Summary
Background
Current literature highlights a gap in precise stroke cost data for Latin America. This study measures the real costs associated with acute ischemic stroke care in Latin America using Time–Driven Activity-Based Costing (TDABC). The findings aim to lay a solid foundation for adopting value-based healthcare (VBHC) strategies in the region.
Methods
The study is an observational, multicenter, international analysis of direct costs and outcomes for patients hospitalised with acute ischemic stroke from December 2021 to December 2022. Data from stroke centres in Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, Peru, and Uruguay were analysed. Costs were stratified by country. Factors such as favourable outcomes based on the modified Rankin Scale (mRS 0–2), clinical risk levels, and treatment interventions were considered for the analysis. Generalized Estimating Equation (GEE) models were utilised to assess the relationship of clinical variables with the total cost per patient.
Findings
A total of 1106 patients were included in the study. Among these patients, 74% received medical treatment alone, 18% received intravenous thrombolysis (IVT), 4% underwent mechanical thrombectomy (MT), and 3% received combined IVT plus MT. The mean cost per patient was I$ 12,203 (SD I$ 15,055), with 49% achieving a favourable functional outcome. Compared to medical treatment alone, MT incurred costs 3.1 times higher, with an incremental cost of I$ 20,418 per patient (p < 0.0001). Across all countries, costs increased according to patients' clinical risk and treatment options, with length of hospital stay emerging as the primary cost driver.
Interpretation
Our study highlights significant disparities in stroke costs across healthcare services in Latin America, influenced by variations in treatment accessibility, patient outcomes, and clinical risk profiles. These findings offer essential insights for shaping health policy decisions to enhance the long-term sustainability of stroke care in the region.
Funding
The project received funding from the World Stroke Organization and Boehringer Ingelheim (BI) IS 0135-0352.
Keywords: Ischemic stroke, Cost, Cost evaluation, Cost impact
Research in context.
Evidence before this study
A systematic literature search was conducted on July 16, 2021, across PubMed, EMBASE, LILACS, and SciELO to identify studies assessing the costs and outcomes of acute ischemic stroke treatment in Latin America. The following search terms were used: (“stroke” OR “acute ischemic stroke” OR “ischemic stroke”) AND (“treatment” OR “acute treatment”) AND (“costs” OR “cost evaluation” OR “cost analysis” OR “economic evaluation” OR “cost-effectiveness analysis” OR “cost-effectiveness”) AND (“Latin America” OR “Latin American countries” OR specific country names in the region). This search identified 589 citations, including 206 from PubMed, 335 from EMBASE, 28 from LILACS, and 20 from SciELO, with 491 non-duplicate articles screened. After applying inclusion criteria—studies focusing on economic assessments of acute ischemic stroke treatment performed in Latin America—18 articles were retrieved and included for review. The search revealed that while some studies provided insights into stroke costs, the literature lacked comprehensive and standardised analyses using robust methodologies like Time–Driven Activity-Based Costing (TDABC). This gap highlighted the need for detailed evaluations to inform healthcare policy and improve resource allocation for stroke care in the region.
Added value of this study
This study is the first to use TDABC to evaluate the direct costs of acute ischemic stroke treatment across eight Latin American countries. By integrating data from public and private stroke centres, the study provides robust evidence on cost variability across treatment modalities and clinical risk profiles. The findings also quantify the economic impact of different treatment approaches, including medical management, intravenous thrombolysis, and mechanical thrombectomy, addressing a critical knowledge gap in regional stroke economics and establishing a foundation for implementing value-based healthcare in Latin America.
Implications of all the available evidence
The evidence presented underscores the urgent need for tailored health policy interventions aimed at reducing the economic burden of stroke care in Latin America. The significant variabilities in treatment costs and outcomes across countries call for a concerted effort to improve treatment accessibility and optimize resource allocation. Policymakers can leverage these insights to enhance the efficiency and sustainability of stroke care systems, ultimately aiming to improve patient outcomes and achieve better value for healthcare expenditures. This study’s methodology and findings could serve as a model for other regions facing similar healthcare challenges.
Introduction
Stroke poses a significant health challenge globally, ranking among the leading causes of death and disability worldwide.1, 2, 3 This burden is particularly pronounced in low- and middle-income countries (LMICs) compared to high-income countries (HICs), where limited healthcare access exacerbates the impact.4 Projections indicate a concerning 50% increase in the number of stroke deaths from 2020 to 2050 in LMICs, with costs estimated to rise from US$891 billion annually to US$2.31 trillion by 2050.5
Despite the well-documented benefits and cost-effectiveness of interventions such as intravenous thrombolysis (IVT) and mechanical thrombectomy (MT), access remains limited, with less than 2% of stroke patients having access in resource-constrained settings.5, 6, 7, 8, 9, 10 Disparities in awareness, healthcare access, and infrastructure between LMICs and HICs present significant obstacles to the widespread adoption of these treatments.10, 11, 12 Local government support plays a pivotal role, as stroke networks rely heavily on budget allocations for infrastructure, healthcare personnel, and the implementation of effective, sustainable programs guided by national policies.12
A literature review indicates a lack of standardised methods for measuring, estimating, and reporting stroke costs.13 In pursuit of more effective, patient-centred, and data-driven care pathways, value-based healthcare (VBHC) has emerged as a promising strategy worldwide.14, 15, 16 Among the prerequisites for implementing and managing care services based on value is the accurate measurement of outcomes and costs. Time–Driven Activity-Based Costing (TDABC) emerges as the gold standard, offering a practical framework for optimising healthcare resource allocation and enhancing stroke care delivery.16, 17, 18, 19
This multicenter study aims to measure stroke costs in Latin American countries, utilising VBHC strategies and TDABC to establish more effective care pathways and enhance resource allocation regionally.
Methods
Study design
A cross-sectional, multicenter international study was undertaken to assess costs and outcomes, drawing patient data from a single designated stroke centre in each of the following countries: Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, Peru, and Uruguay. Data collection commenced with a Pilot Phase spanning from June to December 2021, followed by a validation phase, and extended into the Main Phase from June to December 2022. The study adopted a hospital-centric perspective, encompassing both public (n = 6) and private institutions (n = 2). It employed a methodology integrating microcosting and patient-centred approaches, combining retrospective (Pilot Phase) and prospective (Main Phase) data collection methods. This study was conducted following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines to ensure transparency and consistency in reporting.
Participant criteria and hospital selection
Data collection was centred on hospitalized patients aged 18 years and older diagnosed with acute ischemic stroke during the specified timeframe. Centres were chosen based on convenience, considering factors such as the availability of cost data and robust research infrastructure. Eight stroke centres, certified by the World Stroke Organization (WSO) in the mentioned countries, were selected for their proficiency in stroke care, treating a minimum of 100 stroke patients annually, and possessing clinical research experience, ensuring high-quality data for the current study.
Ethical considerations
Ethical approval was obtained from the coordinating centre, Hospital Moinhos de Vento in Brazil (CAAE: 53723521.8.0000.5330), and the Research Ethics Committees in all participating countries approved the study. Since data acquisition was performed via medical records under hospital protocol, informed consent was waived. Patient confidentiality was safeguarded using numerical coding techniques. Each participating centre omitted sensitive data, such as names and personal identifiers, and assigned unique numerical codes to participants before submitting the information to the coordinating centre. The data was stored in encrypted databases with restricted access, and all personnel were trained in confidentiality and data protection.
Research conduction
The study comprised the Pilot and the Main phase (Supplementary Material I). During the Pilot phase, data were retrospectively collected from these certified hospitals, with 30 consecutive patients per centre. The primary aim of this phase was to evaluate the feasibility of data collection and cost analysis in the hospitals. Subsequently, upon successfully validating the pilot phase data (n = 208), the main phase expanded data collection to an additional 898 patients from these centres, totalling 1106 patients across eight centres from different countries. In this phase, all acute ischemic stroke patients prospectively admitted to the hospitals from June to December 2022 were included. The research coordinating centre provided initial 1-h online training sessions to the financial departments of each centre to ensure accurate data on infrastructure costs, salaries, medications, and devices before the study began. Following this, investigators were trained to collect precise data on professional time per patient, number of exams during hospitalisation, medications, and procedures. If there were any doubts or difficulties, additional meetings were conducted. The coordination team also monitored the quality of cost and effectiveness data through frequent remote monitoring meetings.
Measurement of costs and healthcare outcomes
The methodology systematically evaluated the direct costs associated with acute ischemic stroke treatment across the entire care pathway, employing the TDABC method (Supplementary Material II).16 This method estimated costs using two primary data: the cost capacity rate (CCR in $/unit of time) per resource and the estimated length of time each patient consumed from each resource, in each activity, over its care cycle. Following this methodology, we mapped out the care process and identified the main activities undergone by each patient. We listed all resources and departments involved, estimated the total expenses for each resource, and calculated the hourly capacity and unit cost rate for each resource or department. Then, we analysed the time devoted to each patient by every resource, developed equations for time and costs, calculated the cost per patient, and performed further analyses. In addition to the basic demographic information and stroke subtypes through the TOAST classification (Trial of ORG 10172 in Acute Stroke Treatment), validated stroke scores were used to evaluate healthcare status, including the modified Rankin Score (mRS) at discharge and 3 months and the National Institute for Health Stroke Scale (NIHSS) at admission, in addition to mortality and hospital length of stay.
Mapping of care flow and identification of hospital resources
The study team mapped the care flow based on the hospital's ischemic stroke care routines and protocols, validated by a vascular neurologist. It was assumed that patient flow was similar across all eight centres due to their accreditation by the WSO. Five macrophases were identified across the care pathway: emergency, general ward, angiography, intensive care unit (ICU), and stroke unit. The resources consumed in these phases included professionals' salaries, the physical structure of each department, exams, procedures the patient underwent, medications prescribed, and IVT medications and/or materials used for performing MT, considering the costs of resources available at each centre (Supplementary Material II).
Estimating total costs of hospital resources
For each identified resource, total mean costs were estimated. Labour costs were calculated based on the monthly salary of each professional plus their benefits, considering the volume of consultations across the identified phases. Total costs of hospital infrastructure refer to the fixed monthly costs of the space, such as equipment depreciation, electricity, fees, and other expenses of each department. Data acquisition involved reviewing financial reports from hospital departments. Medication and exam costs are unit costs multiplied by the volume prescribed or performed for each patient, with individual consumption extracted from reviewing medical records and prescriptions. Exams were computed based on the identification in the clinical records. The cost of the thrombolytic agent is estimated by the unit cost of alteplase or tenecteplase and the dosing administered (cost per milligram). The costs of the MT devices refer to the sum of materials costs used per patient during the procedure.
Estimating hourly capacity and unit cost rates
The capacity of each department's space was estimated based on room and bed availability, while the contracted workload determined labour capacity for one month. To account for inherent process losses, 85% of the installed capacity was considered for both resources. Each resource's cost-capacity rate (CCR) can be calculated using the cost per resource and capacity information.
Time allocation per patient and cost equations
The average time for each patient consultation was estimated through employee allocation scales, interviews with professionals, and hospital guidelines. Interviews with department managers and hospital productivity reports determined the time spent in each department per patient. Detailed information on each centre's departmental length of stay can be found in Supplementary Material III.
Calculating the cost per patient
The time spent on each resource was multiplied by its respective CCR to calculate the individual cost per patient. Subsequently, this value was added to the individual cost of medications, exams, and treatments. Specific clinical outcomes, including the mRS and case mix variables such as NIHSS scores and age, were also selected for collection alongside cost data to enable clinical translation. A standardized spreadsheet (Supplementary Material IV) was created for the data collection, allowing the centres to gather patient-level information consistently, ensuring the reliability of the results.
Data estimation
Challenges encountered in post-treatment patient communication, coupled with incomplete financial data in specific centres, necessitated a flexible approach to data estimation. Protocols were devised to address the 1.6% of missing information, ensuring maximal patient inclusion. In the case of clinical data, missing values for NIHSS and mRS scores were extrapolated based on admission and discharge data. Missing NIHSS data at admission were imputed using the NIHSS scores recorded at discharge for the same patients, while missing discharge NIHSS data were imputed using the admission scores. For the missing 3-month mRS data, the mRS scores obtained at discharge were used for the corresponding patients. Financial estimates were derived from the collected sample. For the missing cost data, values were extrapolated using the mean values from patients with complete data from each hospital. For centres in Brazil and Chile, data from previous real-world studies were utilized.20,21 Specifically, for the centres in Brazil and Chile, the studies provided comprehensive insights into the cost dynamics of stroke treatment, enhancing the accuracy of our estimations. Noteworthy adaptations were made for medication details in Chile and Costa Rica centres. The medication costs in the Chilean centre were allocated based on the proportion of each medication's usage at the centre and distributed proportionally to each patient's length of hospital stay. No data on medication usage at the centre in Costa Rica were available, and they were not included in the analysis. The Chilean centre was only a primary stroke centre, and patients undergoing MT were referred to another hospital. Given the complexity of obtaining precise costs for this procedure and considering that MT represented a small proportion of cases in that centre (2%, n = 3), mean values from the referenced studies were employed to approximate these costs.
Risk stratification
Based on the model validated in a previous Brazilian study21 and utilising patient age and NIHSS levels upon hospital admission, the stratification by risk level per treatment was employed to address patient clustering among institutions. This model, previously validated by the Generalized Estimating Equation (GEE) model, categorised patients into low, medium, and high-risk levels. The model confirmed the validity of both the individual and combined effects of NIHSS and age in stratifying risk levels, demonstrating significant associations with favourable outcomes (mRS 0–2) using both univariate and multivariate GEE models. The NIHSS classification criteria and the age cut-off were based on previous studies.22 Specifically, the low-risk level included patients under 70 years with an NIHSS score <8, the medium-risk level encompassed patients under 70 years with an NIHSS score between 8 and 15, as well as those older than 70 with an NIHSS score <8, and the high-risk level included patients older than 70 years with an NIHSS score >8 and those under 70 years with an NIHSS score >15.21
Data analysis
Descriptive cost analyses were conducted to present the results, including mean, standard deviation (SD), median, and interquartile intervals (IQR), considering patients' risk levels and treatment modalities (medical treatment, IVT, MT, and combined IVT plus MT). Purchasing Power Parity and conversion to International Dollars (I$) facilitated comparisons across different countries. Clinical outcomes such as mortality rates, mRS scores at hospital discharge and 3 months post-discharge, and hospitalisation length of stay were also compared across treatments, countries, and risk levels. The consolidated sample data was organised in a Microsoft® Excel spreadsheet. Descriptive analyses were performed, with additional statistical analysis conducted in R to examine potential differences in costs per treatment relative to medical treatment. Generalized Estimating Equation (GEE) models were utilised, with Country as the clustering variable, applying a Gamma distribution and Logarithm link function, and employing an Exchangeable correlation structure to assess the relationship of clinical variables (age, sex, mRS at discharge, hospitalisation length of stay, and risk stratification) with the total cost per patient. Post-hoc pairwise comparisons were made using the Tukey method for multiple comparison adjustments, with a significance level of 0.05 for all analyses.
Role of the funding source
The entities that supported the project did not play any role in the design, analysis, or interpretation of the results of this study.
Results
Hospital characteristics
The study included 8 hospitals, 6 of which were academic public institutions and 2 of which were academic private hospitals. Included public hospitals were in Brazil, Mexico, Costa Rica, Uruguay, Peru, Chile; and private hospitals were in Argentina and Colombia. The hospitals varied in capacity, with the total number of beds as follows: Argentina - 113, Mexico - 126, Peru - 135, Uruguay - 350, Chile - 371, Costa Rica - 645, Colombia - 731, and Brazil - 784. Among these hospitals, 6 were comprehensive stroke centres equipped to perform MT, while the remaining 2 were primary stroke centres providing IVT only, located in Chile and Peru.
Patient characteristics
A total of 1106 patients were included in the study. Most patients, 819 (74%), received medical treatment alone, followed by 203 (18%) who received IVT, 49 (4%) who underwent MT, and 35 (3%) who received IVT followed by MT. The median age was 68 years (IQR 59–77), with 54% male. Upon arrival at the hospital, most patients (38%) were classified as medium risk. The median NIHSS score at hospital admission was 7 (IQR 3–13), and the median mRS score was 2 (IQR 1–4) after 3 months post-discharge. The cause of stroke was undetermined in 33% of cases. The rate of favourable functional outcomes at discharge (mRS 0–2) was 15% in high-risk cases, 55% in moderate-risk cases, and 77% for low-risk patients. The mortality at 3 months was 9% (n = 97). Table 1 provides detailed baseline characteristics of the study sample.
Table 1.
Baseline characteristics and main outcomes of patients with acute ischemic stroke.
| Global | Argentina | Brazil | Chile | Colombia | Costa Rica | Mexico | Peru | Uruguay | |
|---|---|---|---|---|---|---|---|---|---|
| Total patients | 1106 (100%) | 148 (13%) | 157 (14%) | 126 (11%) | 153 (14%) | 197 (18%) | 103 (9%) | 119 (11%) | 103 (9%) |
| Female | 508 (46%) | 71 (48%) | 78 (50%) | 60 (48%) | 75 (49%) | 81 (41%) | 45 (44%) | 51 (43%) | 47 (46%) |
| Mean age | 67 (14) | 70 (14) | 66 (12) | 67 (13) | 72 (14) | 66 (15) | 61 (17) | 65 (15) | 66 (14) |
| NIHSS (Discharge) | |||||||||
| 0–7 | 745 (67%) | 136 (92%) | 117 (75) | 98 (78%) | 104 (68%) | 109 (55%) | 47 (46%) | 65 (55%) | 69 (67%) |
| 8–15 | 327 (21%) | 10 (7%) | 31 (20%) | 20 (16%) | 37 (24%) | 51 (26%) | 31 (30%) | 11 (11%) | 11 (11%) |
| ≥15 | 124 (11%) | 2 (1%) | 9 (6%) | 8 (6%) | 12 (8%) | 37 (19%) | 25 (24%) | 23 (22%) | 23 (22%) |
| Stroke subtype | |||||||||
| Cardioembolic | 266 (24%) | 25 (17%) | 53 (34%) | 9 (7%) | 49 (32%) | 34 (17%) | 38 (37%) | 25 (21%) | 33 (32%) |
| Large vessel atherosclerosis | 236 (21%) | 15 (10%) | 48 (31%) | 42 (33%) | 14 (9%) | 58 (29%) | 14 (14%) | 33 (28%) | 12 (12%) |
| Lacunar | 138 (12%) | 16 (11%) | 13 (8%) | 32 (25%) | 10 (7%) | 22 (11%) | 10 (10%) | 15 (13%) | 20 (19%) |
| Other etiology | 105 (9%) | 19 (13%) | 17 (11%) | 3 (2%) | 11 (7%) | 28 (14%) | 14 (14%) | 8 (7%) | 5 (5%) |
| Undetermined | 361 (33%) | 73 (49%) | 26 (17%) | 40 (32%) | 69 (45%) | 55 (28%) | 27 (26%) | 38 (32%) | 33 (32%) |
| mRS 3 months | |||||||||
| 0 | 269 (24%) | 67 (45%) | 18 (11%) | 29 (23%) | 37 (24%) | 64 (32%) | 12 (12%) | 4 (3%) | 38 (37%) |
| 1 | 192 (17%) | 26 (18%) | 28 (18%) | 29 (23%) | 34 (22%) | 28 (14%) | 23 (22%) | 12 (10%) | 12 (12%) |
| 2 | 165 (15%) | 24 (16%) | 36 (23%) | 11 (9%) | 24 (16%) | 38 (19%) | 17 (17%) | 9 (8%) | 6 (6%) |
| 3 | 162 (15%) | 17 (11%) | 27 (17%) | 33 (26%) | 13 (8%) | 25 (13%) | 14 (14%) | 24 (20%) | 9 (9%) |
| 4 | 150 (14%) | 9 (6%) | 30 (19%) | 14 (11%) | 14 (9%) | 14 (7%) | 15 (15%) | 46 (39%) | 8 (8%) |
| 5 | 71 (6%) | 1 (1%) | 13 (8%) | 5 (4%) | 10 (7%) | 11 (6%) | 12 (12%) | 17 (14%) | 2 (2%) |
| 6 | 97 (9%) | 4 (3%) | 5 (3%) | 5 (4%) | 21 (14%) | 17 (9%) | 10 (10%) | 7 (6%) | 28 (27%) |
| Treatments | |||||||||
| Medical treatment | 819 (74%) | 121 (82%) | 99 (63%) | 100 (79%) | 104 (68%) | 148 (75%) | 71 (69%) | 108 (91%) | 67 (65%) |
| IVT | 203 (18%) | 13 (9%) | 45 (29%) | 23 (18%) | 23 (15%) | 35 (17%) | 28 (27%) | 11 (9%) | 28 (27%) |
| MT | 49 (4%) | 8 (5%) | 10 (6%) | 3 (2%) | 16 (10%) | 5 (3%) | 3 (3%) | NA | 4 (4%) |
| IVT + MT | 35 (3%) | 6 (4%) | 3 (2%) | NA | 10 (7%) | 11 (6%) | 1 (1%) | NA | 4 (4%) |
| Risk levels | |||||||||
| High risk | 351 (32%) | 14 (9%) | 40 (25%) | 30 (24%) | 61 (40%) | 88 (45%) | 47 (46%) | 40 (34%) | 31 (30%) |
| Medium risk | 422 (38%) | 85 (57%) | 67 (43%) | 36 (29%) | 54 (35%) | 68 (35%) | 27 (26%) | 51 (43%) | 34 (33%) |
| Low risk | 333 (30%) | 49 (33%) | 50 (32%) | 60 (48%) | 38 (25%) | 41 (21%) | 29 (28%) | 28 (24%) | 38 (37%) |
IVT = Intravenous Trombolysis; MT = Mechanical Thrombectomy; mRS = modified Rankin Score; SD = Standard Deviation; NA = Not applicable.
Direct costs of acute ischemic stroke treatment
The mean cost per patient was I$ 12,203 (SD I$ 15,055). Treatment modalities (p < 0.0001), mRS at discharge (p < 0.0001), risk levels (p < 0.0001), patient age (p = 0.0185), and hospital length of stay (p < 0.0001) significantly influenced cost variability, while gender showed no significant impact (p = 0.375). IVT incurred an average incremental cost of I$ 5195 per patient (p < 0.0001) compared to medical treatment alone, while MT incurred I$ 20,418 per patient (p < 0.0001) and combined IVT plus MT incurred I$ 19,285 (p < 0.0001). MT and IVT plus MT incurred higher costs than IVT alone (p < 0.0001), as shown in Table 2.
Table 2.
Direct costs associated with treatment modality, mRS scores at discharge, risk levels, and stroke subtypes.
| General (SD) | Medical treatment | IVT | MT | IVT + MT | |
|---|---|---|---|---|---|
| Mean cost per patient | I$ 12,203 (I$ 15,055) | I$ 9735 | I$ 14,930 | I$ 30,153 | I$ 29,019 |
| Labour costs | I$ 4699 (I$ 8573) | I$ 3969 | I$ 5741 | I$ 11,049 | I$ 5907 |
| Structure costs | I$ 5014 (I$ 6272) | I$ 4520 | I$ 5733 | I$ 9290 | I$ 6407 |
| Exams | I$ 1130 (I$ 1272) | I$ 1037 | I$ 1093 | I$ 1558 | I$ 2939 |
| Medicines | I$ 448 (I$ 1883) | I$ 255 | I$ 715 | I$ 599 | I$ 3670 |
| Thrombolysis | I$ 1912 (I$ 1510) | – | I$ 1765 | – | I$ 2764 |
| Materials thrombectomy | I$ 8234 (I$ 2937) | – | – | I$ 7718 | I$ 8487 |
| Mean cost per mRS scale (N) | |||||
| 0 (189; 17%) | I$ 6633 (I$ 5214) | I$ 5464 | I$ 9330 | I$ 16,353 | I$ 18,698 |
| 1 (211; 19%) | I$ 9621 (I$ 10,143) | I$ 7622 | I$ 12,648 | I$ 19,103 | I$ 22,592 |
| 2 (143; 13%) | I$ 11,892 (I$ 13,538) | I$ 9384 | I$ 20,342 | I$ 17,120 | I$ 16,999 |
| 3 (178; 16%) | I$ 13,518 (I$ 13,491) | I$ 11,314 | I$ 16,667 | I$ 20,679 | I$ 39,248 |
| 4 (177; 16%) | I$ 12,900 (I$ 15,115) | I$ 10,792 | I$ 13,257 | I$ 28,292 | I$ 37,796 |
| 5 (137; 12%) | I$ 22,008 (I$ 26,895) | I$ 15,908 | I$ 25,957 | I$ 63,842 | I$ 38,307 |
| 6 (71; 6%) | I$ 11,377 (I$ 9267) | I$ 10,103 | I$ 9779 | I$ 18,667 | I$ 24,491 |
| Mean cost per mRS category (N) | |||||
| 0–2 (543; 49%) | I$ 9179 (I$ 10,079) | I$ 7305 | I$ 13,374 | I$ 17,495 | I$ 20,689 |
| 3–4 (355; 32%) | I$ 13,210 (I$ 14,307) | I$ 11,051 | I$ 15,058 | I$ 24,232 | I$ 38,466 |
| 5–6 (208; 19%) | I$ 18,379 (I$ 23,017) | I$ 14,238 | I$ 18,407 | I$ 44,821 | I$ 32,781 |
| Risk levels | |||||
| High risk | I$ 17,438 (I$ 19,781) | I$ 13,552 | I$ 17,323 | I$ 34,870 | I$ 31,117 |
| Medium risk | I$ 9926 (I$ 12,739) | I$ 8342 | I$ 12,984 | I$ 23,795 | I$ 23,310 |
| Low risk | I$ 9572 (I$ 9673) | I$ 8456 | I$ 13,477 | I$ 18,621 | I$ 30,070 |
| Stroke subtypes | |||||
| Cardioembolic | I$ 15,492 (I$ 19,360) | I$ 11,312 | I$ 17,600 | I$ 36,026 | I$ 33,694 |
| Large vessel atherosclerosis | I$ 13,565 (I$ 15,083) | I$ 11,809 | I$ 18,313 | I$ 21,810 | I$ 26,421 |
| Lacunar | I$ 8165 (I$ 7753) | I$ 7120 | I$ 12,296 | I$ 13,958 | I$ 18,933 |
| Other etiology | I$ 14,860 (I$ 17,644) | I$ 10,843 | I$ 18,014 | I$ 41,602 | I$ 41,224 |
| Undetermined | I$ 9661 (I$ 11,596) | I$ 8074 | I$ 10,693 | I$ 25,834 | I$ 22,976 |
IVT = Intravenous Trombolysis; MT = Mechanical Thrombectomy; mRS = modified Rankin Score; SD = Standard Deviation.
Patients with an mRS score of 5 at discharge incurred the highest cost (I$ 22,008), followed by those with an mRS score of 3 (I$ 13,518), while the mRS 0 category had the lowest mean cost (I$ 6633). Patients with mRS scores of 2 or higher had higher costs than those with mRS 0 (p < 0.0001). Additionally, mRS scores of 4, 5 (p < 0.0001) or 6 (p = 0.037) incurred higher costs than mRS 1, with mRS 5 demonstrating higher costs than all other mRS categories (p < 0.0001). The rate of favourable functional outcomes at discharge (mRS 0–2) was 49%, yielding lower costs than mRS 3–4 (p = 0.0067) and mRS 5–6 (p < 0.0001). Moreover, mRS scores of 3–4 presented lower costs than mRS 5–6 (p = 0.0143).
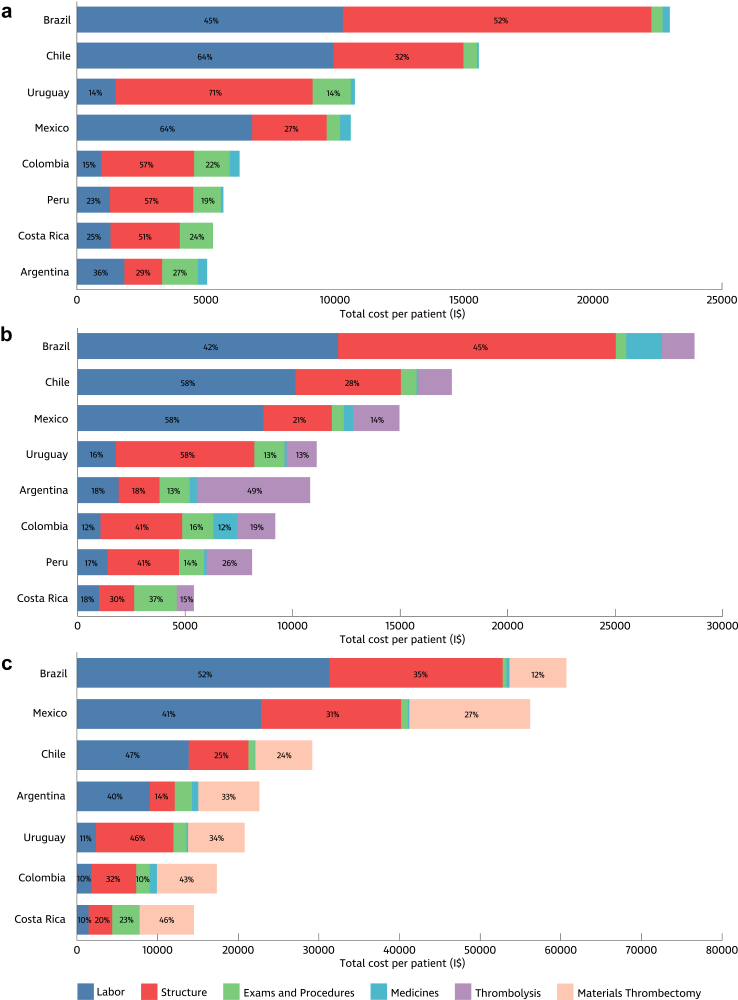
Expanding on our examination of the direct costs associated with acute ischemic stroke treatment, it becomes increasingly apparent that a multitude of factors contribute to the overall financial burden. The most expensive costs identified were hospital structure and labour costs, notably driven by the heightened utilisation of departments such as angiography and the Intensive Care Unit (ICU) for patients undergoing MT (Fig. 1). Compared to other departments, these departments entail higher unit cost rates for infrastructure, attributed to increased depreciation costs and expenses for medical supplies, mechanical ventilation, cardiac monitoring, and renal substitutive therapies, and reflect higher labour costs than the general ward. Moreover, high-risk patients, as well as those receiving MT and combined treatments, required prolonged hospitalisations and more expressive medication use, consequently accounting for the highest mean costs.
Fig. 1.
Cost distribution across treatment types by country. (a) Medical treatment; (b) Intravenous thrombolysis; (c) Mechanical thrombectomy. The contribution of each cost item to the total cost is expressed in percentages. Legends with values below 10% were omitted. I$: International Dollars.
Cost variability by risk level and treatment
Building upon the risk-adjusted cost estimate model established in a prior study,21 patients were categorised into low, medium, and high-risk levels based on age and NIHSS scores upon hospital admission. Refer to the Data Analysis section for the specific methodology.
High-risk patients incurred an average total cost per patient of I$ 17,438 (SD I$ 19,781), whereas medium-risk patients averaged I$ 9926 (SD I$ 12,739), and low-risk patients averaged I$ 9572 (SD I$ 9673). The high-risk level was associated with significantly higher costs than low or medium-risk categories (p < 0.0001). Across all participating countries, mean costs exhibited an upward trend corresponding to patients' clinical risk levels. Treating high-risk patients incurred a substantial cost increase of I$ 7512 compared to medium-risk patients (p < 0.0001) and I$ 7866 compared to low-risk patients (p < 0.0001).
Additionally, this analysis revealed significant disparities in treatment costs among patients across different countries. The Brazilian centre demonstrated the highest mean medical treatment and IVT costs, with expenses reaching I$ 22,978 and I$ 28,688, respectively. With the highest potential for variability, the MT procedure showed the highest cost in Brazil, reaching I$ 60,701, and lowest cost in Costa Rica, at I$ 14,511. The centre in Argentina stood out with the highest cost for the combined treatment (54,906), while the centre in Colombia had the lowest cost (I$ 14,511), as detailed in Table 3. Additionally, specific observations were made regarding treatment access: the centre in Brazil and Mexico incurred notable expenses in MT. In contrast, the centre in Argentina registered higher costs associated with combined treatments. Moreover, limitations in treatment access were identified in certain centres, with Peru lacking access to MT and Chile's essential stroke centre offering solely IVT, with MT cases referred elsewhere.
Table 3.
Differences in costs based on risk stratification levels per treatment.
| Country | Treatments | Risk levels | N | Mean hospital length of stay days (SD) | Mean cost |
|---|---|---|---|---|---|
| All countries | Medical treatment | High risk | 213 (19%) | 16 (18) | I$ 13,552 |
| Medium risk | 333 (30%) | 8 (10) | I$ 8456 | ||
| Low risk | 273 (25%) | 8 (11) | I$ 8342 | ||
| IVT | High risk | 85 (8%) | 12 (12) | I$ 17,323 | |
| Medium risk | 65 (6%) | 8 (7) | I$ 13,477 | ||
| Low risk | 53 (5%) | 8 (8) | I$ 12,984 | ||
| MT | High risk | 30 (3%) | 14 (21) | I$ 34,870 | |
| Medium risk | 15 (1%) | 10 (7) | I$ 18,621 | ||
| Low risk | 4 (0.4%) | 9 (4) | I$ 23,795 | ||
| IVT + MT | High risk | 23 (2%) | 11 (11) | I$ 31,117 | |
| Medium risk | 9 (1%) | 9 (2) | I$ 30,070 | ||
| Low risk | 3 (0.3%) | 8 (3) | I$ 23,310 | ||
| I$ 12,203 | |||||
| Argentina | Medical treatment | High risk | 5 (3%) | 7 (2) | I$ 5839 |
| Medium risk | 73 (49%) | 4 (4) | I$ 4642 | ||
| Low risk | 43 (29%) | 4 (3) | I$ 5655 | ||
| IVT | Medium risk | 8 (5%) | 4 (1) | I$ 9998 | |
| Low risk | 5 (3%) | 4 (2) | I$ 11,957 | ||
| MT | High risk | 5 (3%) | 6 (3) | I$ 20,643 | |
| Medium risk | 3 (2%) | 8 (2) | I$ 25,939 | ||
| IVT + MT | High risk | 4 (3%) | 12 (4) | I$ 49,617 | |
| Medium risk | 1 (1%) | 57 (0) | I$ 91,723 | ||
| Low risk | 1 (1%) | 11 (0) | I$ 39,246 | ||
| I$ 8487 | |||||
| Brazil | Medical treatment | High risk | 17 (11%) | 14 (6) | I$ 31,999 |
| Medium risk | 45 (29%) | 11 (8) | I$ 22,707 | ||
| Low risk | 37 (24%) | 9 (5) | I$ 19,164 | ||
| IVT | High risk | 16 (10%) | 15 (9) | I$ 38,751 | |
| Medium risk | 16 (10%) | 9 (4) | I$ 25,462 | ||
| Low risk | 13 (8%) | 9 (5) | I$ 20,275 | ||
| MT | High risk | 5 (3%) | 20 (8) | I$ 85,661 | |
| Medium risk | 5 (3%) | 10 (5) | I$ 35,741 | ||
| IVT + MT | High risk | 2 (1%) | 17 (1) | I$ 62,315 | |
| Medium risk | 1 (1%) | 38 (0) | I$ 18,145 | ||
| I$ 27,488 | |||||
| Chile | Medical treatment | High risk | 24 (19%) | 26 (23) | I$ 28,374 |
| Medium risk | 30 (24%) | 9 (9) | I$ 13,016 | ||
| Low risk | 46 (37%) | 7 (4) | I$ 10,568 | ||
| IVT | High risk | 5 (4%) | 14 (13) | I$ 21,448 | |
| Medium risk | 5 (4%) | 6 (3) | I$ 12,177 | ||
| Low risk | 13 (10%) | 12 (11) | I$ 17,858 | ||
| MT | High risk | 1 (%) | 41 (0) | I$ 54,265 | |
| Medium risk | 1 (%) | 4 (0) | I$ 13,408 | ||
| Low risk | 1 (%) | 9 (0) | I$ 19,876 | ||
| I$ 16,233 | |||||
| Colombia | Medical treatment | High risk | 34 (22%) | 11 (19) | I$ 6690 |
| Medium risk | 42 (27%) | 6 (7) | I$ 5713 | ||
| Low risk | 28 (18%) | 7 (10) | I$ 6718 | ||
| IVT | High risk | 10 (7%) | 9 (9) | I$ 10,819 | |
| Medium risk | 7 (5%) | 6 (5) | I$ 9063 | ||
| Low risk | 6 (4%) | 3 (2) | I$ 6634 | ||
| MT | High risk | 10 (7%) | 8 (5) | I$ 18,030 | |
| Medium risk | 3 (2%) | 5 (3) | I$ 14,209 | ||
| Low risk | 3 (2%) | 9 (4) | I$ 18,203 | ||
| IVT + MT | High risk | 7 (5%) | 6 (4) | I$ 6924 | |
| Medium risk | 2 (%) | 6 (2) | I$ 3976 | ||
| Low risk | 1 (%) | 8 (0) | I$ 4478 | ||
| I$ 8673 | |||||
| Costa Rica | Medical treatment | High risk | 59 (30%) | 12 (13) | I$ 6924 |
| Medium risk | 53 (27%) | 4 (3) | I$ 3976 | ||
| Low risk | 36 (18%) | 7 (10) | I$ 4478 | ||
| IVT | High risk | 18 (9%) | 5 (4) | I$ 5539 | |
| Medium risk | 10 (5%) | 3 (1) | I$ 4768 | ||
| Low risk | 5 (3%) | 2 (0) | I$ 6225 | ||
| MT | High risk | 4 (2%) | 5 (1) | I$ 15,743 | |
| Medium risk | 1 (1%) | 1 (0) | I$ 9583 | ||
| IVT + MT | High risk | 7 (4%) | 12 (16) | I$ 23,659 | |
| Medium risk | 4 (2%) | 6 (3) | I$ 12,677 | ||
| I$ 6334 | |||||
| Mexico | Medical treatment | High risk | 26 (25%) | 13 (15) | I$ 19,307 |
| Medium risk | 20 (19%) | 8 (26) | I$ 6646 | ||
| Low risk | 25 (24%) | 2 (3) | I$ 4748 | ||
| IVT | High risk | 18 (17%) | 10 (8) | I$ 18,113 | |
| Medium risk | 7 (7%) | 4 (3) | I$ 10,215 | ||
| Low risk | 3 (3%) | 2 (1) | I$ 7187 | ||
| MT | High risk | 3 (3%) | 41 (49) | I$ 56,204 | |
| IVT + MT | Low risk | 1 (1%) | 7 (0) | I$ 31,263 | |
| I$ 13,326 | |||||
| Peru | Medical treatment | High risk | 37 (31%) | 19 (15) | I$ 7121 |
| Medium risk | 45 (38%) | 12 (4) | I$ 4982 | ||
| Low risk | 26 (22%) | 10 (5) | I$ 4824 | ||
| IVT | High risk | 3 (3%) | 23 (11) | I$ 11,454 | |
| Medium risk | 6 (5%) | 11 (4) | I$ 7213 | ||
| Low risk | 2 (2%) | 10 (1) | I$ 5806 | ||
| I$ 5902 | |||||
| Uruguay | Medical treatment | High risk | 11 (11%) | 31 (29) | I$ 20,989 |
| Medium risk | 24 (23%) | 19 (15) | I$ 8932 | ||
| Low risk | 32 (31%) | 19 (24) | I$ 8644 | ||
| IVT | High risk | 15 (15%) | 22 (19) | I$ 11,794 | |
| Medium risk | 7 (7%) | 20 (12) | I$ 11,398 | ||
| Low risk | 6 (6%) | 15 (6) | I$ 9114 | ||
| MT | High risk | 2 (2%) | 15 (13) | I$ 24,210 | |
| Medium risk | 2 (2%) | 24 (4) | I$ 17,394 | ||
| IVT + MT | High risk | 3 (3%) | 18 (11) | I$ 31,965 | |
| Medium risk | 1 (1%) | 15 (0) | I$ 17,459 | ||
| I$ 11,940 |
The numbers in bold are the mean of each category. Above, groups without patient entries are excluded from the table. Low-risk level: patients under 70 years with an NIHSS score <8; Medium-risk level: patients under 70 years with an NIHSS score between 8 and 15, as well as those older than 70 with an NIHSS score <8; High-risk level: patients older than 70 years with an NIHSS score >8 and those with an NIHSS score >15.
IVT = Intravenous Trombolysis; MT = Mechanical Thrombectomy; mRS = modified Rankin Score; SD = Standard Deviation; NA = Not applicable.
Cost drivers and variabilities in stroke centres and treatment modalities
The length of hospital stay is the primary cost driver in stroke centres, directly impacting medication consumption, diagnostic tests, hospital infrastructure usage, and visits from staff. The Argentinian centre achieved the lowest mean times among the observed variabilities across all treatments and risk levels. In contrast, the Uruguayan centre demonstrates longer hospital stays for medical treatment and IVT, while the Brazilian centre notably exhibits prolonged hospitalisation for combined treatment.
The hospital structure emerged as one of the most expensive cost components across countries and treatments, influenced by the in-hospital processes adopted by each centre (Fig. 1). The stroke centres from Argentina, Costa Rica, Uruguay, and Colombia exhibit a similar pattern, characterised by shorter lengths of stay in the emergency department and the proportion of time spent in the ward and stroke units. A different behaviour is encountered in stroke centres in Brazil and Chile, where emergency departments and stroke units concentrate on longer periods of hospitalisation. In addition to the care pathways variabilities observed, structural costs attributed to each unit also contributed to the cost differences measured. For instance, the stroke centre in Chile incurred higher depreciation, energy, and support expenses, while Peru had the lowest depreciation and support supply costs.
Labour costs were a significant cost driver, showing important variabilities across all countries. The centre in Chile has the highest labour cost rate, followed by the centre in Brazil, with the centre in Colombia exhibiting high rates, particularly for specialised professionals. The lowest unit cost rates were observed in the centre in Uruguay for specialised professionals and in the centre in Colombia for non-specialized ones. Among the exams, brain computed tomography scans showed a disparity of up to five times between the lowest cost (Chile, I$ 46) and the highest (Uruguay, I$ 225). The variability in IVT treatment costs follows a similar pattern, with the centre of Costa Rica having the lowest cost (I$ 400) and the centre of Argentina the highest (I$ 1872). Tenecteplase (I$ 1767) was administered to 14 out of 203 IVT patients. Supplementary Material V contains the cost composition information per country, risk level, and therapy for detailed cost information. A table summarizing the costs in medians and interquartile ranges is provided in Supplementary Material VI.
Discussion
This study aimed to evaluate the direct costs of acute ischemic stroke treatment in Latin America using TDABC, highlighting cost variability across countries and treatment modalities. Significant differences were observed, with direct costs ranging from I$ 5902 in Peru to I$ 27,488 in Brazil, underscoring the economic disparities in stroke care across the region. The mean costs for different treatments were as follows: combined IVT plus MT at I$ 29,016, MT alone at I$ 30,153, medical treatment at I$ 9,735, and IVT at I$ 14,930. These findings provide a detailed view of the economic impact of stroke management in Latin America, offering essential insights for policymakers and healthcare providers to enhance care delivery and reduce healthcare system burdens.23,24
Cost variations in acute ischemic stroke care across Latin America can be attributed to differences in care protocols, healthcare systems, access to treatments, professional capacity, varying lengths of hospital stays, stroke severity, and costs of exams, medications, and treatments.25,26 For example, Chile and Brazil demonstrated the highest medical treatment costs, partly due to higher depreciation, energy, and support expenses, and longer periods of hospitalization in emergency departments and stroke units, while Costa Rica had the lowest costs for MT procedures. Argentina had the highest cost for combined treatment, and Colombia had the lowest. Additionally, longer hospital stays, a primary cost driver, were observed in Uruguay, whereas Argentina had shorter stays. Reflecting the unique circumstances of each country's healthcare system, these variations underscore the imperative for targeted interventions to address disparities and enhance healthcare delivery. As treatment complexity rises, fewer patients can receive reperfusion therapies, highlighting the importance of raising public awareness about stroke and emphasizing the need for enhanced coordination between pre-hospital and hospital systems in stroke care.11 Extending the availability of 10 and 20 mg of rtPA in Latin American countries, mirroring the practice in Brazil, can reduce treatment costs minimizing medication wastage.27 Furthermore, despite their higher costs, advanced treatments like MT and combined IVT plus MT have been associated with improved outcomes, as evidenced by higher rates of improvement in NIHSS and mRS scores among recipients.9
Stratifying costs according to risk levels (high, medium, and low) reveals significant variation per patient among countries, offering insights into the correlation between clinical profiles, treatment received, and projected treatment costs. A global concern emerges from the increasing stroke incidence among younger patients, particularly in LMICs.3,4 The predominance of moderate-risk patients, largely due to their younger age rather than NIHSS scores, underscores the urgent need for investments in awareness and prevention efforts to mitigate future stroke burdens over the next 30 years.5 Global collaboration is essential to ensure widespread access to life-saving stroke treatments. Recently, initiatives led by the World Stroke Organization and the Iberoamerican Stroke Society have focused on enhancing stroke care implementation in Latin America. This has been achieved through the collaborative efforts of the Global Stroke Alliance Conferences and the Latin American Stroke Ministerial Meeting,4 reflecting a strong commitment to improving accessibility to stroke prevention, treatment, and rehabilitation. Addressing these disparities through these initiatives is paramount to ensuring equitable access to essential stroke interventions and alleviating the financial strain on healthcare systems in LMICs.28
Implementing risk and outcomes-adjusted mechanisms for reimbursing healthcare systems to optimise public spending necessitates a thorough understanding of the factors influencing cost variations.28 Proposed pilot studies exploring innovative provider payment systems present promising avenues to tackle this challenge.29 Understanding the detailed costs of stroke care has significant implications for the countries included in our study. The findings can inform policy initiatives to optimize resource allocation and improve efficiency for countries with comprehensive public health systems like Chile, Uruguay, and Brazil. For example, Chile can address higher costs from longer hospital stays and structural expenses, while Uruguay can explore reducing unnecessary hospital days. Even in countries with fragmented systems, like Mexico, detailed cost information helps identify high-cost areas and standardise care protocols to improve cost management. Introducing changes to hospital policies, focused on standardising resource utilisation and minimising variation in length of stay, could effectively curb costs and enhance efficiencies in acute stroke management. The efficacy of these strategies is likely to be bolstered by broader policy initiatives currently underway to reform hospital reimbursement systems.29 Collaborative initiatives led by local health authorities should prioritise evidence-based reperfusion therapies within preventive strategies, integrating economic considerations to address evolving stroke challenges, enhance patient outcomes, and mitigate societal and economic burdens. This approach aids decision-making processes by informing resource allocation strategies and underscores the importance of integrating value principles into healthcare financing strategies.
Overall, the findings of this study point to the need for targeted interventions to address disparities in stroke care access and outcomes across Latin America. By leveraging standardised costing methods and VBHC strategies, policymakers and healthcare providers can work collaboratively to optimise resource allocation and improve stroke care delivery. However, further research is warranted to explore the long-term clinical and economic implications of stroke management in the region, considering both direct and indirect costs. Additionally, efforts to enhance access to life-saving interventions like MT must be prioritised to ensure equitable healthcare delivery for all stroke patients, regardless of geographical location or socioeconomic status.
Our study has limitations, including the limited representativeness of the distinct stroke centre (comprehensive vs primary) and hospital (private, public, academic) types. Moreover, it focuses solely on direct and acute hospitalisation costs. Additionally, our study's selection of stroke centres based on convenience may have resulted in a biased sample of hospitals, potentially not fully representing the diversity of stroke care costs in Latin America due to variations in labour costs and infrastructure among different regions. Most of the countries included in this study are classified as having relatively higher economic resources within the region, which may limit the generalisability of our findings to less affluent settings. The inclusion of only one centre per country further restricts the generalisability of the results and prevents an analysis of cost variability across different facility types (public, private, and academic) within individual countries Our study assumed a uniform care flow across all centres. While this facilitated our analysis, it may not fully account for variations in stroke care delivery between different countries and centres. Despite these limitations, the study highlights standardised micro-costing techniques, which are recognised as the gold standard in health economic analyses. The cost results from 8 Latin American countries suggest that the standardised framework employed here could be replicated worldwide, enhancing cost information granularity and facilitating more effective resource distribution policies in LMICs where evidence remains limited. Future research should investigate the variabilities in cost acquisition for specific items like medications and materials and explore socio-determinants of health such as sex, socioeconomic status, race, ethnicity, and age to identify variations in costs and outcomes across the region. Additionally, future studies should examine the implications of indirect costs, long-term follow-up costs, and clinical outcomes associated with stroke management in the region.
Conclusion
This is Latin America's largest real-world data-based focusing on the costs of acute ischemic stroke treatment in Latin America. Our findings provide crucial guidance for healthcare policy and resource allocation, particularly contributing to expanding access to reperfusion treatments. By elucidating the factors driving cost variability and their implications, our findings contribute to the ongoing efforts to enhance the accessibility, affordability, and quality of stroke care in the region. Continued investment in evidence-based research and policy interventions from all stakeholders is key to ensuring equitable access to optimal stroke care and reducing stroke-related disabilities and deaths in Latin America.
Contributors
ACS, APE, GS, CP, LBD, ER, PA, MB and SM conceptualized the structure and design of the manuscript. LBD, APE, MM conducted the main data analysis. LBD wrote the first draft of the manuscript, and ACS, APE, CP, SM, GN, and RN revised all sections and wrote the final version. All other coauthors provided critical intellectual contributions, contributed to the overall structure and concepts, and approved the final version.
ACS, APBSE, and JSS verified the data. ACS and LBD had access to raw data, and APBSE and ACS had the final responsibility for the decision to submit it for publication.
Data sharing statement
The data supporting this study's findings are available from the corresponding author upon reasonable request. All data shared will be anonymized to protect participant privacy.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT (GPT-4 version) to review and ensure clarity and coherence of the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of interests
ACS has received speaker fees from BI and limited grants from the World Stroke Organization and BI. PA has received speaker fees from BI, Ipsen, and Abbott. LAC reports receiving limited grants from the World Stroke Organization and BI/Angels Initiative and consulting and speaker fees from Allm, AstraZeneca, Boehringer Ingelheim, and ISchemaView, outside of this work. RGN reports consulting fees for advisory roles with Anaconda, Biogen, Cerenovus, Genentech, Philips, Hybernia, Hyperfine, Imperative Care, Medtronic, Phenox, Philips, Prolong Pharmaceuticals, Stryker Neurovascular, Shanghai Wallaby, Synchron, and stock options for advisory roles with Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, CrestecBio Inc., Euphrates Vascular, Inc., Vesalio, Viz-AI, RapidPulse and Perfuze. RGN is one of the Principal Investigators of the “Endovascular Therapy for Low NIHSS Ischemic Strokes (ENDOLOW)” trial. Funding for this project is provided by Cerenovus. RGN is the Principal Investigator of the “Combined Thrombectomy for Distal MediUm Vessel Occlusion StroKe (DUSK)” trial. Funding for this project is provided by Stryker Neurovascular. RGN is an investor in Viz-AI, Perfuze, Cerebrotech, Reist/Q’Apel Medical, Truvic, Tulavi Therapeutics, Vastrax, Piraeus Medical, Brain4Care, Quantanosis AI, and Viseon. GS received a modest compensation from the World Stroke Organization for his role of Executive Director/Editor-in-Chief of the World Stroke Academy (WSA). SM reports funding from the Brazilian Ministry of Health for the Resilient and PROMOTE trials and speaker fees from Boehringer Ingelheim, Pfizer, Bayer, Medtronic, Penumbra, Novartis, Novo Nordisk, Servier, Daiichi Sankyo, and Astra Zeneca.
Acknowledgements
We extend our heartfelt gratitude to the entire research team for their unwavering dedication to this project. Special thanks are due to Eder Moreno from the Centro de Investigaciones Clínicas of Fundación Valle del Lili and Mariana Carmona from the Faculty of Health Sciences of the Universidad Icesi for their invaluable assistance in data collection. We also express our deepest appreciation to the patients and their families who participated in this study.
Funding: The project received funding from the World Stroke Organization and Boehringer Ingelheim International (BI) IS 0135-0352. BI had no role in the design, analysis, or interpretation of the results in this study.
Disclaimer: This summary is available in Spanish and Portuguese in the Supplementary Material.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100959.
Appendix ASupplementary data
References
- 1.Johnson C.O., Nguyen M., Roth G.A., et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics—2021 update: a report from the American heart association. Circulation. 2021 doi: 10.1161/cir.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 3.Feigin V.L., Brainin M., Norrving B., et al. World stroke organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- 4.Ouriques Martins S.C., Sacks C., Hacke W., et al. Priorities to reduce the burden of stroke in Latin American countries. Lancet Neurol. 2019;18:674–683. doi: 10.1016/S1474-4422(19)30068-7. [DOI] [PubMed] [Google Scholar]
- 5.Feigin V.L., Owolabi M.O., World Stroke Organization–Lancet Neurology Commission Stroke Collaboration Group Pragmatic solutions to reduce the global burden of stroke: a world stroke organization-lancet neurology commission. Lancet Neurol. 2023;22(12):1160–1206. doi: 10.1016/S1474-4422(23)00277-6. Epub 2023 Oct 9. Erratum in: Lancet Neurol. 2023 Dec;22(12):e13. PMID: 37827183; PMCID: PMC10715732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The national Institute of neurological disorders and stroke rt-PA stroke study group tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M., Menon B.K., van Zwam W.H., et al. HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. Epub 2016 Feb 18. PMID: 26898852. [DOI] [PubMed] [Google Scholar]
- 8.Joo H., Wang G., George M.G. A literature review of the cost-effectiveness of intravenous recombinant tissue plasminogen activator for treating acute ischemic stroke. Stroke Vasc Neurol. 2017;2:73–83. doi: 10.1136/svn-2016-000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Souza A.C., Martins S.O., Polanczyk C.A., et al. Cost-effectiveness of mechanical thrombectomy for acute ischemic stroke in Brazil: results from the RESILIENT trial. Int J Stroke. 2021 doi: 10.1177/17474930211055932. [DOI] [PubMed] [Google Scholar]
- 10.Martins S.C.O., Lavados P., Secchi T.L., et al. Fighting against stroke in Latin America: a joint effort of medical professional societies and governments. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.743732. PMID: 34659101; PMCID: PMC8517273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsay P., Furie K.L., Davis S.M., Donnan G.A., Norrving B. World stroke organization global stroke services guidelines and action plan. Int J Stroke. 2014;9:4–13. doi: 10.1111/ijs.12371. [DOI] [PubMed] [Google Scholar]
- 12.de Souza A.C., Sebastian I.A., Zaidi W.A.W., et al. Regional and national differences in stroke thrombolysis use and disparities in pricing, treatment availability, and coverage. Int J Stroke. 2022;17(9):990–996. doi: 10.1177/17474930221082446. Epub 2022 Mar 18. PMID: 35137645. [DOI] [PubMed] [Google Scholar]
- 13.Rochmah T.N., Rahmawati I.T., Dahlui M., Budiarto W., Bilqis N. Economic burden of stroke disease: a systematic review. Int J Environ Res Public Health. 2021;18(14):7552. doi: 10.3390/ijerph18147552. PMID: 34299999; PMCID: PMC8307880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond M.F., Sculpher M.J., Torrance G.W., O’Brien B.J., Stoddart G.L. 3rd ed. Oxford University Press; Oxford: 2005. Methods for the economic evaluation of health care programmes; p. 396. [Google Scholar]
- 15.Porter M.E. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. Epub 2010 Dec 8. PMID: 21142528. [DOI] [PubMed] [Google Scholar]
- 16.Porter Michael E., Mountford James, Ramdas Kamalini, Takvorian Samuel. Harvard Business School; 2012. Reconfiguring stroke care in north central London. Case 712-496. [Google Scholar]
- 17.Kaplan R., Anderson S. Harvard Business School Publishing Corporation; Boston: 2007. Time-driven activity-based costing: a simpler and more powerful path to higher profits, vol. 82. [PubMed] [Google Scholar]
- 18.da Silva Etges A.P.B., Ruschel K.B., Polanczyk C.A., Urman R.D. Advances in value-based healthcare by the application of time-driven activity-based costing for inpatient management: a systematic review. Value Health. 2020;23(6):812–823. doi: 10.1016/j.jval.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Feeley TW, Mohta NS. Transitioning payment models: fee-for-service to value-based Care. NEJM Catalyst. 2018 https://catalyst.nejm.org Available at: [Google Scholar]
- 20.da Silva Etges A.P.B., Cruz L.N., Notti R.K., et al. An 8-step framework for implementing time-driven activity-based costing in healthcare studies. Eur J Health Econ. 2019;20:1133–1145. doi: 10.1007/s10198-019-01085-8. [DOI] [PubMed] [Google Scholar]
- 21.Etges Ana Paula Beck da Silva, Marcolino M.A.Z., Ogliari L.A., et al. Moving the Brazilian ischaemic stroke pathway to a value-based care: introduction of a risk-adjusted cost estimate model for stroke treatment. Health Pol Plann. 2022;37(9):1098–1106. doi: 10.1093/heapol/czac058. [DOI] [PubMed] [Google Scholar]
- 22.Muchada M., Rubiera M., Rodriguez-Luna D., et al. Baseline National Institutes of Health stroke scale-adjusted time window for intravenous tissue-type plasminogen activator in acute ischemic stroke. Stroke. 2014;45(4):1059–1063. doi: 10.1161/STROKEAHA.113.004307. Epub 2014 Mar 6. PMID: 24603070. [DOI] [PubMed] [Google Scholar]
- 23.Evers S.M., Struijs J.N., Ament A.J., van Genugten M.L., Jager J.H., van den Bos G.A. International comparison of stroke cost studies. Stroke. 2004;35(5):1209–1215. doi: 10.1161/01.STR.0000125860.48180.48. Epub 2004 Apr 8. PMID: 15073405. [DOI] [PubMed] [Google Scholar]
- 24.Isard P.A., Forbes J.F. The cost of stroke to the national service in Scotland. Cerebrovasc Dis. 1992;1/2:47–50. [Google Scholar]
- 25.Kaur P., Kwatra G., Kaur R., Pandian J.D. Cost of stroke in low and middle income countries: a systematic review. Int J Stroke. 2014;9(6):678–682. doi: 10.1111/ijs.12322. Epub 2014 Jul 7. PMID: 25041736. [DOI] [PubMed] [Google Scholar]
- 26.Wei J.W., Heeley E.L., Jan S., et al. Variations and determinants of hospital costs for acute stroke in China. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013041. PMID: 20927384; PMCID: PMC2946911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins S.C., Pontes-Neto O.M., Alves C.V., et al. Brazilian Stroke Network Past, present, and future of stroke in middle-income countries: the Brazilian experience. Int J Stroke. 2013;8(Suppl A100):106–111. doi: 10.1111/ijs.12062. Epub 2013 May 22. PMID: 23692595. [DOI] [PubMed] [Google Scholar]
- 28.de Souza A.C., Lioutas V., Sebastian I., et al. Stroke beyond borders: building strategies to improve stroke care worldwide. Stroke. 2023 doi: 10.1161/STROKEAHA.122.038459. Epub ahead of print. PMID: 37264914. [DOI] [PubMed] [Google Scholar]
- 29.Etges A.P.B.D.S., de Souza A.C., Jones P., et al. Variation in ischemic stroke payments in the USA: a medicare beneficiary study. Cerebrovasc Dis. 2023;53:298–306. doi: 10.1159/000533513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.