Visual Abstract
Abstract
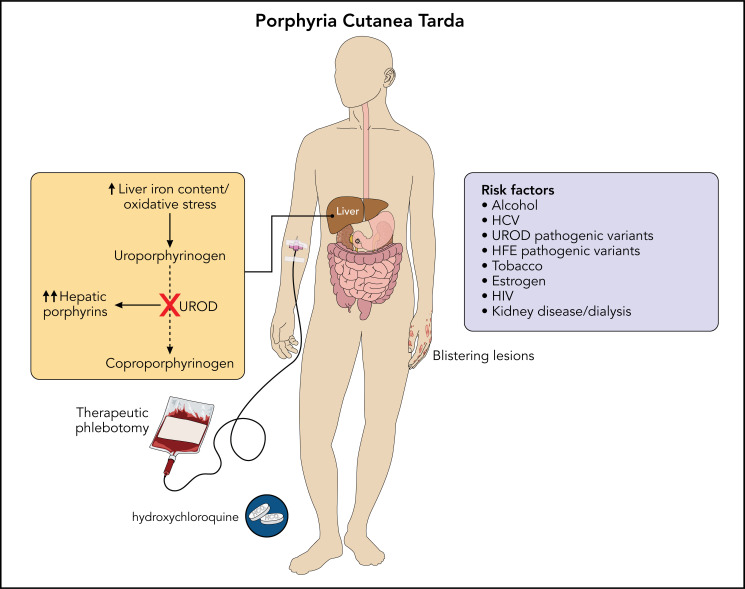
The porphyrias are a group of disorders of heme biosynthesis, each characterized by an enzymatic defect in the heme biosynthetic pathway. Porphyria cutanea tarda (PCT) arises due to the inhibition of uroporphyrinogen decarboxylase (UROD) in the presence of hepatic iron and oxidative stress. Most patients with PCT have evidence of siderosis on liver biopsy, and the disease resolves with iron depletion. PCT manifests as skin fragility, blistering cutaneous lesions on sun-exposed areas, dark urine, and elevated plasma and urine porphyrins. Factors contributing to the development of PCT include alcohol use, hepatitis C virus infection, human immunodeficiency virus, estrogen use, UROD pathogenic variants, and hereditary hemochromatosis. Treatment includes therapeutic phlebotomy to decrease total body iron levels and low-dose hydroxychloroquine, which reduces hepatic porphyrin content. The following review explores the biology of PCT, the critical role of iron in disease pathogenesis, and our approach to the management of these patients.
Learning Objectives
Understand that PCT arises from decreased UROD activity in the liver
Recognize the central role of iron in PCT pathogenesis
Identify effective treatments for PCT, including phlebotomy and hydroxychloroquine
CLINICAL CASE
A 39-year-old woman with a history of anxiety and asthma presented with fluid-filled blisters over the dorsal surfaces of her hands and arms, and dark urine. Her medications included a combined estrogen-progestin oral contraceptive pill and an albuterol inhaler. Skin biopsy was consistent with a subepidermal blister, papillary dermal festooning, and periodic acid-Schiff positivity within blood vessel walls. Urine and plasma porphyrins were elevated, with a predominance of uroporphyrin and heptacarboxyl porphyrin (urine uroporphyrin, 3959 nmol/L [normal <30] and plasma uroporphyrin, 2.0 µg/dL [normal <1.0 µg/dL]), and urine porphobilinogen was within normal limits. The serum ferritin level was 420 µg/L (normal <200), and molecular studies revealed homozygous C282Y pathogenic variants of the HFE gene but no UROD pathogenic variant.
Introduction
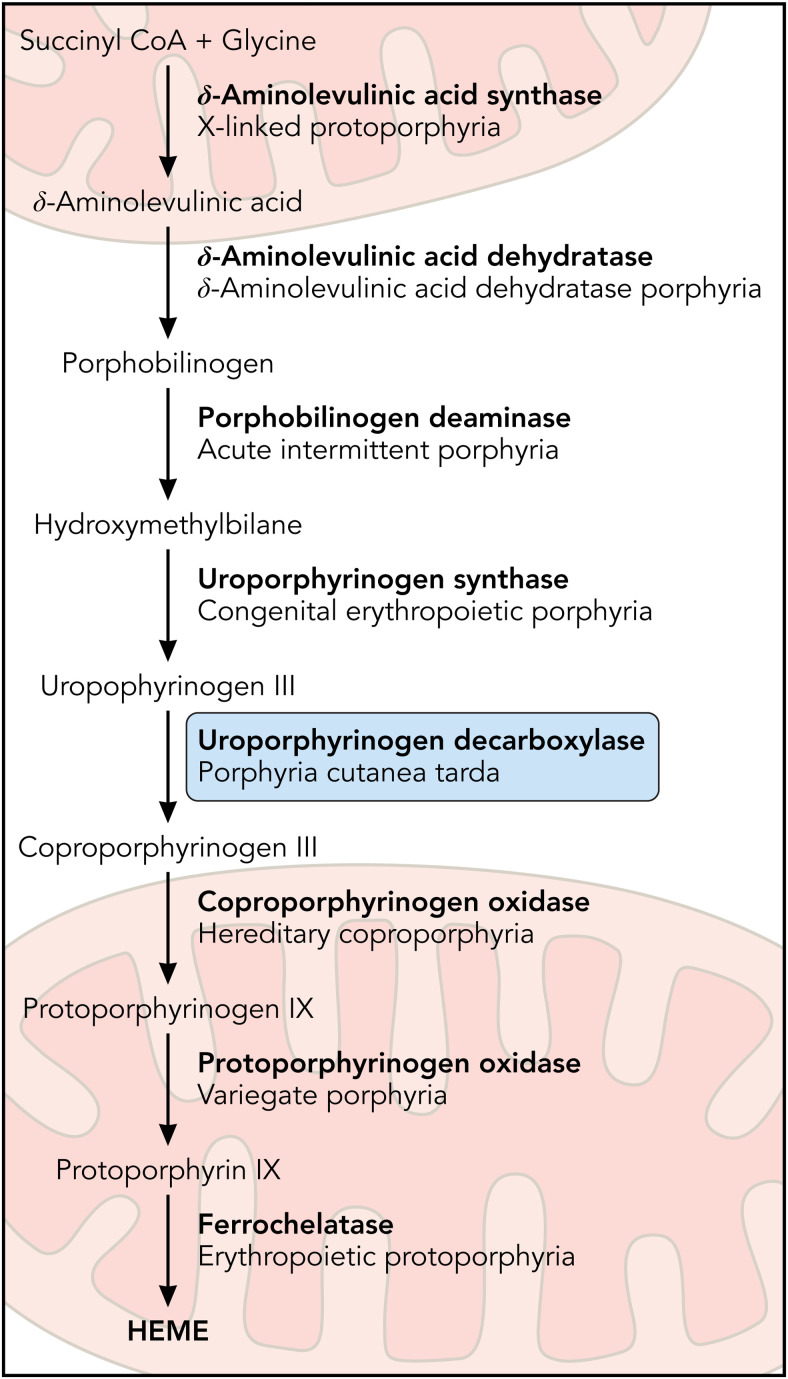
The porphyrias are a group of disorders of heme biosynthesis, each specific to a particular defect in the heme biosynthetic pathway (Figure 1). Porphyria may be categorized clinically as acute hepatic, blistering cutaneous, or nonblistering cutaneous depending on clinical features. They are also classified as erythropoietic or hepatic based on the site of initial accumulation of intermediates of the heme biosynthetic pathway. Porphyria cutanea tarda (PCT) occurs due to inhibition of the fifth enzyme of heme biosynthesis, uroporphyrinogen decarboxylase (UROD). PCT has been estimated to affect 5 to 10 persons per 100 000, usually in mid- or later life.1 Manifestations of PCT include blistering of sun-exposed skin and liver disease due to the accumulation of porphyrins. Contributing environmental factors include alcohol use and hepatitis C virus (HCV) infection.2 PCT usually occurs in the absence of a UROD pathogenic variant, and a heterozygous UROD pathogenic variant alone is not sufficient to cause the disease. Therefore, PCT is considered to be primarily an acquired porphyria, with well-established susceptibility factors associated with disease pathogenesis.
Figure 1.
The heme biosynthetic pathway. Heme biosynthesis occurs via 8 enzymatic steps. The decreased activity of UROD, the fifth step in the pathway, leads to porphyria cutanea tarda. Professional illustration by Patrick Lane, ScEYEnce Studios.
PCT can be classified based on the presence or absence of UROD pathogenic variants and family history. Most cases (~80%) are sporadic (type 1), with no UROD pathogenic variants. A heterozygous UROD pathogenic variant is found in familial (type 2), which is an autosomal dominant condition with low penetrance, such that there is usually no family history of PCT. Rarely, cases occur in more than 1 family member in the absence of a UROD pathogenic variant, which is termed type 3 PCT. HFE mutations or shared environmental factors may be present in such families.3 In all types of PCT, porphyrin accumulation and clinical symptoms of PCT do not occur unless UROD enzymatic activity falls below approximately 20% in the liver. Therefore, even patients with heterozygous UROD pathogenic variant must have additional susceptibility factors to develop and manifest PCT.3-5 Hepatoerythropoietic porphyria (HEP) is an ultrarare form of porphyria caused by homozygous pathogenic UROD variants that usually presents in early childhood with blistering skin lesions, anemia, and hepatosplenomegaly and resembles congenital erythropoietic porphyria.6 Mild cases of HEP may resemble PCT but are readily differentiated by marked elevation in erythrocyte zinc protoporphyrin. Pseudoporphyria is a dermatologic condition with blistering skin lesions identical to those observed in PCT, but without alterations in heme biosynthesis or evidence of porphyrin accumulation.
Pathophysiology
PCT results from the inhibition of hepatic UROD, a cytoplasmic housekeeping enzyme that converts uroporphyrinogen to coproporphyrinogen. This enzyme is encoded by the UROD gene, located on chromosome 1 with 10 exons spanning over 3 kb.7,8 UROD carries out a complex reaction, sequentially decarboxylating the 4 acetyl groups of uroporphyrinogen (an octacarboxyl porphyrin) to hepta-, hexa-, penta-, and finally coproporphyrinogen (a tetracarboxyl porphyrin; Figure 2).3,4 Both uroporphyrinogen I and III isomers are decarboxylated by UROD, but uroporphyrinogen III is preferred because coproporphyrinogen oxidase is specific for coproporphyrinogen III, and the III isomers are intermediates in heme synthesis.9-11
Figure 2.
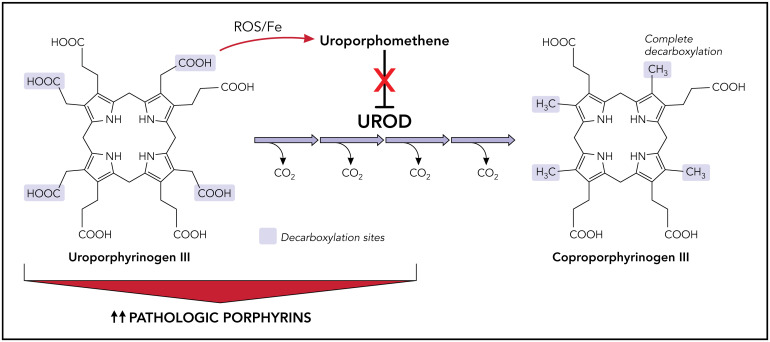
Inhibition of UROD by uroporphomethene leads to the accumulation of porphyrins and manifestations of disease in PCT. Under normal conditions, UROD converts uroporphyrinogen to coproporphyrinogen by a series of 4 sequential decarboxylations. In the presence of iron and free radicals, uroporphyrinogen is partially oxidized, leading to the formation of a uroporphomethene inhibitor of UROD. Decarboxylated uroporphyrinogen intermediates subsequently accumulate and auto-oxidize to their corresponding porphyrins, predominantly uroporphyrins. Photosensitive porphyrins accumulate in the plasma and are responsible for the cutaneous manifestations of PCT. Fe, iron; PCT, porphyria cutanea tarda; UROD, uroporphyrinogen decarboxylase; ROS, reactive oxygen species. Professional illustration by Patrick Lane, ScEYEnce Studios.
The hepatic UROD protein level remains at its genetically determined level in all types of PCT, but hepatic enzyme activity is reduced to less than about 20% of normal, suggesting the presence of an enzyme inhibitor. Phillips and colleagues identified this inhibitor as a uroporphomethene, probably derived from the partial oxidization of uroporphyrinogen.12 At least in murine models, cytochrome P450 enzyme activity is involved in the generation of this inhibitor. Uroporphomethene differs from uroporphyrinogen by a single oxidized bridge carbon, and although it is able to bind strongly to the active site of UROD, it is unable to serve as a substrate.12 That being said, other groups have questioned whether uroporphomethene is in fact a true inhibitor of UROD based on its fragmentation pattern on mass spectrometry.13
When hepatic UROD activity is reduced to less than 20% of normal activity, uroporphyrinogen and the porphyrinogens that are intermediates in its 4-step decarboxylation accumulate in the liver and are auto-oxidized to their corresponding porphyrins.14 After considerable accumulation in the liver, these porphyrins appear in plasma and bile and are excreted in the urine and stool.10 These porphyrins are activated by light exposure (especially at wavelengths near 400 nm) and generate reactive oxygen species that damage sun-exposed skin.14 Further, in UROD-deficient mice, the upregulation of δ-aminolevulinic acid synthase 1 (ALAS-1) by drugs that induce hepatic P450 enzymes and the supplementation of δ-aminolevulinic acid (ALA) in the drinking water have been shown to induce a PCT phenotype.15
Laboratory diagnosis
PCT is diagnosed biochemically by high levels of porphyrins in the plasma and urine, with a predominance of uroporphyrinogen and hepta- and hexa- and pentacarboxyl porphyrins. This pattern of porphyrin elevation is characteristic but not completely specific since uroporphyrin elevation occurs in other porphyrias, and a PCT-like pattern occurs in some patients with variegate porphyria (VP).16 Therefore, analysis of porphyrins in the erythrocytes and feces should be considered. Urine measurements using random urine samples with normalization to creatinine is recommended. Urine porphobilinogen is normal in PCT, and ALA is normal or only mildly increased.3 Plasma fluorescence scanning is useful for rapid differentiation of VP, which has a diagnostic peak at approximately 626 nm. Fecal total porphyrins may be normal or elevated in PCT, and an elevation of fecal isocoproporphyrins is specific for UROD inhibition.16,17
Liver biopsy is seldom indicated in patients with PCT but if done before treatment reveals marked elevations in porphyrin concentrations with red fluorescence when the fresh specimen is illuminated with a Wood's lamp. The pattern of elevation of individual porphyrins is the same as in plasma and urine. Reduced erythrocyte UROD activity suggests familial (type 2) PCT, but genetic testing to identify a familial UROD pathogenic variant is preferred.18 More extended genetic testing may help exclude other cutaneous porphyrias (eg, VP, hereditary coproporphyria, and congenital erythropoietic porphyria) and rare cases of dual porphyria.19 Erythrocyte porphyrins are normal or mildly elevated in PCT but markedly elevated in HEP and congenital erythropoietic porphyria.10 Skin biopsy should reveal subepidermal blisters with papillary dermal festooning and hyalinized blood vessels, often with periodic acid-Schiff positivity. These findings may differentiate other blistering skin diseases but not other blistering cutaneous porphyrias.
Iron and PCT
The association between iron and PCT is well established. Siderosis is detected in up to 90% of patients who undergo liver biopsy, and a reduction in total body iron content by therapeutic phlebotomies leads to disease remission.2,20 Pathogenic variants in the HFE gene found in hereditary hemochromatosis are more common than expected in PCT, even in the absence of marked iron overload.21 For instance, in 1 study of 108 patients with PCT, 19% were homozygous for the HFE C282Y pathogenic variant.2 Other HFE genotypes are reported to occur at higher frequencies in patients with PCT as compared with controls, including compound heterozygous C282Y/H63D.
The pathogenesis of PCT is thought to involve iron-generated free oxygen radicals that oxidize uroporphyrinogen, leading to the formation of a uroporphomethene inhibitor of UROD.10,22 The generation of uroporphomethene is dependent on the amount of iron in the liver in murine models, and in mice with heterozygous UROD knockout, iron overload is sufficient to cause a PCT phenotype.15 However, in the absence of a UROD pathogenic variant and other susceptibility factors for PCT, patients with a homozygous HFE genotype or other conditions that increase total body iron stores (eg, β-thalassemia major) do not develop PCT.23 The cytochrome P450 1A2 has been implicated in PCT pathogenesis in a murine model of PCT24; however, which CYPs might be involved in human PCT has not been conclusively established. Although ALA loading combined with iron enhanced the reduction of UROD activity in mice, factors that induce hepatic ALAS1 are seldom implicated in human PCT.15
Hepcidin, a peptide hormone produced by the liver that serves as a master regulator of iron homeostasis, is thought to be involved in PCT pathogenesis.23 Hepcidin causes degradation of the iron export transporter ferroportin in gut enterocytes and macrophages, and low levels of hepcidin result in increased iron absorption in the gut and increased iron export out of cells such as macrophages and hepatocytes. In various conditions associated with PCT, such as chronic HCV and alcohol use,25,26 hepcidin levels are low compared with controls. However, patients with end-stage kidney disease (ESKD) have high levels of hepcidin and are also at risk of developing PCT.27,28 Ajioka et al found that hepatic expression of HAMP, the gene that encodes hepcidin, is significantly reduced in patients with PCT without ESKD and that hepcidin would also be expected to be reduced.23 However, in a different study of 30 patients with PCT, serum hepcidin levels were unexpectedly higher than in patients with HCV or healthy volunteers and correlated with inflammatory markers such as ferritin and interleukin 6.29 The authors hypothesize that the regulation of hepcidin levels in PCT is complex and multifactorial, depending on factors such as baseline hepatic iron stores, inflammation, and oxidative stress.29
Clinical features and susceptibility factors
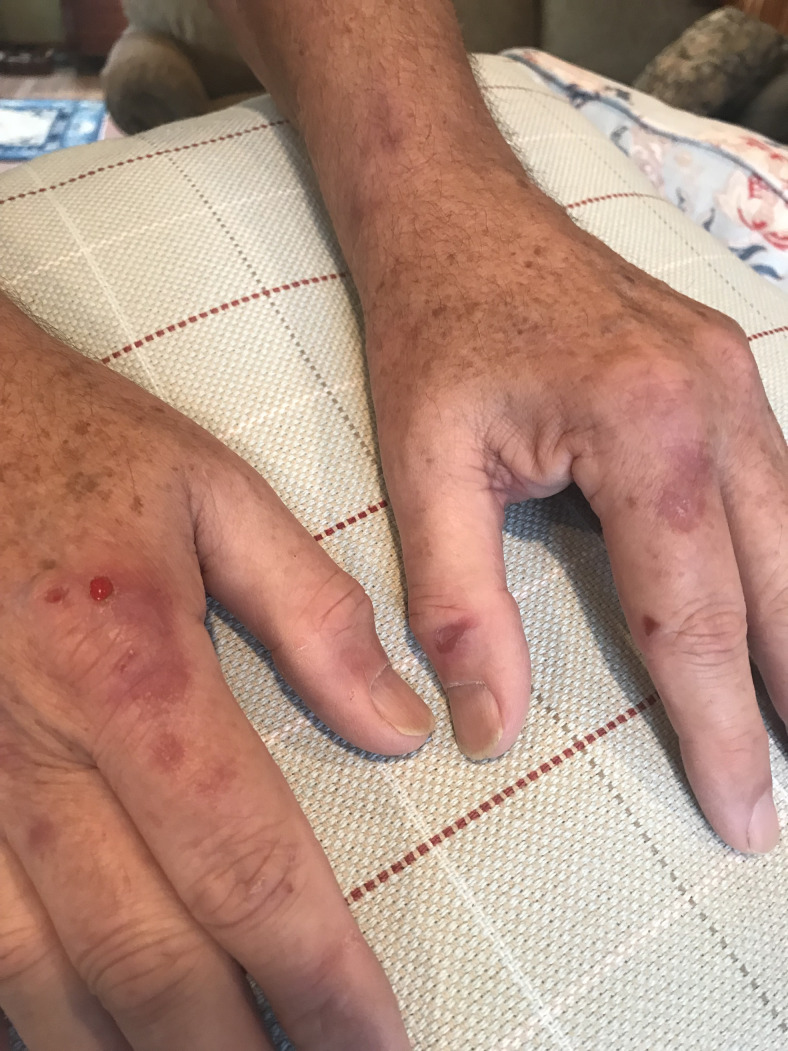
PCT is characterized by skin fragility and blistering on sun- exposed areas, most commonly the dorsal surfaces of the hands, as well as dark urine and hypertrichosis (Figure 3). Patients may report “skin tearing” with only minor trauma, hyper- and hypopigmented scars, scarring alopecia, skin crusting, and milia (raised hardened keratin-filled cysts).30 These lesions are chronic, and patients may not associate them with sun exposure.
Figure 3.
Scarring and blistering lesions on sun-exposed skin in a patient with porphyria cutanea tarda.
PCT is often the result of multiple environmental and inherited factors: UROD pathogenic variants reduce baseline levels of this enzyme, and other factors influence iron and oxidative stress in hepatocytes. The most common environmental or acquired factors are alcohol use, HCV, estrogen use, human immunodeficiency virus (HIV), and smoking. Inherited factors may include UROD and HFE pathogenic variants. In fact, at least 3 of these factors can be identified in most patients with PCT, and risk factor modification can ameliorate and prevent disease recurrence.31
Alcohol consumption decreases hepcidin expression and thereby increases iron absorption, which is mostly carried out by duodenal enterocytes.32 In hepatocytes, alcohol and iron are believed to act synergistically to generate free radicals, which contribute to the formation of the uroporphomethene that inhibits hepatic UROD, as well as cause cellular damage.3,22,33 Although up to 80% of patients with PCT report heavy alcohol consumption,31 only a small minority of patients who report excess alcohol use develop PCT. There is also a strong association between PCT and chronic HCV infection. This viral infection can lead to a reduction in hepcidin levels and generate hepatocyte oxidative damage.34 The successful antiviral treatment of HCV can lead to PCT remission,35 and the availability of effective treatments for HCV is likely to decrease the incidence of PCT in the future. Exogenous estrogen was initially identified as a risk factor for PCT in men treated for prostate cancer,36 and oral estrogen use is common in women who develop PCT. The mechanism of action of estrogens may involve redox cycling and CYPs but has not been fully elucidated.37 Smoking may act by means of CYP1A2 induction. How HIV infection may contribute to the development of PCT is unknown.38,39
PCT is a known complication of ESKD,40 possibly due to iron overload resulting from a number of factors such as erythropoietin deficiency and the administration of exogenous iron. Plasma levels of porphyrins may be higher than in PCT patients with normal kidney function, and porphyrins are difficult to remove via dialysis.41,42 As described below, PCT can be challenging to treat in patients with ESKD.
Treatment
Patients with PCT should be counseled about the modification of risk factors such as alcohol use and smoking, and alternatives to oral estrogens should be considered. It is now established that patients with chronic HCV should receive an effective combination of antiviral agents without additional treatment for PCT unless HCV eradication is not achieved, or the patient has marked iron overload.35 HIV treatment should be optimized before considering treatment of PCT. In other patients, the modification of susceptibility factors is usually inadequate, and additional treatment may be required (see below). Patients should avoid direct sunlight until porphyrin levels are lowered, and protective gloves and clothing are advised. Topical steroid creams may be useful for reducing the pain and inflammation of bullous lesions but may predispose the skin to infection and cause skin thinning. We ensure that all patients with PCT are fully assessed for evidence of chronic liver disease and are vaccinated against hepatitis A and B. Patients with PCT are at increased risk for liver cancer and may benefit from surveillance, especially if they have advanced fibrosis or cirrhosis.
Therapeutic phlebotomy is a highly effective first-line treatment for PCT and is believed to interrupt the iron-dependent generation of the uroporphomethene inhibitor in hepatocytes.43 Patients typically undergo venesection to remove approximately 450 mL of blood every 2 weeks, and this interval is generally adequate to generate replacement erythrocytes and avoid anemia. The treatment target is a ferritin level of approximately 20 ng/mL, but if patients become symptomatic from iron deficiency, a treatment goal closer to about 50 ng/mL may be acceptable. Phlebotomies are stopped when the target ferritin level is achieved (typically after 6-8 sessions), even if skin lesions are still present and porphyrin levels have not entirely normalized, as further iron depletion is not beneficial and causes anemia. Porphyrin levels will continue to decrease and skin fragility and blisters will continue to improve over several months. The recurrence rate after treatment for PCT is not known, but most patients do not relapse and do not require continued treatment, possibly related to the removal of modifiable susceptibility factors. Many more phlebotomies are required in the minority of patients with marked iron overload from hereditary hemochromatosis, and these patients need long-term follow-up. Therapeutic phlebotomy is generally well tolerated but may cause transient lightheadedness and low blood pressure, and it is costly, time-intensive, and may not be accessible for all patients.44 The diagnosis of PCT must be definitively established before treatment, as repeated phlebotomy is not effective for other types of blistering porphyrias or dermatoses.
Low-dose hydroxychloroquine (100 mg orally twice weekly) is an alternative option for patients with PCT who do not have access to or cannot tolerate therapeutic phlebotomy, and who do not have marked iron overload.45 Chloroquine can be used as well, but hydroxychloroquine is preferred because of its superior safety profile. These 4-aminoquinolines are taken up in hepatocyte lysosomes, which then release large amounts of accumulated porphyrins.46 Transient hepatocellular damage ensues but is mild with low-dose hydroxychloroquine, though liver injury can be severe if standard antimalarial or antirheumatic doses are given.47 Treatment is stopped after porphyrin levels have normalized. Compliance with hydroxychloroquine is better compared to repeated phlebotomy, and remission is achieved as rapidly.45 Ophthalmologic clearance is recommended before treatment, although the risk of retinal damage is considered minimal with this low-dose regimen with a duration of less than 1 year. There is some evidence that the PCT relapse rate may be higher with this treatment than with phlebotomy, but detailed studies are lacking.
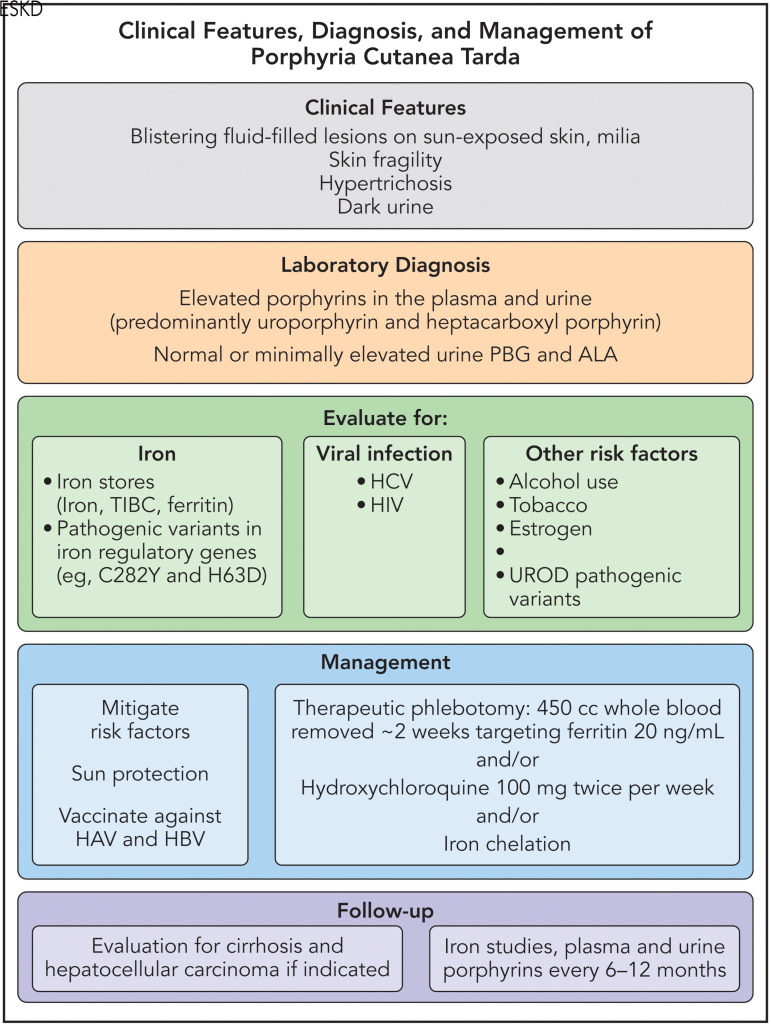
Therapeutic phlebotomy may be challenging in patients with PCT and ESKD who are anemic at baseline. Limited experience suggests that low-dose hydroxychloroquine is not effective because porphyrins that are mobilized from the liver are poorly dialyzed and may be insufficiently eliminated in patients who do not make urine. However, judicious therapeutic phlebotomy can be supported by the administration of recombinant human erythropoietin, and full remission of PCT can be achieved in these patients if the serum ferritin level is sufficiently lowered.48 Another treatment option includes iron chelation, although chelators can be costly and difficult to tolerate.49 Our general approach to the diagnosis and management of PCT can be found in Figure 4.
Figure 4.
Our approach to the diagnosis and management of PCT. HAV, hepatitis A virus; HBV, hepatitis B virus; PBG, porphobilinogen deaminase; TIBC, total iron-binding capacity. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conclusion
PCT is a hepatic porphyria that arises due to the acquired inhibition of hepatic UROD. Susceptibility to developing the disease is increased by a combination of environmental and genetic risk factors that differ among patients. Iron is central to the pathophysiology of PCT, leading to the formation of a UROD inhibitor, present in the liver, that prevents the decarboxylation of uroporphyrinogen to coproporphyrinogen during heme biosynthesis. A reduction in hepatic iron stores via therapeutic phlebotomy is an effective and well-tolerated therapy in the majority of patients.
CLINICAL CASE (continued)
In the above case vignette, the patient stopped her combined oral contraceptive pill and opted for a copper intrauterine device, which allowed for regular menstrual periods. She began phlebotomy with 450 mL of whole blood removed every 2 weeks, and her ferritin level dropped to 50 µg/dL within 8 sessions. Her skin symptoms resolved after 3 months, and her porphyrin levels normalized within 8 months.
Acknowledgments
The authors would like to thank Dr. John Phillips, Dr. Jonathan Carlson, and Dr. Karl Anderson for their critical review of the manuscript and assistance with the figures. Amy K. Dickey and Rebecca K. Leaf are members of the Porphyrias Consortium (PC). The PC is part of the Rare Diseases Clinical Research Network, which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation. The PC is funded under grant number U54DK083909 as a collaboration between NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Dickey also receives funding from an NIH NIAMS K23 grant 1K23AR079586.
Conflict-of-interest disclosure
Rebecca K. Leaf: consultancy: Alnylam Pharma, Recordati Pharma; research funding: Disc Medicine.
Amy K. Dickey: consultancy: Alnylam Pharma, Recordati Pharma; research funding: Disc Medicine.
Off-label drug use
Rebecca K. Leaf: Hydroxychloroquine for treatment of porphyria cutanea tarda.
Amy K. Dickey: Hydroxychloroquine for treatment of porphyria cutanea tarda.
References
- 1.Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med. 2017; 377(9):862-872. [DOI] [PubMed] [Google Scholar]
- 2.Bulaj ZJ, Phillips JD, Ajioka RS, et al.. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria cutanea tarda. Blood. 2000;95(5):1565-1571. [PubMed] [Google Scholar]
- 3.Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128(3):271-281. [DOI] [PubMed] [Google Scholar]
- 4.Felsher BF, Carpio NM, Engleking DW, Nunn AT. Decreased hepatic uroporphyrinogen decarboxylase activity in porphyria cutanea tarda. N Engl J Med. 1982;306(13):766-769. [DOI] [PubMed] [Google Scholar]
- 5.Elder GH, Lee GB, Tovey JA. Decreased activity of hepatic uroporphyrinogen decarboxylase in sporadic porphyria cutanea tarda. N Engl J Med. 1978;299(6):274-278. [DOI] [PubMed] [Google Scholar]
- 6.Elder GH. Porphyria cutanea tarda. Semin Liver Dis. 1998;18(1):67-75. [DOI] [PubMed] [Google Scholar]
- 7.Dubart A, Mattei MG, Raich N, et al.. Assignment of human uroporphyrinogen decarboxylase (URO-D) to the p34 band of chromosome 1. Hum Genet. 1986;73(3):277-279. [DOI] [PubMed] [Google Scholar]
- 8.Romana M, Dubart A, Beaupain D, Chabret C, Goossens M, Romeo PH. Structure of the gene for human uroporphyrinogen decarboxylase. Nucleic Acids Res. 1987;15(18):7343-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips JD, Whitby FG, Kushner JP, Hill CP. Structural basis for tetrapyrrole coordination by uroporphyrinogen decarboxylase. EMBO J. 2003; 22(23):6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips JD. Heme biosynthesis and the porphyrias. Mol Genet Metab. 2019;128(3):164-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP. The uroporphomethene inhibitor causitive for porphyria cutanea tarda (PCT) is generated by oxidation of hydroxymethylbilane (HMB). Blood. 2008; 112(11):3454. [Google Scholar]
- 12.Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2007;104(12):5079-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danton M, Lim CK. Porphomethene inhibitor of uroporphyrinogen decarboxylase: analysis by high-performance liquid chromatography/ electrospray ionization tandem mass spectrometry. Biomed Chromatogr. 2007;21(7):661-663. [DOI] [PubMed] [Google Scholar]
- 14.Poh-Fitzpatrick MB. Pathogenesis and treatment of photocutaneous manifestations of the porphyrias. Semin Liver Dis. 1982;2(2):164-176. [DOI] [PubMed] [Google Scholar]
- 15.Phillips JD. A mouse model of familial porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2001;98(1):259-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Pierro E, De Canio M, Mercadante R, et al.. Laboratory diagnosis of porphyria. Diagnostics (Basel). 2021;11(8):1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder GH. Differentiation of porphyria cutanea tarda symptomatica from other types of porphyria by measurement of isocoproporphyrin in faeces. J Clin Pathol. 1975;28(8):601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Gatti P, Sadílek M, Scott CR, Tureček F, Gelb MH. Direct assay of enzymes in heme biosynthesis for the detection of porphyrias by tandem mass spectrometry. Uroporphyrinogen decarboxylase and coproporphyrinogen III oxidase. Anal Chem. 2008;80(7):2599-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda M, Chen B, Desnick RJ. Recent advances on porphyria genetics: inheritance, penetrance and molecular heterogeneity, including new modifying/causative genes. Mol Genet Metab. 2019;128(3):320-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundvall O. The effect of phlebotomy therapy in porphyria cutanea tarda: its relation to the phlebotomy-induced reduction of iron stores. Acta Med Scand. 1971;189(1-2):33-49. [DOI] [PubMed] [Google Scholar]
- 21.Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139(2):393-408, 408.e1-2408, 408e1. [DOI] [PubMed] [Google Scholar]
- 22.Alla V, Bonkovsky HL. Iron in nonhemochromatotic liver disorders. Semin Liver Dis. 2005;25(4):461-472. [DOI] [PubMed] [Google Scholar]
- 23.Ajioka RS, Phillips JD, Weiss RB, et al.. Down-regulation of hepcidin in porphyria cutanea tarda. Blood. 2008;112(12):4723-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips JD, Kushner JP, Bergonia HA, Franklin MR. Uroporphyria in the Cyp1a2-/- mouse. Blood Cells Mol Dis. 2011;47(4):249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girelli D, Pasino M, Goodnough JB, et al.. Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol. 2009;51(5):845852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dostalikova-Cimburova M, Balusikova K, Kratka K, et al.. Role of duodenal iron transporters and hepcidin in patients with alcoholic liver disease. J Cell Mol Med. 2014;18(9):1840.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz T, Nemeth E.. Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol. 2016;36(2):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallet N, Karras A, Thervet E, Gouya L, Karim Z, Puy H.. Porphyria and kidney diseases. Clin Kidney J. 2018;11(2):191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darwich E, To-Figueras J, Molina-Lõpez RA, et al.. Increased serum hepcidin levels in patients with porphyria cutanea tarda. J Eur Acad Dermatol Venereol. 2013;27(1):e68-e74. [DOI] [PubMed] [Google Scholar]
- 30.Frank J, Poblete-Gutiérrez P.. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24(5):735-745. [DOI] [PubMed] [Google Scholar]
- 31.Egger NG, Goeger DE, Payne DA, Miskovsky EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Dig Dis Sci. 2002;47(2):419-426. [DOI] [PubMed] [Google Scholar]
- 32.Ferrao K, Ali N, Mehta KJ. Iron and iron-related proteins in alcohol consumers: cellular and clinical aspects. J Mol Med (Berl). 2022;100(12): 1673-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27(4):277-284. [PMC free article] [PubMed] [Google Scholar]
- 34.To-Figueras J. Association between hepatitis C virus and porphyria cutanea tarda. Mol Genet Metab. 2019;128(3):282-287. [DOI] [PubMed] [Google Scholar]
- 35.Bonkovsky HL, Rudnick SP, Ma CD, et al.. Ledipasvir/sofosbuvir is effective as sole treatment of porphyria cutanea tarda with chronic hepatitis C. Dig Dis Sci. 2023;68(6):2738-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roenigk HH, Gottlob ME. Estrogen-induced porphyria cutanea tarda: report of three cases. Arch Dermatol. 1970;102(3):260-266. [PubMed] [Google Scholar]
- 37.Wyllie S, Liehr JG. Release of iron from ferritin storage by redox cycling of stilbene and steroid estrogen metabolites: a mechanism of induction of free radical damage by estrogen. Arch Biochem Biophys. 1997;346(2): 180-186. [DOI] [PubMed] [Google Scholar]
- 38.Mansourati FF, Stone VE, Mayer KH. Porphyria cutanea tarda and HIV/AIDS: a review of pathogenesis, clinical manifestations and management. Int J STD AIDS. 1999;10(1):51-56. [DOI] [PubMed] [Google Scholar]
- 39.McAlister F, McClean K, Hamilton PG, Houston S.. Human immunodeficiency virus infection and porphyria cutanea tarda: coexistence of risk factors or causative association? Clin Infect Dis. 1995;20(2):348-351. [DOI] [PubMed] [Google Scholar]
- 40.Poh Fitzpatrick MB, Masullo AS, Grossman ME. Porphyria cutanea tarda associated with chronic renal disease and hemodialysis. Arch Dermatol. 1980;116(2):191-195. [PubMed] [Google Scholar]
- 41.Rodrigues N, Caeiro F, Santana A, Mendes T, Lopes L.. Porphyria cutanea tarda in a patient with end-stage renal disease: a case of successful treatment with deferoxamine and ferric carboxymaltose. Case Rep Nephrol. 22 January 2017;2017:4591871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebril M, Weinkove C, Ead R, McDonald K, Morton R.. Plasma porphyrins in chronic renal failure. Nephron. 1990;55(2):159-163. [DOI] [PubMed] [Google Scholar]
- 43.Walsh JR, Lobitz WC, Mahler DJ, Kingery FAJ. Phlebotomy therapy in cutaneous porphyria: effect on iron and trace metals. Arch Dermatol. 1970; 101(2):167-172. [PubMed] [Google Scholar]
- 44.Elsaid MI, John T, Li Y, et al.. Health care utilization and economic burdens of hemochromatosis in the United States: a population-based claims study. J Manag Care Spec Pharm. 2019;25(12):1377-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singal AK, Kormos-Hallberg C, Lee C, et al.. Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2012;10(12):1402-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kordac V, Jirsa M, Kalab M.. Chloroquine in the treatment of porphyria cutanea tarda. In: Doss M, ed. Diagnosis and Therapy of Porphyrias and Lead Intoxication. Springer; 1978. [Google Scholar]
- 47.Sunkara B, Roofeh D, Silver S, Pearson TL, Ettel M, McCune WJ. The devil's in the dosing: severe drug-induced liver injury in a hydroxychloroquine-naive patient with subacute cutaneous lupus erythematosus and porphyria cutanea tarda. Lupus. 2018;27(8):1383-1386. [DOI] [PubMed] [Google Scholar]
- 48.Anderson KE, Goeger DE, Carson RW, Lee SMK, Stead RB. Erythropoietin for the treatment of porphyria cutanea tarda in a patient on long-term hemodialysis. N Engl J Med. 1990;322(5):315-337. [DOI] [PubMed] [Google Scholar]
- 49.Rocchi E, Cassanelli M, Borghi A, Paolillo F, Pradelli M, Pellizzardi S, Vezzosi A, Gallo E, Baccarani Contri M, Ventura E.. Liver iron overload and desferrioxamine treatment of porphyria cutanea tarda. Dermatologica. 1991;182(1):27-31. [DOI] [PubMed] [Google Scholar]