, 18, which may have resulted in the missing of some patients with HH. In a prospective study, Whitehead et al. reported that more than one-third of all cases with markedly elevated aminotransferase levels were unrecognized by the treating physician[26]. Moreover, ALI and AST have different half-lives, with varying degrees of recovery following a peak[27]. Thus, the selection of varying time points and transaminases may potentially affect the diagnosis of HH and, subsequently, the discrepancies in morbidity observed across studies.
Mortality
In the present study, an early mortality rate of 71 % was observed in the HH patients, with a fluctuating range of 57 % to 95 %. For example, in a study evaluating the etiology, clinical features, and prognosis of HH (defined as a > 10 times the ULN) in the ICU, shock (48 %), cardiac arrest (25 %), and hypoxia (13 %) were the most common causes of HH[6]. However, patients with CA and HH had a markedly elevated mortality rate (72.7 %) compared to the other three categories (38.8 %-52.6 %)[6]. Ischemia-reperfusion after CA leads to the apoptosis of hepatocytes and sinusoidal endothelial cells[28]. Furthermore, prolonged hypoxia generates intracellular reactive oxygen species, as well as xanthine oxidase, and mitochondria, which leads to intracellular oxidative stress[29]. This is why CA has a worse prognosis. On the other hand, given the liver's pivotal role in regulating many essential metabolic, homeostatic, and host defense processes, any dysfunction may have a detrimental impact on the prognosis[30]. As shown in our study, post-CA patients with HH may favor shock, hyperlactatemia, cardiovascular dysfunction, poor neurological prognosis, and increased mortality9, 10, 11, 16, 17, 18.
Our results suggest HH defined as transaminase elevations > 20 times the ULN may serve as a reliable independent risk factor of prognosis for CA patients in terms of liver. Interestingly, the hepatic subcomponent of the Sequential Organ Failure Assessment (SOFA) score is based only on elevated bilirubin levels and may not serve as a robust marker of CA-induced liver injury. As shown in one study, the severity of liver failure as evaluated by the highest hepatic SOFA score within the initial 72-hour period following CA was not related to mortality[31]. In addition, the onset of cholestatic liver dysfunction and jaundice in critically ill patients is usually delayed following their admission to the ICU[8]. Similarly, bilirubin levels between the HH and non-HH groups in the three included studies were not significantly different in the first few days after CA9, 10, 17. In addition, there was mildly elevated bilirubin (median from 8.5 to 16.8 umol/L)9, 10, 17.
On the other hand, acute liver failure (ALF) is commonly diagnosed by measuring the elevation of the INR up to 1.5 times the ULN or the elevation of bilirubin. However, patients with CA and HH had significantly higher mortality (89 % versus 51 % versus 45 %, respectively) and adverse neurologic outcomes (93 % versus 60 % versus 59 %, respectively) compared with those with ALF without HH or those without ALF or HH[17]. In addition, in a study of HH defined by an 8-fold ULN of aminotransferase, there was no statistical difference in mortality between CA patients in the HH and non-HH groups (P = 0.363)[19].
Factors associated with occurrences of HH
The findings of our secondary outcomes indicate that post-CA patients with HH had elevated lactate levels, prolonged recovery of ROSC, and a higher incidence of shock and heart failure. These findings indicate the potential for significant liver damage due to ischemia/reperfusion injury. Moreover, these patients require more robust organ function support, including the administration of vasoactive drugs, MV, and CRRT. These discrepancies may also be regarded as indicators of the severity or a manifestation of the degree of systemic inflammatory response, ischemia, and hypoxia leading to HH. Therefore, these indicators may serve as potential independent predictors of HH development.
Several of the included studies examined the factors associated with the development of HH and found that the time to ROSC[11], shockable rhythm[19], CRRT10, 17, 19, male gender[19], heart failure[11], and lactate level[17] could potentially predict the occurrence of HH after CA. The presence of these indicators can help identify those post-CA patients who require more accurate hemodynamic monitoring and/or optimization of supportive care to minimize further severe liver injury.
Strength and limitations
Our study has several strengths. First, this study is the first meta-analysis to evaluate the impact of HH on the prognosis of post-CA patients. Our findings are consistent with those of previous studies on other patient populations, including patients with respiratory failure, cardiogenic shock, and sepsis[7], which emphasize the adverse clinical outcomes of HH. Thus, our study adds a new evidence population. Second, we comprehensively assessed the mortality in patients with HH after CA, including mortality between HH and non-HH patients and the linear relationship between HH and mortality. third, we performed subgroup and sensitivity analyses based on different potentially influential factors, and the results were reliable. In addition, we explored several secondary clinical outcomes, which support the robustness of our primary outcome.
Our meta-analysis has several limitations. First, the study design precludes us from establishing whether there is a causal relationship between the occurrence of HH and mortality, as well as each of the important clinical outcomes. Second, only studies of HH following CA, defined by transaminase elevations > 20 times the ULN, were included in the analysis. However, a previous meta-analysis suggested that patients with various heterogeneous severe HH defined by 8 times or 10 times the ULN had mortality rates of approximately 49 % and 52 %, which were comparable to the mortality rate of non-HH in the present meta-analysis (52 %)6, 7. Third, the unequal distribution of different underlying diseases in the included studies may also impact the prognosis. We intended to perform subgroup analyses to explore studies based on this diversity but were constrained by an insufficient quantity of data. Fourth, Fourthly, the incidence of HH may be underestimated. There was considerable variation in the time the included studies defined HH patients (48 h after CA to ICU discharge). Therefore, some of the included studies may have missed the high-risk group for early death after cardiac arrest, especially in studies that defined HH in the short term (i.e., 1–3 days) after CA. Finally, although predefined subgroup analyses were conducted, the subgroup results should be interpreted with caution due to the limited number of patients.
Conclusion
In conclusion, our results demonstrate that HH significantly increased mortality in post-CA patients. Moreover, HH is associated with higher lactate levels, longer ROSC time, higher risk of shock, heart failure, poor neurological outcome, and requires more organ function support. It is important to acknowledge the study’s limitations, including its study design and the potential for bias, which may have contributed to the relatively low certainty of our findings. However, these results are meaningful because they help to identify, early detect and optimize corresponding treatment strategies to reduce the risk of this high-mortality disease. Therefore, well-designed research in this area is necessary.
Declarations.
•Ethics approval and consent to participate.
Not applicable.
•Consent for publication
Not applicable.
•Availability of data and materials.
All data generated or analyzed during this study are included in this published article.
•Competing interests.
The authors declare that they have no competing interests.
•Funding.
High Level Chinese Medical Hospital Promotion Project (Funding numbers: HLCMHPP2023091).
•Authors' contributions.
Y-BG contributed to the conception, design, literature search and drafted the manuscript. J-HS and D-XY helped to search the literature, collect the data and performed statistical analyses. H-BH contributed to the conception, design, data interpretation, manuscript revision for critical intellectual content, and study supervision. All authors read and approved the manuscript.
•Acknowledgements.
Not applicable.
•Competing interests.
The authors declare that they have no competing interests.
CRediT authorship contribution statement
Ya-Bei Gao: Writing – original draft, Visualization, Methodology, Formal analysis, Data curation. Jia-Heng Shi: Writing – original draft, Software, Formal analysis, Data curation. Da-Xing Yu: Visualization, Validation, Resources, Methodology. Hui-Bin Huang: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2024.100834.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Dumas F., White L., Stubbs B.A., Cariou A., Rea T.D. Long-term prognosis following resuscitation from out of hospital cardiac arrest: role of percutaneous coronary intervention and therapeutic hypothermia. J Am Coll Cardiol. 2012;60(1):21–27. doi: 10.1016/j.jacc.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Mongardon N., Dumas F., Ricome S., et al. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care. 2011;1(1):45. doi: 10.1186/2110-5820-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen N., Friberg H. Changes in Practice of Controlled Hypothermia after Cardiac Arrest in the Past 20 Years: A Critical Care Perspective. Am J Respir Crit Care Med. 2023;207(12):1558–1564. doi: 10.1164/rccm.202211-2142CP. [DOI] [PubMed] [Google Scholar]
- 4.Sandroni C., Dell'anna A.M., Tujjar O., Geri G., Cariou A., Taccone F.S. Acute kidney injury after cardiac arrest: a systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016;82(9):989–999. [PubMed] [Google Scholar]
- 5.Lemiale V., Dumas F., Mongardon N., et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39(11):1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- 6.Jonsdottir S., Arnardottir M.B., Andresson J.A., Bjornsson H.K., Lund S.H., Bjornsson E.S. Prevalence, clinical characteristics and outcomes of hypoxic hepatitis in critically ill patients. Scand J Gastroenterol. 2021;57(3):311–318. doi: 10.1080/00365521.2021.2005136. [DOI] [PubMed] [Google Scholar]
- 7.Tapper E.B., Sengupta N., Bonder A. The Incidence and Outcomes of Ischemic Hepatitis: A Systematic Review with Meta-analysis. Am J Med. 2015;128(12):1314–1321. doi: 10.1016/j.amjmed.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Henrion J., Schapira M., Luwaert R., Colin L., Delannoy A., Heller F.R. Hypoxic Hepatitis. Medicine. 2003;82(6):392–406. doi: 10.1097/01.md.0000101573.54295.bd. [DOI] [PubMed] [Google Scholar]
- 9.Delignette M.C., Stevic N., Lebosse F., Bonnefoy-Cudraz E., Argaud L., Cour M. Acute liver failure after out-of-hospital cardiac arrest: An observational study. Resuscitation. 2024;197 doi: 10.1016/j.resuscitation.2024.110136. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y.I., Kang M.G., Ko R.E., et al. The Impact of Hypoxic Hepatitis on Clinical Outcomes after Extracorporeal Cardiopulmonary Resuscitation. J Clin Med. 2020;9(9) doi: 10.3390/jcm9092994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roedl K., Spiel A.O., Nurnberger A., et al. Hypoxic liver injury after in- and out-of-hospital cardiac arrest: Risk factors and neurological outcome. Resuscitation. 2019;137:175–182. doi: 10.1016/j.resuscitation.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (clinical Research Ed) 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Method. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ (clinical Research Ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champigneulle B., Geri G., Bougouin W., et al. Hypoxic hepatitis after out-of-hospital cardiac arrest: Incidence, determinants and prognosis. Resuscitation. 2016;103:60–65. doi: 10.1016/j.resuscitation.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Iesu E., Franchi F., Zama Cavicchi F., et al. Acute liver dysfunction after cardiac arrest. PLoS One. 2018;13(11):e0206655. doi: 10.1371/journal.pone.0206655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh S.H., Kim H.J., Park K.N., et al. Hypoxic hepatitis in survivors of out-of-hospital cardiac arrest. Am J Emerg Med. 2015;33(9):1166–1170. doi: 10.1016/j.ajem.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Nam S.H., Choi S.P., Youn C.S., So B.H., Oh Y.M., Park K.N. Hypoxic hepatitis in a postresuscitation state. Journal of the Korean Society of Emergency Medicine. 2009;20(1):65–71. [Google Scholar]
- 20.Huang H., Li H., Chen S., et al. Prevalence and Characteristics of Hypoxic Hepatitis in COVID-19 Patients in the Intensive Care Unit: A First Retrospective Study. Front Med (lausanne) 2020;7 doi: 10.3389/fmed.2020.607206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ucgun I., Ozakyol A., Metintas M., et al. Relationship between hypoxic hepatitis and cor pulmonale in patients treated in the respiratory ICU. Int J Clin Pract. 2005;59(11):1295–1300. doi: 10.1111/j.1742-1241.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 22.Jung C., Fuernau G., Eitel I., et al. Incidence, laboratory detection and prognostic relevance of hypoxic hepatitis in cardiogenic shock. Clin Res Cardiol. 2017;106(5):341–349. doi: 10.1007/s00392-016-1060-3. [DOI] [PubMed] [Google Scholar]
- 23.Choi S.H., Jang H.J., Suh Y.J., et al. Clinical Implication of Hypoxic Liver Injury for Predicting Hypoxic Hepatitis and In-Hospital Mortality in ST Elevation Myocardial Infarction Patients. Yonsei Med J. 2021;62(10):877–884. doi: 10.3349/ymj.2021.62.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuhrmann V., Kneidinger N., Herkner H., et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med. 2011;37(8):1302–1310. doi: 10.1007/s00134-011-2248-7. [DOI] [PubMed] [Google Scholar]
- 25.Vollmar B., Menger M.D. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89(4):1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead M.W., Hawkes N.D., Hainsworth I., Kingham J.G. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129–133. doi: 10.1136/gut.45.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dufour D.R., Lott J.A., Nolte F.S., Gretch D.R., Koff R.S., Seeff L.B. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46(12):2027–2049. [PubMed] [Google Scholar]
- 28.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79(2):115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 29.Cursio R., Gugenheim J., Ricci J.E., et al. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J. 1999;13(2):253–261. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz P., de Garibay A., Kortgen A., Leonhardt J., Zipprich A., Bauer M. Critical care hepatology: definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit Care. 2022;26(1):289. doi: 10.1186/s13054-022-04163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts B.W., Kilgannon J.H., Chansky M.E., et al. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Crit Care Med. 2013;41(6):1492–1501. doi: 10.1097/CCM.0b013e31828a39e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.