Abstract
Study objectives
To evaluate the capability and accuracy of magnetocardiography (MCG) to identify patients with ischemic chest pain from those with non-ischemic pain and to verify normalcy in the MCG in healthy subjects.
Design
We studied 133 patients (mean age 59 ± 14 years, 69 % male) with chronic or acute chest pain syndrome and 63 healthy subjects (mean age 41.7 ± 12.2 years, 51 % male) using unshielded cryogenically cooled MCG systems (Cardiomag Imaging Inc., 9 and 36 channels) in a general clinical setting. Scan time was 90 s to 6 min. Interventions: The MCG data were processed with the same automated analysis software and results were immediately available. All patients were chest pain free at the time of scanning.
Results
A diagnosis of ischemic chest pain was established in 41 % after non-invasive and invasive testing. Rest MCG was normal in all healthy subjects. An abnormal rest MCG was strongly associated with ischemic chest pain, p < 0.0001 (sensitivity of 86 %, specificity of 80 %, positive (PPV) and negative predictive value (NPV) of 75 % and 89 %, respectively). In comparison, the sensitivity, specificity, PPV and NPV of stress SPECT was 93 %, 72 %, 77 % and 91 %, respectively.
Conclusion
Resting MCG is a rapid risk-free method for the detection of ischemic chest pain without the use of radiation or contrast with results comparable with stress SPECT.
Keywords: Ischemia, Coronary artery disease, Angina, Magnetocardiography
1. Introduction
For over four decades, there has been extensive research on the development and clinical utilization of multichannel magnetometers suitable for clinical magnetocardiography (MCG) in many cardiac diseases and disorders within several fields of cardiology [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. MCG detects the weak magnetic fields (pico-femto [10-12-10-15] Tesla, pT-fT, range) generated by the heart's electrical currents. MCG technology has evolved significantly over the last 6 decades from simple coils [10] to cryogenically cooled superconducting quantum interference devices (SQUID) [11], and more recently using optically pumped magnetometers (OPM) [12,13], as well as solid state and solid state atomic magnetometry technologies that are operated at room temperature and require far less maintenance than SQUID systems [14,15].
OPM systems have been studied relatively recently and rather extensively for applications in MCG. Although some OPM systems have intrinsic sensitivity on par with that of SQUIDs, head-to-head comparison of SQUID and OPM systems [12] have shown OPM systems (and their intrinsic sensitivities) to be more prone to external noise thus requiring advances in noise mitigation technology and the use of artificial intelligence and machine learning [16,17] to achieve the signal quality of SQUID systems, especially in unshielded environment. Development of unshielded portable and even wearable MCG systems are currently within reach [18]. Table 1 summarizes the comparison of the most common technologies used in MCG.
Table 1.
Comparison of technologies commonly used in MCG.
| Shielded | Unshielded | Room temp | Sensitivity Intrinsic x10−15 T |
Noise floor / Hz1/2 Unshielded x10−15T |
Gradiometer | Cost | Maint. | |
|---|---|---|---|---|---|---|---|---|
| SQUID | ✓ | ✓ | − | <10−3 | >102 | ✓ | ++++ | ++++ |
| OPM | ✓ | ✓ | ✓ | <10−1 | <103 | ✓ | ++++ | ++ |
| Fluxgate | ✓ | ✓ | ✓ | >103 | >104 | ✓ | ++/+++ | + |
| QFlux | ✓ | ? | ✓ | > 101 | − | ? | ? | ? |
| NVD | ✓ | ✓ | ✓ | <10−1 | <6 × 102 | ? | ++ | ++ |
| Coil | ✓ | ✓ | ✓ | >104 | >105 | ✓ | + | + |
Despite the commercial availability of several MCG devices, clinical use has been limited by operational practicalities including the need for expensive shielded rooms and uncertainties regarding the role of MCG in existing diagnostic pathways. Several small, mostly case-control studies, and studies performed in shielded rooms have been published. The patient population would often be highly selected and varying parameters have been utilized in interpreting the MCG maps [[19], [20], [21], [22], [23], [24], [25], [26]].
With the advent of machine learning and new sensor technologies operating at room temperature, several recent studies have shown promise for the detection of ischemia [[27], [28], [29]]. Early and accurate diagnosis of ischemic heart disease (IHD) remains challenging. Patients with a chief complaint of chest pain result in approximately 10.5 million annual US emergency department (ED) visits and ranks as one of the most common chief complaints [30]. Chest pain patients commonly have normal 12‑lead ECG and cardiac enzymes and further testing involving stress and/or radiation are needed [31,32].
In healthy subjects, the spatial features of the magnetic field maps will be similar at specific times during depolarization and repolarization, i.e. demonstrating a “stable pattern” [33,34]. With impaired coronary blood flow the magnetic field map demonstrates an “unstable pattern”, which can be quantified [1,2,3,19,20,35]. Animal studies demonstrate alterations in MCG produced by coronary occlusion [[36], [37], [38], [39]]. Reversible ST-T changes on the MCG occurs as early as 20 s after coronary occlusion in dogs and rabbits.
The reporting of this study is triggered by the current growing interest in clinical applications of magnetocardiography. The present study utilized an unshielded cryogenically cooled MCG system as an intermediate step between shielded cryogenically cooled systems and unshielded room temperature MCG systems currently under development. The data presented aim to report the utility of unshielded MCG in a clinical environment as well as reporting the data quality needed in upcoming designs [20,40,41]. SQUID magnetometry is one of the most sensitive and well-developed modalities in which issues of noise (external and internal, including radio frequency [RF] noise) have been addressed and treated to a great extent thus representing magnetometry “gold” standard in fT and sub-fT regime [[42], [43], [44], [45], [46]].
Our study aims to show that the predictive accuracy of MCG is comparable with that of myocardial perfusion scans in a standard clinical practice, but acquired contactless in a few minutes and without ionizing radiation or any other risk to the patients. This study also aims to show that unshielded MCG with clinically relevant signal quality is possible and probably the preferable choice for clinical application at scale in hospital environments [1].
Our study was completed more than a decade ago with our patients prospectively enrolled, but the data has not been published. With the renewed interest in MCG in clinical practice we have reviewed and analyzed our data and now presenting the complete study.
2. Methods
2.1. Study population
We studied 133 chest pain patients with or without known IHD. Of these, 99 had acute chest pain presentation and were studied after presentation to the ED at a large hospital in the US with the 9-channel MCG, and another 34 patients had stable angina and was studied at a large University Hospital in Italy, using the 36-channel device. In addition, we included 63 healthy non-smoking asymptomatic volunteers with no hypertension, diabetes, or heart disease to confirm the previously assessed normality range of MCG parameters [20]. Informed consent was obtained in accordance with the institutional review boards.
2.2. Inclusion criteria
Patients with chest pain undergoing clinical workup for ischemia.
2.3. Exclusion criteria
Hemodynamic instability, ST-elevation myocardial infarction (MI), 3° atrio-ventricular block, atrial flutter, left bundle branch block, and pacemakers or internal cardiac defibrillators (due to magnetic interference). Patients with sternal wires, mechanical heart valves, and stents were NOT excluded.
2.4. Procedures
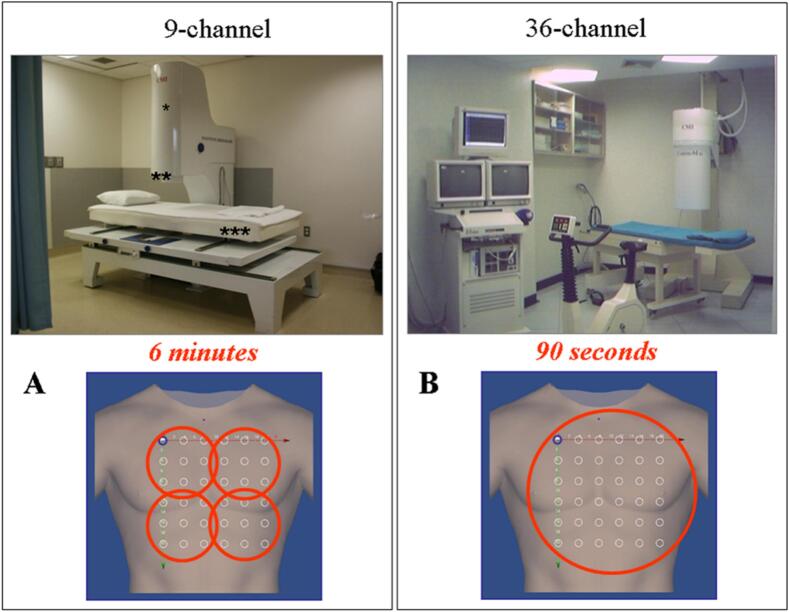
12‑lead ECG and blood pressure were obtained. MCG recordings were obtained with CardioMag Imaging, Inc., (CMI), 9-or 36-channel system (Fig. 1). These unshielded systems consist of SQUID systems (2nd order gradiometer, liquid helium cooled, sensitivity <30 fT/Hz1/2). The two systems were constructed with the same technology and sensor parameters/geometry so that their MCG recordings are equivalent. MCG data was recorded, from a 36-point (6 × 6) grid uniformly covering an area of 20 cm × 20 cm, sequentially at 4 pre-defined bed positions for 90 s at each position for a total imaging time of 6 min with the 9-channel device, and with a single shot of 90 s for the 36-channel device. The reproducibility of MCG mapping carried out with the two systems in the same patients has been demonstrated [47,48].
Fig. 1.
(A) 9-channel MCG. * Sensor tower containing the cryostat with nine SQUIDs; ** Gantry tower containing the cryostat and SQUID electronics; *** Couch for sequential mapping from four positions over the chest (also shown at the bottom). (B) 36-channel MCG. This system maps the same grid points with a single recording.
SQUID = super conducting quantum interference device.
Cardiovascular history and risk factors were recorded. Patients were classified as having ischemic chest pain as part of the standard of care when results of functional testing or coronary angiography were abnormal (elevated troponin I, perfusion defects on stress testing, or ≥ 70 % luminal diameter stenosis in major epicardial vessels).
2.5. Data interpretation
The methods used to analyze the ECG's and the MCG data have been previously described [20]. MCG data were processed and interpreted with a proprietary automated MCG program using Effective Magnetic Vector (EMV) analysis [49]. The magnitude and strength of motion of the EMV can be described by seven predefined parameters analyzing the trajectory, angle deviation, and frontal angle of the ascending and descending limbs of the T-wave (Table 2). If any of the seven parameters, which are weighted equally, is outside the normal range, the patient is classified as having repolarization indicative of ischemia.
Table 2.
MCG⁎ analysis parameters.
| Parameter | Unit | Abnormal value range |
|---|---|---|
| Pre-peak repolarization | ||
| MCG angle (frontal plane) | Degrees | ≥ − 15 or ≤ −110 |
| MCG trajectory | Centimeters | ≥ 7.5 |
| MCG angular deviation | Radians | ≥ 1.0 |
| Post-peak repolarization | ||
| MCG angle (frontal plane) | Degrees | ≥ − 22 or ≤ −100 |
| MCG trajectory | Centimeters | ≥ 5.0 |
| MCG angular deviation | Radians | ≥ 0.7 |
| Pre to post orientation change in MCG angle | Degrees | ≥ −12 or ≤ −35 |
Magnetocardiography.
2.6. Statistics
Continuous variables were presented as mean ± standard deviation. Categorical variables were assessed by Fisher's exact test. Continuous variables were assessed by Student's t-test or analysis of variance (ANOVA). In ANOVA models, homogeneity of variance was assessed by Levine's test. When there was evidence against homogeneity of variance, Welch's ANOVA was used to confirm the standard ANOVA results. Post hoc comparisons were made using Dunnett's t-tests. Stepwise logistic regression was used to determine variables associated with ischemia. Logistic regression (specifically the log likelihood statistic −2 Log L) was used to evaluate the incremental improvement of MCG over ECG in the prediction of ischemia. Differences in related proportions (sensitivity and specificity) were assessed using McNemar's test. A p-value of <0.05 was considered significant. All statistical tests were performed using the software package SAS version 9.1 (SAS Institute, Cary, NC).
3. Results
3.1. Patient demographics
We studied 133 patients (69 % male), average age 59.1 ± 13.7 years. Patients were angina-free during MCG scanning. Patient characteristics are shown in Table 3. Also studied were 63 healthy volunteers (mean age 41.7 ± 12.2 years, 51 % male).
Table 3.
Patient characteristics.
| (n = 133) | |
|---|---|
| Age, mean ± STD* (years) | 59.1 ± 13.7 |
| Male (%) | 69.2 |
| SBP† (mmHg) | 126.6 +/− 20.2 |
| DBP‡ (mmHg) | 73.3 +/− 11.7 |
| HR§ (bpm) | 67.6 +/− 11.8 |
| BMI║(kg/m2) | 28.4 +/− 5.7 |
| Atypical chest pain (%) | 39.1 |
| CCS# Class 1–2 angina (%) | 15.8 |
| CCS# Class 3 angina (%) | 7.6 |
| CCS# Class 4 angina (%) | 32.2 |
| NonQ MI** (%) | 8.5 |
| LV EF†† (%) | 58.2 +/− 10.8 |
| Hypertension (%) | 63.6 |
| Diabetes (%) | 25.2 |
| Smoking (%) | 38.5 |
| Hypercholesterolemia (%) | 72.7 |
| Family History (%) | 40.6 |
| Prior MI** (%) | 31.8 |
| Prior CABG‡‡ (%) | 10.7 |
| Prior PCI§§ (%) | 29.0 |
* = Standard Deviation; † = Systolic blood pressure; ‡ = Diastolic blood pressure; § = Heart rate; ║ = Body mass index; # = Canadian cardiovascular society classification; ** = myocardial infarction; †† = left ventricular ejection fraction; ‡‡ = coronary artery bypass graft surgery; §§ = percutaneous coronary intervention.
3.2. Clinical data
All healthy subjects and 97 patients (72.9 %) had normal 12‑lead ECG. Serial troponin I was normal in 90.0 % of patients. Coronary angiography was performed in half the patients (50.4 %, n = 67, 44 with acute chest pain and 23 with stable angina) and stress SPECT was completed in 86 (64.7 %, 67 with acute chest pain and 19 with stable angina). The vast majority (85.7 %, n = 114) had either stress SPECT or coronary angiography. FFR and iFR were utilized at the discretion of the interventional operators but was not mandated. Based on their clinical results, 41.4 % (n = 55) were determined to have ischemic chest pain. These patients were older, had more hypercholesterolemia, history of prior MI, percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) than the non-ischemic group (p < 0.0001, 0.01, <0.0001, 0.002, and 0.004, respectively).
3.3. MCG results
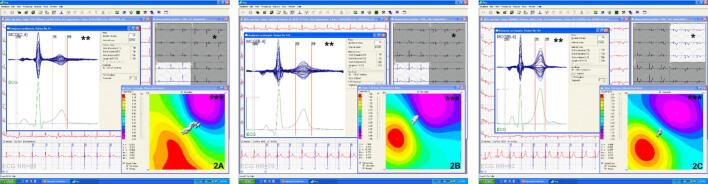
An abnormal MCG was found in 63 (47.4 %) patients. They were older (p = 0.004), had more hypertension (p = 0.004), hypercholesterolemia (p = 0.04), history of CABG (p = 0.02) and a lower left ventricular ejection fraction (55.3 % vs. 61.3 %, p = 0.003) than the group with normal MCG. By univariate analysis, an abnormal MCG, age, hypercholesterolemia, prior MI, CABG, and PCI were significantly associated with ischemic chest pain (p < 0.0001, p < 0.0001, 0.01, < 0.0001, 0.004, and 0.002, respectively). Stepwise logistic regression analysis demonstrated that an abnormal MCG had the strongest relationship with ischemic chest pain (OR 29.2, p < 0.0001), followed by age (p = 0.0008) and prior MI (OR 6.6, p = 0.001). All healthy volunteers had normal MCG. Figs. 2A-C show instantaneous pictures of MCG time traces and magnetic dipole field maps in patients with ischemic chest pain, non-ischemic pain, and a healthy volunteer.
Fig. 2.
A) Magnetic field map imaging of a 41 year old male with known IHD, new class IV angina and non-ischemic 12‑lead ECG. LVEF was 51 % with inferior wall motion abnormality. Coronary angiography of patient 2A demonstrated 2 vessel CAD. MCG obtained same day was abnormal (increased dynamic motion of the effective dipole vector as indicated by the vectors shown in white). B) 50 year old male with body mass index of 36.2 kg/m2, atypical chest pain and normal 12‑lead ECG. Stress nuclear scan showed 15 % reversible defect inferiorly. Cardiac catheterization revealed no CAD. MCG performed on the same day was normal. (Stable motion -no dispersion- of the effective dipole vector as indicated by the vectors shown in white). C) MCG of a 40 year old healthy female demonstrating a very stable dipole vector during repolarization. All images show the MCG time traces of individual channels (*), superposition of MCG traces of the same beat acquired on all channels; red vertical lines show the repolarization epoch between T3 and T4 (**), and the magnetic field topography maps (***). The white vectors demonstrate the magnetic dipole vector trajectory during ventricular repolarization.
The vectors (indicated by solid white arrow sequences, each representing a single timepoint within the T3 and T4 interval (repolarization epoch) in the ischemic patient (Fig. 2A) show dispersion in repolarization which is also apparent in the lack of clear dipolar distribution in magnetic field map (there is no clear circular red pole); the non-ischemic patient (Fig. 2B) and the normal healthy subject (Fig. 2C) show no dispersion and clear dipolar distribution in magnetic field map. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Diagnostic value of MCG
An abnormal MCG had a sensitivity of 86 %, specificity of 80 %, positive predictive value (PPV) of 75 %, and a negative predictive value (NPV) of 89 % for the detection of ischemic pain (Fig. 3). Assuming that the healthy subjects have no significant CAD (and therefore are non-ischemic), including these subjects increase the specificity and NPV to 89 % and 94 %, respectively. The MCG correctly classified the patients in 82 % (88 % when including controls) compared with 83 % for stress SPECT. In patients with acute chest pain the MCG sensitivity was 89 % and NPV was 92 % (89 % and 90 % for stress SPECT, respectively).
Fig. 3.
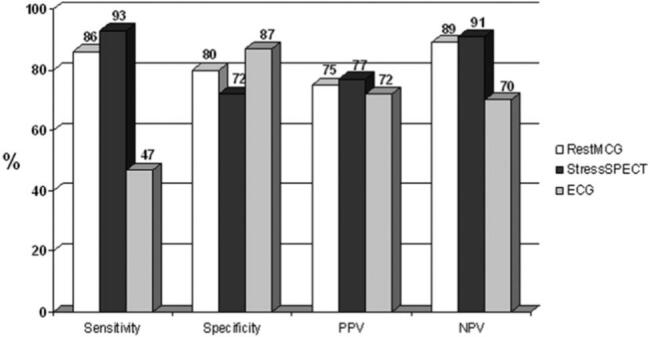
Diagnostic value of rest MCG (white bars), stress SPECT (black bars) and 12‑lead ECG (grey bars) for the diagnosis of ischemia.
MCG = Magnetocardiography; SPECT = single photon emission tomography; ECG = Electrocardiography; PPV = Positive Predictive Value; NPV = Negative Predictive Value.
The MCG sensitivity, specificity, PPV and NPV for detecting obstructive CAD in acute chest pain patients were 92 %, 83 %, 89 %, and 88 %, respectively. In 55 patients with known IHD the sensitivity was 86 %, specificity 90 %, PPV 94 %, and NPV 77 %, compared to 90 %, 50 %, 90 %, and 50 %, respectively for stress SPECT. A correct classification by MCG was seen in 87 % compared with 83 % for stress SPECT. In subjects without known history of IHD, a normal MCG had a very high predictive value for excluding ischemia (NPV = 97 %). In subjects with non-ischemic 12‑lead ECG and negative troponin I, the NPV was 95 %. In patients younger than 55 years, the sensitivity of MCG was 92 %, specificity 80 %, and NPV 97 % (99 % if including the younger healthy controls). MCG had 100 % sensitivity and NPV in low to intermediate-risk patients (compared with 31 % and 80 % for 12‑lead ECG). There was a significant incremental value to MCG imaging over ECG, while there was no added value of ECG over MCG.
4. Discussion
This study shows that MCG can be used to diagnose ischemic chest pain (association p < 0.0001) with high diagnostic accuracy (correct classification in 82 % to 88 %). The results of rest MCG were similar to stress SPECT for sensitivity and specificity. The sensitivity of MCG increased when evaluating acute chest pain patients (89 % to 92 %). MCG was accurate in excluding ischemia: A NPV of 89 % in all subjects with chest pain increased to 95 % to 100 % when evaluating subjects without known history of IHD, those with normal ECG and troponin, as well as younger patients, and those with low to intermediate risk.
Exposure to ionizing radiation is contraindicated for children and women of child-bearing age and should be minimized, especially in younger patients. The corresponding extra-risk in a lifetime of fatal cancer is 1 in 2000 exposed patients for a sestamibi stress and 1 in 1000 for a thallium scan. Lifetime cancer risk estimate for CT coronary angiography is 1 in 143 for a 20-year old woman [[50], [51], [52], [53]].
A meta-analysis study by Agarwal et al. showed that “the pooled test characteristics for MCG -sensitivity 83%, specificity 77%- are approaching the most sensitive existing noninvasive modalities for diagnosing CAD” [54]. This study also showed significant heterogeneity in sensitivity and specificity “unrelated to study level covariates of clinical presentation (stable CAD vs. acute coronary syndrome), setting where test was performed (shielded vs. unshielded) and study quality (high versus low)”. All MCG studies in this meta-analysis utilized SQUID-based systems. Similar detailed pooled analysis with the inclusion of additional parameters and instrumentation that utilize various modalities will be helpful in understanding and stratifying the observed heterogeneities in MCG studies [2,3].
Two studies of 101 and 83 patients demonstrated MCG sensitivity and specificity of 75 % to 84 % for the detection of CAD and non-Q MI [22,23]. Both these studies (using shielded SQUID systems) were performed in magnetically shielded rooms and did not have pre-defined MCG analysis endpoints. Park et al studied 264 high-risk acute chest pain patients using unshielded (SQUID) MCG [24]. Only 75 % had an acceptable signal-to-noise ratio. The study by this group also found a high PPV of the MCG for the detection of CAD utilizing a subjective visual analysis, while an automated analysis program that differs from ours was much less accurate. Li et al studied 101 patients with known CAD and 116 healthy volunteers using a 7-channel unshielded (SQUID) MCG [25]. They looked at 3 parameters, the Rmax, Ratio of Rmax to Tmax, and the average angle during the ST segment. They excluded patients with left ventricular dysfunction, left ventricular hypertrophy, electrolyte and acid-base disturbances, as well as those with arrhythmia or bundle branch block or atrial fibrillation. While the authors concluded that MCG performed better than 12 lead ECG and resting echo, only 74 % of the patients with CAD had an abnormal MCG.
The heterogeneity of MCG findings has been reported in studies using various modalities as well. Recently, Mace et al. published data from a multicenter trial, enrolling 390 intermediate risk chest pain patients (Heart Score ≥ 3) presenting to the emergency room utilizing a room temperature MCG, CardioFLux [29]. A total of 89 patients (23 %) had uninterpretable MCGs due to noise/metallic interference. Using standard clinical practice and testing as the endpoint for ischemia, 42/301 (14 %) of patients were diagnosed with ischemia. They found similar sensitivity of MCG as non-invasive testing (66.7 % for both) but specificity for MCG was lower at 57.1 % compared with 89.9 %. Time to completion of the clinical workup was significantly different between MCG and standard testing: 3.18 h versus 22.71 h. In addition, a very recent preliminary multicenter study, carried out with the same CardioFlux OPM-system, has tested the ability of MCG to detect coronary microvascular dysfunction (CMD) in patients with angina and non-obstructive coronary artery disease, using invasive coronary flow reserve detection as a diagnostic standard. The results suggest that 90-s of shielded contactless OPM-MCG scan can detect CMD (with 68 % sensitivity and 65 % specificity), without the need for ionizing radiation or invasive coronary procedures [55]. On the contrary, another clinical study using a portable room temperature unshielded MCG device (VitalScan MCG) reported unsatisfactory results, failing to rule out acute coronary syndrome (ACS) in a cohort suspected of ACS [56].
The sensitivity of coil- and most flux-gate-based sensors are in pT/Hz1/2 regime; MCG requires sensor sensitivity and resolution in the low fT/Hz1/2 regime for diagnosis and to extract cardiac signal features needed for many cardiac abnormalities [57,58]. As mentioned, OPM systems have sensor sensitivity in the femto-T and atto-T region [59], however, susceptibility to external and instrumentation noise has been a limiting factor in the development of OPM-based MCG systems for unshielded clinical recording [1,12]. However, recent advances in gradiometer-free and optical gradiometry noise suppression techniques [18,60,61], as well as implementation of machine learning and artificial intelligence routines [16,62] hold promise to address some of the confounds associated with noise.
There are several possible explanations for the high sensitivity of the resting magnetic field map measurements, making MCG a useful tool for the detection of IHD without the need for stress provocation: MCG is most sensitive to tangential currents and ischemia and obstructive CAD which interfere with the normal activation and deactivation sequence increase the contribution of tangential currents. In addition, MCG detects vortex currents that may arise at the border zones between normal and abnormal myocardium [63]. MCG is also much less sensitive to tissue conductivities and is free of confounds associated with skin-electrode contact impedance [23,58]. Thus, changes in MCG may appear earlier in the cascade of ischemia than wall motion abnormalities, ECG changes, troponin elevations, or changes in differential blood flow as detected by SPECT causing the MCG to be abnormal when the patients are presumed non-ischemic by current standard methods.
Our study has some limitations: We only analyzed the T-wave. The accuracy of MCG may improve by evaluating other parts of the cardiac cycle [[21], [22], [23]]. However, this was not incorporated into our software program, and we avoided any subjective classification of the MCG to prevent bias. MCG may be abnormal in some subjects with hypertensive heart disease, as well as in other conditions such as mitral valve prolapse, non-ischemic dilated cardiomyopathy, and inflammatory cardiomyopathy [7,41,64,65]. We did see a decrease in specificity in subjects with a history of hypertension, which was also observed with stress SPECT (MCG specificity 66 %, SPECT specificity 67 %). Detection of ischemia as well as its exclusion in this population requires further studies. Furthermore, we did not study patients using high sensitivity troponin or PET perfusion scans. We also did not attempt localization of the ischemic areas because that was not an endpoint of the present study protocol. However, MCG has high spatial accuracy in localizing cardiac sources [66], which has provided bi-dimensional imaging of ischemic areas, well in agreement with that of PET imaging [67].
In conclusion, rest MCG detects repolarization abnormalities consistent with ischemia and excludes the presence of ischemia in non-cardiac patients and volunteers with results available immediately. The findings of this study indicate that MCG is at least as accurate as SPECT. Thus, early use of MCG in the triage of chest pain patients after the first normal 12‑lead ECG and troponin I is particularly promising. Furthermore, as there is no radiation exposure with MCG it is an attractive alternative to stress SPECT and CT coronary angiography. It will be important for further development and inclusion of this technology in mainstream cardiology to achieve data quality accuracies with room temperature devices similar to that of SQUID-based systems.
Our results suggest that MCG can potentially be used as a complementary tool to existing diagnostic algorithms for diagnosis of CAD in patients presenting with chest pain with normal or nonspecific ECG. The very high negative predictive value with a normal MCG (Fig. 3) would result in early discharge, decreased length of stay, and avoidance of further testing. On the other hand, a positive MCG increases the probability of CAD, a conclusion that will be reached faster and with less risk than with any current methodology. To improve the specificity of the MCG more data and utilization of AI and machine learning will be needed.
Ethical statement
The authors certify that have abided by all ethical standards outlined in: https://www.elsevier.com/about/policies-and-standards/publishing-ethics#4-duties-of-authors.
CRediT authorship contribution statement
Kirsten Tolstrup: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Massoud Akhtari: Writing – review & editing, Writing – original draft, Investigation. Donatella Brisinda: Writing – review & editing, Methodology. Anna M. Meloni: Writing – review & editing, Methodology. Robert J. Siegel: Writing – review & editing, Supervision, Resources, Methodology, Investigation. Riccardo Fenici: Writing – review & editing, Supervision, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
CardioMag Imaging (CMI) provided partial salary support for research nurse at Cedars-Sinai Medical Center.
References
- 1.Brisinda D., Fenici P., Fenici R. Clinical magnetocardiography: the unshielded bet-past, present, and future. Front Cardiovasc Med. 2023 Aug;10(10) doi: 10.3389/fcvm.2023.1232882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Her A.Y., Dischl D., Kim Y.H., Kim S.W., Shin E.S. Magnetocardiography for the detection of myocardial ischemia. Front Cardiovasc Med. 2023 Jul;7(10) doi: 10.3389/fcvm.2023.1242215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camm A.J., Henderson R., Brisinda D., Body R., Charles R.G., Varcoe B., Fenici R. Clinical utility of magnetocardiography in cardiology for the detection of myocardial ischemia. J. Electrocardiol. 2019 Nov-Dec;57:10–17. doi: 10.1016/j.jelectrocard.2019.07.009. (Epub 2019 Jul 25) [DOI] [PubMed] [Google Scholar]
- 4.Strand S.A., Strasburger J.F., Wakai R.T. Fetal magnetocardiogram waveform characteristics. Physiol. Meas. 2019 Mar 22;40(3) doi: 10.1088/1361-6579/ab0a2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wikswo J., Fairbank W. Application of superconducting magnetometers to the measurement of the vector magnetocardiogram. IEEE Trans. Magn. 1977;13(1):354–357. doi: 10.1109/TMAG.1977.1059333. [DOI] [Google Scholar]
- 6.Donofrio M.T., Moon-Grady A.J., Hornberger L.K., Copel J.A., Sklansky M.S., Abuhamad A., Cuneo B.F., Huhta J.C., Jonas R.A., Krishnan A., Lacey S., Lee W., Michelfelder E.C., Sr., Rempel G.R., Silverman N.H., Spray T.L., Strasburger J.F., Tworetzky W., Rychik J. American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the Young and Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular and Stroke Nursing. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014 doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 7.Yang S., Yang K., Zhang L., Ren Y., Liu L., Zhang H. Case report: optical pumped magnetometer magnetocardiography as a potential method of therapy monitoring in fulminant myocarditis. CVIA. 2024;9(1) doi: 10.15212/CVIA.2024.0031. [DOI] [Google Scholar]
- 8.Tamaki W., Tsuda E., Hashimoto S., Toyomasa T., Fujieda M. Magnetocardiographic recognition of abnormal depolarization and repolarization in patients with coronary artery lesions caused by Kawasaki disease. Heart Vessel. 2019 Oct;34(10):1571–1579. doi: 10.1007/s00380-019-01383-4. Epub 2019 Mar 25. Erratum in: Heart Vessels. 2019 Oct;34(10):1580. doi: 10.1007/s00380-019-01409-x. [DOI] [PubMed] [Google Scholar]
- 9.Brala D., Thevathasan T., Grahl S., Barrow S., Violano M., Bergs H., Golpour A., Suwalski P., Poller W., Skurk C., Landmesser U., Heidecker B. Application of magnetocardiography to screen for inflammatory cardiomyopathy and monitor treatment response. J. Am. Heart Assoc. 2023 Feb 21;12(4) doi: 10.1161/JAHA.122.027619. (Epub 2023 Feb 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baule G.M., McFee R. Detection of the magnetic field of the heart. Am. Heart J. 1963 Jul;66:95–96. doi: 10.1016/0002-8703(63)90075-9. [DOI] [PubMed] [Google Scholar]
- 11.Cohen D., Edelsack E.A., Zimmerman J.E. Magnetocardiograms taken inside a shielded room with a superconducting point-contact magnetometer. Appl. Phys. Lett. 1970;16:278–280. doi: 10.1063/1.1653195. [DOI] [Google Scholar]
- 12.Fenici Riccardo, Bison Georg, Wynands Robert, Brisinda Donatella, Meloni A.M., Weis Antoine. 2004. Comparison of Magnetocardiographic Mapping with SQUID-based and Laser-pumped Magnetometers in Normal Subjects. Development of an Optical Cardio-magnetometer. Dissertation hdl.handle.net/10807/17620. [Google Scholar]
- 13.Shah V.K., Wakai R.T. A compact, high performance atomic magnetometer for biomedical applications. Phys. Med. Biol. 2013 Nov 21;58(22):8153–8161. doi: 10.1088/0031-9155/58/22/8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai K., Kuwahata A., Nishitani D., et al. Millimetre-scale magnetocardiography of living rats with thoracotomy. Commun. Phys. 2022;5:200. doi: 10.1038/s42005-022-00978-0. [DOI] [Google Scholar]
- 15.Bunkov Y.M., Kuzmichev A.N., Safin T.R., Vetoshko P.M., Belotelov V.I., Tagirov M.S. Quantum paradigm of the foldover magnetic resonance. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-87196-w. Article number: 7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao R., Zhang S., Huang X., Tao M., Ma J., Ma S., Zhang C., Zhang T., Tang F., Lu J., Shen C., Xie X. Magnetocardiography-based ischemic heart disease detection and localization using machine learning methods. IEEE Trans. Biomed. Eng. 2019 Jun;66(6):1658–1667. doi: 10.1109/TBME.2018.2877649. [DOI] [PubMed] [Google Scholar]
- 17.Senthilnathan S., Shenbaga Devi S., Sasikala M., Satheesh S., Selvaraj R.J. The role of beat-by-beat cardiac features in machine learning classification of ischemic heart disease (IHD) in magnetocardiogram (MCG) Biomed Phys Eng Express. 2024 May 7;10(4) doi: 10.1088/2057-1976/ad40b1. [DOI] [PubMed] [Google Scholar]
- 18.Xiao W., Sun C., Shen L., Feng Y., Liu M., Wu Y., Liu X., Wu T., Peng X., Guo H. A movable unshielded magnetocardiography system. Sci. Adv. 2023 Mar 29;9(13) doi: 10.1126/sciadv.adg1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenici R., Brisinda D., Venuti A., Sorbo A.R. Thirty years of clinical magnetocardiography at the Catholic University of Rome: diagnostic value and new perspectives for the treatment of cardiac arrhythmias. Int. J. Cardiol. 2013 Oct 12;168(5):5113–5115. doi: 10.1016/j.ijcard.2013.07.238. [DOI] [PubMed] [Google Scholar]
- 20.Tolstrup K., Madsen B.E., Ruiz J.A., Greenwood S.D., Camacho J., Siegel R.J., Gertzen H.C., Park J.W., Smars P.A. Non-invasive resting magnetocardiographic imaging for the rapid detection of ischemia in subjects presenting with chest pain. Cardiology. 2006;106(4):270–276. doi: 10.1159/000093490. [DOI] [PubMed] [Google Scholar]
- 21.On K., Watanabe S., Yamada S., Takeyasu N., Nakagawa Y., Nishina H., Morimoto T., Aihara H., Kimura T., Sato Y., Tsukada K., Kandori A., Miyashita T., Ogata K., Suzuki D., Yamaguchi I., Aonuma K. Integral value of JT interval in magnetocardiography is sensitive to coronary stenosis and improves soon after coronary revascularization. Circ. J. 2007 Oct;71(10):1586–1592. doi: 10.1253/circj.71.1586. [DOI] [PubMed] [Google Scholar]
- 22.Lim H.K., Chung N., Kim K., Ko Y.G., Kwon H., Lee Y.H., Kim J.M., Joung B., Kim J.B., Yu K.K., Cho J.R., Kim I.S., Park Y.K. Can magnetocardiography detect patients with non-ST-segment elevation myocardial infarction? Ann. Med. 2007;39(8):617–627. doi: 10.1080/07853890701538040. [DOI] [PubMed] [Google Scholar]
- 23.Gapelyuk A., Wessel N., Fischer R., Zacharzowsky U., Koch L., Selbig D., Schütt H., Sawitzki B., Luft F.C., Dietz R., Schirdewan A. Detection of patients with coronary artery disease using cardiac magnetic field mapping at rest. J. Electrocardiol. 2007 Sep-Oct;40(5):401–407. doi: 10.1016/j.jelectrocard.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Park J.W., Hill P.M., Chung N., Hugenholtz P.G., Jung F. Magnetocardiography predicts coronary artery disease in patients with acute chest pain. Ann. Noninvasive Electrocardiol. 2005 Jul;10(3):312–323. doi: 10.1111/j.1542-474X.2005.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Che Z., Quan W., Yuan R., Shen Y., Liu Z., Wang W., Jin H., Lu G. Diagnostic outcomes of magnetocardiography in patients with coronary artery disease. Int. J. Clin. Exp. Med. 2015;8(2):2441–2446. Feb 15. [PMC free article] [PubMed] [Google Scholar]
- 26.Chaikovsky I., Hailer B., Sosnytskyy V., Lutay M., Mjasnikov G., Kazmirchuk A., Bydnyk M., Lomakovskyy A., Sosnytskaja T. Predictive value of the complex magnetocardiographic index in patients with intermediate pretest probability of chronic coronary artery disease: results of a two-center study. Coron. Artery Dis. 2014 Sep;25(6):474–484. doi: 10.1097/MCA.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi-Roudsari S., Al-Shimary A., Varcoe B., Byrom R., Kearney L., Kearney M. A portable prototype magnetometer to differentiate ischemic and non-ischemic heart disease in patients with chest pain. PLoS One. 2018 Jan 19;13(1) doi: 10.1371/journal.pone.0191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beadle R., McDonnell D., Ghasemi-Roudsari S., Unitt L., Parker S.J., Varcoe B.T.H. Assessing heart disease using a novel magnetocardiography device. Biomed Phys Eng Express. 2021 Feb 23;7(2) doi: 10.1088/2057-1976/abe5c5. [DOI] [PubMed] [Google Scholar]
- 29.Mace S.E., Peacock W.F., Stopyra J., Mahler S.A., Pearson C., Pena M., Clark C. Accelerated magnetocardiography in the evaluation of patients with suspected cardiac ischemia: the MAGNETO trial. Am Heart J Plus. 2024 Feb;29(40) doi: 10.1016/j.ahjo.2024.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Hospital Ambulatory Medical Care Survey: 2021 Emergency Department Summary Tables. 2021. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf Published online.
- 31.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., Blankstein R., Boyd J., Bullock-Palmer R.P., Conejo T., Diercks D.B., Gentile F., Greenwood J.P., Hess E.P., Hollenberg S.M., Jaber W.A., Jneid H., Joglar J.A., Morrow D.A., O’Connor R.E., Ross M.A., Shaw L.J. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 doi: 10.1161/CIR.0000000000001029. Epub 2021 Oct 28. Erratum in: Circulation. 2021 Nov 30;144(22):e455. doi: 10.1161/CIR.0000000000001047. Erratum in: Circulation. 2023 Dec 12;148(24):e281. doi: 10.1161/CIR.0000000000001198. PMID: 34709879. [DOI] [PubMed] [Google Scholar]
- 32.Virani S.S., Newby L.K., Arnold S.V., Bittner V., Brewer L.C., Demeter S.H., Dixon D.L., Fearon W.F., Hess B., Johnson H.M., Kazi D.S., Kolte D., Kumbhani D.J., LoFaso J., Mahtta D., Mark D.B., Minissian M., Navar A.M., Patel A.R., Piano M.R., Rodriguez F., Talbot A.W., Taqueti V.R., Thomas R.J., van Diepen S., Wiggins B., Williams M.S., Peer Review Committee Members 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023 Aug 29;148(9):e9–e119. doi: 10.1161/CIR.0000000000001168. Epub 2023 Jul 20. Erratum in: Circulation. 2023 Sep 26;148(13):e148. doi: 10.1161/CIR.0000000000001183. Erratum in: Circulation. 2023 Dec 5;148(23):e186. doi: 10.1161/CIR.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 33.Takala P., Hänninen H., Montone J., Mäkijärvi M., Nenonen J., Oikarinen L., Simeliu K., Toivonen L., Katil T. Magnetocardiographic and electrocardiographic exercise mapping in healthy subjects. Ann. Biomed. Eng. 2001 Jun;29(6):501–509. doi: 10.1114/1.1376388. [DOI] [PubMed] [Google Scholar]
- 34.Brisinda D., Meloni A.M., Fenici P., Fenici R. Unshielded multichannel magnetocardiographic study of ventricular repolarization in healthy subjects. Biomed. Tech. 2004;48:165–167. [Google Scholar]
- 35.Shin E.S., Park J.W., Lim D.S. Magnetocardiography for the diagnosis of non-obstructive coronary artery disease. Clin. Hemorheol. Microcirc. 2018;69:9. doi: 10.3233/CH-189106. [DOI] [PubMed] [Google Scholar]
- 36.Cohen D., Kaufman L.A. Magnetic determination of the relationship between the S-T segment shift and the injury current produced by coronary artery occlusion. Circ.Res. 1975;36:414–424. doi: 10.1161/01.res.36.3.414. [DOI] [PubMed] [Google Scholar]
- 37.Fischer R., Gapelyuk A., Wessel N., Gruner K., Gruner A., Müller D., Dietz R., Schirdewan A. Time course of changes in cardiac magnetic fields mapping after myocardial infarction in rats. Int. Congr. Ser. 2007;1300:484–487. [Google Scholar]
- 38.Brazdeikis A., Chu C.W., Cherukuri P., Litovsky S., Naghavi M. Changes in magnetocardiogram patterns of infarcted-reperfused myocardium after injection of superparamagnetic contrast media. Neurol.Clin.Neurophysiol. 2004;Nov30:16. [PubMed] [Google Scholar]
- 39.Monteiro E.C., Penna S.D., Donato L.D., Luzio S.D., Pasquarelli A., Erné S.N., Romani G.L. The study of steady magnetic fields associated with primary and secondary ST shift in ischaemic rabbit hearts. Physiol. Meas. 1997;18:191–200. doi: 10.1088/0967-3334/18/3/004. [DOI] [PubMed] [Google Scholar]
- 40.Fenici R., Brisinda D., Meloni A.M., Fenici P. First 36-channel system for clinical magnetocardiography in unshielded hospital laboratory for cardiac electrophysiology. International Journal of Bioelectromagnetism. 2003;5(1):80–83. http://ee.tut.fi/rgi/ijbem/volume5/number1/032.htm [Google Scholar]
- 41.Brisinda D., Meloni A.M., Fenici. First 36-channel magnetocardiographic study of CAD patients in an unshielded laboratory for interventional and intensive cardiac care. LNCS. 2023;2674:122–131. doi: 10.1007/3-540-44883-7_13. [DOI] [Google Scholar]
- 42.Sun L., Hämäläinen M.S., Okada Y. Noise cancellation for a whole-head magnetometer-based MEG system in hospital environment. Biomed Phys Eng Express. 2018;4(5) doi: 10.1088/2057-1976/aad627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chwala A., Kingman J., Stolz R., Schmelz M., Zakosarenko V., Linzen S., Bauer F., Starkloff M., Meyer M., Meyer H.-G. Noise characterization of highly sensitive SQUID magnetometer systems in unshielded environments. Supercond. Sci. Technol. 2013;26 doi: 10.1088/0953-2048/26/3/035017. [DOI] [Google Scholar]
- 44.Kesavaraja C., Sengottuvel S., Patel Rajesh, Khan Pathan Fayaz, Swain Pragyna Parimita, Gireesan K. Measurement, processing and analysis of stressMagnetocardiograms. J. Phys. Conf. Ser. 2021;1921 doi: 10.1088/1742-6596/1921/1/012102. 012102IOP Publishing. [DOI] [Google Scholar]
- 45.Moser J., Bensaid S., Kroupi E., Schleger F., Wendling F., Ruffini G., Preißl H. Evaluating complexity of fetal MEG signals: a comparison of different metrics and their applicability. Front. Syst. Neurosci. 2019;27(13):23. doi: 10.3389/fnsys.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.H Storm J.-H., Hömmen P., Höfner N., Körber R. Detection of body noise with an ultra-sensitive SQUID system. Meas. Sci. Technol. 2019;30 doi: 10.1088/1361-6501/ab3505. [DOI] [Google Scholar]
- 47.Brisinda D., Meloni A.M., Fenici R. Clinical multichannel MCG in the unshielded hospital environment. Neurol. Clin. Neurophysiol. 2004 Nov;30(2004):8. (PMID: 16015715) [PubMed] [Google Scholar]
- 48.Fenici R.R., Brisinda D., Nenonen J., Morana G., Fenici P. First MCG multichannel instrumentation operating in an unshielded hospital laboratory for multimodal cardiac electrophysiology. Preliminary experience. Biomed. Tech. 2001;46(2):219–222. doi: 10.1515/bmte.2001.46.s2.219. [DOI] [Google Scholar]
- 49.Bakharev Alexander A. 2001. PCT Application Based on U.S. Prov. Appl. No.: 60/228,640. Title: Ischemia Identification, Quantification and Partial Localization in MCG. [Google Scholar]
- 50.Picano E. Economic and biological costs of cardiac imaging. Cardiovasc. Ultrasound. 2005;25(3):13. doi: 10.1186/1476-7120-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 52.Einstein A.J., Moser K.W., Thompson R.C., Cerqueria M.D., Henzlova M.J. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 53.Brenner D.J., Hall E.J. Computed tomography--an increasing source of radiation exposure. N. Engl. J. Med. 2007;29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal R., Saini A., Alyousef T., Umscheid C.A. Magnetocardiography for the diagnosis of coronary artery disease: a systematic review and meta-analysis. Ann. Noninvasive Electrocardiol. 2012;17(4):291–298. doi: 10.1111/j.1542-474X.2012.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashokprabhu N., Ziada K., Daher E., Cho L., Schmidt C.W., Roca Y., Palmer C., Kaur S., Henry T.D., Pepine C.J., Quesada O. Evaluation of coronary microvascular dysfunction using magnetocardiography: a new application to an old technology. American Heart Journal Plus: Cardiology Research and Practice. 2024;44 doi: 10.1016/j.ahjo.2024.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodacre S., Walters S.J., Qayyum H., Coffey F., Carlton E., Coats T., Glazebrook W., Unitt L. Diagnostic accuracy of the magnetocardiograph for patients with suspected acute coronary syndrome. Emerg. Med. J. 2021;38(1):47–52. doi: 10.1136/emermed-2020-210396. [DOI] [PubMed] [Google Scholar]
- 57.Hänninen H., Takala P., Korhonen P., Oikarinen L., Mäkijärvi M., Nenonen J., Katila T., Toivonen L. Features of ST segment and T-wave in exercise-induced myocardial ischemia evaluated with multichannel magnetocardiography. Ann. Med. 2002;34(2):120–129. doi: 10.1080/07853890252953518. [DOI] [PubMed] [Google Scholar]
- 58.Hänninen H., Holmström M., Vesterinen P., Karvonen M., Väänänen H., Oikarinen L., Mäkijärvi M., Nenonen J., Lauerma K., Katila T., Toivonen L. (2006) Magnetocardiographic assessment of healed myocardial infarction. Ann. Noninvasive Electrocardiol. 2006 Jul;11(3):211–221. doi: 10.1111/j.1542-474X.2006.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savukov I., Kim Y.J., Shah V., Boshier M.G. High-sensitivity operation of single-beam optically pumped magnetometer in a kHz frequency range. Meas. Sci. Technol. 2017;28 doi: 10.1088/1361-6501/aa58b4. [DOI] [Google Scholar]
- 60.Cooper R.J., Prescott D.W., Lee G.J., Sauer K.L. RF atomic magnetometer array with over 40 dB interference suppression using electron spin resonance. J. Magn. Reson. 2018;296:36–46. doi: 10.1016/j.jmr.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 61.Gerginov V., Pomponio M., Knappe S. Scalar magnetometry below 100 fT/Hz1/2 in a microfabricated cell. IEEE Sensors J. 2020;PP(99):1-1 doi: 10.1109/JSEN.2020.3002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohsen A., Al-Mahdawi M., Fouda M.M., Oogane M., Ando Y., Fadlullah Z.M. ICC, IEEE International Conference on Communications (ICC), Dublin, Ireland. Vol. 2020. 2020. AI aided noise processing of spintronic based IoT sensor for Magnetocardiography application; pp. 1–6. [DOI] [Google Scholar]
- 63.Dutz S., Bellemann M., Leder U., Haueisen L. Passive vortex currents in magneto- and electrocardiography: comparison of magnetic and electric signal strengths Phys. La Medicina Biologica. 2006;51:145. doi: 10.1088/0031-9155/51/1/011. [DOI] [PubMed] [Google Scholar]
- 64.Comani S., Gallina S., Lagatta A., Orlandi M., Morana G., DeLuzio S., Brisinda D., DeCaterina R., Fenici R., Romani G.L. Concentric remodeling detection by magnetocardiography in patients with recent onset arterial hypertension. PACE. 2004;27(6 Pt 1):709–718. doi: 10.1111/j.1540-8159.2004.00518.x. [DOI] [PubMed] [Google Scholar]
- 65.Brisinda D., Meloni A.M., Fenici P., Fenici R. Unshielded multichannel magnetocardiographic study of ventricular repolarization abnormalities in patients with mitral valve prolapse. Biomed. Tech. 2004;48(2):128–130. [Google Scholar]
- 66.Kawakami S., Takaki H., Hashimoto S., Kimura Y., Nakashima T., Aiba T., Kusano K.F., Kamakura S., Yasuda S., Sugimachi M. Utility of high-resolution magnetocardiography to predict later cardiac events in nonischemic cardiomyopathy patients with normal QRS duration. Circ. J. 2016 Dec 22;81(1):44–51. doi: 10.1253/circj.CJ-16-0683. [DOI] [PubMed] [Google Scholar]
- 67.Suwalski P., Golpour A., Musigk N., Wilke F., Landmesser U., Heidecker B. Case report: recurrence of inflammatory cardiomyopathy detected by magnetocardiography. Front Cardiovasc Med. 2023;10(September):1–7. doi: 10.3389/fcvm.2023.1225057. [DOI] [PMC free article] [PubMed] [Google Scholar]