Abstract
Oxidation is a fundamental transformation in synthesis. Developing facile and effective aerobic oxidation processes under ambient conditions is always in high demand. Benefiting from its high energy and good penetrability, ionizing radiation can readily produce various reactive species to trigger chemical reactions, offering another option for synthesis. Here, we report an ionizing radiation-induced aerobic oxidation strategy to synthesize oxygen-containing compounds. We discovered that molecular oxygen (O2) could be activated by reactive particles generated from solvent radiolysis to produce solvent-derived peroxyl radicals (RsolOO·), which facilitated the selective oxidation of sulfides and phosphorus(iii) compounds at room temperature without catalysts. Density functional theory (DFT) calculations further revealed that multiple RsolOO· enable the oxidation reaction through an oxygen atom transfer process. This aerobic oxidation strategy broadens the research scope of radiation-induced chemical transformations while offering an opportunity to convert nuclear energy into chemical energy.
Solvent-derived peroxyl radicals (RsolOO·), a new group of reactive oxygen species (ROS), enable the radiation-induced aerobic oxidation strategy.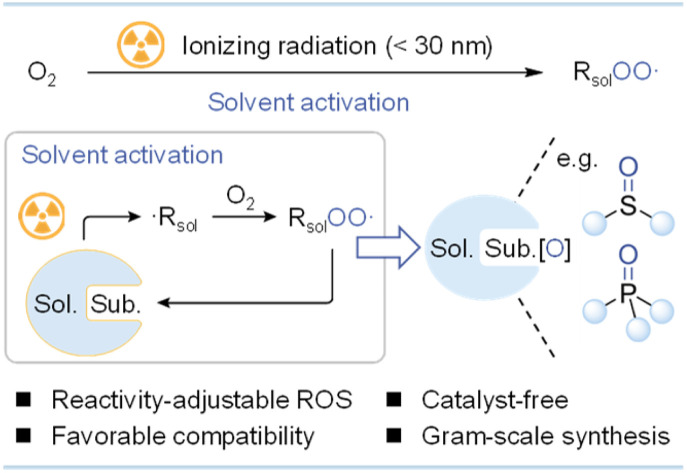
Introduction
Oxidation reactions are among the most widespread transformations in synthesis.1 With the growing interest in green synthesis and sustainable chemistry, molecular oxygen (O2) is regarded as an ideal oxidant due to its good natural abundance and high cost-effectiveness, making it a superior choice for synthesizing oxygen-containing compounds. Utilizing oxygen instead of classical oxidants not only eliminates the need for stoichiometric reagents, but also avoids harsh reaction conditions and the production of potentially toxic waste.2 Emerging as an environmentally friendly approach, the photochemical aerobic oxidation strategy under ambient conditions has garnered extensive attention in recent years and continues to evolve.3 In this strategy, triplet oxygen with relatively low reactivity is usually activated by photosensitizers (PSs, e.g., transition metal complexes and π-conjugated aromatic dyes) or electron donor–acceptor (EDA) complexes to produce singlet oxygen (1O2) or superoxide anion radicals (O2˙−) for subsequent reactions (Scheme 1a).4 Nonetheless, the diversity of reactive oxygen species (ROS) remains limited, and the incorporation of catalysts could compromise the cost-effectiveness and functional group compatibility.5 Therefore, developing facile and effective methods that enable aerobic oxidation is always in high demand.
Scheme 1. Solvent-derived peroxyl radicals enable radiation-induced aerobic oxidation. UV = ultraviolet, Sol. = solvent, and Sub. = substrate.
Ionizing radiation (e.g., γ-ray photons), the main form of nuclear energy utilization,6 has been widely applied in wastewater treatment,7 material fabrication,8 and radiotherapy.9 These high-energy particles can uniformly and effectively produce highly reactive species, which can overcome activation barriers for subsequent reactions under ambient conditions. Furthermore, ionizing radiation possesses good penetrability and industrial accessibility, holding promise for large-scale production.10 Recently, ionizing radiation has been harnessed to produce chemical feedstocks such as hydrogen,11 methanol,12 acetic acid,13 and ammonia,14 demonstrating the feasibility of nuclear energy-induced chemical transformations. To the best of our knowledge, radiation-induced aerobic oxidation has not yet been developed. Diverging from photochemical aerobic oxidation processes, in which photoresponsive groups harness ultraviolet-visible light energy to activate O2, solvent molecules with the largest proportion predominantly absorb energy and trigger O2 activation in radiation-induced aerobic oxidation reactions.15 Upon irradiation, stable solvent molecules can be converted into solvated electrons (esol−), hydrogen radicals (H·), and solvent-derived radicals (·Rsol) with a homogeneous distribution within tens of nanoseconds.16 In the presence of air, ·Rsol with a high radiolytic yield can rapidly activate O2 (k > 109 L mol−1 s−1) to generate solvent-derived peroxyl radicals (RsolOO·).17 As a group of ROS that have received little attention yet commonly exist in the radiolysis of various solvents, reactivity-adjustable RsolOO· are anticipated to enable a broad array of aerobic oxidation reactions (Scheme 1b).
Herein, we used γ-ray radiation from a 60Co source to investigate whether ionizing radiation could induce aerobic oxidation processes. The results revealed that multiple RsolOO· could successfully promote the oxidation of sulfides and phosphorus(iii) compounds at ambient temperature and pressure without any catalysts. This aerobic oxidation strategy exhibits favorable functional group compatibility and is suitable for gram-scale synthesis, demonstrating that ionizing radiation can enable selective oxidation processes for synthesizing chemical feedstocks rather than unselectively degrading organics to carbon dioxide and water as in wastewater treatment. It provides an effective approach for organic synthesis and opens broad prospects for the valuable utilization of nuclear energy.
Results and discussion
Optimization of the reaction conditions
Sulfoxide is a ubiquitous structural building block widely applied in natural products, pharmaceuticals, agrochemicals, food additives, and other chemical feedstocks.18 Normally, sulfoxides are produced through the oxidation of the corresponding sulfides with the help of various oxidants, such as m-chloroperbenzoic acid, oxone, hydrogen peroxide, etc.19 To validate our hypothesis, we first selected methylphenyl sulfide (1a) as the model compound to investigate radiation-induced aerobic oxidation.
The initial reaction was carried out in different solvents under an air atmosphere and irradiated with γ-rays at room temperature with a dose rate of 110 Gy min−1 (1 Gy = 1 J kg−1) (Tables 1 and S1, ESI†). To our delight, acetonitrile (CH3CN) as the solvent gave the highest yield of (methylsulfinyl)benzene (2a), reaching 95% after 15 h of irradiation (Table 1, entry 1). Methanol (CH3OH), acetone, and ethyl acetate (EtOAc) could also facilitate the oxidation of 1a in moderate yields (Table 1, entries 2–4), while tetrahydrofuran (THF), cyclohexane, dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), toluene, and dichloromethane (CH2Cl2) were not conducive to the reaction (Table 1, entries 5–10). Considering that the addition of water can inhibit the conversion of sulfoxides to sulfones through hydrogen bond interactions, we then evaluated a mixed solvent of CH3CN and water (Table 1, entries 11 and 12, and Table S2, ESI†).20 However, with the increase in the proportion of water, the yield correspondingly decreased, which could be attributed to the decreased solubility of the substrate and the reduced concentration of the primary oxidizing species. In addition, hydroxyl radicals which are potent oxidants produced from water radiolysis tended to cause overoxidation of organics, thus reducing the yield. The dose rate had almost no effect on the reaction performance when the total absorbed dose was fixed (Table S3, ESI†). Of note, the reaction time played a vital role in determining the product distribution (Table S4, ESI†). We found that increasing the reaction time from 2 h to 15 h would raise the yield of 2a from 27% to 95%. However, when the reaction time exceeded 15 h, 2a started to convert to methylphenyl sulfone (3a), indicating that it is feasible to selectively obtain sulfoxides or sulfones by modulating the reaction time. When 2a was used as the substrate, 3a could also be obtained with the other reaction conditions unchanged, further demonstrating that sulfoxides served as necessary intermediates in the oxidation of sulfides to sulfones (Table S5, ESI†).
Table 1. Optimization of the reaction conditionsa.

| ||
|---|---|---|
| Entry | Solvent | Yieldb (%) |
| 1 c | CH 3 CN | 95 |
| 2 | CH3OH | 87 |
| 3 | EtOAc | 62 |
| 4 | Acetone | 75 |
| 5 | THF | 18 |
| 6 | Cyclohexane | 22 |
| 7 | DMSO | Trace |
| 8 | DMF | 21 |
| 9 | Toluene | 24 |
| 10 | CH2Cl2 | n.d. |
| 11 | CH3CN/H2O (4/1) | 72 |
| 12 | CH3CN/H2O (1/4) | 16 |
Reaction conditions: 1a (0.3 mmol) in solvent (15 mL) with γ-ray radiation (dose rate: 110 Gy min−1, measured using a Fricke dosimeter) at room temperature and under an air atmosphere for 15 h.
Determined by gas chromatography-mass spectrometry (GC-MS) using 1,3,5-trimethoxybenzene as the internal standard.
The optimized reaction condition. n.d. = no detected.
Substrate scope
Based on the optimized reaction conditions, we further evaluated the substrate scope of radiation-induced aerobic oxidation using various sulfides. Aryl methyl sulfide derivatives with different substituents were examined first. As shown in Fig. 1, both electron-donating groups and electron-withdrawing groups were tolerated. Aryl methyl sulfides with electron-donating groups (e.g., alkyl, alkoxy, and trifluoromethoxy) (1b–1d) were converted to the corresponding sulfoxides (2b–2d) in moderate yields. Similarly, aryl methyl sulfides with electron-withdrawing groups (e.g., halogen, nitro, cyano, acetyl, and trifluoromethyl) (1e–1s) could also be converted to sulfoxides (2e–2s) in satisfactory yields. High functional group compatibility was shown by the successful preservation of various groups in products, including trifluoromethoxy (2d), nitro (2n), cyano (2o), boronic pinacol ester (Bpin, 2p), and trifluoromethyl groups (2s). Additionally, the number and positional properties of the functional groups have a negligible effect on the reactivity (2i–2m). Next, we investigated the oxidation efficiency of naphthyl or heteroaryl methyl sulfides. To our satisfaction, sulfides bearing naphthalene (2t), thiophene (2u and 2v), pyridine (2w), pyrimidine (2x), thiazole (2y), or benzothiazole (2z) were also accommodated by the radiation-induced aerobic oxidation system. It is worth mentioning that the sulfur-containing heteroaromatic rings remained unoxidized, showing that this oxidation strategy is chemoselective.
Fig. 1. The substrate scope of radiation-induced aerobic oxidation of sulfides. Reaction conditions: 1 (0.3 mmol) in CH3CN (15 mL) with γ-ray radiation (dose rate: 110 Gy min−1, measured using a Fricke dosimeter) at room temperature and under an air atmosphere for 15 h. All yields are isolated. aReaction time: 30 h. bReaction time: 7 h.
Subsequently, we expanded the substrate scope to other aryl alkyl sulfides with different chain lengths, isomeric structures, and functional groups. They could all be oxidized to sulfoxides with alkyl motifs retained in moderate yields (2aa–2af). Apart from aryl alkyl sulfides, a series of diaryl sulfides and dialkyl sulfides were evaluated. The relatively low reactivity of diaryl sulfides is attributed to the conjugation effect of benzene rings (2ag–2ak). Considering that thianthrene contains two sulfur atoms, we found that only mono-oxygenation sulfoxide (2aj) could be obtained. In contrast to diaryl sulfides, dialkyl sulfides could be transformed into sulfoxides (2al and 2am) in excellent yields in a shorter reaction time. The above results indicate that electronic effects of the substituents linked to the sulfur atom play an important role in the conversion efficiency. The conversion of sulfides containing electron-donating groups was easier than those bearing electron-withdrawing groups. In addition, diaryl sulfides required a longer reaction time than aryl alkyl sulfides and dialkyl sulfides due to the decreased electron density on the sulfur atom.
Encouraged by the satisfactory results above, we further verified the applicability of this oxidation strategy for synthesizing bioactive molecules and pharmaceuticals. A potential anticancer drug sulforaphane (2an) was obtained in 71% yield with its sensitive isothiocyanate group preserved.21 Moreover, the corresponding sulfoxide of etoricoxib (2ao), a cyclo-oxygenase (COX)-2 inhibitor, could also be prepared in 52% yield.22
Similar to the sulfur atoms in sulfides, the phosphorus atoms in phosphorus(iii) compounds also tend to accept oxygen atoms and be oxidized to form the corresponding phosphorus(v) compounds, which are crucial for the synthesis of organophosphorus compounds.23 As shown in Fig. 2, aryl alkyl phosphine oxides with different alkyl motifs (5a–5f), triaryl phosphine oxides with different substituents (5g–5p), and tricyclohexylphosphine oxide (5q) could all be readily obtained with excellent yields in a much shorter reaction time. Alkene (5e), furan ring (5o), and pyridine ring (5p) were well preserved after the reaction. Of note, 5g was successfully obtained at a gram-scale level (91%, 1.26 g), suggesting that this protocol is promising for scale-up synthesis. Moreover, this radiation-induced aerobic oxidation strategy could also be applied to the synthesis of phosphinates, phosphonates, and phosphates (5r–5u).
Fig. 2. The substrate scope of radiation-induced aerobic oxidation of phosphorus(iii) compounds. Reaction conditions: 4 (0.3 mmol) in CH3CN (15 mL) with γ-ray radiation (dose rate: 110 Gy min−1, measured using a Fricke dosimeter) at room temperature and under an air atmosphere for 3 h. All yields are isolated. aReaction time: 100 min. bReaction time: 220 min. cGram-scale synthesis.
Mechanistic investigation
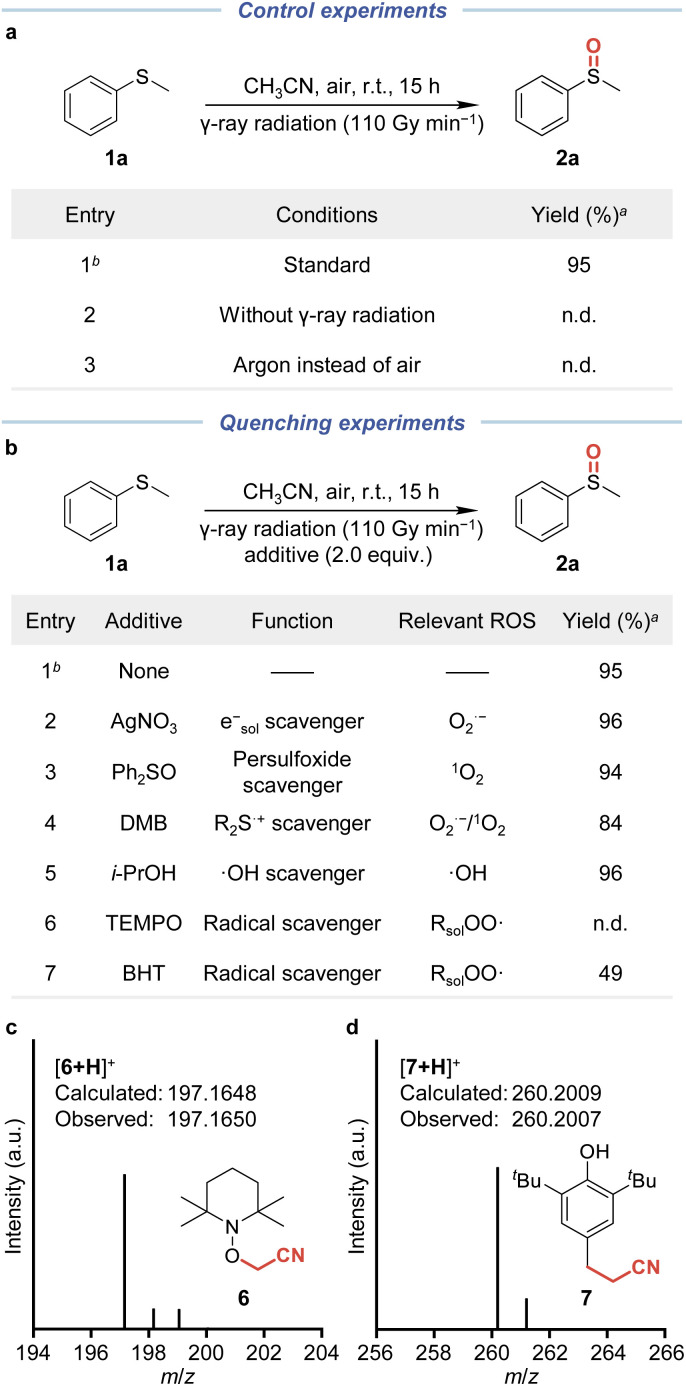
We next turned our attention to the mechanism of radiation-induced aerobic oxidation of sulfides and phosphorus(iii) compounds. All mechanistic studies were conducted with 1a as the model substrate and CH3CN as the solvent. According to the results of control experiments, both γ-ray radiation and O2 were essential for the success of this oxidation strategy (Fig. 3a). Then, we introduced the ROS fluorescent probe 2′,7′-dichlorodihydrofluorescein (DCFH) to the system and observed an increased fluorescence signal with prolonged irradiation time, supporting the generation of ROS in this oxidation process (Fig. S2, ESI†).
Fig. 3. Experiments for mechanistic investigation. (a) Control experiments for mechanistic studies. (b) Quenching experiments for investigating the key ROS. (c) High-resolution mass spectrometry of the adduct between TEMPO and ·CH2CN. (d) High-resolution mass spectrometry of the adduct between BHT and ·CH2CN. aDetermined by GC-MS using 1,3,5-trimethoxybenzene as the internal standard. bStandard conditions: 1a (0.3 mmol) in CH3CN (15 mL) with γ-ray radiation (dose rate: 110 Gy min−1, measured using a Fricke dosimeter) at room temperature and under an air atmosphere for 15 h. n.d. = no detected.
To further investigate the key ROS, a series of quenching experiments were performed by introducing various scavengers (Fig. 3b). When an electron quencher (e.g., AgNO3) was added, there was no significant difference in the reaction performance, demonstrating that esol− and O2˙− derived from esol− were not the primary reactive species. Similarly, we utilized the O2˙−-specific probe nitrotetrazolium blue chloride (NBT) to monitor the superoxide species, and the unabated absorbance at 260 nm revealed the absence of O2˙− (Fig. S3, ESI†). By introducing diphenyl sulfoxide (Ph2SO), we could rule out the formation of a persulfoxide intermediate between sulfide and 1O2 in that the reaction performance remained essentially unchanged and only a trace amount of diphenyl sulfone (Ph2SO2) formed between persulfoxide and Ph2SO (Fig. S4, ESI†).24 Meanwhile, the reaction performance was hardly affected when deuterated acetonitrile (CD3CN) was used as the solvent, where the lifetime of 1O2 is longer [τΔ(CD3CN/CH3CN) = 1625/82 μs] (Fig. S7, ESI†).25 Therefore, we speculated that 1O2 also played a minor role in this oxidation process. The addition of 1,4-dimethoxybenzene (DMB) as a sulfide radical cation (R2S˙+) scavenger also did not significantly suppress the reaction, suggesting that R2S˙+ might not be as important as they are in photocatalytic aerobic oxidation driven by O2˙− or 1O2.26 Hydroxyl radicals (·OH) were excluded by introducing 2-propanol (i-PrOH) as the scavenger. Furthermore, when 2,2,6,6-tetramethyl-1-piperinedinyloxy (TEMPO) or butylated hydroxytoluene (BHT) was added as the radical trapping reagent, the oxidation of 1a was severely suppressed, and the adduct with the cyanomethyl radical (·CH2CN) 6 or 7 could be observed by high-resolution mass spectrometry (HRMS) (Fig. 3c, d, and S5, ESI†). These results indicated that a ·CH2CN-mediated radical process may be involved.
Based on the above results, we propose that the radiation-induced aerobic oxidation process differs from most photocatalytic processes mediated by O2˙− or 1O2. Solvent-derived peroxyl radicals (e.g., cyanomethylperoxyl radicals, ·OOCH2CN) generated from the rapid combination of O2 and ·Rsol (e.g., ·CH2CN) may enable this oxidation process.
To further unravel the mechanism, density functional theory (DFT) calculations were performed on the model substrate 1a with CH3CN as the solvent. The proposed mechanism and calculated results are shown in Fig. 4. Upon irradiation with 60Co γ-rays, CH3CN is initially ionized to generate e− and radical cations (CH3CN˙+) along the tracks. After the subsequent rapid processes such as spur expansion, geminate recombination, and intra-track reaction, ·CH2CN becomes the predominant reactive species in the solution within tens of nanoseconds.27 Therefore, the cleavage of the C–H bond is not the rate-determining step, which is consistent with the findings of kinetic studies (Fig. S7, ESI†). According to previous studies and the calculated results, once ·CH2CN is formed, it reacts with O2 to produce ·OOCH2CN smoothly (k = 1.3 × 109 L mol−1 s−1), which is spontaneous (Fig. 4a and S8, ESI†).28 The ·OOCH2CN then undergoes the oxygen atom transfer (OAT) process with 1a to yield 2aviaTS1a/2a with the Gibbs activation energy (ΔG≠) and the Gibbs reaction energy (ΔG) being 23.3 kcal mol−1 and −15.5 kcal mol−1, respectively. By contrast, it is more difficult for ·OOCH2CN to undergo the same process with 2a to produce 3aviaTS2a/3a, where ΔG≠ and ΔG are 26.6 kcal mol−1 and −33.5 kcal mol−1. These results reveal that sulfoxides are kinetic products and sulfones are thermodynamic products, which is consistent with the relationship between product distribution and reaction time (Table S4, ESI†). Moreover, the second oxygen atom of ·OOCH2CN is unlikely to undergo the OAT process, because the values of ΔG≠ for the reaction of cyanomethoxy radicals (·OCH2CN) with 1a and 2a are as high as 35.3 kcal mol−1 and 33.1 kcal mol−1, respectively, indicating that the ·OCH2CN pathway is unfavorable compared to the ·OOCH2CN pathway (Fig. 4b). All of the transition state geometries were determined by intrinsic reaction coordinate (IRC) analysis (Fig. S9, ESI†).
Fig. 4. Mechanism proposal and DFT calculations. (a) The radiolysis of CH3CN and the formation of ·OOCH2CN. (b) Gibbs energy profile for the radiation-induced aerobic oxidation of 1a to 2a and 3avia ·OCH2CN (left) or ·OOCH2CN (right) was calculated at the B3LYP-D3/6-311+G(d,p)/SMD(acetonitrile) level of theory. (c) Distortion–interaction analysis for transition states in the Gibbs energy profile was calculated at the B3LYP-D3/6-311+G(d,p)/SMD(acetonitrile) level of theory.
Distortion–interaction analysis was also performed on these four transition states to explain why ·OOCH2CN is more conducive to undergoing the OAT process (Fig. 4c and Table S8, ESI†).29 Compared to the other three transition states, the total distortion energy (ΔE≠dist, the energy required to distort substrate and oxidizing species into the transition state geometry) of TS1a/2a is 26.8 kcal mol−1, which is higher than that of TS2a/3a and  . However, the interaction energy (ΔE≠int, the energy released when the two fragments form a complete transition state) of TS1a/2a is down to −15.1 kcal mol−1, which compensates for the relatively higher ΔE≠dist. Therefore, the activation energy (ΔE≠, the sum of ΔE≠dist and ΔE≠int) of TS1a/2a is the lowest (11.7 kcal mol−1), suggesting that TS1a/2a is relatively stable and the transformation from 1a to 2avia ·OOCH2CN is more favorable.
. However, the interaction energy (ΔE≠int, the energy released when the two fragments form a complete transition state) of TS1a/2a is down to −15.1 kcal mol−1, which compensates for the relatively higher ΔE≠dist. Therefore, the activation energy (ΔE≠, the sum of ΔE≠dist and ΔE≠int) of TS1a/2a is the lowest (11.7 kcal mol−1), suggesting that TS1a/2a is relatively stable and the transformation from 1a to 2avia ·OOCH2CN is more favorable.
Similar to ·CH2CN, hydroxymethyl radicals (·CH2OH), cyclohexyl radicals (·C6H11), and tetrahydrofuran-2-yl radicals (·C4H7O) generated from solvent radiolysis could also react with O2 rapidly to produce the corresponding peroxyl radicals, which then promote the oxidation of 1a to 2a through the OAT process.30 The results of control experiments and radical trapping experiments showed that γ-ray radiation, O2, and ·Rsol also play important roles in radiation-induced aerobic oxidation mediated by other several solvents (Tables S6, S7 and Fig. S6, ESI†). As shown in Fig. 5a, as the peroxyl radicals change from ·OOCH2CN, hydroxymethylperoxyl radicals (·OOCH2OH), and cyclohexylperoxyl radicals (·OOCy) to tetrahydrofuran-2-peroxy radicals (·OOTHF), the value of ΔG≠ increases from 23.3 kcal mol−1 to 34.0 kcal mol−1, which indicates that the OAT process becomes difficult to proceed. This trend is also consistent with the reaction performance shown in Table 1. The yield of 2a decreases with the increase in Gibbs activation energy, indicating that the oxygen atom transfer ability of RsolOO· is a key factor affecting the reaction performance. Distortion–interaction analysis reveals that the interaction between the substrate and RsolOO· is particularly important for the stabilization of the transition state (Fig. 5b and Table S8, ESI†). When the values of ΔE≠dist are nearly identical, the value of ΔE≠int in CH3CN (−15.1 kcal mol−1) or CH3OH (−15.9 kcal mol−1) is significantly lower compared to that in cyclohexane (−8.7 kcal mol−1) or THF (−8.8 kcal mol−1), contributing to a more stable transition state and a lower ΔE≠ (11.7 kcal mol−1). The unfavorable interaction between ·OOCy or ·OOTHF and 1a may be due to the steric hindrance brought by the cyclohexyl or tetrahydrofuran-2-yl group (Fig. 5c). Therefore, the efficacy of the radiation-induced aerobic oxidation strategy heavily relies on the solvent type and the reactive species generated from solvent radiolysis.
Fig. 5. Multiple solvent-derived peroxyl radicals enable the radiation-induced aerobic oxidation strategy. (a) Gibbs energy profile for the radiation-induced aerobic oxidation of 1a to 2avia different RsolOO· was calculated at the B3LYP-D3/6-311+G(d,p)/SMD level of theory. CyH = cyclohexane. (b) Distortion–interaction analysis for transition states in the Gibbs energy profile was calculated at the B3LYP-D3/6-311+G(d,p)/SMD level of theory. (c) Optimized geometries of transition states.
Conclusions
In summary, we presented an ionizing radiation-induced aerobic oxidation strategy that enables the selective oxidation of sulfides and phosphorus(iii) compounds at room temperature without catalysts. This strategy exhibited a wide substrate scope and favorable functional group compatibility. Through mechanistic experiments and DFT calculations, we established that highly active RsolOO· produced from solvent radiolysis, rather than O2˙−, 1O2, and ·OH, serve as the primary oxidizing species and facilitate the oxidation through an OAT process. Notably, RsolOO· are commonly generated during the radiolysis of various solvents in the presence of O2, making it easy to adjust the reaction performance by choosing the appropriate solvent. Combined with the increasing accessibility and strong penetrability of ionizing radiation, our aerobic oxidation strategy is expected to have a wider range of applications in various oxidation reactions and scale-up synthesis, presenting a fresh avenue for nuclear energy utilization.
Data availability
The ESI† includes details of optimization studies, substrate scope studies, mechanistic studies, and computational studies. Characterization data, NMR spectra, and Cartesian coordinates of all optimized geometries are included as well.
Author contributions
Z. L. conceived the study. Y. X., assisted by B. M., W. L. and J. L., performed optimization studies and substrate scope studies. Y. X., assisted by B. M. and Z. T., performed mechanistic studies. Y. X., assisted by B. M. and Z. S., performed computational studies. Y. X., B. M. and Z. L. analyzed the data. Z. L. wrote the paper with inputs from all authors. All authors discussed the results and commented on the paper.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank J. Li and M. Zhai at Peking University for providing the 60Co source. We thank the facility support from the Analytical Instrumentation Center of Peking University. We thank the technical assistance from the High-Performance Computing Platform of Peking University. This study was funded by the National Natural Science Foundation of China (Grant No. 22225603 and 22306005), the Ministry of Science and Technology of the People's Republic of China (Grant No. 2021YFA1601400), the Beijing Municipal Natural Science Foundation (Grant No. Z200018) and Changping Laboratory to Z. L.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc05558f
Notes and references
- (a) Punniyamurthy T. Velusamy S. Iqbal J. Chem. Rev. 2005;105:2329–2364. doi: 10.1021/cr050523v. [DOI] [PubMed] [Google Scholar]; (b) Ma S. Liu J. Li S. Chen B. Cheng J. Kuang J. Liu Y. Wan B. Wang Y. Ye J. Yu Q. Yuan W. Yu S. Adv. Synth. Catal. 2011;353:1005–1017. doi: 10.1002/adsc.201100033. [DOI] [Google Scholar]; (c) Jiang X. Zhang J. Ma S. J. Am. Chem. Soc. 2016;138:8344–8347. doi: 10.1021/jacs.6b03948. [DOI] [PubMed] [Google Scholar]
- (a) Shi Z. Zhang C. Tang C. Jiao N. Chem. Soc. Rev. 2012;41:3381–3430. doi: 10.1039/C2CS15224J. [DOI] [PubMed] [Google Scholar]; (b) Liang Y. Wei J. Qiu X. Jiao N. Chem. Rev. 2018;118:4912–4945. doi: 10.1021/acs.chemrev.7b00193. [DOI] [PubMed] [Google Scholar]; (c) Tang C. Qiu X. Cheng Z. Jiao N. Chem. Soc. Rev. 2021;50:8067–8101. doi: 10.1039/D1CS00242B. [DOI] [PubMed] [Google Scholar]
- Zhang X. Rakesh K. P. Ravindar L. Qin H.-L. Green Chem. 2018;20:4790–4833. doi: 10.1039/C8GC02382D. [DOI] [Google Scholar]
- (a) Schultz D. M. Yoon T. P. Science. 2014;343:1239176. doi: 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wei J. Meng J. Zhang C. Liu Y. Jiao N. Nat. Commun. 2024;15:1886. doi: 10.1038/s41467-024-45866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolia E. Gkizis P. L. Nikitas N. F. Kokotos C. G. Green Chem. 2022;24:4108–4118. doi: 10.1039/D2GC00799A. [DOI] [Google Scholar]
- (a) Parsons J. Buongiorno J. Corradini M. Petti D. Science. 2019;363:105. doi: 10.1126/science.aaw5304. [DOI] [PubMed] [Google Scholar]; (b) Ramirez-Corredores M. M. Diaz L. A. Gaffney A. M. Zarzana C. A. Renew. Sustain. Energy Rev. 2021;150:111450. doi: 10.1016/j.rser.2021.111450. [DOI] [Google Scholar]
- (a) Ponomarev A. V. Ershov B. G. Environ. Sci. Technol. 2020;54:5331–5344. doi: 10.1021/acs.est.0c00545. [DOI] [PubMed] [Google Scholar]; (b) Londhe K. Lee C.-S. Zhang Y. Grdanovska S. Kroc T. Cooper C. A. Venkatesan A. K. ACS EST Eng. 2021;1:827–841. doi: 10.1021/acsestengg.0c00222. [DOI] [Google Scholar]
- (a) Zhang Z. Cui X. Yuan W. Yang Q. Liu H. Xu H. Jiang H.-L. Inorg. Chem. Front. 2018;5:29–38. doi: 10.1039/C7QI00577F. [DOI] [Google Scholar]; (b) Guo K. Baidak A. Yu Z. J. Mater. Chem. A. 2020;8:23029–23058. doi: 10.1039/D0TA06742C. [DOI] [Google Scholar]; (c) Chen J. Zhang M. Shu J. Yuan M. Yan W. Bai P. He L. Shen N. Gong S. Zhang D. Li J. Hu J. Li R. Wu G. Chai Z. Yu J. Wang S. Angew. Chem., Int. Ed. 2021;60:14858–14863. doi: 10.1002/anie.202103766. [DOI] [PubMed] [Google Scholar]; (d) Chen J. Zhang M. Zhang S. Cao K. Mao X. Zhang M. He L. Dong X. Shu J. Dong H. Zhai F. Shen R. Yuan M. Zhao X. Wu G. Chai Z. Wang S. Angew. Chem., Int. Ed. 2022;61:e202212532. doi: 10.1002/anie.202212532. [DOI] [PubMed] [Google Scholar]
- (a) Ni K. Lan G. Song Y. Hao Z. Lin W. Chem. Sci. 2020;11:7641–7653. doi: 10.1039/D0SC01949F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fu Q. Li H. Duan D. Wang C. Shen S. Ma H. Liu Z. Angew. Chem., Int. Ed. 2020;59:21546–21552. doi: 10.1002/anie.202005612. [DOI] [PubMed] [Google Scholar]; (c) Ding Z. Guo Z. Zheng Y. Wang Z. Fu Q. Liu Z. J. Am. Chem. Soc. 2022;144:9458–9464. doi: 10.1021/jacs.2c02521. [DOI] [PubMed] [Google Scholar]; (d) Guo Z. Hong H. Zheng Y. Wang Z. Ding Z. Fu Q. Liu Z. Angew. Chem., Int. Ed. 2022;61:e202205014. doi: 10.1002/anie.202205014. [DOI] [PubMed] [Google Scholar]; (e) Duan D. Han Y. Tu Z. Guo H. Zhang Z. Shi Y. Li J. Sun Q. Chen J. Li Z. Liu T. Cui D. Liu Z. CCS Chem. 2023;5:2589–2602. doi: 10.31635/ccschem.023.202202488. [DOI] [Google Scholar]; (f) Fu Q. Gu Z. Shen S. Bai Y. Wang X. Xu M. Sun P. Chen J. Li D. Liu Z. Nat. Chem. 2024;16:1348–1356. doi: 10.1038/s41557-024-01501-4. [DOI] [PubMed] [Google Scholar]; (g) Luo T. Jiang X. Fan Y. Yuan E. Li J. Tillman L. Lin W. Natl. Sci. Rev. 2024;11:nwae167. doi: 10.1093/nsr/nwae167. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Fu Q. Zhang S. Shen S. Gu Z. Chen J. Song D. Sun P. Wang C. Guo Z. Xiao Y. Gao Y. Q. Guo Z. Liu Z. Nat. Biomed. Eng. 2024;8:1425–1435. doi: 10.1038/s41551-024-01239-x. [DOI] [PubMed] [Google Scholar]
- (a) Zhang M. Chen J. Zhang S. Zhou X. He L. Sheridan M. V. Yuan M. Zhang M. Chen L. Dai X. Ma F. Wang J. Hu J. Wu G. Kong X. Zhou R. Albrecht-Schmitt T. E. Chai Z. Wang S. J. Am. Chem. Soc. 2020;142:9169–9174. doi: 10.1021/jacs.0c03941. [DOI] [PubMed] [Google Scholar]; (b) Chen J. Zhang M. Shu J. Liu S. Dong X. Li C. He L. Yuan M. Wu Y. Xu J. Zhang D. Ma F. Wu G. Chai Z. Wang S. J. Am. Chem. Soc. 2023;145:23651–23658. doi: 10.1021/jacs.3c07778. [DOI] [PubMed] [Google Scholar]
- Hu C. Cheng L. Zhou L. Jiang Z. Gan P. Cao S. Li Q. Chen C. Wang Y. Mostafavi M. Wang S. Ma J. J. Am. Chem. Soc. 2023;145:5578–5588. doi: 10.1021/jacs.3c00547. [DOI] [PubMed] [Google Scholar]
- Hu C. Jiang Z. Wu Q. Cao S. Li Q. Chen C. Yuan L. Wang Y. Yang W. Yang J. Peng J. Shi W. Zhai M. Mostafavi M. Ma J. Nat. Commun. 2023;14:4767. doi: 10.1038/s41467-023-40418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Fang F. Sun X. Liu Y. Huang W. J. Am. Chem. Soc. 2024;146:8492–8499. doi: 10.1021/jacs.3c14632. [DOI] [PubMed] [Google Scholar]; (b) Mu B.-S. Zhang Y. Peng M. Tu Z. Guo Z. Shen S. Xu Y. Liang W. Wang X. Wang M. Ma D. Liu Z. Angew. Chem., Int. Ed. 2024;63:e202407443. doi: 10.1002/anie.202407443. [DOI] [PubMed] [Google Scholar]
- Mu B.-S. Xu Y. Tu Z. Zhang Y. Liang W. Li J. Wang X. Shen S. Chen J. Liu Z. Natl. Sci. Rev. 2024;11:nwae302. doi: 10.1093/nsr/nwae302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh E. Sanche L. Chem. Rev. 2012;112:5578–5602. doi: 10.1021/cr300063r. [DOI] [PubMed] [Google Scholar]
- Jonah C. D. and Rao B. S. M., Radiation Chemistry: Present Status and Future Trends, Elsevier Science, Amsterdam, 2001 [Google Scholar]
- (a) Maillard B. Ingold K. U. Scaiano J. C. J. Am. Chem. Soc. 1983;105:5095–5099. doi: 10.1021/ja00353a039. [DOI] [Google Scholar]; (b) Boyd S. L. Boyd R. J. Barclay L. R. C. J. Am. Chem. Soc. 1990;112:5724–5730. doi: 10.1021/ja00171a008. [DOI] [Google Scholar]
- (a) Jacob C. Nat. Prod. Rep. 2006;23:851–863. doi: 10.1039/B609523M. [DOI] [PubMed] [Google Scholar]; (b) Wang N. Saidhareddy P. Jiang X. Nat. Prod. Rep. 2020;37:246–275. doi: 10.1039/C8NP00093J. [DOI] [PubMed] [Google Scholar]
- (a) Yu B. Liu A.-H. He L.-N. Li B. Diao Z.-F. Li Y.-N. Green Chem. 2012;14:957–962. doi: 10.1039/C2GC00027J. [DOI] [Google Scholar]; (b) Chakravarthy R. D. Ramkumar V. Chand D. K. Green Chem. 2014;16:2190–2196. doi: 10.1039/C3GC42245C. [DOI] [Google Scholar]; (c) Lang X. Leow W. R. Zhao J. Chen X. Chem. Sci. 2015;6:1075–1082. doi: 10.1039/C4SC02891K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lang X. Hao W. Leow W. R. Li S. Zhao J. Chen X. Chem. Sci. 2015;6:5000–5005. doi: 10.1039/C5SC01813G. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li Y. Rizvi S. A.-e.-A. Hu D. Sun D. Gao A. Zhou Y. Li J. Jiang X. Angew. Chem., Int. Ed. 2019;58:13499–13506. doi: 10.1002/anie.201906080. [DOI] [PubMed] [Google Scholar]; (f) Skolia E. Gkizis P. L. Kokotos C. G. ChemPlusChem. 2022;87:e202200008. doi: 10.1002/cplu.202200008. [DOI] [PubMed] [Google Scholar]
- Pitchen P. Dunach E. Deshmukh M. N. Kagan H. B. J. Am. Chem. Soc. 1984;106:8188–8193. doi: 10.1021/ja00338a030. [DOI] [Google Scholar]
- Clarke J. D. Dashwood R. H. Ho E. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. J. Jarvis B. Keating G. M. Drugs. 2002;62:2637–2651. doi: 10.2165/00003495-200262180-00006. [DOI] [PubMed] [Google Scholar]
- Zhang J.-Q. Han L.-B. J. Org. Chem. 2024;89:2090–2103. doi: 10.1021/acs.joc.3c02398. [DOI] [PubMed] [Google Scholar]
- (a) Jensen F. Greer A. Clennan E. L. J. Am. Chem. Soc. 1998;120:4439–4449. doi: 10.1021/ja973782d. [DOI] [Google Scholar]; (b) Baciocchi E. Giacco T. D. Elisei F. Gerini M. F. Guerra M. Lapi A. Liberali P. J. Am. Chem. Soc. 2003;125:16444–16454. doi: 10.1021/ja037591o. [DOI] [PubMed] [Google Scholar]
- Jensen R. L. Arnbjerg J. Ogilby P. R. J. Am. Chem. Soc. 2010;132:8098–8105. doi: 10.1021/ja101753n. [DOI] [PubMed] [Google Scholar]
- Zou X.-N. Zhang D. Luan T.-X. Li Q. Li L. Li P.-Z. Zhao Y. ACS Appl. Mater. Interfaces. 2021;13:20137–20144. doi: 10.1021/acsami.1c03083. [DOI] [PubMed] [Google Scholar]
- Grills D. C. Lymar S. V. Phys. Chem. Chem. Phys. 2018;20:10011–10017. doi: 10.1039/C8CP00977E. [DOI] [PubMed] [Google Scholar]
- (a) Mosseri S. Neta P. Meisel D. Radiat. Phys. Chem. 1990;36:683–687. doi: 10.1016/1359-0197(90)90162-B. [DOI] [Google Scholar]; (b) Imamura T. Sumiyoshi T. Takahashi K. Sasaki Y. J. Phys. Chem. 1993;97:7786–7791. doi: 10.1021/j100131a058. [DOI] [Google Scholar]
- (a) Liu F. Paton R. S. Kim S. Liang Y. Houk K. N. J. Am. Chem. Soc. 2013;135:15642–15649. doi: 10.1021/ja408437u. [DOI] [PubMed] [Google Scholar]; (b) Chen S. Huang X. Meggers E. Houk K. N. J. Am. Chem. Soc. 2017;139:17902–17907. doi: 10.1021/jacs.7b08650. [DOI] [PubMed] [Google Scholar]
- (a) Choi S. U. Lichtin N. N. J. Am. Chem. Soc. 1964;86:3948–3953. doi: 10.1021/ja01073a009. [DOI] [Google Scholar]; (b) Kuruc J. Šeršeň F. J. Radioanal. Nucl. Chem. 1990;145:197–204. doi: 10.1007/BF02202025. [DOI] [Google Scholar]; (c) Freeman G. R. J. Chem. Phys. 1960;33:71–78. doi: 10.1063/1.1731137. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ESI† includes details of optimization studies, substrate scope studies, mechanistic studies, and computational studies. Characterization data, NMR spectra, and Cartesian coordinates of all optimized geometries are included as well.