Abstract
This review elucidates the pivotal role of pyroptosis, triggered by gut microbiota, in the development of multiple sclerosis (MS), emphasizing its significance within the gut-brain axis. Our comprehensive analysis of recent literature reveals how dysbiosis in the gut microbiota of MS patients—characterized by reduced microbial diversity and shifts in bacterial populations—profoundly impacts immune regulation and the integrity of the central nervous system (CNS). Pyroptosis, an inflammatory form of programmed cell death, significantly exacerbates MS by promoting the release of inflammatory cytokines and causing substantial damage to CNS tissues. The gut microbiota facilitates this detrimental process through metabolites such as short-chain fatty acids and neuroactive compounds, or self-structural products like lipopolysaccharides (LPS), which modulate immune responses and influence neuronal survival. This review highlights the potential of modulating gut microbiota to regulate pyroptosis, thereby suggesting that targeting this pathway could be a promising therapeutic strategy to mitigate inflammatory responses and preserve neuronal integrity in patients with MS.
Keywords: multiple sclerosis, gut microbiota, pyroptosis, gut-brain axis, neuroinflammation, therapeutic targets
Introduction
Multiple sclerosis (MS) is a chronic, debilitating autoimmune disease primarily affecting the central nervous system (CNS), leading to severe neurological deficits. It emerges as a frequently observed neurological condition, exhibiting an annual incidence rate of 2.1 per 10` individuals [95% CI: 2.09, 2.12].1 The pathogenesis of MS is marked by complex interactions that result in inflammation and demyelination throughout the brain and spinal cord.2 In MS, the disease process begins with focal lymphocyte infiltration that damages myelin and axons.3 Initially, this inflammation is transient and may even be accompanied by some myelin regeneration,4 though such regeneration is not sustained. As a result, patients early in the disease course often experience neurological dysfunctions which may temporarily resolve. Over time, the pathology evolves from these initial symptoms to a more severe and chronic state.5 The hallmark of this demyelinating disease is the formation of sclerotic plaques,6 representing the end stage of a cascade of pathological changes including ongoing inflammation,7 repeated cycles of demyelination8 and remyelination,9 depletion of oligodendrocytes,10 astrocytosis,11 and extensive neuronal and axon degeneration.12 The varied symptoms of MS, such as visual and sensory disturbances, muscle weakness, coordination problems, and sometimes severe cognitive decline, reflect the complex and multifaceted nature of this pathology. Moreover, the progression of MS is highly individualized and remains unpredictable, complicating management and prognosis.12 Effective management of MS aims to improve quality of life and ensure patient independence through a combination of strategies.13 These include early and continuous immunomodulatory treatments, targeted rehabilitation to enhance functional capabilities, and the deployment of assistive devices that aid mobility, all designed to more effectively manage symptoms.14 Despite the availability of these interventions, completely halting the progression of MS poses a substantial challenge. To specifically address the inflammation associated with relapsing forms of MS, eight FDA-approved agents are currently available.15 These therapies target various components of the immune system, each with the goal of reducing and preventing further inflammatory activity.16,17 Among the disease-modifying therapies (DMTs) are injectable treatments such as interferons and glatiramer acetate, oral medications including fingolimod, dimethyl fumarate,18 and teriflunomide,19 and infusion therapies like natalizumab,20 alemtuzumab,21 and ocrelizumab.22 Despite these diverse options, the complete prevention of MS progression remains elusive, underscoring the need for continued innovation in treatment approaches.
Recent scientific research has underscored a profound link between human gut microbiota, dietary patterns, and the onset of chronic degenerative diseases, indicating that variations in the microbiota’s composition and function are associated with various chronic inflammatory mechanisms.23 This is particularly evident in MS, where the gut-brain axis—characterized by bidirectional communication between the gut microbiota and the CNS—becomes a critical factor in the disease’s pathogenesis. In MS patients, distinct microbiota profiles have been identified,24 marked by a decreased abundance of beneficial bacteria such as those in the Clostridia XIVa and IV clusters, and an increased presence of potentially harmful bacteria that contribute to inflammation and autoimmunity.25 This dysbiosis disrupts immune homeostasis, enhances the permeability of both the intestinal and blood-brain barriers (BBB), and triggers autoimmune reactions against CNS components.26
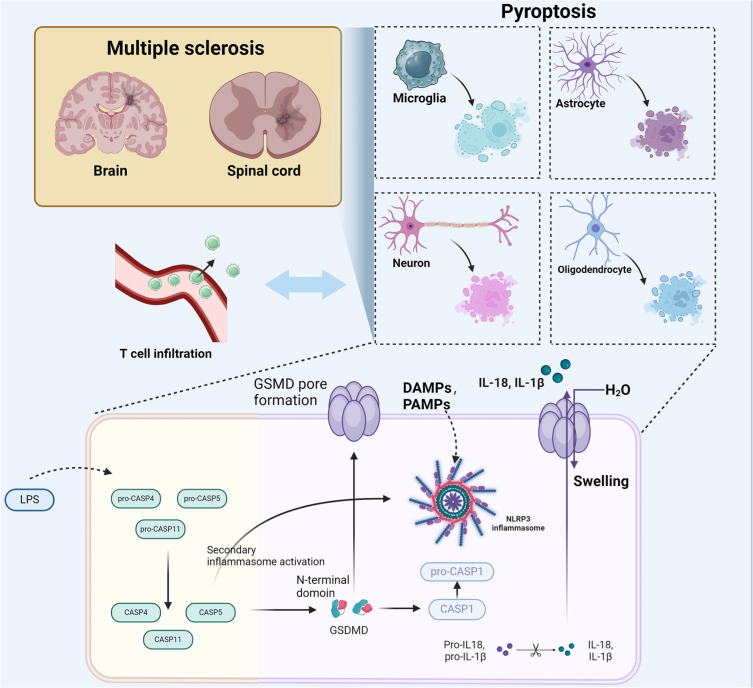
Pyroptosis, an intensely inflammatory type of programmed cell death marked by the activation of caspase-1 or caspase-11, plays a critical role in exacerbating the pathology of MS27 (Figure 1). Triggered by various inflammatory signals, this process leads to cellular rupture and the emission of pro-inflammatory cytokines like interleukin 1β (IL-1β) and interleukin 18 (IL-18), which amplify the inflammatory response within the CNS.28 Recent studies have highlighted that gut microbiota can induce pyroptosis, contributing to severe inflammatory conditions like intestinal inflammation, cancer, and sepsis, and particularly impacting MS by exacerbating local and systemic inflammation. This dysregulation disrupts cellular and neurological functions essential for CNS integrity, thereby accelerating the progression of MS.29
Figure 1.
The role of pyroptosis in exacerbating MS pathology. The activation pathways involving DAMPs, PAMPs, and LPS, leading to inflammasome activation. This process triggers Gasdermin D-mediated cell death in neurons, astrocytes, oligodendrocytes, and microglia, exacerbating inflammation and autoimmunity in MS.
The review begins with an introduction to the relationship between Multiple Sclerosis (MS) and cell pyroptosis, examining emerging evidence that suggests gut microbiota-induced pyroptosis significantly contributes to the pathogenesis of MS. It explores how microbial imbalances and related pathways can lead to CNS involvement through pyroptotic mechanisms. Delving deeper, the review elucidates the cascade of events triggered by such imbalances and emphasizes the potential of gut microbiota modulation as a therapeutic strategy. This approach not only underscores the intricate connections between gut microbiota and MS but also suggests promising avenues for intervention to mitigate the progression of the disease.
The Overview of Pyroptosis
Pyroptosis
Pyroptosis is a recently discovered form of programmed cell death, distinct in its evolutionary development and mechanism from apoptosis or necrosis. This form of cell death has gained increasing attention, due to its significant biological and medical relevance. Pyroptosis is generally categorized into apoptotic-like programmed death and non-apoptotic death, with the latter typically involving necrosis. As a distinctive type of programmed cell death, pyroptosis has recently been recognized for its unique features and mechanisms.28,30
In 1996, Chen et al31 were the first to observe caspase-1-dependent cell death in mouse cells during experimental studies, although at that time this type of cell death was not fully understood and was often mistakenly identified as apoptosis. It was not until 2001 that Cookson et al32 used the the term ‘pyroptosis’ to characterize caspase-1-dependent programmed cell death, bringing significant attention to this unique form of cell death.
Pyroptosis is triggered by various inflammatory factors that induce the formation of pores in the cell membrane, a process mediated by caspase-1 (Figure 2).33 Research has shown that the caspase enzymes involved in pyroptosis vary among different organisms; in mice, the primary enzymes are caspase-1 and caspase-11, while in humans, they are caspase-1, caspase-4, and caspase-5. Depending on the dependency factors and mediation mechanisms, pyroptosis can be divided into classical and non-classical pathways.34
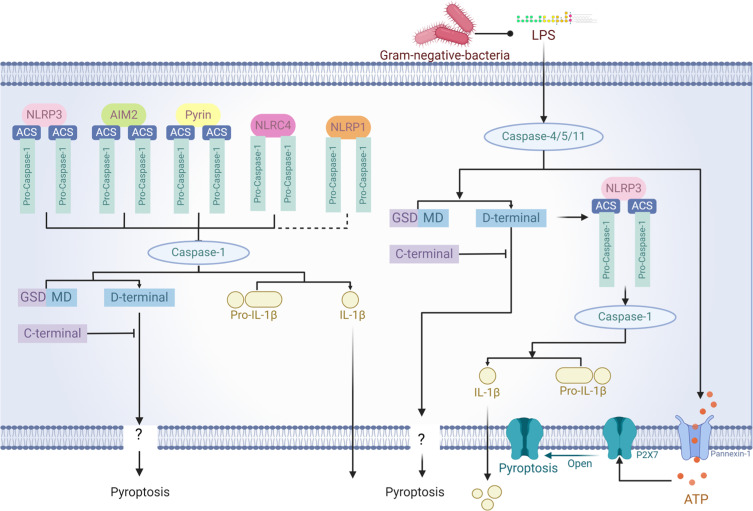
Figure 2.
Inflammasomes in Pyroptosis. NLRP3, AIM2, Pyrin, NLRC4, and NLRP1 inflammasomes activate caspase-1 and caspase-4/5/11, which cleave GSDMD. This releases its N-terminal domain to form membrane pores, initiating pyroptosis. Additionally, caspase-1 converts pro-IL-1β to IL-1β, enhancing inflammation, while ATP via P2X7 receptors facilitates this process.
Classical Pathway
The classical pathway of pyroptosis primarily relies on the activation of caspase-1, with inflammasomes playing a crucial role. Inflammasomes are large molecular complexes, approximately 700 kDa in size, consist of NOD-like receptors (NLRs), the precursor of caspase-1 (pro-caspase-1), and the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD). These complexes are key in activating pro-caspase-1, thereby inducing pyroptosis. The most studied inflammasomes include NLRP-1, NLRP-3, NLRC-4, AIM-2, and PYRIN, each mediating distinct pyroptotic activation pathways.
NLRP-1 primarily responds to muramyl dipeptide (MDP), a component of bacterial cell walls. NLRP-3 reacts to various stimuli including cell stressors like asbestos, silica, and several viral transfections that produce aftermath such as uric acid crystals. NLRP-4 mainly addresses stimuli related to alterations in the cellular genetic makeup.35,36 Extensive research has been conducted on NLRP-3, which consists of the NLRP-3 complex, ASC, and pro-caspase-1. Upon stimulation by pathogens or exogenous agents, NLRP-3 triggers the aggregation of reactive oxygen species, initiating the formation of the activation complex. This process involves the interaction of CARD domains (caspase activation and recruitment domain) within the complex, linking NLRP-3 with pro-caspase-1. This stimulates the release of pro-caspase-1’s enzymatic activity, cleaving it into the p10 and p20 subunits to form an active caspase-1. This enzyme then processes and activates the precursors of IL-1β and IL-18, turning them into active cytokines. These cytokines, in turn, stimulate a broad immune response, leading to various37 inflammatory mediators and driving acute inflammatory responses. Additionally, NLRP-1 and NLRC-4 can also directly interact with caspase-1 to exert their effects.38
NLRP1 Inflammasome
The NLRP1 inflammasome plays a crucial role in immune responses against pathogens and cellular damage. It is notably activated by Bacillus anthracis’ lethal factor, triggering caspase-1-mediated pyroptosis.39 This configuration underscores the inflammasome’s capacity to induce inflammatory cell death, essential in combating pathogens and in autoimmune disease defense.
A specific mutation in murine NLRP1a (Q593P) exacerbates autoimmune responses by enhancing caspase-1 and IL-1β activation, resulting in more severe symptoms in models like lymphocytic choriomeningitis virus (LCMV) infection.40 Conversely, mice lacking Nlrp1a exhibit improved recovery from immune challenges, suggesting that NLRP1 modulation could influence disease outcomes.
NLRP1’s role extends beyond infections to include neuroinflammatory conditions such as MS, Alzheimer’s disease, and brain trauma, where it can trigger pyroptosis. In MS, dysregulated NLRP1 activation exacerbates the inflammatory environment, contributing to tissue damage and symptom severity.41 Targeting the NLRP1 pathways could, therefore, offer therapeutic potential to mitigate the effects of MS by controlling pyroptosis and associated inflammation.42
NLRP3 Inflammasome
The NLRP3 inflammasome is integral to the pathophysiology of MS mediating inflammatory responses and pyroptosis—a highly inflammatory form of programmed cell death. It is activated by various damage-associated molecular patterns (DAMPs), ATP, ROS, and calcium flux. Upon activation, NLRP3 facilitates the oligomerization and recruitment of the adaptor protein ASC and pro-caspase-1 to the inflammasome complex, thus promoting the assembly and activation of pro-caspase-1.43,44 This assembly leads to the conversion of pro-IL-1β and pro-IL-18 into their active forms, amplifying inflammatory responses essential for MS progression.45 Activated caspase-1 also promotes the cleavage of gasdermin D, resulting in pore formation in the cell membrane, exacerbating cell lysis, and enhancing the secretion of inflammatory cytokines.46
Furthermore, the interaction between Toll-like receptor 4 (TLR4) signaling and NLRP3 inflammasome activation is critical.47 Signals via TLR4 lead to the recruitment of adaptor proteins like MyD88 and TRIF, which activate kinases such as RIPK1 and proteins like FADD. These molecules facilitate Caspase-8 interactions, enhancing NLRP3 activation and IL-1β production, thus bridging innate immune recognition with inflammatory cell death in MS.48 Additionally, Caspase-8 can independently cleave gasdermin D, directly initiating pyroptosis and underscoring a complex network of pathways converging on NLRP3 to drive inflammation and neurodegeneration in MS.49
NLRC4 Inflammasome
The NLRC4 inflammasome, integral to the immune response, plays a critical role in pyroptosis through its activation of caspase-1. This activation enables the processing and release of inflammatory cytokines such as IL-1β and IL-18, essential for managing the inflammatory response in MS. The activation mechanism of the NLRC4 inflammasome is distinguished by its interaction with NLR family, apoptosis inhibitory protein (NAIP) proteins, which detect bacterial elements like flagellin and components of the type III secretion system (T3SS). These interactions promote the oligomerization of NLRC4 and the recruitment of ASC and caspase-1, leading to the assembly of the inflammasome complex that initiates pyroptosis.
AIM2 Inflammasome
The AIM2 inflammasome is triggered by the binding of double-stranded DNA (dsDNA) to its HIN200 domain,50 leading to the recruitment of the PYD domains and subsequent assembly of the inflammasome complex.51 This complex includes ASC and pro-caspase-1, ultimately resulting in caspase-1 activation and the initiation of the pyroptotic cell death pathway, marked by the release of IL-1β and other pro-inflammatory cytokines, which are crucial for MS pathogenesis.52
Pyrin
Pyrin is composed of a PYD domain, a B-box, and a coiled-coil domain, which facilitate its role in detecting modifications induced by specific bacterial toxins. These toxins, including Clostridium difficile’s TcdB, Burkholderia cenocepacia’s C3, and Bordetella pertussis’s PT,53 activate Rho GTPases, essential for the assembly of the Pyrin inflammasome. Once activated, Pyrin recruits the adaptor protein ASC via its PYD domain to form the inflammasome. This complex subsequently activates caspase-1, culminating in the processing and release of the pro-inflammatory cytokine IL-1β.54
Non-Classical Pathway
Non-classical pyroptosis primarily depends on the activation of caspase-4/5/11, a process triggered by endogenous polysaccharides such as LPS. Studies have shown that, following LPS treatment, caspase-11 in macrophages binds specifically to LPS, thereby activating the enzyme and inducing pyroptotic cell death along with the secretion of IL-1β. This occurs through the localization of TLR4 and the non-structural protein pannexin-1 on the cell membrane; in humans, recognition by the TLR4/MD2 complex allows caspase-4/5 to bind effectively and initiate the downstream signaling pathway that leads to inflammatory responses and cell death.55
Gasdermin D
Members of the Gasdermin family, particularly Gasdermin D (GSDMD), play a pivotal role in both classical and non-classical pyroptosis pathway.34 The activation of GSDMD involves the cleavage of its C-terminal, which enables the release of caspase-1’s enzymatically active subunits, p10 and p20. These subunits facilitate the further cleavage and release of GSDMD’s N-terminal, which forms pores in the cell membrane.56 These pores are responsible for the morphological changes characteristic of pyroptosis, leading to cell lysis and the propagation of pyroptosis. Studies have demonstrated that the N-terminal of GSDMD, once cleaved, plays a crucial role in enhancing the cytotoxic effects associated with this process. Furthermore, research by Shao et al has identified Gasdermin E (GSDME), another member of the Gasdermin family, which also triggers cell death through pyroptosis.37,57
The Role of Pyroptosis in MS
MS is an autoimmune disorder marked by chronic inflammation and characterized by the invasion of myelin-reactive CD4+ T cells into the nervous system. This infiltration leads to demyelination, attacking oligodendrocytes58 and disrupting communication between the CNS and peripheral nervous system (PNS), which results in a spectrum of mental, physical, and psychiatric issues. Currently, there is no definitive cure for MS, and it typically reduces patient life expectancy by 5–10 years.59 Further research has revealed heterogeneity in the disease response to treatments such as interferon-beta (IFN-β), which has been a standard therapy for over two decades but is only effective in two-thirds of MS patients. This variability underscores the complex nature of MS and suggests potential differences in inflammasome involvement across various Experimental Autoimmune Encephalomyelitis (EAE) models and in human MS.
When compared with healthy individuals, MS patients demonstrate significantly higher levels of caspase-1 and IL-18. Clinically, relapsing MS patients often show elevated levels of NLRP3, caspase-1, and IL-1β in their peripheral blood mononuclear cells (PBMCs) relative to healthy individuals.60 Activation of NLRP3 in these cells initiates a cascade that shifts their cytokine profile toward a TH17 phenotype.61 This finding is supported by notable increases in caspase-1 mRNA in brain samples from MS patients, with immunohistochemical analysis confirming significant caspase-1 activity in both recent and long-standing MS lesions, but not in healthy brain tissue. Furthermore, an increase in caspase-1 activity has been documented in microglia and mononuclear cells near vascular regions, and uniquely, in oligodendrocytes within lesions.62 Recent investigations have also shown a surge in NLRP3 inflammasome activity in patients unresponsive to treatments like fingolimod, and studies have suggested that IL-1β activation by macrophages could be mitigated by IFN-β, linking reduced NLRP3-mediated inflammation with this treatment.60 In conditions marked by inflammasome activation, notably in MS, elevated levels of IL-18 and IL-1β are consistently observed in cerebrospinal fluid (CSF).63
The EAE model, involving injections of myelin oligodendrocyte glycoprotein peptide (MOG) in adjuvant, serves as a prevalent model for simulating MS. This model excels in elucidating immune responses, as it facilitates the recruitment of MOG-specific T cells into the nervous system.64 Research has identified two distinct subsets of EAE based on their dependence on the NLRP3 inflammasome. Intriguingly, it was found that interferon-beta (IFN-β), a common therapeutic agent used in treating MS, is ineffective in models of EAE that are induced in a manner independent of the NLRP3 inflammasome.65,66 It has been suggested that IFN-β could suppress IL-1β activation by macrophages, indicating a selective reduction of NLRP3-dependent EAE with IFN-β therapy. These observations support the notion that IFN-β could specifically target the NLRP3 inflammasome, along with IL-18 and IL-1β, providing potential therapeutic benefits in MS.67 Prior to the recognition of inflammasome pathways, elements such as caspase-1, IL-18, and IL-1β had been established as crucial in EAE progression. Resistance to EAE is observed in mice lacking these genes, underscoring the role of pyroptosis in the disease process.68–70 More aggressive immunization approaches, like those using heat-killed mycobacteria (Mtb), demonstrate significant effects in Nlrp3-/- and Asc-/- mice, particularly when lower doses of Mtb are used.67 Contemporary research underscores the NLRP3 inflammasome’s pivotal role in MS, indicating that mice deficient in NLRP3 show diminished IL-18 production and milder symptoms. In EAE models, increased expression of NLRP3 has been observed, particularly during disease progression. Mice with the Nlrp3 gene knocked out not only show a delayed disease course and reduced severity but also exhibit less astrogliosis and fewer infiltrating inflammatory cells AE.71 In another experimental model of MS Nlrp3-/-, animals have lower oligodendrocyte loss and demyelination.72 In a pertussis toxin (PTX)-induced EAE model, IL-1β activation necessitates TLR4 and the pyrin-dependent inflammasome for pro-IL-1β conversion. Once activated, IL-1β induces stromal cells to produce IL-6, enhancing leukocyte migration and adhesion. Transgenic mice with MOG-specific T cell receptors, specifically Pyrin-/- 2D2, show reduced EAE occurrences, exhibiting milder symptoms and delayed disease progression after PTX administration.73 Additionally, studies have indicated that the NLRP3 inflammasome, through IL-18 expression, may promote EAE by affecting leukocyte migration and adhesion.71 Similarly, Nlrp3 -/- mice display a reduction in IL-18 levels and disease severity. These findings indicate that inflammasomes, and the pyroptosis they initiate, significantly contribute to the disease’s exacerbation.64 Nlrp3 expression in the spinal cord increases, whereas mice lacking Nlrp3 exhibit a protracted disease progression with less severe pathology, characterized by diminished astrogliosis and fewer infiltrating inflammatory cells. Additionally, compared to control animals, mice subjected to EAE show elevated IL-18 levels, and those deficient in IL-18 mirror the delayed disease progression seen in Nlrp3-/- mice, suggesting that the Nlrp3 pathway may exacerbate EAE through IL-18 activity.64,71 Caspase-1 plays a critical role in the initial phase of the immune responses that exacerbate EAE. This enzyme is crucial in triggering the inflammation typical of the acute phase of relapsing-remitting MS, not only by enabling the production and release of pro-inflammatory cytokines such as IL-1β and IL-18 but also by initiating a series of immune reactions that intensify neurological damage. Despite differences in the exact localization and intensity of IL-1β expression between EAE and MS, the staining patterns in both conditions suggest that IL-1β is predominantly expressed in affected tissues rather than peripherally.74 NLRP3 inflammasome is activated and contributes to cognitive deficits in EAE mice.75
Activation of Inflammatory Responses
Pyroptosis, unlike apoptosis which sequesters and disposes of cellular debris quietly, actively releases intracellular contents including cytokines such as IL-1β, IL-18,76 and TNF-α into the extracellular matrix.28,48 Pyroptosis is characterized by the explosive rupture of cell membranes, driven by the action of inflammatory caspases like caspase-1, −4, −5, and −11 that cleave gasdermin D, facilitating the formation of membrane pores. The resultant release of cellular contents into the extracellular space is a significant inflammatory event, triggering further immune responses. These cytokines are powerful agents that not only recruit additional immune cells to the site of damage but also amplify the inflammatory response. This is particularly notable in MS, where elevated levels of caspase-1, IL-1β, and IL-18 in lesions underscore the NLRP3 inflammasome’s critical role in disease pathology.62,77
Evidence suggests that NLRP3 inflammasome activation, particularly in microglia, is instrumental in both the onset and progression of MS by mobilizing activated T cells that exacerbate CNS inflammation.78 The process is especially critical in MS, where myeloid cells such as microglia and macrophages undergo pyroptosis. Upon activation, these cells release inflammatory mediators that heighten CNS responses, potentially leading to further neurological complications.29,48,79 The structural disruption caused by cleaved gasdermin D contributes to cellular rupture, releasing not just IL-1β and IL-18 but also TNF-α, intensifying the inflammatory milieu.80–82 Crucial inflammatory cytokines, such as TNF-α, can induce pyroptosis, perpetuating and amplifying the inflammatory response.82 This cascade can compromise the BBB, possibly enhancing the infiltration of immune cells into the CNS and intensifying neuroinflammation.83
During the early stages of MS, the disruption of CNS barriers facilitates the entry of peripheral immune cells, with IL-1β playing a pivotal role in degrading CNS structural defenses.84,85 This degradation, alongside the secretion of additional pro-inflammatory cytokines, catalyzes further infiltration of peripheral immune cells.86 CNS astrocytes and endothelial cells also participate actively in this process, with activated astrocytes releasing cytokines that weaken endothelial tight junctions. Moreover, chemokines from these astrocytes attract more leukocytes into the CNS, contributing to the progression of MS.87 Microglia, activated by IL-1β and functioning as antigen-presenting cells (APCs), amplify this neuroinflammation by attracting and activating infiltrated CD4+ T cells, which in turn stimulate microglia further.88 Current research indicates that microglia, along with astrocytes and CD4+ T cells, are key players in NLRP3 inflammasome activation within MS, correlating strongly with the extent of demyelination observed in patients
Involvement in Autoimmune Responses
Pyroptosis plays a pivotal role in the autoimmune dynamics of MS by releasing self-antigens that activate autoreactive immune cells, thereby exacerbating the disease’s progression.89,90 This form of cell death, characterized by the rupture of cells and the ensuing release of contents, including myelin components and other CNS-specific proteins, is crucial as it triggers the immune system to react against the body’s own neural structures.89,91 APCs such as dendritic cells and macrophages are instrumental in this context. They capture and process released self-antigens, presenting them on MHC class II molecules which is critical in linking innate and adaptive immunity. This presentation activates T cells that are specifically sensitized to CNS antigens, marking a crucial phase in the autoimmune sequence typical of MS.92,93
The activation of T cells in the CNS, which then release cytokines aggravating the inflammatory response, is mediated through both traditional and novel NLRP3 inflammasome pathways, including one involving caspase-8.71,94 This results in a heightened release of IL-1β, which supports the persistence of autoimmune Th17 cells.95 Remarkably, this activity has been observed within Th17 cells themselves, not just in microglia. Th17 cells are noted for their role in intensifying autoimmune and inflammatory reactions that propel MS,96 with these cells specifically producing pro-inflammatory cytokines like IL-17 that activate the NLRP3 inflammasome.29,97 Further, the expression of Nlrp3 in APCs is vital for triggering TH17 and TH1 cells in response to CNS antigens.64 Moreover, the presence of ASC and NLRP3 within autoreactive Th17 cells has been linked to ongoing inflammation in the CNS due to their continuous release of IL-1β.95,98 Interestingly, the lack of Nlrp3 and the adaptor protein ASC results in reduced expression of chemokines and chemokine receptors such as Ccr6 and Ccr2, thus decreasing the migration of these T cells into the CNS and potentially dampening the inflammatory response in models lacking Nlrp3 and ASC post-MOG administration.71
This is particularly significant in progressive MS, highlighting the crucial role of the immune regulation by CD4+ T cells, which differentiate into memory and regulatory T (Treg) cells.99 Although Treg cells generally suppress inflammasome activation and reduce inflammatory responses, excessive IL-1β production has been shown to counteract this suppression, potentially reversing the autoimmune moderation facilitated by Treg cells.
Damage to Neurons and Oligodendrocytes
Pyroptosis in MS significantly affects the CNS by releasing neurotoxic elements detrimental to neurons and oligodendrocytes, which are essential for myelin production. When pyroptosis occurs, the resulting cellular rupture releases not only inflammatory cytokines but also free radicals, further exacerbating the inflammatory milieu within the CNS. This is particularly damaging to oligodendrocytes that are tasked with myelin sheath maintenance and repair. Damage to these cells is a critical factor in MS pathogenesis, leading to axon degeneration and progressing neurological symptoms. In MS lesions, oligodendrocytes exhibit upregulation of pyroptosis mediators like GSDMD, and studies show that inhibiting inflammasomes can mitigate damage to these cells in EAE.48
Oligodendrocytes’ vulnerability to the inflammatory milieu created by pyroptosis significantly impedes myelin repair and production, accelerating demyelination. Neurons are also adversely affected by this enhanced inflammatory response, disrupting neural communication and contributing to long-term disabilities in MS patients. Moreover, stressed neurons and oligodendrocytes release self-antigens and DAMPs, triggering further immune responses and perpetuating inflammation and neural damage.
Interactions with Other Cell Death Pathways
The complex interplay between pyroptosis and other cell death mechanisms, such as apoptosis, ferroptosis, and necroptosis, adds significant complexity to the pathological processes of diseases like MS, intensifying cellular damage and inflammatory responses.
Apoptosis and Pyroptosis: Caspase-3, well-known for its role in apoptosis, can also initiate pyroptosis by cleaving GSDME. Intriguingly, GSDME can modulate apoptosis by inhibiting caspase-3 mediated pathways, showcasing a reciprocal relationship between these cell death processes.100,101 Additionally, caspase-8 can trigger pyroptosis through the activation of GSDMD under specific conditions, illustrating the intersection within the PANoptosome complex that integrates components from both apoptosis and pyroptosis pathways, thereby enhancing the complexity of cellular responses to stress and damage.102
Ferroptosis and Pyroptosis: Characterized by iron accumulation and extensive lipid peroxidation, ferroptosis closely interacts with pyroptosis.103 Activation of the NLRP3 inflammasome and caspase-1 can initiate pyroptosis, while the release of the N-terminal fragment of GSDMD during pyroptosis not only disrupts cell membranes but also promotes lipid peroxidation, enhancing ferroptosis.90 Moreover, studies show that lipid peroxidation itself can drive Gasdermin D-mediated pyroptosis, linking these processes even more directly and suggesting a pivotal role for lipid peroxides in propagating cell death.104
Necroptosis and Pyroptosis: MLKL, the primary executor of necroptosis, activates the NLRP3 inflammasome via potassium efflux, facilitating pyroptosis.105 This activation leads to the release of the GSDMD-N fragment during pyroptosis, further promoting necroptosis. Similarly, RIPK3, another key necroptosis mediator, triggers the NLRP3 inflammasome, enhancing pyroptotic activity.106 MLKL’s role extends to initiating innate immune responses by activating NLRP3 and caspase-1, which are crucial for IL-1β secretion and inflammation.106 Additionally, during viral infections like influenza, MLKL and caspase-8 drive inflammatory cell death, highlighting their significance in immune defense and pathology.107
Gut Microbiota Regulation of MS Through Pyroptosis
Microbiota Dysbiosis in MS
Research into MS consistently shows that MS patients exhibit reduced gut microbial diversity compared to healthy individuals,23,108,109 along with notable alterations in specific bacterial phyla or genera24,110 (Table 1). Despite challenges such as variability in sample size, participant heterogeneity, study design, control types, geographical location, sequencing platforms, and targeted regions of the 16S rRNA gene, these findings remain robust.111 Beneficial bacteria like Bifidobacterium, Prevotella, and Bacillus are found in lower abundances in MS patients. Bifidobacterium is recognized for its anti–inflammatory properties and its role in maintaining gut barrier integrity.110 Prevotella is associated with a plant-based diet and reduced inflammation.112 In contrast, there is an increase in potentially harmful bacteria, such as certain members of the Firmicutes phylum and Proteobacteria, which are linked to inflammation and dysbiosis in various autoimmune diseases.23,24,110,113–116 The fluctuations in these bacteria types become more pronounced during MS relapses. A cohort study by Chen et al found within the MS patient cohort, there was a trend toward reduced species richness among patients with active disease, whereas those in remission showed similarities to healthy controls.115 Jangi et al performed a secondary analysis comparing treated and untreated MS patients and noted that certain genera such as Prevotella and Sutterella were reduced in untreated patients but returned to normal levels with treatment. Furthermore, the genus Sarcina was only reduced in treated patients.113 Consistent with this, recent research has linked Akkermansia to reduced disability in patients with progressive relapsing MS (pwMS).117 This further supports the connection between gut microbiota and MS pathogenesis.
Table 1.
Microbioota Changes in MS
| Subjects | Change in Abundance (P- Phyla, F- family, G-genus) | ||
|---|---|---|---|
| MS | Control | ||
| 31 | 36 | Blautia (G), Dorea (G), Pedobacter (G) and Flavobacterium (G), Pseudomonas (G), Mycoplana (G), Haemophilus (G↑ | 115 |
| Prevotella (G), Parabacteroides (G), Coprobacillus (G), Haemophilus (G), Adlercreutzia (G), Collinsella (G), Lactobacillus (G)↓ | |||
| 60 | 43 | Akkermansia (G), Methanobrevibacter (G)↑ | 113 |
| Butyricimonas (G) Prevotella (G) ↓ | |||
| 18 | 17 | Bilophila (G), Desulfovibrio (G), Christensenellaceae (F) ↑ | 118 |
| Lachnospiraceae (F), Ruminococcaceae (F), Lachnospiraceae (F) ↓ | |||
| 23 | 43 | Proteobacteria (P), Actinobacteria (P) ↑ | 119 |
| Bacteriophages (P), Proteobacteria (P)↓ | |||
| 45 | 44 | Verrucomicrobiales (P) and in Akkermansia at the genus level Adlercreutzia (G), Blautia (G), Holdemania (G), and Dorea (G)↑ | 120 |
| Prevotella (G), Slackia (G), Lachnospira (G), and Dialister (G)↓ | |||
| 150 | 150 | Coprobacillus (G), Lachnospiraceae (F), Coprobacillus (G), Peptoniphilus (G) and Varibaculum (G)↑ | 112 |
| Blautia (G), Firmicutes (G), Faecalibacterium (G), Fusicatenibacter (G) Clostridium (G)↓ | |||
Note: “↑” for increase, “↓” for decrease.
Impact of Gut Microbiota on Pyroptosis in MS
Metabolic Byproducts
The link between gut microbiota and MS via pyroptosis is significantly influenced by metabolic byproducts known as short-chain fatty acids (SCFAs), including acetate (C2), propionate (C3), and butyrate (C4). These SCFAs are produced by beneficial bacteria within the phyla Firmicutes, Bacteroidetes, and Actinobacteria during the fermentation of dietary fibers.121
In MS patients, notable declines in populations of beneficial bacteria such as Bifidobacterium, Prevotella, and Bacillus correlate with reduced levels of these neuroprotective SCFAs.122,123 These acids are pivotal for modulating gene expression and mitigating oxidative stress, processes that could potentially decelerate the progression of MS.124–126 Butyrate and propionate are crucial for maintaining the integrity of the gut barrier.127 This loss in barrier function facilitates the translocation of triggers such as LPS from the gut into the CNS, exacerbating neuroinflammation and advancing disease progression.128
However, the relationship between SCFAs and the pyroptosis s complex and context-dependent. Under normal conditions, SCFAs generally inhibit the activation of the NLRP3 inflammasome and reduce the secretion of L-1β and IL-18.129,130 However, under inflammatory conditions, SCFAs can act as danger signals that activate the NLRP3 inflammasome in immune cells. Notably, butyrate and propionate can activate the NLRP3 inflammasome in the presence of TLR agonists.131 Additionally, butyrate has been shown to potentiate the activation of the inflammasome induced by Enterococcus faecalis lipoteichoic acid through the inhibition of histone deacetylases.132 This activation of the NLRP3 inflammasome leads to the increased production of pro-inflammatory cytokines and can trigger pyroptosis in CNS cells, contributing to the demyelination and neurodegeneration characteristic of MS. Understanding the dual role of SCFAs, as both protective agents under normal conditions and potential exacerbators of inflammation under disease conditions, is critical.
Bacterial Structures
The connection between bacterial structures, such as LPS found in gram-negative bacteria, and pyroptosis is increasingly recognized as a significant factor in the pathogenesis of MS.133 Once LPS crosses the BBB, it triggers the activation of the NLRP3 inflammasome within the CNS.134–136 Recent studies have shifted focus from the traditionally implicated TLR4 to caspase-11 as the initiator of GSDMD activation, which is crucial for disrupting the BBB in response to circulating LPS or during septic conditions.133 This activation results in neuronal cell death and the release of pro-inflammatory cytokines, exacerbating neuroinflammation and accelerating MS progression.
Moreover, emerging research has identified a significant lung-brain axis, indicating that lung infections—a known risk factor for MS—can influence disease progression through microbial interactions. For instance, the administration of neomycin, which increases LPS-enriched bacterial phyla, can modulate immune responses in the brain. This modulation is particularly evident in microglial cells, which may adopt a type-I-interferon-primed state, altering their response to autoimmune stimuli and potentially mitigating MS symptoms.137
Peptidoglycan (PGN), another bacterial cell wall component found in both gram-positive and negative species, has been detected in brain tissue lesions of patients with MS.138 PGN and its fragments are highly pro-inflammatory, signaling through TLRs, NLRs, and specialized PGN recognition proteins (PGLYRP1-4). Specifically, PGN’s component GlcNAc inhibits mitochondrial hexokinase release and activates the NLRP3 inflammasome, leading to the cleavage of pro-IL-1β into mature IL-1β by caspase-1.119,139 PGN was immunodetected in phagocytes within demyelinating lesions from MS patients and in non-human primate models of MS, and was found concentrated in lesions.140
Additionally, other bacterial structures and exogenous substances like nigericin, a known potassium ionophore, are known to activate the NLRP3 inflammasome,141 underscoring the need for further detailed research in bacterial structures.
Neurotransmitter Effects
The influence of neurotransmitters on pyroptosis within the CNS, particularly in conditions like MS, is closely tied to the composition and function of the gut microbiota. Gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the CNS, is produced by gut bacteria such as Lactobacillus and Bifidobacterium.142,143 GABA reduces neuronal excitability through the hyperpolarization of neurons, thus decreasing the likelihood of neuronal cell death. By modulating the immune response and reducing inflammation, GABA might indirectly suppress NLRP3 inflammasome activation, a key driver of pyroptosis in MS.144 Purified antigen-presenting cells APCs have been shown to respond to GABAergic drug treatment by reducing the production of pro-inflammatory cytokines like IL-1β and IL-6, closely linked to pyroptosis.145
Conversely, imbalances in serotonin and dopamine, influenced by gut microbiota, may increase neuronal excitability and vulnerability, thereby raising the risk of pyroptosis.146 This risk is further underscored by evidence supporting the potential therapeutic benefits of selective serotonin reuptake inhibitors (SSRIs), which may attenuate pyroptosis by stabilizing neurotransmitter levels. Additionally, acetylcholine (ACh), a neurotransmitter released by the vagus nerve’s efferent fibers, has been demonstrated in vitro to inhibit the production of pro-inflammatory cytokines in macrophages, leading to the “cholinergic anti-inflammatory hypothesis”.147 Decreased levels of ACh in both serum and CSF of patients, coupled with increased levels of pro-inflammatory cytokines such as IL-1β and IL-17, further illustrate the complex interplay between neurotransmitters and inflammatory processes in MS.148 Such neurotransmitter imbalances can lead to heightened neuronal damage in MS, emphasizing the dual role of neuroactive compounds in either protecting against or exacerbating neuronal injury.
Immune Modulation
The differentiation and balance between regulatory T cells (Tregs) and Th17 cells play a crucial role in immune regulation, significantly influencing MS progression. Both Tregs and Th17 cells can either alleviate or exacerbate MS, with their development being deeply influenced by the gut microbiota.149,150 For example, the generation of encephalitogenic Th cells—key players in MS pathogenesis—depends on the presence of pertussis toxin (PTX) during immunization, highlighting the microbiota’s role in immune system priming.151
In germ-free (GF) mice, a reduction in Th17 cells is observed. These cells are a subset of CD4+ effector T cells that produce cytokines like IL-17A, IL-17F, IL-22, and GM-CSF. While Th17 cells are essential for defense against bacterial and fungal infections, they contribute to destructive inflammation in autoimmune diseases such as MS.152 Elevated levels of IL-17 mRNA have been observed in the blood and CSF of MS patients, underlining the role of Th17 cells in MS pathophysiology.153 Certain microbial components, such as segmented filamentous bacteria (SFB), promote the differentiation of pro-inflammatory Th17 cells, which exacerbate autoimmune responses.152 Additionally, the aryl hydrocarbon receptor (AhR), when bound to microbiota-derived ligands, further drives Th17 cell production.154 Th17 cells, in turn, activate inflammatory pathways, including the NLRP3 inflammasome and caspase-1, leading to pyroptosis and inflammatory cell death, both of which are characteristic of MS pathology.155
Conversely, commensal bacteria such as Bacteroides fragilis contribute to immune homeostasis by promoting Treg development through molecules like polysaccharide A (PSA).156 These Tregs are essential for maintaining immune tolerance and preventing autoimmune responses, key factors in MS pathogenesis.157–159 The presence of beneficial gut bacteria enhances immune tolerance, potentially reducing autoimmune activity and limiting inflammation and pyroptosis in the CNS. Additionally, a study investigating the transfer of miR-30d to promote the abundance of Akkermansia found that Treg induction could alleviate symptoms of EAE.160 In mouse models, Akkermansia administration improved EAE symptoms by decreasing γδ T cells that produce RORγt+ and IL-17, further highlighting the role of gut microbiota in regulating inflammatory responses in MS.161
Therapeutic Potential of Modulating Gut Microbiota
The critical role of the gut microbiota in the pathogenesis of MS underscores the potential of modulating its composition as a promising therapeutic strategy (Table 2). This modulation can influence the gut-brain axis and, consequently, the inflammatory and neurodegenerative processes central to MS, including the regulation of pyroptosis in the central nervous system (CNS).
Table 2.
Clinical Trials of Modulating Gut Microbiota for MS
| Study Detail | Intervention | Results | |
|---|---|---|---|
| Relapsing-remitting MS (n=9), healthy controls (n=13); evaluations prior to, at discontinuation, and three months post-therapy. |
VSL3 Probiotic: 3×1011 CFU /g, 8 strains— 4 Lactobacillus, 3 Bifidobacterium, 1 Streptococcus. |
MS:Intermediate monocytes↓ MS:HLA-DR on dendritic cells↓ MS:Anti-inflammatory response halted post-probiotic |
162 |
| Relapsing-remitting MS patients on interferon beta-1α: probiotic (n=30); placebo comparison(n=30) |
Probiotic: 2×109 CFU /g, L. acidophilus, L. casei, B. bifidum, L. fermentum. |
Probiotic: EDSS↓Probiotic: high-sensitivity CRP serum level↓ | 163 |
| Relapsing-remitting MS patients on interferon beta-1α: probiotic (n=24); placebo comparison(n=24) |
Probiotic:2×109 CFU /g, B. infantis, B. lactis, L. reuteri, L. casei, L. plantarum, L. fermentum |
Probiotic: EDSS↓Probiotic: IL-6↓, IL-10↑ | 164 |
| Secondary progressive MS (n=1) | FMT from her partner | EDSS score stabilized | 165 |
| MS (n=179), healthy controls (n=68); |
SCFAs: propionic acid for 14 days | Tregs induction and function↑ | 166 |
Note: “↑” for increase, “↓” for decrease.
Abbreviation: EDSS, Expanded Disability Status Scale.
Fecal Microbiota Transplantation (FMT) and Probiotic Supplementation
Research utilizing MS mouse models has shown that transplanting gut microbiota from healthy donors can alleviate disease symptoms, highlighting the profound impact of gut microbiota on MS progression. In contrast, microbiota from MS patients can exacerbate the condition, indicating the potential of gut microbiota modulation in reversing or mitigating disease symptoms. Probiotic supplementation further supports this approach by introducing beneficial bacteria that restore microbial balance, enhance gut barrier integrity, and reduce the translocation of pro-inflammatory agents.128,163,167–169 These interventions aim to re-establish a healthy microbial environment that can directly influence immune regulation and neuroinflammation.170,171 Experiments using MS mouse models have demonstrated that transplanting gut microbiota from healthy donors can alleviate disease symptoms, whereas microbiota from MS patients exacerbates them.172–174 This finding further underscores the significant impact of gut microbiota on the development and progression of MS.
SCFAs as Therapeutic Agents
Research is exploring the use of microbial metabolic products, such as SCFAs, as potential therapeutic targets for MS.175 SCFAs, primarily produced by gut bacteria during the fermentation of dietary fibers, have been shown to possess anti-inflammatory properties and the ability to strengthen the intestinal barrier.124 Utilizing these metabolic products could provide a novel means to modulate immune responses and reduce neuroinflammation in MS patients, offering a non-invasive and potentially effective strategy to manage and possibly ameliorate the course of the disease.
The metabolic products of gut microbiota, particularly SCFAs like butyrate and propionate, are gaining attention as potential therapeutic targets for MS.124 Produced during the fermentation of dietary fibers, SCFAs possess anti-inflammatory properties and strengthen the intestinal barrier, thereby modulating immune responses and reducing neuroinflammation.123 Utilizing SCFAs could offer a novel, non-invasive strategy to manage MS by suppressing key inflammatory pathways, such as the NLRP3 inflammasome, thus potentially reducing pyroptosis in the CNS.176 A high-fiber diet has been shown to alter gut microbiota and increase serum levels of interleukin-18 (IL-18).122,123
Dietary Modifications
Adjusting dietary intake to enhance the production of beneficial metabolites like SCFAs involves increasing fiber-rich foods that support the growth of beneficial gut bacteria and reducing inflammation-promoting foods. Such dietary modifications not only support gut health but also enhance systemic immune regulation, which is crucial in controlling the inflammatory responses associated with MS. The NLRP3 inflammasome is controlled by mitochondrial ROS and oxidized mitochondrial DNA (mtDNA), is inhibited by ketone bodies. Both ketogenic and fasting diets have shown potential in improving MS outcomes through the modulation of NLRP3, with proven effectiveness in both animal models and humans with neurodegenerative diseases.177
Conclusion
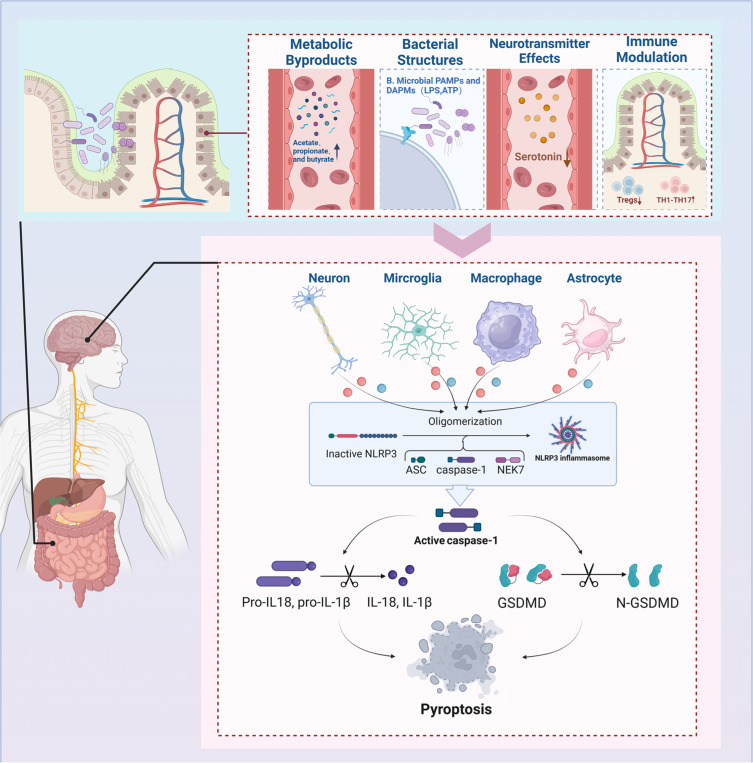
As the scientific community continues to explore the complexities of MS, the role of gut microbiota in its pathogenesis—particularly through the mechanism of pyroptosis, a highly inflammatory form of programmed cell death—is gaining recognition. This review highlights the significant impact that gut microbiota dysregulation has on the inflammatory milieu of MS, highlighting how such imbalances can exacerbate symptoms and hasten disease progression by activating pyroptotic pathways. (Figure 3). The complex relationship between gut microbiota and pyroptosis offers critical insights into the pathogenesis of MS and highlights the potential of microbiota-centered therapies for developing novel, non-invasive treatment strategies. The manipulation of gut microbiota, through strategies such as dietary adjustments, probiotic supplementation, and FMT, presents a promising therapeutic frontier. These interventions aim to restore microbial balance, suppress detrimental inflammation, and enhance neuronal protection, addressing the complex interplay between microbial communities and neuroimmune responses that is critical in MS pathogenesis. The effectiveness of these interventions and their precise mechanisms—particularly how they influence pyroptosis and the disease trajectory—must be validated through rigorous clinical trials.
Figure 3.
Gut-Brain Axis and Pyroptosis in MS. Gut microbiota impact MS through pyroptosis via four mechanisms: 1) Metabolic byproducts like acetate, 2) Bacterial lipopolysaccharides (LPS), 3) Neurotransmitter modulation (serotonin, GABA), and 4) Immune interactions (Treg and Th17 cells). These pathways facilitate inflammasome assembly, IL-1β and IL-18 production, and GSDMD cleavage, intensifying pyroptosis and MS progression.
While the therapeutic interventions targeting gut microbiota show promise for MS, the complexity of these interactions highlights the need for detailed studies to validate the efficacy and mechanisms fully. It is crucial to recognize that despite the potential of manipulating the gut microbiota to influence MS progression, our understanding of the exact pathways, especially how they relate to pyroptosis, remains incomplete. Future research should not only focus on identifying specific microbiota strains that benefit MS management but also explore the temporal dynamics of microbiota changes in relation to disease progression. This will help to determine the most effective timing for therapeutic interventions. Additionally, given the role of pyroptosis in exacerbating MS symptoms through inflammatory responses, further investigations into how gut microbiota influence this process could lead to novel therapeutic strategies. Researchers should also consider the potential for developing microbiota-based therapies that could modulate pyroptosis directly or through intermediary pathways. By addressing these complex relationships through a comprehensive, holistic approach, the scientific community can overcome current challenges and maximize the therapeutic potential of microbiota-centered interventions, potentially revolutionizing MS treatment paradigms.
Funding Statement
This study was supported by Heluo Young Talents Support Program (2024HLTJ08), Joint Fund of Henan Provincial Science and Technology R&D Project (242103810034) and Henan Provincial Young and Middle-aged Health Science and Technology Innovation Leading Talent Training Program (LJRC2024019).
Disclosure
The authors declare that they have no competing interests.
References
- 1.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020;26:1816–1821. doi: 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghasemi N, Razavi S, Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017;19(1):1–10. doi: 10.22074/cellj.2016.4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Noort JM, Baker D, Kipp M, Amor S. The pathogenesis of multiple sclerosis: a series of unfortunate events. Clinical and Experimental Immunology. 2023;214:1–17. doi: 10.1093/cei/uxad075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brück W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252 Suppl 5:v3–9. doi: 10.1007/s00415-005-5002-7 [DOI] [PubMed] [Google Scholar]
- 5.Lopez JA, Denkova M, Ramanathan S, Dale RC, Brilot F. Pathogenesis of autoimmune demyelination: from multiple sclerosis to neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody-associated disease. Clin transl immunol. 2021;10(7):e1316. doi: 10.1002/cti2.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olek MJ. Multiple Sclerosis. Ann Intern Med. 2021;174:ITC81–96. doi: 10.7326/AITC202106150 [DOI] [PubMed] [Google Scholar]
- 7.Vasileiou ES, Fitzgerald KC. Multiple sclerosis pathogenesis and updates in targeted therapeutic approaches. Curr Allergy Asthma Rep. 2023;23:481–496. doi: 10.1007/s11882-023-01102-0 [DOI] [PubMed] [Google Scholar]
- 8.Lubetzki C, Stankoff B. Demyelination in multiple sclerosis. Handb Clin Neurol. 2014;122:89–99. doi: 10.1016/B978-0-444-52001-2.00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccarelli O, Barkhof F, Bodini B, et al. Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. Lancet Neurol. 2014;13:807–822. doi: 10.1016/S1474-4422(14)70101-2 [DOI] [PubMed] [Google Scholar]
- 10.Loma I, Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011;9:409–416. doi: 10.2174/157015911796557911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadelmann C, Wegner C, Brück W. Inflammation, demyelination, and degeneration — recent insights from MS pathology. Biochimica Et Biophysica Acta. 2011;1812:275–282. doi: 10.1016/j.bbadis.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 12.Shah A, Panchal V, Patel K, et al. Pathogenesis and management of multiple sclerosis revisited. Disease-a-Month. 2023;69:101497. doi: 10.1016/j.disamonth.2022.101497 [DOI] [PubMed] [Google Scholar]
- 13.Amatya B, Khan F, Galea M. Rehabilitation for people with multiple sclerosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2019;2019:CD012732. doi: 10.1002/14651858.CD012732.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases. 2015;3:545–555. doi: 10.12998/wjcc.v3.i7.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15..Rommer PS, Milo R, Han MH, et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019:10. doi: 10.3389/fimmu.2019.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson D, Moreo N. Disease-modifying therapies in multiple sclerosis: overview and treatment considerations. Fed Pract. 2016;33:28–34. [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelsztejn A. Multiple sclerosis: overview of disease-modifying agents. Perspect Medicin Chem. 2014;6:65–72. doi: 10.4137/PMC.S13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bomprezzi R. Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: an overview. Ther Adv Neurol Disord. 2015;8:20–30. doi: 10.1177/1756285614564152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller AE. An updated review of teriflunomide’s use in multiple sclerosis. Neurodegener Dis Manag. 2021;11:387–409. doi: 10.2217/nmt-2021-0014 [DOI] [PubMed] [Google Scholar]
- 20.Horga A, Tintoré M. Natalizumab for relapsing-remitting multiple sclerosis. Neurologia. 2011;26:357–368. doi: 10.1016/S2173-5808(11)70082-7 [DOI] [PubMed] [Google Scholar]
- 21.Katsavos S, Coles A. Alemtuzumab as treatment for multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8:a032029. doi: 10.1101/cshperspect.a032029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin M, Zhang J, Zhang Y, Luo J, Shi S. Ocrelizumab for multiple sclerosis. Cochrane Database Syst Rev. 2022;5:CD013247. doi: 10.1002/14651858.CD013247.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altieri C, Speranza B, Corbo MR, Sinigaglia M, Bevilacqua A. Gut-microbiota, and multiple sclerosis: background, evidence, and perspectives. Nutrients. 2023;15:942. doi: 10.3390/nu15040942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantoni C, Lin Q, Dorsett Y, et al. Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine. 2022;76:103798. doi: 10.1016/j.ebiom.2021.103798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buga AM, Padureanu V, Riza A-L, Oancea CN, Albu CV, Nica AD. The gut–brain axis as a therapeutic target in multiple sclerosis. Cells. 2023;12:1872. doi: 10.3390/cells12141872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bronzini M, Maglione A, Rosso R, et al. Feeding the gut microbiome: impact on multiple sclerosis. Front Immunol. 2023;14:1176016. doi: 10.3389/fimmu.2023.1176016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Man SM, Karki R, Briard B, et al. Differential roles of caspase-1 and caspase-11 in infection and inflammation. Sci Rep. 2017;7:45126. doi: 10.1038/srep45126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Wan T, Gao X, et al. Microglia pyroptosis: a candidate target for neurological diseases treatment. Front Neurosci. 2022;16:922331. doi: 10.3389/fnins.2022.922331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias EE, Lyons B, Muruve DA. Gasdermins and pyroptosis in the kidney. Nat Rev Nephrol. 2023;19:337–350. doi: 10.1038/s41581-022-00662-0 [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Smith MR, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 32.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3 [DOI] [PubMed] [Google Scholar]
- 33.Doitsh G, Galloway NLK, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Sun Q, Zhong X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–955.e20. doi: 10.1016/j.cell.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 35.Sauer J-D, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao EA, Mao DP, Yudkovsky N, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- 38.Van Opdenbosch N, Gurung P, Vande Walle L, Fossoul A, Kanneganti T-D, Lamkanfi M. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun. 2014;5:3209. doi: 10.1038/ncomms4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724 [DOI] [PubMed] [Google Scholar]
- 40.Mamantopoulos M, Ronchi F, Van Hauwermeiren F, et al. Nlrp6- and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. Immunity. 2017;47:339–348.e4. doi: 10.1016/j.immuni.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 41.Pontillo A, Laurentino W, Crovella S, Pereira AC. NLRP1 haplotypes associated with leprosy in Brazilian patients. Infect Genet Evol. 2013;19:274–279. doi: 10.1016/j.meegid.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 42.Eisenbarth SC, Flavell RA. Innate instruction of adaptive immunity revisited: the inflammasome. EMBO Mol Med. 2009;1:92–98. doi: 10.1002/emmm.200900014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanneganti T-D, Özören N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517 [DOI] [PubMed] [Google Scholar]
- 45.Cui Y, Yu H, Bu Z, Wen L, Yan L, Feng J. Focus on the role of the NLRP3 inflammasome in multiple sclerosis: pathogenesis, diagnosis, and therapeutics. Front Mol Neurosci. 2022;15:894298. doi: 10.3389/fnmol.2022.894298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galloway DA, Carew SJ, Blandford SN, et al. Investigating the NLRP3 inflammasome and its regulator miR-223-3p in multiple sclerosis and experimental demyelination. J Neurochem. 2022;163:94–112. doi: 10.1111/jnc.15650 [DOI] [PubMed] [Google Scholar]
- 47.Unterberger S, Mullen L, Flint MS, Sacre S. Multiple TLRs elicit alternative NLRP3 inflammasome activation in primary human monocytes independent of RIPK1 kinase activity. Front Immunol. 2023;14:1092799. doi: 10.3389/fimmu.2023.1092799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48..McKenzie BA, Mamik MK, Saito LB, et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci U S A. 2018;115:E6065–74. doi: 10.1073/pnas.1722041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai Z, Liu W-C, Chen X-Y, Wang X, Li J-L, Zhang X. Gasdermin D-mediated pyroptosis: mechanisms, diseases, and inhibitors. Front Immunol. 2023;14:1178662. doi: 10.3389/fimmu.2023.1178662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bürckstümmer T, Baumann C, Blüml S, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702 [DOI] [PubMed] [Google Scholar]
- 51.Corrales L, Woo S-R, Williams J, McWhirter SM, Dubensky TW, Gajewski TF. Antagonism of the STING pathway via activation of the AIM2 inflammasome by intracellular DNA. J Immunol. 2016;196:3191–3198. doi: 10.4049/jimmunol.1502538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin Q, Sester DP, Tian Y, et al. Molecular mechanism for p202-mediated specific inhibition of AIM2 inflammasome activation. Cell Rep. 2013;4:327–339. doi: 10.1016/j.celrep.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H, Yang J, Gao W, et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449 [DOI] [PubMed] [Google Scholar]
- 54.Kim ML, Chae JJ, Park YH, et al. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J Exp Med. 2015;212:927–938. doi: 10.1084/jem.20142384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabrielli L, Capitoli A, Bini D, et al. Recent approaches to novel antibacterials designed after LPS structure and biochemistry. Curr Drug Targets. 2012;13:1458–1471. doi: 10.2174/138945012803530242 [DOI] [PubMed] [Google Scholar]
- 56.Liu X, Zhang Z, Ruan J, et al. Inflammasome - activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018;495:1418–1425. doi: 10.1016/j.bbrc.2017.11.156 [DOI] [PubMed] [Google Scholar]
- 58.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Confavreux C, Compston A. The natural history of multiple sclerosis. McAlpine’s Multiple Sclerosis. 2006;183–272. doi: 10.1016/B978-0-443-07271-0.50006-9 [DOI] [Google Scholar]
- 60..Malhotra S, Hurtado-Navarro L, Pappolla A, et al. Increased NLRP3 inflammasome activation and pyroptosis in patients with multiple sclerosis with fingolimod treatment failure. Neurol Neuroimmunol Neuroinflamm. 2023;10:e200100. doi: 10.1212/NXI.0000000000200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peelen E, Damoiseaux J, Muris A-H, et al. Increased inflammasome related gene expression profile in PBMC may facilitate T helper 17 cell induction in multiple sclerosis. Mol Immunol. 2015;63:521–529. doi: 10.1016/j.molimm.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 62.Ming X, Li W, Maeda Y, et al. Caspase-1 expression in multiple sclerosis plaques and cultured glial cells. J Neurol Sci. 2002;197:9–18. doi: 10.1016/s0022-510x(02)00030-8 [DOI] [PubMed] [Google Scholar]
- 63.de Jong BA, Huizinga TWJ, Bollen ELEM, et al. Production of IL-1β and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J Neuroimmunol. 2002;126:172–179. doi: 10.1016/S0165-5728(02)00056-5 [DOI] [PubMed] [Google Scholar]
- 64.Gris D, Ye Z, Iocca HA, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malhotra S, Río J, Urcelay E, et al. NLRP3 inflammasome is associated with the response to IFN-β in patients with multiple sclerosis. Brain. 2015;138:644–652. doi: 10.1093/brain/awu388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66..Inoue M, Williams KL, Oliver T, et al. Interferon-β therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal. 2012;5:ra38. doi: 10.1126/scisignal.2002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo W, Liu W, Jin B, et al. Asiatic acid ameliorates dextran sulfate sodium-induced murine experimental colitis via suppressing mitochondria-mediated NLRP3 inflammasome activation. Int Immunopharmacol. 2015;24:232–238. doi: 10.1016/j.intimp.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 68.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379 [DOI] [PubMed] [Google Scholar]
- 69.Furlan R, Martino G, Galbiati F, et al. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol. 1999;163:2403–2409. doi: 10.4049/jimmunol.163.5.2403 [DOI] [PubMed] [Google Scholar]
- 70.Shi F-D, Takeda K, Akira S, Sarvetnick N, Ljunggren H-G. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-γ by NK cells. J Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099 [DOI] [PubMed] [Google Scholar]
- 71.Inoue M, Williams KL, Gunn MD, Shinohara ML NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Nat Acad Sci. 2012;109:10480–10485. doi: 10.1073/pnas.1201836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jha S, Srivastava SY, Brickey WJ, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73..Dumas A, Amiable N, Vaccari JPDR, et al. The inflammasome pyrin contributes to pertussis toxin-induced IL-1β synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLOS Pathogens. 2014;10:e1004150. doi: 10.1371/journal.ppat.1004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burm SM, Peferoen LAN, Zuiderwijk-Sick EA, et al. Expression of IL-1β in rhesus EAE and MS lesions is mainly induced in the CNS itself. J Neuroinflamm. 2016;13:138. doi: 10.1186/s12974-016-0605-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hou B, Zhang Y, Liang P, et al. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 2020;11:1–16. doi: 10.1038/s41419-020-2565-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang W-X, Huang P, Hillert J. Increased expression of caspase-1 and interleukin-18 in peripheral blood mononuclear cells in patients with multiple sclerosis. Mult Scler. 2004;10:482–487. doi: 10.1191/1352458504ms1071oa [DOI] [PubMed] [Google Scholar]
- 78.Olcum M, Tastan B, Kiser C, Genc S, Genc K. Microglial NLRP3 inflammasome activation in multiple sclerosis. Adv Protein Chem Struct Biol. 2020;119:247–308. doi: 10.1016/bs.apcsb.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 79.McKenzie BA, Fernandes JP, Doan MAL, Schmitt LM, Branton WG, Power C. Activation of the executioner caspases-3 and −7 promotes microglial pyroptosis in models of multiple sclerosis. J Neuroinflamm. 2020;17:253. doi: 10.1186/s12974-020-01902-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yi Y-S. Caspase-11 noncanonical inflammasome: a novel key player in murine models of neuroinflammation and multiple sclerosis. Neuroimmunomodulation. 2021;28:195–203. doi: 10.1159/000516064 [DOI] [PubMed] [Google Scholar]
- 81.Wu J, Lin S, Chen W, et al. TNF-α contributes to sarcopenia through caspase-8/caspase-3/GSDME-mediated pyroptosis. Cell Death Discov. 2023;9:76. doi: 10.1038/s41420-023-01365-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Loo G, Bertrand MJM. Death by TNF: a road to inflammation. Nat Rev Immunol. 2023;23:289–303. doi: 10.1038/s41577-022-00792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Govindarajan V, de Rivero Vaccari JP, Keane RW. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J Neuroinflamm. 2020;17:260. doi: 10.1186/s12974-020-01944-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kermode AG, Thompson AJ, Tofts P, et al. Breakdown of the blood-brain barrier precedes symptoms and other mri signs of new lesions in multiple sclerosis: pathogenetic and clinical implicationS. Brain. 1990;113:1477–1489. doi: 10.1093/brain/113.5.1477 [DOI] [PubMed] [Google Scholar]
- 85.Paul C, Bolton C. Inhibition of blood-brain barrier disruption in experimental allergic encephalomyelitis by short-term therapy with dexamethasone or cyclosporin A. Int J Immunopharmacol. 1995;17:497–503. doi: 10.1016/0192-0561(95)00034-Y [DOI] [PubMed] [Google Scholar]
- 86.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochimica Et Biophysica Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 87.Argaw AT, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S. The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis. Prog Neurobiol. 2015;127–128:1–22. doi: 10.1016/j.pneurobio.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.You R, He X, Zeng Z, Zhan Y, Xiao Y, Xiao R. Pyroptosis and its role in autoimmune disease: a potential therapeutic target. Front Immunol. 2022;13:841732. doi: 10.3389/fimmu.2022.841732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang D, Li Y, Du C, et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J Transl Med. 2022;20:363. doi: 10.1186/s12967-022-03566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhaiban S, Al-Ani M, Elemam NM, Al-Aawad MH, Al-Rawi Z, Maghazachi AA. Role of peripheral immune cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Sci. 2021;3:12. doi: 10.3390/sci3010012 [DOI] [Google Scholar]
- 92.Deets KA, Nichols RD, Rauch I, Vance RE. Pyroptosis-dependent and -independent cross-priming of CD8+ T cells by intestinal epithelial cell-derived antigen. BioRxiv. 2021. doi: 10.1101/2021.07.08.451636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W, Zhang L, Sun Z. Eliciting pyroptosis to fuel cancer immunotherapy: mechanisms and strategies. Cancer Biol Med. 2022;19:948–964. doi: 10.20892/j.issn.2095-3941.2022.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antonopoulos C, Russo HM, El Sanadi C, et al. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem. 2015;290:20167–20184. doi: 10.1074/jbc.M115.652321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin BN, Wang C, Zhang C, et al. T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis. Nat Immunol. 2016;17:583–592. doi: 10.1038/ni.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen L, Ruan G, Cheng Y, Yi A, Chen D, Wei Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front Immunol. 2023;13:1055914. doi: 10.3389/fimmu.2022.1055914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li -L-L, Dai B, Sun Y-H, Zhang -T-T. The activation of IL-17 signaling pathway promotes pyroptosis in pneumonia-induced sepsis. Ann Transl Med. 2020;8:674. doi: 10.21037/atm-19-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98..Arbore G, West EE, Spolski R, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao Y, Vent-Schmidt J, McGeough MD, et al. Tr1 cells, but not Foxp3+ regulatory T cells, suppress NLRP3 inflammasome activation via an IL-10–dependent mechanism. J Immunol. 2015;195:488–497. doi: 10.4049/jimmunol.1403225 [DOI] [PubMed] [Google Scholar]
- 100.Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:1–11. doi: 10.1038/s41420-020-00349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L, Wang S, Zhou W. Balance cell apoptosis and pyroptosis of caspase-3-activating chemotherapy for better antitumor therapy. Cancers. 2023;15:26. doi: 10.3390/cancers15010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Samir P, Malireddi RKS, Kanneganti T-D. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10:238. doi: 10.3389/fcimb.2020.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng Y, Huang Y, Xu Y, Sang L, Liu X, Li Y. Ferroptosis, pyroptosis and necroptosis in acute respiratory distress syndrome. Cell Death Discov. 2023;9:1–12. doi: 10.1038/s41420-023-01369-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang R, Zeng L, Zhu S, et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108.e4. doi: 10.1016/j.chom.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18:2114–2127. doi: 10.1038/s41423-021-00740-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106..Conos SA, Chen KW, De Nardo D, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. 2017;114:E961–9. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lei X, Chen Y, Lien E, Fitzgerald KA. MLKL-driven inflammasome activation and caspase-8 mediate inflammatory cell death in influenza A virus infection. mBio. 2023. doi: 10.1128/mbio.00110-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rutsch A, Kantsjö JB, Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. 2020;11:604179. doi: 10.3389/fimmu.2020.604179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ordoñez-Rodriguez A, Roman P, Rueda-Ruzafa L, Campos-Rios A, Cardona D. Changes in gut microbiota and multiple sclerosis: a systematic review. Int J Environ Res Public Health. 2023;20:4624. doi: 10.3390/ijerph20054624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thirion F, Sellebjerg F, Fan Y, et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023;15:1. doi: 10.1186/s13073-022-01148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.iMSMS Consortium. Household paired design reduces variance and increases power in multi-city gut microbiome study in multiple sclerosis. Mult Scler. 2020;1352458520924594. doi: 10.1177/1352458520924594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou X, Baumann R, Gao X, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185:3467–3486.e16. doi: 10.1016/j.cell.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. doi: 10.1038/ncomms12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gandy KAO, Zhang J, Nagarkatti P, Nagarkatti M. The role of gut microbiota in shaping the relapse-remitting and chronic-progressive forms of multiple sclerosis in mouse models. Sci Rep. 2019;9:6923. doi: 10.1038/s41598-019-43356-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. doi: 10.1038/srep28484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vacaras V, Muresanu DF, Buzoianu AD, et al. The role of multiple sclerosis therapies on the dynamic of human gut microbiota. J Neuroimmunol. 2023;378:578087. doi: 10.1016/j.jneuroim.2023.578087 [DOI] [PubMed] [Google Scholar]
- 117.Cox LM, Maghzi AH, Liu S, et al. The gut microbiome in progressive multiple sclerosis. Ann Neurol. 2021;89:1195–1211. doi: 10.1002/ana.26084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tremlett H, Fadrosh DW, Faruqi AA, et al. Gut microbiota composition and relapse risk in pediatric MS: a pilot study. J Neurol Sci. 2016;363:153–157. doi: 10.1016/j.jns.2016.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Branton WG, Lu JQ, Surette MG, et al. Brain microbiota disruption within inflammatory demyelinating lesions in multiple sclerosis. Sci Rep. 2016;6:37344. doi: 10.1038/srep37344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ventura RE, Iizumi T, Battaglia T, et al. Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci Rep. 2019;9:16396. doi: 10.1038/s41598-019-52894-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fusco W, Lorenzo MB, Cintoni M, et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. 2023;15:2211. doi: 10.3390/nu15092211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trend S, Leffler J, Jones AP, et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci Rep. 2021;11:5244. doi: 10.1038/s41598-021-84881-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Olsson A, Gustavsen S, Nguyen TD, et al. Serum short-chain fatty acids and associations with inflammation in newly diagnosed patients with multiple sclerosis and healthy controls. Front Immunol. 2021;12:661493. doi: 10.3389/fimmu.2021.661493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barcutean L, Maier S, Burai-Patrascu M, Farczadi L, Balasa R. The immunomodulatory potential of short-chain fatty acids in multiple sclerosis. Int J Mol Sci. 2024;25:3198. doi: 10.3390/ijms25063198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu X-F, Shao J-H, Liao Y-T, et al. Regulation of short-chain fatty acids in the immune system. Front Immunol. 2023;14:1186892. doi: 10.3389/fimmu.2023.1186892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saadh MJ, Ahmed HM, Alani ZK, et al. The role of gut-derived short-chain fatty acids in multiple sclerosis. Neuromol Med. 2024;26:14. doi: 10.1007/s12017-024-08783-4 [DOI] [PubMed] [Google Scholar]
- 127.Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol Biochem. 2018;49:190–205. doi: 10.1159/000492853 [DOI] [PubMed] [Google Scholar]
- 128.Ghezzi L, Cantoni C, Pinget GV, Zhou Y, Piccio L. Targeting the gut to treat multiple sclerosis. J Clin Invest. 2021;131:e143774. doi: 10.1172/JCI143774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129..Xiao N, He W, Chen S, et al. Protective effect of egg yolk lipids against dextran sulfate sodium-induced colitis: the key role of gut microbiota and short-chain fatty acids. Mol Nutr Food Res. 2024;68:e2400048. doi: 10.1002/mnfr.202400048. [DOI] [PubMed] [Google Scholar]
- 130.Yi C, Sun W, Ding L, et al. Short-chain fatty acids weaken ox-LDL-induced cell inflammatory injury by inhibiting the NLRP3/caspase-1 pathway and affecting cellular metabolism in THP-1 cells. Molecules. 2022;27:8801. doi: 10.3390/molecules27248801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131..Wang W, Dernst A, Martin B, et al. Butyrate and propionate are microbial danger signals that activate the NLRP3 inflammasome in human macrophages upon TLR stimulation. Cell Rep. 2024:43. doi: 10.1016/j.celrep.2024.114736. [DOI] [PubMed] [Google Scholar]
- 132.Park O-J, Ha Y-E, Sim J-R, et al. Butyrate potentiates Enterococcus faecalis lipoteichoic acid-induced inflammasome activation via histone deacetylase inhibition. Cell Death Discov. 2023;9:1–10. doi: 10.1038/s41420-023-01404-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei C, Jiang W, Wang R, et al. Brain endothelial GSDMD activation mediates inflammatory BBB breakdown. Nature. 2024. doi: 10.1038/s41586-024-07314-2 [DOI] [PubMed] [Google Scholar]
- 134.Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao K, Pi Y, Mu C-L, Farzi A, Liu Z, Zhu W-Y. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: aromatic amino acids linking the microbiota-brain axis. J Neurochem. 2019;149:641–659. doi: 10.1111/jnc.14709 [DOI] [PubMed] [Google Scholar]
- 136.Zhu F, Guo R, Wang W, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020;25:2905–2918. doi: 10.1038/s41380-019-0475-4 [DOI] [PubMed] [Google Scholar]
- 137.Hosang L, Canals RC, van der Flier FJ, et al. The lung microbiome regulates brain autoimmunity. Nature. 2022;603:138–144. doi: 10.1038/s41586-022-04427-4 [DOI] [PubMed] [Google Scholar]
- 138.Schrijver IA, van Meurs M, Melief M-J, et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain. 2001;124:1544–1554. doi: 10.1093/brain/124.8.1544 [DOI] [PubMed] [Google Scholar]
- 139.Laman JD, Bert A, Power C, Dziarski R. Bacterial Peptidoglycan as a Driver of Chronic Brain Inflammation. Trends Mol Med. 2020;26:670–682. doi: 10.1016/j.molmed.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 140.Kriesel JD, Bhetariya P, Wang Z-M, Renner D, Palmer C, Fischer KF. Spectrum of microbial sequences and a bacterial cell wall antigen in primary demyelination brain specimens obtained from living patients. Sci Rep. 2019;9:1387. doi: 10.1038/s41598-018-38198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Armstrong H, Bording-Jorgensen M, Chan R, Wine E. Nigericin promotes NLRP3-independent bacterial killing in macrophages. Front Immunol. 2019;10. doi: 10.3389/fimmu.2019.02296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Braga JD, Thongngam M, Kumrungsee T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis. Npj Sci Food. 2024;8:16. doi: 10.1038/s41538-024-00253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duranti S, Ruiz L, Lugli GA, et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci Rep. 2020;10:14112. doi: 10.1038/s41598-020-70986-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akyuz E, Celik BR, Aslan FS, Sahin H, Angelopoulou E. Exploring the role of neurotransmitters in multiple sclerosis: an expanded review. ACS Chem Neurosci. 2023;14:527–553. doi: 10.1021/acschemneuro.2c00589 [DOI] [PubMed] [Google Scholar]
- 145.Bhat R, Axtell R, Mitra A, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Nat Acad Sci 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsuchida Y, Hatao F, Fujisawa M, et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60:638–647. doi: 10.1136/gut.2010.227546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Di Bari M, Reale M, Di Nicola M, et al. Dysregulated homeostasis of acetylcholine levels in immune cells of RR-multiple sclerosis patients. Int J Mol Sci. 2016;17:2009. doi: 10.3390/ijms17122009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun CY, Yang N, Zheng ZL, Liu D, Xu QL. T helper 17 (Th17) cell responses to the gut microbiota in human diseases. Biomed Pharmacother. 2023;161:114483. doi: 10.1016/j.biopha.2023.114483 [DOI] [PubMed] [Google Scholar]
- 150.Kim KS. Regulation of T cell repertoires by commensal microbiota. Front Cell Infect Microbiol. 2022;12. doi: 10.3389/fcimb.2022.1004339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ronchi F, Basso C, Preite S, et al. Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1β production by myeloid cells. Nat Commun. 2016;7:11541. doi: 10.1038/ncomms11541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152..Lamas B, Hernandez-Galan L, Galipeau HJ, et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci Transl Med. 2020;12:eaba0624. doi: 10.1126/scitranslmed.aba0624. [DOI] [PubMed] [Google Scholar]
- 153.Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206 [DOI] [PubMed] [Google Scholar]
- 154.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881 [DOI] [PubMed] [Google Scholar]
- 155.Pandiyan P, Bhaskaran N, Zou M, Schneider E, Jayaraman S, Huehn J. Microbiome dependent regulation of Tregs and Th17 cells in mucosa. Front Immunol. 2019;10. doi: 10.3389/fimmu.2019.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cheng H, Guan X, Chen D, Ma W. The Th17/Treg cell balance: a gut microbiota-modulated story. Microorganisms. 2019;7:583. doi: 10.3390/microorganisms7120583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tan J, Taitz J, Sun SM, Langford L, Ni D, Macia L. Your regulatory T cells are what you eat: how diet and gut microbiota affect regulatory T cell development. Front Nutr. 2022;9:878382. doi: 10.3389/fnut.2022.878382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Nat Acad Sci. 2010;107(27):12204–12209. doi: 10.1073/pnas.0909122107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu S, Rezende RM, Moreira TG, et al. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host Microbe. 2019;26:779–794.e8. doi: 10.1016/j.chom.2019.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cox LM, Maghzi AH, Liu S, et al. Gut microbiome in progressive multiple sclerosis. Ann Neurol. 2021;89:1195–1211. doi: 10.1002/ana.26084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tankou SK, Regev K, Healy BC, et al. Investigation of probiotics in multiple sclerosis. Mult Scler. 2018;24:58–63. doi: 10.1177/1352458517737390 [DOI] [PubMed] [Google Scholar]
- 163.Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245–1249. doi: 10.1016/j.clnu.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 164.Salami M, Kouchaki E, Asemi Z, Tamtaji OR. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J Func Foods. 2019;52:8–13. doi: 10.1016/j.jff.2018.10.023 [DOI] [Google Scholar]
- 165.Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e459. doi: 10.1212/NXI.0000000000000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180:1067–1080.e16. doi: 10.1016/j.cell.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 167.Tankou SK, Regev K, Healy BC, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83:1147–1161. doi: 10.1002/ana.25244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rahimlou M, Nematollahi S, Husain D, Banaei-Jahromi N, Majdinasab N, Hosseini SA. Probiotic supplementation and systemic inflammation in relapsing-remitting multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Front Neurosci. 2022;16:901846. doi: 10.3389/fnins.2022.901846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Asghari KM, Dolatkhah N, Ayromlou H, Mirnasiri F, Dadfar T, Hashemian M. The effect of probiotic supplementation on the clinical and para-clinical findings of multiple sclerosis: a randomized clinical trial. Sci Rep. 2023;13:18577. doi: 10.1038/s41598-023-46047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Borody T, Leis S, Campbell J, Torres M, Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS): 942. Off J Ame College Gastroenterol. 2011;106:S352. [Google Scholar]
- 171.Smits LP, Bouter KEC, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058 [DOI] [PubMed] [Google Scholar]
- 172.Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114:10713–10718. doi: 10.1073/pnas.1711235114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Berer K, Gerdes LA, Cekanaviciute E, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114:10719–10724. doi: 10.1073/pnas.1711233114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li K, Wei S, Hu L, et al. Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediators Inflamm. 2020;2020:2058272. doi: 10.1155/2020/2058272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Golpour F, Abbasi-Alaei M, Babaei F, et al. Short chain fatty acids, a possible treatment option for autoimmune diseases. Biomed Pharmacother. 2023;163:114763. doi: 10.1016/j.biopha.2023.114763 [DOI] [PubMed] [Google Scholar]
- 176.Dominguez-Mozo MI, Perez-Perez S, Villarrubia N, et al. Herpesvirus antibodies, vitamin D and short-chain fatty acids: their correlation with cell subsets in multiple sclerosis patients and healthy controls. Cells. 2021;10:119. doi: 10.3390/cells10010119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim DY, Hao J, Liu R, Turner G, Shi F-D, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012;7:e35476. doi: 10.1371/journal.pone.0035476 [DOI] [PMC free article] [PubMed] [Google Scholar]