Visual Abstract
Abstract
End-of-life (EOL) care is a critical part of sickle cell disease (SCD) management. However, barriers to high-quality EOL care remain, including (1) disease-related barriers (prior opioid exposure, risk of vaso-occlusive crises, chronic conditions with conflicting needs, and limitations of receiving disease-directed therapy on hospice); (2) communication-related barriers (challenges of identifying and responding to religious and spiritual concerns, limited health literacy, and previous health care system experience); (3) systemic issues (social determinants of health, structural racism, and mistrust of the medical system). However, palliative care and interdisciplinary collaboration can overcome many of these barriers. In addition, we can improve EOL care by accounting for opioid exposures, multimodal symptom management, and exploring (1) who people want involved in decision-making, (2) the role of religion and spirituality in decision-making, and (3) previous experiences with EOL. Systemic barriers can be addressed through the social determinants of health screening, minimizing financial burdens of care, and building longitudinal relationships with people with SCD. This requires the continued education of SCD providers about primary palliative care and palliative care providers about SCD. With such strategies, high-quality EOL care is possible for this vulnerable population.
Learning Objectives
Describe disease-related, communication-related, and systemic barriers to high-quality end-of-life care for people with sickle cell disease.
Identify strategies to providing high-quality end-of-life care for people with sickle cell disease.
Explore communication strategies to elicit decision-making and end-of-life preferences.
CLINICAL CASE
Mr. Jones was a 37-year-old with sickle cell disease (SCD) complicated by end-stage renal disease (ESRD), was on hemodialysis, and was hospitalized for community-acquired pneumonia with concern for acute chest syndrome (ACS). Given his oxygen requirement, an exchange transfusion was ordered. The team discontinued home methadone and started patient-controlled analgesia, though at doses appropriate for an opiate-naive patient, leading to inadequate pain control. Exchange transfusion was delayed due to difficulty finding compatible blood; he was intubated and transferred to the intensive care unit (ICU). After several days, he was unable to be weaned off the ventilator. When the ICU team met with the health care proxy to discuss tracheostomy and gastrostomy-tube placement, his proxy was overwhelmed.
Introduction
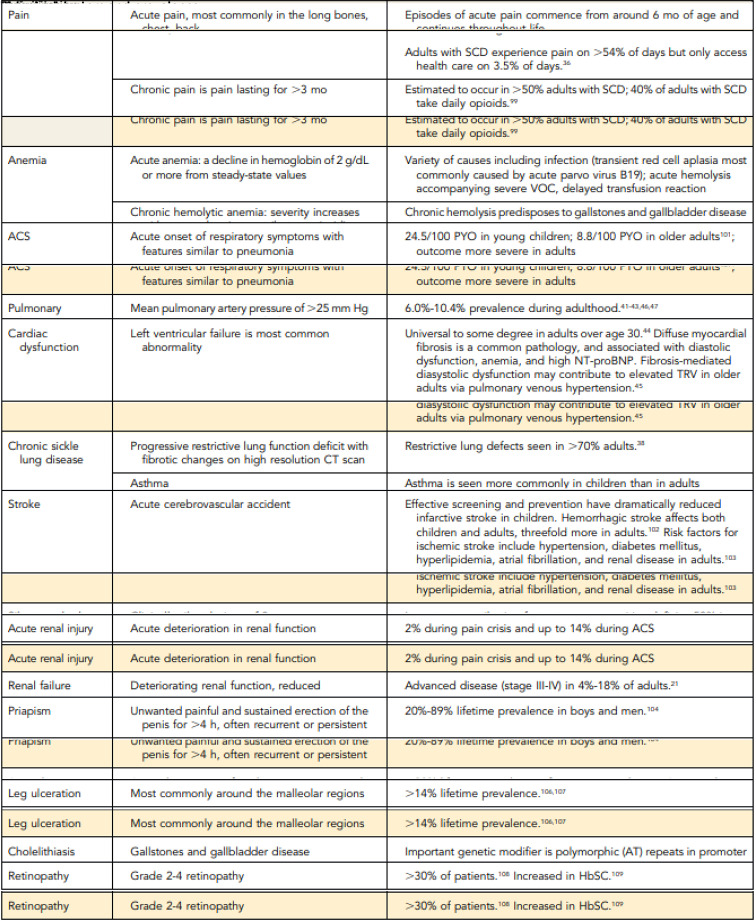
SCD afflicts millions worldwide,1 most of whom identify as Black.2 Despite advances in treatment, people with SCD have a reduced life expectancy of 53 years and experience suffering and organ dysfunction from both acute (eg, pain crises) and chronic (eg, pulmonary hypertension) complications (Table 1).3-5 Therefore, advance care planning (ACP) and end-of-life (EOL) care are critical components of SCD management.
Table 1.
Complications of SCD
|
CT, computed tomographic; HbSC, hemoglobin SCD; NT-proBNT, N-terminal pro-B-type natriuretic peptide; PYO, person-years of observation; TRV, tricuspid regurgitation velocity.
Adapted from Payne Thein and Howard.5
Here we (1) briefly describe patterns of EOL care in SCD; (2) discuss barriers to providing high-quality EOL care for this population, including disease-related, communication-related, and systemic barriers; and (3) identify strategies to mitigate these barriers, including palliative care (PC) (Figure 1). EOL care for SCD is underresearched; therefore, we discuss the limited literature, extrapolate from other diseases, and rely on expert opinion. We also use the clinical vignette to highlight the challenges and opportunities in EOL care for people with SCD. Although SCD is a global disease,1 we focus on EOL care for eople with SCD in the United States, which number over 100 000.2
Figure 1.
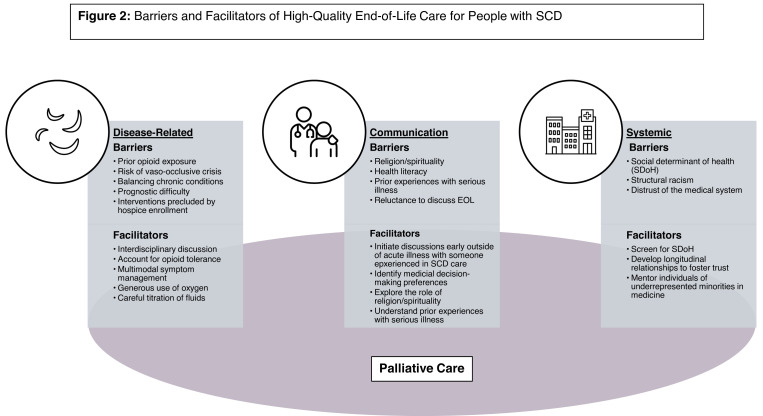
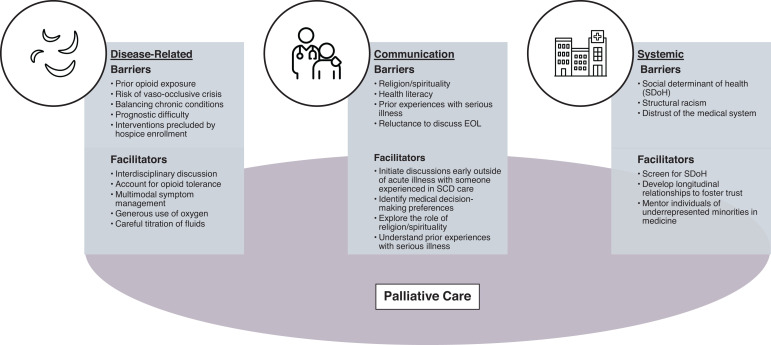
Barriers and facilitators of high-quality EOL care for people with SCD.
Patterns of EOL care
As the management of acute complications of SCD has improved, so has life expectancy, which has led to more patients dying of chronic complications.6 Younger patients tend to die of acute complications (eg, infection), while older patients die from chronic complications (eg, ESRD, pulmonary hypertension).7 Common causes of death include cardiac (eg, congestive heart failure), respiratory (eg, ACS, pneumonia, pulmonary embolism), renal (eg, ESRD), and multiorgan failure. Iron overload, depression, and pulmonary hypertension are independent predictors of early mortality.4 While cancer is an uncommon cause of death (<1%),4,8 the increased use of allogeneic hematopoietic stem cell transplant (HSCT) may increase the risk of secondary malignancies and graft-versus-host disease. Furthermore, the acute and long-term complications of gene therapy are under study and may also shift patterns of EOL care.
Most people with SCD die in acute care settings (hospital [63%] and emergency department [15%]) and have high inpatient utilization in the year preceding death, with an average of 42 hospitalized days over 5 admissions.9
There are a lack of data about PC utilization in people with SCD. The limited literature explores inpatient PC consults: fewer than 1% of people admitted with SCD receive PC, although rates have been increasing over time.10 Hospice utilization among people with SCD is likely low given the pattern of health care utilization prior to death, but to our knowledge, this remains unstudied.
Barriers to quality EOL care
Here we focus on common disease-related, communication, and systemic barriers to caring for people with SCD at the EOL.
Disease-related barriers
Of the numerous disease-related barriers to providing high- quality EOL care for people with SCD, the most prevalent include (1) prior opioid exposure, (2) the risk of vaso-occlusive crises (VOCs), (3) the challenges of managing multiple chronic conditions, and (4) the limitations of hospice in providing key aspects of SCD care.
Opioids are a cornerstone of pain and dyspnea management at the EOL. Although there is debate about the best practices in opioid use for people with SCD,11 many are not opioid naive. About half of people with SCD have at least 1 opioid prescription every year, with a mean of 4 opioid prescriptions annually.12,13 People with opioid tolerance, including people with SCD, require higher opioid doses than opioid-naive people for symptom management, putting them at risk for poorly controlled pain and/or dyspnea when opioids are underdosed.14 Additionally, these high opioid doses increase the risk of dose-dependent side effects (eg, sedation), particularly in the context of liver and kidney dysfunction.
Further, chronic pain is common among people with SCD.15 It can be important to address if pain is acute vs chronic for 2 reasons. First, chronic pain may be better addressed with nonopioid therapies (eg, gabapentinoids, serotonin-norepinephrine reuptake inhibitors). Second, some causes of acute pain are reversible if the etiology is known, and hence, acute pain may require a further workup. VOC pain is a hallmark of SCD. Many parts of the EOL trajectory are known triggers for VOCs, including stress, dehydration, hypoxia, and steroids. Because people with SCD are at increased risk of pain crises at the EOL, it can be challenging to determine whether it is due to VOC pain crises, chronic pain, or other etiologies.
Given the chronic complications associated with SCD (Table 1),3-5 the management of 1 condition may conflict with another. For instance, people with SCD are at risk of chronic kidney disease. Depending on the etiology, additional fluids or fluid restriction may be recommended; both pose a risk to those with SCD. Fluid overload can further compromise a tenuous respiratory status, and dehydration increases the risk of VOC. The many complications of SCD coupled with the acute or chronic nature of the disease make prognostic uncertainty a challenge. Therefore, knowing when to initiate EOL decision-making and to recommend hospice is difficult, particularly in the era of improved outcomes with HSCT and gene therapy.
Finally, when people enroll in hospice, they frequently must stop disease-directed therapy for their hospice-qualifying condition unless they have concurrent care. Concurrent care allows people to enroll in hospice while receiving disease-directed therapy; it is mainly limited to children on Medicaid and the Veterans Administration system. In people with SCD, what patients can receive on hospice may be limited and varies significantly between hospice-qualifying conditions (SCD or a SCD-related complications), hospice agencies, and payors. In some instances, adults with SCD receiving disease-directed therapies may delay hospice enrollment, particularly due to concerns about stopping hemodialysis and transfusions. In ESRD the cessation of hemodialysis decreases survival.16 Patients on hemodialysis may therefore have to make a difficult choice between shortened survival and hospice services.16 Additionally, individuals receiving chronic transfusions or red cell apheresis to mitigate recurrent VOCs put themselves at increased risk of acute VOC complications when they stop transfusions to enroll in hospice. Although the importance of providing transfusion support for patients with hematologic malignancies on hospice is a frequent topic of research and debate,17 the challenges of stopping transfusions for people with SCD have received less attention.
Communication challenges
Although people with SCD are not a monolith, some communication considerations apply to many Black people and others with SCD. That is not to say that everything we discuss below applies to everyone with SCD or that it does not apply to patients without SCD.
First, religion and spirituality (R/S) are intrinsic to how individuals with serious illness cope, especially among individuals of cultural minorities.18,19 In particular, R/S frequently influence EOL decision-making for Black patients and families.20,21 However, physician- and nurse-reported barriers to providing spiritual care include inadequate training to assess for spiritual needs and a lack of time and private space to discuss these matters in the outpatient setting.22 Thus, clinicians may inadequately identify R/S considerations in EOL decisions for people with SCD.
Limited health literacy is associated with poor health outcomes, many of which are critical for high-quality EOL care, including health care communication,23,24 illness understanding, and engagement in ACP. People living in areas with limited health literacy are more likely to receive medically intense EOL care and less likely to enroll in hospice.25 Due to a variety of social factors, low health literacy disproportionately impacts racial and ethnic minorities, including Black patients.26-28 Limited health literacy is further magnified by language barriers and SCD-related cognitive disorders.29
Finally, people with SCD often have extensive experience with the health care system, which informs medical decision-making.30 For instance, individuals requiring mechanical intubation for acute respiratory failure related to ACS may not realize its implications in chronic respiratory failure from advanced pulmonary hypertension. Conversely, experiences with therapy-related adverse effects may discourage someone from accepting other interventions. Additionally, many people with SCD may know others with SCD or other chronic illnesses; their experiences with PC, hospice, and other aspects of EOL care invariably influence medical decision-making and openness to discussing EOL care preferences.
Systemic issues
We would be remiss in discussing challenges in providing high-quality EOL care for people with SCD without discussing social determinants of health (SDoH), structural racism, and distrust of the medical system. SDoH are the “conditions in the environments where people are born, live, learn, work, play, worship, and age that affect a wide range of health functioning and quality-of-life outcomes and risks.” Black patients are more likely to have adverse SDoH,31 which have implications for EOL.32 In particular, people with adverse SDoH may struggle with medication costs as well as transportation costs and may not have people who can meet their informal caregiving needs. Closely related to SDoH is structural racism, or “the structure, policies, practices, and norms resulting in differential access to the goods, services, and opportunities of society by race.”33 Structural racism can impact access to care, patient-family interactions with the health care system, and toxic stress,34 all central to palliative and EOL care.
Years of unethical research practices and suboptimal care provided to the Black community due to SDoH, structural racism, and other issues have led to distrust of the medical system.35,36 Trust is necessary at the EOL if patients are to believe a clinician who says no curative options are available or for a patient to feel comfortable engaging in ACP, including telling clinicians their hopes and goals, preferences, and worries regarding EOL care. A barrier to trust is that most clinicians do not look like the patients with SCD. When clinicians resemble their patients, patients are more likely to disclose symptoms, adhere to recommendations, and reveal treatment preferences,37-39 all necessary for high-quality EOL care. However, only 5.7% of US physicians are Black.40
Facilitators of quality EOL care
Fortunately, these barriers are not insurmountable. In particular, a palliative approach to EOL care (with or without subspecialty PC) can help address many of these issues. In addition to PC, we lay out other strategies for addressing the barriers highlighted above (Figure 1).
Palliative care
PC addresses physical, psychological, social, and R/S distress to improve quality of life for patients and their families.41 Although the EOL is a core component of PC, a palliative approach to care is appropriate for anyone with chronic conditions, including SCD. Early PC is associated with decreased symptom burden, improved patient quality of life,42 and decreased patient, family, and provider distress.43 Therefore, PC involvement should be considered early in the disease trajectory. PC may help people with SCD with symptom management, goal setting in the context of shortened life expectancy, and coping with chronic illnesses. Inherent to PC is the concept of “total pain,” wherein 4 components (physical, emotional, social, and spiritual suffering) underlie the experience of total pain.44 Although PC can benefit many with SCD and there are calls for PC integration in SCD care,45,46 guidelines are needed to identify when and how PC should be integrated within existing models of SCD care. To standardize the process, we propose potential triggers for a PC consult for patients with SCD (Table 2). Additionally, although SCD providers have positive perceptions of PC,47 little is known about people with SCD's perceptions of and willingness to engage with PC. And little is known about the PC capacity for and experience with caring for people with SCD.
Table 2.
Potential triggers for PC consult for people with SCD
|
|
|
|
|
|
|
|
As to who should provide PC for people with SCD, PC can be delivered by those without advanced PC training, such as general practitioners and hematologists (primary PC), or by those with subspecialty PC training (secondary PC). As most studies about the benefits of PC focus on secondary PC, clinicians caring for patients with SCD should have a low threshold for consulting PC. For those patients who do not need or have access to specialty PC or are hesitant about receiving PC, SCD clinicians must develop excellent primary PC skills. There are multiple primary PC training courses.48-50
Disease-related facilitators
Addressing many of the disease-related considerations requires interdisciplinary collaboration. First, working with pharmacists experienced in chronic opioid management and/or specialty PC can help ensure adequate symptom management. Second, discussions with subspecialists can address the complex management of multiple chronic diseases and can weigh in on the implications on management and prognosis and decision- making in EOL care. Third, depending on whether life-sustaining therapies are due to the hospice-qualifying condition or not, some hospice agencies may continue those therapies. For instance, if a patient qualifies for hospice due to SCD and is on dialysis due to complications from diabetes, they may not be able to continue dialysis on hospice. However, such discussions are nuanced and will require conversations with PC and hospice providers to ascertain what is available. Finally, as mentioned above, PC providers may have limited experience with people with SCD. Therefore, providing guidance on what to expect, how to manage VOCs, and having open, ongoing communication between hematology and PC/hospice is essential.
Beyond interdisciplinary collaboration, EOL care planning for people with SCD should address symptom management, incorporating pharmacologic and nonpharmacologic strategies. Opioids should be dosed based on prior exposure. Adjunctive treatments can be considered for specific pain syndromes (eg, nonsteroidal anti-inflammatory drugs, acetaminophen for somatic pain; gabapentinoids, serotonin-norepinephrine reuptake inhibitors for neuropathic pain). Non-pharmacologic therapies include fans, massage, mindfulness, oxygen. Fluids should be administered cautiously accounting for other co-morbidities.
Communication strategies
Exploring medical decision-making preferences is essential to EOL care. Some patients may not be ready to discuss preferences initially due to their experiences with death and illness or their cultural beliefs and values, and some preferences may change over time. Therefore, it is essential to discuss preferences early with a familiar provider who understands the severity of the disease and/or complications. Preferences should be revisited over time as a patient's health changes, preferably during a period of relative wellness rather than during an acute illness. Since general evidence-based practices in difficult conversations and medical decision-making (eg, responding to emotion and exploring values and goals) are outside the scope of this manuscript,30,51 we focus on 3 core areas that are particularly relevant for people with SCD, including (1) who they want involved with medical decision-making, (2) the role R/S play in their decision-making, and (3) their previous experiences with serious illness. Because exploring these areas is challenging and may be new for many clinicians, each section starts with a useful question, and Table 3 includes additional suggestions.
Table 3.
Example questions for exploring key preferences for EOL
| Who they want to have involved in decision-making |
|
| Religion/spirituality |
|
| Prior experiences with serious illness |
|
Denotes questions or statements from Vitaltalk (https://www.vitaltalk.org/).
Who they want involved in decision-making: “When I have medical updates to share with you, who would you like to have there with you?” Most people with SCD in the United States identify with cultural minorities, who tend to make medical decisions collectively, which contrasts with the model of autonomous decision-making encouraged by the US medical system.52 Patients may want individuals from their families or communities involved. Therefore, clinicians must know who should be present when information is disclosed or when medical decisions are needed and then facilitate their involvement (eg, flexible scheduling, permitting extra people at appointments, allowing individuals to call into appointments). Similarly, clinicians need to know who patients want to make decisions for them should they be unable to articulate their wishes.
The role of R/S in medical decision-making: “How may your faith affect how you make decisions about your health?” As above, it is important to know if individuals want anyone from their congregation and/or faith community to be available for psychosocial support or medical decision-making.53 Further, it is important to discuss how R/S may impact decisions (eg, hoping for a miracle) so providers can match language and tailor recommendations appropriately as well as assess for R/S distress. As many providers may feel uncomfortable addressing some of these issues,22 it is essential that providers partner with chaplains and other spiritual care providers to address unmet needs.18
Previous experiences with serious illness: “Have you experienced the loss of a loved one?” Providers cannot know how previous experiences may shape decision-making without asking. Therefore, asking about previous experiences, particularly with PC and hospice, is essential. Only then can providers appropriately address any concerns and make EOL recommendations consistent with their preferences.
Systemic solutions
Some of the systemic issues can feel overwhelming, yet providers can take pragmatic steps to counter them. Growing evidence supports universal screening for SDoH in clinical settings, including which tools to use and how to fit screening into routine care.54,55 Therefore, SCD centers that are not routinely screening for SDoH should consider how to integrate screening into practice, including at the EOL. In the meantime, providers can work to minimize the financial burden of medical care (eg, scheduling subspecialist visits on the same day, offering telehealth) and partner with social workers to address unmet needs. Finally, providers may need more time and effort to build trust with patients who do not look like themselves; continuity of care and building longitudinal relationships is critical—including with the primary hematology team and specialty PC. Further, educational initiatives that support developing clinicians sensitive to our patients' needs and the development of a workforce that looks like our patients are essential.
CLINICAL CASE (continued)
Let us revisit how Mr. Jones' experience could have been different by addressing some of these considerations.
When his nephrologist recommended hemodialysis, Mr. Jones was reluctant. His hematologist referred him to specialty PC to discuss goals of care. Mr. Jones was unsure what another physician had to offer but agreed. After establishing rapport, the PC clinician learned the following.
Mr. Jones lived alone and was on disability. He was otherwise independent in his activities of daily living and hoped to remain in this state of health for as long as possible.
He felt he was doing well until he learned about the need for hemodialysis.
Mr. Jones acknowledged that he had difficulty understanding medical information and relied on his neighbor to assist with decision-making due to several SCD-related strokes. However, his neighbor was married with children and worked full time, which left her little time to attend visits.
He identified as Christian and attended weekly services when he had transportation. His pastor called him weekly for support.
With Mr. Jones' permission, his medical team scheduled a call with his neighbor; she agreed to be named as his health care proxy. Although she was shocked about the recommendation for hemodialysis, it confirmed what she had already sensed about Mr. Jones' overall health. Mr. Jones began hemodialysis and tolerated it well for several months.
Ultimately, he was admitted with community-acquired pneumonia complicated by ACS. While awaiting exchange transfusion, patient-controlled analgesia was appropriately dosed based on his methadone regimen with the help of PC. The medical team, including PC, alerted Mr. Jones' neighbor and called Mr. Jones' pastor to offer support. When he met his proxy, he reaffirmed his goal to remain independent but to prioritize symptom management should his condition become irreversible. He was transferred to the ICU and intubated. After 1 week without improvement, the ICU team met with his neighbor to discuss tracheostomy and gastrostomy tube placement. Recalling Mr. Jones' goals, she elected a terminal extubation for him. Opioids and sedatives were dosed, his pastor said a blessing, and he was extubated. He died peacefully minutes later, surrounded by his neighbor, pastor, and friends from church.
Conclusion
EOL care is central to caring for people with SCD and requires acknowledging medical, communication, and systemic challenges. Although we propose strategies to address these challenges, more research is needed in this area, particularly around how people with SCD define high-quality EOL care, how best to integrate PC into SCD care, and how HSCT and gene therapies impact EOL care for people with SCD. Meanwhile, with primary and secondary PC, interdisciplinary collaboration, and attention to SDoH, high-quality EOL care is possible.
Conflict-of-interest disclosure
Rushil V. Patel: no competing financial interests to declare.
Emily E. Johnston: no competing financial interests to declare.
Off-label drug use
Rushil V. Patel: nothing to disclose.
Emily E. Johnston: nothing to disclose.
References
- 1.Centers for Disease Control and Prevention. Data and statistics on sickle cell disease. Sickle cell disease (SCD). Accessed 5 June 2024. https://www.cdc.gov/sickle-cell/data/index.html.
- 2.Committee on Addressing Sickle Cell Disease. Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. National Academies Press; 2020. [PubMed] [Google Scholar]
- 3.Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387(10037):2565-2574. [DOI] [PubMed] [Google Scholar]
- 4.Njoku F, Pugh N, Brambilla D, et al.. Mortality in adults with sickle cell disease: results from the sickle cell disease implementation consortium (SCDIC) registry. Am J Hematol. 2024;99(5):900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thein SL, Howard J.. How I treat the older adult with sickle cell disease. Blood. 2018;132(17):1750-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao B, Johnson KM, Ramsey SD, Bender MA, Devine B, Basu A.. Long-term survival with sickle cell disease: a nationwide cohort study of Medicare and Medicaid beneficiaries. Blood Adv. 2023;7(13):3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne AB, Mehal JM, Chapman C, et al.. Trends in sickle cell disease-related mortality in the United States, 1979 to 2017. Ann Emerg Med. 2020; 76(3S):S28-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamideh D, Alvarez O.. Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer. 2013;60(9):1482-1486. [DOI] [PubMed] [Google Scholar]
- 9.Johnston EE, Adesina OO, Alvarez E, et al.. Acute care utilization at end of life in sickle cell disease: highlighting the need for a palliative approach. J Palliat Med. 2020;23(1):24-32. [DOI] [PubMed] [Google Scholar]
- 10.Nwogu-Onyemkpa E, Dongarwar D, Salihu HM, et al.. Inpatient palliative care use by patients with sickle cell disease: a retrospective cross- sectional study. BMJ Open. 2022;12(8):e057361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osunkwo I, O'Connor HF, Saah E.. Optimizing the management of chronic pain in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2020;2020(1):562-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J, Zhou J, Saraf SL, Gordeuk VR, Calip GS. Characterization of opioid use in sickle cell disease. Pharmacoepidemiol Drug Saf. 2018;27(5):479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang HA, Barner JC, Richards KM, Bhor M, Paulose J, Kutlar A.. Association between vaso-occlusive crises and opioid prescriptions among patients with sickle cell disease: a retrospective claims-based study. J Health Econ Outcomes Res. 2020;7(1):94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adebola A, Duncan N.. Acute pain management in patients with opioid tolerance. Accessed 1 May 2024. https://www.uspharmacist.com/article/acute-pain-management-in-patients-with-opioid-tolerance.
- 15.Smith WR, Penberthy LT, Bovbjerg VE, et al.. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94-101. [DOI] [PubMed] [Google Scholar]
- 16.Schell JO, Johnson DS. Challenges with providing hospice care for patients undergoing long-term dialysis. Clin J Am Soc Nephrol. 2021;16(3):473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odejide OO, Steensma DP. Patients with haematological malignancies should not have to choose between transfusions and hospice care. Lancet Haematol. 2020;7(5):e418-e424. [DOI] [PubMed] [Google Scholar]
- 18.Balboni TA, VanderWeele TJ, Doan-Soares SD, et al.. Spirituality in serious illness and health. JAMA. 2022;328(2):184-197. [DOI] [PubMed] [Google Scholar]
- 19.Derlega VJ, Janda LH, Miranda J, Chen IA, Goodman BM III, Smith W.. How patients' self-disclosure about sickle cell pain episodes to significant others relates to living with sickle cell disease. Pain Med. 2014;15(9):1496-1507. [DOI] [PubMed] [Google Scholar]
- 20.Johnson J, Hayden T, True J, et al.. The impact of faith beliefs on perceptions of end-of-life care and decision making among African American Church members. J Palliat Med. 2016;19(2):143-148. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes RL, Elwood B, Lee SC, Tiro JA, Halm EA, Skinner CS. The desires of their hearts: the multidisciplinary perspectives of African Americans on end-of-life care in the African American community. Am J Hosp Palliat Care. 2017;34(6):510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balboni MJ, Sullivan A, Enzinger AC, et al.. Nurse and physician barriers to spiritual care provision at the end of life. J Pain Symptom Manage. 2014;48(3):400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kripalani S, Jacobson TA, Mugalla IC, Cawthon CR, Niesner KJ, Vaccarino V.. Health literacy and the quality of physician-patient communication during hospitalization. J Hosp Med. 2010;5(5):269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynia MK, Osborn CY. Health literacy and communication quality in health care organizations. J Health Commun. 2010;15(suppl 2):102-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Q, Shi K, Hung P, Wang SY. Associations between health literacy and end-of-life care intensity among Medicare beneficiaries. Am J Hosp Palliat Care. 2021;38(6):626-633. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry SI, Herrin J, Phillips C, et al.. Racial disparities in health literacy and access to care among patients with heart failure. J Card Fail. 2011;17(2): 122-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rikard RV, Thompson MS, McKinney J, Beauchamp A.. Examining health literacy disparities in the United States: a third look at the National Assessment of Adult Literacy (NAAL). BMC Public Health. 2016;16(1):975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muvuka B, Combs RM, Ayangeakaa SD, Ali NM, Wendel ML, Jackson T.. Health literacy in African-American communities: barriers and strategies. Health Lit Res Pract. 2020;4(3):e138-e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishkin AD, Prince EJ, Leimbach EJ, Mapara MY, Carroll CP. Psychiatric comorbidities in adults with sickle cell disease: a narrative review. Br J Haematol. 2023;203(5):747-759. [DOI] [PubMed] [Google Scholar]
- 30.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4): 256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill L, Ndugga N, Artiga S.. Key data on health and health care by race and ethnicity. KFF. 15 March 2023. Accessed 1 May 2024. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/.
- 32.Office of Disease Prevention and Health Promotion. Social determinants of health—healthy people 2030. Accessed 22 February 2023. https://health.gov/healthypeople/priority-areas/social-determinants-health.
- 33.Jones CP. Confronting institutionalized racism. Phylon. 2002;50(1/2):7-22. [Google Scholar]
- 34.Umaretiya PJ, Wolfe J, Bona K.. Naming the problem: a structural racism framework to examine disparities in palliative care. J Pain Symptom Manage. 2022;63(5):e461-e463. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cult Divers. 2007;14(2):56-60. [PubMed] [Google Scholar]
- 36.Miller F, Miller P.. Transgenerational trauma and trust restoration. AMA J Ethics. 2021;23(6):E480-E486. [DOI] [PubMed] [Google Scholar]
- 37.Fennell ML. Racial disparities in care: looking beyond the clinical encounter. Health Serv Res. 2005;40(suppl 6, pt 1):1713-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray B, Stoddard JJ. Patient-physician pairing: does racial and ethnic congruity influence selection of a regular physician? J Community Health. 1997;22(4):247-259. [DOI] [PubMed] [Google Scholar]
- 39.Escarce JJ. How does race matter, anyway? Health Serv Res. 2005;40(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Association of American Medical Colleges. 2022. physician specialty report data highlights. Accessed 1 May 2024. https://www.aamc.org/data-reports/workforce/data/2022-physician-specialty-report-data-highlights.
- 41.Mittelberger J. The value of palliative care. Center to Advance Palliative Care. Accessed 7 April 2024. https://www.capc.org/the-case-for-palliative-care/.
- 42.Temel JS, Greer JA, Muzikansky A, et al.. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742. [DOI] [PubMed] [Google Scholar]
- 43.Casarett D, Shreve S, Luhrs C, et al.. Measuring families' perceptions of care across a health care system: preliminary experience with the family assessment of treatment at end of life short form (FATE-S). J Pain Symptom Manage. 2010;40(6):801-809. [DOI] [PubMed] [Google Scholar]
- 44.Mehta A, Chan LS. Understanding of the concept of “total pain.” 2008; 10(1):26-32. [Google Scholar]
- 45.Wilkie DJ, Johnson B, Mack AK, Labotka R, Molokie RE. Sickle cell disease: an opportunity for palliative care across the life span. Nurs Clin North Am. 2010;45(3):375-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamin L. Pain management in sickle cell disease: palliative care begins at birth? Hematol Am Soc Hematol Educ Program. 2008;2008(1):466-474. [DOI] [PubMed] [Google Scholar]
- 47.Nwogu-Onyemkpa E, Saleem N, Amspoker A.. Sickle cell disease providers' perspectives on palliative care. J Pain Symptom Manage. 2024;67(5): e539-e540. [Google Scholar]
- 48.VitalTalk. Accessed 5 June 2024. https://www.vitaltalk.org/.
- 49.Calton BA, Alvarez-Perez A, Portman DG, Ramchandran KJ, Sugalski J, Rabow MW. The current state of palliative care for patients cared for at leading US cancer centers: the 2015 NCCN palliative care survey. J Natl Compr Canc Netw. 2016;14(7):859-866. [DOI] [PubMed] [Google Scholar]
- 50.Elk R, Barnett MD, Tucker RO, et al.. African American communities speak to healthcare providers: clinical practice outcomes. J Pain Symptom Manag. 2024;67(5):e558. [Google Scholar]
- 51.Cain CL, Surbone A, Elk R, Kagawa-Singer M.. Culture and palliative care: preferences, communication, meaning, and mutual decision making. J Pain Symptom Manage. 2018;55(5):1408-1419. [DOI] [PubMed] [Google Scholar]
- 52.Hobbs GS, Landrum MB, Arora NK, et al.. The role of families in decisions regarding cancer treatments. Cancer. 2015;121(7):1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elk R, Emanuel L, Hauser J, Bakitas M, Levkoff S.. Developing and testing the feasibility of a culturally based tele-palliative care consult based on the cultural values and preferences of southern, rural African American and white community members: a program by and for the community. Health Equity. 2020;4(1):52-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power-Hays A, Li S, Mensah A, Sobota A.. Universal screening for social determinants of health in pediatric sickle cell disease: a quality- improvement initiative. Pediatr Blood Cancer. 2020;67(1):e28006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magoon V. Screening for social determinants of health in daily practice. Fam Pract Manag. 2022;29(2):6-11. [PubMed] [Google Scholar]