Abstract
Sentinel lymph node (SLN) mapping has been shown to be important for staging in dogs with mast cell tumors (MCTs). Despite this, many patients are referred to an oncologist after surgical intervention has been carried out. It is unknown whether lymphatic drainage patterns are altered by surgery and whether postoperative SLN mapping can be reliably conducted. The objective of this study was to compare lymphatic drainage patterns from MCT sites before and after surgical removal to determine whether the SLN changes following tumor excision. Twenty-nine client-owned dogs with 31 cytologically diagnosed MCTs were prospectively enrolled, with 14 dogs (N = 15 MCTs) completing the study. Preoperative SLN mapping was conducted using radiographic indirect lymphography (IL). Water-soluble iodinated contrast (WIC) medium was injected peritumorally using a 4-quadrant technique and digital radiography was then used to assess lymphatic drainage patterns. Orthogonal projections were obtained every 1 to 2 min until the SLN was visualized, up to 20 min post-injection. Dogs were re-evaluated 2 to 5 wk postoperatively and radiographic IL was carried out again using the same protocol as previously described with WIC injected around the surgical scar line in a 4-quadrant technique. An SLN was identified for 15 MCTs in 14 dogs preoperatively and in 13/15 MCTs postoperatively. Sixteen dogs with 16 MCTs did not have postoperative lymphography and did not complete the study. Agreement between preoperative and postoperative SLNs was a complete match in 7/15 MCTs, a partial match in 5/15 MCTs, and no match in 3/15 MCTs. A negative IL study was obtained in 2/15 MCTs postoperatively. Complete agreement between preoperative and postoperative SLN identification was detected in 46.7% of cases and there was no agreement in 20% of cases. Surgical intervention did not change the time to SLN identification when carrying out radiographic IL. Thus, surgical removal of MCTs affects lymphatic drainage and can alter the SLN(s) detected. Clinicians should be aware of this finding and interpret results of postoperative lymph node staging with caution.
Résumé
La cartographie des ganglions sentinelles (SLN) s’est avérée importante pour la stadification chez les chiens atteints de mastocytomes (TMC). Malgré cela, de nombreux patients sont référés à un oncologue après une intervention chirurgicale. On ne sait pas si les schémas de drainage lymphatique sont modifiés par la chirurgie et si la cartographie postopératoire des SLN peut être réalisée de manière fiable. L’objectif de cette étude était de comparer les schémas de drainage lymphatique des sites de TMC avant et après l’ablation chirurgicale afin de déterminer si le SLN change après l’excision de la tumeur. Vingt-neuf chiens appartenant à des clients et présentant 31 TMC diagnostiqués par cytologie ont été recrutés de manière prospective, 14 chiens (N = 15 TMC) ayant terminé l’étude. La cartographie préopératoire des SLN a été réalisée à l’aide d’une lymphographie indirecte radiographique (IL). Un milieu de contraste iodé hydrosoluble (WIC) a été injecté par voie péritumorale à l’aide d’une technique à 4 quadrants et une radiographie numérique a ensuite été utilisée pour évaluer les schémas de drainage lymphatique. Des projections orthogonales ont été obtenues toutes les 1 à 2 minutes jusqu’à ce que le ganglion sentinelle soit visualisé, jusqu’à 20 minutes après l’injection. Les chiens ont été réévalués 2 à 5 semaines après l’opération et une IL radiographique a été réalisée à nouveau en utilisant le même protocole que celui décrit précédemment avec du WIC injecté autour de la ligne de cicatrice chirurgicale dans une technique à 4 quadrants. Un ganglion sentinelle a été identifié pour 15 MCT chez 14 chiens avant l’opération et dans 13/15 MCT après l’opération. Seize chiens avec 16 MCT n’ont pas eu de lymphographie postopératoire et n’ont pas terminé l’étude. La concordance entre les ganglions sentinelles préopératoires et postopératoires était une correspondance complète dans 7/15 MCT, une correspondance partielle dans 5/15 MCT et aucune correspondance dans 3/15 MCT. Une étude IL négative a été obtenue dans 2/15 MCT après l’opération. Une concordance complète entre l’identification préopératoire et postopératoire du ganglion sentinelle a été détectée dans 46,7 % des cas et aucune concordance n’a été constatée dans 20 % des cas. L’intervention chirurgicale n’a pas modifié le délai d’identification du ganglion sentinelle lors de la réalisation d’une IL radiographique. Ainsi, l’ablation chirurgicale des MCT affecte le drainage lymphatique et peut modifier le ou les ganglions sentinelles détectés. Les cliniciens doivent être conscients de ce résultat et interpréter les résultats de la stadification postopératoire des ganglions lymphatiques avec prudence.
(Traduit par Docteur Serge Messier)
Introduction
Mast cell tumors (MCTs) are the most common cutaneous tumors in dogs and account for up to 20% of all canine skin tumors (1–3). The biologic behavior of MCTs is variable, ranging from low to high metastatic potential (1,3–5). Although histologic grading provides valuable information in regard to prognosis, up to 15% of dogs with low-grade cutaneous MCTs have metastatic disease at the time of diagnosis (4,5). Mast cell tumors frequently metastasize to lymph nodes first and then to the spleen, liver, and other visceral organs (1,2).
In a study by Warland et al (2), staging was carried out for 220 dogs with MCTs and 31% of dogs were reported as having metastasis to the lymph node based on local lymph node aspiration. No dog had or developed distant metastasis in the absence of lymph node metastasis (2). Lymph node involvement has been shown to be a strong predictive factor in dogs with MCTs and the utility of further staging in the absence of lymph node metastasis is low (2,3,6,7).
The sentinel lymph node (SLN) is the first lymph node within a lymphatic basin to which a tumor drains, the presence or absence of tumor cells in the SLN is predictive of metastasis for dogs with MCTs. In some tumors, multiple lymphatic drainage patterns are seen, which results in the presence of more than 1 sentinel lymph node (8–10). Sentinel lymph node mapping can therefore help identify dogs that would benefit from additional staging or adjuvant treatment for their disease.
Sentinel lymph nodes are not necessarily the closest anatomic regional lymph nodes (8,11–17). In a recent study in which lymphoscintigraphy was used to identify SLNs in dogs with MCTs, 40% of dogs had SLNs that differed from the closest regional lymph node (13). Similar results were seen in a study by Lapsley et al (16), in which computed tomography (CT) indirect lymphography (IL) was used for SLN mapping, with the conclusion that the SLN differed from the locoregional lymph node in 27% of cases and ultimately changing treatment recommendations in 40% of dogs. Ferrari et al (18) reported an even greater discrepancy using SLN mapping in 30 MCTs, with 63% of SLNs not corresponding to the expected regional lymph node.
The findings of these studies demonstrate the importance of SLN mapping for staging of dogs with MCTs. Although multiple methods of SLN mapping have been described, the use of a water-soluble iodinated contrast (WIC) medium and digital radiography has been shown to be an inexpensive and reliable option that is readily available not only at specialty veterinary hospitals, but also at first-opinion veterinary practices (12,14,19,20). In a recent study evaluating SLN identification in healthy beagle dogs, radiographic indirect lymphography (IL) identified an SLN in 7/8 dogs (19). Another study revealed a 77.9% diagnostic or partially diagnostic rate of SLN identification using IL (20).
Many dogs are referred to specialty veterinary hospitals for staging and treatment recommendations following excision of an MCT by their primary-care veterinarian. The reliability of postoperative staging in these dogs is complicated by the limited information available on the effect of surgery on lymphatic drainage. Hlusko et al (21) used the canine brachium as a simulated tumor model to study the effect of surgery on SLN mapping using lymphoscintigraphy. Agreement between preoperative and postoperative SLN mapping was complete in 50% of cases and partial in 50%, with more SLNs identified during the postoperative studies. As several types of cancer are known to promote lymphangiogenesis (8,22), tumor excision may alter lymphatic drainage patterns, precluding accurate identification of the SLN in dogs following MCT excision.
Few studies report the feasibility and results of postoperative SLN mapping in dogs after MCT excision. In a study using IL to identify the SLN in dogs, 3 dogs underwent SLN mapping for cutaneous MCTs that had been incompletely excised by the primary-care veterinarian (14). It was reported that 2 of these 3 cases had metastasis to the SLN on histopathology (14), which highlights the important usefulness of IL for SLN mapping.
Another study evaluated the effect of surgery on contrast-enhanced ultrasound for SLN mapping in dogs with MCTs, with 38.7% of dogs having had previously excised MCTs. When comparing these cases with treatment-naive dogs, a significantly lower number of SLN(s) was identified in the dogs with previously excised MCTs. To the authors’ knowledge, these are the only studies that directly evaluate postoperative lymph node staging of patients following removal of an MCT, but direct comparison to preoperative status was not assessed.
The objective of this study was to prospectively compare lymphatic drainage patterns from primary mast cell tumor (MCT) sites before and after surgical removal of MCTs to determine if the sentinel lymph node (SLN) changes after the tumor has been removed. We hypothesized that lymphatic drainage and the lymph node identified using SLN mapping would be altered by surgical intervention.
Materials and methods
Patient selection
Dogs were prospectively enrolled between August 2020 and June 2022 at a single institution and all procedures were approved by the Institutional Animal Care and Use Committee at Auburn University. Client-owned dogs presenting for surgical removal of a cytologically confirmed cutaneous or subcutaneous MCT were enrolled. Dogs were excluded if they had previously undergone surgery for excision of the primary MCT or if there was evidence of metastatic disease at the time of presentation and owners had elected to have the metastatic lymph node removed. All patients underwent a physical examination, serum chemistry, complete blood (cell) count (CBC), urinalysis, and thoracic radiographs at the time of presentation.
Procedures
Sentinel lymph node mapping was carried out on all dogs using indirect lymphography (IL) with digital radiography. All studies were conducted by either a small animal surgery resident (CLB), faculty surgeon (KCH), a Board-certified medical oncologist (NB), or a Board-certified surgeon (BMM). All lymphography studies were then interpreted by 1 Board-certified radiologist (RCC).
All dogs were sedated for imaging with dexmedetomidine (Dexdomitor; Orion Corporation, Espoo, Finland), 2.5 to 10 μg/kg body weight (BW) intravenously (IV) and butorphanol (Torbugesic; Zoetis, Kalamazoo, Michigan, USA), 0.2 to 0.4 mg/kg BW, IV, with specific doses determined by the attending clinician. Data monitored throughout sedation included heart rate, respiratory rate, and temperature. At the conclusion of the study, the dexmedetomidine was reversed with an equal volume of atipamezole (Antisden; Orion Corporation), 2.5 to 10 μg/kg BW administered intramuscularly (IM).
Preoperative SLN mapping was carried out for each dog using 4 mL of WIC medium (Iopamadol; Isovue, Bracco Diagnostics, Monroe Twp., New Jersey, USA), 370 mg/mL iodine, injected peritumorally into the tissues in a 4-quadrant technique, with the total dose volume split equally among the 4 quadrants as described in previous studies (11,13,14,23). The area was then gently massaged for approximately 30 s (24). Digital radiography (Luminos-Agile; Siemens, Munich, Germany) was then used to assess the lymphatic drainage patterns from the primary tumor site.
Radiographic images were obtained immediately after contrast injection, continuing every 1 to 2 min, up to 20 min post-injection, until a sentinel lymph node(s) (SLN) was identified. Both lateral and ventrodorsal (VD) radiographs were carried out in order to appropriately assess the lymphatics and lymph nodes. All personnel involved in positioning of animals and obtaining radiographs were required to wear dosimetry badges to measure exposure levels of radiation. Lymphography was considered unsuccessful if the contrast was not identified within the SLN after 20 min.
Once the SLN was identified, it was sampled by means of a fine needle aspirate and cytology was carried out by a Board-certified clinical pathologist to evaluate for the presence of metastatic disease. Ultrasound guidance was used to sample lymph nodes as needed. Dogs without evidence of metastatic disease on lymph node cytology were eligible for continued enrollment in the study.
If metastatic disease was present, an abdominal ultrasound with fine-needle aspirates of the liver and spleen was recommended for complete staging. In addition, removal of affected lymph node(s) was recommended for dogs with metastatic disease to the SLN at the time of tumor removal. Dogs with a negative lymphography study or with a metastatic SLN were not eligible to continue to the second phase of the study for postoperative lymphography imaging.
Dogs continuing in the study underwent surgery for primary tumor removal. Anesthesia protocols were determined by a Board-certified anesthesiologist. Surgery was carried out by a supervised small animal surgery resident or Board-certified surgeon, with the margins of tumor removal determined at the discretion of the surgeon, but aiming for 2- to 3-cm margins. All masses were submitted for histopathology to achieve a diagnosis, tumor grade, and margin evaluation. All dogs were hospitalized after surgery and received routine postoperative care as deemed appropriate by the attending clinician.
Dogs continuing to the second phase of the study were reevaluated 2 to 5 wk after surgery and SLN mapping was carried out again. Lymphography was conducted with 4 mL of the WIC medium injected subcutaneously and circumferentially around the surgical scar using the same 4-quadrant technique. The area of injection was then gently massaged for 30 s. Digital radiography was used to assess the lymphatic drainage patterns immediately post-contrast injection and continuing every 1 to 2 min, up to 20 min post-injection or until the contrast was visualized within the SLN.
Data analysis
Agreement between the SLN(s) and the locoregional lymph node (LRLN), as determined using previously reported lymphosomes (9), was designated as a match (complete agreement), partial match (partial agreement), or no match (no agreement). The SLN(s) identified using SLN mapping were compared before and after surgery to determine if the SLN changed following surgery. Data evaluated included time to SLN identification (if present), anatomic location of the SLN(s), and number of SLNs identified for all SLN mapping studies.
Preoperative and postoperative lymphography studies were then compared to evaluate changes in anatomic location of the SLN, number of SLNs identified, time to SLN identification, and agreement in SLNs between the studies. Agreement was determined as no agreement if the preoperative and postoperative SLN(s) were different, complete agreement if the preoperative and postoperative SLN(S) were the same, and partial agreement if multiple SLNs were identified either preoperatively or postoperatively and some, but not all, were the same. Descriptive statistics were used to analyze the data. Testing for normality was done using a Shapiro-Wilk test and time to SLN identification was evaluated using a Mann-Whitney U-test.
Results
Study population
Twenty-nine dogs with a total of 31 mast cell tumors (MCTs) were initially enrolled in the study. Two dogs developed a second mast cell tumor 2 and 9 mo after their initial enrollment. There were 15 females (12 spayed) and 14 males (12 neutered), with a median age of 7.7 y (range: 2 to 13 y) and a median body weight of 27.9 kg (range: 8.8 to 46.6 kg). Breeds included 5 American Staffordshire terriers, 5 mixed-breed dogs, 4 boxers, 3 golden retrievers, 3 pugs, 2 Australian shepherds, and 1 each of Rottweiler, Great Dane, rat terrier, French bulldog, Belgian malinois, vizsla, and Great Pyrenees.
MCT characteristics
Ten tumors were in the hip or upper thigh, 6 in the thorax region, 5 in the head and neck region, 5 in the brachium region, 2 in the pelvic limb, 2 in the abdomen, and 1 in the perianal region. Grade was determined to be Kiupel low grade/Patnaik Grade I in 2 MCTs, Kiupel low grade/Patnaik Grade II in 18 MCTs, and Kiupel high grade/Patnaik Grade III in 1 MCT. Six MCTs were not graded as they were subcutaneous, although mitotic counts ranged from 0/10 to 12/10 per high power field. Four dogs did not undergo surgery after preoperative lymphography due to lack of owner compliance. Of the 15 MCTs in dogs that completed the study, 13 were completely excised on histopathology and 2 were incompletely excised.
On preoperative palpation, it was determined that 22 MCTs were cutaneous and 9 were subcutaneous. Seven of the 9 preoperative subcutaneous tumors were dismissed from the study, 3 due to a negative preoperative lymphography study, 3 due to a lymphadenectomy carried out due to suspected metastasis, and 1 due to lack of owner follow-up. On histopathologic analysis of all surgical cases (27 total), 21 MCTs were cutaneous and 6 were subcutaneous (Table I). Location status differed between preoperative palpation and histopathology in 6/27 cases (22.2%).
Table I.
Locating tumors by preoperative palpation versus histopathology. Further distinction is made between mast cell tumors on dogs that were enrolled in the study to completion and those on dogs that were dismissed.
| Preoperative palpation | Histopathology | |||
|---|---|---|---|---|
|
|
|
|||
| Subcutaneous | Cutaneous | Subcutaneous | Cutaneous | |
| Completed (n) | 2 | 13 | 2 | 13 |
| Dismissed (n) | 6 (1) | 6 (3) | 4 | 8 |
| Total (N) | 9 | 22 | 6 | 21 |
Note: Cases listed in parenthesis include the 4 dogs in which surgery was not carried out and are therefore omitted from the histopathology results.
Preoperative indirect lymphography
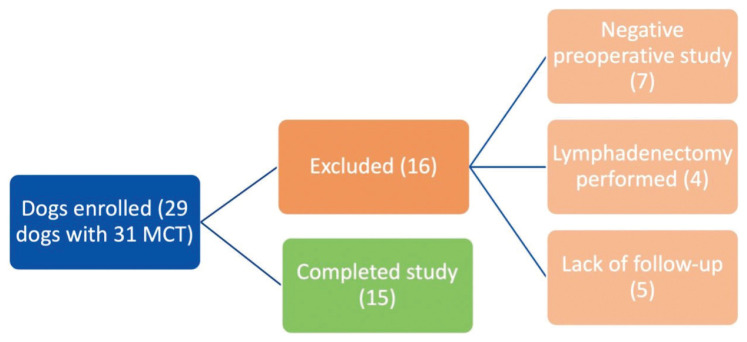
Preoperative SLN mapping was carried out without complications in all dogs and an SLN(s) successfully identified in 24/31 lymphography studies (77.4%). The average and median time to SLN identification for preoperative studies was 2.75 and 1 min, respectively (range: 0 to 17 min). One to 3 SLN(s) were identified on SLN mapping studies. Agreement between the SLN(s) and the locoregional lymph node (LRLN) was a complete match in 15/24 MCTs (62.5%), a partial match in 8/24 MCTs (33.3%), and no match in 1/24 MCTs (4.2%). In all dogs with a partial match, SLN mapping revealed the suspected LRLN, as well as 1 to 2 additional SLNs. After preoperative SLN mapping, 16 MCTs were excluded from the study, 7 due to a negative preoperative lymphography study, 4 due to a lymphadenectomy carried out due to suspected metastasis, and 5 due to lack of owner follow-up (Figure 1).
Figure 1.
Flow diagram depicting all dogs enrolled in the study and exclusion criteria for 14 dogs. MCT — Mast cell tumor.
The following were the cytology results of all SLN(s) for dogs remaining in the study: 8 were either consistent with a normal lymph node, 8 with a reactive lymphoid hyperplasia, and 1 with aspiration of perinodal fat; or 1 was a nondiagnostic sample. Cytology was not carried out in 2 cases as the lymph node was too small to safely aspirate. One of these cases was a medial iliac lymph node and the other was an axillary lymph node. Of the 4 dogs in which a lymphadenectomy was carried out due to a suspected metastatic SLN on cytology, 2 were confirmed metastatic on histopathology (1 with no HN score provided that had few mast cells on cytology and 1 with an HN2 score with an overtly metastatic lymph node on cytology) and 2 that were non-metastatic, HN0 (both showed rare mast cells on cytology).
Postoperative indirect lymphography
Fourteen dogs with a total of 15 MCTs completed the study (Figure 1). An SLN(s) was identified preoperatively in 15/15 MCTs and postoperatively in 13/15 MCTs. Complete agreement was seen in 7/15 MCTs (46.7%), partial agreement in 5/15 MCTs (33.3%), and no agreement in 3/15 MCTs (20%) (Table II). Two of the cases with no agreement were due to negative postoperative lymphography studies and 2 with partial agreement were MCTs that were incompletely excised on histopathology, with both having 1 SLN preoperatively and 2 SLNs postoperatively. One to 3 SLN(s) were identified on SLN mapping studies. Figure 2 shows an example of a postoperative IL study.
Table II.
Sentinel lymph node mapping data of all 15 mast cell tumors on dogs that completed the study.
| Case | MCT location | Preoperative SLN | Time (min) | Postoperative SLN | Time (min) | Agreement |
|---|---|---|---|---|---|---|
| 1 | Ventral thorax | Left axillary | 1 | Negative | — | None |
| 2 | Right pinna | Right superficial cervical | 2 | Right superficial cervical | 2 | Complete |
| 3 | Right perianal region | Right medial iliac | 3 | Right medial iliac | 5 | Partial (1 pre-, 2 post-) |
| Right superficial inguinal | 5 | |||||
| 4 | Left hip | Left medial iliac | 5 | Left medial iliac | 1 | Partial (1 pre-, 3 post-) |
| Left superficial inguinal | 1 | |||||
| Left aortic | 1 | |||||
| 5 | Right flank fold | Right superficial inguinal | 4 | Right superficial inguinal | 2 | Complete |
| Right medial iliac | 17 | Right medial iliac | 2 | |||
| 6 | Right rostrodorsal muzzle | Mandibular | 0 | Mandibular | 0 | Complete |
| 7 | Left distal brachium | Left superficial cervical | 5 | Left superficial cervical | 1 | Complete |
| 8 | Caudoventral thorax | Right axillary | 1 | Right axillary | 5 | Partial (2 pre-, 1 post-) |
| Left axillary | 1 | |||||
| 9 | Right caudo-lumbar region | Right medial iliac | 0 | Left medial iliac | 5 | None |
| Right axillary | 10 | |||||
| 10 | Left thigh | Left superficial inguinal | 0 | Left superficial inguinal | 0 | Partial (2 pre-, 1 post-) |
| Left medial iliac | 0 | |||||
| 11 | Right ventral abdomen | Right superficial inguinal | 0 | Right superficial inguinal | 1 | Complete |
| Right medial iliac | 1 | Right medial iliac | 9 | |||
| 12 | Left caudolateral thorax | Left axillary | 2 | Left axillary | 8 | Complete |
| 13 | Left ear base | Left superficial cervical | 0 | Left superficial cervical | 2 | Partial (1 pre-, 2 post-) |
| Left medial retropharyngeal | 2 | |||||
| 14 | Right cranio-proximal thigh | Right medial iliac | 3 | Negative | — | None |
| 15 | Right cervical region | Right superficial cervical | 0 | Right superficial cervical | 5 | Complete |
SLN — Sentinel lymph node; MCT — Mast cell tumor.
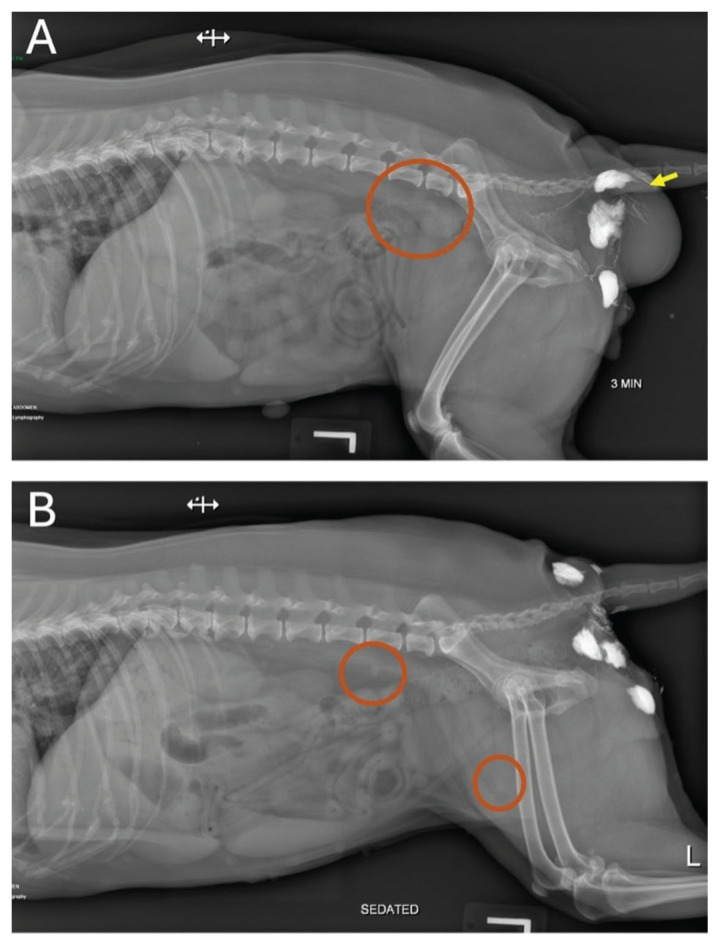
Figure 2.
Lymphography images from a dog with a mast cell tumor (MCT) in the right perianal region preoperatively (A) and 20 d after surgery (B). A — Left lateral radiograph of the caudal abdomen conducted 3 min after injection of water-soluble iodinated contrast (WIC) medium (Iopamadol) around an MCT in the right perianal region (arrow). Contrast uptake can be seen within the afferent lymphatics surrounding the tumor and within the right medial iliac lymph node (circled). B — Lateral radiograph of the caudal abdomen taken 5 min after injection of WIC medium at the scar line in the right perianal region. Contrast uptake can be seen within the lymphatics in the perineum, with uptake noted in the right medial iliac lymph node and right superficial inguinal lymph node (circled). This denotes a patient with partial agreement.
The average and median time to SLN identification for postoperative studies was 3 and 2 min, respectively (range: 0 to 9 min). No difference was seen in time to SLN identification between preoperative and postoperative SLN mapping studies (P = 0.234).
Discussion
The results of this study provide evidence that surgery alters the findings of SLN mapping in dogs with tumors. In dogs with partial agreement between SLN mapping studies, the number of SLNs identified preoperatively or postoperatively was inconsistent, as some dogs had more SLNs identified preoperatively, and some had more identified postoperatively. Lastly, the time to SLN identification varied throughout the studies, with no significant difference noted between preoperative and postoperative SLN mapping studies. Given these findings, the reliability of staging MCT dogs postoperatively must be considered cautiously, as it could increase the risk of falsely identifying the SLN or missing additional SLNs.
The SLN identified was compared with the locoregional lymph node (LRLN) determined using the canine lymphatic territories previously reported (9). A complete match rate was seen in 62.5% of cases and 1 dog had no match. These results are similar to previously reported differences of 27 to 63% between the SLN and LRLN with MCTs (13,16,18). A benefit of SLN mapping was its ability to identify multiple SLNs in 33.3% dogs, which would have been missed if relying solely on the LRLN for identification. Since the exact drainage patterns between different lymphocentrums are unknown, it is possible the additional lymph nodes could represent second tier lymph nodes as opposed to true sentinel lymph nodes.
Although some authors define the SLN as the first lymph node in a lymphatic pathway to uptake contrast, lymphangiogenesis and the recruitment of a larger number of lymph nodes has been seen as a result of neoplasia (22,25). Alterations in lymphatic drainage patterns may therefore result in additional lymphatic channels that directly lead to a presumed second tier lymph node independently.
Some MCTs showed drainage via separate lymphatic pathways, such as an MCT on the thigh that identified both the left popliteal lymph node and left superficial inguinal lymph node as SLNs. Uptake of a contralateral lymph node was also identified in 1 dog. These results highlight the importance of SLN mapping to identify additional and possibly metastatic draining lymph nodes that may alter prognosis and treatment.
Radiographic indirect lymphography (IL) has been shown to be a reliable, cost-effective, and easily accessible method for carrying out SLN mapping, with success rates reported from 77.9 to 96.6% (12,14,19,20,26). These previously referenced studies all included the use of a lipid soluble contrast agent, with some reporting the use of a WIC medium as unsuccessful (8,25,27). Despite this, successful use of a WIC medium for radiographic IL was recently reported by Hlusko et al (19), with successful SLN identification seen in 8/9 dogs.
In the present study, a success rate of 77.4% was attained with the use of a WIC medium, with 7 dogs having a negative preoperative study. Further review of tumors with a non-diagnostic study revealed that most were smaller in size, ~1 cm or less in diameter, with none larger than 3 cm. The effect of tumor size on the success of SLN mapping is presently not understood. The exact distance from the tumor for contrast injection to allow for the best lymphography study is currently not known.
Tumor location with respect to negative IL studies was variable, as 3 were subcutaneous, 4 were dermal, and all were at different anatomic locations on the body. There was therefore no definite conclusion as to why these studies were negative. Given the smaller tumor size, however, it is possible that lymphatics were less established, and it was therefore not possible to visualize the uptake of contrast.
Another possibility is the site of the contrast injection, either cutaneous or subcutaneous, in regard to the tumors. As previously discussed, palpation of tumors is not a reliable way to determine depth of MCT (16), and therefore this could have resulted in the contrast injection being incorrectly administered subcutaneously for dermal tumors or vice versa. Regardless, indirect lymphography with use of WIC medium is a quick, reliable, and cost-effective method of SLN mapping with a low patient risk that can be carried out in many general practices under light sedation and without the need for advanced equipment.
Sentinel lymph node (SLN) mapping is an important diagnostic test used to stage and treat many cancers in humans. Aberrant lymphatic drainage has been identified on repeat SLN mapping in patients with recurrent breast cancer (28–32). In a recent meta-analysis by Ahmed et al (30), the rate of aberrant drainage was 25.7%, with the rate being significantly greater when the original surgery included axillary lymph node dissection. Although this finding is not unexpected, as altered drainage would be anticipated after a lymphadenectomy is conducted, aberrant drainage was still noted in 17.4% of cases in which an SLN biopsy alone was carried out (31).
Further evidence of the effect of surgical intervention on lymphatics can be seen in women suffering from lymphedema after breast-conserving surgery. On comparison of lymphography patterns in these patients with healthy controls, collateral axillary lymphatic drainage was noted in 90% of the lymphedema patients and in none of the controls (33). Lastly, lymphatic drainage has been evaluated 1 and 6 mo after abdominoplasty, with only 15% of cases exhibiting the same drainage pathway as seen preoperatively (34). As 40% of dogs in our study had altered lymphatic drainage postoperatively, these results support these findings.
The ability to determine cutaneous versus subcutaneous location of a tumor based on preoperative palpation alone has been questioned, with Lapsley et al (16) noting a 35% disagreement after histopathologic analysis. On evaluation of the 27 surgical cases in our study, a smaller discrepancy was seen, as 22.2% (6/27) disagreement was noted between palpation and histopathology (Table I).
There was a higher number of palpable subcutaneous tumors (N = 7) within the MCT group dismissed from the study as opposed to dogs that completed the study (n = 2), which raises the question of whether indirect lymphography (IL) was less successful in subcutaneous tumors. Further review of the 7 cases revealed that only 3 of those tumors resulted in a negative study, as 3 of those dogs went on to receive a lymphadenectomy and 1 never had surgery due to lack of owner follow-through. Furthermore, 1 of the 3 presumed subcutaneous tumors with a negative SLN mapping study was noted to be cutaneous on histopathology. Thus, based on this study, subcutaneous location of tumors did not appear to influence the success rate of SLN mapping using radiographic IL.
A limitation of this study was the small overall sample size. Given that this was a prospective clinical study, the overall dropout rate was higher than anticipated due to both lack of owner follow-through and the recommendation for lymphadenectomy in some cases. It is therefore difficult to generalize based on the results and further studies are needed.
As this study was carried out at 1 institution, there were subtle alterations in lymphography technique and interpretation of SLN mapping may vary among a larger population of clinicians. Ultimately, a multi-institutional study would help not only to improve overall case numbers, but also to eliminate any possible bias or differences seen in lymphography technique or surgical intervention.
Another limitation noted was the difficulty in standardizing the lymphography studies. Although all studies were carried out by 1 of the authors using the same technique for sedation and contrast injection, it was difficult to standardize radiographic timing. Since many different tumor locations were involved, some dogs required more repositioning between radiographs to better evaluate and follow the lymphatic contrast uptake. Although all studies were completed in less than 17 min, it was noted that contrast uptake was faster in some dogs than in others, so that radiographs were taken at a quicker rate in order to map lymphatic flow and identify the SLN. Given the differences in contrast uptake and the need for patient repositioning, timing for radiographic acquisition could not be standardized.
Similarly, patient positioning among all lymphography studies depended on the tumor location. As it has been previously reported that patient positioning may impact lymphatic drainage, it is possible that the different positioning of the dogs in this study impacted drainage (11). Despite this, since each patient acted as its own control, positioning was similar for both preoperative and postoperative studies and likely did not greatly impact SLN mapping results.
Based on the results of this study, surgery appears to affect lymphatic drainage patterns, which resulted in alterations seen on SLN mapping from the surgical scar line. A complete agreement between studies done before and after surgery was seen in less than half of cases and there was no agreement in 20% of cases. Time to SLN identification did not appear to be affected by surgery and the number of SLNs identified did not vary between studies done before and after surgery.
Although additional studies are needed to evaluate the effect of surgical intervention on lymphatic drainage, the accuracy of retroactive lymph node staging in dogs with previously excised MCTs using SLN mapping alone should be questioned.
Acknowledgments
This study was funded by Auburn University College of Veterinary Medicine, Department of Clinical Sciences. The authors thank Elizabeth Stickelmaier, LVT, for assisting with enrollment and finances for the study.
Funding Statement
This study was funded by Auburn University College of Veterinary Medicine, Department of Clinical Sciences. The authors thank Elizabeth Stickelmaier, LVT, for assisting with enrollment and finances for the study
References
- 1.Welle MM, Bley CR, Howard J, Rüfenacht S. Canine mast cell tumours: A review of the pathogenesis, clinical features, pathology and treatment. Vet Dermatol. 2008;19:321–339. doi: 10.1111/j.1365-3164.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 2.Warland J, Amores-Fuster I, Newbury W, Brearley M, Dobson J. The utility of staging in canine mast cell tumours. Vet Comp Oncol. 2014;12:287–298. doi: 10.1111/vco.12012. [DOI] [PubMed] [Google Scholar]
- 3.Krick EL, Billings AP, Shofer FS, Watanabe S, Sorenmo KU. Cytological lymph node evaluation in dogs with mast cell tumours: Association with grade and survival. Vet Comp Oncol. 2009;7:130–138. doi: 10.1111/j.1476-5829.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 4.Stefanello D, Buracco P, Sabattini S, et al. Comparison of 2- and 3-category histologic grading systems for predicting the presence of metastasis at the time of initial evaluation in dogs with cutaneous mast cell tumors: 386 cases (2009–2014) J Am Vet Med Assoc. 2015;246:765–769. doi: 10.2460/javma.246.7.765. [DOI] [PubMed] [Google Scholar]
- 5.Sledge DG, Webster J, Kiupel M. Canine cutaneous mast cell tumors: A combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet J. 2016;215:43–54. doi: 10.1016/j.tvjl.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Weishaar KM, Thamm DH, Worley DR, Kamstock DA. Correlation of nodal mast cells with clinical outcome in dogs with mast cell tumour and a proposed classification system for the evaluation of node metastasis. J Comp Pathol. 2014;151:329–338. doi: 10.1016/j.jcpa.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Murphy S, Sparkes AH, Blunden AS, Brearley MJ, Smith KC. Effects of stage and number of tumours on prognosis of dogs with cutaneous mast cell tumours. Vet Rec. 2006;158:287–291. doi: 10.1136/vr.158.9.287. [DOI] [PubMed] [Google Scholar]
- 8.Patsikas MN, Karayannopoulou M, Kaldrymidoy E, et al. The lymph drainage of the neoplastic mammary glands in the bitch: A lymphographic study. Anat Histol Embryol. 2006;35:228–234. doi: 10.1111/j.1439-0264.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 9.Suami H, Yamashita S, Soto-Miranda MA, Chang DW. Lymphatic territories (lymphosomes) in a canine: An animal model for investigation of postoperative lymphatic alterations. PLoS One. 2013;8:e69222. doi: 10.1371/journal.pone.0069222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier Q, Thierry F, Longo M, et al. Contrast-enhanced ultrasound for sentinel lymph node mapping in the routine staging of canine mast cell tumours: A feasibility study. Vet Comp Oncol. 2021;19:451–462. doi: 10.1111/vco.12647. [DOI] [PubMed] [Google Scholar]
- 11.Majeski SA, Steffey MA, Fuller M, Hunt GB, Mayhew PD, Pollard RE. Indirect computed tomographic lymphography for iliosacral lymphatic mapping in a cohort of dogs with anal sac gland adenocarcinoma: Technique description. Vet Radiol Ultrasound. 2017;58:295–303. doi: 10.1111/vru.12482. [DOI] [PubMed] [Google Scholar]
- 12.Patsikas MN, Papadopoulou PL, Charitanti A, et al. Computed tomography and radiographic indirect lymphography for visualization of mammary lymphatic vessels and the sentinel lymph node in normal cats. Vet Radiol Ultrasound. 2010;51:299–304. doi: 10.1111/j.1740-8261.2009.01657.x. [DOI] [PubMed] [Google Scholar]
- 13.Worley DR. Incorporation of sentinel lymph node mapping in dogs with mast cell tumours: 20 consecutive procedures. Vet Comp Oncol. 2014;12:215–226. doi: 10.1111/j.1476-5829.2012.00354.x. [DOI] [PubMed] [Google Scholar]
- 14.Brissot HN, Edery EG. Use of indirect lymphography to identify sentinel lymph node in dogs: A pilot study in 30 tumours. Vet Comp Oncol. 2017;15:740–753. doi: 10.1111/vco.12214. [DOI] [PubMed] [Google Scholar]
- 15.Herring ES, Smith MM, Robertson JL. Lymph node staging of oral and maxillofacial neoplasms in 31 dogs and cats. J Vet Dent. 2002;19:122–126. doi: 10.1177/089875640201900301. [DOI] [PubMed] [Google Scholar]
- 16.Lapsley J, Hayes GM, Janvier V, et al. Influence of locoregional lymph node aspiration cytology vs sentinel lymph node mapping and biopsy on disease stage assignment in dogs with integumentary mast cell tumors. Vet Surg. 2021;50:133–141. doi: 10.1111/vsu.13537. [DOI] [PubMed] [Google Scholar]
- 17.Grimes JA, Secrest SA, Wallace ML, Laver T, Schmiedt CW. Use of indirect computed tomography lymphangiography to determine metastatic status of sentinel lymph nodes in dogs with a pre-operative diagnosis of melanoma or mast cell tumour. Vet Comp Oncol. 2020;18:818–824. doi: 10.1111/vco.12592. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari R, Chiti LE, Manfredi M, et al. Biopsy of sentinel lymph nodes after injection of methylene blue and lymphoscintigraphic guidance in 30 dogs with mast cell tumors. Vet Surg. 2020;49:1099–1108. [Google Scholar]
- 19.Hlusko KC, Cole R, Tillson DM, et al. Sentinel lymph node detection differs when comparing lymphoscintigraphy to lymphography using water soluble iodinated contrast medium and digital radiography in dogs. Vet Radiol Ultrasound. 2020;61:659–666. doi: 10.1111/vru.12908. [DOI] [PubMed] [Google Scholar]
- 20.Haas S, Linden D, Cole R, Smith A, Schleis S, Matz B. Indirect lymphography for sentinel lymph node detection in dogs with mast cell tumors. Can Vet J. 2023;64:142–148. [PMC free article] [PubMed] [Google Scholar]
- 21.Hlusko KC, Cole R, Tillson DM, et al. The effect of surgery on lymphoscintigraphy drainage patterns from the canine brachium in a simulated tumor model. Vet Surg. 2020;49:1118–1124. doi: 10.1111/vsu.13473. [DOI] [PubMed] [Google Scholar]
- 22.Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soultani C, Patsikas MN, Karayannopoulou M, et al. Assessment of sentinel lymph node metastasis in canine mammary gland tumors using computed tomographic indirect lymphography. Vet Radiol Ultrasound. 2017;58:186–196. doi: 10.1111/vru.12460. [DOI] [PubMed] [Google Scholar]
- 24.Grimes JA, Reed RA, Beale C, Secrest SA. Effect of contrast agent viscosity and massage on success of computed tomography lymphangiography with aqueous contrast for sentinel lymph node identification in healthy dogs. Vet Comp Oncol. 2021;19:587–592. doi: 10.1111/vco.12698. [DOI] [PubMed] [Google Scholar]
- 25.Pereira CT, Rahal SC, de Carvalho Balieiro JC, Ribeiro AA. Lymphatic drainage on healthy and neoplasic mammary glands in female dogs: Can it really be altered? Anat Histol Embryol. 2003;32:282–290. doi: 10.1046/j.1439-0264.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- 26.De Bonis A, Collivignarelli F, Paolini A, et al. Sentinel lymph node mapping with indirect lymphangiography for canine mast cell tumour. Vet Sci. 2022;9:484. doi: 10.3390/vetsci9090484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaude JV, Abrams RM, Daly JW. Percutaneous indirect lymphography with a new experimental contrast medium: A preliminary report. Angiology. 1978;29:162–168. doi: 10.1177/000331977802900210. [DOI] [PubMed] [Google Scholar]
- 28.Tokmak H, Kaban K, Muslumanoglu M, Demirel M, Aktan S. Management of sentinel node re-mapping in patients who have second or recurrent breast cancer and had previous axillary procedures. World J Surg Oncol. 2014;12:205. doi: 10.1186/1477-7819-12-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folli S, Falco G, Mingozzi M, et al. Repeat sentinel lymph node biopsy in patients with ipsilateral recurrent breast cancer after breast-conserving therapy and negative sentinel lymph node biopsy: A prospective study. Minerva Chir. 2016;71:73–79. [PubMed] [Google Scholar]
- 30.Ahmed M, Baker R, Rubio IT. Meta-analysis of aberrant lymphatic drainage in recurrent breast cancer. Br J Surg. 2016;103:1579–1588. doi: 10.1002/bjs.10289. [DOI] [PubMed] [Google Scholar]
- 31.Maaskant-Braat AJ, Voogd AC, Roumen RM, Nieuwenhuijzen GA. Repeat sentinel node biopsy in patients with locally recurrent breast cancer: A systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2013;138:13–20. doi: 10.1007/s10549-013-2409-1. [DOI] [PubMed] [Google Scholar]
- 32.Newman EA, Cimmino VM, Sabel MS, et al. Lymphatic mapping and sentinel lymph node biopsy for patients with local recurrence after breast-conservation therapy. Ann Surg Oncol. 2006;13:52–57. doi: 10.1245/ASO.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Heydon-White A, Suami H, Boyages J, Koelmeyer L, Peebles KC. Assessing breast lymphoedema following breast cancer treatment using indocyanine green lymphography. Breast Cancer Res Treat. 2020;181:635–644. doi: 10.1007/s10549-020-05661-y. [DOI] [PubMed] [Google Scholar]
- 34.Bassalobre M, Liebano RE, da Silva MP, et al. Changes in the pattern of superficial lymphatic drainage of the abdomen after abdominoplasty. Plast Reconstr Surg. 2022;149:1106e–1113e. doi: 10.1097/PRS.0000000000009114. [DOI] [PubMed] [Google Scholar]