Abstract
Background
Postpartum hemorrhage is considered a risk factor for pregnancy-associated complement-mediated hemolytic uremic syndrome (CM-HUS; previously known as atypical hemolytic uremic syndrome) but has not been systematically studied.
Objectives
To systematically examine the role of postpartum hemorrhage in precipitating CM-HUS and to describe the characteristics of postpartum hemorrhage-associated CM-HUS, its prognosis and recommended management.
Methods
A systematic review of individual participant data from case series and reports in addition to a case series from our institution. Search terms were “thrombotic microangiopathy,” “atypical hemolytic uremic syndrome,” and “complement mediated” combined with “pregnancy,” “postpartum,” and/or “postpartum hemorrhage”. Cases of thrombotic microangiopathy other than CM-HUS were excluded. Outcomes were clinical and laboratory characteristics of postpartum hemorrhage-associated CM-HUS, treatment, and outcomes.
Results
Thirty-three studies comprising 48 women with postpartum hemorrhage-associated CM-HUS and 3 patients from our institution were included in the study. Most women presented at term (28/45; 62%), delivered by cesarean section (21/41; 51%), and had pregnancy complications, mainly preeclampsia (16/51; 31%) or fetal demise (9/51; 18%). Hematological and renal abnormalities usually appeared within the first 24 hours postdelivery. The median platelet count was 46 × 109/L (IQR, 26-72), and the median maximal lactate dehydrogenase was 2638 U/L (IQR, 1620-3588). Renal function normalized in 20/23 (87%) women treated with C5 inhibitors with or without plasma exchange; in 7/11 (63%) women treated with plasma exchange alone, but only in 3/17 (18%) patients treated with supportive care. Patients treated with C5 inhibitors and/or plasma exchange achieved significantly better renal outcomes compared with supportive care alone (P < .001).
Conclusion
CM-HUS is a rare complication following postpartum hemorrhage and occurs mainly in women with preeclampsia and/or following cesarean section. Patients treated with C5 inhibitors and/or plasma exchange had a better renal prognosis compared with patients who received supportive treatment alone.
Keywords: postpartum hemorrhage, pregnancy, Complement C5 inhibitors, atypical hemolytic uremic syndrome, thrombotic microangiopathies, pregnancy complications, postpartum period, treatment outcome, acute kidney injury, Parturition
Essentials
-
•
Complement-mediated hemolytic uremic syndrome (CM-HUS) is a rare postpartum hemorrhage sequela.
-
•
We performed a systematic review of participants’ data from published CM-HUS cases.
-
•
Postpartum hemorrhage-associated CM-HUS appeared often after preeclampsia or cesarean sections.
-
•
C5 inhibitors and/or plasma exchange led to better renal outcomes compared with supportive care.
1. Introduction
The early postpartum period is a known risk factor for different forms of thrombotic microangiopathy (TMA). This occurs especially following delivery complications due to complement dysregulation [[1], [2], [3]]. TMA is caused by endothelial cell injury leading to microangiopathic hemolytic anemia, thrombocytopenia, and microvascular thrombi, which may lead to end-organ damage in the kidneys, the brain, and the heart [4].
The differential diagnosis of postpartum TMA includes hemolysis, elevated liver enzymes, and proteinuria (HELLP) syndrome, disseminated intravascular coagulopathy (DIC), immune thrombotic thrombocytopenic purpura (iTTP), and complement-mediated hemolytic uremic syndrome (CM-HUS; previously known as an atypical hemolytic uremic syndrome) [5,6]. The TMA syndromes have an overlapping clinical presentation yet require different treatment modalities. Significant strides have been made in the management of different types of TMA in recent years. The introduction of C5 inhibitors has revolutionized the outcomes of pregnancy-associated CM-HUS [[7], [8], [9]], emphasizing the need for an accurate and timely diagnosis [8,10]. However, reaching a diagnosis of CM-HUS remains elusive and requires the exclusion of other causes of postpartum TMA and, therefore, may result in treatment delays. This diagnostic challenge is further confounded by postpartum hemorrhage, which is an independent risk factor for DIC and acute kidney injury.
Postpartum hemorrhage is defined as blood loss of 1000 mL or as hemorrhage associated with signs or symptoms of hypovolemia within 24 hours after delivery [11,12]. The incidence of postpartum hemorrhage has been reported to gradually increase in recent decades and is currently estimated at 1% to 5% of deliveries in high-income countries [11,13,14]. As a result, it remains a leading cause of maternal mortality worldwide [[13], [14], [15], [16]].
Significant bleeding has not been suggested to be a precipitating factor for developing CM-HUS in nonobstetric settings [17].
Inspired by 3 cases occurring in our institution, we aimed to systematically examine the role of postpartum hemorrhage in precipitating CM-HUS and describe the characteristics of postpartum hemorrhage-associated atypical hemolytic uremic syndrome, its prognosis, and recommended management.
2. Methods
2.1. Case series
We present 3 cases of CM-HUS following postpartum hemorrhage from a single center in the years 2016-2018 and discuss the diagnostic process, administered treatments, and outcomes. The study was approved by the institutional review board in the Rabin Medical Center, Israel, study number RMC-0200-20. All case series participants provided written informed consent.
2.2. Systematic literature review—study design and search strategies
We performed a systematic review of individual participants’ data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participant Data statement [18]; PROSPERO registration number CRD42021271388. The search strategy aimed to identify cases of TMA diagnosed following postpartum hemorrhage that does not correspond to previously described types of TMA, namely HELLP, DIC, and iTTP. We searched Medline, Cochrane Library, ClinicalTrials.gov, Web of Science, and the EMBASE database, restricted to the English language, using the following terms and their alternative spellings: “thrombotic microangiopathy,” “atypical hemolytic uremic syndrome,” and “complement-mediated hemolytic uremic syndrome” in combination with “pregnancy,” “post-partum,” and/or “postpartum hemorrhage/haemorrhage” [7] (last accessed December 2023).
For each of the identified studies, we manually reviewed individual participants’ data and selected patients who fulfilled our predefined inclusion and exclusion criteria, namely patients who experienced postpartum hemorrhage and presented with hemolytic anemia (elevated lactate dehydrogenase [LDH], decreased hemoglobin [Hb] and low haptoglobin, and/or schistocytes on blood smear), thrombocytopenia, and renal failure (creatinine > 1.5 g/dL and/or oliguria). We further searched the respective reference lists to identify additional case series or case reports. Exclusion criteria included other types of TMA concurrent with CM-HUS, namely DIC, HELLP, and iTTP. These were excluded based on the diagnosis reached in the source publication.
2.3. Study selection, data collection, and risk of bias
A.G.S. and M.S. used Rayyan [19] to screen and assess all titles and abstracts for inclusion and consulted G.S. in cases of disagreement. Articles were excluded if they were not relevant to the study question or failed to present individual case data. Data recorded from case reports included the year and type of publication, patient characteristics (age, medical history, and parity), pregnancy characteristics (gestational age, mode of delivery, pregnancy, and delivery complications, particularly preeclampsia), TMA presentation (timing postdelivery, laboratory values, ADAMTS-13 levels, and genetic testing) and management (blood products transfusions, plasma-exchange, corticosteroids, C5 inhibitors, and hemodialysis), and maternal and neonatal outcomes. Laboratory indices were extracted as peak values for creatinine and LDH and nadir values for Hb and platelet counts. Maternal outcomes were determined according to the final report in the examined manuscript; where it was unclear, we corresponded with the authors. Unavailable data were listed as not available (NA).
Risk of bias was assessed using the Joanna Brigs Institute critical appraisal tool for case reports [20] and the Institute of Health Economics Quality Appraisal Checklist for Case Series Studies [21]. Statistical testing was performed using a chi-square test with significance at P < .05. Data were analyzed using IBM SPSS 25 statistics.
3. Results
3.1. Case series
3.1.1. Patient 1 (2016)
A 32-year-old woman, gravida 3, para 1, with a medical history significant for bariatric surgery (2015), presented to the emergency room at 39 weeks of gestation due to elevated blood pressure (BP) of 170/110 mm Hg and abnormal fetal monitoring, and was diagnosed with pregnancy-induced hypertension. No clinical or laboratory signs of preeclampsia or HELLP were observed. Labor induction was initiated, but fetal distress led to an emergency cesarean section (CS). Postoperatively, a drop in Hb level and significantly prolonged clotting times raised the suspicion of postpartum hemorrhage and DIC. Urgent laparotomy revealed uterine atony and massive bleeding, and despite mass transfusion, she remained hemodynamically unstable and anuric, necessitating a hysterectomy. Oliguria persisted despite the administration of fluids, and as Hb and platelet count continued to drop 12 hours after the hysterectomy, she was diagnosed with TMA accompanied by severe renal failure (Table 1). At this time, there was no evidence of active bleeding nor signs of active DIC, BP was normal, and complement levels were low, supporting the diagnosis of pregnancy-associated CM-HUS. Levels of terminal membrane attack complex and complement factor H autoantibodies were NA in our institution at the time.
Table 1.
Laboratory parameters of 3 patients with postpartum hemorrhage-associated complement-mediated hemolytic uremic syndrome.
| Laboratory value | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Hemoglobin nadir (g/dL) | 4.0 | 8.5 | 7.8 |
| Platelet count nadir (×109/L) | 77 | 30 | 15 |
| Creatinine peak (mg/dL) | 3.4 | 4.3 | 4.9 |
| LDH peak (U/L) | 2280 | 3300 | 3500 |
| Schistocytes on peripheral blood smear | Yes | Yes | Yes |
LDH, lactate dehydrogenase.
The patient underwent hemodialysis once and received 4 cycles of daily plasma exchange, with gradual improvement in hematological and renal parameters. The workup for other causes of TMA was negative. Hb and platelet count stabilized on postoperative day 11, and plasma creatinine levels returned to normal within 3 weeks. No antihypertensive medication was required, and she was not treated with C5 inhibitors due to clinical improvement. Genetic testing was not performed.
3.1.2. Patient 2 (2016)
A 29-year-old woman, gravida 4, para 3, with a history of bariatric surgery and heterozygosity for factor (F)V Leiden, received enoxaparin and aspirin during pregnancy. Routine BP monitoring was normal. Oligohydramnios at 36 weeks led to an elective CS performed at 37 weeks gestation. Postsurgery, she experienced massive vaginal bleeding requiring a total hysterectomy. On postoperative day 1, she developed anemia, thrombocytopenia of 30 × 109/L, elevated LDH, and nonoliguric acute renal failure (Table 1). No coagulation abnormalities were noted, and she received 3 packed cell transfusions. With suspected postpartum TMA, ADAMTS-13 activity was normal; she underwent daily plasma exchange for 4 days. Workup for paroxysmal nocturnal hemoglobinuria and antiphospholipid syndrome were negative. Hemolysis gradually subsided, but renal function continued to deteriorate despite normal urine output. Dialysis was not indicated. Renal function normalized 3 weeks post-CS.
The differential diagnosis for acute renal failure, in this case, included a combination of acute tubular necrosis secondary to hypovolemia and contrast nephropathy (after uterine arteries embolization) or pregnancy-associated CM-HUS. Normal complement levels and the abrupt onset of renal failure, in combination with a markedly increased LDH and hematological abnormalities, led us to suspect pregnancy-associated CM-HUS. Since spontaneous improvement was noted, we did not initiate eculizumab. Genetic testing was not performed.
3.1.3. Patient 3 (2018)
A 35-year-old woman, gravida 1, para 0, underwent a termination of pregnancy at 27 weeks due to a chromosome 8 deletion detected on amniocentesis. Following feticide and labor induction, she experienced significant vaginal bleeding, and a laboratory diagnosis of DIC was made based on prolonged clotting times and a steep decrease in fibrinogen levels. An exploratory laparotomy revealed uterine atony with signs of hemorrhage from the placental bed. Postsurgery, fibrinogen levels normalized, but she developed oliguric acute kidney injury, platelet counts and Hb decreased, and LDH was significantly elevated. TMA was suspected, and she underwent 3 courses of plasma exchange. ADAMTS-13 activity was normal, C3 and C4 levels were low, and testing for Shiga toxin was negative. Urine output improved following plasma exchange, but hematological and renal laboratory values worsened (Table 1).
A diagnosis of pregnancy-associated CM-HUS was made, and eculizumab was initiated at an induction dose of 900 mg/wk, resulting in an improvement in Hb, platelet count, and LDH. Eculizumab was continued during a subsequent pregnancy without relapse of CM-HUS. Eculizumab was successfully tapered down and eventually discontinued after 2 years of treatment following negative genetic testing for complement abnormalities.
3.2. Systematic literature review of individual participant data
3.2.1. Description of included studies
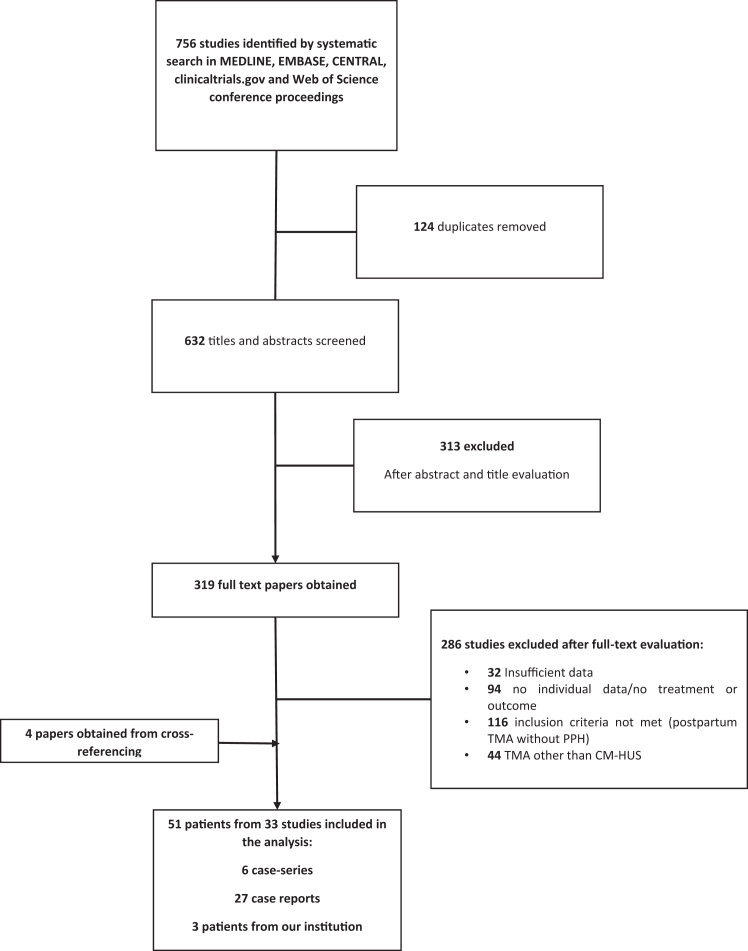
We identified 756 studies using our search strategy. Thirty-three articles were included, reporting on 48 unique cases of postpartum hemorrhage-associated CM-HUS. Study search flowchart and reasons for exclusion are presented in Figure 1. In total, 51 patients were included in the analysis (48 identified by the systematic review and 3 cases from our institution, described in the case series above). Patients’ characteristics, presentation, treatment, and clinical course are summarized in Table 2 [1,4,5,8,10,[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]].
Figure 1.
Study selection flowchart. CM-HUS, complement-mediated hemolytic uremic syndrome; TMA, thrombotic microangiopathy; PPH, postpartum hemorrhage.
Table 2.
Patients’ characteristics.
| Patient no. | First author (publication y) | Age (y) | G | Gest. wk | Preeclampsiaa | Delivery type (indication for CS) | TMA diagnosis (d) | Hgb (gr/L) | Max LDH ( μ//L) | PLT (×109/L) | Cr max (gr/dL) | Plasma exchange | Dialysis | C5 inhibitors | Renal outcome, days to normalization |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Current study | 32 | 3 | 38 | Yes | Emergency CS (fetal distress) | POD1 | 4.1 | 1726 | 77 | 2.95 | Yes | Yes | No | CR, POD14 |
| 2 | Current study | 29 | 4 | 37 | No | Elective CS (oligohydramnios) | POD1 | 8.5 | 3000 | 30 | 4.3 | Yes | No | No | CR, POD4 |
| 3 | Current study | 35 | 1 | 27 | No | Vaginal | Delivery | 7.8 | 2500 | 35 | 3 | Yes | No | Yes | CR, POD21 |
| 4 | Catarci et al. (2023) [22] | 35 | 1 | 36 | No | Elective CS | POD1 | 7.1 | 2356 | 20 | 4.7 | No | No | Yes | CR, NA |
| 5 | Cody et al. (2023) [23] | 13 | 1 | 37 | Yes | Vaginal | POD1 | 7.1 | 2679 | 100 | 2.24 | No | Yes | Yes | CKD |
| 6 | Harazim et al. (2023) [24] | 35 | 4 | 40 | No | Vaginal | POD3 | NA | 2100 | 83 | 7.5 | No | Yes | Yes | CKD |
| 7 | Ghazanfar et al. (2022) [25] | 36 | NA | 36 | No | CS (breech presentation) | POD3 | 6.6 | 2856 | 70 | 6.4 | Yes | No | Yes | CR |
| 8 | Hasan et al. (2022) [26] | 24 | 4 | 39 | No | Vaginal | POD5 | 7 | 2051 | 28 | 3.6 | Yes | Yes | No | Died |
| 9 | Markin and Shatylovych (2022) [27] | 37 | 4 | 27 | No | Vaginal | POD2 | 4.8 | 1514 | 70 | 3.4 | Yes | Yes | Yes | ESRD, POD28 |
| 10 | Guzzo et al. (2021) [28] | 50 | 1 | 41 | No | Elective CS (induction failure) | POD9 | 6 | 1340 | 102 | 1.3 | No | Yes | Yes | CR, POD60 |
| 11 | So et al. (2021) [29] | 27 | 1 | 36 | No | Emergency CS (obstructed labor) | Delivery | 8.9 | 3490 | 87 | 1.26 | No | Yes | Yes | CR, POD28 |
| 12 | Gackler et al. (2021) [8] | 24 | 1 | 38 | No | Emergency CS (fetal distress) | POD6 | NA | NA | NA | 3.1 | NA | Yes | Yes | CR, NA |
| 13 | 43 | 1 | 35 | Yes | Emergency CS | POD1 | NA | NA | NA | 0.57 | NA | Yes | Yes | CR, NA | |
| 14 | Fakhouri et al. (2020) [10] | 27 | 1 | 33 | Yes | Emergency CS (fetal distress) | Delivery | 9.8 | 1120 | 39 | 3.8 | No | No | No | CR, POD21 |
| 15 | Wang et al. (2021) [30] | 29 | 2 | 40 | Yes | Emergency CS (HELLP) | NA | 5.7 | 3001 | 23 | 9.34 | Yes | Yes | No | CKD |
| 16 | 38 | 2 | 39 | Yes | Vaginal | NA | 4.9 | 3706 | 38 | 10.7 | No | Yes | No | ESRD | |
| 17 | 31 | 1 | 39 | Yes | Urgent CS (HELLP) | NA | 6.7 | 2020 | 40 | 13 | No | Yes | No | CKD | |
| 18 | Kim et al. (2019) [31] | 29 | 1 | 39 | No | Vaginal | POD4 | NA | NA | NA | Oliguria | Yes | Yes | Yes | CR, POD11 |
| 19 | Nnanoma et al. (2019) [32] | 26 | 6 | NA | No | Vaginal | NA | <12 | 2607 | 56 | 4.47 | Yes | No | Yes | CR, NA |
| 20 | Ramachandran et al. (2019) [33] | 29 | NA | 38 | No | NA | NA | 4.9 | 3687 | 8 | 3.6 | No | Yes | No | Died |
| 21 | 23 | NA | 30 | Yes | NA | NA | 4.5 | 2045 | 58 | 5.1 | No | Yes | No | ESRD | |
| 22 | 26 | NA | 38 | No | NA | NA | 7.1 | 2912 | 101 | 1.9 | No | Yes | No | CKD | |
| 23 | 29 | NA | 38 | Yes | NA | NA | 8.7 | 3752 | 77 | 4.7 | No | Yes | No | ESRD | |
| 24 | 38 | NA | 35 | Yes | NA | NA | 6.9 | 4031 | 48 | 2.6 | No | Yes | No | ESRD | |
| 25 | 22 | NA | 38 | No | NA | NA | 7.4 | 3816 | 91 | 3.1 | No | Yes | No | ESRD | |
| 26 | 29 | NA | 38 | Yes | NA | NA | 6.4 | 2046 | 23 | 5 | No | Yes | No | Died | |
| 27 | 24 | NA | 38 | No | NA | NA | 5.2 | 8390 | 35 | 10 | No | Yes | No | ESRD | |
| 28 | 21 | NA | 36 | No | NA | NA | 6.5 | 1282 | 22 | 6 | No | Yes | No | ESRD | |
| 29 | Kumar et al. (2019) [34] | 25 | 4 | 39 | No | Elective CS | POD4 | 6.8 | 1323 | 70 | 4.2 | Yes | Yes | Yes | CR, POD35 |
| 30 | Huerta et al. (2018) [1] | 41 | 2 | NA | No | Emergency CS (vaginal bleeding) | POD 28 | 5.3 | 1977 | 60 | HD | Yes | Yes | No | ESRDb |
| 31 | 35 | 1 | NA | No | CS | POD 1 | 5.3 | 7487 | 26 | HD | Yes | Yes | Yes | CR, NA | |
| 32 | 28 | 1 | NA | No | CS | POD 1 | 5.2 | 7183 | 20 | 4.55 | Yes | No | Yes | CR, NA | |
| 33 | Gaggl et al. (2018) [35] | 20 | 1 | 20 | Yes | CS | Delivery | <12d | NA | <150 | >1.5 | Yes | No | No | CR, POD21c |
| 34 | Shivarov and De Vitta (2018) [36] | 35 | NA | 37 | No | Emergency CS (obstructed labor and fetal distress) | POD1 | 6.9 | 1324 | 62 | 7.2 | Yes | Yes | Yes | CR, POD20 |
| 35 | Gately et al. (2017) [37] | 32 | 1 | 40 | No | Vaginal | Delivery | <12 | 3160 | 44 | >1.5 | Yes | Yes | Yes | CR, POD14 |
| 36 | Chua et al. (2017) [38] | 29 | NA | 37 | Yes | Vaginal | POD2 | 6.8 | 620 | 70 | >1.5 | Yes | No | Yes | CR, POD28 |
| 37 | Frimat et al. (2016) [39] | 39 | NA | 41 | No | NA | NA | 8.7 | NA | 75 | 2 | No | Yes | No | CKD |
| 38 | 40 | >3 | 38 | No | NA | NA | 6.7 | 2152 | 58 | 3.3 | No | Yes | No | CKD | |
| 39 | 33 | NA | 38 | No | NA | NA | 9.3 | 1324 | 57 | 1 | No | Yes | No | CKD | |
| 40 | Asif et al. (2017) [40] | 33 | NA | 33 | No | Emergency CS (abruptio placenta) | Delivery | 6.7 | 2670 | 39 | >1.5 | Yes | Yes | Yes | CR, POD14 |
| 41 | Kyung (2016) [4] | 37 | NA | 37 | Yes | Emergency CS (fetal distress) | POD1 | <12 | >1000 | <150 | >1.5 | No | Yes | Yes | CR, POD14-21 |
| 42 | Canigral et al. (2014) [41] | 32 | 1 | NA | No | Urgent CS (anemia, thrombocytopenia, and renal failure) | Delivery | 7 | 7183 | 20 | >1.5 | Yes | No | Yes | CR, POD 14 |
| 43 | Zschiedrich et al. (2013) [42] | 31 | NA | 41 | No | Vaginal | POD3 | 6.6 | 2800 | 30 | 7.1 | Yes | Yes | Yes | CR, NA |
| 44 | Dawsari and Jazieh (2011) [43] | 22 | 3 | 39 | Yes | Vaginal | POD3 | 7.6 | 2756 | 36 | 3.9 | Yes | No | Yes | CR, POD14 |
| 45 | Habek et al. (2007) [44] | 37 | 4 | 35 | No | Emergency CS (placenta percreta, massive vaginal bleeding) | POD7 | 6.1 | NA | 72 | 4.7 | Yes | No | No | CR, NA |
| 46 | Yamanaka et al. (2005) [45] | 34 | >2 | 14 | No | D&C (fetal demise) | POD1 | 7.4 | 900 | 10 | 1.7 | Yes | Yes | No | CR, POD14 |
| 47 | Rosen et al. (2005) [5] | 25 | 2 | 38 | No | Emergency CS (obstructed labor) | POD3 | 7.9 | 6380 | 9 | 6.42 | Yes | Yes | No | CKD |
| 48 | Anacleto et al. (2003) [46] | 17 | 1 | 33 | Yes | Emergency CS (abruption placenta) | Delivery | 6.7 | 593 | 23 | 11.7 | No | Yes | No | CR, POD43 |
| 49 | Chen et al. (2002) [47] | 28 | 1 | 39 | No | Emergency CS (fetal distress) | POD3 | 4.2 | 1440 | 77 | 2.4 | Yes | No | No | CR, POD7 |
| 50 | Wu et al. (2002) [48] | 32 | 3 | 34 | No | Emergency CS (abruptio placenta) | POD3 | 6.8 | 5830 | 22 | 6 | Yes | Yes | No | CR, POD19 |
| 51 | Pajor et al. (1993) [49] | 32 | 2 | 24 | No | Vaginal | POD1 | 6.4 | 2895 | 80 | 9.4 | No | Yes | No | CR, POD60 |
CKD, chronic kidney disease; CR, complete remission; Cr, creatinine; CS, cesarean section; D&C, dilatation and curettage; ESRD, end-stage renal disease; G, gravida; Gest. wk, gestational week; HD, hemodialysis; HELLP, hemolysis, elevated liver enzyme levels, and low platelet levels; Hgb, hemoglobin post TMA diagnoses; LDH, lactate dehydrogenase; NA, not available; PLT, platelets; POD, postdelivery day; TMA, thrombotic microangiopathy.
Including HELLP syndrome.
Lost 2 renal transplants due to relapses.
Two normal subsequent pregnancies with preventative treatment.
Where values of anemia, thrombocytopenia, and/or renal injury were not stated, the standard definition appears.
3.2.2. Patient characteristics and presentation
Patients’ ages ranged between 13 and 50 (median, 31; IQR, 26-35) years; medical history was available for 25/51 (49%) patients, which was generally unremarkable, except for 2 cases of obesity requiring a bariatric surgery (patients 1 and 2), heavy smoking in patient number 3, asthma in patients number 3, 7, and 51, heterozygosity to FV Leiden (patient number 2), and past abortions in patient number 38. Patient number 11 had paraplegia due to meningocele and ventriculoperitoneal shunt. Two patients (numbers 29 and 33) had a known diagnosis of CM-HUS.
Gravida status was provided for 33/51 (64.7%) women, among which 17 (52%) were nulliparous, 5 (15%) gravida 2, 3 gravida 3 (9%), and 8 gravida ≥ 4 (24%). One patient (number 4) had monochorionic diamniotic twins. Gestational age was provided for 45/51 (88%) patients; most (28/45, 62%) presented at term, and only 2 presented at postterm. The diagnosis of CM-HUS was often preceded by a pregnancy complication: preeclampsia (including HELLP syndrome) in 16/51 (31%) patients; abruptio placenta in 4; premature rupture of membranes and placenta accreta each in 1 patient; and oligohydramnios and gestational diabetes were each reported in 1 patient. Ten women presented with fetal abnormalities, namely fetal demise (patients 3, 9, 36, 40, 44, 46, 48, 50, and 51), fetal growth restriction (patient 2), and a chromosomal abnormality (patient 3). CS, particularly emergent CS, was the most common mode of delivery employed in 21/41 (51%) patients where data were available.
Six patients were documented to receive tranexamic acid (patients 3-10, 14, and 37-39). Four patients received a loading dose of 2 g (patients 10, 14, and 37-38), and 1 patient received a loading dose followed by maintenance (10 g/8 h).
Hematological and renal abnormalities appeared within the first 24 hours postdelivery in most patients (20/37; 54%), with 1 patient presenting as late as 4 weeks postpartum. The median platelet count was 46 × 109/L (IQR, 26-72), and the median maximal LDH was 2638 U/L (IQR, 1620-3588). ADAMTS-13 was normal for all 25 women tested (patients 1-13, 15-19, 29, 34-36, 40, and 42-43).
3.2.3. Risk of bias
Case reports are low in the hierarchy of evidence, as they are inherently predisposed to a high risk of bias, including reporting bias [50]. However, using the Joanna Brigs Institute and the Institute of Health Economics, we assess the risk of bias as medium-high.
3.2.4. Treatment and clinical course
In the diagnostic process, 14 women underwent imaging using computed tomography or magnetic resonance imaging (patients 11, 14, 20-28, and 37-40), and 7 women underwent kidney biopsy (patients 5, 15-17, 29, 33, and 50). In the imaging group, 12 were diagnosed with renal cortical necrosis (RCN; all belonging to the Ramachandran et al. [33] and Frimat et al. [39] case series; patients 20-28 and 37-39). For both remaining patients (patients 10 and 14), imaging did not reveal RCN. Among the 7 patients who had kidney biopsy results, all manifested findings were compatible with TMA.
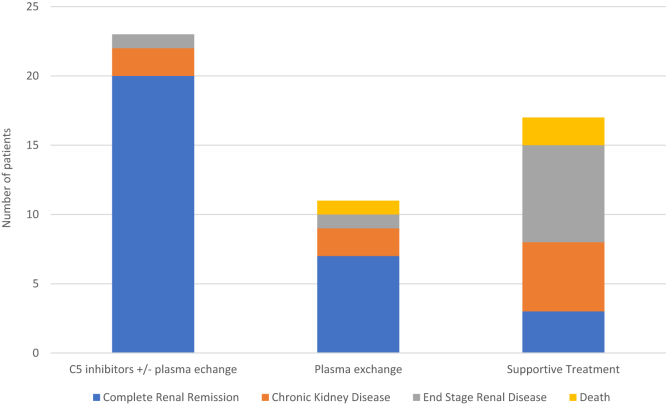
Plasma exchange was administered for 26/49 (53%) women, with a number of cycles ranging from 3 to 27 (data available for 13 women). Hemodialysis was performed in 38/51 (74%) patients. Thirty-eight cases from 26 publications were published after the introduction of eculizumab in 2011 [1,4,8,10,[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43],51]. Among these, 23 women were treated with C5 inhibitors (eculizumab = 21, ravulizumab = 2 [8]), 20 of whom achieved complete renal remission. Among the 3 remaining patients, 2 (patients 5 and 6) had impaired renal function at the time of discharge without follow-up information, and patient 9 chose to discontinue eculizumab shortly after discharge and subsequently presented with end-stage renal disease (ESRD). Information on the duration of eculizumab treatment was available for 12 patients (Table 3). Of note, 15/23 women treated with C5 inhibitors also underwent plasma exchange. Seven out of 11 (63%) patients treated with plasma exchange without C5 inhibitors experienced complete renal remission. One patient (number 8) died. Seventeen patients who were not treated with either plasma exchange or C5 inhibitors had a worse outcome (P < .001), with only 3 women (18%) achieving complete normalization of renal function and 12 (70%) patients developing chronic kidney disease, among which 7 women (41%) progressed to ESRD, and 2 women (12%) died (Figure 2). Patients treated with C5 inhibitors and/or plasma exchange achieved significantly better renal outcomes compared with the supportive treatment (P < .001). Patients treated with plasma exchange alone also achieved better renal outcomes compared with supportive treatment alone (P = .038). The addition of C5 inhibitors to plasma exchange did not demonstrate a statistically significant difference (P = .12). However, the limited group sizes should be mentioned in this regard.
Table 3.
Duration of C5 inhibitors treatment.
| Patient no. | Discontinued/ongoing | Treatment duration | Renal outcome, last follow-up |
|---|---|---|---|
| 3 | Discontinued | 36 months postpartum | CR, NA |
| 4 | Discontinued | POD40 | CR, NA |
| 5 | Discontinued | 12 months postpartum | CKD, discharge |
| 9 | Discontinueda | POD14 | ESRD, 12 months |
| 10 | Discontinued | 6 dosesb | CR, 24 months |
| 12 | Ongoing | POD183 | CR, POD183 |
| 13 | Ongoing | POD183 | CR, POD183 |
| 18 | Discontinued | POD90 | CR, 12 months |
| 35 | Ongoing | POD20 | CR, POD20 |
| 36 | Discontinued | 4 doses | CR, POD20 |
| 40 | Ongoing | POD14 | CR, POD14 |
| 42 | Discontinued | 6 months postpartum | CR, 12 months |
CKD, chronic kidney disease; CR, complete remission; ESRD, end-stage renal disease; NA, not available; POD, postdelivery day.
By patient’s choice.
When CH50 reached below 10%, and hemodialysis could be discontinued.
Figure 2.
Patient outcomes according to treatment group.
Of note, 12/17 nontreated patients were presented in 2 case series studies focusing on patients diagnosed with RCN [33,39]. Overall, among 51 patients, 30 (59%) achieved normal renal function, 9 (18%) remained with chronic kidney disease, 9 (18%) progressed to ESRD, and 3 (6%) patients died. Hematological response was available for 41 patients, with stabilization or normalization achieved in all cases.
Among 15 women tested for genetic complement abnormalities, 7 (46%) exhibited variations. Specifically, in C3 (patients 33 and 29), complement factor H (patients 6, 30, 33, and 29), and complement factor I (patients 23 and 35) and modifications of uncertain significance (patients 12, 13, and 36).
4. Discussion
Postpartum hemorrhage is a recognized risk factor for pregnancy-associated CM-HUS but has not been systematically studied. We reviewed 51 cases of pregnancy-associated CM-HUS following postpartum hemorrhage and proposed it as a distinct complication, especially in the presence of CS and/or preeclampsia. Our review suggests that treatment with plasma exchange, eculizumab, or both may improve renal prognosis compared with no treatment. Since early intervention improves renal prognosis, it is crucial to promptly identify pregnancy-associated CM-HUS HUS after postpartum hemorrhage [52].
Our findings diverge from recently published recommendations by the International Working Group on pregnancy-related thrombotic microangiopathies. This group concluded that postpartum hemorrhage-associated TMA is a distinct entity that does not require treatment and that postpartum hemorrhage should be excluded prior to diagnosing pregnancy-associated CM-HUS [10]. However, this recommendation was based on a single retrospective series of 18 patients presenting with RCN following postpartum hemorrhage and tranexamic acid administration [39], in whom CM-HUS was not considered in the differential diagnosis [33]. All 12 patients with RCN in our cohort were from these case series [33,39] and received supportive treatment only, resulting in poor outcomes. Importantly, tranexamic acid, implicated in RCN pathogenesis by Frimat et al. [39], was administered at higher-than-standard doses in this case series. RCN is one of the pathological findings associated with CM-HUS [53,54] but does not typically present with additional clinical and laboratory findings, such as observed in pregnancy-associated CM-HUS.
The characteristics of postpartum hemorrhage-associated CM-HUS in our study resemble those previously described for pregnancy associated-CM-HUS [1,7,55,56]. The development of TMA in our cohort was often preceded by preeclampsia and CS and manifested up to 48 hours postdelivery. Genetic testing identified complement abnormalities in 7 of 15 CM-HUS patients, similar to previously published rates in pregnancy-associated CM-HUS [1,57,58].
The pathogenesis of the association between postpartum hemorrhage and TMA remains unclear, with hypotheses suggesting a bidirectional influence. Endothelial damage and microvascular stress from hypovolemia during surgery, including CS, might trigger TMA [5,[59], [60], [61]], and the removal of the placenta, which inhibits complement, might also contribute to TMA development [62]. Complement dysregulation has been implicated in the pathogenesis of preeclampsia [63,64], suggesting an explanation for the high prevalence of preeclampsia in this and previous cohorts [1,30,33].
The medical background was NA for the majority of patients, but patients 1 and 3 from our institution both had a history of bariatric surgery with marked weight reduction, which, although not previously reported to precipitate TMA, might cause endothelial damage [65]. Eleven cases (24%) occurred following fetal complications, particularly intrauterine fetal death, in line with the 28% reported by Gupta et al. [7]. Fetal demise is considered a risk factor for DIC [66], but 6 of 9 patients with intrauterine fetal death in our cohort did not exhibit coagulation abnormalities, suggesting other factors may contribute as well.
The true incidence of CM-HUS among the postpartum TMAs is not known. While postpartum hemorrhage is common, postpartum hemorrhage associated with CM-HUS is rare. To assist differentiation between CM-HUS and other causes of TMA, Burwick et al. [67] suggested a higher LDH and a more pronounced renal failure. The median LDH value we observed was 2638 (230-480 U/L, IQR 1620-3588), which represents a 5-fold rise compared with the upper limit of LDH considered normal. Moreover, acute renal failure requiring hemodialysis is rare in iTTP and highly suggestive of CM-HUS [68].
The prognosis of CM-HUS, including pregnancy-associated CM-HUS, has improved significantly since the introduction of complement inhibitors [57,69]. Eculizumab [37,57,70] and ravulizumab [8] have been shown to be safe in pregnancy and the postpartum period, with no adverse effects reported in the fetus. Eculizumab has never been found in the milk of the treated mothers, and only small amounts of eculizumab were found in cord blood in a small percentage of cases [[70], [71], [72]]. The safety of eculizumab in postpartum, in combination with the dramatically improved outcomes, further stresses the importance of an accurate diagnosis of this TMA subtype and timely initiation of treatment. Kaufeld et al. [73] recently reached similar conclusions when comparing patients with CM-HUS postpartum with and without prior postpartum hemorrhage. This is further exemplified by the case of a woman with known CM-HUS, who had a significant difference in the rate of both hematological and renal normalization between early and late initiation of eculizumab in 2 subsequent pregnancies [34].
Of note, patients who were treated with plasma exchange alone also had improved renal prognosis compared with no treatment. Plasma exchange is known to benefit approximately 35% of patients with CM-HUS [74]. This might be due to the role of plasma exchange in the replacement of mutated with nonmutated factors and the removal of pathogenic autoantibodies such as antifactor H and other triggers (eg, cytokines) of endothelial dysfunction and platelet hyperaggregability [58,[75], [76], [77]].
Several limitations of our study ought to be addressed. We observed high heterogeneity in diagnostic criteria and treatment approaches across medical centers. In addition, the cases of postpartum hemorrhage and CM-HUS in which C5 inhibitors and plasmapheresis had a beneficial effect are more likely to be reported, contributing to a publication bias [50]. There is no standard method to estimate the volume of blood loss during postpartum hemorrhage [78]; as a result, we could not compare this parameter across publications. The cases stretch over a period of 20 years, during which multiple changes to practices in diagnosis and treatment were introduced, and many of the cases do not report on genetic testing and/or C5 inhibitors. In addition, most of the patients in the nontreated group originated from 2 case series where patients were diagnosed with RCN. We could not precisely define risk factors for postpartum hemorrhage-associated CM-HUS due to the lack of a control cohort of patients with pregnancy-associated CM-HUS without postpartum hemorrhage or patients with postpartum hemorrhage without CM-HUS. This comparison has recently been applied by Kaufeld et al. [73], who presented similar findings. Finally, some of the women experienced other potential triggers of TMA, namely preeclampsia, CS, the administration of tranexamic acid, and hysterectomy. Despite these limitations, mainly due to the rarity of this syndrome, our collected studies represent the best available evidence. Further research is needed to improve understanding of the possible association between postpartum hemorrhage and CM-HUS and to optimize management strategies.
5. Conclusions
Postpartum hemorrhage-associated CM-HUS is a life- and kidney-threatening syndrome. If acute renal failure occurs after postpartum hemorrhage, consider pregnancy-associated CM-HUS as a potential diagnosis and conduct the appropriate workup. Recognizing this complication is crucial for the timely treatment and administration of C5 inhibitors, which may improve outcomes. Genetic testing can be considered to detect hereditary pathogenic mutations, allowing for tailored counseling for the patient and her family regarding future pregnancies.
Acknowledgments
We thank our patients for their willingness to take part in this study and wish them the best of health. We extend our gratitude to Dr Ana Huerta, Dr Justin Chua, and Dr Ryan Gately, who have previously investigated TMA in pregnancy and kindly shared their data and insights with us.
Funding
No funding was received for this study.
Author contributions
A.G.S. designed the study, performed the literature search, analyzed and wrote the manuscript; A.L. and P.R. contributed to the study design and critically revised the manuscript; S.O.Z., A.W., P.S., and M.D. contributed to the case series and critically revised the manuscript; M.S. participated in study selection and analysis; E.N.H. assisted in study design, search strategy definition, and critically revised the manuscript; G.S. conceived, designed, analyzed, wrote the manuscript, and supervised the study. All authors approved the manuscript before submission.
Relationship disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Michelle Sholzberg
References
- 1.Huerta A., Arjona E., Portoles J., Lopez-Sanchez P., Rabasco C., Espinosa M., et al. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int. 2018;93:450–459. doi: 10.1016/j.kint.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Fakhouri F., Roumenina L., Provot F., Sallée M., Caillard S., Couzi L., et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21:859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George J.N., Nester C.M., McIntosh J.J. Syndromes of thrombotic microangiopathy associated with pregnancy. Hematol Am Soc Hematol Educ Program. 2015;2015:644–648. doi: 10.1182/asheducation-2015.1.644. [DOI] [PubMed] [Google Scholar]
- 4.Adamski J. Thrombotic microangiopathy and indications for therapeutic plasma exchange. Hematol Am Soc Hematol Educ Program. 2014;2014:444–449. doi: 10.1182/asheducation-2014.1.444. [DOI] [PubMed] [Google Scholar]
- 5.Rosen M., Brauer K.I., Alperin J.B., Hankins G.D., Saade G. Postpartum hemorrhagic shock resulting in thrombotic thrombocytopenic purpura–hemolytic uremic syndrome. J Matern Fetal Neonatal Med. 2003;13:208–210. doi: 10.1080/jmf.13.3.208.210. [DOI] [PubMed] [Google Scholar]
- 6.Lim M.Y., Abou-Ismail M.Y., Branch D.W. Differentiating and managing rare thrombotic microangiopathies during pregnancy and postpartum. Obstet Gynecol. 2023;141:85–108. doi: 10.1097/AOG.0000000000005024. [DOI] [PubMed] [Google Scholar]
- 7.Gupta M., Govindappagari S., Burwick R.M. Pregnancy-associated atypical hemolytic uremic syndrome: a systematic review. Obstet Gynecol. 2020;135:46–58. doi: 10.1097/AOG.0000000000003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gäckler A., Schönermarck U., Dobronravov V., La Manna G., Denker A., Liu P., et al. Efficacy and safety of the long-acting C5 inhibitor ravulizumab in patients with atypical hemolytic uremic syndrome triggered by pregnancy: a subgroup analysis. BMC Nephrol. 2021;22:5. doi: 10.1186/s12882-020-02190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haninger N., Gaggl M., Aigner C., Renate K., Prohaszka Z., Böhmig G.A., et al. Pregnancy and delivery outcomes in patients with complement gene variant mediated thrombotic microangiopathy [abstract] Nephrol Dial Transplant. 2020;35(suppl 3):gfaa142. doi: 10.1093/ndt/gfaa142.P0231. [DOI] [Google Scholar]
- 10.Fakhouri F., Scully M., Provot F., Blasco M., Coppo P., Noris M., et al. Management of thrombotic microangiopathy in pregnancy and postpartum: report from an international working group. Blood. 2020;136:2103–2117. doi: 10.1182/blood.2020005221. [DOI] [PubMed] [Google Scholar]
- 11.van Stralen G., von Schmidt auf Altenstadt J.F., Bloemenkamp K.W., van Roosmalen J., Hukkelhoven C.W. Increasing incidence of postpartum hemorrhage: the Dutch piece of the puzzle. Acta Obstet Gynecol Scand. 2016;95:1104–1110. doi: 10.1111/aogs.12950. [DOI] [PubMed] [Google Scholar]
- 12.Bienstock J.L., Eke A.C., Hueppchen N.A. Postpartum hemorrhage. N Engl J Med. 2021;384:1635–1645. doi: 10.1056/NEJMra1513247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reale S.C., Easter S.R., Xu X., Bateman B.T., Farber M.K. Trends in postpartum hemorrhage in the United States from 2010 to 2014. Anesth Analg. 2020;130:e119–e122. doi: 10.1213/ANE.0000000000004424. [DOI] [PubMed] [Google Scholar]
- 14.Schutte J.M., Steegers E.A., Schuitemaker N.W., Santema J.G., de Boer K., Pel M., et al. Rise in maternal mortality in the Netherlands. BJOG. 2010;117:399–406. doi: 10.1111/j.1471-0528.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 15.Bateman B.T., Berman M.F., Riley L.E., Leffert L.R. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–1373. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Pregnancy mortality surveillance system. Maternal and infant health. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm [accessed December 1, 2024].
- 17.Jokiranta T.S. HUS and atypical HUS. Blood. 2017;129:2847–2856. doi: 10.1182/blood-2016-11-709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart L.A., Clarke M., Rovers M., Riley R.D., Simmonds M., Stewart G., et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 19.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. In: Joanna Briggs Institute reviewer’s manual. Aromataris E., Lockwood C., Porritt K., Pilla B., Jordan Z., editors. The Joanna Briggs Institute; 2017. Systematic reviews of etiology and risk. [Google Scholar]
- 21.Moga C., Guo B., Harstall C. Development of a quality appraisal tool for case series studies using a modified Delphi technique. Edmonton AB: Institute of Health Economics. 2012:1–71. [Google Scholar]
- 22.Catarci S., Zanfini B.A., Di Muro M., Capone E., Frassanito L., Santantonio M.T., et al. A case report of an atypical haemolytic uremic syndrome in pregnancy: something wicked this way comes. BMC Anesthesiol. 2023;23:94. doi: 10.1186/s12871-023-02066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cody E., Claes D., Taylor V., Erkan E. Pregnancy associated TMA in 13-year-old patient successfully treated with Eculizumab: case report. BMC Nephrol. 2022;23:147. doi: 10.1186/s12882-022-02766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harazim M., Šenkyřík M., Buliková A., Stehlíková O., Doubek M. Postpartum atypical hemolytic uremic syndrome. Diagnostic importance of flow cytometry. Ann Clin Case Reports. 2023;8:2457. doi: 10.25107/2474-1655.2457. [DOI] [Google Scholar]
- 25.Ghazanfar H., Nawaz I., Allena N., Ashraf S., Saad M., Ali N. A case of atypical hemolytic uremic syndrome in a pregnant patient. Cureus. 2022;14 doi: 10.7759/cureus.25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan N.Y., Rugaan A.S., Ali N.A.M., Iqbal M. A rare case of post-partum hemorrhage with refractory thrombotic microangiopathy, and hepatic infarction: a diagnostic dilemma with high mortality. Gynecol Obstet Reprod Med. 2022;28:277–281. [Google Scholar]
- 27.Markin L., Shatylovych K. Postpartum renal thrombotic microangiopathy: a turn-based differential diagnosis. Wiad Lek. 2022;75:128–131. [PubMed] [Google Scholar]
- 28.Guzzo G., Kissling S., Pantaleo G., Pascual M., Sadallah S., Teta D. Complement activation and blockade in massive post-partum haemorrhage, thrombotic microangiopathy and acute kidney injury: a case report. BMC Nephrol. 2021;22:252. doi: 10.1186/s12882-021-02456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.So S., Fischer E., Gangadharan Komala M., Bose B. Postpartum atypical hemolytic uremic syndrome: evaluating thrombotic microangiopathy in the pregnant woman. Obstet Med. 2021;14:105–108. doi: 10.1177/1753495X20926043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Liu C.Y., Yang Y., Zou G.M., Zhuo L., Han S.H., et al. Acute kidney injuries induced by thrombotic microangiopathy following severe hemorrhage in puerperants: a case series and literature review. Am J Transl Res. 2021;13:6182–6190. [PMC free article] [PubMed] [Google Scholar]
- 31.Kim D., D’Souza S., Mather A., Roxburg S. Society of Obstetric Medicine of Australia and New Zealand Annual Scientific Meeting. SAGE Publications; 2019. Two cases of postpartum atypical haemolytic uraemic syndrome in the pre- and post-eculizumab era; p. 26. [Google Scholar]
- 32.Nnanoma C., Adeboye A., Angeli D., Mody M., Lefkowitz H. Rare case of postpartum atypical HUS [abstract] Am J Kidney Dis. 2019;73:710–711. [Google Scholar]
- 33.Ramachandran R., Nayak S., Anakutti H.P., Yadav A.K., Nada R., Jain V., et al. Postpartum renal cortical necrosis is associated with atypical hemolytic uremic syndrome in developing countries. Kidney Int Rep. 2019;4:420–424. doi: 10.1016/j.ekir.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar D., King M., Jim B., Acharya A. Case report: recurrent case of pregnancy-induced atypical haemolytic uremic syndrome (P-aHUS) BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-226571. bcr-2018-226571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaggl M., Aigner C., Csuka D., Szilágyi Á., Prohászka Z., Kain R., et al. Maternal and fetal outcomes of pregnancies in women with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2018;29:1020–1029. doi: 10.1681/ASN.2016090995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivarov H., De Vitta M.V. Postpartum associated atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2018;29:792. [Google Scholar]
- 37.Gately R., San A., Kurtkoti J., Parnham A. Life-threatening pregnancy-associated atypical haemolytic uraemic syndrome and its response to eculizumab. Nephrology (Carlton) 2017;22(suppl 1):32–35. doi: 10.1111/nep.12938. [DOI] [PubMed] [Google Scholar]
- 38.Chua J., Paizis K., He S.Z., Mount P. Suspected atypical haemolytic uraemic syndrome in two post-partum patients with foetal-death in utero responding to eculizumab. Nephrology (Carlton) 2017;22(suppl 1):18–22. doi: 10.1111/nep.12935. [DOI] [PubMed] [Google Scholar]
- 39.Frimat M., Decambron M., Lebas C., Moktefi A., Lemaitre L., Gnemmi V., et al. Renal cortical necrosis in postpartum hemorrhage: a case series. Am J Kidney Dis. 2016;68:50–57. doi: 10.1053/j.ajkd.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Asif A., Nayer A., Haas C.S. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J Nephrol. 2017;30:347–362. doi: 10.1007/s40620-016-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cañigral C., Moscardó F., Castro C., Pajares A., Lancharro A., Solves P., et al. Eculizumab for the treatment of pregnancy-related atypical hemolytic uremic syndrome. Ann Hematol. 2014;93:1421–1422. doi: 10.1007/s00277-013-1970-3. [DOI] [PubMed] [Google Scholar]
- 42.Zschiedrich S., Prager E.P., Wolfgang Kuehn E. Successful treatment of the postpartum atypical hemolytic uremic syndrome with eculizumab. Ann Intern Med. 2013;159:76. doi: 10.7326/0003-4819-159-1-201307020-00023. [DOI] [PubMed] [Google Scholar]
- 43.Dawsari G.A., Jazieh A.R. Post-partum hemolytic uremic syndrome: a case report and review of the literature. J Applied Hematol. 2011;2:46–50. [Google Scholar]
- 44.Habek D., Petrović D., Vidović D., Gudelj G. Placenta praevia percreta with silent uterine incomplete rupture complicated with puerperal haemolytic-uremic syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;131:103–105. doi: 10.1016/j.ejogrb.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Yamanaka Y., Takeuchi K., Oomori S., Oda N., Ashitani N., Maruo T. Two cases of clinically suspected thrombotic thrombocytopenic purpura/hemolytic uremic syndrome in the puerperium. Acta Obstet Gynecol Scand. 2005;84:920–921. doi: 10.1111/j.0001-6349.2005.0414c.x. [DOI] [PubMed] [Google Scholar]
- 46.Anacleto F.E., Cifra C.L., Elises J.S. Postpartum hemolytic uremic syndrome in a 17-year-old Filipina primigravid. Pediatr Nephrol. 2003;18:1283–1285. doi: 10.1007/s00467-003-1261-7. [DOI] [PubMed] [Google Scholar]
- 47.Chen M.J., Tien H.F., Ho H.N. Treatment of thrombotic microangiopathy in pregnancy with exchange: a report of two cases. J Formos Med Assoc. 2002;101:859–863. [PubMed] [Google Scholar]
- 48.Wu V.C., Lin S.L., Tsai C.C., Tien H.F. Postpartum hemolytic uremic syndrome following abruptio placenta: report of a case. J Formos Med Assoc. 2002;101:868–870. [PubMed] [Google Scholar]
- 49.Pajor A., Hintalan A., Bakos L., Lintner F. Postpartum hemolytic uremic syndrome following placental abruption. Eur J Obstet Gynecol Reprod Biol. 1993;49:201–204. doi: 10.1016/0028-2243(93)90271-d. [DOI] [PubMed] [Google Scholar]
- 50.Daly J., Willis K., Small R., Green J., Welch N., Kealy M., et al. A hierarchy of evidence for assessing qualitative health research. J Clin Epidemiol. 2007;60:43–49. doi: 10.1016/j.jclinepi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Kyung, Sungwon Rachel Miller J The diagnostic challenges of differentiating TTP-HUS and HELLP syndrome in an anuric post-partum patient. Proc UCLA Healthc. 2016;20 [Google Scholar]
- 52.Scully M., Neave L. Etiology and outcomes: thrombotic microangiopathies in pregnancy. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prakash J., Sen D., Kumar N.S., Kumar H., Tripathi L.K., Saxena R.K. Acute renal failure due to intrinsic renal diseases: review of 1122 cases. Ren Fail. 2003;25:225–233. doi: 10.1081/jdi-120018723. [DOI] [PubMed] [Google Scholar]
- 54.Wang R., Liu X., Li W., Tan Y., Qiu J., Su T. Pregnancy-associated renal cortical necrosis and nonenhanced functional magnetic resonance imaging: a case series. Kidney Med. 2023;5 doi: 10.1016/j.xkme.2023.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kozlovskaya N.L., Korotchaeva Y.V., Bobrova L.A. Adverse outcomes in obstetric-atypical haemolytic uraemic syndrome: a case series analysis. J Matern Fetal Neonatal Med. 2019;32:2853–2859. doi: 10.1080/14767058.2018.1450381. [DOI] [PubMed] [Google Scholar]
- 56.Kozlovskaya N.L., Korotchaeva Y.V., Shifman E.M., Kudlay D.A. Pregnancy-associated atypical hemolytic-uremic syndrome: is pregnancy to blame or its complications? Vopr Ginekol Akusherstva i Perinatol. 2020;19:81–91. [Google Scholar]
- 57.Bruel A., Kavanagh D., Noris M., Delmas Y., Wong E.K.S., Bresin E., et al. Hemolytic uremic syndrome in pregnancy and postpartum. Clin J Am Soc Nephrol. 2017;12:1237–1247. doi: 10.2215/CJN.00280117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fakhouri F., Frémeaux-Bacchi V., Loirat C. Atypical hemolytic uremic syndrome: from the rediscovery of complement to targeted therapy. Eur J Intern Med. 2013;24:492–495. doi: 10.1016/j.ejim.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Chang J.C., El-Tarabily M., Gupta S. Acute thrombotic thrombocytopenic purpura following abdominal surgeries: a report of three cases. J Clin Apher. 2000;15:176–179. doi: 10.1002/1098-1101(2000)15:3<176::aid-jca4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 60.Moake J.L., Turner N.A., Stathopoulos N.A., Nolasco L.H., Hellums J.D. Involvement of large plasma von Willebrand Factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986;78:1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farkas P., Csuka D., Mikes B., Sinkovits G., Réti M., Németh E., et al. Complement activation, inflammation and relative ADAMTS13 deficiency in secondary thrombotic microangiopathies. Immunobiology. 2017;222:119–127. doi: 10.1016/j.imbio.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Holmes C.H., Simpson K.L., Okada H., Okada N., Wainwright S.D., Purcell D.F., et al. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55) Eur J Immunol. 1992;22:1579–1585. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y., Kong L.R., Ge Q., Lu Y.Y., Hong M.N., Zhang Y., et al. Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J Cell Mol Med. 2017;22:1034–1046. doi: 10.1111/jcmm.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaught A.J., Gavriilaki E., Hueppchen N., Blakemore K., Yuan X., Seifert S.M., et al. Direct evidence of complement activation in HELLP syndrome: a link to atypical hemolytic uremic syndrome. Exp Hematol. 2016;44:390–398. doi: 10.1016/j.exphem.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borzì A.M., Buscemi C., Corleo D., Randazzo C., Rosafio G., Pantuso G., et al. Endothelial function in obese patients treated with bariatric surgery. Diabetes Metab Syndr Obes. 2020;13:247–256. doi: 10.2147/DMSO.S230684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero R., Kadar N., Vaisbuch E., Hassan S.S. Maternal death following cardiopulmonary collapse after delivery: amniotic fluid embolism or septic shock due to intrauterine infection? Am J Reprod Immunol. 2010;64:113–125. doi: 10.1111/j.1600-0897.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burwick R.M., Moyle K.A., Gupta M. Pregnancy-associated atypical hemolytic uremic syndrome: some answers [abstract] Am J Obstet Gynecol. 2019;220:S397–S398. [Google Scholar]
- 68.Coppo P., Wolf M., Veyradier A., Bussel A., Malot S., Millot G.A., et al. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br J Haematol. 2006;132:66–74. doi: 10.1111/j.1365-2141.2005.05837.x. [DOI] [PubMed] [Google Scholar]
- 69.Scully M., Rayment R., Clark A., Westwood J.P., Cranfield T., Gooding R., et al. A British Society for Haematology Guideline: diagnosis and management of thrombotic thrombocytopenic purpura and thrombotic microangiopathies. Br J Haematol. 2023;203:546–563. doi: 10.1111/bjh.19026. [DOI] [PubMed] [Google Scholar]
- 70.Kelly R.J., Höchsmann B., Szer J., Kulasekararaj A., de Guibert S., Röth A., et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2015;373:1032–1039. doi: 10.1056/NEJMoa1502950. [DOI] [PubMed] [Google Scholar]
- 71.Miyasaka N., Miura O., Kawaguchi T., Arima N., Morishita E., Usuki K., et al. Pregnancy outcomes of patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab: a Japanese experience and updated review. Int J Hematol. 2016;103:703–712. doi: 10.1007/s12185-016-1946-x. [DOI] [PubMed] [Google Scholar]
- 72.Sarno L., Tufano A., Maruotti G.M., Martinelli P., Balletta M.M., Russo D. Eculizumab in pregnancy: a narrative overview. J Nephrol. 2019;32:17–25. doi: 10.1007/s40620-018-0517-z. [DOI] [PubMed] [Google Scholar]
- 73.Kaufeld J.K., Kühne L., Schönermarck U., Bräsen J.H., von Kaisenberg C., Beck B.B., et al. Features of postpartum hemorrhage-associated thrombotic microangiopathy and role of short-term complement inhibition. Kidney Int Rep. 2024;9:919–928. doi: 10.1016/j.ekir.2024.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noris M., Caprioli J., Bresin E., Mossali C., Pianetti G., Gamba S., et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loirat C., Garnier A., Sellier-Leclerc A.L., Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36:673–681. doi: 10.1055/s-0030-1262890. [DOI] [PubMed] [Google Scholar]
- 76.Licht C., Greenbaum L.A., Muus P., Babu S., Bedrosian C.L., Cohen D.J., et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaidya A., Shastry S., Mohan G., Prethika P.A. Role of therapeutic plasma exchange in managing complement mediated thrombotic microangiopathy – case series. Transfus Clin Biol. 2022;29:84–88. doi: 10.1016/j.tracli.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Hancock A., Weeks A.D., Lavender D.T. Is accurate and reliable blood loss estimation the “crucial step” in early detection of postpartum haemorrhage: an integrative review of the literature. BMC Pregnancy Childbirth. 2015;15:230. doi: 10.1186/s12884-015-0653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]