Abstract
Objective
To retrospectively compare the efficacy of Sacubitril/Valsartan and Benazepril in the treatment of heart failure in patients following acute myocardial infarction.
Methods
A retrospective analysis of clinical data was conducted for 103 patients with heart failure following acute myocardial infarction admitted to our hospital from January 2021 to January 2024. All patients met complete inclusion and exclusion criteria. Based on the treatment interventions received, they were divided into a control group (n=51) and an observation group (n=52). All patients received percutaneous coronary intervention (PCI) and conventional drug treatment upon admission. The control group received additional treatment with benazepril, while the observation group received Sacubitril/Valsartan on top of the baseline treatment. A comparison was made between the two groups in terms of clinical treatment outcomes, cardiac function indicators [left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDD), left ventricular ejection fraction (LVEF)], levels of inflammatory markers [high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6)], N-terminal pro-B-type natriuretic peptide (NT-proBNP), incidence of adverse reactions, major adverse cardiac events (MACEs), and 6-minute walking distance (6MWD).
Results
No patients were lost to follow-up. After six months of treatment, the observation group demonstrated significantly greater improvements in left ventricular function parameters (LVESV, LVEDD, and LVEF) and reductions in inflammatory markers (hs-CRP, IL-6) and NT-proBNP levels compared to the control group (P < 0.05). The observation group also had a significantly lower incidence of major adverse cardiac events (MACEs) (11.54% vs 31.37%, P < 0.05) and a greater improvement in 6-minute walking distance (P < 0.05). The incidence of adverse reactions was comparable between the two groups (P > 0.05).
Conclusion
Sacubitril/Valsartan is a safe and effective treatment for heart failure post-AMI, offering significant improvements in cardiac function, inflammatory response, exercise capacity, and a reduction in MACE risk.
Keywords: sacubitril/valsartan, heart failure following acute myocardial infarction, efficacy, cardiac function
Introduction
Heart failure is a condition characterized by the insufficient pumping of blood by the heart, leading to inadequate perfusion of tissues throughout the body. It is a common and severe complication following acute myocardial infarction (AMI).1 The cardiac damage and functional impairment caused by AMI often result in the loss of myocardial contraction and relaxation functions, ultimately progressing to heart failure. This condition not only reduces patients’ quality of life but also significantly increases the risk of recurrent cardiovascular events and mortality.2 In clinical practice, angiotensin-converting enzyme inhibitors (ACEIs) like benazepril have been widely used for managing heart failure. By inhibiting the renin-angiotensin-aldosterone system (RAAS), ACEIs help reduce cardiac preload and afterload, improving cardiac function and lowering mortality in patients with heart failure.3,4 However, despite these benefits, the therapeutic effects of ACEIs may be insufficient for certain patient populations, underscoring the need for more effective treatment modalities.
Excessive activation of the RAAS is a key mechanism driving ventricular remodeling in heart failure.5 Both ACEIs and angiotensin receptor blockers (ARBs) have demonstrated efficacy in improving outcomes in patients with heart failure following AMI.6 Recently, the PARADIGM-HF study highlighted that sacubitril/valsartan, an angiotensin receptor-neprilysin inhibitor (ARNI), significantly improved prognosis in patients with heart failure with reduced ejection fraction (HFrEF) compared to the ACEI enalapril.7 This dual-acting ARNI not only suppresses RAAS but also enhances natriuretic peptide activity, which collectively contributes to improved clinical outcomes.8
While sacubitril/valsartan has shown promise in managing HFrEF, its role in treating heart failure following AMI remains less established. Existing studies suggest it may offer specific benefits over conventional therapies such as ACEIs, including better cardiac function, lower inflammatory markers, and reduced risk of major adverse cardiac events (MACEs). However, further research is needed to comprehensively evaluate its efficacy and safety in this context.
Study Objective and Hypothesis: This study aims to compare the clinical efficacy and safety of sacubitril/valsartan versus benazepril in treating heart failure following AMI. It is hypothesized that sacubitril/valsartan will yield superior outcomes, including improved cardiac function parameters, reduced inflammatory markers, and lower MACE incidence, without significantly increasing adverse reactions.
By retrospectively analyzing clinical data from 103 patients treated between January 2021 and January 2024, this study seeks to provide evidence-based insights to inform individualized treatment plans and improve the quality of care for patients with heart failure post-AMI. Improved management of heart failure in this population could have profound long-term benefits, including reduced rehospitalization rates, better quality of life, and lower mortality.
Objects and Methods
Study Population
A retrospective analysis was conducted on clinical data from 103 patients with heart failure after acute myocardial infarction admitted to our hospital from January 2021 to January 2024. Inclusion criteria: ① Patients diagnosed with heart failure after acute myocardial infarction through clinical tests. ② Patients who underwent percutaneous coronary intervention (PCI) following acute myocardial infarction and had an ejection fraction (EF) <50% on echocardiography during hospitalization. ③ Patients who exhibited postoperative signs of cardiac dysfunction with NT-proBNP >300μg/L. ④ Patients aged ≥18 years, of any gender. ⑤ Availability of complete and authentic clinical data for analysis. Exclusion criteria: ① Patients who did not undergo PCI treatment. ② Patients with EF ≥50% on echocardiography during hospitalization after PCI. ③ Patients with severe organ dysfunction. ④ Patients with allergies or contraindications to the medications or methods used in this study. ⑤ Patients with cognitive or consciousness disorders.
Data Collection and Validation
Data were collected by trained medical professionals using a standardized protocol to ensure consistency. Patient records were reviewed using electronic medical records (EMR) systems, and data accuracy was validated through cross-referencing clinical notes and laboratory reports. Any discrepancies or missing information were resolved through consultation with attending physicians.
Based on the treatment interventions received, patients were divided into a control group (n=51) and an observation group (n=52). All patients received PCI and conventional drug treatment upon admission. Patients in the control group received benazepril treatment on this basis, while patients in the observation group received sacubitril/valsartan treatment in addition.
Methods
All included patients underwent PCI treatment within the acute myocardial infarction time window (12 h) after admission, followed by coronary angiography and stent placement. Routine health education was provided to explain precautions during treatment, and conventional drug therapy, including inotropic agents, diuretics, anti-sympathetic nerve remodeling, antiplatelet aggregation, and lipid-lowering, was administered as needed.
Control Group
Patients in the control group received benazepril treatment with oral benazepril hydrochloride tablets (produced by Beijing Novartis Pharma Ltd., National Medical Products Administration approval number H20000292), with a dosage of 10 mg per dose, once daily. The treatment duration was 6 months.
Observation Group
Patients in the observation group received sacubitril/valsartan treatment, taking sacubitril/valsartan sodium tablets (produced by Beijing Novartis Pharma Ltd., National Medical Products Administration approval number J20171054) orally. The initial oral dose was 50 mg per dose, twice daily, with dose adjustments every 2–4 weeks based on blood pressure levels until reaching a target dose of 200 mg, twice daily. The treatment duration was 6 months.
Observation Indicators
(1) Clinical Efficacy Rate:9 Marked effect: Complete disappearance of clinical symptoms, improvement of heart function grade by ≥2 levels after treatment. Effective: Significant improvement of clinical symptoms, improvement of heart function grade by ≥1 level after treatment. Ineffective: No significant improvement in clinical symptoms and heart function grade or even further deterioration after treatment. Total efficacy rate = (Marked effect + Effective) cases / Total cases × 100%.
(2) Levels of Heart Function Indicators: Before and after treatment, using the Philips IE Elite echocardiography diagnostic instrument, left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDD), and left ventricular ejection fraction (LVEF) were measured in patients.
(3) Levels of Inflammatory Factor Indicators: Before and after treatment, 5 mL of fasting venous blood was collected from each patient in the morning, and serum was obtained by routine centrifugation. Enzyme-linked immunosorbent assay (ELISA) was used to detect serum levels of high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6).
(4) N-terminal pro-B-type natriuretic peptide (NT-proBNP) Levels: Before and after treatment, serum was collected from each patient (method same as above), and the NT-proBNP levels were determined using electrochemiluminescence.
(5) Occurrence of Adverse Reactions: Adverse reactions observed in this study included dizziness, nausea, hypotension, hyperkalemia, changes in renal function, bradycardia, etc. The occurrence of these adverse reactions was uniformly recorded by relevant medical staff in our hospital.
(6) Major adverse cardiac events (MACEs):10 A 6-month follow-up was conducted to document outcomes, including myocardial infarction, readmission rate for heart failure, occurrence of ventricular tachycardia or frequent ventricular premature contractions, and cardiac-related death. These outcome data were uniformly recorded by relevant medical staff in our hospital.
(7) 6-Minute Walk Test (6MWD) Level: After completion of treatment, the 6MWD was used to assess exercise endurance. A quiet and well-ventilated corridor was selected, a starting point was marked, and a distance of 30 meters was indicated. Patients were informed of the test purpose and method, and they were instructed to walk back and forth as quickly as possible during the 6-minute test. If any discomfort such as shortness of breath occurred, patients could adjust their breathing and walking speed. The 6-minute walking distance, heart rate, blood oxygen saturation, and other relevant data were then recorded.
Statistical Analysis
GraphPad Prism 8 was used for graphical representation, and SPSS 22.0 was employed for data analysis. For continuous data, mean and standard deviation were used to describe their distribution, and the t-test was applied for statistical analysis. For categorical data, frequency and percentage were used to describe their distribution, and the chi-square test was employed for statistical analysis. A significance level of P<0.05 was considered statistically significant.
Results
Baseline Data Comparison
The baseline characteristics of the two groups showed no statistically significant differences (P > 0.05), indicating comparability. See Table 1 for details.
Table 1.
Comparison of Baseline Data
| Control (n=51) | Observation (n=52) | t/x² | P | |
|---|---|---|---|---|
| Gender | – | – | 0.318 | 0.572 |
| Male | 41 (80.39%) | 44 (84.62%) | – | – |
| Female | 10 (19.61%) | 8 (15.38%) | – | – |
| Age (years) | 48.64±9.85 (45.94, 51.34) | 47.96±9.97 (45.25, 50.67) | 0.348 | 0.728 |
| BMI (kg/m²) | 24.26±1.91 (23.73, 24.79) | 24.07±2.02 (23.51, 24.63) | 0.490 | 0.625 |
| Onset to Intervention Time (h) | 6.15±5.87 (4.51,7.79) | 5.93±6.02 (4.27,7.59) | 0.187 | 0.851 |
| SBP (mmHg) | 120.15±17.26 (115.34,125.00) | 119.42±15.97 (115.01,123.83) | 0.222 | 0.824 |
| DBP (mmHg) | 76.34±15.38 (72.05, 80.63) | 74.96±16.03 (70.52, 79.40) | 0.445 | 0.656 |
| Comorbidities | – | – | – | – |
| Hypertension | 19 (37.25%) | 21 (40.38%) | 0.106 | 0.744 |
| Diabetes | 6 (11.76%) | 8 (15.38%) | 0.287 | 0.592 |
| Myocardial Infarction Site | – | – | 0.083 | 0.772 |
| Anterior Wall | 39 (76.47%) | 41 (78.85%) | – | – |
| Inferior Wall | 12 (23.53%) | 11 (21.15%) | – | – |
| Stents Implanted (number) | 1.78±0.92 (1.524, 2.036) | 1.83±0.89 (1.584, 2.076) | 0.280 | 0.779 |
Comparison of Clinical Efficacy Rate
The total effective rate of treatment in the control group was 72.55%, while in the observation group, it was 92.31%, indicating a significantly higher total effective rate in the observation group than in the control group (P<0.05). See Table 2 for details.
Table 2.
Comparison of Clinical Efficacy Rate
| Group | n | Marked Effect Effect | Effective | Ineffective | Total Efficacy Rate |
|---|---|---|---|---|---|
| Control | 51 | 21 | 15 | 12 | 79.31% |
| Observation | 52 | 30 | 9 | 3 | 94.92% |
| x² | – | – | – | – | 6.372 |
| P | – | – | – | – | 0.011 |
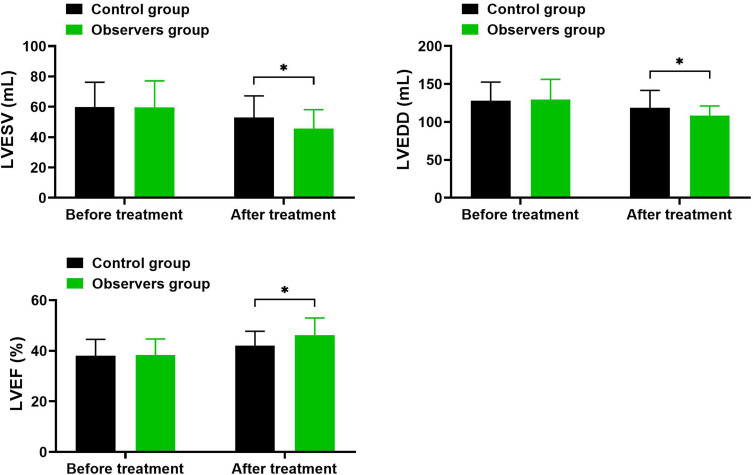
Comparison of Left Ventricular Function Parameters Before and After Treatment: Changes in LVESV, LVEDD, and LVEF Across Groups
As shown in Table 3 and Figure 1, Before treatment, there were no significant differences in LVESV, LVEDD, and LVEF levels between the two groups (P > 0.05). After treatment, both groups demonstrated significant reductions in LVESV and LVEDD levels (P < 0.05) and a significant increase in LVEF (P < 0.05). Additionally, the improvements in these parameters were significantly greater in the observation group compared to the control group (P < 0.05).
Table 3.
Comparison of Left Ventricular Function Parameters Before and After Treatment: Changes in LVESV, LVEDD, and LVEF Across Groups
| Indicator | Control (n=51) | Observation (n=52) | t/x² | P | |
|---|---|---|---|---|---|
| Before Treatment | LVESV (mL) | 59.93±16.27 (55.39, 64.47) | 59.67±17.43 (54.87, 64.47) | 0.078 | 0.939 |
| After Treatment | 52.84±14.36 (48.87, 56.81) | 45.69±12.41 (42.23, 49.15) | 3.343 | 0.001 | |
| Before Treatment | LVEDD (mm) | 128.19±24.36 (121.38, 134.00) | 129.54±26.69 (122.15, 136.93) | 0.123 | 0.903 |
| After Treatment | 118.96±22.65 (112.68, 125.24) | 108.37±12.65 (104.87, 111.87) | 2.353 | 0.025 | |
| Before Treatment | LVEF (%) | 38.06±6.45 (36.26, 39.86) | 38.34±6.32 (36.59, 40.09) | 0.097 | 0.929 |
| After Treatment | 42.07±5.63 (40.51, 43.63) | 46.12±6.87 (44.22,48.02) | 1.319 | 0.015 |
Figure 1.
Changes in LVESV, LVEDD, and LVEF in the Control and Observation Groups Before and After Treatment.
Notes: *Indicates intergroup comparison with P<0.05.
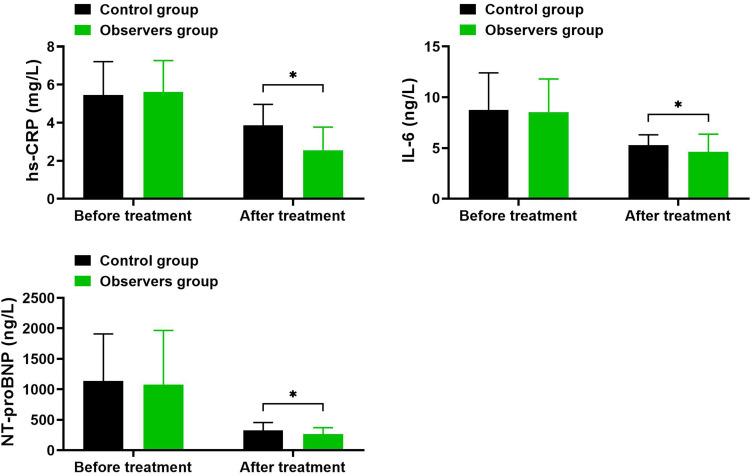
Comparison of Inflammatory Marker and NT-proBNP Levels Before and After Treatment in SV and Control Groups
As depicted in Table 4 and Figure 2, before treatment, there were no significant differences between the two groups in hs-CRP, IL-6, and NT-proBNP levels (P > 0.05). After treatment, levels of hs-CRP, IL-6, and NT-proBNP significantly decreased in both groups, with the observation group showing greater reductions compared to the control group (P < 0.05).
Table 4.
Comparison of Inflammatory Marker and NT-proBNP Levels Before and After Treatment in SV and Control Groups
| Indicator | Control (n=51) | Observation (n=52) | t/x² | P | |
|---|---|---|---|---|---|
| Before Treatment | hs-CRP (mg/L) | 5.45±1.76 (4.96, 5.94) | 5.61±1.65 (5.15, 6.07) | 1.336 | 0.381 |
| After Treatment | 3.87±1.09 (3.57, 4.17) | 2.54±1.23 (2.20, 2.88) | 0.513 | 0.018 | |
| Before Treatment | IL-6 (pg/mL) | 8.76±3.65 (7.28, 10.24) | 8.54±3.27 (7.08, 9.99) | 0.934 | 0.73 |
| After Treatment | 5.29±1.02 (5.01, 5.57) | 4.63±1.74 (4.15, 5.11) | 1.116 | 0.019 | |
| Before Treatment | NT-proBNP (pg/mL) | 1138.31±772.21 (921.68, 1354.32) | 1079.52±886.62 (834.28, 1323.72) | 1.421 | 0.749 |
| After Treatment | 331.37±125.70 (296.21, 365.79) | 267.42±104.19 (238.33, 295.67) | 0.518 | 0.024 |
Figure 2.
Comparison of Inflammatory Marker and NT-proBNP Levels Before and After Treatment in SV and Control Groups.
Notes: *Indicates intergroup comparison with P<0.05.
Comparison of Adverse Reactions
The incidence of adverse reactions in the control group was 19.61%, while in the observation group, it was 15.38%. There was no significant difference in the incidence of adverse reactions between the two groups (P=0.572>0.05). See Table 5 for details.
Table 5.
Comparison of Adverse Reactions
| Adverse Reactions | Control (n=51) | Observation (n=52) | x² | P |
|---|---|---|---|---|
| Dizziness and Nausea | 3 (5.88%) | 4 (7.69%) | – | – |
| Hypotension | 3 (5.88%) | 2 (3.85%) | – | – |
| Hyperkalemia | 2 (3.92%) | 1 (1.92%) | – | – |
| Renal Function Changes | 1 (1.96%) | 1 (1.92%) | – | – |
| Bradycardia | 1 (1.96%) | 0 (0.00%) | – | – |
| Total Incidence | 10 (19.61%) | 8 (15.38%) | 0.318 | 0.572 |
Comparison of Clinical Outcomes and 6MWD Levels
In the control group, the incidence of recurrent myocardial infarction was 1 (1.96%), ventricular tachycardia or frequent premature ventricular contractions was 6 (11.76%), heart failure readmission was 9 (17.65%), and sudden cardiac death was 0 (0.00%), resulting in an overall MACE incidence of 16 (31.37%). In the observation group, the incidence of recurrent myocardial infarction was 1 (1.92%), ventricular tachycardia or frequent premature ventricular contractions was 4 (7.69%), heart failure readmission was 1 (1.92%), and sudden cardiac death was 0 (0.00%), leading to an overall MACE incidence of 6 (11.54%). The difference between the two groups was statistically significant (P =0.014< 0.05). The 6-minute walking distance (6MWD) was significantly higher in the observation group compared to the control group (P=0.009<0.05). See Table 6 for details.
Table 6.
Comparison of Major MACE Incidence and 6MWD Levels Between the Two Groups
| Clinical Outcomes | Control (n=51) | Observation (n=52) | t/x² | P |
|---|---|---|---|---|
| Recurrent Myocardial Infarction | 1 (1.96%) | 1 (1.92%) | ||
| Ventricular Tachycardia/PVCs | 6 (11.76%) | 4 (7.69%) | ||
| Heart Failure Readmission | 9 (17.65%) | 1 (1.92%) | ||
| Sudden Cardiac Death | 0 (0.00%) | 0 (0.00%) | ||
| Overall MACE incidence | 16 (31.37%) | 6 (11.54%) | 6.031 | 0.014 |
| 6MWD (meters) | 367.82±112.43 | 425.39±108.76 | 2.641 | 0.009 |
Discussion
Acute myocardial infarction (AMI) occurs when thrombosis within the coronary arteries leads to myocardial cell ischemia, hypoxia, necrosis, and subsequent impairment of myocardial function.11 Even with coronary intervention, approximately 30% of patients may undergo cardiac remodeling, a crucial pathological basis for the development of heart failure following AMI.12 Therefore, comprehensive management of various risk factors contributing to heart failure is crucial. This includes addressing coronary ischemia, arrhythmias, blood pressure monitoring, glucose control, and achieving optimal lipid levels. Following myocardial ischemia, myocardial cells and the extracellular matrix experience damage, energy depletion, neurohumoral imbalances, activation of the fibrinolytic system, and expression of cytokines such as IL-6, triggering immune and inflammatory responses, ultimately leading to left ventricular remodeling.13 Even after coronary intervention, the possibility of left ventricular remodeling persists, especially in cases of extensive myocardial infarction.14 The treatment of chronic heart failure requires comprehensive intervention and management, with the “golden triangle” treatment approach (including β-blockers, ACE inhibitors or ARBs, and diuretics) being a cornerstone for heart failure prevention and treatment. However, despite the high usage rates of the “golden triangle”, patients with refractory heart failure still face increased risks of death and rehospitalization within one year.15 In addition to antagonizing the renin-angiotensin-aldosterone system (RAAS) and the sympathetic system, the natriuretic peptide system plays a critical role in left ventricular remodeling. Sacubitril/valsartan, besides inhibiting RAAS, also suppresses the natriuretic peptide system, dilates blood vessels, reduces sympathetic nerve activity, and exerts effects such as diuresis and vasodilation, which are significant in inhibiting myocardial cell hypertrophy and fibrosis associated with ventricular remodeling.16 Previous studies have indicated that compared to angiotensin-converting enzyme inhibitors, sacubitril/valsartan significantly reduces the risks of cardiovascular death, heart failure readmission, and all-cause mortality in heart failure patients, while also markedly improving symptoms and quality of life.17
To further clarify the efficacy of sacubitril/valsartan in the treatment of heart failure after acute myocardial infarction (AMI), this study retrospectively analyzed clinical data from a group of patients who underwent percutaneous coronary intervention (PCI) for AMI-induced heart failure at our hospital. The results of this study revealed that the total effective rate of treatment in the control group was 72.55%, while in the observation group, it was 92.31%, significantly higher than that in the control group (P<0.05). After treatment, the levels of left ventricular end-systolic volume (LVESV) and left ventricular end-diastolic volume (LVEDD) in the observation group were significantly lower than those in the control group, and the left ventricular ejection fraction (LVEF) was significantly higher than that in the control group (P<0.05). The adverse reaction rate in the control group was 19.61%, and in the observation group, it was 15.38%, with no significant difference between the two groups (P>0.05). The occurrence rates of recurrent myocardial infarction, ventricular tachycardia/frequent ventricular premature contractions, and sudden cardiac death showed no significant difference between the two groups (P>0.05). The observation group exhibited a significantly lower heart failure rehospitalization rate and a significantly higher 6-minute walk distance (6MWD) compared to the control group (P<0.05). These findings, consistent with previous relevant studies,18–20 suggest that compared to enalapril, sacubitril/valsartan more significantly improves left ventricular function in patients, further increases patients’ exercise tolerance, and does not increase adverse reactions or cardiovascular events. Therefore, it is speculated that, on the basis of optimized standard drug therapy, early use of sacubitril/valsartan may provide greater benefits to patients with heart failure after acute myocardial infarction.
NT-proBNP is a cardiac hormone and the precursor molecule of B-type natriuretic peptide (BNP). Ventricular cells release BNP in response to stretching or myocardial cell damage, and BNP is then enzymatically cleaved into two fragments, namely active BNP and inactive NT-proBNP.21 Therefore, measuring NT-proBNP levels is commonly used as an indicator to assess cardiac stress, load, and the severity of heart failure. The elevation of NT-proBNP is closely associated with increased ventricular pressure caused by myocardial injury.22 It is worth noting that many diseases other than heart failure, such as infections, inflammatory lung diseases, cancer, etc., may also lead to an increase in NT-proBNP levels. NT-proBNP has been proven to be an effective marker for predicting adverse outcomes in heart failure patients. Although active BNP has a protective effect on the heart during heart failure attacks, the circulating levels of BNP or NT-proBNP reflect unstable hemodynamics and neurohormonal activation.23 During heart failure attacks, systemic inflammatory response syndrome may also occur, and the levels of inflammatory biomarkers in the body of heart failure patients increase with the progression of the disease. Research24 suggests that the pro-inflammatory state may contribute to the occurrence and development of heart failure, affecting not only myocardial function but also causing dysfunction in other organs and tissues, leading to a series of complications in heart failure patients, including cachexia and anemia. hs-CRP and IL-6 are commonly used clinical inflammation indicators, and they significantly increase in heart failure patients, with an increase as decompensation occurs, possibly closely related to the deterioration of cardiac function and reduced exercise tolerance in heart disease.25,26 Elevated levels of hs-CRP and IL-6 reflect inflammation and immune imbalance in heart failure, predicting adverse disease outcomes. The results of this study showed that after treatment, the levels of hs-CRP, IL-6, and NT-proBNP in the observation group were significantly lower than those in the control group (P<0.05). Similar to previous relevant research results, this suggests that the application of sacubitril/valsartan in the treatment of heart failure after acute myocardial infarction can effectively reduce NT-proBNP levels and inflammation reactions in patients.
Limitations and Areas for Future Research
While this study presents promising results, several important limitations must be considered: ① Small Sample Size: The relatively small sample size in this study (n=51 for the control group and n=52 for the observation group) may limit the statistical power and generalizability of the findings. A larger sample size could help confirm the results and provide more robust evidence. ② Retrospective Design and Potential Bias: The study’s retrospective design inherently introduces the risk of information bias and treatment selection bias. The lack of random assignment means that patient characteristics (eg, comorbidities, prior medications) could influence the results. A prospective randomized controlled trial (RCT) would offer more reliable evidence by controlling for these variables. Additionally, propensity score matching could be employed to reduce bias in future studies. ③ Control of Confounding Factors: While we did attempt to match baseline characteristics between groups, unmeasured confounding factors such as lifestyle, other medications, or the severity of coronary artery disease could still impact outcomes. Future studies should aim to explicitly control for these factors to ensure the observed effects are due to sacubitril/valsartan rather than other variables. ④ Follow-up Duration: The relatively short follow-up period in this study (typically a few months) limits our ability to evaluate long-term effects of sacubitril/valsartan on heart failure progression and patient survival. Extended follow-up could better capture the durability of the treatment effects, including long-term mortality, rehospitalization rates, and quality of life. ⑤ Safety Profile in Complex Populations: While no significant differences were observed in adverse reactions between groups, the safety profile of sacubitril/valsartan in patients with specific comorbid conditions (eg, renal insufficiency, diabetes, hypertension) warrants further investigation. Additional research focusing on these subgroups will be crucial to understanding the full safety profile of this therapy in a broader patient population.
Future Directions
Future research should focus on randomized controlled trials with larger and more diverse patient populations to validate these findings. Multi-center studies would also increase the generalizability of results, addressing the broader applicability of sacubitril/valsartan in heart failure treatment post-AMI. Additionally, longer follow-up durations will be essential to assess the long-term efficacy and safety of sacubitril/valsartan in heart failure patients. Ultimately, understanding the impact of sacubitril/valsartan in real-world clinical settings, including patients with multiple comorbidities, will further solidify its role as a critical therapy for heart failure management after AMI.
Conclusion
The application of sacubitril/valsartan in the treatment of heart failure after acute myocardial infarction demonstrates promising effectiveness. Compared to benazepril, sacubitril/valsartan can further enhance patient outcomes, improve cardiac remodeling and function, reduce NT-proBNP levels and inflammatory responses, decrease the risk of heart failure rehospitalization, enhance exercise tolerance, and importantly, the use of sacubitril/valsartan does not increase the risk of adverse reactions or cardiovascular events in patients. This suggests a higher level of drug safety, making it worthy of clinical promotion and application.
Funding Statement
Study on the efficacy of Sackubactril Valsartan in the treatment of acute anterior myocardial infarction complicated with HFmrEF. Id: LSFGG-2024016
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bahit MC, Kochar A, Granger CB. Post-Myocardial Infarction Heart Failure. JACC Heart Fail. 2018;6(3):179–186. doi: 10.1016/j.jchf.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 2.Jenča D, Melenovský V, Stehlik J, et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021;8(1):222–237. doi: 10.1002/ehf2.13144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffman M, Guillot E, Blondel T, et al. Clinical efficacy of a benazepril and spironolactone combination in dogs with congestive heart failure due to myxomatous mitral valve disease: the BEnazepril Spironolactone Study (BESST). J Vet Intern Med. 2021;35(4):1673–1687. doi: 10.1111/jvim.16155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper TE, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease. Cochrane Database Syst Rev. 2023;7(7):Cd007751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyse A, Manhaeghe L, Mahieu E, et al. Sacubitril/valsartan in heart failure and end-stage renal insufficiency. ESC Heart Fail. 2019;6(6):1331–1333. doi: 10.1002/ehf2.12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann DL, Givertz MM, Vader JM, et al. Effect of Treatment With Sacubitril/Valsartan in Patients With Advanced Heart Failure and Reduced Ejection Fraction: a Randomized Clinical Trial. JAMA Cardiol. 2022;7(1):17–25. doi: 10.1001/jamacardio.2021.4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray JJ, Packer M, Desai AS, et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077 Epub 2014 Aug 30. PMID: 25176015. [DOI] [PubMed] [Google Scholar]
- 8.Jackson AM, Jhund PS, Anand IS, et al. Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. Eur Heart J. 2021;42(36):3741–3752. doi: 10.1093/eurheartj/ehab499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abazid RM, Khalaf HH, Sakr HI, et al. Effects of Ramadan fasting on the symptoms of chronic heart failure. Saudi Med J. 2018;39(4):395–400. doi: 10.15537/smj.2018.4.22011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aakre EK, Aakre KM, Flaatten H, Hufthammer KO, Ranhoff AH, Jammer I. High-Sensitivity Cardiac Troponin T and Frailty Predict Short-Term Mortality in Patients ≥75 Years Undergoing Emergency Abdominal Surgery: a Prospective Observational Study. Anesth Analg. 2024;139(2):313–322. doi: 10.1213/ANE.0000000000006845 [DOI] [PubMed] [Google Scholar]
- 11.Wang XY, Zhang F, Zhang C, et al. The Biomarkers for Acute Myocardial Infarction and Heart Failure. Biomed Res Int. 2020;2020:2018035. doi: 10.1155/2020/2018035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz S, Hundertmark MJ, Schulz-Menger J, et al. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43(27):2549–2561. doi: 10.1093/eurheartj/ehac223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Buono MG, Garmendia CM, Seropian IM, et al. Heart Failure After ST-Elevation Myocardial Infarction: beyond Left Ventricular Adverse Remodeling. Curr Probl Cardiol. 2023;48(8):101215. doi: 10.1016/j.cpcardiol.2022.101215 [DOI] [PubMed] [Google Scholar]
- 14.Harrington J, Butler J. Heart failure after myocardial infarction: glass emptier than full. Eur J Heart Fail. 2023;25(8):1225–1227. doi: 10.1002/ejhf.2961 [DOI] [PubMed] [Google Scholar]
- 15.Luo L, Yang X, Tang K, et al. Efficacy of three novel drugs in the treatment of heart failure: a network meta-analysis. Medicine. 2022;101(29):e29415. doi: 10.1097/MD.0000000000029415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieske B, Wachter R, Shah SJ, et al. Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: the PARALLAX Randomized Clinical Trial. JAMA. 2021;326(19):1919–1929. doi: 10.1001/jama.2021.18463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang H, Zhang J, Zhang X, et al. Effects of sacubitril/valsartan in patients with heart failure and chronic kidney disease: a meta-analysis. Eur J Pharmacol. 2020;884:173444. doi: 10.1016/j.ejphar.2020.173444 [DOI] [PubMed] [Google Scholar]
- 18.Mann DL, Greene SJ, Givertz MM, et al. Sacubitril/Valsartan in Advanced Heart Failure With Reduced Ejection Fraction: rationale and Design of the LIFE Trial. JACC Heart Fail. 2020;8(10):789–799. doi: 10.1016/j.jchf.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang HY, Feng A-N, Fong M-C, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74(4):372–380. doi: 10.1016/j.jjcc.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Huetteman AT, Reyes EA, et al. Effects of Sacubitril-Valsartan in Patients With Various Types of Heart Failure: a Meta-analysis. J Cardiovasc Pharmacol. 2023;81(6):434–444. doi: 10.1097/FJC.0000000000001421 [DOI] [PubMed] [Google Scholar]
- 21.Cao Z, Jia Y, Zhu B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int J Mol Sci. 2019;20(8):1820. doi: 10.3390/ijms20081820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham JW, Myhre PL. NT-proBNP Response to Heart Failure Therapies: an Imperfect Surrogate. J Am Coll Cardiol. 2021;78(13):1333–1336. doi: 10.1016/j.jacc.2021.07.045 [DOI] [PubMed] [Google Scholar]
- 23.Semenov AG, Feygina EE. Standardization of BNP and NT-proBNP Immunoassays in Light of the Diverse and Complex Nature of Circulating BNP-Related Peptides. Adv Clin Chem. 2018;85:1–30. [DOI] [PubMed] [Google Scholar]
- 24.Murphy SP, Kakkar R, McCarthy CP, et al. Inflammation in Heart Failure: JACC State-of-The-Art Review. J Am Coll Cardiol. 2020;75(11):1324–1340. doi: 10.1016/j.jacc.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 25.Gui XY, Rabkin SW. C-Reactive Protein, Interleukin-6, Trimethylamine-N-Oxide, Syndecan-1, Nitric Oxide, and Tumor Necrosis Factor Receptor-1 in Heart Failure with Preserved Versus Reduced Ejection Fraction: a Meta-Analysis. Curr Heart Fail Rep. 2023;20(1):1–11. doi: 10.1007/s11897-022-00584-9 [DOI] [PubMed] [Google Scholar]
- 26.Sugiura T, Nawaz S, Ferrell BE, Yoshida T. Induced Pluripotent Stem Cells: a New Dawn for the Treatment of Ischemic Cardiomyopathy. Innov Discov. 2024;1(4):31. doi: 10.53964/id.2024031 [DOI] [Google Scholar]