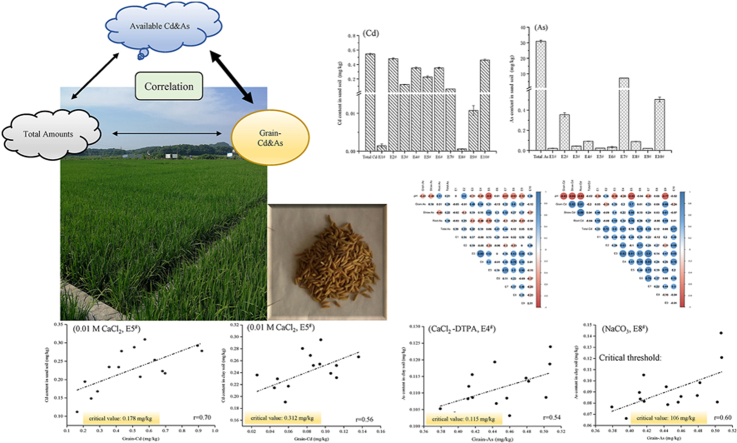
Abstract
Cadmium (Cd) and arsenic (As) contamination risk in paddy soils has raised global concern. In order to scientifically and objectively evaluate the bioavailability of soil Cd, As and the risk of Cd or As threshold in contaminated farmland, this study was conducted to investigate different types of extractants for their potential extraction efficiency of Cd and As. Soils from two different parent materials in Hunan, Yueyang and Yiyang, typical double-cropping rice production areas in the south of China, were used as test soils. The extraction capabilities of 10 extractants (ultrapure water, 0.1 mol/L HCl, 1.0 mol/L NH4OAc, CaCl2-DTPA, 0.01 mol/L CaCl2, 0.1 mol/L CaCl2, 0.5 mol/L NaH2PO4, 0.05 mol/L NaHCO3, 0.1 mol/L NaNO3, 0.1 mol/L HNO3), were compared for their extraction capabilities of soil available Cd and As. Meanwhile, the content of Cd and As in plants issues and grains of rice was monitored during harvest, and the Cd, As content correlation between extracted forms and rice was analyzed. The results showed that the HCl, CaCl2, HNO3, and CaCl2-DTPA solutions exhibited high extraction efficiency for Cd (42.2–88.4 %); for As, NaH2PO4, HCl, and HNO3 have the extraction efficiency (0.85–23.4 %). The concentration of Cd extracted by 0.01 mol/L CaCl2 was significantly positively correlated with Cd levels in rice. The potential risk extraction threshold of CaCl2 in sandy loam soil was 0.178 mg/kg, while it was 0.312 mg/kg in clay soil. The concentration of As extracted by CaCl2-DTPA and 0.05 mol/L NaHCO3 in clay soil was significantly positively correlated with As levels in rice, the potential risk extraction thresholds were 0.115 mol/L and 0.106 mg/kg, respectively. These investigations indicated that the heavy metals extraction methods by 0.01 mol/L CaCl2, CaCl2-DTPA, and 0.05 mol/L NaHCO3 could reflect the Cd and As pollution degree in farmland and suggest their potential to serve as methods for assessing the risk of Cd and As pollution in sandy loam and clay paddy soil.
Keywords: Cadmium, Arsenic, Bioavailability, Rice, Extraction efficiency, Farmland soil, Risk threshold
Graphical abstract
Highlights
-
•
The content of Cd & As in rice was positively correlated with the available Cd & As abundance in soil.
-
•
HCl, DTPA, CaCl2, and HNO3 showed high extraction efficiency for Cd (>60 %).
-
•
NaH2PO4 showed high extraction efficiency for As (13.4–23.4 %).
-
•
The positive correlation between CaCl2 or NaHCO3 extracted Cd-As and in rice content.
-
•
The critical risk values of Cd-As in sandy and clay soil are studied and calculated.
1. Introduction
Heavy metal cadmium (Cd) and metalloid arsenic (As) are common toxic elements in paddy soil, whose point-exceeding rate ranked the first and the third inorganic pollutants [1,2]. Once in the farmland, they are easily absorbed and accumulated by crops and difficult to degrade, thus causing almost irreversible soil pollution [3,4]. Recent nationwide surveys indicate that 16 % of soil samples, and 19 % of agricultural soils, are contaminated according to China's soil environmental quality limits, mainly with heavy metals and metalloids. The exceedance rates of Cd and As pollution points are 7.0 % and 2.7 %, respectively [[5], [6], [7]]. Comparisons with other regions of the world show that the current status of soil contamination, based on total contaminant concentrations, is not worse in China. However, the concentrations of some heavy metals in Chinese soils appear to be increasing at much greater rates [7]. The severe pollution of Cd and As in farmland poses a significant threat to the safety of agricultural products and human health. Compared to the other gramineous food crops, rice was easier to accumulate As or Cd by transporting them through the roots [8,9]. With the deepening research on heavy metal forms, people realized that the ecological risk posed by heavy metals primarily depends on their forms and the proportion of bioavailable forms [10,11]. In contrast, the total amount of heavy metals in soil only represents the storage abundance of the element, which can't fully reflect the level of crops that can be utilized or soil polluted [12]. Therefore, based on the Cd and As concentration in crop, scientifically evaluating the bioavailability of field paddy Cd and As, predicting the potential risk threshold of soil for Cd and As in farmland rice, which is great significance for the safe use of paddy soil.
The migration and transformation of different forms of heavy metals in soil significantly affect their bioavailability [13]. In general, water-soluble Cd and exchangeable Cd are easily absorbed and utilized by corps. Carbonate-bound forms are easily released with the decreased of pH value, iron-manganese oxidized forms, and organic-bound forms were be decomposed under strong oxidizing conditions, while residual state could be stable in the soil [14,15]. Inorganic states are the main forms of arsenic in soil, under oxidation condition, arseniate is the abundant existing species, and arsenite is the predominant form with reducing environment. Similarly, inorganic arsenic compounds are the main types absorption and accumulation by plant [16,17]. The key to determining the effective form of soil pollution elements lies in the selection of extractants and the determination of extraction conditions. At present, the common soil effective Cd extractants include DTPA, HCl, and neutral salts. DTPA is widely used and which suitable for the determination of trace elements in neutral calcareous soils [11,13,14]. Some studies also shown that dilute hydrochloric acid is fit for acidic Cd polluted soils assessment. However, there are fewer studies on the extraction methods of effective As in soil. Some scholars believed that soils with pH less than 6.5, 0.05 mol/L HCl is recommended as the extractant, and 0.5 mol/L NaHCO3 is recommended for higher pH (>6.5) [18,19]. 0.1 mol/L HCl, 1.0 mol/L NH4OAc and 0.5 mol/L NaHCO3 (pH 8.5) also has been reported to be suitable for the extraction of available arsenic from soil [20]. The effectiveness of heavy metal extraction in soils polluted by different types of Cd and As varies due to differences in soil parent material, soil properties, and pollution sources. The principles, capabilities, and the correlation between extraction quantity and the concentration of metals in crops vary with different leaching agents.
Hunan, as a major production area of double-season rice in southern China, face a concentrated and significant problem of soil Cd and As pollution, with some areas exceeding the national standard limits (GB15618-2018, for paddy soil, Cd, 0.3–0.8 mg/kg, As, 20–30 mg/kg, varies with soil pH). At present, there are a variety of methods for extracting effective Cd and As, and the correlation with the concentration of Cd and As in rice is controversial. This study selected two types of typical Cd and As compound polluted farmland in Yueyang and Yiyang, Hunan, which are major areas for double cropping rice production, using locally recommended rice varieties as test crops. The study compared the relationship between the concentrations of effective Cd and As in soil extracted by 10 different common extraction methods, including various types and polarities of extractants, neutral salts, monobasic acid, pure water, phosphates, and carbonates. The effects of these extractants on the extraction of soil-active cadmium and arsenic, as well as the concentrations of Cd and As in rice, have been widely discussed and remain controversial. This research aims to screen suitable methods for extracting effective Cd and As in soil, and to provide a basis for the safe production of crops in typical acidic farmland contaminated with Cd and As in the south, which will also aid in the evaluation of soil remediation technologies.
2. Materials and methods
2.1. Samples of test soil
A total of 30 samples were collected from farmland around the mildly Cd and As polluted soil in Hunan province, specifically from Bai Ni Lake in Yueyang (referred to as Yueyang Soil) and Heshan District in Yiyang (referred to as Yiyang Soil), the main production areas of double-season rice in southern China. The soil parent material of these soils is mainly river lacustrine deposits and slope wash deposits, with deep soil layers, fertile soil, and rich trace elements. The basic physical and chemical properties of the two types of test soils are listed in Table 1.
Table 1.
Basic physical and chemical properties of the tested soil.
| EX-Al |
EX- Ca |
EX- Mg |
EX- K |
EX- Na |
CEC |
pH | Total Cd |
Total As |
||
|---|---|---|---|---|---|---|---|---|---|---|
| (cmol/kg) | (mg/kg) | |||||||||
| Yue-yang Soil (sandy loam) |
S1 | 0.37 | 6.2 | 1.3 | 0.28 | 0.19 | 10.1 | 5.3 | 0.543 | 33.8 |
| S2 | 0.28 | 5.6 | 1.3 | 0.36 | 0.13 | 9.1 | 5.2 | 0.481 | 32.4 | |
| S3 | 0.23 | 6.4 | 1.6 | 0.28 | 0.13 | 10.3 | 5.2 | 0.612 | 31.6 | |
| S4 | 0.12 | 7.4 | 1.6 | 0.33 | 0.16 | 10.3 | 5.7 | 0.588 | 31.9 | |
| S5 | 0.12 | 6.6 | 1.7 | 0.29 | 0.16 | 10.9 | 5.7 | 0.565 | 30.4 | |
| S6 | 0.14 | 6.6 | 1.6 | 0.28 | 0.13 | 10.1 | 5.6 | 0.592 | 33.5 | |
| S7 | 0.12 | 8.8 | 2.1 | 0.34 | 0.13 | 11.5 | 6.2 | 0.509 | 29.8 | |
| S8 | 0.12 | 7.5 | 1.8 | 0.34 | 0.13 | 10.6 | 6.1 | 0.565 | 31.5 | |
| S9 | 0.12 | 8.1 | 1.9 | 0.32 | 0.16 | 10.7 | 6.2 | 0.567 | 26.3 | |
| S10 | 0.12 | 7.7 | 1.8 | 0.30 | 0.13 | 10.6 | 5.9 | 0.530 | 35.7 | |
| S11 | 0.14 | 7.0 | 1.7 | 0.26 | 0.11 | 11.7 | 5.8 | 0.485 | 26.9 | |
| S12 | 0.12 | 7.3 | 1.8 | 0.29 | 0.16 | 10.0 | 5.9 | 0.516 | 28.0 | |
| S13 | 0.14 | 8.1 | 1.9 | 0.33 | 0.11 | 11.0 | 6.0 | 0.513 | 31.1 | |
| S14 | 0.22 | 6.8 | 1.6 | 0.32 | 0.11 | 10.5 | 5.9 | 0.504 | 31.7 | |
| S15 |
0.12 |
8.5 |
1.9 |
0.23 |
0.13 |
10.9 |
6.1 |
0.606 |
30.3 |
|
| Yi-yang Soil (clay loam) | S16 | 1.90 | 4.3 | 1.2 | 0.30 | 0.14 | 13.2 | 4.8 | 0.505 | 34.8 |
| S17 | 2.72 | 4.3 | 1.0 | 0.30 | 0.08 | 13.9 | 4.7 | 0.497 | 36.8 | |
| S18 | 3.28 | 3.7 | 1.0 | 0.32 | 0.11 | 13.2 | 4.7 | 0.470 | 39.4 | |
| S19 | 0.79 | 6.3 | 1.5 | 0.24 | 0.14 | 13.4 | 5.0 | 0.555 | 39.2 | |
| S20 | 1.52 | 5.3 | 1.6 | 0.32 | 0.08 | 13.1 | 5.0 | 0.516 | 36.5 | |
| S21 | 1.33 | 5.5 | 1.5 | 0.27 | 0.14 | 13.3 | 5.1 | 0.482 | 35.8 | |
| S22 | 0.36 | 6.5 | 1.7 | 0.29 | 0.14 | 13.3 | 5.2 | 0.473 | 33.9 | |
| S23 | 0.49 | 6.5 | 1.7 | 0.31 | 0.08 | 13.4 | 5.3 | 0.492 | 38.5 | |
| S24 | 0.46 | 6.6 | 1.6 | 0.31 | 0.14 | 13.7 | 5.3 | 0.522 | 39.0 | |
| S25 | 0.98 | 6.0 | 1.6 | 0.29 | 0.14 | 14.0 | 5.2 | 0.540 | 36.5 | |
| S26 | 0.47 | 7.4 | 1.6 | 0.26 | 0.17 | 13.2 | 5.4 | 0.551 | 39.2 | |
| S27 | 0.81 | 5.8 | 1.5 | 0.36 | 0.17 | 13.2 | 5.1 | 0.514 | 35.6 | |
| S28 | 0.51 | 8.0 | 1.9 | 0.38 | 0.08 | 13.1 | 5.6 | 0.537 | 37.9 | |
| S29 | 0.51 | 7.2 | 1.9 | 0.37 | 0.11 | 13.1 | 5.5 | 0.536 | 38.0 | |
| S30 | 0.60 | 6.0 | 1.5 | 0.27 | 0.17 | 12.6 | 5.1 | 0.502 | 35.9 | |
Note: S1-S15 were Yueyang Soil, the soil texture is mainly sandy loam, and landform is wash plain. S16-S30 were Yiyang Soil, the soil texture is mainly claying loam, and landform is hilly; EX-means exchangeable forms.
Leaching Agents: 1) Ultrapure water (labeled as E1#) [14]; 2) 0.1 mol/L HCl solution (labeled as E2#): take 8.3 mL HCl (GR, 37 %) and dilute to 1 L with water [14]; 3) 1.0 mol/L NH4OAc solution (labeled as E3#): weigh 77.08 g NH4OAc, dissolve, and dilute to 1 L with purified water, the solution pH value is 7.03 [21]; 4) CaCl2-DTPA solution (labeled as E4#): dissolve 1.967 g DTPA in 14.92 g triethanolamine and a small amount of water, then dissolve 1.47 g CaCl2·2H2O in water, dilute to about 950 mL, then adjust the solution pH to 7.30 by HCl solution, finally dilute to 1 L with water [13]; 5) 0.01 mol/L CaCl2 (labeled as E5#): weigh 1.11 g CaCl2, dissolve and dilute to 1 L with water (pH value is 7.01) [11]; 6) 0.1 mol/L CaCl2 (labeled as E6#): weigh 11.1 g CaCl2, dissolve and dilute to 1 L with water (pH value is 7.10) [12]; 7) 0.5 mol/L NaH2PO4 (labeled as E7#): weigh 57.98 g NaH2PO4, dissolve and dilute to 1 L with water [11]; 8) 0.05 mol/L NaHCO3 (labeled as E8#): weigh 4.2 g NaHCO3, dissolve in 900 mL of water, adjust the solution pH to 8.50, and dilute to 1 L with water [19]; 9) 0.1 mol/L NaNO3 (labeled as E9#): weigh 8.50 g NaNO3, dissolve and dilute to 1 L with water (pH value is 7.12) [22]; 10) 0.1 mol/L HNO3 solution (labeled as E10#): take 6.25 mL HNO3 (GR, 65 %) and dilute to 1 L with water.
The reagents were purchased from Sinopharm Group, and the water used in the experiment was ultra-pure water filtered through a membrane. All the experimental vessels were soaked in 10 % nitric acid overnight before use.
2.2. Field experiment
The field trials of rice were conducted in different sub-areas within the test regions (Yiyang and Yueyang). According to local fertilizer practices, 600 kg/ha compound fertilizer (15-15-15, N-P-K) was applied as base fertilizer. This was followed by top dressing with urea and potassium fertilizer. Field water and fertilizer management were conducted uniformly across the test areas. After the rice matured, samples were collected. The early rice variety “Xiang Zao Xian 45” was used in the experiment.
2.3. Sampling and analytical methods
Sample Collection 30 topsoil samples (0–15 cm) were collected from the sub-areas in different field areas for analysis. Each sample was a composite of multi points. Plant residues and other non-soil components were sieved out. The soil samples were air-dried indoors, screened with a 1.0 mm mesh, and the sifted soil was used for analysis. During the mature stage of rice, the whole rice plant was harvested. The plant samples were washed with tap water and deionized water, then fixed for 30 min at 105 °C. After that, they were dried at 80 °C until reaching a constant weight. The rice roots, straws, and brown rice were ground into powder for further analysis.
Soil sample analysis Soil samples were air dried and ground for analysis of their characteristics. Soil pH was measured in a 1:2.5 (w/v) ratio of soil to deionized water using a pH meter (FE28, Mettler Toledo, Switzerland). For the determination of available Cd and As in the soil: 5.0 g of air-dried sifted soil was placed in a 100 mL wide-mouth bottle, 25.0 mL of the above-mentioned ten extractants were added (soil-liquid ratio was 1:5), and the mixture was extracted in a constant temperature reciprocating shaker at uniform constant temperature (25 ± 0.5 °C) at 180 rpm for 2 h, then the filtrate was collected. Total Cd and As in soil samples were digested using HNO3 and HCl in a ratio of 1:3 [23]. The concentrations of Cd and As in digestion solutions were determined via inductively coupled plasma mass spectrophotometry (i Cap-Q ICP-MS, Thermo Fisher Scientific, US).
Plant sample analysis After grinding, about 0.3 g of plant samples were digested with a mixture of 10 mL HNO3 and H2O2 (4:1) [24]. Pre-digested at room temperature for a night, the digestion tubes were placed in a microwave digestion furnace (CEM Mars 6, US) and the temperature was increased in steps from 60 to 180 °C, and hold up to 2 h. Samples were removed until mixture digestion solution volumes reduced to 2 mL, the digested suspensions were filtered through filter and adjusted to 50 mL with deionized water. Then, the concentrations of Cd and As were determined by ICP-MS.
For quality assurance and control, standard reference materials (n = 3, certified reference material GBW07385 for soil and GBW10049 for plant, China) were included in the digestion procedure and sample analysis. During analysis, two analytical blanks, two standard reference materials and two calibration verification standards were included in every 40 samples. The test conditions of ICP-MS are listed in Table 2.
Table 2.
The analytical parameters of the inductively coupled plasma mass spectrometer.
| No. | Parameters | Value |
|---|---|---|
| 1 | Detection mode | anti-interference (KED) |
| 2 | Gas flow rate in the collision reaction tank (He) | 4.5 mL/min |
| 3 | The cooling gas flow rate (Ar) | 14 L/min |
| 4 | The internal standard | 103Rh |
| 5 | A recovery rate of internal standard | 90%–105 % |
| 6 | Dwell time for each element | 0.01s |
| 7 | Number of sweeps | 80–120 |
| 8 | Runtime of each sample for once | around 5 s |
| 9 | Main runs | 3 times |
2.4. Statistical analysis
The soil extraction efficiency of the extraction agents (SEE) was calculated using the following equation:
The coefficient of variation of soil extractant (CVS) was calculated using the following equation:
Data were shown as means ± standard deviations in three independent replicates. The experimental data were statistically processed using Origin Pro 2017, SPSS 20, and R software. A one-way analysis of variance (ANOVA) using the Duncan and Pearson method, P<0.05) was conducted to analyze the significance and correlation of various parameters under different treatments.
3. Results
3.1. Extraction efficiency of Cd by different leaching agents
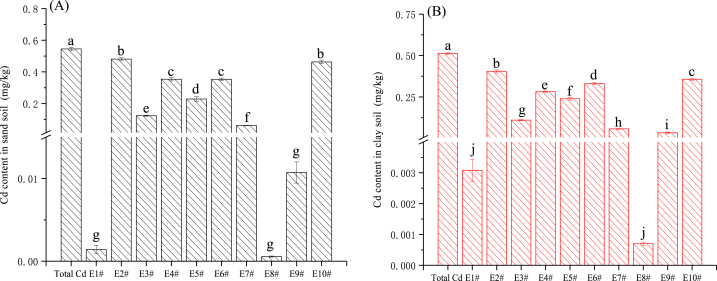
As shown in Fig. 1(A, B) and Table 3, each extractant shows different extraction efficiencies for Cd in the same type of farmland soil. Among them, for the sandy loam (YueYang Soil, 5.2 < pH < 6.2), the performance is E2#, E10# > E4#, E6# > E5# > E3# > E7# > E9#, E1#, E8# (P<0.05). The extraction efficiency of E2# is the highest at 88.4 %, followed by E10# (85.0 %), while E4#, E5#, and E6# all exceed 42 %. The extraction rates of E1# and E8# are the lowest (less than 1 %). From the perspective of the extraction coefficient of variation, E1# was the highest at 129.92 %, followed by E8# and E9# (exceeding 45 %). The extraction coefficient of variation for E2#, E10#, and E6# are all less than 6.6 %, and the extraction coefficient of variation for E7#, E3#, and E4# are relatively stable (see Fig. 1).
Fig. 1.
Extraction concentration of available cadmium from soil by each extractant (mg/kg), A: sandy soils; B: clay soils. Total Cd: Total cadmium content in soil, E1#: ultrapure water, E2#: 0.1 mol/L HCl, E3#: 1.0 mol/L NH4OAc, E4#: CaCl2-DTPA, E5#: 0.01 mol/L CaCl2, E6#: 0.1 mol/L CaCl2, E7#: 0.5 mol/L NaH2PO4, E8#: 0.05 mol/L NaHCO3, E9#: 0.1 mol/L NaNO3, E10#: 0.1 mol/L HNO3. Data were presented as means ± SE with significant differences by Duncan test, P < 0.05. The lowercase letters represent significant differences between treatments (the same below).
Table 3.
The extraction efficiency and variation coefficient of soil available Cd by each extractant.
| Treatment | Yue-yang Soil(sandy loam) |
Yi-yang Soil(clay loam) |
||
|---|---|---|---|---|
| extraction ratio (%) | variable coefficient (%) | extraction ratio (%) | variable coefficient (%) | |
| E1# | 0.25 ± 0.085 f | 129.92 | 0.59 ± 0.068 h | 44.32 |
| E2# | 88.4 ± 1.27 a | 5.58 | 78.8 ± 1.54 a | 7.57 |
| E3# | 22.6 ± 0.68 d | 11.67 | 21.5 ± 0.77 f | 13.89 |
| E4# | 65.2 ± 2.05 b | 12.19 | 51.9 ± 0.78 d | 5.82 |
| E5# | 42.2 ± 2.72 c | 25.01 | 46.8 ± 1.75 e | 14.53 |
| E6# | 64.9 ± 1.05 b | 6.27 | 64.4 ± 1.01 c | 6.11 |
| E7# | 11.3 ± 0.31 e | 10.69 | 11.2 ± 0.48 g | 16.65 |
| E8# | 0.10 ± 0.014 f | 52.44 | 0.14 ± 0.011 h | 26.36 |
| E9# | 1.98 ± 0.23 f | 45.56 | 6.67 ± 0.77 g | 44.91 |
| E10# | 85.0 ± 1.45 a | 6.60 | 69.4 ± 1.23 b | 6.86 |
Note: the lowercase letters represent significant differences (P<0.01, n = 15) between treatments in the same column.
Similarly, for clay loam (Yiyang soil, 4.7 < pH < 5.6), it also shows that E2# > E10# > E6# > E4# > E5# > E3# > E7# > E9# > E1# > E8# (P<0.05), the changing trend is basically consistent with the river and lake sediment soil. The extraction efficiency of E2# is the highest at 78.8 %, followed by E10# (69.4 %), while E4#, E5#, and E6# all exceed 46 %, E1#, E8# have the lowest extraction rates (less than 1 %). From the perspective of the extraction coefficient of variation, E9#, E1# were the highest, all exceeding 44 %. The extraction coefficient of variation for E2#, E4#, E6#, and E10# are all less than 7.6 %, and the E3#, E5#, and E7# are also relatively stable.
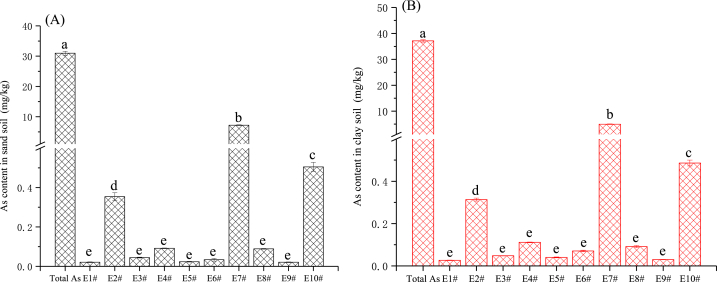
3.2. Extraction efficiency of As by different leaching agents
Fig. 2A, B and Table 4 indicated that for the two different types of soils, the extraction efficiencies of the 10 extractants are ranges as E7# > E10# > E2# > E1#、E3#、E4#、E5#、E6#、E8#、E9# (P<0.01). The extraction efficiency of E7# ranges from 13.4 to 23.4 %, followed by E10# (1.31–1.64 %) and E2# (0.85–1.15 %), the others are all below 1 %, among which E1# and E9# are the lowest (0.067–0.081 %). From the perspective of extraction coefficient of variation, E6# shows the highest value in sandy loam soil at 81.71 %. The extraction coefficient of variation of other extractions in the two soils are all below 30 %, among which E4# and E7# are below 10 %. The results indicated that the extraction efficiency of these extractants is relatively stable.
Fig. 2.
Extraction concentration of available arsenic from soil by each extractant (mg/kg), A: sandy soils; B: clay soils. Data were presented as means ± SE with significant differences by Duncan test, P < 0.05.
Table 4.
The extraction efficiency and variation coefficient of soil available As by each extractant.
| Treatment | Yue-yang Soil(sandy loam) |
Yi-yang Soil(clay loam) |
||
|---|---|---|---|---|
| extraction ratio (%) | variable coefficient (%) | extraction ratio (%) | variable coefficient (%) | |
| E1# | 0.068 ± 0.005 d | 28.81 | 0.071 ± 0.004 d | 19.85 |
| E2# | 1.15 ± 0.065 c | 22.00 | 0.85 ± 0.017 c | 8.06 |
| E3# | 0.144 ± 0.0072 d | 19.46 | 0.129 ± 0.003 d | 7.82 |
| E4# | 0.297 ± 0.0065 d | 8.50 | 0.30 ± 0.005 d | 6.46 |
| E5# | 0.074 ± 0.005 d | 28.34 | 0.108 ± 0.006 d | 22.68 |
| E6# | 0.107 ± 0.022 d | 81.71 | 0.189 ± 0.009 d | 19.53 |
| E7# | 23.4 ± 0.47 a | 7.75 | 13.4 ± 0.29 a | 8.47 |
| E8# | 0.289 ± 0.0084 d | 11.29 | 0.25 ± 0.013 d | 21.11 |
| E9# | 0.067 ± 0.0048 d | 27.30 | 0.081 ± 0.0027 d | 13.23 |
| E10# | 1.64 ± 0.075 b | 17.77 | 1.31 ± 0.038 b | 11.35 |
Note: the lowercase letters represent significant differences (P<0.01, n = 15) between treatments in the same column.
3.3. Comparison of ten Assays for Cd bioavailability analysis of available Cd in soil
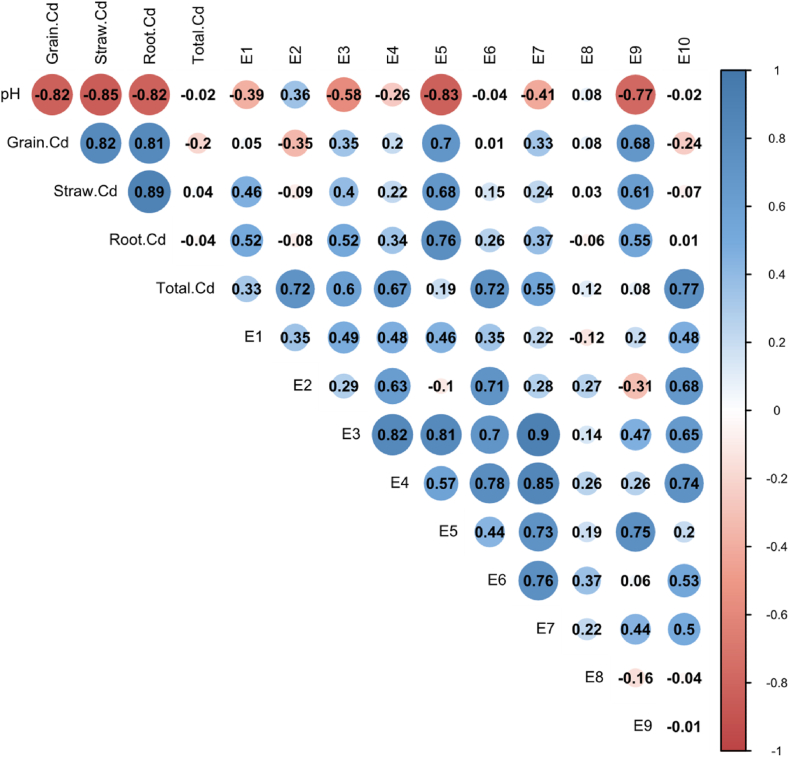
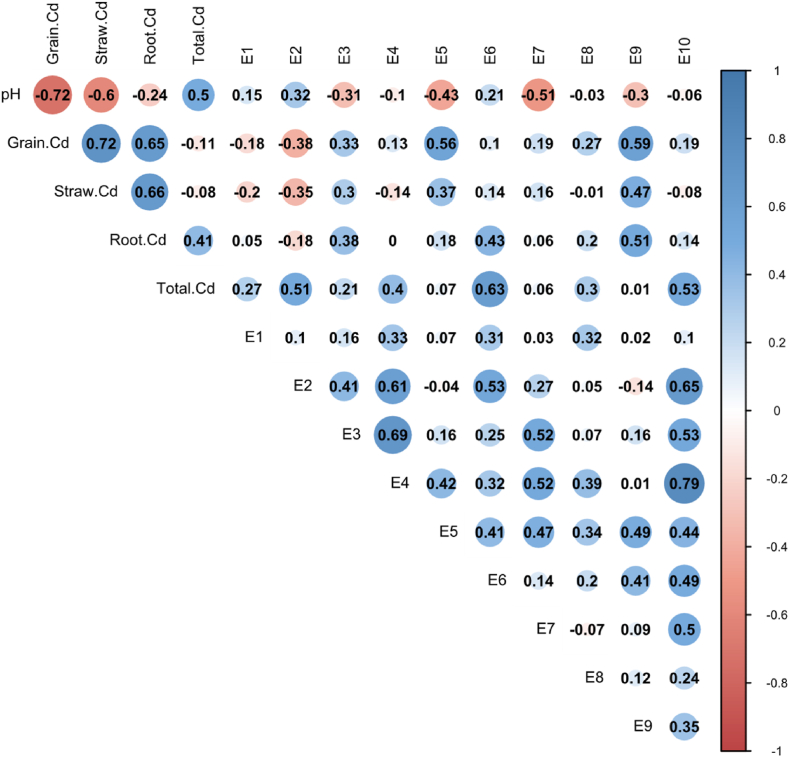
The Cd content in rice and the Cd concentrations extracted in different forms show different correlations (Fig. 3). Detailed data on Cd content in various tissues and organs of rice can be found in S1. Cd content of GBW10049 and GBW07385 is 0.209 mg/kg and 0.286 mg/kg, respectively, which conforms to the quality control sample setting range (0.19 ± 0.02 mg/kg; 0.28 ± 0.02 mg/kg). The rice Cd is extremely significantly positively correlated with the Cd in the straw and root system (r-straw Cd = 0.82/0.72, r-root Cd = 0.81/0.65, P<0.01). However, there is no significant correlation between grain Cd and the total amount of Cd in the soil. The concentration of extracted Cd in all states with the total Cd in the soil, with extractions by E2#, E6#, and E10# being significantly positively correlated (sandy loam: r2# = 0.72, r6# = 0.72, r10# = 0.77, P<0.01; clay loam: r2# = 0.51, r6# = 0.63, r10# = 0.53, P<0.05).
Fig. 3.
Correlation analysis of pH、Total Cd、paddy rice Cd and different extraction states Cd content in paddy soil. A: sandy soils; B: clay soils. Grain. Cd: Cadmium content in brown rice, Straw. Cd: Cadmium content in Stem and leaf, Root. Cd: Cadmium content in root. The Person analysis was used to test the results (the same below).
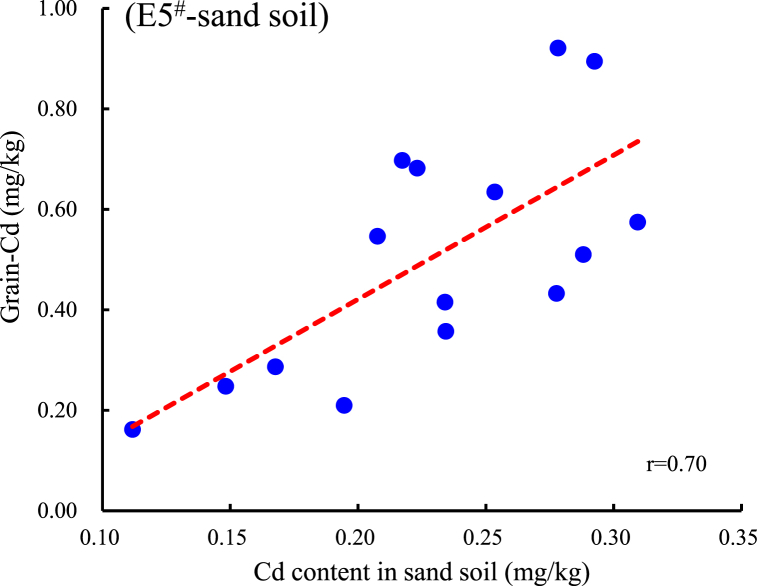
In sandy loam (Yueyang soil), Cd concentration extracted by E5# and E9# are extremely significantly positively correlated with the grain Cd (r5# = 0.70, r9# = 0.68, P<0.01), and are significantly positively correlated with the Cd in crop straws, and roots (r5#-straw Cd = 0.68, r5#-root Cd = 0.76, P<0.01; r9#-straw Cd = 0.61, r9#-root Cd = 0.55, P<0.05). And the Cd concentration extracted by E5# is extremely significantly positively correlated with the E3#, E4#, E7#, and E9# to varying degrees. The soil pH value is significantly negatively correlated with the E5#, E9#, and E3# extracted Cd contents (Fig. 3A). In clay loam (Yiyang soil), the E5# and E9# extracted Cd contents are significantly positively correlated with the grain Cd content (r5# = 0.56, r9# = 0.59, P<0.05), and show a positive correlation trend with the Cd content in the straws, and root system (r5# = 0.37, 0.18; r9# = 0.47, 0.51, Fig. 3B). While the soil pH value is not significantly correlated with the extracted Cd content in each state, except E5# and E7# (r5 = −0.43, r = −0.51).
3.4. Comparison of ten Assays for As bioavailability analysis of available As in soil
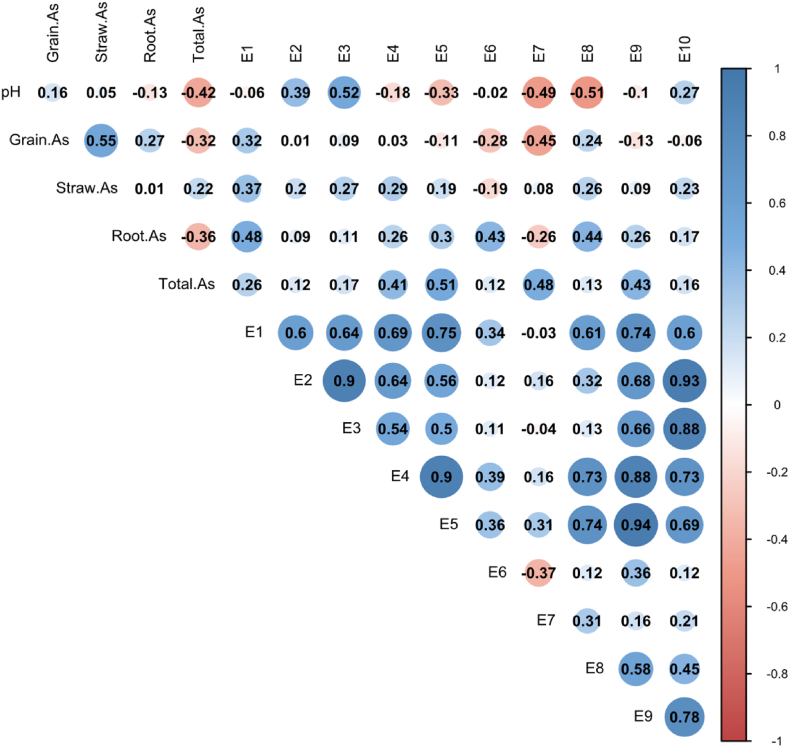
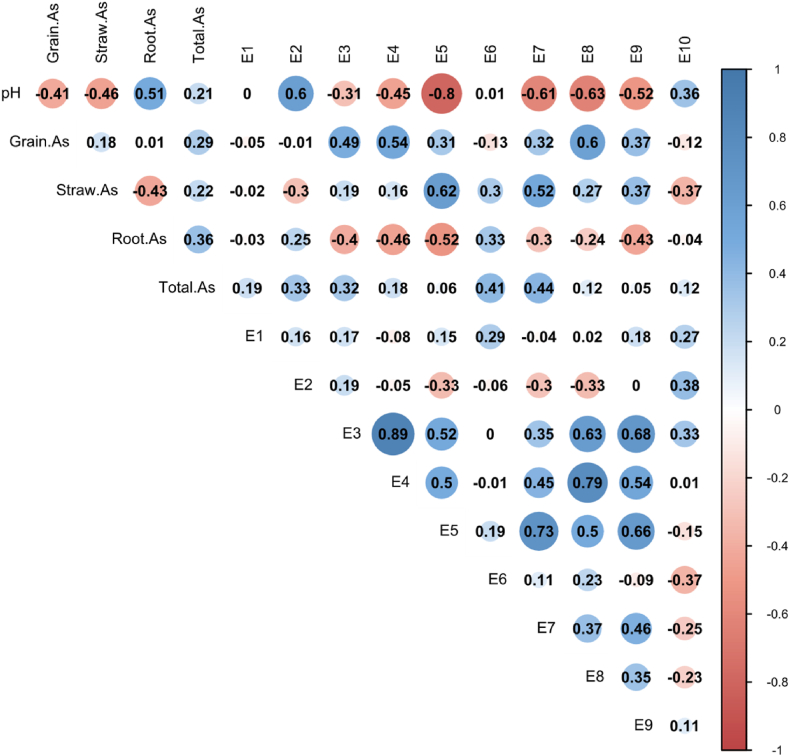
Similarly, As content of GBW10049 and GBW07385 is 0.577 mg/kg and 9.81 mg/kg, respectively, which accords with the quality control sample setting range (0.53 ± 0.11 mg/kg; 9.3 ± 0.8 mg/kg). The grain As content is not significantly correlated with the total As in the soil, however, the As concentration in all extracted states increases with the total As in the soil. Under different soil texture conditions, the As content of each extracted state shows different correlations with grain As (Fig. 4), and the detailed data of As concentration in various tissues and organs of rice can be found in S2.
Fig. 4.
Correlation analysis of pH、Total As、paddy rice As and different extraction states As content in paddy soil. A: sandy soils; B: clay soils. The Person analysis was used to test the results.
In the sandy loam (Yueyang soil), the grain As is not significantly correlated with the extracted arsenic content in all forms, however, there is a positive correlation with E1# and E8# (r1# = 0.32, r8# = 0.24). The soil pH value is significantly positively correlated with E3# (r3# = 0.52, P<0.05) and significantly negatively correlated with E8# (r8# = -0.51, P<0.05, Fig. 4A). The As content extracted by E4# is significantly positively correlated with E1#, E2#, E3#, E5#, E8#, E9#, and E10# to varying degrees, and E8# extracted As content is significantly positively correlated with E1#, E4#, E5#, E9# and E10#.
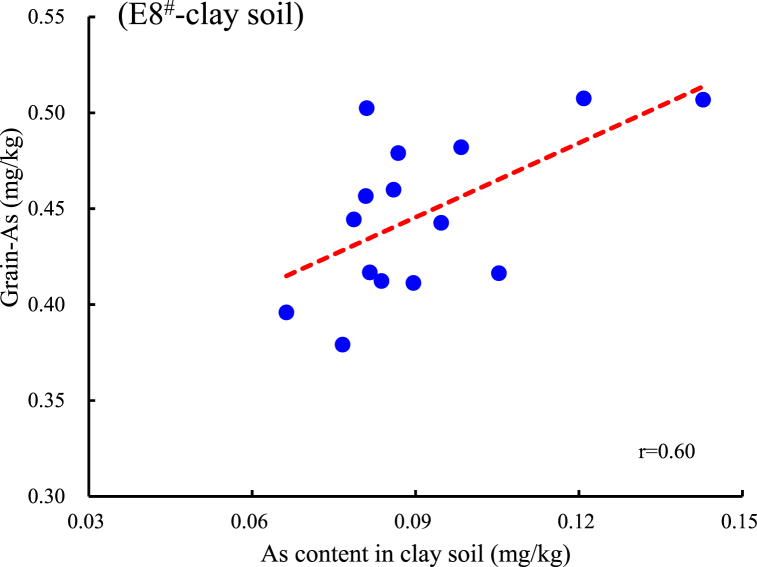
In the clay loam (Yiyang soil), the E4# and E8# extracted As concentration is significantly positively correlated with the grain As (r4# = 0.54, r8# = 0.60, P<0.05). The soil pH value is significantly positively correlated with E2# (r2# = 0.60, P<0.05), and is significantly negatively correlated with E5#, E7#, E8#, and E9# (r5# = -0.80, r7# = -0.61, r8# = -0.63, r9# = -0.52, P<0.05, Fig. 4B). The As concentration extracted by E4# is significantly positively correlated with the E3#, E8#, and E9# extracted As to varying degrees, and the E8# extracted As is significantly positively correlated with the E3# and E4#.
3.5. Analysis on control threshold of extracted Cd and As
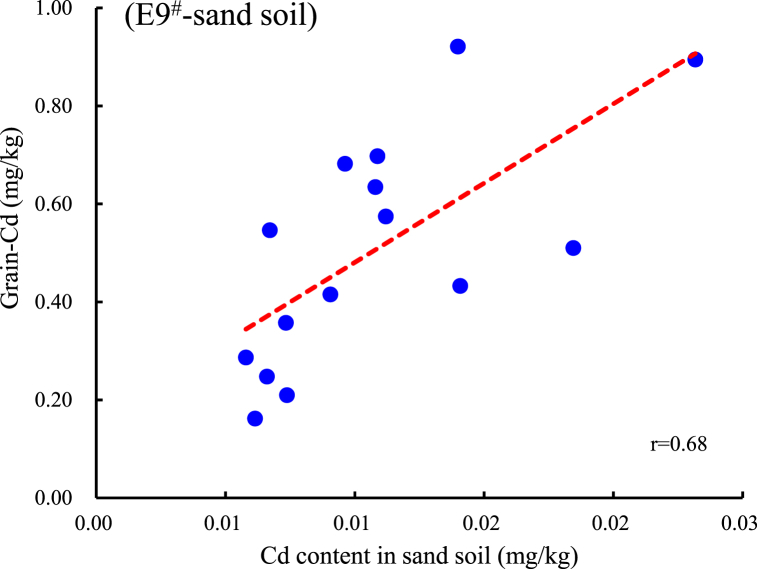
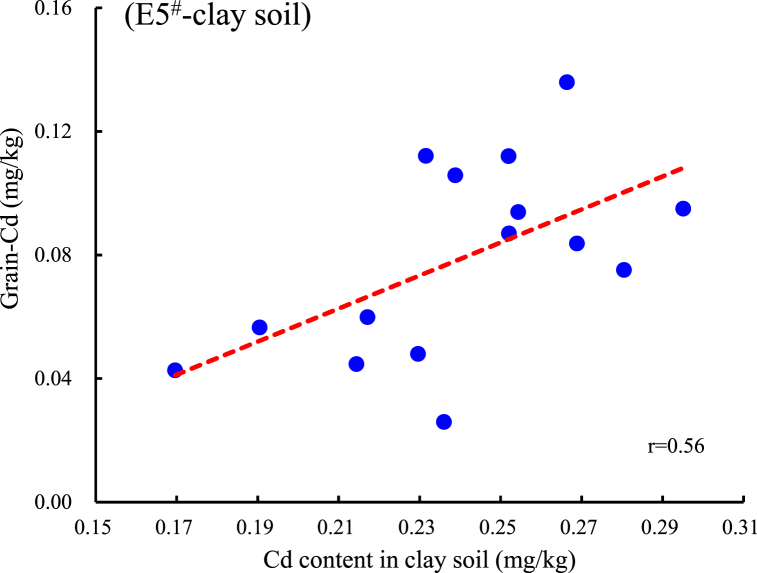
Based on the correlation analysis between different extracted concentration of Cd and As and those in grain, the regression equations for extraction that are significantly correlated with grain Cd and As were statistically calculated (P<0.05, Table 5, Fig. 5A–F). The current national standard (GB2762-2022) sets no limit on total As content in brown rice (only for inorganic arsenic at 0.35 mg/kg), this study refers to the limit for As in cereal crops (0.5 mg/kg) for calculation. The limit for Cd in rice is 0.2 mg/kg [25]. Therefore, using 0.2 mg/kg Cd and 0.5 mg/kg As in brown rice as the critical standards, the corresponding control threshold concentrations for the extracted states were calculated. Table 5 shows that when the Cd extraction value exceeds 0.178 mg/kg by E5# or exceeds 0.00638 mg/kg by E9# in sandy loam, there may be a risk of Cd contamination in the rice grain. When the Cd extraction value by E5# exceeds 0.312 mg/kg or that by the E9# exceeds 0.0695 mg/kg in clay loam, there may be a risk of Cd contamination in the grain. Similarly, when the As extraction value exceeds 0.115 mg/kg by E4# or exceeds 0.106 mg/kg by the E8# in clay loam, there may be a risk of As contamination. There is no significant positive correlation between the As content in rice and the As extracted by E1# and E8# in sandy soil, therefore, the threshold value is not recommended. These two extraction reagents could be used as references for evaluating As activity of sandy soil.
Table 5.
Linear relationship between typical extraction methods and Cd/As contents in rice and the threshold limit value analysis.
| Leaching Agents | sandy loam |
clay loam |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd |
Cd |
As |
||||||||||
| Regression equation | r | p | Threshold limit value (mg/kg) | Regression equation | r | p | Threshold limit value (mg/kg) | Regression equation | r | p | Threshold limit value (mg/kg) | |
| E4# | / | / | / | / | / | / | / | / | y = 0.0782x + 0.0764 | 0.54 | <0.05 | 0.115 |
| E5# | y = 0.1686x + 0.1441 | 0.70 | <0.01 | 0.178 | y = 0.597x + 0.1929 | 0.56 | <0.05 | 0.312 | / | / | / | / |
| E8# | / | / | / | / | / | / | / | / | y = 0.2754× - 0.0317 | 0.60 | <0.05 | 0.106 |
| E9# | y = 0.0144x + 0.0035 | 0.68 | <0.01 | 0.00638 | y = 0.2908x + 0.0113 | 0.59 | <0.05 | 0.0695 | / | / | / | / |
Fig. 5.
Correlation and regression equation analysis of several typical reagents with significant positive correlation between Cd and As in brown rice. A, B: Correlation between Cd content in sand soil extracted by E5# or E9# and grain-Cd; C, D: Correlation between Cd content in clay soil extracted by E5# or E9# and grain-Cd; E, F: Correlation between As content in clay soil extracted by E4# or E8# and grain-As.
4. Discussion
Various forms of heavy metals exist in the soil environment, and different types of extractants exhibit varying extraction efficiencies for Cd and As in soils of different textures. The extraction efficiency of elements is also influenced to some extent by different extraction times and soil-liquid ratios [26,27]. The optimization of extraction methods of available Cd and As in soil and the selection of extractants which can objectively reflect the pollution levels in paddy soil are of great significance to the assessment of farmland pollution risk. This study compared and analyzed the effects of different leaching agents on Cd and As in two types of soil textures (sandy loam and clay loam) under controlled extraction conditions.
For soil Cd activity, the 0.1 mol/L HCl solution (E2#), CaCl2-DTPA solution(E4#), 0.01 mol/L CaCl2(E5#), 0.1 mol/L CaCl2(E6#), and 0.1 mol/L HNO3(E10#) show higher Cd extraction efficiency, with average extraction rates of 69 % and 62 %, respectively (Table 3). Meanwhile, the extraction coefficient of variation is low, and the extraction effect is relatively stable, and the extraction ability of the above extractants for Cd is consistent with previous research results [28,29]. The enhancement of Cd activity of soil by dilute acid has been widely reported [[30], [31], [32]]: 1) dilute acid solutions act as strong substitution agents with a high capacity to dissolve heavy metals in soil. Hydrogen ions in the environment promoted the dissolution of Cd2+ and improved the activity of Cd in soil. 2) a large amount of H+ destroy the lattice structure of the soil, corrodes other substances other than silicates, and a large number of bioactive and non-bioactive Cd ions in the soil are released. 3) chloride ions in dilute acids an activating effect on Cd2+ in the soil, Cl− forms a complex with Cd2+, moving Cd2+ from the solid phase to the liquid phase, thus increasing the Cd concentrations in the soil solution. 4) dilute acid promotes the release of soil organic matter, the soil macromolecular organic matter functional groups bind with Cd2+ through complexation, thus reducing the soil's adsorption of metal elements.
As a neutral strong chelating agent, DTPA has strong chelating ability to Cd2+. DTPA salt exchanges with Cd2+, promoting the release of soil Cd2+ and increasing the extraction efficiency of bioavailable Cd. Simultaneously, DTPA salt could reduce the dissolution of carbonate in the soil, thereby decreasing the displacement of Ca2+ by other metal ions [33], which results in a decrease in the leaching of non-active Cd. Indeed, the results of this study indicated that as a neutral salt extractant, CaCl2 mainly displaces exchangeable Cd in the soil according to ion exchange. Act as a neutral salt extractant with similar chemical properties, the extraction capacity of 3# (NH4OAc), 4# (DTPA), 5# (CaCl2), and 9# (NaNO3) exhibited a strong positive correlation (Fig. 3).
In comparison to the extraction efficiency of soil-available Cd, the extraction rate of soil-available As is lower. The extraction rate of soil-available As for both types of test soil is highest with 0.5 mol/L NaH2PO4 (E7#) and 0.1 mol/L HNO3 (E10#) or 0.1 mol/L HCl (E2#) (Table 4). Anionic exchange was the main chemical mechanism for phosphate to extract arsenate. 1) Phosphorus, as the same main group element of As, has similar chemical properties, which competes with As for adsorption sites in soil colloids. 2) H2PO4− significantly reduces the adsorption capacity of soil colloids for As. Compared with AsO43−, phosphate can be strongly adsorbed and fixed by iron oxide, alumina and calcium oxide in acidic soil. 3) Compared with sulfate, chloride, nitrate, and carbonate anions, phosphate shows an order of magnitude advantage in the extraction efficiency of arsenic. The adsorption selectivity of soil phosphoric acid is higher than that of arsenate, so phosphoric acid can effectively extract the arsenate adsorbed by soil. Some studies shows that dilute acid solution can be used as an effective As extractor for acidic soils, and NaHCO3 is better for alkaline soils [34,35]. In this study, dilute HCl and dilute HNO3 are extractants second only to NaH2PO4, both with good extraction effect stability. As a monadic strong acid, similar chemical properties make the extraction capacity of hydrochloric acid and nitric acid significantly positive correlation (Fig. 4A).
Obviously, the objective and scientific selection of extractants for the soil available element requires a comprehensive consideration of their extraction capacity and the correlation with biological accumulation [36]. It's worth noting that there is no correlation between the rice Cd and the total soil Cd, nor with strong extractants like 0.1 mol/L HCl (E2#) and 0.1 mol/L HNO3 (E10#, Fig. 3). Homoplastically, there is no correlation between rice As and the total amount of soil, nor with the three extractants with higher As extraction efficiency (E7#, E10#, E2#) in test two types field soils (Fig. 4). This phenomenon prompts us to consider whether there is a certain balance between Cd levels in crops and the extraction efficiency of extractants or the total amount in soil. Under the destruction of the soil lattice by strong acidity, strong chelating ability, or the substitution of phosphoric acid with the main group, some non-biological active heavy metal ions were excessively released. And the less active Cd or As in the soil has a weak migration ability and can't be absorbed and utilized by plants. Thus, the concentration of heavy metals absorbed and accumulated by plants doesn't have a direct relationship with the total amount or high extraction states in soil. Rather, it depends on specific extraction agents and methods. To test this hypothesis, the concentration of Cd and As in rice was compared with that in neutral salt extraction. Our belief is that the low concentration of neutral salt and bicarbonate extracted Cd/As plays an important role in the accumulation about Cd and As in grain.
Our study revealed several important findings that supported our hypothesis. Firstly, in sandy loam soil, 0.01 mol/L CaCl2 (E5#) and 0.1 mol/L NaNO3 (E9#) extracted Cd concentrations are significantly positively correlated with Cd in rice, straws, and roots (Fig. 3, Fig. 5A,B), and two extractants also show a significant positive correlation with grain Cd in clay loam soil (Fig. 3, Fig. 5C,D). Secondly, in clay loam soil, grain As is significantly positively correlated with CaCl2-DTPA (E4#) and 0.05 mol/L NaHCO3 (E8#) extracted states (Fig. 4, Fig. 5E,F).
Some studies indicate that CaCl2-DTPA reagent is primarily used to extract available ions such as Cd, lead, iron, copper, zinc, and nickel from neutral soils, showing a good correlation with metal content in crops [11,37]. Based on the results of this experiment, which suggesting that: 1) the main forms of Cd absorbed by plants are water-soluble and exchangeable states, consistent with the components extracted by the neutral salt solution CaCl2, while the Cd extracted by dilute acid solutions includes some non-bioavailable forms. 2) The composition of Cd forms in naturally contaminated soils is complex, with varying capacities for metal adsorption across different soil types. In general, sandy loam soils with a higher proportion of sand grains have larger gaps between soil particles and weaker adsorption buffering capacity than clay loam soils [38]. 3) In acidic soils, the hydroxyl groups in DTPA displace the biologically active free arsenate radicals to form complexes with DTPA. At the same time, a certain concentration of HCO3− can displace arsenate from the fixed matrix surface of soil As [39], and the As be displaced may be highly bioactive. Although the extraction efficiency of the above two extractants is relatively low, the extracted As concentration from acid clay loam is positively correlated with grain As, effectively reflecting the level of bioavailable As in soil.
In addition, according to the pollution limits of Cd and As concentrations in rice, the critical thresholds for extraction of effective extractants in two kinds of soil were analyzed. The 0.01 mol/L CaCl2 (E5#) extraction method is suitable for assessing the safety risks of typical cadmium-contaminated acidic paddy soils. With a limit value of 0.2 mg/kg for brown rice Cd concentration, the critical threshold for CaCl2 extracted Cd in sandy loam soil is 0.178 mg/kg, and for clay loam soil, it is 0.312 mg/kg. These experimental results are consistent with previous studies, although some research data slightly higher than ours [29]. This may be due to differences in metal adsorption caused by different pH levels and soil textures. Meanwhile, it is worth paying attention to, based on the extraction efficiency of each extraction agent, the corresponding total soil Cd levels are 0.422 mg/kg and 0.667 mg/kg respectively. According to the “Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land (Trial)" (GB 15618-2018), both levels indicate a risk of pollution. Obviously, the safe utilization of Cd in these soils is in a critical state, rather than a risk state. There is also a correlation coefficient between total soil Cd content and grain Cd content lower than those for the two types of available Cd. Similarly, CaCl2-DPTA (E4#) and 0.05 mol/L NaHCO3 (E8#) extraction methods are suitable for assessing the safety risk of typical arsenic-contaminated paddy clay loam soils. With a limit value of 0.5 mg/kg for brown rice As concentration, the critical thresholds for CaCl2-DPTA and NaHCO3 extracted As in clay loam soil are 0.115 mg/kg and 0.106 mg/kg, respectively (Table 5). Based on the extraction efficiency of each extraction agent, the total soil As contents range from 38.6 to 42.5 mg/kg, which belongs to exist the pollution risk, but doesn't reach the risk control standard (Fig. 1). Likewise, based on the grain As, the safe utilization of As in these soils is in a critical state, rather than a risk state. The correlation coefficient between total soil As and grain As also lower than those for the two types of available As. Hence, assessing the levels of available elements, rather than total heavy metal content, provides a more scientific and objective evaluation of the environmental risks to food safety posed by Cd and As pollution in paddy fields.
The limitations of the present study include: 1) The experiments were conducted on only two soil types, warranting further investigation into the bioavailability of Cd and As in soil with varying textures and pH values. 2)The critical thresholds for soil in this study await further collection and analysis of samples from various types, textures, and pollution degree to assess soil risk more scientifically and accurately.
5. Conclusion
In conclusion, our study suggests that evaluating the abundance of available metals in soil using scientific analysis methods is important for the safe production of crops in Cd-As contaminated farmland. In two types of soils with combined Cd and As contamination, four extractants including HCl, CaCl2, HNO3, and CaCl2-DTPA showed high Cd extraction efficiency and stability, with extraction rates from 42.2 to 88.4 %. Three extractants including 0.5 mol/L NaH2PO4, HCl, HNO3 showed high As extraction efficiency, with an extraction rate of 0.85–23.4 %. The concentrations of Cd and As in rice showed no significant correlation with the total amounts in the soil. The Cd extracted by 0.01 mol/L CaCl2 and 0.1 mol/L NaNO3 showed significant correlations with grain Cd, with CaCl2 demonstrating significantly better extraction efficiency and lower coefficient of variation. The critical threshold of extraction by CaCl2 risk may be 0.178 mg/kg in sandy loam soil (5.2 < pH < 6.2) and 0.312 mg/kg in clay loam soil (4.7 < pH < 5.6). The extractable As content in clay loam by CaCl2-DTPA and 0.05 mol/L NaHCO3 showed a significant correlation and stability with the As content in rice. The risk extraction threshold values are 0.115 mg/kg for the former and 0.106 mg/kg for the latter.
CRediT authorship contribution statement
Di Guan: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition, Formal analysis, Data curation. Xionghui Ji: Project administration, Funding acquisition. Saihua Liu: Visualization. Shan Chen: Formal analysis. Yunhe Xie: Formal analysis. Jiamei Wu: Writing – review & editing, Visualization.
Data availability statement
All the relevant data are included in the manuscript and the supplementary document.
Funding
This work was supported by the National and Regional Joint Fund (U21A20291), the National Key Research and Development Program of China (Grant No.2022YFD1700105, 2022YFD1700103) and the Agricultural Science and Technology Innovation Fund Project of Hunan Province, China (2024CX130).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the support from the Key Laboratory of Agro-Environment in Midstream of Yangtze Plain, Ministry of Agriculture, P. R. China and the Key Laboratory of Prevention, Control and Remediation of Soil Heavy Metal Pollution in Hunan Province.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40910.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Jiang Y., Yi X.T., Liu M.Y., Liu B.B., Zhou H., Zeng P., Liao B.H., Gu J.F. Dynamic responses of soil enzymes at key growth stages in rice after the in-situ remediation of paddy soil contaminated with cadmium and arsenic. Sci. Total Environ. 2022;830 doi: 10.1016/j.scitotenv.2022.154633. [DOI] [PubMed] [Google Scholar]
- 2.Guarín D., Lopez J.M.M., Libohova Z.L., Bolanos J.B., Maximova S.N., Guiltinan M.J., Sipargo J., Silva M.D., Fernandez A., Drohan P. Accumulation of cadmium in soils, litter and leaves in cacao farms in the North Sierra Nevada de Santa Marta, Colombia. Geoderma Region. 2024;36 doi: 10.1016/j.geodrs.2024.e00762. [DOI] [Google Scholar]
- 3.Roy M., McDonald L.M. Metal uptake in plants and health risk assessments in metal contaminated smelter soils. Land Degrad. Dev. 2015;26(8):785–792. doi: 10.1002/ldr.2237. [DOI] [Google Scholar]
- 4.Iwuala E., Olajide O., Abiodun I., Odjegba V., Utoblo O., Ajewole T., Oluwajobi A., Uzochukwu S., Uzochukwu S. Silicon ameliorates cadmium (Cd) toxicity in pearl millet by inducing antioxidant defense system. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong X.L., Lian W.L., Tian S., Yu Q.Y., Guo Z.L., Zhang X., Hu C., Yuan Y., Fan Y.Q., Liu Z.W., Zheng J.F., Bian R.J., Li L.Q., Pan G.X. Utilizing ragweed and oyster shell derived biochar as an effective stabilizer for the restoring Cd and Pb-contaminated soil. Geoderma Region. 2024;37 doi: 10.1016/j.geodrs.2024.e00762. [DOI] [Google Scholar]
- 6.China M. Ministry of Environmental Protection and Ministry of Land and Resources of the People’s Republic of China; Beijing, China: 2014. The Ministry of Land and Resources Report on the National Soil Contamination Survey. [Google Scholar]
- 7.Zhao F.J., Ma Y., Zhu Y.G., Tang Z., McGrath S.P. Soil contamination in China: current status and mitigation strategies. Environ. Sci. Technol. 2015;49(2):750–759. doi: 10.1021/es5047099. [DOI] [PubMed] [Google Scholar]
- 8.Sui F.Q., Chang J.D., Tang Z., Liu W.J., Huang X.Y., Zhao F.J. Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil. 2018;433:377–389. doi: 10.1007/s11104-018-3849-5. [DOI] [Google Scholar]
- 9.Guan D., Wu J.M., Xie Y.H., Huang X., Ji X.H. Double prevention of cadmium uptake by iron and zinc in rice seedling - a hypotonic study. J. Soil Sci. Plant Nutr. 2024;24:318–330. doi: 10.1007/s42729-023-01528-5. [DOI] [Google Scholar]
- 10.Wang P., Chen H.P., Kopittke P.M., Zhao F.J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019;249:1038–1048. doi: 10.1016/j.envpol.2019.03.063. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Zhang R.C., Pan B., Qiu H., Wang J., Zhang J.Y., Niu X.K., He L.P., Qian W.M., Peijnenburg W.J.G.M. Uptake of heavy metals by crops near a mining field: pathways from roots and leaves. Chemosphere. 2023;322 doi: 10.1016/j.chemosphere.2023.138215. [DOI] [PubMed] [Google Scholar]
- 12.Tang X.J., Shen H.R., Chen M., Yang X., Yang X., Yang D., Wang F., Chen Z.Z., Liu X.M., Wang H.L., Xu J.M. Achieving the safe use of Cd- and As-contaminated agricultural land with an Fe-based biochar: a field study. Sci. Total Environ. 2020;706 doi: 10.1016/j.scitotenv.2019.135898. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y.L., Zeng H.W., Zhou H.L., Zhang S.J., Tie B.Q., Zeng Q.R., Chen A.W., Luo S. Assessing the influence of contaminated rice straw decomposition on the speciation of cadmium and arsenic in a naturally contaminated soil. J. Soils Sediments. 2023;23:1415–1427. doi: 10.1007/s11368-022-03409-3. [DOI] [Google Scholar]
- 14.Wang Y.M., Wang S.W., Wang C.Q., Zhang Z.Y., Zhang J.Q., Meng M., Li M., Uchimiya M., Yuan X.Y. Simultaneous immobilization of soil Cd(Ⅱ) and As(Ⅴ) by Fe-modified biochar. Int. J. Environ. Res. Publ. Health. 2020;17(3):827. doi: 10.3390/ijerph17030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan D., Wu J.M., Xie Y.H., Chen S., Chen J., Peng H., Ji X.H. Effects of iron-based silicon salts on fraction and transformation of cadmium and arsenic in soil environment. China Environ. Sci. 2022;42(4):1803–1811. http://www.zghjkx.com.cn/CN/Y2022/V42/I4/1803 [Google Scholar]
- 16.Voigt D.E., Brantley S.L., Hennet R.J.C. Chemical fixation of arsenic in contaminated soils. Appl. Geochem. 1996;11:633–643. doi: 10.1016/S0883-2927(96)00009-1. [DOI] [Google Scholar]
- 17.Ali W., Mao K., Zhang H., Junaid M., Xu N., Rasool A., Feng X.B., Yang Z.G. Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J. Hazard Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122720. [DOI] [PubMed] [Google Scholar]
- 18.Woolson E.A., Axley J.H., Kearney P.C. Correlation between available soil arsenic, estimated by six methods, and response of corn (Zea mays L.) Soil Sci. Soc. Am. J. 1971;35(1):101–105. doi: 10.2136/sssaj1971.03615995003500010030x. [DOI] [Google Scholar]
- 19.Peryea F.J. Evaluation of five soil tests for predicting responses of apple trees planted in lead arsenate-contaminated soil. Commun. Soil Sci. Plant Anal. 2002;33:243–257. doi: 10.1081/CSS-120002391. [DOI] [Google Scholar]
- 20.Huang R.Q., Wang G., Tang R.Y., Liao S.Q., Chen Y.H. Extraction method for available arsenic in acid soils. Journal of Agro-Environment Science. 2005;24(3):10–615. [Google Scholar]
- 21.Zhu Q.H., Huang D.Y., Liu S.L., Luo Z.C., Zhu H.H., Zhou B., Lei M., Rao Z.X., Cao X.L. Assessment of single extraction methods for evaluation the immobilization effect of amendments on cadmium in contaminated acidic paddy soil. Plant Soil Environ. 2012;58(2):98–103. doi: 10.17221/358/2011-PSE. [DOI] [Google Scholar]
- 22.Gupta A.K., Sinha S. Assessment of single extraction methods for the prediction of bioavailability of metals to Brassica juncea L. Czern. (var. Vaibhav) grown on tannery waste contaminated soil. J. Hazard Mater. 2007;149(1):144–150. doi: 10.1016/j.jhazmat.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 23.Desrosiers M., Gagnon C., Masson S., Martel L., Babut M.P. Relationships among total recoverable and reactive metals and metalloid in St. Lawrence River sediment: bioaccumulation by chironomids and implications for ecological risk assessment. Sci. Total Environ. 2008;389(1):101–114. doi: 10.1016/j.scitotenv.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y.Y., Zhang Z.Y., Liang H.T., Gu M.H., Shen F.K., Shohag M.J.I., Li X.F. In vivo-in vitro correlations for the assessment of cadmium bioavailabity in vegetables. J. Agric. Food Chem. 2021;69(41):12295–12304. doi: 10.1021/acs.jafc.1c03284. [DOI] [PubMed] [Google Scholar]
- 25.GB 2762-2022, Food Safety National Standard and Food Pollutant Limits [S].
- 26.Wang S.L., Nan Z.R., Liu X.W., Zhang G.Z., Zhao Z.J. Availability and speciation of Cu, Zn, and Pb added to irrigated desert soil. Pol. J. Environ. Stud. 2010;19(4):865–869. doi: 10.1017/S0032247409008626. [DOI] [Google Scholar]
- 27.Gil D.M., Luchsinger H.A., Garcia G.P., Alonso J., Lobo M.C. Selecting efficient methodologies for estimation of as and Hg availability in a brownfield. Environ. Pollut. 2021;270 doi: 10.1016/j.envpol.2020.116290. [DOI] [PubMed] [Google Scholar]
- 28.Bakircioglu D., Kurtulus Y.B., Ibar H. Comparison of extraction procedures for assessing soil metal bioavailability of to wheat grains. Clean. 2011;39(8):728–734. doi: 10.1002/clen.201000501. [DOI] [Google Scholar]
- 29.Li J.H., Nie D.T., Liu M.N., Mao X.Y., Liao Z.W., Chen X. Comparison of Cd bioavailability determination methods and the risk control value of Cd for typical Cd contaminated paddy soils in Guangdong. J. Agric. Resour. Econ. 2021;38(6):1094–1101. doi: 10.13254/j.jare.2021.0549. 2021. [DOI] [Google Scholar]
- 30.Zhong S.X., Fang L.P., Li X.M., Liu T.X., Wang P., Gao R.C., Chen G.J., Yin H.M., Yang Y., Huang F., Li F.B. Roles of chloride and sulfate ions in controlling cadmium transport in soil-rice system as evidenced by the Cd isotope fingerprint. Environ. Sci. Technol. 2023;57(46):17920–17929. doi: 10.1021/acs.est.3c04132. [DOI] [PubMed] [Google Scholar]
- 31.Guo J.X., Chen M.F., Huang Y.X., Xie S.C., Zhang X.H., Zuo T.T., Hu C., Wang G. Chloride application weakens cadmium immobilization by lime in paddy rice soil. Ecotoxicol. Environ. Saf. 2022;241 doi: 10.1016/j.ecoenv.2022.113761. [DOI] [PubMed] [Google Scholar]
- 32.Nystrand M.I., Osterholm P. Metal species in a boreal river system affected by acid sulfate soils. Appl. Geochem. 2013;31:133–141. doi: 10.1016/j.apgeochem.2012.12.015. [DOI] [Google Scholar]
- 33.Wu J.H., Song Q.M., Zhou J.Y., Wu Y.X., Liu X.W., Liu J.J., Zhou L.L., Wu Z.H., Wu W.C. Cadmium threshold for acidic and multi-metal contaminated soil according to Oryza sativa L. cadmium accumulation: influential factors and prediction model. Ecotoxicol. Environ. Saf. 2021;208 doi: 10.1016/j.ecoenv.2020.111420. [DOI] [PubMed] [Google Scholar]
- 34.Woolson E.A., Axley J.H., Kearney P.C. Correlation between available soil arsenic, estimated by six methods, and response of corn (Zea mays L.) Soil Sci. Soc. Am. J. 1971;35(1):101. doi: 10.2136/sssaj1971.03615995003500010030x. [DOI] [Google Scholar]
- 35.Alam M.G.M., Tokunaga S., Maekawa T. Extraction of arsenic in a synthetic arsenic-contaminated soil using phosphate. Chemosphere. 2001;43:1035–1041. doi: 10.1016/S0045-6535(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Liu H.Y., Liu N., Jiang Z.M., Wei S.Q. Screening and evaluation of the methods for the determination of the available lead (Pb) and cadmium (Cd) in farmland Soil. J. Environ. Sci. (China) 2021;42(7):3494–3506. doi: 10.13227/j.hjkx.202012011. [DOI] [PubMed] [Google Scholar]
- 37.Lu H.L., Li K.W., Nkoh J.N., Shi Y.X.X., He X., Hong Z.N., Xu R.K. Effects of the increases in soil pH and Ph buffering capacity induced by crop residue biochars on available Cd contents in acidic paddy soils. Chemosphere. 2022;301 doi: 10.1016/j.chemosphere.2022.134674. [DOI] [PubMed] [Google Scholar]
- 38.Zanutel M., Bielders C.L. Contrasted effects of biochar application on interrill erosion depending on age, application rate and soil type. Geoderma Reg. 2023;34 doi: 10.1016/j.geodrs.2023.e00706. [DOI] [Google Scholar]
- 39.Frau F., Addari D., Atzei D., Biddau R., Cidu R., Rossi A. Influence of major anions on As(Ⅴ) adsorption by synthetic 2-line ferrihydrite. Kinetic investigation and XPS study of the competitive effect of bicarbonate. Water Air Soil Pollut. 2010;205(1–4):25–41. doi: 10.1007/s11270-009-0054-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the relevant data are included in the manuscript and the supplementary document.