Abstract
Sphingosine 1-phosphate (S1P) is a lysosphingolipid with antiatherogenic properties, but mechanisms underlying its effects remain unclear. We here investigated atherosclerosis development in cholesterol-rich diet–fed LDL receptor–deficient mice with high or low overexpression levels of S1P receptor 1 (S1P1) in macrophages. S1P1-overexpressing macrophages showed increased activity of transcription factors PU.1, interferon regulatory factor 8 (IRF8), and liver X receptor (LXR) and were skewed toward an M2-distinct phenotype characterized by enhanced production of IL-10, IL-1RA, and IL-5; increased ATP-binding cassette transporter A1– and G1–dependent cholesterol efflux; increased expression of MerTK and efferocytosis; and reduced apoptosis due to elevated B cell lymphoma 6 and Maf bZIP B. A similar macrophage phenotype was observed in mice administered S1P1-selective agonist KRP203. Mechanistically, the enhanced PU.1, IRF8, and LXR activity in S1P1-overexpressing macrophages led to downregulation of the cAMP-dependent PKA and activation of the signaling cascade encompassing protein kinases AKT and mTOR complex 1 as well as the late endosomal/lysosomal adaptor MAPK and mTOR activator 1. Atherosclerotic lesions in aortic roots and brachiocephalic arteries were profoundly or moderately reduced in mice with high and low S1P1 overexpression in macrophages, respectively. We conclude that S1P1 signaling polarizes macrophages toward an antiatherogenic functional phenotype and countervails the development of atherosclerosis in mice.
Keywords: Inflammation, Vascular biology
Keywords: Atherosclerosis, Lipoproteins, Macrophages
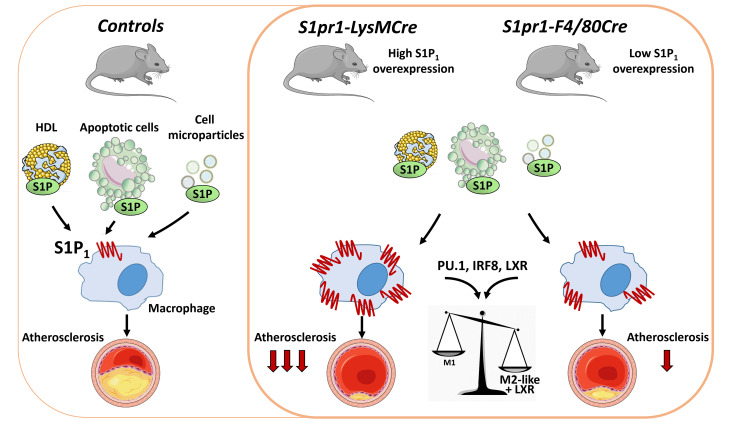
Sphingosine-1-phosphate (S1P) signaling mediated by S1P receptor type 1 polarizes macrophages towards an anti-atherogenic functional phenotype and countervails the development of atherosclerosis in mice.
Introduction
Sphingosine 1-phosphate (S1P) is a membrane-derived lysosphingolipid with multiple regulatory roles in physiology (1–3). Formation of S1P from sphingosine is catalyzed by sphingosine kinases (SK1 and SK2), and its breakdown to sphingosine or to 2-hexadecenal and phosphoethanolamine is mediated by S1P phosphatase or lyase, respectively (1–3). The S1P formed by SKs either binds to intracellular target proteins or is exported from cells to act as a ligand of 5 S1P-specific G protein–coupled receptors (S1P1–5) (2, 3). The physiological effects associated with S1P receptor activation are determined by the specific G protein coupling. S1P1 couples with the inhibitory G protein α subunit (Gαi), which inhibits adenylate cyclase and lowers intracellular cAMP. In addition, Gi activates kinases such as AKT, PKCα and ε, and ERK1/2 (2–4). The signaling through S1P1 promotes beneficial processes in the vasculature, including endothelial proliferation and survival, endothelial barrier stabilization, and amelioration of endothelial dysfunction (2, 3, 5). Furthermore, acting via S1P1, S1P interferes with proliferation, migration, and activation of lymphocytes and monocytes/macrophages and regulates their recruitment to inflamed tissues (3, 6). These modulatory activities explain the aggravation of inflammation in S1P1 deficiency (7, 8) and account for favorable effects exerted by S1P1 agonists in animal models of inflammatory diseases (2, 3, 9).
Erythrocytes, platelets, and endothelial cells are the major sources of S1P in plasma, in which it binds to albumin or apolipoprotein M (apoM) found in a subpopulation of HDL (9, 10). This apoM+ HDL-bound S1P was suggested to contribute to atheroprotective effects attributed to HDL in epidemiological studies (10–12). S1P in plasma correlates with HDL in a concentration range, in which this lipoprotein protects against atherosclerosis, and lower levels of both, total and HDL-bound S1P, were noted in patients with coronary artery disease (13–15). However, studies on the effects of S1P on atherosclerosis in animal models led to disparate results. Synthetic S1P mimetics such as FTY720 — an S1P1,3,4,5 pan-agonist — and KRP203 — a specific S1P1 agonist — diminished lesions in LDL receptor–deficient (Ldlr–/–) models, improved endothelial function, and suppressed macrophage activation (16, 17). Likewise, elevating S1P levels by eliminating SK2 or S1P lyase in hematogenous cells blunted atherosclerosis in Ldlr–/– mice (18, 19). Conversely, S1pr1 deletion in endothelial cells or macrophages enhanced plaque development in mice (8, 20). By contrast, reduced atherosclerotic lesion formation was observed in apolipoprotein E–deficient (Apoe–/–) mice deficient in S1pr2 (21). Moreover, S1P elevation in Apoe–/– mice overexpressing apoM augmented aortic root but not aortic arch atherosclerosis, and this effect was abolished in uremia (22). Thus, the involvement of S1P in atherosclerosis depends on the S1P receptor involved, the location of lesions, and the experimental setting. In the present study, we attempted to resolve the controversy over the identity of S1P receptors and their cellular targets mediating atheroprotective effects. Toward this aim, we generated mouse lines with high or low overexpression levels of S1P1 in macrophages. Our results demonstrate that the amplification of S1P1 signaling produces a distinct subset of M2-like macrophages with antiinflammatory and antiatherogenic properties and ameliorates atherosclerosis development in Ldlr–/– mice. Since atheroprotective effects related to S1P1 overexpression were preserved in Ldlr–/– mice lacking apoM, our findings suggest that at least with respect to macrophages S1P binding to HDL is redundant for its beneficial effects in atherosclerosis.
Results
S1pr1-LysMCre and S1pr1-F4/80Cre mice overexpress S1P1 in macrophages.
To amplify S1P1 signaling in target cells, double-transgenic mice expressing mouse S1P1 in mononuclear cells were constructed by crossing 2 lines. The S1pr1-KI line carries a transgenic cassette in the Rosa 26 locus harboring the mouse S1pr1 cDNA and separated from the CAG promoter by a lox-Stop-lox insert (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.158127DS1). S1pr1-KI mice were crossed to either LysMCre or F4/80Cre mice expressing Cre recombinase under the control of the lysozyme M or F4/80 promoter, respectively. In the offspring the lox-Stop-lox insert is excised in Cre-expressing cells, which induces cDNA expression driven by the CAG promoter. ImmGen Skyline tool for the lysozyme M promoter–driven Cre expression predicted highest activities in peritoneal macrophages (PMs) and neutrophils and slightly lower activities in tissue-resident macrophages (lung, adipose tissue, bone marrow) (Supplemental Figure 1B). With respect to the F4/80 promoter, the highest Cre expression predicted in PMs was 40% lower compared with the lysozyme M promoter, whereas Cre expressions predicted in tissue-resident macrophages were 70%–80% lower. No Cre expression was predicted for the F4/80 promoter in neutrophils and for both lysozyme M and F4/80 promoters in T cells (Supplemental Figure 1B). Accordingly, we observed strongly enhanced S1pr1 mRNA expression in PMs and neutrophils from S1pr1-LysMCre mice and a weaker expression in S1pr1-F4/80Cre mice, whereas no differences were observed in T cells (Supplemental Figure 1C). In addition, a 3- to 6-fold increase in S1P1 on the macrophage or neutrophil cell surface was noted in double-transgenic lines and in the S1pr1-LysMCre line, respectively (Supplemental Figure 1C).
S1P1 overexpression retards atherosclerosis and alters plaque composition.
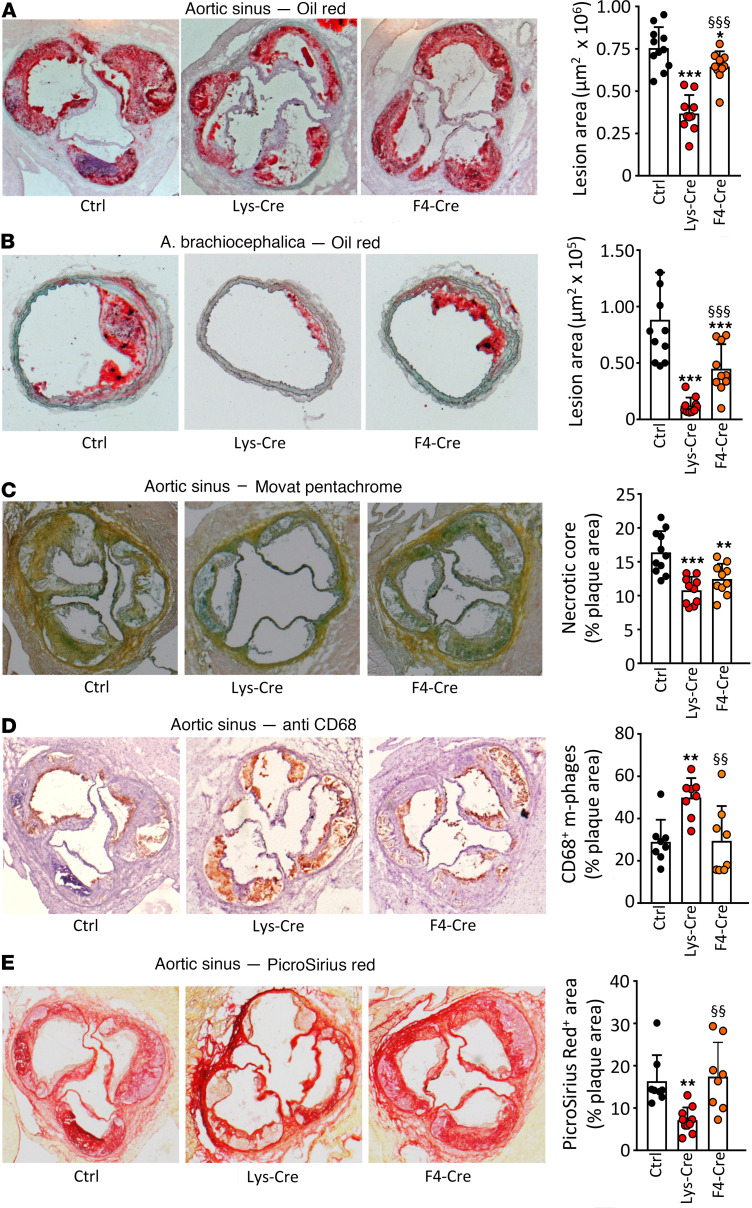
Atherosclerotic lesions were quantified in Ldlr–/– mice transplanted with S1pr1-KI, S1pr1-LysMCre, or S1pr1-F4/80Cre bone marrow (BM) and fed an atherogenic diet for 14 weeks. Lesion areas were profoundly reduced in aortic roots (~50%) and brachiocephalic arteries (~90%) in S1pr1-LysMCre–transplanted mice but only attenuated in aortic roots and roughly halved in brachiocephalic arteries from S1pr1-F4/80Cre chimeras (Figure 1, A and B). In addition, necrotic core area was significantly reduced, in both S1pr1-LysMCre– and S1pr1-F4/80Cre–transplanted mice (Figure 1C). Analysis of aortic root lesions yielded an increase of CD68-positive macrophage content in S1pr1-LysMCre but not S1pr1-F4/80Cre chimeras (Figure 1D). Conversely, the collagen content assessed by Picrosirius red staining was lower in S1pr1-LysMCre– but not S1pr1-F4/80Cre–transplanted mice (Figure 1E). The analysis of collagen-degrading proteases in PMs revealed no consistent mRNA expression pattern that could account for the reduced collagen amount in atherosclerotic plaques (Supplemental Figure 2).
Figure 1. S1P1 overexpression in macrophages and monocytes retards atherosclerotic lesion development and alters plaque morphology in Ldlr–/– mice.
Aortic root (A and C–E) and brachiocephalic arteries (B) from WD-fed Ldlr–/– mice transplanted with S1pr1-KI (Ctrl, n = 11), S1pr1-LysMCre (Lys-Cre, n = 10), or S1pr1-F4/80Cre (F4-Cre, n = 10) BM were used for morphometry (A and B) or stained for necrotic core analysis (Movat pentachrome, C), macrophages (anti-CD68, D), or collagen (Picrosirius red, E). Bar graphs show the necrotic core extent or the macrophage or collagen content in plaques expressed as the percentage of lesion area. * - P < 0.05, ** - P < 0.01, *** - P < 0.001 (Lys-Cre vs. Ctrl or F4-Cre vs. Ctrl), §§ - P < 0.01, §§§ - P < 0.001 (Lys-Cre vs. F4-Cre, 1-way ANOVA except B: Kruskal-Wallis h test).
S1P1 overexpression affects leukocyte count but not body weight and plasma lipid profile.
Transplanted mice on Western diet (WD) showed expanded monocyte and B cell (B220+) and reduced T cell (CD3+) populations (Supplemental Table 1). Irrespective of dietary treatment, S1P1 overexpression controlled by the lysozyme M, but not the F4/80, promoter increased monocyte and neutrophil counts. Further, we observed reduced erythrocyte numbers, hematocrit, and hemoglobin in both S1pr1-LysMCre and S1pr1-F4/80Cre mice and decreased platelets in S1pr1-LysMCre mice on WD. Lymphocyte count and subpopulations were not affected. No differences in body weight and plasma lipid levels including S1P were observed between WD-fed Ldlr–/– mice transplanted with BM from S1pr1-KI, S1pr1-LysMCre, or S1pr1-F4/80Cre (Supplemental Table 2).
S1P1 overexpression in macrophages activates PU.1 and interferon regulatory factor 8.
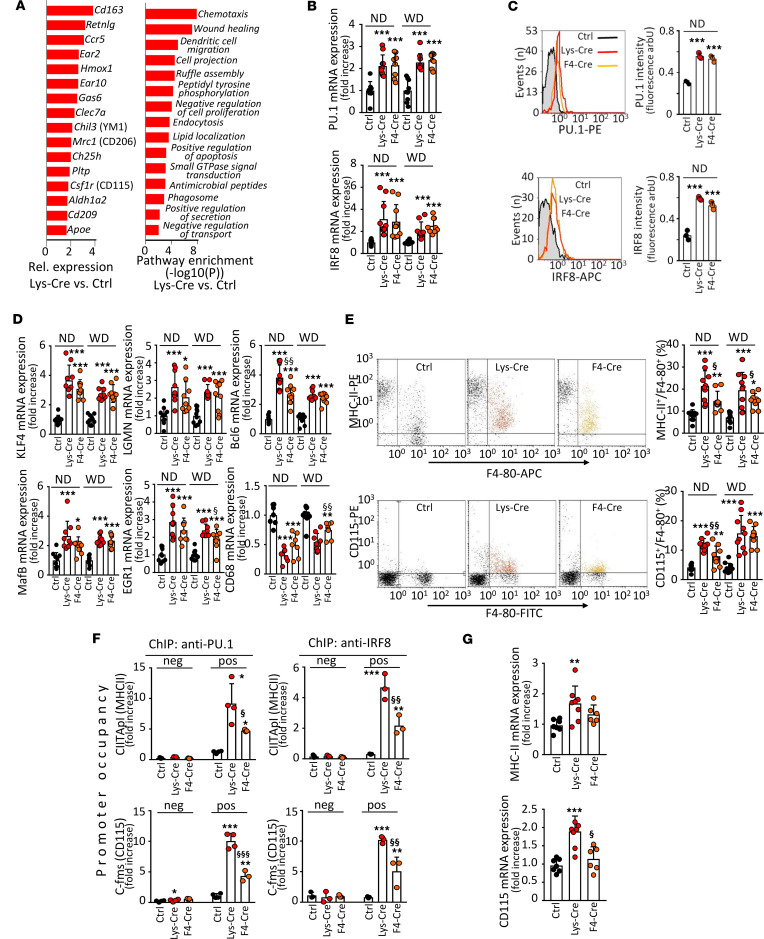
To characterize the transcriptional response to S1P1 overexpression, we performed gene expression analysis on PMs using oligonucleotide microarrays. mRNA expression levels of several genes involved in antiinflammatory macrophage polarization were increased in cells from S1pr1-LysMCre mice (Figure 2A). These findings were corroborated in a pathway and process enrichment analysis. Upregulated transcripts in macrophages from S1pr1-LysMCre mice revealed significant enrichment for antiinflammatory phenotype-associated functions such as wound healing (Figure 2A). Since several antiinflammatory genes identified in our analysis are directly or indirectly controlled by the transcription factors IRF8 and PU.1 (23, 24), we next assessed the effect of S1P1 overexpression on IRF8 and PU.1. Both mRNA and protein levels of Irf8/IRF8 and Spi1/PU.1 were increased in PMs from S1pr1-LysMCre and S1pr1-F4/80Cre mice (Figure 2, B and C). In addition, we detected elevated mRNA expression of several atheroprotective genes coordinately regulated by PU.1 and IRF8 (23) in cells overexpressing S1P1 (Figure 2D). By contrast, the mRNA expression of Cd68, which is negatively controlled by PU.1/IRF8 or PU.1/IRF4 complexes, was downregulated by S1P1 overexpression (Figure 2D). As IRF4 levels were not altered in PMs from S1pr1-LysMCre and S1pr1-F4/80Cre mice (not shown), we attribute this effect to IRF8. Notably, S1P1-overexpressing cells showed an increased surface presence of MHC class II (MHC-II) and CD115 (Figure 2E), whose promoters are canonical targets of PU.1. To investigate transcriptional activities of PU.1 and IRF8, we used chromatin immunoprecipitation (ChIP) to assess their binding to the class II transactivator (Ciita = Mhc2ta) and the Csfmr (= Cd115) gene promoters. Both PU.1 and IRF8 occupancies at – 0.2 kb and – 74 bp binding sites in Mhc2ta and Cd115 promoters, respectively, were increased in PMs from S1pr1-LysMCre and S1pr1-F4/80Cre mice (Figure 2F). We also observed increased Mhc2ta and Cd115 mRNA expression levels in aortas from these animals (Figure 2G).
Figure 2. S1P1 overexpression in macrophages enhances expression and activation of PU.1 and interferon regulatory factor-8.
PMs from either S1pr1-KI (Ctrl, n = 7–10), S1pr1-LysMCre (Lys-Cre, n = 7–10), or S1pr1-F4/80Cre (F4-Cre, n = 7–10) on normal diet (ND) or Ldlr–/– mice transplanted with S1pr1-KI (n = 10), S1pr1-LysMCre (n = 9), or S1pr1-F4/80Cre (n = 9) BM on WD. (A) Gene expression in PMs from Lys-Cre and Ctrl mice (n = 3–4 for each group) assessed with microarrays. Left panel: expression pattern showing elevated genes controlled by PU.1/interferon regulatory factor-8 (IRF8) (colony-stimulating factor-1 receptor [Csf1r], Clec7a, Mrc1) and liver X receptor (LXR) (phospholipid transfer protein [Pltp], Ch25h, Apoe) in S1pr1-LysMCre mice. Right panel: enrichment analysis of upregulated transcripts in S1pr1-LysMCre mice. Chil3, chitinase 3-like; Hmox, heme oxygenase. (B) Pu1 and Irf8 expression by quantitative PCR (qPCR). mRNA normalized to Gapdh and shown relative to S1pr1-KI. (C) Intracellular stainings for PU.1 (top panels) and IRF8 (bottom panels) analyzed by flow cytometry (n = 3 for each group). (D) qPCR of PU.1 and IRF8 signature genes. (E) CD115 and MHC-II analyzed by flow cytometry. (F) PU.1 and IRF8 occupancy at Cfms (Cd115) and Mhc2ta (CIITApI, MHC-II) promoters analyzed by ChIP. Primers amplifying at –0.2 kb and +4.5 kb for Cfms and –74 bp and –3.0 kb for CIITApI used as positive binding sites and negative controls (n = 3–4 for each group). (G) CD115 and MHC-II mRNA expression in aortas of WD-fed Ldlr–/– mice receiving S1pr1-KI, S1pr1-LysMCre, or S1pr1-F4/80Cre BM. * - P < 0.05, ** - P < 0.01, *** - P < 0.001 (Lys-Cre vs. Ctrl or F4-Cre vs. Ctrl), § - P < 0.05, §§ - P < 0.01, §§§ - P < 0.001 (Lys-Cre vs. F4-Cre, 1-way or 2-way ANOVA except B IRF8 and D CIITApI/anti-PU.1: Kruskal-Wallis h test).
S1P1 overexpression promotes antiinflammatory macrophage phenotype.
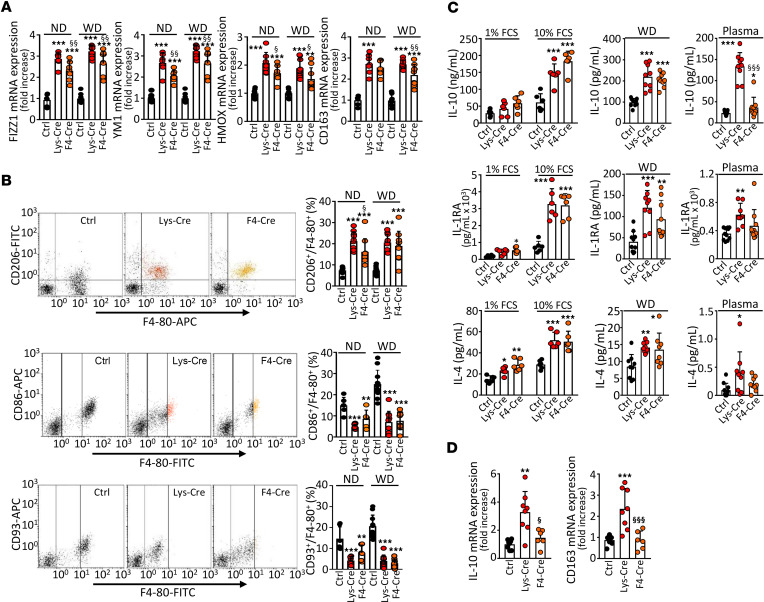
Transcription factors Krüppel-like factor 4 (KLF4) and Maf bZIP B (MAFB) control an antiinflammatory phenotype (25, 26). As mRNA expression levels of both, Klf4 and Mafb, were elevated in S1P1-overexpressing PMs (Figure 2D), we next investigated macrophage polarization markers in S1P1-knockin mice. We detected enhanced mRNA expression of genes belonging to an antiinflammatory phenotype signature, including resistin-like α (Retnla, Fizz1), Chi3l3 (Ym1), hemoglobin scavenger receptor (Cd163), and Hmox1, in S1pr1-LysMCre and S1pr1-F4/80Cre PMs (Figure 3A). In addition, these cells showed elevated cell surface presence of mannose receptor 1 (CD206; Figure 3B). By contrast, cell surface markers of pro-inflammatory macrophage phenotype CD86 and CD93 were downregulated in PMs overexpressing S1P1 (Figure 3B). To further elucidate the functional characteristics of S1pr1-LysMCre and S1pr1-F4/80Cre PMs, we measured antiinflammatory and pro-inflammatory cytokines in cell supernatants. Production of IL-10, IL-1RA, and IL-4 was increased in PMs from S1pr1-LysMCre and S1pr1-F4/80Cre mice (Figure 3C). In parallel, we detected elevated plasma levels of IL-10, IL-1RA, and IL-4 in S1pr1-LysMCre mice on WD (Figure 3C). Moreover, mRNA levels of Cd163 and Il10 were increased in the aortic arches of Ldlr–/– mice transplanted with S1pr1-LysMCre or S1pr1-F4/80Cre BM (Figure 3D). By contrast, production of pro-inflammatory cyto- and chemokines, including TNF-α, IL-6, KC1/GROα, and CCL5/RANTES, tended to be lower in S1P1-overexpressing PMs exposed for 24 hours to agonists of Toll-like receptor 2 (TLR-2; peptidoglycan [PGN], 0.02 μg/mL) or TLR-3 (polyinosinic–polycytidylic acid [pIC], 0.05 μg/mL; Supplemental Figure 3A). Concomitantly, lower levels of TNF-α, IL-6, KC1/GROα, and CCL5/RANTES were noted in plasma from S1pr1-LysMCre or S1pr1-F4/80Cre mice (Supplemental Figure 3B).
Figure 3. S1P1 overexpression in macrophages promotes antiinflammatory polarization.
PMs from either S1pr1-KI (Ctrl, n = 6–10), S1pr1-LysMCre (Lys-Cre, n = 6–10), or S1pr1-F4/80Cre (F4-Cre, n = 6–9) on ND or Ldlr–/– mice transplanted with S1pr1-KI (n = 9–11), S1pr1-LysMCre (n = 9–10), or S1pr1-F4/80Cre (n = 9–10) BM on WD. (A) qPCR of antiinflammatory signature genes. mRNA normalized to Gapdh and presented relative to S1pr1-KI. (B) CD206 (antiinflammatory marker) and CD86 and CD93 (pro-inflammatory markers) analyzed by flow cytometry. (C) PMs incubated for 24 hours in media containing 1% FCS (ND-fed mice, n = 6 for each group, left panels) or 10% FCS (ND- and WD-fed mice, left and central panels). Cytokines in media and plasmas (WD-fed mice, n = 8–11 for each group, central and right panels) determined by ELISA. (D) Cytokine mRNA expression in aortas by qPCR (n = 6–8 for each group). * - P < 0.05, ** - P < 0.01, *** - P < 0.001 (Lys-Cre vs. Ctrl or F4-Cre vs. Ctrl, § - P < 0.05, §§ - P < 0.01, §§§ - P < 0.001 (Lys-Cre vs. F4-Cre, 1-way ANOVA except C IL-10/Plasma and C IL-4/Plasma: Kruskal-Wallis h test).
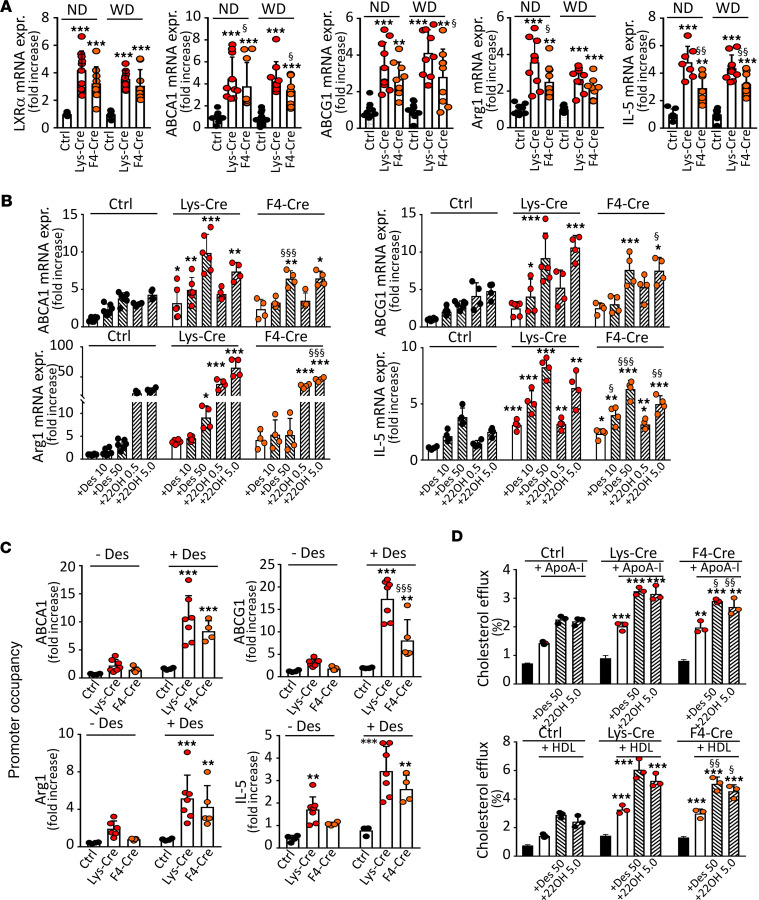
S1P1 overexpression in macrophages activates LXR.
In addition to IRF8- and PU.1-controlled genes, S1pr1-LysMCre PMs showed an increased expression of the liver X receptor–dependent (LXR-dependent) genes Apoe and Pltp (Figure 2A). Therefore, we investigated the effect of S1P1 overexpression on LXR activity. We found elevated mRNA expression of Lxr and its target genes ATP-binding cassette transporters A1 (Abca1) and G1 (Abcg1), arginase 1 (Arg1), and interleukin-5 (Il5) in S1pr1-LysMCre and S1pr1-F4/80Cre PMs (Figure 4A). Furthermore, the excessive mRNA expression of these genes was upregulated by LXR ligands desmosterol or 22-hydroxycholesterol with retinoic acid (22OH/RA, Figure 4B). In addition, the cell surface expression of LXR targets CD226 and CD244 was upregulated in S1pr1-LysMCre and S1pr1-F4/80Cre PMs (Supplemental Figure 4A). To assess LXR activity, we evaluated its binding to target gene promoters by ChIP. LXR promoter occupancy at –85 bp, –0.1 kb, –0.7 kb, and –0.2 kb binding sites in Abca1, Abcg1, Arg1, and Il5, respectively, was enhanced in macrophages from S1pr1-LysMCre and S1pr1-F4/80Cre mice and augmented by desmosterol (Figure 4C). Because of the elevated LXR activity, S1P1-overexpressing macrophages displayed increased cholesterol efflux to apoA-I or HDL, respectively (Figure 4D). In addition, these cells secreted more IL-5 in response to desmosterol or 22OH/RA, and elevated IL-5 was found in the plasma from S1pr1-LysMCre and S1pr1-F4/80Cre mice (Supplemental Figure 4B).
Figure 4. S1P1 overexpression in macrophages enhances expression and activation of LXRα.
PMs from either S1pr1-KI (Ctrl, n = 7–10), S1pr1-LysMCre (Lys-Cre, n = 7–10), or S1pr1-F4/80Cre (F4-Cre, n = 7–10) mice on ND or Ldlr–/– mice transplanted with S1pr1-KI (n = 10), S1pr1-LysMCre (n = 9), or S1pr1-F4/80Cre (n = 9) BM on WD. (A) qPCR of Lxra and LXR signature genes. mRNA normalized to Gapdh and presented relative to S1pr1-KI. (B) PMs from ND-fed mice incubated for 24 hours in media with desmosterol (Des, 10 or 50 μmol/L) or 22-hydroxycholesterol/9-cis-retinoic acid (22OH, 0.5 and 5.0 μg/mL). qPCR of LXR signature genes (n = 4–6 for each group). (C) LXR occupancy at Abca1, Abcg1, Arg1, and Il5 promoters by ChIP in PMs from ND-fed mice incubated for 24 hours with or without desmosterol (50 μmol/L). Primers amplifying at –85 bb and –4.0 kb for Abca1, –0.1 kb and –3.2 kb for Abcg1, –0.7 kb and –1.6 kb for Arg1, and –0.2 kb and –1.0 kb for Il5 used as positive binding sites and negative controls (n = 4–7 for each group). (D) PMs from ND-fed mice loaded with [1,2-3H]-cholesterol (n = 3 for each group) were incubated for 4 hours with apoA-I (10.0 μg/mL) or HDL (12.5 μg/mL). * - P < 0.05, ** - P < 0.01, *** - P < 0.001 (Lys-Cre vs. Ctrl or F4-Cre vs. Ctrl), § - P < 0.05, §§ - P < 0.01, §§§ - P < 0.001 (Lys-Cre vs. F4-Cre, 1-way or 2-way ANOVA except A Lxra/WD and A Abca1/ND: Kruskal-Wallis h test).
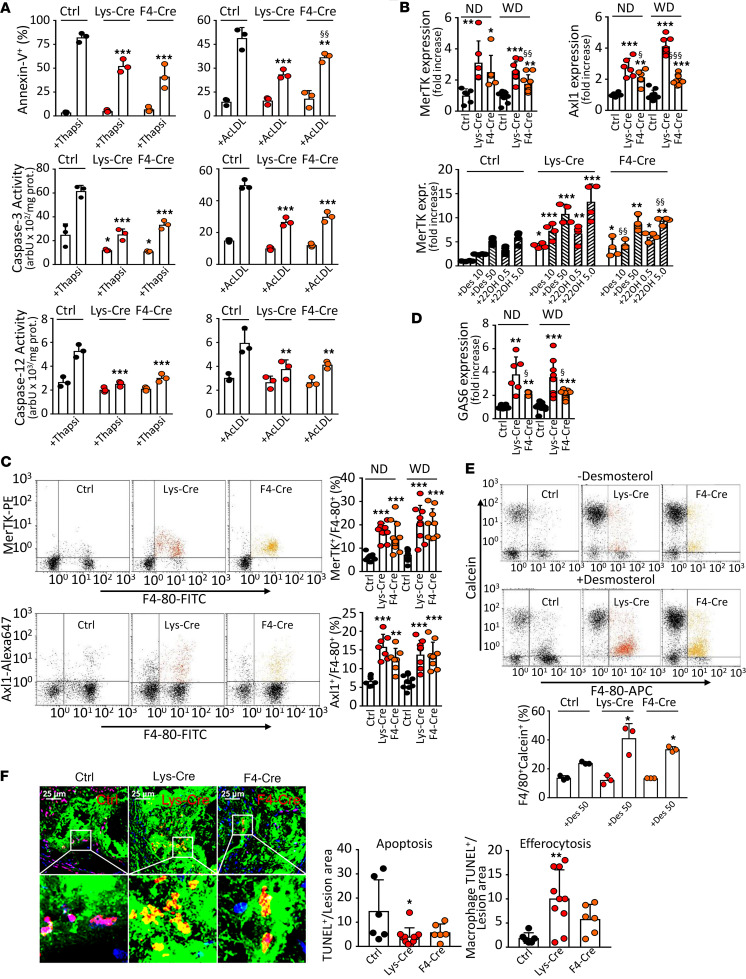
S1P1 overexpression in macrophages inhibits apoptosis and promotes efferocytosis.
Since the mRNA expression of antiapoptotic genes Mafb and Bcl6 was increased in S1P1-overexpressing macrophages (Figure 2D), we examined the macrophage propensity to undergo ER stress–induced apoptosis. The transmembrane phosphatidylserine shift and caspase-3 activity were determined in PMs after ER stress induction with thapsigargin/fucoidan or cholesterol loading. In addition, the ER stress–induced caspase-12 activity was determined. All indicators were attenuated in S1pr1-LysMCre and S1pr1-F4/80Cre mice (Figure 5A). Moreover, the tendency toward lower caspase-3 activity in S1P1-overexpressing macrophages was reversed by the BCL6 inhibitor 79-6 or the MafB modulator bortezomib (Supplemental Figure 5). As the reduced apoptosis is coupled to more effective efferocytosis, we subsequently examined whether efferocytosis is altered in S1P1-overexpressing macrophages. We found that the mRNA expression of Mertk and Axl1 — receptors responsible for the apoptotic cell ingestion and controlled by LXR and MafB, respectively (27, 28), was elevated in S1pr1-LysMCre and S1pr1-F4/80Cre PMs (Figure 5, B and C). In addition, both desmosterol and 22OH/RA augmented the mRNA expression of the LXR target Mertk in these cells (Figure 5B). Likewise, S1P1 overexpression enhanced the mRNA expression of growth arrest-specific 6 (Gas6), the bridging molecule between MerTK and apoptotic cells (Figure 5D). Consequently, desmosterol enhanced efferocytosis in S1P1-overexpressing macrophages as inferred from the increased ingestion of apoptotic RAW264.7 cells (Figure 5E). In addition, S1P1 overexpression affected apoptosis and efferocytosis in atherosclerotic lesions. We detected fewer macrophages with TUNEL-positive own nuclei but more macrophages with TUNEL-positive ingested nuclei within aortic root lesions, pointing to attenuated apoptosis but more efficient efferocytosis in S1pr1-LysMCre and S1pr1-F4/80Cre mice (Figure 5F).
Figure 5. S1P1 overexpression in macrophages inhibits ER stress–dependent apoptosis and enhances efferocytosis.
PMs from S1pr1-KI (Ctrl, n = 6–10), S1pr1-LysMCre (Lys-Cre, n = 6–10), or S1pr1-F4/80Cre (F4-Cre, n = 6–10) mice on ND or Ldlr–/– mice transplanted with S1pr1-KI (n = 10–11), S1pr1-LysMCre (n = 9), or S1pr1-F4/80Cre (n = 9) BM on WD. (A) PMs from ND-fed mice exposed for 24 hours to thapsigargin/fucoidan (Thapsi, 0.5 μmol/L and 25.0 μg/mL) or acetylated LDL (AcLDL, 100.0 μg/mL). Percentage of apoptotic (annexin V positive) cells and caspase-3 and -12 activities (n = 3 for each group). (B) qPCR of Mertk and Axl1 mRNA normalized to Gapdh and presented relative to S1pr1-KI. Lower panel: Mertk in PMs from ND-fed mice incubated for 24 hours with desmosterol (10 or 50 μmol/L) or 22-hydroxycholesterol/9-cis-retinoic acid (0.5 and 5.0 μg/mL, n = 4 for each group). (C) MerTK and Axl1 analyzed by flow cytometry. (D) qPCR of Gas6. (E) Dot plots showing efferocytosis of apoptotic RAW264.7 cells (ATCC) by PMs from ND-fed mice incubated for 24 hours with or without desmosterol (50 μmol/L). RAW264.7 cells and PMs were labeled with calcein and anti–F4/80-FITC (n = 3 for each group). (F) Aortic root section images with apoptotic cells labeled by TUNEL (red), macrophages by anti–MOMA-2 (green), and nuclei by DAPI (blue). Apoptotic cells appear violet (red on blue), and efferocytotic cells appear yellow (red on green, n = 5–10 for each group). The side of the square inset box measures 36 µm. * - P < 0.05, ** - P < 0.01, *** - P < 0.001 (Lys-Cre vs. Ctrl or F4-Cre vs. Ctrl), § - P < 0.05, §§ - P < 0.01, §§§ - P < 0.001 (Lys-Cre vs. F4-Cre, 1-way or 2-way ANOVA except B Axl1/WD, D ND and WD, and F Apoptosis: Kruskal-Wallis h test).
S1P1 agonist KRP203 emulates the effect of S1P1 overexpression on the functional phenotype in macrophages.
To strengthen the evidence underscoring the S1P1 effect on macrophage polarization, we treated both wild-type (WT) and Ldlr–/– mice on atherogenic diet with KRP203 — an S1P1 agonist with antiatherogenic properties (16) — and assessed their peritoneal macrophage phenotype. KRP203 substantially reduced absolute peripheral leukocyte and relative lymphocyte count in both mouse strains, verifying the treatment efficacy, but had no effect on body weight and plasma lipids in Ldlr–/– mice (Supplemental Figure 6, A and B). As shown in Supplemental Figure 6, C and D, changes in the mRNA expression of PU.1/IRF8-dependent genes (Bcl6, Klf4, Mhc2ta, and Cd115), the cell surface expression of polarization markers (CD206 and CD86), and the mRNA expression of LXR-dependent genes (Abca1, Abcg1, Mertk, and Cd244) in PMs from both WT and Ldlr–/– mice treated with KRP203 fully recapitulated the expression patterns seen in S1P1-overexpressing macrophages. In addition, the mRNA expression of Cd68 was reduced in PMs from mice administered KRP203 (Supplemental Figure 6D). A similar pattern was exhibited in PMs exposed in vitro to the active S1P1 ligand KRP203-phosphate (1.0 μmol/L) for 24 hours and was further enhanced by the concomitant overexpression of S1P1 (Supplemental Figure 6E).
We were concerned that the Cre transgene might affect the animal phenotype, translating into some beneficial changes in PM function in S1P1-overexpressing hematopoietic chimeras. However, we observed no differences between WT (C57BL/6), S1pr1-KI, and LysM-Cre mice with respect to the functional macrophage phenotype (Supplemental Figure 7).
S1P1 overexpression attenuates atherosclerosis and produces an antiatherogenic macrophage phenotype in Apom–/– Ldlr–/– mice.
To investigate whether binding to HDL is a prerequisite for S1P to unfold antiatherogenic activity, we employed Apom–/– Ldlr–/– mice, which lack the S1P chaperone apoM. As shown in Supplemental Figure 8, apoM deficiency did not abolish the favorable effect of S1P1 overexpression on the lesion area or macrophage phenotype (Supplemental Figure 8, A–D).
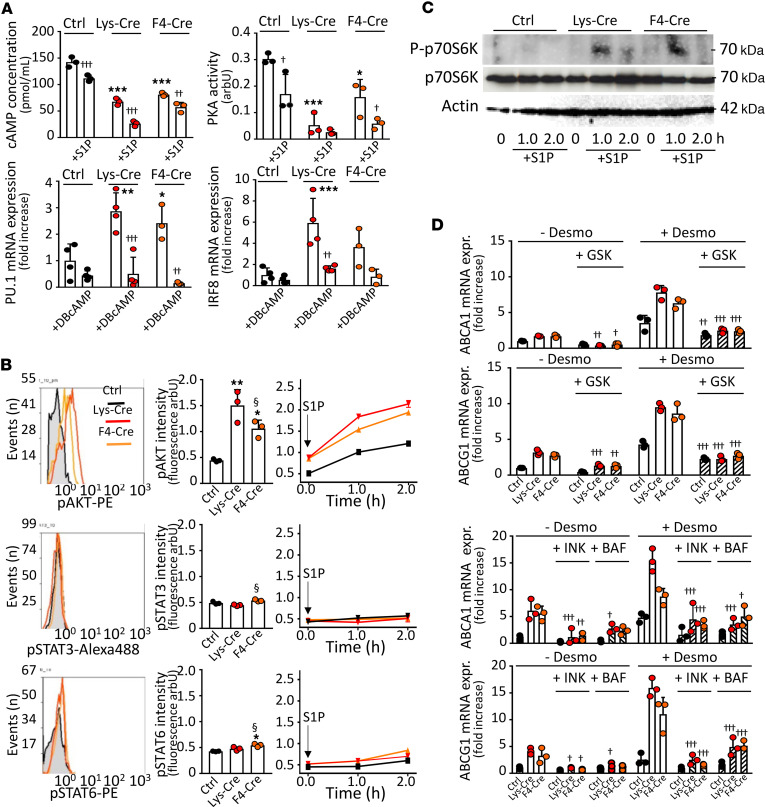
S1P1 signals in macrophages via PKA and AKT.
Finally, we investigated signaling pathways accounting for the antiatherogenic phenotype in S1P1-overexpressing macrophages. Since PU.1 and LXR activities are inversely and directly regulated by PKA as well as AKT and mechanistic target of rapamycin complex 1 (mTORC1) (29–31), respectively, we examined the effect of S1P on these kinases. Both the PKA activity and concentration of its upstream regulator cAMP were lower in S1pr1-LysMCre and S1pr1-F4/80Cre PMs, and this was potentiated by exogenous S1P (Figure 6A). By contrast, basal and S1P-stimulated AKT activities were increased in S1P1-overexpressing macrophages (Figure 6B). Similarly, S1P treatment stimulated mTORC1 in S1pr1-LysMCre and S1pr1-F4/80Cre PMs, as indicated by the p70S6 kinase phosphorylation (Figure 6C). In addition, phosphorylation of the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) — a well-known mTOR substrate — was enhanced in S1pr1-LysMCre PMs (Supplemental Figure 9A). Preincubating PM with the PKA activator DBcAMP suppressed the elevated Spi1 (PU.1) and Irf8 mRNA expression (Figure 6A). Similarly, the elevated mRNA expression of LXR targets Abca1 and Abcg1 was abolished by inhibitors of AKT (GSK690693) and mTORC1 (INK128) (Figure 6D). In addition, Abca1 and Abcg1 mRNA expression was suppressed by bafilomycin (Figure 6D), which indirectly inhibits the late endosomal/lysosomal adaptor MAPK and mTOR activator 1 (Lamtor-1) — the scaffolding protein for mTORC1 activation and the element connecting the signaling between AKT, mTORC1, and LXR (31). The involvement of AKT was further verified using A-674563, a synthetic inhibitor of AKT1, which completely abolished desmosterol-mediated induction of Abca1 and Abcg1 mRNA expression in S1pr1-LysMCre and S1pr1-KI PMs, whereas CCT-128930, a specific inhibitor of AKT2, showed only weak inhibitory effects (Supplemental Figure 9B). In contrast with AKT, basal and S1P-stimulated activities of STAT3 and STAT6, which are involved in macrophage polarization (see Discussion), were unchanged in PMs from S1P1-overexpressing macrophages (Figure 6B).
Figure 6. Stimulatory effects of S1P1 overexpression are mediated by PKA and AKT.
PMs from S1pr1-KI (Ctrl, n = 3–4), S1pr1-LysMCre (Lys-Cre, n = 3–4), or S1pr1-F4/80Cre (F4-Cre, n = 3–4) mice on ND were established in culture. (A) Cells were exposed to S1P (1.0 μmol/L) for 2 hours (upper panels) or dibutyryl-cAMP (DBcAMP) (0.25 mmol/L) for 24 hours (lower panels). cAMP levels and PKA activity were measured using enzyme immunoassay or Pep-Tag assay. Pu1 and Irf8 expressions were analyzed by qPCR. (B and C) PMs were analyzed for kinase activities or exposed to S1P (1.0 μmol/L) for indicated times. Intracellular stainings for phospho-AKT, phospho-STAT3, and phospho-STAT6 analyzed by flow cytometry (B). For mTOR1 activity, PMs lysates probed with antibodies against total and phosphorylated (P) p70S6 kinase (C). Blots representative for 2 independent experiments. (D) Cells were exposed for 30 minutes to GSK690693 (10.0 μmol/L), INK128 (0.2 μmol/L), or bafilomycin (1.0 μmol/L) prior to incubation with desmosterol (50 μmol/L) for 24 hours. Abca1 and Abcg1 genes analyzed by qPCR. Shown are results from 3 independent experiments. † - P < 0.05, †† - P < 0.01, ††† - P < 0.001 with vs. without treatment with activator/inhibitor, * - P < 0.05, ** - P < 0.01, *** - P < 0.001 (Lys-Cre vs. Ctrl or F4-Cre vs. Ctrl), § - P < 0.05 (Lys-Cre vs. F4-Cre, 1-way or 2-way ANOVA).
S1P1 overexpression exerts marginal effects on neutrophil plaque content and function in S1pr1-LysMCre mice.
In addition to macrophages, lysozyme M promoter–driven Cre also shows significant activity in neutrophils, which led to substantially increased S1p1 mRNA and S1P1 cell surface expression in neutrophils isolated from the S1pr1-LysMCre BM as compared with the S1pr1-KI BM (Supplemental Figure 1C). Therefore, we investigated the effects of increased neutrophil S1P1 expression on neutrophil function and cell content in atherosclerotic lesions.
We observed a slight reduction in neutrophil content in aortic roots from S1pr1-LysMCre–transplanted Ldlr–/– mice (Supplemental Figure 10A). In addition, IL-10 and KC1/GROα production in response to lipopolysaccharide (LPS) was lower in S1pr1-LysMCre neutrophils (Supplemental Figure 10B). However, shedding of L selectin and ROS generation in response to LPS or PMA, both sensitive indicators of neutrophil function, were comparable in S1pr1-LysMCre and S1pr1-KI mice (Supplemental Figure 10, B–D).
Discussion
Previous studies revealed that S1P signaling protects against atherosclerosis (16–20), but the identity of the S1P receptor subtype mediating this effect remained enigmatic. Studies employing S1P mimetics in mouse atherosclerosis models led to discrepant results, which was attributed to inadequate specificity or poorly defined side effects. Recently, accelerated lesion progression was reported in mice with endothelial or myeloid S1P1 deficiency (8, 20). However, the effect of cell-specific S1P1 upregulation on atherosclerosis was not examined. Here, we generated myeloid and macrophage-specific S1P1-knockin mouse lines with high and low S1P1 overexpression in macrophages, respectively, and used them as BM donors to produce Ldlr–/– chimeras prone to vascular lesion development. Analysis of atherosclerosis revealed reduced lesion areas in both lines, albeit the protective effects were more pronounced in S1pr1-LysMCre with high S1P1 overexpression than in S1pr1-F4/80Cre mice with low S1P1 overexpression. In addition, S1P1 overexpression blunted necrotic core formation, which critically depends on the macrophage apoptosis and removal of cellular debris within plaques. Accordingly, both S1pr1-LysMCre and S1pr1-F4/80Cre mice exhibited diminished apoptosis within atherosclerotic lesions along with an increased total and efferocytosis-positive macrophage number. These findings combined with the lower collagen amount in S1pr1-LysMCre lesions that could not be attributed to the increased expression of collagen-degrading proteases in macrophages point to slower advancement of lesions in S1P1-overexpressing chimeras. Collectively, these data for the first time to our knowledge provide unequivocal evidence that the amplification of S1P1-mediated signaling in macrophages protects against the development of atherosclerosis.
Although both the LysM and F4/80 promoters direct gene expression exclusively in the myeloid lineages, the 2 S1P1-overexpressing chimeras presented with quantitatively distinct phenotypes. While S1pr1-LysMCre mice showed a dramatic reduction of atherosclerosis combined with marked blood count alterations, the respective effects were moderate in S1pr1-F4/80Cre mice. Two explanations may account for this finding. First, considerably lower F4/80 than LysM promoter activity was predicted in silico in PMs, and this effect was even more pronounced in tissue-resident macrophages (see Supplemental Figure 1B). Accordingly, we found roughly 20%–50% weaker gene expressions, promoter occupancies, and/or functional cell responses in S1pr1-F4/80Cre as compared with S1pr1-LysMCre macrophages. These findings are congruent with previously published empirical results demonstrating that Cre expression from the F4/80 promoter is lower than from the LysM promoter in PMs and to an even greater extent in tissue-resident macrophages, which are primarily involved in the development of vascular lesions (32). Thus, the less pronounced antiatherogenic effect seen in S1pr1-F4/80Cre mice could be related to the quantitatively lesser enhancement of S1P1 signaling in macrophages in this mouse line. Second, our in silico analysis predicted marked LysM, but not F4/80, promoter activity in neutrophils in addition to macrophages. As neutrophils are now firmly identified as important players in the pathogenesis of atherosclerosis, the favorable antiatherogenic effect of S1P1 overexpression in these cells cannot be entirely dismissed. Of note, apoM-bound S1P has been recently shown to inhibit the formation of neutrophil extracellular traps and to increase the survival rate in a mouse model of LPS-induced sepsis (33). On the other hand, S1P1 seems to potentiate the neutrophil recruitment to chronically inflamed tissues, and inhibition of S1P1 signaling promotes the resolution rather than the exacerbation of neutrophilic inflammation (34, 35). With respect to atherosclerosis, little evidence could be found supporting the attenuating effect of S1P1 signaling in neutrophils on vascular lesion development in myeloid S1pr1-deficient mice (20). Our present results regarding the potential impact of neutrophil S1P1 on atherosclerosis remain inconclusive. While we observed a slight but significant reduction of neutrophil counts in aortas from S1pr1-LysMCre–transplanted mice, these cells were mainly localized in the adventitia rather than intima, making their impact on the atherosclerosis development questionable. In addition, while some proinflammatory neutrophil functions, such as cyto- and chemokine production, were reduced in cells obtained from S1pr1-LysMCre–transplanted mice, other important indicators of neutrophil activation (ROS production, L selectin shedding) were comparable to control cells. Notwithstanding this, BM transplantation from S1pr1-F4/80Cre mice with only moderate enhancement of S1P1 signaling exclusively in macrophages was sufficient to produce a measurable antiatherogenic effect in Ldlr–/– mice. This strongly supports the notion that the major antiatherogenic effect attributable to S1P1 takes place in macrophages and that potential antiatherogenic effects of S1P1 overexpression in neutrophils — if any — have only auxiliary character.
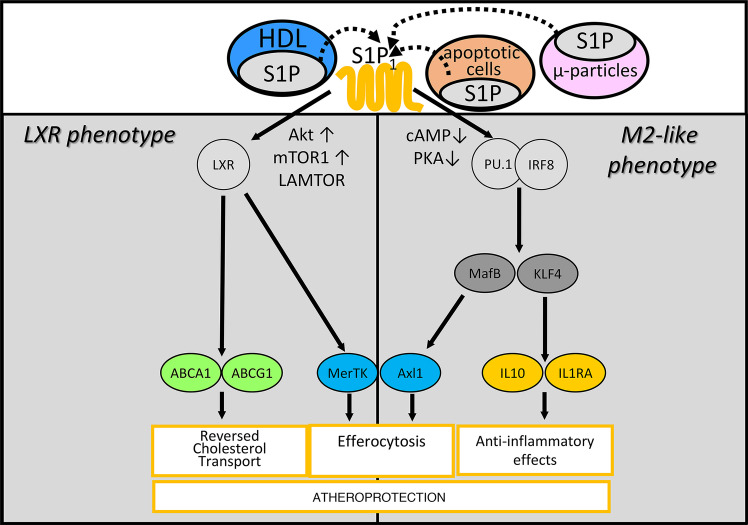
Our findings demonstrate a unique effect of S1P1 overexpression on the functional phenotype of macrophages. Previous studies indicated the role of S1P-induced signaling in promoting antiinflammatory macrophage polarization corresponding to the classical antiinflammatory M2 phenotype. For instance, the expression of pro-inflammatory genes (inducible nitric oxide synthase, cyclooxygenase) and the production of inflammatory cytokines were reduced in LPS-stimulated macrophages pretreated with S1P, while canonical markers defining the M2 phenotype (Arg1, Ym1, Il10, Cd163, Cd206) were upregulated (36–38). We here partially recapitulate these findings with respect to lower pro-inflammatory cyto- and chemokine production. Moreover, we extend them to define what we believe to be a novel, previously unrecognized functional phenotype distinct from the M2 polarization, which emerges both in S1P1-overexpressing macrophages and macrophages from mice treated with S1P1 agonist KRP203 as a result of the concomitant PU.1/IRF8 and LXRα activation. Previous studies documented that PU.1, acting in concert with transcription factors, such as IRF8, IRF4, STAT6, or HOXA3, promotes the expression of M2 polarization markers (Arg1, Ym1, Fizz/Retnla) and drives macrophages toward a corresponding phenotype distinguished by the increased production of MHC-II, CD206, IL-1RA, and IL-4 (23, 39–42). In addition, PU.1 controls transcription factors KLF4 and MafB, which support the sustained M2 macrophage polarization (25, 26). In our experimental setting, macrophages overexpressing S1P1 or treated with KRP203 acquired similar phenotypic features. However, reprogramming of macrophages toward the classical M2 phenotype was repeatedly shown to downregulate rather than upregulate LXRα-dependent gene expression (43, 44). Accordingly, macrophages generated by exposure to IL-4 displayed diminished, not increased, cholesterol efflux capacity owing to the reduced expression of Lxra and Abca1 (44). In a remarkable contrast, both S1P1 overexpression and KRP203 treatment led to activation of LXRα, which entailed the upregulation of Abca1, Abcg1, Il5, and Mertk. Overall, the concomitant activation PU.1/IRF8 and LXRα generated a unique macrophage phenotype not identical with the M2 one, bundling several antiatherogenic mechanisms (Figure 7). First, due to enhanced expression of PU.1, KLF4, MafB, and LXRα, these cells acquired antiinflammatory properties, including the increased secretion of IL-10, IL-1RA, and IL-5, aiding to resolve inflammation and protect against atherosclerosis. Second, owing to the augmented LXRα activity, S1P1-overexpressing macrophages upregulated cholesterol transporters Abca1 and Abcg1 and cholesterol efflux capacity, which likely reduced plaque cholesterol burden. Third, the increased expression of Bcl6 and Mafb attenuated the macrophage propensity to undergo ER stress–induced apoptosis, which translated into enhanced macrophage survival within atherosclerotic lesions. Fourth, due to the LXR- and MafB-dependent enhancement of Mertk and Axl1 expression, these macrophages could also be expected to effectively efferocytize apoptotic cells, a process governing necrotic core formation and late-stage lesion progression. Collectively, the present findings support the contention that S1P1 acts as master regulator of a unique functional macrophage phenotype unifying multiple atheroprotective mechanisms (Figure 7). The emergence of such a phenotype in S1pr1-LysMCre and to a lesser extent in S1pr1-F4/80Cre mice provides a consistent explanation for the attenuated plaque formation in these animals.
Figure 7. Proposed molecular mechanisms underlying atheroprotective effects of S1P1 signaling in macrophages.
Two signaling pathways are triggered by S1P upon interaction with S1P1 in macrophages: First, lowering intracellular cAMP and PKA activity enhances the function of IRF8 and PU.1, which facilitates the development of an M2-like macrophage phenotype characterized by the increased production of antiinflammatory cytokines. Second, stimulation of AKT and mTOR1 fosters LXR activity and thereby promotes ABCA1– and G1–dependent reversed cholesterol transport. By elevating MerTK and Axl1 both pathways facilitate efferocytosis. The combined effect is the attenuation of the development of atherosclerotic lesions.
Our findings provide additional mechanistic insights into signaling pathways linking S1P1 with the molecular machinery involved in the development of the atheroprotective phenotype in macrophages (Figure 7). By coupling to the trimeric Gi protein, S1P1 initiates signaling cascades, which in addition to the G protein class-defining inhibition of adenylate cyclase includes the activation of AKT (1–3). Accordingly, reduced cAMP concentration and PKA activity as well as increased AKT activity were seen in macrophages from S1pr1-LysMCre and S1pr1-F4/80Cre mice. By contrast, STAT3, which is activated by S1P1 in several tumor cell lines (45), remained quiescent in S1P1-overexpressing macrophages. Likewise, S1P1 overexpression did not affect STAT6, which participates in the development of the M2 phenotype in macrophages exposed to IL-4 (46). Previous studies reported decreased PU.1 promoter binding in macrophages upon elevated intracellular cAMP and PKA activation (29, 30), which is congruent with the reversal of increased PU.1/IRF8 expression in S1P1-overexpressing macrophages exposed to the cAMP mimetic DBcAMP. We assume that the diminished cAMP content coupled to the enhanced PU.1 and IRF8 expression in these cells translated into the antiinflammatory macrophage phenotype and reduced atherosclerosis in S1pr1-LysMCre and S1pr1-F4/80Cre mice. Moreover, previous reports associated AKT activation in macrophages with diverse antiatherogenic effects. For instance, AKT promotes macrophage survival by inhibiting caspase-3 or stimulating the transcriptional activation of antiapoptotic genes (47). Moreover, impaired efferocytosis was observed in macrophages obtained from LDL-related protein 1–deficient mice characterized by a defective AKT activation (47, 48). Finally, upregulation of AKT activity promotes antiinflammatory macrophage polarization (48). Out of 3 AKT isoforms expressed in macrophages, only AKT1 was unequivocally identified as atheroprotective, whereas AKT2 seems to exert some pro-atherogenic activity, as its loss in hematopoietic cells reduces plaque development in Ldlr–/– mice (47, 49). These findings are in line with our study, which shows that the potentially antiatherogenic signaling cascade triggered by S1P1 activation can be primarily linked to AKT1. Of note, AKT was identified as a triggering component of the signaling cascade encompassing mTORC1, the lysosomal adaptor Lamtor-1, and LXR, culminating in the M2 polarization of macrophages (31). In line with these findings, we observed that LXR activation in S1P1-overexpressing macrophages was dependent on AKT, mTORC1, and Lamtor-1, as it was abolished by respective inhibitors. Collectively, our findings point to AKT — most likely AKT1 — as an important mediator of the antiatherogenic effect exerted by S1P1 in macrophages.
Previous studies revealed that only apoM+ HDL-bound and not albumin-bound S1P augments the barrier function and inhibits leukocyte adhesion and apoptosis in endothelial cells via S1P1 (8, 11, 12). However, in our study, the transplantation of Ldlr–/– mice with S1P1-overexpressing BM reduced atherosclerosis and promoted antiatherogenic macrophage phenotype regardless of apoM expression. This finding does not support the contention that S1P mediates antiatherogenic effects of HDL in macrophages, though it may be required for the atheroprotective action in endothelial cells. Alternatively, other S1P chaperones in HDL might account for its antiatherogenic action in the absence of apoM. In this context, it is of interest that apoA-IV, which is a constituent of an HDL subfraction, was recently demonstrated to bind and present S1P to its receptors (50). In addition, Pltp was proposed to act as an S1P binding protein in plasma (51). Finally, it cannot be excluded that atheroprotective effects of non-HDL-bound S1P, which is present in atherosclerotic lesions as a component of apoptotic bodies or endothelially derived microparticles, becomes particularly evident under conditions of S1P1 overexpression. Clearly, further studies in alternative animal models are necessary to delineate the contribution of S1P to the antiatherogenic potential of HDL.
In conclusion, our study documents that the amplification of S1P1-dependent signaling in monocytes and macrophages countervails the lesion development in a mouse model of atherosclerosis. The underlying molecular mechanism involves the emergence of what we believe to be a novel macrophage phenotype, in which the parallel activation of transcription factors PU.1/IRF8 and LXR orchestrates several antiatherogenic pathways, including enhanced secretion of antiinflammatory cytokines, cholesterol disposal and efferocytosis, as well as reduced ER stress–induced apoptosis. Further investigations will be required to understand whether the atheroprotective mechanisms of S1P identified here may contribute to the beneficial effect of this lysosphingolipid on cardiovascular risk inferred from observational studies in humans.
Methods
Sex as a biological variable.
Our study examined female mice because larger and more pronounced atherosclerotic lesions in female compared with male Ldlr–/– mice are expected on a C57BL/6J background (52).
Animals.
C57Bl/6J-Gt(ROSA)26Sortm1(S1pr1)Geno mice (referred to as S1pr1-KI) were generated by Genoway by knocking in the floxed mouse S1pr1 transgene into embryonic stem cells as described (53). Briefly, the mouse S1pr1 cDNA controlled by the CAG promoter was engineered to contain a neomycin-stop cassette between promoter and the cDNA. Hence, the S1pr1 cDNA was only expressed following its Cre-mediated removal. The construct was introduced into the Rosa 26 locus. For S1P1 overexpression in monocytic cells, S1pr1-KI were crossed to B6.129P2-Lyz2tm1(cre)Ifo/J mice (Jackson Laboratory; crosses referred to as S1pr1-LysMCre) or B6.129P2-Adgre1tm1(cre)Kpf mice (from K. Pfeffer, University of Düsseldorf, Düsseldorf, Germany; crosses referred to as S1pr1-F4/80Cre). Female B6.129S7-Ldlrtm1Her/J mice (Jackson Laboratory, referred to as Ldlr–/–, 6 to 8 weeks of age) or Ldlr–/– crossed to apoM-lacking Apomtm1Cchr mice (provided in-house, referred to as Apom–/– Ldlr–/–, 6 to 8 weeks of age) underwent BM aplasia by irradiation (11 Gy) before the transplantation with S1pr1-KI, S1pr1-LysMCre, or S1pr1-F4/80Cre BM. Thereafter, animals were put on a WD (0.5% cholesterol, 21% fat; Altromin) for 14 weeks. C57Bl/6J WT mice (Charles River Laboratories) received i.p. injections of 0.075 mg KRP203 (Novartis) twice weekly for 4 weeks. For euthanasia, mice were anesthetized with 5% (v/v) isoflurane introduced via a vaporizer followed by exsanguination by heart puncture.
Materials and analytical procedures.
The detailed description of analytical procedures and materials used can be found in the online supplement (Supplemental Methods and Supplemental Tables 3–5).
Statistics.
Data are presented as means ± SD from at least 3 independent determinations. The distribution normality was assessed either with Smirnov-Kolmogorov or with Shapiro-Wilk tests. Comparisons between 2 groups were performed with Student’s 2-tailed t test or Mann-Whitney test for normally and non-normally distributed populations, respectively. Comparisons between 3 or more groups were performed with 1- or 2-way ANOVA with Holm-Šidák test for pairwise post hoc comparisons or Kruskal-Wallis h test with Conover test for pairwise post hoc comparisons for normally and non-normally distributed populations, respectively. P values less than 0.05 were considered significant.
Study approval.
All experiments conformed to the guidelines from directive 2010/63/EU and were approved by the local animal protection authorities (LANUV, Recklinghausen, Germany, permissions 84-02.04.2015.A505 and 81-02.04.2022.A329).
Data availability.
Values for all data points in graphs are reported in the Supporting Data Values.xls file. The microarray data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-14469. Other data from this study are available upon reasonable request.
Author contributions
FP, RB, and JRN initiated and designed the study; FP, ES, RF, JF, LA, GV, EP, MDS, GL, BH, MF, and AHL performed experiments; FP, GV, RB, and JRN performed the statistical analyses, interpreted the results, and drafted the manuscript; AHL, JR, BEK, FR, TR, CC, and MS helped interpret the results, critically read the manuscript, and provided important resources and methodological aid. FP, MS, RB, MF, and JRN acquired funding.
Supplementary Material
Acknowledgments
This project was supported by grants NO406/3-1 and BU2263/3-1 from Deutsche Forschungsgemeinschaft (DFG) to JRN and RB, DFG — Project number 209933838 — Collaborative Research Center 1052 “Obesity Mechanisms” (SFB-1052/B07) to RB, grant IDEAS RBID08777T from the Italian Ministry of Education, Universities and Research to JRN and MS and grants GR-2011-02346974 from the Italian Ministry of Health and FIL2016-competitive section from the University of Parma to FP. The expert technical assistance of Beate Schulte, Cornelia Richter-Elsenheimer, and Bärbel Schell is gratefully acknowledged.
Version 1. 11/12/2024
In-Press Preview
Version 2. 12/20/2024
Electronic publication
Version 3. 12/26/2024
Text spacing ommision in title: the space between “1
Version 4. 01/28/2025
Figures 2, 3, and 5 had parts of the image shifted. They now appear correctly
Funding Statement
to Jerzy-Roch Nofer
to Ralph Burkhardt
to Ralph Burkhardt
to Jerzy-Roch Nofer and Manuela Simoni
to Francesco Poti
to Francesco Poti
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2024, Potì et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2024;9(24):e158127.https://doi.org/10.1172/jci.insight.158127.
Contributor Information
Francesco Potì, Email: francesco.poti@unipr.it.
Enrica Scalera, Email: enricascalera@gmail.com.
Renata Feuerborn, Email: Renataantonina.Feuerborn@ukmuenster.de.
Josephine Fischer, Email: zessi-josi@hotmail.de.
Lilli Arndt, Email: lilli.arndt@medizin.uni-leipzig.de.
Georg Varga, Email: varga@uni-muenster.de.
Evangelia Pardali, Email: liapardali@gmail.com.
Matthias D. Seidl, Email: seidl@uni-muenster.de.
Manfred Fobker, Email: manfred.fobker@ukmuenster.de.
Gerhard Liebisch, Email: gerhard.liebisch@klinik.uni-regensburg.de.
Bettina Hesse, Email: hesseb@gmx.net.
Alexander H. Lukasz, Email: alexander-henrik.lukasz@ukmuenster.de.
Jan Rossaint, Email: rossaint@uni-muenster.de.
Beate E. Kehrel, Email: kehrel@uni-muenster.de.
Frank Rosenbauer, Email: frank.rosenbauer@ukmuenster.de.
Thomas Renné, Email: t.renne@uke.de.
Christina Christoffersen, Email: Christina.Christoffersen@regionh.dk.
Manuela Simoni, Email: manuela.simoni@unimore.it.
Ralph Burkhardt, Email: ralph.burkhardt@ukr.de.
Jerzy-Roch Nofer, Email: nofer@uni-muenster.de.
References
- 1.Kano K, et al. Lysophospholipid mediators in health and disease. Annu Rev Pathol. 2022;17:459–483. doi: 10.1146/annurev-pathol-050420-025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannavo A, et al. Sphingosine kinases and sphingosine 1-phosphate receptors: signaling and actions in the cardiovascular system. Front Pharmacol. 2017;8:556. doi: 10.3389/fphar.2017.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelbrecht E, et al. Lysolipids in vascular development, biology, and disease. Arterioscler Thromb Vasc Biol. 2021;41(2):564–584. doi: 10.1161/ATVBAHA.120.305565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marciniak A, et al. An update on sphingosine-1-phosphate receptor 1 modulators. Bioorg Med Chem Lett. 2018;28(23–24):3585–3591. doi: 10.1016/j.bmcl.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li N, Zhang F. Implication of sphingosine-1-phosphate in cardiovascular regulation. Front Biosci. 2016;21(7):1296–1131. doi: 10.2741/4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoki M, et al. Sphingosine-1-Phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediators Inflamm. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry HM, et al. Endothelial sphingosine 1‑phosphate receptor‑1 mediates protection and recovery from acute kidney injury. J Am Soc Nephrol. 2016;27(11):3383–3393. doi: 10.1681/ASN.2015080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvani S, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8(389):ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stepanovska B, Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res. 2020;154:104170. doi: 10.1016/j.phrs.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Christoffersen C, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108(23):9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen PM, et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016;30(6):2351–2359. doi: 10.1096/fj.201500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz M, et al. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler Thromb Vasc Biol. 2017;37(1):118–129. doi: 10.1161/ATVBAHA.116.308435. [DOI] [PubMed] [Google Scholar]
- 13.Karuna R, et al. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 2011;219(2):855–863. doi: 10.1016/j.atherosclerosis.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Sattler K, et al. Defects of high-density lipoproteins in coronary artery disease caused by low sphingosine-1-phosphate content: correction by sphingosine-1-phosphate-loading. J Am Coll Cardiol. 2015;66(13):1470–1485. doi: 10.1016/j.jacc.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Soltau I, et al. Serum-sphingosine-1-phosphate concentrations are inversely associated with atherosclerotic diseases in humans. PLoS One. 2016;11(12):e0168302. doi: 10.1371/journal.pone.0168302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potì F, et al. KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R-/- mice. Arterioscler Thromb Vasc Biol. 2013;33(7):1505–1512. doi: 10.1161/ATVBAHA.113.301347. [DOI] [PubMed] [Google Scholar]
- 17.Nofer JR, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115(4):501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 18.Bot M, et al. Dietary carbohydrates modulate Candida albicans biofilm development on the denture surface. PLoS One. 2013;8(5):e64645. doi: 10.1371/journal.pone.0063360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feuerborn R, et al. Elevating endogenous sphingosine-1-phosphate (S1P) levels improves endothelial function and ameliorates atherosclerosis in low density lipoprotein receptor-deficient (LDL-R-/-) mice. Thromb Haemost. 2018;118(8):1470–1480. doi: 10.1055/s-0038-1666870. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez L, et al. Sphingosine-1-Phosphate receptor 1, expressed in myeloid cells, slows diet-induced atherosclerosis and protects against macrophage apoptosis in Ldlr KO Mice. Int J Mol Sci. 2017;18(12):2721. doi: 10.3390/ijms18122503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skoura A, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(1):81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosteen MH, et al. Effects of apolipoprotein M in uremic atherosclerosis. Atherosclerosis. 2017;265:93–101. doi: 10.1016/j.atherosclerosis.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Kurotaki D, et al. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood. 2013;121(10):1839–1849. doi: 10.1182/blood-2012-06-437863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feinberg MW, et al. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26(18):4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao X, et al. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121(7):2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H. The transcription factor MafB promotes anti-inflammatory M2 polarization and cholesterol efflux in macrophages. Sci Rep. 2017;7(1):7591. doi: 10.1038/s41598-017-07381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato M, et al. MafB enhances efferocytosis in RAW264.7 macrophages by regulating Axl expression. Immunobiology. 2018;223(1):94–100. doi: 10.1016/j.imbio.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Park SY, et al. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by γ-ray radiation. Br J Pharmacol. 2013;168(2):421–431. doi: 10.1111/j.1476-5381.2012.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zasłona Z, et al. Protein kinase A inhibition of macrophage maturation is accompanied by an increase in DNA methylation of the colony-stimulating factor 1 receptor gene. Immunology. 2016;149(2):225–237. doi: 10.1111/imm.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura T, et al. Polarization of M2 macrophages requires Lamtor-1 that integrates cytokine and amino-acid signals. Nat Commun. 2016;7:13130. doi: 10.1038/ncomms13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abram CL, et al. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurano M, et al. Apolipoprotein M bound sphingosine 1-phosphate suppresses NETosis through activating S1P1 and S1P4. Biomed Pharmacother. 2023;166:115400. doi: 10.1016/j.biopha.2023.115400. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, et al. Neutrophil recruitment mediated by sphingosine 1-phosphate (S1P)/S1P receptors during chronic liver injury. Cell Immunol. 2021;359:104243. doi: 10.1016/j.cellimm.2020.104243. [DOI] [PubMed] [Google Scholar]
- 35.Perez DA, et al. Inhibition of the sphingosine-1-phosphate pathway promotes the resolution of neutrophilic inflammation. Eur J Immunol. 2019;49(7):1038–1051. doi: 10.1002/eji.201848049. [DOI] [PubMed] [Google Scholar]
- 36.Hughes JE, et al. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102(8):950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SJ, et al. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell Signal. 2014;26(10):2249–2258. doi: 10.1016/j.cellsig.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Ono Y, et al. Sphingosine 1-phosphate (S1P) in the peritoneal fluid skews M2 macrophage and contributes to the development of endometriosis. Biomedicines. 2021;9(11):1519. doi: 10.3390/biomedicines9111519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pourcet B, et al. LXRα regulates macrophage arginase 1 through PU.1 and interferon regulatory factor 8. Circ Res. 2011;109(5):492–501. doi: 10.1161/CIRCRESAHA.111.241810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Sadoun H, et al. Enforced expression of Hoxa3 inhibits classical and promotes alternative activation of macrophages in vitro and in vivo. J Immunol. 2016;197(3):872–884. doi: 10.4049/jimmunol.1501944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czimmerer Z, et al. Extensive and functional overlap of the STAT6 and RXR cistromes in the active enhancer repertoire of human CD14+ monocyte derived differentiating macrophages. Mol Cell Endocrinol. 2018;471:63–74. doi: 10.1016/j.mce.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 42.Qian F, et al. The transcription factor PU.1 promotes alternative macrophage polarization and asthmatic airway inflammation. J Mol Cell Biol. 2015;7(6):557–567. doi: 10.1093/jmcb/mjv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czimmerer Z, et al. The transcription factor STAT6 mediates direct repression of inflammatory enhancers and limits activation of alternatively polarized macrophages. Immunity. 2018;48(1):75–90. doi: 10.1016/j.immuni.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chinetti-Gbaguidi G, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ Res. 2011;108(8):985–995. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagahashi M, et al. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv Biol Regul. 2014;54:112–120. doi: 10.1016/j.jbior.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7(6):485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linton MF, et al. Akt signaling in macrophage polarization, survival, and atherosclerosis. Int J Mol Sci. 2019;20(11):2703. doi: 10.3390/ijms20112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yancey PG, et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol. 2010;30(4):787–795. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babaev VR, et al. Loss of 2 Akt (protein kinase B) isoforms in hematopoietic cells diminished monocyte and macrophage survival and reduces atherosclerosis in Ldl receptor-null mice. Arterioscler Thromb Vasc Biol. 2019;39(2):156–169. doi: 10.1161/ATVBAHA.118.312206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obinata H, et al. Identification of ApoA4 as a sphingosine 1-phosphate chaperone in ApoM- and albumin-deficient mice. J Lipid Res. 2019;60(11):1912–1921. doi: 10.1194/jlr.RA119000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng J, et al. PLTP deficiency-mediated atherosclerosis regression could be related to sphinogosine-1-phosphate reduction. Atherosclerosis. 2022;356:53–55. doi: 10.1016/j.atherosclerosis.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van der Vaart JI, et al. Atherosclerosis: an overview of mouse models and a detailed methodology to quantify lesions in the aortic root. Vasc Biol. 2024;6(1):e230017. doi: 10.1530/VB-23-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velagapudi S, et al. Apolipoprotein M and sphingosine-1-phosphate receptor 1 promote the transendothelial transport of high-density lipoprotein. Arterioscler Thromb Vasc Biol. 2021;41(10):e468–e479. doi: 10.1161/ATVBAHA.121.316725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Values for all data points in graphs are reported in the Supporting Data Values.xls file. The microarray data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-14469. Other data from this study are available upon reasonable request.