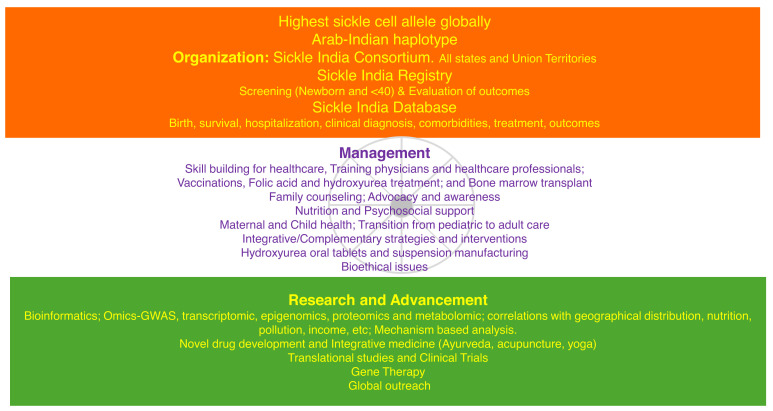
Visual Abstract
Abstract
India, the most populous nation in the world, also has a high frequency of the sickle hemoglobin (HbS) allele globally. The Arab Indian HbS haplotype in India is characterized by a relatively high percentage of fetal Hb, with widely varying frequencies of α-thalassemia. Hence, sickle cell disease (SCD) in India was perceived to be mild. Advances in the past decade in screening and SCD management have revealed that the severity of SCD in India is comparable to many other parts of the world. Clinical features in India include vaso-occlusive crisis, acute chest syndrome, avascular necrosis, renal involvement, stroke, etc, at a relatively young age. Once a fatal disease of childhood, the majority of patients born with SCD are expected to survive into adulthood, largely because of improvements in comprehensive care programs including newborn screening, penicillin prophylaxis, transcranial Doppler, and hydroxyurea therapy. Several centers are performing hematopoietic stem cell transplants successfully for SCD. To address the urgent need to control and manage SCD in India's population, the Government of India launched the National Sickle Cell Anaemia Elimination Mission, with significant funding for large-scale measures to screen, treat, counsel, educate, and develop technologies and novel therapies and gene therapies.
Learning Objectives
Summarize the current status of SCD in India, which has a high percentage of fetal hemoglobin accompanied by mild to severe complications
Discuss the milestones and progress in understanding the SCD phenotype, accelerated by the National Sickle Cell Anaemia Elimination Mission 2023
CLINICAL CASE
A 16-year-old girl with HbSS (sickle homozygous) receiving comprehensive care since diagnosis at newborn screening (NBS) had a persistent HbF level of 21.8%. Hydroxyurea (HU) was started at the age of 10 years, and the dose was escalated to 20 mg/kg. Transcranial Doppler was normal. She presented with fever and respiratory distress with hypoxia, with pulse oximetry revealing an oxygen saturation of 92%; hemoglobin [Hb], 7.9 g/dL; a white blood cell count, 18 200; a platelet count of 72.00; bilateral lung infiltrates on chest x-ray; and negative blood cultures. She received oxygen, a packed red cell transfusion, and antibiotics. On day 5, the patient developed left hemiparesis, and magnetic resonance imaging of the brain revealed acute infarcts in the left parietal lobe. She developed bilateral lower-extremity deep vein thromboses, which were managed with anticoagulants. The patient was discharged without residual neurological damage. She continues to be followed up in the sickle cell clinic.
Introduction
Sickle cell disease (SCD) is a global disorder, with the highest number of births in Nigeria and the Democratic Republic of the Congo, followed by India, with the third-highest number of births with HbSS disease.1-3 Sickle cell trait (SCT) was first described in the aboriginal communities of Southern India, and the focus was on the anthropological investigation of potential ancient migrations between India and Africa (Figure 1). The severity of the sickle cell phenotype and disease prevalence in India were underestimated.4,5 Because of the high percentage of HbF in persons with SCD in India, the disease was mistakenly considered mild.6,7 We trace its progress from these uncertain beginnings to the current status of SCD prevalence, morbidity, mortality, management, and the recent impressive efforts of the Government of India (GOI) to screen, manage, and control SCD through its National Sickle Cell Anaemia Elimination Mission (NSCAEM) 2023 (Figures 2 and 3).8,9 These developments are driven and sustained by the federal and state governments and partnership with for-profit and nonprofit institutions, industry, and academia to mitigate the suffering from this debilitating condition in India. The progress in SCD care in India has the potential to positively impact other low- and middle-income countries globally.
Figure 1.
Historic milestones in the detection and progress of SCD in India. AS, heterozygous; CSIR, Council of Scientific and Industrial Research; HSCT, hematopoietic stem cell transplantation.
Figure 2.
Various components of the Sickle Cell Management Program of the NSCAEM 2023. Adapted with permission from the Ministry of Health and Family Welfare9 and the Ministry of Tribal Affairs, GOI, Government of India; POC, point of care.
Figure 3.
Consort diagram showing the well-organized Sickle Cell Management Program of the NSCAEM 2023. AB-HWC, Ayushman Bharat-Health and Wellness Center; CZE, capillary zone electrophoresis; HPLC, high-performance liquid chromatography; IEF, isoelectric focusing. Adapted with permission from the Ministry of Health and Family Welfare.9
History of SCD and diversity of the βS-globin gene haplotypes in India (Figure 1)
In 1952, Lehmann and Cutbush first reported the presence of sickle cell trait in the aboriginal communities of Southern India.10 Dunlop and Mazumder reported the presence of HbS in migrant tribal workers in the eastern states of Bihar and Odisha and in the tea gardens of Assam in the Northeast.11 Findings of the βS-globin (βS) trait formed the basis of a hypothesized population exchange between India and Africa and the milder phenotype. Subsequently, it was reported that SCD also affected nonaboriginal populations,12 with significant morbidity and mortality despite high HbF amid the heterogenous presentation of SCD in the culturally and geographically diverse landscape of India (Figures 4 and 5).5,13-16 It is not clear if the origin of the βS mutation in India is related to migration from Africa or the Middle East or represents an independently arising mutation in areas where malaria is endemic in India.17
Figure 4.
Map of India showing states with a high and partial prevalence of SCD. Reproduced with permission from Prevention, management and elimination of sickle cell disease: a strategic road map, Ministry of Tribal Affairs, GOI, Government of India.
Figure 5.
Regional distribution of SCD among the tribal population of India. Reproduced with permission from the Ministry of Health and Family Welfare.9 AA, without sickle; AS, heterozygous/trait.
In late 20th century, studies first documented the severity of the clinical phenotype of SCD in India. Kar et al reported that the sickle cell gene was widespread and presented with moderate to severe anemia, vaso-occlusive episodes (86.5%-89.36%), splenic sequestration (8.43%-12.76%), crippling avascular bone necrosis (5.7%-35.08%), and osteomyelitis (5%).18-25 Jain et al in Nagpur observed that a significant proportion of children with SCD from central India presented with severe symptoms and required regular medical attention.5,13-16,26-36
The major β-globin haplotype in India is the Arab Indian, but other haplotypes also exist in lower proportions. In a newborn cohort in Nagpur, out of the 150 infants with HbSS, 141 (94.0%) were linked to the AI haplotype.28 Similar genotypic prevalences were reported in a cohort of 100 subjects from Chhattisgarh in central India.29
Since SCD was most prevalent in aboriginal (scheduled tribes) and disadvantaged communities, it is likely that many remained undiagnosed and/or did not survive because of lack of access to care, which may have led to the poor understanding of the disease phenotype. Recent studies have revealed that SCD is not confined to these communities.
Early studies of disease phenotype following NBS
NBS was initiated in early 2000 and revealed the high frequency of sickle cell mutation in central India.29,33,37-42 Since then the NBS cohort has been followed by the Government Medical College, Nagpur, with comprehensive multidisciplinary care including pneumococcal prophylaxis and immunization, HU, and screening for cerebral blood flow velocity proteinuria, retinopathy, avascular necrosis and cholelithiasis. Of 2178 newborns screened with a mother positive for βS (targeted NBS), 104 were sickle homozygous (SS), 7 sickle β-thalassemia, 5 sickle DPunjab, and 1978 SCT.28 Pain, severe anemia requiring blood transfusions, and acute febrile illness were the major complications with 59.7, 45.1, and 42.6 cases/100 person-years.29 Some of the barriers to follow-up included low literacy and seasonal migration for work.
Comparison of SCD cohorts in India with clinical phenotypes in other parts of the world
Generalizing the clinical features of SCD in India may be challenged by the tremendous genetic, cultural, socioeconomic, and geographical diversity of India and limited access to care. We conducted a single-center study in Nagpur in a large cohort of 833 children to examine the rate of complications and define the phenotype of SCD in India and to determine whether clinical phenotype was actually milder in aboriginal tribes.15 We compared the rate of complications per 100 person-years for 1954 person-years to those reported by the Cooperative Study of Sickle Cell Disease.15 The rates of vaso-occlusive crises, severe anemia, stroke, splenic sequestration, and meningitis were more frequent in Nagpur compared to the Cooperative Study of Sickle Cell Disease. Notably, non-aboriginals presented with more frequent complications compared to aboriginal tribal populations.
We also compared the disease phenotype in the long term follow-up of children in Nagpur with Jamaica.16 Compared to the Jamaican cohort, the Nagpur cohort had lesser α-thalassemia; higher HbF, hypersplenism, and persistent splenomegaly; and lesser dactylitis (Table 1). There were also high admission rates for febrile illness and marked anemia in the Nagpur cohort. Invasive pneumococcal disease occurred in 10% of Jamaicans but was not seen in Nagpur, perhaps because the Nagpur cohort received a pneumococcal vaccine and penicillin prophylaxis from birth until 5 years of age.
Table 1.
Comparison of HbF between Jamaican and Central Indian cohorts of persons with SCD
| Untransformed percentage mean HbF | Transformed mean difference, 95% CI | P | ||
|---|---|---|---|---|
| Age in years | Jamaica | Nagpur Central India | ||
| 1 year | 15.5 | 25.2 | 2.51, 2.35-2.68 | <0.001 |
| 2 years | 12.1 | 22.5 | 2.58, 2.42-2.73 | <0.01 |
| 3 years | 11.2 | 22 | 0.89, 0.18-1.60 | <0.05 |
Adapted with permission from Jain et al.16
Despite high HbF levels, there is a prevalence of the severe SCD phenotype in India, but to the best of our knowledge, no data are available to explain this. We speculate that in addition to HbF other genetic and epigenetic factors—environmental factors such as social determinants of health, health care availability/ utilization, concomitant infections and infestations, nutrition, environmental exposure, maternal health, etc, and biological factors such as hemolysis, hydration, and coexistence with other diseases may modulate disease severity. There is an urgent need to unravel the paradox of both very high HbF and mild disease and severe disease in the presence of high HbF.
Malaria is endemic in many regions of India, particularly those with large SCD cohorts, and may contribute to disease severity. While sickle cell trait is known be protective against malaria, individuals with SCD may have more severe manifestations of malaria. High mortality has been reported in patients with HbSS with Plasmodium falciparum malaria.43 Further clinical and epidemiological studies are indicated to determine the effect of malaria on the morbidity and mortality of SCD in India. Preliminary data show a high rate of mortality and serious complications among patients with SS, with those contracting P. falciparum developing fatal outcomes and serious complications.
Current status of comprehensive care
Prevention of pneumococcal infections
Immunization against Streptococcus pneumoniae and Haemophilus influenzae infections has improved survival and decreased the morbidity associated with invasive pneumococcal disease and H. influenzae.
Prenatal screening for SCD
Prenatal screening for SCD using chorionic villus sampling and amniocentesis is available at GOI's expense. However, utilization of these services may be impacted by limited access to care in geographically remote populations.
Social support and special protection
Thalassemia, hemophilia, and SCD are the 3 hematological disorders included in the Rights of Persons with Disabilities (RPWD) Act of 2016. Those with 40% and above disability are entitled to accommodation at school and work, including time flexibility, extra leave for medical care, relaxation in attendance requirements, extra time for timed exams, financial assistance for medical expenses, and consideration of employment in proximity to comprehensive care. The RPWD act also mandates nondiscrimination, the right to equality, and life with dignity and respect and provides incentives to employers to employ individuals with a disability. The act also establishes preferences in admission to courses of higher education.
Disease-modifying therapies
Hydroxyurea
Hydroxyurea (HU) has been used in patients with SCD in India since 2008.34-36,44-48 HU is manufactured in India by several pharmaceutical companies and is readily available there. HU is dispensed free of charge through the government health system and is commercially available at a very low cost for a 200- or 500-mg/tablet or capsule. A 500-mg dose costs from approximately Rs 2.5 to Rs 15 (equivalent to US $0.03 to $0.18). An oral liquid formulation of HU that is stable at room temperature is being manufactured in India at a sale price of Rs 600 (USD $7.18)/100 mL. This formulation offers a major benefit for treating children in remote areas in the tropical climates of India and Africa.
HU has been shown to be effective in decreasing painful episodes, blood transfusion requirements, and rates of hospitalization. Initially, a low fixed dose of HU at 10 mg/kg/d was started in persons with severe SCD. Several studies from different regions in India found that HU treatment from 6 months to 24 months led to a statistically significant increase in total Hb, HbF, MCV, MCH, and MCHC levels and a significant reduction in HbS, leukocytes, platelets, reticulocytes, total serum bilirubin, and lactate dehydrogenase; it also decreases hemolysis, improves anemia, leads to fewer VOCs and hospitalization, and reduces the transfusion requirement.34,36,45,47,49-51
Currently, HU is offered to all patients with SS starting at 2 years of age, irrespective of symptoms. Most of the published literature from India reports that a low and fixed dose of HU (10 mg/kg/d) in children affected with severe disease leads to a significant reduction in recurrent painful episodes, the need for frequent blood transfusions, and the frequency of hospitalization, along with other sickle cell anemia–related comorbidities. This may in part be due to high basal levels of HbF. However, dose escalation is undertaken only if there is no reduction in pain crises and depending upon the requirement for blood transfusions. A multicentric randomized trial is ongoing to assess the efficacy of 10 mg/kg/d vs 20 mg/kg/d and dose escalation of HU in the treatment of SCD.
Crizanlizumab is currently available in India, and L-glutamine is expected to become available shortly.
Curative therapies
Hematopoietic cell transplantation
Hematopoietic cell transplantation (HCT) has steadily grown in India over the last 4 decades. Currently, there are 114 HCT centers in the country, of which 89 report results to a bone marrow transplant registry.52 To date, over 27 000 HCT procedures, including 16 000 allogeneic HCTs, have been performed, with 65% of the HCTs occurring in adults. Thalassemia is prevalent in many ethnic groups in India but has no predilection for disadvantaged communities. There are several available programs to support HCT for thalassemia, which has led to the initiation and success of HCT for SCD. It is encouraging to note that novel strategies have been used in customizing the standard HCT protocols in India for SCD.53 Nationally, complete data from different centers are not yet available for transplants in SCD patients from India, but efforts to provide regular updates are being organized through the NSCAEM registry. There is increasing interest among patients and health care providers in considering transplants for SCD. Thus far, a significant number of patients have traveled from Africa to India to receive HCT. Pre- and post-HCT care, which is critical for successful outcomes, may be limited in their home countries. Resources to meet the demand for bone marrow transplants in Africa are urgently needed.54 Therefore, a global strategy is required to harness the technically advanced resources in India in synergy with other low- and middle-income countries to successfully utilize curative therapies in a cost-effective manner.
Clinical trials
Approximately 47 clinical trials are registered in the Clinical Trial Registry India for SCD, including but not limited to HU, haploidentical transplant conditioning regimens, decitabine- tetrahydrouridine, crizanlizumab, and ayurvedic and integrative medications/strategies such as yoga, omega-3 fatty acids, nutraceuticals, plant extracts, and homeopathic interventions (https://ctri.nic.in/Clinicaltrials/pubview2.php). Of 18 ongoing clinical trials for SCD, 12 are randomized controlled trials. With large numbers of patients with SCD and medical services in private and academic hospitals, India has the potential to serve as a source for conducting interventional clinical trials.
A vision for the future and a national initiative
The NSCAEM, implemented since 2023, focuses on addressing the significant health challenges posed by SCD, with special emphasis on tribal populations of the country.26 The mission is designed to improve the care of all SCD patients and to lower the prevalence of the disease through a multifaceted coordinated approach toward screening and awareness strategies with the ambitious target of eliminating SCD as a public health problem in India before 2047 (Figures 2 and 3).9
The mission includes support for increasing awareness of the disease in the community, implementing mass screening activities for early identification, building a strong network for diagnosis and treatment and a robust monitoring system, strengthening the existing primary health care mechanism to incorporate SCD-related strategies, forming primary, secondary, and tertiary health care teams, and creating cost-effective intensive interventions at quaternary care facilities. The main pillars of the mission are i) health promotion through awareness generation and premarital genetic counseling, ii) prevention by universal screening and early detection, iii) holistic management and continuing care at primary, secondary, and tertiary health care levels, (iv) treatment facilities at tertiary and quaternary health care facilities, and (v) implementation of a patient support system and community engagement to encourage adoption. Mass screening has been undertaken in 17 high-prevalence states with the goal of screening 70 million individuals aged under 40 years in 3 years. To date 36 million individuals have been screened, and 150 000 individuals with SCD and 1 million individuals with sickle cell trait have been detected. Screening metrics are updated in real time to a live public online portal to guide resource allocation at the district, state, and national level (https://sickle.nhm.gov.in/home/guest_dashboard) (Figure 6).
Figure 6.
Dashboard showing updated sickle cell screening data in real time. Reproduced with permission from the Ministry of Health and Family Welfare,9 accessed 7 July 2024, https://sickle.nhm.gov.in/home/guest_dashboard.
All individuals screened are provided with a standardized color-coded identity card with holes punched according to their sickle cell status that also reveals reproductive risk in a health literacy-friendly format (Figure 7). All pregnant women attending antenatal clinics are screened for SCD; if both partners test positive, chorionic villus sampling or amniocentesis is offered free of charge.
Figure 7.
Sickle cell cards for every individual showing their status as normal, as a carrier, or as a person with SCD. The blue is for males and the pink for females. “Front of the card bears the special QR code and identifying information specific to the individual. Back of the card shows the possible outcomes of conception between 2 individuals depending upon their status. For matchmaking the couple's cards are held together against the light and the coinciding holes show the possibility of having a child with Trait or SCD.” Reproduced with permission from the Ministry of Health and Family Welfare.9
Research support for gene therapies
While disease-modifying therapies such as HU have been shown to prevent or reduce complications, gene therapies offer the hope of a transformative therapy moving toward a cure. The approval by the US Food and Drug Administration of Lyfgenia and Casgevy offers the hope of wide implementation of these therapies.55 Serious concerns remain about the feasibility and cost of gene therapies in low- and middle-income countries.56 It is encouraging to note that the GOI has already made major investments in research to develop gene therapies for SCD in India.57
Conclusions
India is home to a large proportion of those with the βS allele globally. Heterogeneity in geography, cultural and ethnic diversity, and variability in the health system contributes to the challenges of providing quality SCD care. The severity of disease phenotype is similar to that described elsewhere in the world, though there may be variations among ethnic groups. India has seen tremendous progress in newborn and population screening, comprehensive care, pneumococcal prophylaxis, and disease- modifying therapy with HU, all available free in government-run clinics. Government and philanthropic support is available for curative therapies, and research is ongoing to develop gene therapies. There is a large capacity to manufacture disease- modifying drugs, safe blood products, vaccines, and genomic therapies at low cost and to scale. Political will at the federal and state levels has led to the initiation of the largest comprehensive effort anywhere for alleviating SCD at the personal to the population level, with the ambitious goal of eliminating SCD as a public health problem by 2047.
Implications for clinical case
The patient developed acute chest syndrome despite receiving comprehensive care per the current standard, a high baseline level of HbF and a moderate dose of HU. She was able to access timely quality treatment including transfusions, antibiotics, and supportive care. Stroke has been observed in patients with severe acute chest syndrome. Deep vein thrombosis likely resulted from the immobilization related to her severe hospital course. She may be a candidate for escalation of HU dosing, but recurrent thrombocytopenia (platelets <80 000) prevented her health care team from accelerating the dose. HCT or gene therapy at a regional center of excellence may be considered.
Acknowledgments
We thank Dr Donovan Argueta, University of California, Irvine, CA, for manuscript preparation and submission; Dr Mihir Gupta, Yale School of Medicine, New Haven, CT, for help with figures and critical review of the manuscript; Dr Graham Serjeant, Sickle Cell Trust, Kingston, Jamaica, for comparison between Indian and Jamaican cohorts; Dr Debojyoti Chakraborty and Dr Sivaprakash Ramalingam, Council of Scientific and Industrial Research—Institute of Genomics and Integrative Biology, New Delhi, for insights on gene therapy efforts in India; Vinita Srivastava, Ministry of Health and Family Welfare, GOI, for input on the NSCAEM; Dr Aishwarya Mahalle, for word processing; Dr Reena Das, Postgraduate Institute, Chandigarh, India, for a critical review of the manuscript.
Kalpna Gupta received research support from a Susan Samueli Scholar Award from Susan Samueli Integrative Health Institute, University of California, Irvine, CA.
Conflict-of-interest disclosure
Kalpna Gupta: no competing financial interests to declare.
Lakshmanan Krishnamurti: no competing financial interests to declare.
Dipty Jain: no competing financial interests to declare.
Off-label drug use
Kalpna Gupta: nothing to diclose.
Lakshmanan Krishnamurti: nothing to disclose.
Dipty Jain: nothing to disclose.
References
- 1.GBDSCD Collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000-2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023;10(8):e585-e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hockham C, Bhatt S, Colah R, et al.. The spatial epidemiology of sickle-cell anaemia in India. Sci Rep. 2018;8(1):17685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piel FB, Patil AP, Howes RE, et al.. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishore RR, Gupta M, Gupta K. A new era dawns on sickle cell disease in India. Indian J Med Res. 2023;157(6):491-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain D, Mohanty D. Clinical manifestations of sickle cell disease in India: misconceptions and reality. Curr Opin Hematol. 2018;25(3):171-176. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MH, Sebastiani P. Genetic modifiers of sickle cell disease. Am J Hematol. 2012;87(8):795-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. [DOI] [PubMed] [Google Scholar]
- 8.Majhi MM, Mishra A, Garg S, Kumar R. How healthy is the health budget of Amrit Kaal: 2023-24? J Family Med Prim Care. 2024;13(1):5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health and Family Welfare, Government of India. National Sickle Cell Anaemia Elimination Mission 2023. Accessed 13 July 2024. https://sickle.nhm.gov.in/.
- 10.Lehmann H, Cutbush M. Sickle-cell trait in southern India. Br Med J. 1952;1(4755):404-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunlop KJ, Mozumder UK. The occurrence of sickle cell anaemia among a group of tea garden labourers in Upper Assam. Ind Med Gaz. 1952;87(9): 387-391. [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla RN, Solanki BR. Sickle-cell trait in Central India. Lancet. 1958; 1(7015):297-298. [DOI] [PubMed] [Google Scholar]
- 13.Jain D, Italia K, Sarathi V, Ghoshand K, Colah R. Sickle cell anemia from central India: a retrospective analysis. Indian Pediatr. 2012;49(11):911-913. [DOI] [PubMed] [Google Scholar]
- 14.Jain D, Bagul AS, Shah M, Sarathi V. Morbidity pattern in hospitalized under five children with sickle cell disease. Indian J Med Res. 2013;138(3): 317-321. [PMC free article] [PubMed] [Google Scholar]
- 15.Jain D, Arjunan A, Sarathi V, et al.. Clinical events in a large prospective cohort of children with sickle cell disease in Nagpur, India: evidence against a milder clinical phenotype in India. Pediatr Blood Cancer. 2016;63(10):1814-1821. [DOI] [PubMed] [Google Scholar]
- 16.Jain D, Tokalwar R, Upadhye D, Colah R, Serjeant GR. Homozygous sickle cell disease in Central India and Jamaica: a comparison of newborn cohorts. Indian J Med Res. 2020;151(4):326-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labie D, Srinivas R, Dunda O, et al.. Haplotypes in tribal Indians bearing the sickle gene: evidence for the unicentric origin of the beta S mutation and the unicentric origin of the tribal populations of India. Hum Biol. 1989;61(4):479-491. [PubMed] [Google Scholar]
- 18.Kar BC. Clinical profile of sickle cell trait. J Assoc Physicians India. 2002;50:1368-1371. [PubMed] [Google Scholar]
- 19.Dash BP, Kar BC. Priapism is rare in sickle cell disease in India. J Assoc Physicians India. 2000;48(2):255. [PubMed] [Google Scholar]
- 20.Kar BC. Splenectomy in sickle cell disease. J Assoc Physicians India. 1999;47(9):890-893. [PubMed] [Google Scholar]
- 21.Kar BC, Devi S. Clinical profile of sickle cell disease in Orissa. Indian J Pediatr. 1997;64(1):73-77. [DOI] [PubMed] [Google Scholar]
- 22.Mohapatra BN, Dash BP, Kar BC. Serum immunoglobulins in sickle cell disease. J Assoc Physicians India. 1993;41(7):418-419. [PubMed] [Google Scholar]
- 23.Kar BC. Sickle cell disease in India. J Assoc Physicians India. 1991;39(12):954-960. [PubMed] [Google Scholar]
- 24.Kar BC, Agrawal KC, Panda A. Sickle haemoglobin, G-6PD deficiency and malaria in western Orissa. J Assoc Physicians India. 1990;38(8): 555-557. [PubMed] [Google Scholar]
- 25.Kulozik AE, Thein SL, Kar BC, Wainscoat JS, Serjeant GR, Weatherall DJ. Raised Hb F levels in sickle cell disease are caused by a determinant linked to the beta globin gene cluster. Prog Clin Biol Res. 1987;251:427-439. [PubMed] [Google Scholar]
- 26.Jain D, Gupta M, Madkaikar M, et al.. Sickle cell disease in India: current status and progress. Lancet Haematol. 2024;11(5):e322-e323. [DOI] [PubMed] [Google Scholar]
- 27.Odame I, Jain D. Sickle cell disease: progress made and challenges ahead. Indian J Med Res. 2020;151(6):505-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upadhye D, Jain D, Nadkarni A, Ghosh K, Colah R. Red cell indices and hemoglobin profile of newborn babies with both the sickle gene and alpha thalassaemia in Central India. Indian J Hematol Blood Transfus. 2019;35(1):109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Upadhye DS, Jain DL, Trivedi YL, Nadkarni AH, Ghosh K, Colah RB. Neonatal screening and the clinical outcome in children with sickle cell disease in Central India. PLoS One. 2016;11(1):e0147081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upadhye D, Jain D, Trivedi Y, Nadkarni A, Ghosh K, Colah R. Influence of single nucleotide polymorphisms in the BCL11A and HBS1L-MYB gene on the HbF levels and clinical severity of sickle cell anaemia patients. Ann Hematol. 2016;95(7):1201-1203. [DOI] [PubMed] [Google Scholar]
- 31.Jain D, Warthe V, Dayama P, et al.. Sickle cell disease in Central India: a potentially severe syndrome. Indian J Pediatr. 2016;83(10):1071-1076. [DOI] [PubMed] [Google Scholar]
- 32.Mohanty D, Mukherjee MB, Colah RB, et al.. Spectrum of hemoglobinopathies among the primitive tribes: a multicentric study in India. Asia Pac J Public Health. 2015;27(2):NP562-NP571. [DOI] [PubMed] [Google Scholar]
- 33.Jain DL, Sarathi V, Upadhye D, et al.. Newborn screening shows a high incidence of sickle cell anemia in Central India. Hemoglobin. 2012;36(4): 316-322. [DOI] [PubMed] [Google Scholar]
- 34.Jain DL, Sarathi V, Desai S, Bhatnagar M, Lodha A. Low fixed-dose hydroxyurea in severely affected Indian children with sickle cell disease. Hemoglobin. 2012;36(4):323-332. [DOI] [PubMed] [Google Scholar]
- 35.Italia KY, Jijina FF, Jain D, et al.. The effect of UGT1A1 promoter polymorphism on bilirubin response to hydroxyurea therapy in hemoglobinopathies. Clin Biochem. 2010;43(16-17):1329-1332. [DOI] [PubMed] [Google Scholar]
- 36.Italia K, Jain D, Gattani S, et al.. Hydroxyurea in sickle cell disease—a study of clinico—pharmacological efficacy in the Indian haplotype. Blood Cells Mol Dis. 2009;42(1):25-31. [DOI] [PubMed] [Google Scholar]
- 37.Colah RB, Mehta P, Mukherjee MB. Newborn screening for sickle cell disease: Indian experience. Int J Neonatal Screen. 2018;4(4):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Italia Y, Krishnamurti L, Mehta V, et al.. Feasibility of a newborn screening and follow-up programme for sickle cell disease among South Gujarat (India) tribal populations. J Med Screen. 2015;22(1):1-7. [DOI] [PubMed] [Google Scholar]
- 39.Upadhye DS, Jain DL, Trivedi YL, Nadkarni AH, Ghosh K, Colah RB. Newborn screening for haemoglobinopathies by high performance liquid chromatography (HPLC): diagnostic utility of different approaches in resource-poor settings. Clin Chem Lab Med. 2014;52(12):1791-1796. [DOI] [PubMed] [Google Scholar]
- 40.Patel J, Serjeant GR. Newborn screening for sickle cell disease in India: the need for defining optimal clinical care. Indian J Pediatr. 2014;81(3):229-230. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh K, Colah R, Manglani M, et al.. Guidelines for screening, diagnosis and management of hemoglobinopathies. Indian J Hum Genet. 2014;20(2): 101-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panigrahi S, Patra PK, Khodiar PK. Neonatal screening of sickle cell anemia: a preliminary report. Indian J Pediatr. 2012;79(6):747-750. [DOI] [PubMed] [Google Scholar]
- 43.Purohit P, Mohanty PK, Patel S, Das P, Panigrahi J, Das K. Comparative study of clinical presentation and hematological indices in hospitalized sickle cell patients with severe Plasmodium falciparum malaria. J Infect Public Health. 2018;11(3):321-325. [DOI] [PubMed] [Google Scholar]
- 44.Sethy S, Panda T, Jena RK. Beneficial effect of low fixed dose of hydroxyurea in vaso-occlusive crisis and transfusion requirements in adult HbSS patients: a prospective study in a tertiary care center. Indian J Hematol Blood Transfus. 2018;34(2):294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deshpande SV, Bhatwadekar SS, Desai P, et al.. Hydroxyurea in sickle cell disease: our experience in Western India. Indian J Hematol Blood Transfus. 2016;32(2):215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dehury S, Purohit P, Patel S, et al.. Low and fixed dose of hydroxyurea is effective and safe in patients with HbSβ(+) thalassemia with IVS1-5(G→C) mutation. Pediatr Blood Cancer. 2015;62(6):1017-1023. [DOI] [PubMed] [Google Scholar]
- 47.Jain DL, Apte M, Colah R, et al.. Efficacy of fixed low dose hydroxyurea in Indian children with sickle cell anemia: a single centre experience. Indian Pediatr. 2013;50(10):929-933. [DOI] [PubMed] [Google Scholar]
- 48.Vaibhav D, Panda PS, Debata I, Sinha AK. Morbidity pattern and impact of hydroxyurea therapy among sickle cell patients in Raipur district of Chhattisgarh. J Family Med Prim Care. 2024;13(5):1825-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel S, Purohit P, Mashon RS, et al.. The effect of hydroxyurea on compound heterozygotes for sickle cell-hemoglobin D-Punjab—a single centre experience in eastern India. Pediatr Blood Cancer. 2014;61(8):1341-1346. [DOI] [PubMed] [Google Scholar]
- 50.Patel DK, Mashon RS, Patel S, Das BS, Purohit P, Bishwal SC. Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India. Hemoglobin. 2012;36(5):409-420. [DOI] [PubMed] [Google Scholar]
- 51.Pandey A, Kaur H, Borah S, et al.. A systematic review on hydroxyurea therapy for sickle cell disease in India. Indian J Med Res. 2022;156(2):299-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair V, Yanamandra U, Nazneen PS. Hematopoietic cell transplantation landscape in India. Med J Armed Forces India. 2023;79(6):621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kharya G, Bakane AN, Rauthan AM. Pretransplant myeloid and immune suppression, reduced toxicity conditioning with posttransplant cyclophosphamide: initial outcomes of novel approach for matched unrelated donor hematopoietic stem cell transplant for hemoglobinopathies. Pediatr Blood Cancer. 2021;68(4):e28909. [DOI] [PubMed] [Google Scholar]
- 54.Makani J, Cavazzana M, Gupta K, et al.. Blood diseases in Africa: redressing unjust disparities is an urgent unmet need. Am J Hematol. 2022;97(12): 1505-1506. [DOI] [PubMed] [Google Scholar]
- 55.Barak M, Hu C, Matthews A, Fortenberry YM. Current and future therapeutics for treating patients with sickle cell disease. Cells. 2024;13(10):848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doxzen KW, Adair JE, Fonseca Bazzo YM, et al.. The translational gap for gene therapies in low- and middle-income countries. Sci Transl Med. 2024;16(746):eadn1902. [DOI] [PubMed] [Google Scholar]
- 57.Gupta P, Goswami SG, Kumari G, et al.. Development of pathophysiologically relevant models of sickle cell disease and β-thalassemia for therapeutic studies. Nat Commun. 2024;15(1):1794. [DOI] [PMC free article] [PubMed] [Google Scholar]