Abstract
Dermal white adipose tissue (dWAT), distinguished by its origin from cells within the dermis and independence from subcutaneous fat tissue, has garnered significant attention for its non-metabolic functions. Characterized by strong communication with other components of the skin, dWAT mediates the proliferation and recruitment of various cell types by releasing adipogenic and inflammatory factors. Here, we focus on the modulatory role of dWAT at different stages during wound healing, highlighting its ability to mediate the adipocyte-to-myofibroblast transition which plays a pivotal role in the physiology and pathology processes of skin fibrosis, scarring, and aging. This review highlights the regulatory potential of dWAT in modulating wound healing processes and presents it as a target for developing therapeutic strategies aimed at reducing scarring and enhancing regenerative outcomes in skin-related disorders.
Keywords: Wound healing, Dermal white adipose tissue, Skin, Regeneration
1. Introduction
Skin, the largest organ of the human body, is composed of the epidermis, the dermis, and the hypodermis or subcutaneous adipose layer [[1], [2], [3]]. As the first barrier against external stimuli, skin is highly susceptible to injuries that can result in fibrotic and non-functional scars during wound healing, affecting millions of people worldwide each year [4,5].
Notably, adipose tissue, situated within the dermal layer of the skin, is gaining recognition as a significant contributor to tissue repair [6]. In addition to storing and mobilizing lipids in response to systemic nutritional and metabolic demands, adipose tissue actively participates in maintaining skin homeostasis [7,8]. As one of the important components of adipose tissue, dermal white adipose tissue (dWAT) resides beneath and is partially integrated with the reticular dermis, playing a crucial role in the wound healing process [6,9,10]. Despite this, dWAT in the skin serves indispensable metabolic roles such as energy storage, thermal insulation, adipokine secretion, and mechanical support [11,12]. In the past decade, alongside its traditional metabolic functions, the nonmetabolic roles of dWAT have garnered increasing attention, including modulating inflammation, facilitating the hair cycle, and tissue remodeling. Research has focused on its ability to undergo periodic remodeling in accordance with the hair cycle, highlighting the intricate signaling interplay between hair follicles and dWAT [13]. It has been suggested that deficiencies in dWAT function may disrupt the hair cycle [13,14]. Furthermore, recent findings have underscored dWAT's involvement in wound healing, attributed to the release of adipocyte-derived lipids that facilitate macrophage recruitment and revascularization [15,16], as well as the mediation of fibroblast function to promote tissue regeneration rather than scarring. In this review, we aim to underscore the pivotal role of dWAT in skin protection, wound repair, and regeneration.
2. Dermal adipose tissue and wound healing
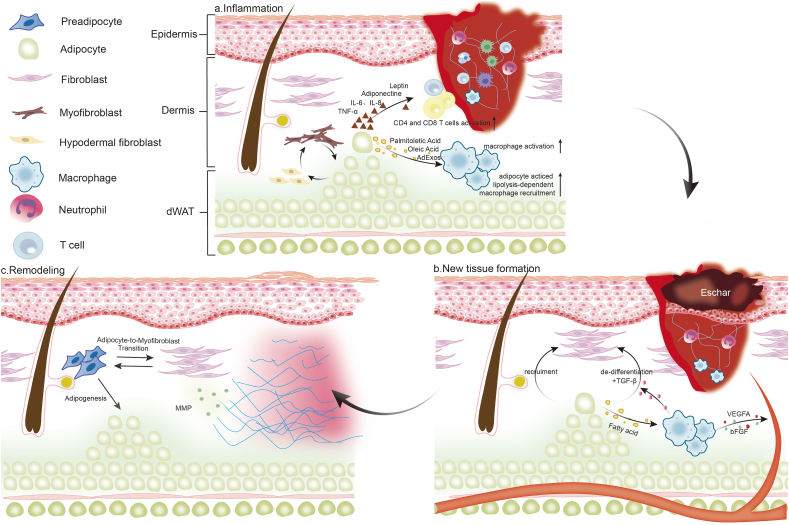
The process of wound repair typically unfolds through three distinct but interrelated stages: inflammation, new tissue formation, and remodeling [15]. The inflammatory phase, which spans up to 48 h post-injury, is characterized by the formation of fibrin clots within an anoxic environment, facilitating the recruitment and proliferation of migrating cells [17,18]. Subsequently, the proliferative phase unfolds over days to weeks, during which the initial clot transforms into granulation tissue, aided by chemoattractant derived from inflammatory cells that promote the migration of cells such as adipose-derived stem cells (ADSC) and fibroblasts into the wound [19]. Finally, the remodeling stage extends for a year or more, during which disorganized collagen III is gradually replaced by collagen type I, and blood vessels regress as endothelial cells, macrophages, and myofibroblasts either undergo apoptosis or exit the injury site [20,21]. Throughout these stages, characterized by persistence and overlap, dWAT plays a crucial role through paracrine signaling and cellular transition between myofibroblast and adipocyte.
2.1. Inflammation phase
During the inflammatory phase, the continuous infiltration of neutrophils, monocytes, and lymphocytes stands out as a hallmark [18]. Immunocytes represent the second most abundant cellular component within adipose tissue, following adipocytes. The delicate equilibrium and reciprocal interactions between adipocytes and immunocytes play pivotal roles in sustaining metabolic and immune homeostasis. For instance, when adipose tissue undergoes a state of chronic but low-grade inflammation, it becomes infiltrated by type 1 macrophages and T cells, thereby eliciting the production of pro-inflammatory mediators that disrupt metabolic functionality, culminating in metabolic dysregulation [22]. Conversely, excessive adipose accumulation and concomitant insulin resistance are intricately linked to alterations in both the quantity and functionality of immunocytes, notably the augmentation and activation of type 1 macrophages and T cells [23,24]. When the skin is injured, adipose cells facilitate the activation and progression of the inflammatory response through various mechanisms.
2.1.1. Adipocytes orchestrate macrophage function
Researchers have demonstrated that the loss of dermal adipocytes leads to a significant delay in skin wound healing, accompanied by impaired recruitment of inflammatory macrophages. Further studies have revealed that dWAT upregulate breakdown of triglyceride, resulting in the release of fatty acids, including palmitoleic acid and oleic acid, around the wound site. This, in turn, attracts and activates pro-inflammatory macrophages, expediting wound vascularization. Interestingly, the molecular mechanisms through which dermal adipocytes influence macrophage phenotype transition via metabolic products remain unclear [25]. Future investigations will be required to elucidate how adipocytes regulate macrophage activity in dWAT, potentially involving mechanisms such as exosomes and metabolic programming.
The activation of lipolysis is associated with increased lipid mobilization and increased numbers of adipose tissue macrophages. Adipocyte-derived lipids might be directly signaling to macrophages because macrophages express multiple fatty acids (FAs) receptors and transporters, and inflammatory macrophages rely on glycolysis for energy [26]. Interestingly, heat-inactivated, adipocyte-conditioned media increases monocyte/macrophage migration in vitro, demonstrating that lipids can promote macrophage migration [27]. Researchers observed that inhibiting dermal adipocyte lipolysis decreases the number of medium-chain FAs that could promote macrophage migration through GPR84 signaling [25,28]. Mechanistically, upon activation by fatty acids (FAs), GPR84 primarily couples with Gi/o proteins in the cell membrane, leading to the phosphorylation of Akt, ERK1/2, and NF-κB-p65 in macrophages. This signaling cascade enhances the expression of various pro-inflammatory cytokines, including TNFα, IL-6, and IL-12B, as well as chemokines such as CCL2, CCL5, and CXCL1, particularly in LPS-treated macrophages [29].
Macrophages are key cells that mediate the innate immunity of neutrophils and the adaptive immunity of T cells. Adipose tissue regulates macrophage function by secreting relevant factors, which orchestrate the changes during the healing phases. The polarized state of macrophages can be classified into two subsets: classically activated (M1) and alternatively activated (M2). M1 macrophages are characterized by high expression of genes associated with pro-inflammatory cytokines and oxidative stress, including TNF-alpha, IL-6, MCP-1, and iNOS. In contrast, M2 macrophages express genes encoding arginase-1, Mrc1 (CD206), Ym1, and IL-10, which is an anti-inflammatory cytokine [30]. dWAT contains a higher proportion of M2-type macrophages compared to skin tissue, marked by CD163 expression and the absence of CD11c. Additionally, dWAT is rich in ADSCs, which promote the polarization of macrophages towards the pro-repair M2 phenotype. This polarization alleviates excessive inflammatory responses and oxidative stress during tissue damage, creating a favorable environment for healing [31]. Transplanting ADSCs into wound beds can alter the phenotype of wound bed macrophages, inducing TGF-β(1)-dependent angiogenesis, fibroblast differentiation, and granulation tissue formation, thereby enhancing overall tissue repair [32]. The adipocytes, as the primary components of dWAT, play a crucial role in controlling inflammation and regulating immune responses. They secrete adipokines—such as inflammatory factors (IL-1β and IL-17) and fibrotic factors (TGF-β1). For instance, the adipokine leptin promotes the production of pro-inflammatory cytokines and activates CD4+ and CD8+ T cells [33]. Conversely, the adipokine adiponectin has an anti-inflammatory effect by inhibiting tumor necrosis factor-alpha expression in monocytes, suppressing macrophage and T-cell proliferation, and reducing IL-10 production [33]. Future studies elucidating the mechanisms by which FAs drive macrophage recruitment to tissues may lead to therapies for multiple inflammatory conditions.
2.1.2. Adipocytes trigger inflammation via cytokines and adipokines
Adipocytes exhibit a multifaceted response to tissue injury, playing crucial roles beyond mere release of fatty acid. In addition to their capacity for antigen recognition via pattern recognition receptors and adaptor proteins, adipocytes contribute significantly to the inflammatory milieu by secreting an array of inflammatory cytokines and chemokines, notably interleukin-6 (IL-6), interleukin-8 (IL-8), and various adipokines [34]. Among these, leptin and adiponectin emerge as key adipokines with distinct immunomodulatory functions. While leptin promotes the production of proinflammatory cytokines and activates both CD4 and CD8 T cells [35,36], adiponectin exerts anti-inflammatory effects by inhibiting tumor necrosis factor-alpha (TNF-α) expression and suppressing the proliferation of macrophages and T cells [37].
Following skin tissue damage, inflammation, or bacterial infection, adipose tissue undergoes localized expansion, termed reactive adipogenesis. In response to Staphylococcus aureus skin infection, the expanded dermal fat depot synthesizes cathelicidin [38]. Among these antimicrobial peptides, human LL-37, expressed in neutrophils and inflamed skin keratinocytes, serves as a prominent mediator of innate defense responses [39]. Besides their antimicrobial properties, antimicrobial peptides, including cathelicidins, facilitate skin healing by promoting re-epithelialization [40]. This observation aligns with previous research indicating that adipocytes release a plethora of adipogenic and inflammatory factors, thereby orchestrating the inflammatory cascade following injury [41].
In summary, the inflammatory phase is critical for initiating the wound healing process, with dWAT playing a central role in modulating immune cell recruitment and activation. By releasing fatty acids and cytokines, dWAT influences macrophage function, which is vital for the subsequent transition to the new tissue formation phase.
2.2. New tissue formation phase
2.2.1. dWAT contributes to the recruitment of fibroblasts
After the hemostasis and the clearance of foreign pathogens by immune cells, the second stage consists of the proliferation and migration of different cells. Fibroblasts play a key role in promoting wound healing, which undergoes marked changes in gene expression and phenotype. Most of the fibroblasts are stimulated by macrophages and attracted from the edge of the wound and bone marrow, and some differentiate into myofibroblasts [42,43]. During this process, dWAT serves important functions, such as the recruitment of fibroblasts and the transition mechanism of myofibroblasts [44]. Inhibition of adipogenesis results in impaired wound healing with the reduction of fibroblast migration, demonstrating the indispensable role of dWAT and intercellular communication between adiposes and fibroblasts during wound healing [45].
2.2.2. dWAT contributes to the transition of fibroblasts
Following hemostasis and the clearance of foreign pathogens by immune cells, the subsequent phase of wound healing involves the proliferation and migration of various cell types. Fibroblasts, in particular, play a pivotal role in driving wound healing processes, marked by significant changes in gene expression and cellular phenotype. Cytokine stimulation triggers the activation of most fibroblasts, prompting their migration from the wound's periphery, with some originating from fascial tissue [42,43]. Throughout this intricate process, dWAT assumes crucial functions, including the recruitment of fibroblasts and the orchestration of myofibroblast transition. Inhibition of adipogenesis leads to impaired wound healing, characterized by reduced fibroblast migration, underscoring the essential role of the interplay between adipose tissues and fibroblasts in the wound healing cascade [45].
Prior studies have indicated a potential shared lineage relationship between fibroblasts and adipocytes, suggesting their derivation from a common fibro/adipogenic progenitor during skeletal muscle regeneration [46,47]. Notably, investigations employing transgenic lineage-tracing mice have provided compelling evidence supporting the hypothesis that dermal adipocytes may serve as progenitors for myofibroblasts in fibrotic conditions. Specifically, in murine models of bleomycin-induced fibrosis, genetically labeled adipocytes underwent a transition characterized by the loss of adipocytic markers and the acquisition of myofibroblast features [48].
In the realm of wound healing, research has unveiled the potential of dermal adipocytes to migrate into the wound bed, undergo dedifferentiation and assume a fibroblast-like phenotype. Studies by Shook et al. demonstrated that following complete lipid depletion, dermal white adipocytes undergo a process of dedifferentiation, transforming into myofibroblasts that migrate to the injury site and contribute to wound healing through the secretion of extracellular matrix(ECM) components [25]. A recent study published in Cell Metabolism indicates that adipocytes from both sWAT and dWAT serve as an important cell source for early wound repair [49]. This phenomenon of adipocyte dedifferentiation has also been observed in various organ systems, including mammary tissue and hair follicle cycling [50,51]. Furthermore, investigations have highlighted that the wound microenvironment stimulates dermal adipocyte-derived fibroblasts at the wound margin to re-enter the cell cycle. These collective findings underscore the remarkable cellular plasticity of dermal adipocytes in promoting tissue repair, challenging the traditional notion of adipocytes as terminally differentiated cells [25,52].
Myofibroblasts actively participate in wound healing and regeneration, significantly impacting its outcome due to their dual characteristics of fibroblast-like secretory activity and smooth muscle cell-like contractile function [53]. Myofibroblasts can contract the edges of the wound and interact with fibroblasts and produce ECM through their contractile properties, excessive collagen deposition, and secretion of profibrotic cytokines. Studies have identified the myofibroblast as a plastic cell type that can reprogram and generate adipocytes and contribute to scar formation [20]. As previously implicated, mature adipocytes acquire a fibroblast-like transcriptional signature after wound signals, the dermal adipocyte-to-myofibroblast transformation supports the key role of dWAT in the pathogenesis of fibrosis and scarring of the skin. This further suggests that inhibition or reversal of adipocyte transdifferentiation into myofibroblasts, enhanced survival of adipocyte stem cells and their progeny, and expression of anti-fibrotic cytokines are potential strategies for effective anti-fibrotic therapy [48].
Myofibroblasts actively contribute to wound healing and regenerative outcomes, exerting a significant impact due to their dual characteristics of fibroblast-like secretory activity and smooth muscle-like contractile function [5]. Through mechanical contraction signals, myofibroblasts engage with fibroblasts and drive the production of abnormal ECM characterized by excessive collagen deposition and profibrotic cytokine secretion. Notably, research has recognized myofibroblasts as a versatile cell type capable of being reprogrammed into adipocytes to potentially rescue scar formation [20]. As previously observed, mature adipocytes undergo a shift toward a fibroblast-like transcriptional profile in response to wound signaling, underscoring the significance of dermal adipocyte-to-myofibroblast transformation in the pathogenesis of fibrosis and scar formation. This suggests that inhibiting or reversing the differentiation of adipocytes into myofibroblasts, promoting the survival of adipocyte stem cells and their progeny, or reprogramming myofibroblasts into adipocytes may represent potential strategies for effective anti-fibrotic therapy.
In the proliferative phase, factors secreted by macrophages, such as transforming growth factor-β (TGF-β), induce the transdifferentiation of fibroblasts into myofibroblasts [5]. In the process of fibroblast-myofibroblast transition, TGF-β1 signals through the Smad2/3 pathway, leading to the upregulation of Smad target genes, including various ECM molecules and integrin receptors. These integrin receptors can bind to the arginylglycylaspartic acid (RGD) peptide within the latent TGF-β complex, exposing a cleavage site that facilitates the release of active TGF-β. This mechanism establishes a feed-forward loop, perpetuating the process through the site-specific activation of TGF-β once it is initiated [54].
ADSC play a vital role in wound healing and remodeling due to their multipotent capability. However, TGF-β-mediated myofibroblast differentiation of ADSC exacerbated skin fibrosis. Adipocyte dedifferentiation into myofibroblasts, known as the adipocyte-myofibroblast transition (AMT), plays a significant role in the development of skin fibrosis [48]. This process is largely driven by TGF-β signaling, leading to the loss of adipogenesis markers and the accumulation of α-SMA-positive myofibroblasts. Both adipose progenitor cells and mature adipocytes can contribute to AMT, with mature adipocytes dedifferentiating into PDGFRα+ preadipocytes as an intermediate step [51]. Strategies to reverse this process include inhibiting TGF-β signaling and utilizing PPARγ agonists or activators of the adiponectin receptor signaling pathway to block the fibrotic response [55,56]. Additionally, adiponectin plays an anti-fibrotic role by regulating TGF-β and CTGF signaling [57,58], highlighting the therapeutic potential of activating adiponectin receptors to alleviate fibrosis. Remodeling of the dermis is essential in the wound healing process to avoid the formation of depressed scars. Adipocytes can repopulate skin wounds after inflammation and during fibroblast and endothelial cell recruitment. During this process, adipocyte precursor cells proliferate and mature adipocytes repopulate skin wounds after inflammation and concurrently with fibroblast migration [45].
In the process of AMT, Wnt-β-catenin plays a stabilizing role. The Wnt/β-catenin pathway is implicated in the pathogenesis of systemic sclerosis and drives fibrosis by promoting fibroblast activation while inhibiting adipocyte differentiation. In transgenic mice expressing persistently active β-catenin in platelet-derived growth factor receptor alpha-positive (PDGRα+) progenitor cells, there is a notable attrition of the dWAT layer and an increase in fibrotic dermis [59]. This process is characterized by a decrease in adipocyte differentiation markers (e.g., FABP4, perilipin, CCAAT/enhancer-binding protein α) and the replacement of dWAT with fibrotic tissue. Specifically, the continuous activation of β-catenin in PDGRα+/Sca1+ fibroblasts indicate a significant impairment of adipogenesis, which facilitates the transition of progenitor cells into fibrotic fibroblasts rather than adipocytes [60]. Therefore, the Wnt/β-catenin pathway is critical in driving fibrosis by suppressing adipogenesis and promoting the proliferation of fibrotic fibroblasts. PPARγ is the main regulator of adipogenesis and is essential for the differentiation of adipocytes. When PPARγ expression is inhibited via the Wnt/β-catenin signaling pathway, adipocytes convert into fibroblasts. This transition adversely affects regenerative outcomes, as PPARγ activation typically promotes adipocyte differentiation and supports tissue repair and regeneration [61,62]. In healthy tissue, activated PPARγ enhances the regenerative capacity of dWAT, contributing to adipose tissue stability and functionality. Thus, PPARγ serves as a key factor in fostering regenerative outcomes, counteracting the profibrotic effects of the Wnt/β-catenin pathway.
During the proliferative phase, the wound bed undergoes a dynamic process wherein adipocyte precursor cells repopulate, concomitant with the recruitment of fibroblasts and endothelial cells, ultimately leading to the formation of dWAT. This cellular rearrangement is orchestrated, in part, by the secretion of cytokines by macrophages, with TGF-β being a prominent player. Notably, TGF-β facilitates the transdifferentiation of fibroblasts into myofibroblasts, thereby contributing to tissue remodeling. Moreover, TGF-β exerts a regulatory influence on ADSC within the dWAT microenvironment, promoting their differentiation towards a myofibroblast phenotype. Conversely, the action of bFGF opposes this process, mitigating the transformation of ADSC into myofibroblasts.
2.2.3. dWAT interactions with keratinocytes and endothelial cells
Subsequent to the inflammatory phase, fibroblasts and endothelial cells migrate to the wound area, where they produce vascularized connective tissue and fresh tissue with a granulation appearance. Concurrently, keratinocytes proliferate from the wound edges towards the center, thereby covering the wound with a new epidermal layer. dWAT not only interacts with fibroblasts but also plays a significant role in the behavior of keratinocytes and endothelial cells, which are crucial in the wound healing process. dWAT influences epithelialization by affecting keratinocyte migration. During the healing process of skin defects, the subcutaneous fascia migrates to fill the wound bed [63], aided by various cell types including fibroblasts in the fascia, adipocyte-derived myofibroblasts, local fibroblasts, and dermal myofibroblasts. Collectively, these cells remodel the ECM [25], creating a supportive scaffold that promotes the migration and proliferation of keratinocytes. dWAT also plays a pivotal role in promoting angiogenesis, particularly through the action of ADSCs present within the tissue. ADSCs contribute to wound healing by secreting a variety of growth factors, including vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), which are crucial for endothelial cell proliferation, migration, and new blood vessel formation. Notably, ADSCs enhance the release of these growth factors under hypoxic conditions, which are common in damaged tissues [64,65].
The ability of dWAT to regulate fibroblast, keratinocyte and endothelial cell activity as well as ECM deposition becomes critical as the wound transitions from the inflammatory phase to the phase of new tissue formation. This coordinated process ensures effective wound closure and sets the stage for the final phase of dermal remodeling.
2.3. Remodeling phase
In the final phase of tissue repair, characterized by programmed cell death of endothelial cells, macrophages, and myofibroblasts, fibrotic masses predominantly composed of collagen and other ECM proteins develop [15]. Notably, myofibroblasts play a pivotal role in this process by secreting matrix metalloproteinases (MMPs), which facilitate the gradual replacement of type III collagen with type I collagen within immature scars, thereby enhancing the mechanical strength of the healed tissue [66]. However, dysregulation of collagen metabolism can lead to the formation of keloids, characterized by abnormal collagen fiber structures and excessive ECM production, significantly impacting patient outcomes [67]. Interestingly, dWAT exhibits a spatial distribution primarily in scar-prone areas, suggesting potential involvement in the pathogenesis of fibrosis. During Bleomycin-induced dermal fibrosis in murine models, adiponectin-positive precursor cells, typically restricted to dWAT, disperse throughout the damaged dermis over time [48]. These cells lose their adipocyte-specific markers and express myofibroblast markers, indicating a phenotypic transition possibly related to the fibrotic response [44]. The absence of dWAT and reduced lipogenesis are consistent features observed in skin fibrosis, highlighting the potential significance of dWAT in modulating fibrotic processes.
Concurrently, dWAT exhibits regenerative potential, as evidenced by the possible transition of adipocytes from myofibroblasts. Recent investigations have elucidated the capacity of wound myofibroblasts to undergo lineage conversion towards the adipocyte lineage [25]. These findings underscore the prospect, post-injury, to steer tissue regeneration rather than scarring by promoting the transformation of myofibroblasts into adipocytes. Intriguingly, genetic lineage tracing studies have indicated that wound adipocytes do not undergo conversion into fibrogenic myofibroblasts [68]. While a comprehensive comprehension of the impact of dermal adipocytes on tissue remodeling remains imperative, these observations bear significance, illustrating the potential utility of dWAT as a strategy for mitigating or potentially reversing scarring, with broader implications for organ systems beyond the skin.
The remodeling phase marks the culmination of wound healing, where dWAT may not only influence fibrosis but also contribute to tissue regeneration by transitioning myofibroblasts back to adipocytes. Understanding this plasticity is key to developing therapies aimed at reducing scarring and promoting regenerative healing (Fig. 1).
Fig. 1.
The dynamic role of dWAT in three stages of wound healing. (a) Inflammation phase: dWAT influences the recruitment and activation of macrophages through the release of fatty acids, producing inflammatory cytokines, chemokines and adipokines to mediate the inflammation phase; (b) New tissue formation phase: dWAT contributes to the recruitment of fibroblasts and the transition of myofibroblasts; (c) Remodeling Phase: The adipocyte-to-myofibroblast transition associated with dWAT may promote tissue regeneration rather than scarring. dWAT, dermal white adipose tissue; IL-6, interleukin-6; IL-8, interleukin-8; TNF-α, tumor necrosis factor-alpha; TGF-β, transforming growth factor-β; VEGFA, vascular endothelial growth factor A; bFGF, basic fibroblast growth factor; MMP, matrix metalloproteinase.
3. Aging adipose and wound healing
Affected by various internal and external factors such as the change in hormone levels and environmental conditions, tissue integrity, function, and regenerative capacity decline with age resulting in a delayed healing process and non-healing wounds [42]. As far back as 1916, research has recognized that the healing of skin wounds is delayed in the elderly, and this has been reinforced by much clinical and experimental evidence to date [69]. With age, the structure of each layer of skin changes to varying degrees. As the skin ages, dWAT undergoes changes that affect immune responses, angiogenesis, and fibrosis. The aging process increases M1 pro-inflammatory macrophages and decreases M2 macrophages [70], leading to a chronic inflammatory state that hinders tissue repair and promotes fibrosis. Studies have shown that in the epidermis, the papilla and bulge at the epidermal-dermal junction become flat with age, and the keratinocyte proliferation activity and migration rate decrease, leading to skin aging and thinning of the epidermal layer [71]. The density and volume fraction of collagen in the dermis will decrease significantly, the degree of disorder will increase, and the elastin form will be disordered, which is manifested as a decrease in skin elasticity [72]. Aging also impairs angiogenesis in dWAT. Reduced levels of VEGF and other angiogenic factors in dWAT lead to poor blood vessel formation, delaying wound healing due to insufficient oxygen and nutrient supply to the tissue [73]. As we age, the total volume of subcutaneous adipose tissue decreases and its thermoregulatory function becomes progressively weaker. At the same time, as adipose tissue can play a key role in buffering stress, the skin of elderly patients is more susceptible to the effects of high mechanical pressure, local tissue ischemia, and ulcer formation. Age-related fibrosis is exacerbated by the AMT, driven by TGF-β signaling, which converts adipocytes into myofibroblasts. This leads to excessive collagen deposition and fibrosis, worsening the skin's ability to heal [74]. Several studies have shown that although healthy elderly people can achieve normal wound healing, disrupted age-related changes are present to some extent ats every stage of wound healing. Therefore, the contribution of dWAT in the healing process of older cutaneous wounds deserves additional attention.
3.1. Aging excaberates inflammatory response via adipocyte-macrophage interactions
Aged individuals exhibit a chronic inflammation state in the whole body, which is known as inflammaging [75]. Inflammaging resulted in overactivated immune response in aged wound sites, which is characterized as overexpression of pro-inflammatory cytokines and prolonged inflammation stage [76]. Senescent cells are known to accumulate in various tissues as part of the aging process, including dWAT [77]. This accumulation is often attributed to factors such as oxidative stress, chronic inflammation, and cellular damage, which are prevalent in aging tissues [78]. Recent evidence indicates that a higher accumulation of senescent cells in adipose tissue may play a role in promoting the pro-inflammatory phenotype associated with aging, which is essential for the effective clearance of these cells [79]. With aging, dWAT expresses higher levels of adiponectin and inflammatory factors, along with lower levels of leptin, which resulted in adipocyte hypertrophy [80]. Enlarged adipocytes lead to tissue hypoxia which inhibits angiogenesis and promoting wound fibrosis [81]. Furthermore, with the aging process, the level of adipocyte apoptosis increases, and the released DAMPs and various inflammatory factors like TNF-α, CXCL1, and interleukins collectively recruit macrophages [82]. Macrophages are core participants in the SASP within aging WAT tissue [79]. Aging adipose macrophages predominantly exhibit an M1 phenotype, characterized by high expression of the JNK and TLR pathways [83]. Recent studies showed that aging macrophages dysregulated adipose metabolism and wound healing mainly through elevated IL-6 and IL-33 [84]. Besides, senescent adipose macrophages are also characterized by the breakdown and mobilization of fatty acids. Elevated expression of catecholamine-degrading enzymes in adipose macrophages impedes the transmission of signals for triglyceride breakdown to adipocytes. Notably, this process is notably dependent on the nod-like receptor pyrin domain-containing 3 (NLRP3) inflammasome [85]. This inflammasome, secreted by macrophages, can be activated by various pathogen- or damage-associated molecular patterns [86]. Studies have shown that deletion of NLRP3 can ameliorate age-related alterations in visceral white adipose tissue (vWAT) [87]. Conversely, inflammatory factors produced by senescent macrophages, such as IL-1β, promote insulin resistance in adipose tissue, exacerbating adipose hypertrophy and consequently leading to tissue hypoxia and cell apoptosis [88]. On the other hand, senescent macrophages promote the differentiation of adipocyte progenitors into myofibroblasts, contributing to wound fibrosis [52]. Therefore, the interaction between macrophages and adipocytes plays a crucial role in all stages of wound healing, with inflammaging presenting a promising therapeutic target.
3.2. Aged dWAT dysregulates fibroblast function in proliferation phase
The huge structural change of the aging skin, especially in the structure of the dermis which is mainly composed of fibroblasts and ECM impairs the proliferation phase of wound healing. During aging, fibroblast density is reduced while cell volume enlarges to fill the space. Salzer et al. [89] proved that skin fibroblasts acquired an adipogenic profile with aging and transformed into adipogenic lineage cells, leading to decreased cell density and ECM expression. Corresponding to this result, another research identified that dWAT abundance increases with age [51]. Kruglikov et al. found that dWAT modifications induced by extrinsic or intrinsic aging affect fibroblast function. With age, both the number and the size of adipocytes increase, which is also seen in obesity [90]. Enlarged adipocytes negatively control dermal fibroblast function through activation of toll-like receptors (TLRs) by secreted free fatty acids (FFA) [91]. The bigger cells also lead to decreased nutrient transport and probably accelerate metabolic disorders and inhibit cell signaling. Severe hypertrophy makes it harder for oxygen to get into cells and leads to an oxygen-poor environment, which is also why obesity causes cells to lack oxygen [92]. Furthermore, with the thinning of capillaries and significantly reduced blood flow, blood vessel density in elderly skin decreases [93]. Other studies indicated VEGF expression and vascular density in mouse adipose tissue decreased in an age-dependent manner, this is worsened by inadequate compensation from the blood vessels [94,95]. To conclude, decreased fibroblast activity, hypoxia and ischemia impede the wound healing process, all of which can be closely regulated by dWAT.
3.3. Aging adipose enhances fibrosis in the remodeling phase of wound healing
The remodeling phase of wound healing exhibits less variability in an aging milieu compared to the earlier stages [96]. In aged skin, there is an upregulation in the expression of MMPs, leading to heightened collagen degradation [3]. Aging skin manifests three distinct morphological alterations: a modest reduction in dermal thickness, a significant expansion of dWAT at the dermal-subcutaneous interface, and a notable decline in sWAT [97]. Salzer et al. [89] observed reduced expression of ECM-related genes, particularly collagen and glycosaminoglycan enzymes, in aging dermal fibroblasts, juxtaposed with heightened expression of markers associated with immune response and adipogenesis, including adipocyte differentiation. In aging individuals, senescent fibroblasts exhibit a senescence-associated phenotype that replaces the normal fibroblastic phenotype, leading to reduced fibronectin secretion and exacerbating tissue fibrosis [98]. These changes in aged dermal fibroblasts may culminate in diminished fibrotic capacity during wound healing and the acquisition of pro-adipogenic characteristics [99]. Concurrently, there is an elevation in the activity of proteolytic enzymes, a decrease in collagen synthesis, and a delay in collagen remodeling in aged skin. Senescent fibroblasts exhibit a senescence-associated secretory phenotype (SASP), characterized by the production of high levels of MMPs, which exacerbates tissue degradation and ECM dysfunction [100]. On the other hand, ECM dysfunction and tissue fibrosis lead to decreased mobility of resident memory cells, causing cell membrane damage. This results in the release of damage-associated molecular patterns (DAMPs), which in turn trigger tissue inflammation [101]. Consequently, aging skin manifests wrinkles and loss of elasticity due to disorganized and weakened collagen bundles. These structural alterations heighten the susceptibility of older individuals to injury and disease. Intriguingly, aging may entail adipose tissue fibrosis, as evidenced by increased collagen density in VWAT in aged mice [95]. In summary, the diminished collagen synthesis and remodeling capacity in aging skin impede wound healing; however, the metabolic and expansionary dynamics of adipose tissue may serve to mitigate excessive fibrosis post-wound healing.
4. Conclusion and future perspective
In this comprehensive review, our aim was to elucidate the pivotal role of dWAT across various stages of wound healing, an aspect overlooked in current literature. As experimental methodologies continue to advance, the significance of dWAT in diverse processes such as hair follicle cycling and wound healing has garnered increasing attention. Nonetheless, the molecular mechanisms underlying dWAT-mediated wound healing remain elusive.
Characterized as a distinctive adipose depot, dWAT exhibits intricate communication with other constituents of the skin. Through the secretion of fatty acids and inflammatory factors, dWAT orchestrates the proliferation and recruitment of diverse cell populations crucial for wound healing. Significantly, the signaling crosstalk between dWAT and these cells is bidirectional, necessitating ongoing feedback from external stimuli to modulate dWAT responsiveness. Particularly in the context of aging and obesity-induced microenvironments, sustained and exacerbated inflammatory responses precipitate metabolic dysregulation within dWAT. However, the precise mechanisms through which the equilibrium of interactions between dWAT and surrounding cells is disrupted, and whether this imbalance engenders a reinforcing feedback loop, remain enigmatic. Consequently, a comprehensive understanding of the intricate interplay between dWAT and neighboring cells is imperative for delineating the mechanisms governing wound healing.
The plasticity of dWAT has emerged as a focal point of investigation, diverging from the conventional perspective of adipocytes as terminally differentiated cells. Recent findings have illuminated a transition mechanism between myofibroblasts and adipocytes, notably occurring concomitantly with the pathogenesis of skin fibrosis and scarring [48,50,51]. This phenomenon aligns with observations that dWAT is predominantly found in regions prone to scarring, while its presence is notably absent in dermal areas where scarring is rare, such as the scalp, oral mucosa, and skin of early fetus [17,67,102]. Consequently, further elucidation of the transition mechanism and exploration of strategies to inhibit the dedifferentiation of dWAT hold promise for advancing anti-scarring and antifibrotic interventions.
Moreover, the distinct physiological architectures of mouse and human dWAT warrant investigation. In humans, dWAT directly interfaces with sWAT due to the absence of the panniculus carnosus. Studies have indicated a reduction in dWAT in patients with systemic scleroderma, accompanied by marked downregulation of adiponectin expression compared to normal control skin [103]. While dWAT across various species share a high degree of structural similarity in other aspects, the debate persists regarding the equivalent role of human dWAT in wound healing. Therefore, further research endeavors are essential to elucidate the functional significance of human dWAT in the context of wound healing.
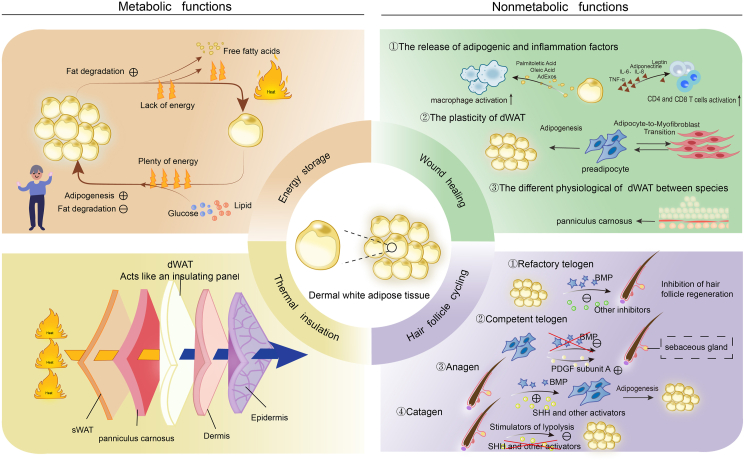
As skin undergoes aging processes, dWAT undergoes multifaceted changes. These age-associated alterations extend beyond metabolic disturbances to encompass immune cell infiltration, diminished vasculature, and heightened fibrotic tendencies. These pathological transformations collectively impact the efficacy of skin wound healing. To explore promising therapeutic modalities for age-related conditions, further investigations are imperative to gain a comprehensive understanding of dWAT dynamics and its age-related changes (Fig. 2).
Fig. 2.
Metabolic and nonmetabolic functions of dWAT. dWAT performs various metabolic functions like energy storage, thermal insulation, and mechanical support, as well as non-metabolic functions such as regulating hair follicle cycling and skin wound healing. BMP, bone morphogenetic protein; PDGF, platelet-derived growth factor; SHH, sonic hedgehog; sWAT, subcutaneous white adipose tissue.
Recent advancements underscore the potential of dWAT as a therapeutic target in various clinical applications, including wound healing, scar prevention, and anti-aging therapies. The differentiating role of ADSC in aging has been highlighted in various studies [[104], [105], [106]]. ADSC are capable of differentiating into multiple cell types, such as adipocytes, chondrocytes, and osteoblasts, which makes them essential for tissue regeneration. In aging tissues, ADSCs play a critical role by replenishing damaged or aging cells and promoting tissue repair. In the context of secretion potential, ADSC-exosomes (ADSC-exo) can be internalized by fibroblasts and influence immune cells indirectly, promoting wound healing by activating the PI3K/AKT and ERK pathways, which aid in reepithelialization, collagen deposition, and neovascularization [107]. ADSCs-exo under hypoxia has advantages in promoting angiogenesis and bone healing compared with that under normorxia. Studies have shown that Hypoxic ADSC-exosomes (HypADSCs-exo) promotes fibroblast proliferation and migration by activating PI3K/AKT pathway, which can accelerate healing of diabetic wounds [108]. High-throughput sequencing analyses revealed that hypoxic exosomes exhibit differential expression of microRNAs (miRNAs), with 215 miRNAs upregulated and 369 downregulated compared to normoxic exosomes. Key upregulated miRNAs, including miR-21-3p, miR-126-5p, and miR-31-5p, play vital roles in promoting fibroblast proliferation and migration, while downregulated miRNAs like miR-99b and miR-146a contribute to the regulation of the immune response [109,110]. Notably, HypADSCs-exo induced proliferation, collagen metabolism and migration through PI3K/AKT signaling pathway in human skin fibroblasts. Additionally, microvesicles derived from ADSCs can enhance angiogenesis and promote rapid wound closure. The ERK pathway is particularly important for cellular functions such as proliferation and migration. In the context of wound healing, Adipose stem cell-derived microvesicles (ASC-MVs) enhanced the expression of proliferation-associated genes, including cyclins and c-Myc, which facilitate cell cycle progression [111]. Moreover, ASC-MVs were found to significantly increase the expression of angiogenic factors like VEGFA and PDGFA in endothelial cells, contributing to improved blood supply in wound beds [112]. Therapeutic strategies using exosomal and microvesicle delivery systems have demonstrated anti-inflammatory and antioxidant effects, offering promising avenues for reducing age-related oxidative stress and fibrosis.
Current research on dWAT in wound healing has primarily relied on animal models, particularly transgenic mice, which have revealed key insights into dWAT's role in cell differentiation and fibrosis. While these models are effective, they have limitations due to species differences, particularly the presence of the panniculus carnosus in rodents, which is absent in humans. Additionally, lineage tracing techniques offer valuable data on cell fate but lack sufficient resolution to fully understand the molecular mechanisms underlying dWAT plasticity. Future research should prioritize developing human-based models, such as 3D skin cultures and organoids, to better replicate human dWAT biology. Investigating the plasticity of dWAT, especially the reversible transitions between adipocytes and myofibroblasts, could unlock therapeutic strategies to enhance wound healing and reduce scarring. Advanced tools like single-cell RNA sequencing and proteomics will help identify the molecular pathways involved, particularly those related to TGF-β and adiponectin signaling. Finally, exploring the translational potential of therapies targeting dWAT, including ADSC therapies and anti-fibrotic treatments, could significantly impact clinical wound healing and fibrosis treatment.
In summation, the pivotal role of dWAT in the diverse phases of wound healing continues to unfold, particularly in modulating inflammatory responses and scar formation. Recent research focusing on the transition between dermal white adipocytes and myofibroblasts underscores the intricate involvement of dWAT in both youthful and aged skin physiology. Advancing our comprehension of dWAT functionality holds promise for the development of novel interventions targeting wound healing and age-related ailments.
Authors' contribution
Zhongyu Wu: writing-original draft preparation. Zhanqi Wang: writing-review and editing. Dongyang Wang, Feng Zhou, Guorui Zhang and Shan Wei: provided additional scientific information. Tao Chen: visualization. Yingying Wu: Supervision, Writing – review & editing, Funding acquisition. All authors have reviewed and consented to the published version of the manuscript.
Data availability statement
This is a review article, and no new data were generated or analyzed in this study. Therefore, data sharing does not apply in this article.
Funding
This work was supported by the Research and Development Project of West China Hospital of Stomatology of Sichuan University (no.LCYJ2023-DL-4). All authors gave their final approval and agree to be accountable for all aspects of the work.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Bellu E., Medici S., Coradduzza D., Cruciani S., Amler E., Maioli M. Nanomaterials in skin regeneration and rejuvenation. Int J Mol Sci. 2021;22:7095. doi: 10.3390/ijms22137095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwiecien K., Zegar A., Jung J., Brzoza P., Kwitniewski M., Godlewska U., et al. Architecture of antimicrobial skin defense. Cytokine Growth Factor Rev. 2019;49:70–84. doi: 10.1016/j.cytogfr.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Blair M.J., Jones J.D., Woessner A.E., Quinn K.P. Skin structure-function relationships and the wound healing response to intrinsic aging. Adv Wound Care. 2020;9:127–143. doi: 10.1089/wound.2019.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascharak S., Talbott H.E., Januszyk M., Griffin M., Chen K., Davitt M.F., et al. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell. 2022;29:315–327.e6. doi: 10.1016/j.stem.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driskell R.R., Jahoda C.A.B., Chuong C.-M., Watt F.M., Horsley V. Defining dermal adipose tissue. Exp Dermatol. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane H., Lynch L. Innate immune control of adipose tissue homeostasis. Trends Immunol. 2019;40:857–872. doi: 10.1016/j.it.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Zwick R.K., Guerrero-Juarez C.F., Horsley V., Plikus M.V. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metabol. 2018;27:68–83. doi: 10.1016/j.cmet.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker G.E., Marzullo P., Verti B., Guzzaloni G., Maestrini S., Zurleni F., et al. Subcutaneous abdominal adipose tissue subcompartments: potential role in rosiglitazone effects. Obesity. 2008;16:1983–1991. doi: 10.1038/oby.2008.326. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B., Horsley V. Unravelling hair follicle-adipocyte communication. Exp Dermatol. 2012;21:827–830. doi: 10.1111/exd.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasza I., Kühn J.-P., Völzke H., Hernando D., Xu Y.G., Siebert J.W., et al. Contrasting recruitment of skin-associated adipose depots during cold challenge of mouse and human. J Physiol. 2022;600:847–868. doi: 10.1113/JP280922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasza I., Suh Y., Wollny D., Clark R.J., Roopra A., Colman R.J., et al. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y., Hu Z., Chen J., Zhang J., Fan Z., Qu Q., et al. Feasibility of adipose-derived therapies for hair regeneration: insights based on signaling interplay and clinical overview. J Am Acad Dermatol. 2023;89:784–794. doi: 10.1016/j.jaad.2021.11.058. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero-Juarez C.F., Plikus M.V. Emerging nonmetabolic functions of skin fat. Nat Rev Endocrinol. 2018;14:163–173. doi: 10.1038/nrendo.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 16.Merrick D., Seale P. Skinny fat cells stimulate wound healing. Cell Stem Cell. 2020;26:801–803. doi: 10.1016/j.stem.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Berman B., Maderal A., Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 18.Wang P.-H., Huang B.-S., Horng H.-C., Yeh C.-C., Chen Y.-J. Wound healing. J Chin Med Assoc. 2018;81:94–101. doi: 10.1016/j.jcma.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Su W.-H., Cheng M.-H., Lee W.-L., Tsou T.-S., Chang W.-H., Chen C.-S., et al. Nonsteroidal anti-inflammatory drugs for wounds: pain relief or excessive scar formation? Mediat Inflamm. 2010;2010 doi: 10.1155/2010/413238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plikus M.V., Guerrero-Juarez C.F., Ito M., Li Y.R., Dedhia P.H., Zheng Y., et al. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai H.-W., Wang P.-H., Tsui K.-H. Mesenchymal stem cell in wound healing and regeneration. J Chin Med Assoc. 2018;81:223–224. doi: 10.1016/j.jcma.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Chung K.-J., Nati M., Chavakis T., Chatzigeorgiou A. Innate immune cells in the adipose tissue. Rev Endocr Metab Disord. 2018;19:283–292. doi: 10.1007/s11154-018-9451-6. [DOI] [PubMed] [Google Scholar]
- 23.Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 24.Bapat S.P., Myoung Suh J., Fang S., Liu S., Zhang Y., Cheng A., et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528:137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shook B.A., Wasko R.R., Mano O., Rutenberg-Schoenberg M., Rudolph M.C., Zirak B., et al. Dermal adipocyte lipolysis and myofibroblast conversion are required for efficient skin repair. Cell Stem Cell. 2020;26:880–895.e6. doi: 10.1016/j.stem.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Curto E., Milligan G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem Pharmacol. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Sohn J.H., Lee Y.K., Han J.S., Jeon Y.G., Kim J.I., Choe S.S., et al. Perilipin 1 (Plin1) deficiency promotes inflammatory responses in lean adipose tissue through lipid dysregulation. J Biol Chem. 2018;293:13974–13988. doi: 10.1074/jbc.RA118.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recio C., Lucy D., Purvis G.S.D., Iveson P., Zeboudj L., Iqbal A.J., et al. Activation of the immune-metabolic receptor GPR84 enhances inflammation and phagocytosis in macrophages. Front Immunol. 2018;9:1419. doi: 10.3389/fimmu.2018.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsango S., Milligan G. Regulation of the pro-inflammatory G protein-coupled receptor GPR84. Br J Pharmacol. 2024;181:1500–1508. doi: 10.1111/bph.16098. [DOI] [PubMed] [Google Scholar]
- 30.Fujisaka S., Usui I., Nawaz A., Takikawa A., Kado T., Igarashi Y., et al. M2 macrophages in metabolism. Diabetol Int. 2016;7:342–351. doi: 10.1007/s13340-016-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H., Shang Q., Pan Z., Bai Y., Li Z., Zhang H., et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67:235–247. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 32.Jackson W.M., Nesti L.J., Tuan R.S. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai T., Autieri M.V., Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320:C375–C391. doi: 10.1152/ajpcell.00379.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kredel L., Batra A., Siegmund B. Role of fat and adipokines in intestinal inflammation. Curr Opin Gastroenterol. 2014;30:559–565. doi: 10.1097/MOG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 35.Kiernan K., MacIver N.J. The role of the adipokine leptin in immune cell function in health and disease. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.622468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palhinha L., Liechocki S., Hottz E.D., Pereira JA. da S., de Almeida C.J., Moraes-Vieira P.M.M., et al. Leptin induces proadipogenic and proinflammatory signaling in adipocytes. Front Endocrinol. 2019;10:841. doi: 10.3389/fendo.2019.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L., Guerrero-Juarez C.F., Hata T., Bapat S.P., Ramos R., Plikus M.V., et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinoshita M., Ogawa Y., Hama N., Ujiie I., Hasegawa A., Nakajima S., et al. Neutrophils initiate and exacerbate Stevens-Johnson syndrome and toxic epidermal necrolysis. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.aax2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carretero M., Escámez M.J., García M., Duarte B., Holguín A., Retamosa L., et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 41.Schäffler A., Schölmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010;31:228–235. doi: 10.1016/j.it.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Woodley D.T. Distinct fibroblasts in the papillary and reticular dermis: implications for wound healing. Dermatol Clin. 2017;35:95–100. doi: 10.1016/j.det.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Portou M.J., Baker D., Abraham D., Tsui J. The innate immune system, toll-like receptors and dermal wound healing: a review. Vasc Pharmacol. 2015;71:31–36. doi: 10.1016/j.vph.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Li Y., Long J., Zhang Z. Insights into the unique roles of dermal white adipose tissue (dWAT) in wound healing. Front Physiol. 2024;15 doi: 10.3389/fphys.2024.1346612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt B.A., Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joe A.W.B., Yi L., Natarajan A., Le Grand F., So L., Wang J., et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uezumi A., Ito T., Morikawa D., Shimizu N., Yoneda T., Segawa M., et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 48.Marangoni R.G., Korman B.D., Wei J., Wood T.A., Graham L.V., Whitfield M.L., et al. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67:1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai J., Quan Y., Zhu S., Jiayan Lin null, Zhang Q., Liu J., et al. The browning and mobilization of subcutaneous white adipose tissue supports efficient skin repair. Cell Metabol. 2024;36:1287–1301.e7. doi: 10.1016/j.cmet.2024.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q.A., Song A., Chen W., Schwalie P.C., Zhang F., Vishvanath L., et al. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metabol. 2018;28:282–288.e3. doi: 10.1016/j.cmet.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z., Shao M., Hepler C., Zi Z., Zhao S., An Y.A., et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest. 2019;129:5327–5342. doi: 10.1172/JCI130239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shook B.A., Wasko R.R., Rivera-Gonzalez G.C., Salazar-Gatzimas E., López-Giráldez F., Dash B.C., et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science. 2018;362 doi: 10.1126/science.aar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gabbiani G., Ryan G.B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 54.Noom A., Sawitzki B., Knaus P., Duda G.N. A two-way street – cellular metabolism and myofibroblast contraction. NPJ Regen Med. 2024;9:15. doi: 10.1038/s41536-024-00359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita T., Lakota K., Taniguchi T., Yoshizaki A., Sato S., Hong W., et al. An orally-active adiponectin receptor agonist mitigates cutaneous fibrosis, inflammation and microvascular pathology in a murine model of systemic sclerosis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-29901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshizaki A., Yanaba K., Iwata Y., Komura K., Ogawa A., Akiyama Y., et al. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol. 2010;185:2502–2515. doi: 10.4049/jimmunol.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reinke L., Lam A.P., Flozak A.S., Varga J., Gottardi C.J. Adiponectin inhibits Wnt co-receptor, Lrp6, phosphorylation and β-catenin signaling. Biochem Biophys Res Commun. 2016;470:606–612. doi: 10.1016/j.bbrc.2016.01.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masui Y., Asano Y., Shibata S., Noda S., Aozasa N., Akamata K., et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. J Eur Acad Dermatol Venereol. 2012;26:354–360. doi: 10.1111/j.1468-3083.2011.04077.x. [DOI] [PubMed] [Google Scholar]
- 59.Mastrogiannaki M., Lichtenberger B.M., Reimer A., Collins C.A., Driskell R.R., Watt F.M. β-Catenin stabilization in skin fibroblasts causes fibrotic lesions by preventing adipocyte differentiation of the reticular dermis. J Invest Dermatol. 2016;136:1130–1142. doi: 10.1016/j.jid.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei J., Melichian D., Komura K., Hinchcliff M., Lam A.P., Lafyatis R., et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P., et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Distler J.H.W., Györfi A.-H., Ramanujam M., Whitfield M.L., Königshoff M., Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15:705–730. doi: 10.1038/s41584-019-0322-7. [DOI] [PubMed] [Google Scholar]
- 63.Correa-Gallegos D., Jiang D., Christ S., Ramesh P., Ye H., Wannemacher J., et al. Patch repair of deep wounds by mobilized fascia. Nature. 2019;576:287–292. doi: 10.1038/s41586-019-1794-y. [DOI] [PubMed] [Google Scholar]
- 64.Alexaki V.-I., Simantiraki D., Panayiotopoulou M., Rasouli O., Venihaki M., Castana O., et al. Adipose tissue-derived mesenchymal cells support skin reepithelialization through secretion of KGF-1 and PDGF-BB: comparison with dermal fibroblasts. Cell Transplant. 2012;21:2441–2454. doi: 10.3727/096368912X637064. [DOI] [PubMed] [Google Scholar]
- 65.Huang S.-P., Hsu C.-C., Chang S.-C., Wang C.-H., Deng S.-C., Dai N.-T., et al. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg. 2012;69:656–662. doi: 10.1097/SAP.0b013e318273f909. [DOI] [PubMed] [Google Scholar]
- 66.Kasuya A., Tokura Y. Attempts to accelerate wound healing. J Dermatol Sci. 2014;76:169–172. doi: 10.1016/j.jdermsci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Lee H.J., Jang Y.J. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. 2018;19:711. doi: 10.3390/ijms19030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalgudde Gopal S., Dai R., Stefanska A.M., Ansari M., Zhao J., Ramesh P., et al. Wound infiltrating adipocytes are not myofibroblasts. Nat Commun. 2023;14:3020. doi: 10.1038/s41467-023-38591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding X., Kakanj P., Leptin M., Eming S.A. Regulation of the wound healing response during aging. J Invest Dermatol. 2021;141:1063–1070. doi: 10.1016/j.jid.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Gather L., Nath N., Falckenhayn C., Oterino-Sogo S., Bosch T., Wenck H., et al. Macrophages are polarized toward an inflammatory phenotype by their aged microenvironment in the human skin. J Invest Dermatol. 2022;142:3136–3145.e11. doi: 10.1016/j.jid.2022.06.023. [DOI] [PubMed] [Google Scholar]
- 71.Biniek K., Kaczvinsky J., Matts P., Dauskardt R.H. Understanding age-induced alterations to the biomechanical barrier function of human stratum corneum. J Dermatol Sci. 2015;80:94–101. doi: 10.1016/j.jdermsci.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Kobayashi T., Naik S., Nagao K. Choreographing immunity in the skin epithelial barrier. Immunity. 2019;50:552–565. doi: 10.1016/j.immuni.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L.-J., Chen S.X., Guerrero-Juarez C.F., Li F., Tong Y., Liang Y., et al. Age-related loss of innate immune antimicrobial function of dermal fat is mediated by transforming growth factor beta. Immunity. 2019;50:121–136.e5. doi: 10.1016/j.immuni.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu M., Lu F., Feng J. Aging and homeostasis of the hypodermis in the age-related deterioration of skin function. Cell Death Dis. 2024;15:443. doi: 10.1038/s41419-024-06818-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X., Li C., Zhang W., Wang Y., Qian P., Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Targeted Ther. 2023;8:239. doi: 10.1038/s41392-023-01502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh A., Schurman S.H., Bektas A., Kaileh M., Roy R., Wilson D.M., et al. Aging and inflammation. Cold Spring Harb Perspect Med. 2024;14:a041197. doi: 10.1101/cshperspect.a041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muris Consortium Tabula. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020;583:590–595. doi: 10.1038/s41586-020-2496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith U., Li Q., Rydén M., Spalding K.L. Cellular senescence and its role in white adipose tissue. Int J Obes. 2021;45:934–943. doi: 10.1038/s41366-021-00757-x. [DOI] [PubMed] [Google Scholar]
- 79.Matacchione G., Perugini J., Di Mercurio E., Sabbatinelli J., Prattichizzo F., Senzacqua M., et al. Senescent macrophages in the human adipose tissue as a source of inflammaging. Geroscience. 2022;44:1941–1960. doi: 10.1007/s11357-022-00536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joffin N., Jaubert A.-M., Durant S., Barouki R., Forest C., Noirez P. Citrulline counteracts overweight- and aging-related effects on adiponectin and leptin gene expression in rat white adipose tissue. Biochim Open. 2015;1:1–5. doi: 10.1016/j.biopen.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Von Bank H., Kirsh C., Simcox J. Aging adipose: depot location dictates age-associated expansion and dysfunction. Ageing Res Rev. 2021;67 doi: 10.1016/j.arr.2021.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindhorst A., Raulien N., Wieghofer P., Eilers J., Rossi F.M.V., Bechmann I., et al. Adipocyte death triggers a pro-inflammatory response and induces metabolic activation of resident macrophages. Cell Death Dis. 2021;12:579. doi: 10.1038/s41419-021-03872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu B., Huang L., Cao J., Li L., Wu W., Chen X., et al. Adipose tissue macrophages in aging-associated adipose tissue function. J Physiol Sci. 2021;71:38. doi: 10.1186/s12576-021-00820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benvie A.M., Lee D., Steiner B.M., Xue S., Jiang Y., Berry D.C. Age-dependent Pdgfrβ signaling drives adipocyte progenitor dysfunction to alter the beige adipogenic niche in male mice. Nat Commun. 2023;14:1806. doi: 10.1038/s41467-023-37386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Camell C.D., Sander J., Spadaro O., Lee A., Nguyen K.Y., Wing A., et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 2017;550:119–123. doi: 10.1038/nature24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strowig T., Henao-Mejia J., Elinav E., Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 87.Chen Y., Ye X., Escames G., Lei W., Zhang X., Li M., et al. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023;28:51. doi: 10.1186/s11658-023-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bing C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015;4:149–152. doi: 10.4161/21623945.2014.979661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salzer M.C., Lafzi A., Berenguer-Llergo A., Youssif C., Castellanos A., Solanas G., et al. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell. 2018;175:1575–1590.e22. doi: 10.1016/j.cell.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 90.Kruglikov I.L., Scherer P.E. Skin aging: are adipocytes the next target? Aging (Albany NY) 2016;8:1457–1469. doi: 10.18632/aging.100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ezure T., Amano S. Negative regulation of dermal fibroblasts by enlarged adipocytes through release of free fatty acids. J Invest Dermatol. 2011;131:2004–2009. doi: 10.1038/jid.2011.145. [DOI] [PubMed] [Google Scholar]
- 92.Halberg N., Khan T., Trujillo M.E., Wernstedt-Asterholm I., Attie A.D., Sherwani S., et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bentov I., Reed M.J. The effect of aging on the cutaneous microvasculature. Microvasc Res. 2015;100:25–31. doi: 10.1016/j.mvr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimoda Y., Matsuo K., Ono K., Soma Y., Ueyama T., Matoba S., et al. Aging differentially alters the expression of angiogenic genes in a tissue-dependent manner. Biochem Biophys Res Commun. 2014;446:1243–1249. doi: 10.1016/j.bbrc.2014.03.098. [DOI] [PubMed] [Google Scholar]
- 95.Donato A.J., Henson G.D., Hart C.R., Layec G., Trinity J.D., Bramwell R.C., et al. The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: evidence of significant multisystem dysfunction. J Physiol. 2014;592:4083–4096. doi: 10.1113/jphysiol.2014.274175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sgonc R., Gruber J. Age-related aspects of cutaneous wound healing: a mini-review. Gerontology. 2013;59:159–164. doi: 10.1159/000342344. [DOI] [PubMed] [Google Scholar]
- 97.Zhang W., Qu J., Liu G.-H., Belmonte J.C.I. The ageing epigenome and its rejuvenation. Nat Rev Mol Cell Biol. 2020;21:137–150. doi: 10.1038/s41580-019-0204-5. [DOI] [PubMed] [Google Scholar]
- 98.Schnabl B., Purbeck C.A., Choi Y.H., Hagedorn C.H., Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 99.Walendzik K., Bukowska J., Kopcewicz M., Machcinska S., Gimble J.M., Gawronska-Kozak B. Age, diet and epidermal signaling modulate dermal fibroblasts' adipogenic potential. Int J Mol Sci. 2020;21:8955. doi: 10.3390/ijms21238955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levi N., Papismadov N., Solomonov I., Sagi I., Krizhanovsky V. The ECM path of senescence in aging: components and modifiers. FEBS J. 2020;287:2636–2646. doi: 10.1111/febs.15282. [DOI] [PubMed] [Google Scholar]
- 101.Moreau J.-F., Pradeu T., Grignolio A., Nardini C., Castiglione F., Tieri P., et al. The emerging role of ECM crosslinking in T cell mobility as a hallmark of immunosenescence in humans. Ageing Res Rev. 2017;35:322–335. doi: 10.1016/j.arr.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Waasdorp M., Krom B.P., Bikker F.J., van Zuijlen P.P.M., Niessen F.B., Gibbs S. The bigger picture: why oral mucosa heals better than skin. Biomolecules. 2021;11:1165. doi: 10.3390/biom11081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varga J., Marangoni R.G. Systemic sclerosis in 2016: dermal white adipose tissue implicated in SSc pathogenesis. Nat Rev Rheumatol. 2017;13:71–72. doi: 10.1038/nrrheum.2016.223. [DOI] [PubMed] [Google Scholar]
- 104.Setiawan A.M., Kamarudin T.A., Abd Ghafar N. The role of BMP4 in adipose-derived stem cell differentiation: a minireview. Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.1045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nguyen H.P., Lin F., Yi D., Xie Y., Dinh J., Xue P., et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev Cell. 2021;56:1437–1451.e3. doi: 10.1016/j.devcel.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naderi N., Combellack E.J., Griffin M., Sedaghati T., Javed M., Findlay M.W., et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. 2016;14:112–124. doi: 10.1111/iwj.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J., Wu H., Peng Y., Zhao Y., Qin Y., Zhang Y., et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol. 2021;19:202. doi: 10.1186/s12951-021-00942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J., Wu H., Peng Y., Zhao Y., Qin Y., Zhang Y., et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol. 2021;19:202. doi: 10.1186/s12951-021-00942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Casado-Díaz A., Quesada-Gómez J.M., Dorado G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol. 2020;8:146. doi: 10.3389/fbioe.2020.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang D., Xuan J., Zheng B.-B., Zhou Y.-L., Lin Y., Wu Y.-S., et al. Metformin improves functional recovery after spinal cord injury via autophagy flux stimulation. Mol Neurobiol. 2017;54:3327–3341. doi: 10.1007/s12035-016-9895-1. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J., Chen C., Hu B., Niu X., Liu X., Zhang G., et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through erk1/2 signaling. Int J Biol Sci. 2016;12:1472–1487. doi: 10.7150/ijbs.15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ren S., Chen J., Duscher D., Liu Y., Guo G., Kang Y., et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther. 2019;10:47. doi: 10.1186/s13287-019-1152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article, and no new data were generated or analyzed in this study. Therefore, data sharing does not apply in this article.