Abstract
The objective of this study was to evaluate whether supplementation with probiotics over a 2-week period stabilizes the gut microbiota in dogs following prolonged cefovecin treatment. A significant number of clinical veterinarians prescribe oral probiotics to dogs in conjunction with systemic antibiotics with the intention of protecting against gut dysbiosis. The effects of antibiotics and probiotics in dogs have not been extensively studied, however, and the optimal treatment for gut dysbiosis remains uncertain. To investigate the impact of cefovecin and probiotics on the gut microbiota, 12 healthy companion dogs that underwent surgical castration were included in the study. The dogs were administered cefovecin immediately after surgery. Of the 12 dogs, 7 dogs were supplemented with oral probiotics for 2 wk after cefovecin treatment (probiotic group), whereas the other 5 dogs were not supplemented with probiotics (non-probiotic group). Fecal samples were collected from each dog before and 2 wk after cefovecin treatment and subjected to 16S rRNA gene sequencing using the Illumina platform. We noted that cefovecin induced changes in bacterial diversity of the gut microbiota, with the Shannon index values of the non-probiotic group decreasing significantly, whereas those of the probiotic group remained stable (P = 0.025). Our findings suggest that supplementation with oral probiotics is recommended for preventing cefovecin-induced gut dysbiosis in dogs.
Résumé
L’objectif de cette étude était d’évaluer si une supplémentation en probiotiques sur une période de 2 semaines stabilise le microbiote intestinal chez les chiens après un traitement prolongé à la céfovécine. Un nombre significatif de vétérinaires cliniciens prescrivent des probiotiques oraux aux chiens en association avec des antibiotiques systémiques dans le but de les protéger contre la dysbiose intestinale. Les effets des antibiotiques et des probiotiques chez les chiens n’ont cependant pas été étudiés de manière approfondie et le traitement optimal de la dysbiose intestinale reste incertain. Pour étudier l’impact de la céfovécine et des probiotiques sur le microbiote intestinal, 12 chiens de compagnie en bonne santé ayant subi une castration chirurgicale ont été inclus dans l’étude. Les chiens ont reçu de la céfovécine immédiatement après la chirurgie. Sur les 12 chiens, 7 chiens ont reçu un supplément de probiotiques oraux pendant 2 semaines après le traitement à la céfovécine (groupe probiotique), tandis que les 5 autres chiens n’ont pas reçu de supplément de probiotiques (groupe non probiotique). Des échantillons de selles ont été prélevés sur chaque chien avant et 2 semaines après le traitement à la céfovécine et soumis au séquençage du gène ARNr 16S à l’aide de la plateforme Illumina. Nous avons constaté que la céfovécine induisait des changements dans la diversité bactérienne du microbiote intestinal, les valeurs de l’indice de Shannon du groupe sans probiotique diminuant significativement, tandis que celles du groupe probiotique restaient stables (P = 0,025). Nos résultats suggèrent qu’une supplémentation en probiotiques oraux est recommandée pour prévenir la dysbiose intestinale induite par la céfovécine chez les chiens.
(Traduit par Docteur Serge Messier)
Gut microbiota is defined as the collective of all living microorganisms, including bacteria, fungi, protozoa, and viruses within the gut (1). The gut microbiota plays a crucial role in host health, including with development, digestion, behavior, and functioning of the immune system (2,3). A reduction in microbial diversity or an imbalance in microbiota composition, which is termed dysbiosis, is closely associated with pathogenic conditions, including chronic enteropathy and colorectal cancer (4,5). Gut dysbiosis can be caused by several factors, including dietary changes, pathogenic conditions, or antibiotic therapy, with antibiotic use being one of the major factors underlying gut dysbiosis in humans (6).
Probiotics are defined as “live microorganisms, such as Lactobacillus spp. and Bifidobacterium spp., that provide health benefits to their hosts when administered in appropriate quantities (7). In recent years, probiotics are being consumed as supplements because of their beneficial effects for the host. Probiotics can help restore bacterial balance in the digestive system and lead to better digestive health after normal function has been disrupted by factors such as stress, infection, disease, or antibiotic therapy (8,9). However, the protective effect of probiotics on gut dysbiosis induced by antibiotics has not been elucidated in dogs.
A previous study investigating changes in the gut microbiota of dogs following perioperative cefazolin treatment and the protective effect of probiotics during a 48-hour period reported no change in the dysbiosis index in dogs following administration of perioperative antibiotics, anesthesia, and surgery (10). In the present study, we evaluated whether supplementation with probiotics over a 2-week period stabilizes the gut microbiota in dogs following prolonged cefovecin treatment.
Twelve healthy companion dogs that underwent surgical castration were included in this study. All dogs underwent a comprehensive physical examination, blood tests, and chest X-rays. Written, informed consent was obtained from the owners before enrolment in the study. Fecal samples were collected from the dogs by a veterinarian using a swab before and 2 wk after cefovecin treatment for gut microbiota analysis.
The dogs were injected with cefovecin (Zoetis, Parsippany, New Jersey, USA) [8 mg/kg body weight (BW)] immediately after surgery as a prophylactic treatment. After the surgery, 7 dogs (probiotic group) were supplemented daily with 2 g of oral probiotics (Estien, Seoul, South Korea) following meals. The 2-gram dose of probiotics contained the following 5 bacterial species: Bifidobacterium bifidum [> 1 × 1010 colony-forming units (CFUs)]; Bifidobacterium longum (> 5 × 108 CFUs); Lactobacillus acidophilus (> 2 × 109 CFUs); Lactobacillus casei (> 2.5 × 109 CFUs); and Enterococcus faecium (> 3 × 109 CFUs). The other 5 dogs (non-probiotic group) were not supplemented with probiotics.
Fecal samples were collected using a DNA/RNA Shield Collection Tube with Swab (Zymo Research, Tustin, California, USA). All samples were stored at −80°C for further analysis. Genomic DNA was extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and the extracted DNA was quantified using Quant-iT PicoGreen (Invitrogen, Waltham, Massachusetts, USA).
We amplified the V3–V4 hypervariable region (~469 bp) of the 16S rRNA gene using 2 universal primers with adapter overhang sequences: V3-F, 5′-TCGTCGGCAGCGTCAGATGTGT ATAAGAGACAGCCTACGGGNGGCWGCAG-3′; and V4-R, 5′-GT CTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTA CHVGGGTATCTAATCC-3′.
The amplicons were sequenced using the Illumina MiSeq platform (San Diego, California, USA). Raw Illumina MiSeq data were classified using an index sequence and a paired-end FASTQ file was created for each sample. The FASTQ files were submitted to the Sequence Read Archive (SRA) at the National Center for Biotechnology Information (NCBI) (accession no. SRP414109). The DADA2 plugin in QIIME2 (version 2021.8) (Garratt-Callahan, Burlingame. California, USA) was used to denoise amplicon sequences. The collected amplicon sequence variants (ASVs) were used for microbial diversity and relative abundance analyses using the QIIME2 (11) and Microbiome Analyst packages in R (R Foundation, Vienna, Austria) (https://www.r-project.org/) (12).
Alpha diversity, which is microbial diversity within a single sample, is expressed by 2 major indices: richness and evenness. Richness refers to the number of species inhabiting a given area and evenness describes the distribution of abundance or proportions of each taxon in the community. Alpha diversity within each sample was analyzed using the Shannon diversity index (13), considering both richness and evenness.
Linear mixed-effects (LME) modelling was then used to validate whether administration of probiotics caused significant differences in alpha diversity over time after antibiotic administration. In the LME model, we set the probiotic treatment as fixed effects and age and weight as random effects. Statistical significance was set at P < 0.05.
Beta diversity refers to the composition measured by the species change between 2 given areas. Beta diversity between the samples before and after cefovecin treatment was analyzed using the unweighted UniFrac distance and the Bray Curtis dissimilarity index (14). The unweighted UniFrac distance was obtained by calculating the presence/absence of species by coupling phylogenetic extension (14), whereas the Bray Curtis dissimilarity index was obtained by examining both the abundance of microbes shared between 2 samples and the number of microbes found in each. Beta diversity between groups was compared with a permutational multivariate analysis of variance (PERMANOVA) and no statistical significance was established. Statistical significance was set at P < 0.05.
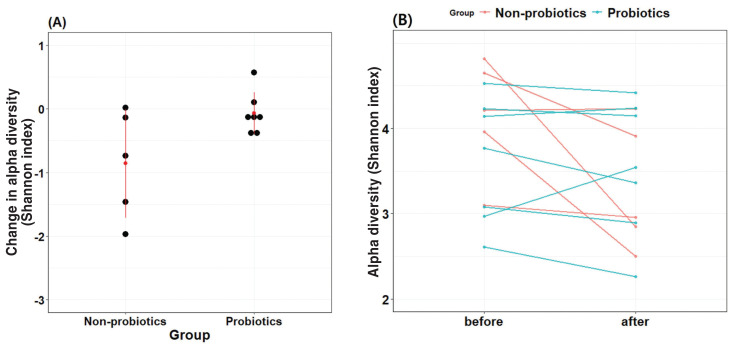
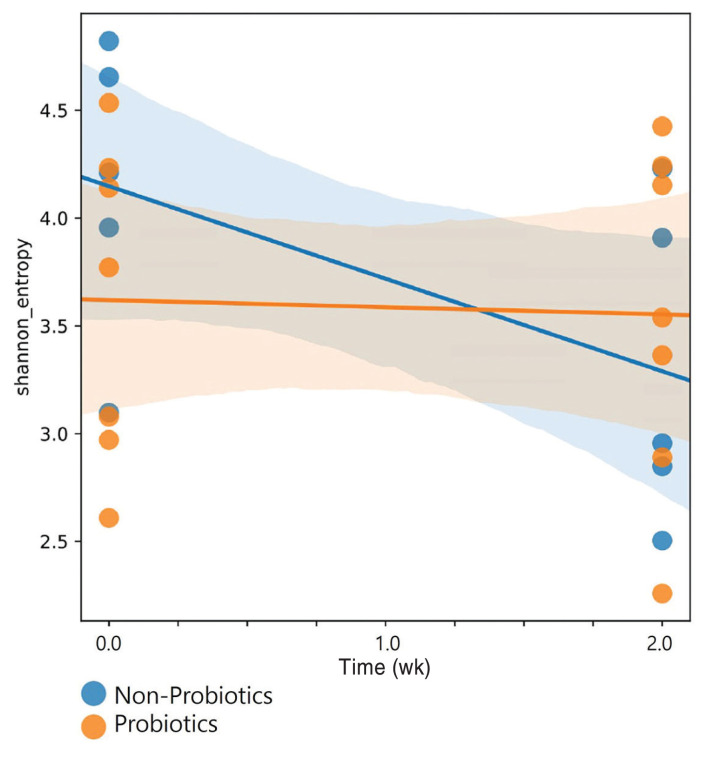
The change in Shannon diversity index before and after antibiotic administration in the probiotic group was not greater than that in the non-probiotic group (Figure 1). In the LME model, the Shannon index values of the non-probiotic group significantly decreased following antibiotic treatment, which indicates a loss of microbial diversity. In contrast, the Shannon index values of the probiotic group remained stable, indicating that probiotic supplementation helped maintain microbial diversity by preventing this decline (Figure 2).
Figure 1.
Comparison of changes in alpha diversity between the probiotics and non-probiotics groups based on the Shannon index. A — The red dot and red line represent a mean value and 1 standard deviation distance from the mean, respectively. The graphs show that, compared to the probiotics group, the non-probiotics group exhibited a greater decrease on average in the Shannon index. B — The Shannon index in both groups before and after probiotic supplementation. Individual “before” and “after” values are presented as connected dot plots.
Figure 2.
Comparison of changes in alpha diversity between the probiotics and non-probiotics groups as per the Shannon index with the linear mixed-effects (LME) model. The non-probiotic group exhibited a decreasing tendency in the Shannon index, whereas the probiotic group maintained similar levels in the Shannon index during antibiotic treatment (total 2 wk). (P = 0.025).
The estimated change in Shannon index across time and probiotic treatment was 0.198 (P = 0.025), which was significant (Table I). This suggests that probiotic supplementation helps to prevent antibiotic-induced declines in gut microbial diversity and assists in maintaining its stability.
Table I.
Results of linear mixed-effects (LME) model estimation.
| Fixed effects | Random effects | ||||
|---|---|---|---|---|---|
|
| |||||
| Intercept | Treatment | Time | Time × Treatment | ||
| Shannon index | 4.194 (0.000) | −0.554 (0.122) | −0.215 (0.001) | 0.198 (0.025) | Age, Weight, Age × Weight included |
Note: Numbers in table represent coefficient estimates from estimation of LME model.
P-values are shown in parentheses.
These results suggest that cefovecin treatment induced changes in the bacterial diversity (alpha diversity) in the gut microbiota of dogs and that probiotic supplementation is potentially effective in attenuating those changes. There were not statistical significances in beta diversity.
Antibiotic treatment can cause an imbalance in the gut microbiota. Broad-spectrum antibiotics can affect the relative abundance of approximately 30% of taxa in the gut microbiota in humans (2), causing a rapid and significant reduction in the microbial diversity of the gut microbiota (2,15). Recovery from antibiotic-induced dysbiosis depends on the resilience of the bacterial species, however, and reestablishment of the original microbiota composition is challenging. Microbiota alterations induced by antibiotics can persist for months or even years in humans (15–17).
Cefovecin, a third-generation cephalosporin, exhibits broadspectrum anti-microbial activity. It is administered subcutaneously and has a long elimination half-life (5.5 d in dogs), which allows 14-day dosing intervals in dogs (18). In the present study, cefovecin treatment reduced the alpha diversity of the gut microbiota, similar to that reported with the use of tylosin and metronidazole in previous studies. Healthy dogs that were administered tylosin (7 d of treatment) or metronidazole (2 wk of treatment) exhibited decreased alpha diversity, which did not fully recover even after antibiotics had been discontinued for over 4 wk (19,20).
The present study is limited by the small sample size, as well as the fact that only 1 antibiotic (cefovecin) was considered. Nonetheless, it serves as an important preliminary field trial and proof-of-principle and its findings should be validated in clinical trials with a larger sample size, emphasizing the use of probiotic supplementation with different antibiotics.
Based on the alpha diversity analyses carried out in the present study, we conclude that cefovecin treatment induced gut dysbiosis in dogs; however, no evidence of severe dysbiosis was found in dogs that were supplemented with probiotics following cefovecin treatment. The findings of this study suggest that probiotics may prevent cefovecin-induced gut dysbiosis in dogs by helping to maintain intrinsic gut microbiota.
References
- 1.Suchodolski JS. Dysbiosis and the use of pre-, pro- and synbiotics. In: Bruyette D, editor. Clinical Small Animal Internal Medicine. 1st ed. Hokoben, New Jersey: John Wiley & Sons; 2020. pp. 621–626. [Google Scholar]
- 2.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondo E, Marliani G, Accorsi PA, Cochi M, DiLeone A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet J. 2019;9:253–258. doi: 10.4314/ovj.v9i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng M, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013;7:1256–1261. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francino MP. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front Microbiol. 2016;6:1543. doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 8.Kelley RL, Minikhiem D, Kiely B, et al. Clinical benefits of probiotic canine-derived Bifidobacterium animalis strain AHC7 in dogs with acute idiopathic diarrhea. Vet Ther. 2009;10:121–130. [PubMed] [Google Scholar]
- 9.Schmitz SS. Value of probiotics in canine and feline gastroenterology. Vet Clin North Am Small Anim Pract. 2021;51:171–217. doi: 10.1016/j.cvsm.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Lucchetti B, Lane SL, Koenig A, Good J, Suchodolski JS, Brainard BM. Effects of a perioperative antibiotic and veterinary probiotic on fecal dysbiosis index in dogs. Can Vet J. 2021;62:240–246. [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. [Google Scholar]
- 14.Lozupone C, Knight R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl Eniron Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 17.De La Cochetière MF, Durand T, Lepage P, Bourreillé A, Galmiche JP, Doré J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol. 2005;43:5588–5592. doi: 10.1128/JCM.43.11.5588-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stegemann M, Sherington J, Blanchflower S. Pharmacokinetics and pharmacodynamics of cefovecin in dogs. J Vet Pharmacol Ther. 2006;29:501–511. doi: 10.1111/j.1365-2885.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 19.Manchester AC, Webb CB, Blake AB, et al. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J Vet Intern Med. 2019;33:2605–2617. doi: 10.1111/jvim.15635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilla R, Gaschen FP, Barr JW, et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J Vet Intern Med. 2020;34:1853–1866. doi: 10.1111/jvim.15871. [DOI] [PMC free article] [PubMed] [Google Scholar]