Visual Abstract
Abstract
The management of immune thrombocytopenia (ITP) is continuously evolving with the development and introduction of newer therapies and a better understanding of the disease. Corticosteroids still represent the cornerstone of first-line treatment. Patients who fail to achieve remission with a short course of corticosteroids require subsequent therapy. Most guidelines recommend starting with either a thrombopoietin receptor agonist (TPO-RA), rituximab, or fostamatinib since these agents have been investigated in randomized trials and have well-characterized efficacy and safety profiles. Patients' involvement to reach a shared decision regarding choice of therapy is essential as these treatments have different modes of administration and mechanisms of action. Less than 10% will fail to respond to and/or be intolerant of multiple second-line therapeutic options and thus be considered to have refractory ITP and require a third-line therapeutic option. Such patients may require drugs with different targets or a combination of drugs with different mechanisms of action. Combining a TPO-RA and an immunomodulatory agent may be an appropriate approach at this stage. Many studies have been conducted during the last 2 decades investigating the efficacy and safety of combinations strategies for first and later lines of therapies. Yet none of these are recommended by current guidelines or have gained wide acceptance and consensus.
Learning Objectives
Describe the current ITP treatment paradigm, which is centered mainly on sequential monotherapy
Compare the efficacy and safety of combination therapy vs monotherapy for up-front and subsequent treatment of ITP
CLINICAL CASE
A 23-year-old woman was diagnosed with immune thrombocytopenia (ITP) 6 weeks after a COVID infection. She responded to a 6-week course of prednisolone. Her platelet count increased from 3 to 260 × 109/L. Twelve months later she was admitted with a platelet count of 5 × 109/L, mucocutaneous bleeding, and menorrhagia. She received prednisolone and IV immunoglobulin (IVIg), resulting in a transient increase in the platelet count to 38 × 109/L. Three days later, she was readmitted with epistaxis and a platelet count of 8 × 109/L. Other conditions were excluded, and a diagnosis of primary ITP was confirmed. After discussing available options and following her wish to become pregnant, the physician and patient agreed on rituximab. Due to a persistently low platelet count of <10 × 109/L after rituximab, avatrombopag was initiated.
The management of ITP is continuously evolving with the development of new therapies, improved understanding of pathophysiology, and expanding knowledge of health-related quality of life and disease burden.
Current guidelines emphasize the need for patient involvement and shared decision-making.1,2 The management of ITP should start by defining treatment goals—a process that should be based on patient education and dialogue to reach a common understanding of the expectations and goals of treatment. Our main goals when treating ITP are to prevent severe bleeding by achieving and maintaining a hemostatically “safe” and stable platelet count with minimal toxicity, to improve health-related quality of life (HRQoL), and whenever possible, to induce lasting treatment-free remission. A recent survey has identified important differences between patients' and physicians' goals and perceptions of ITP. Apart from remission, the 3 top treatment goals identified by patients were healthy platelet counts, prevention of episodes of worsening ITP, and increased energy levels.3
Because of the lack of reliable biomarkers to facilitate a personalized approach to treatment, current strategies are still based on sequential administration of single agents based on factors described in Table 1. Switching to the next drug is indicated because of suboptimal response, toxicity, inconvenience, or occasionally cost.
Table 1.
Factors determining treatment selection
| Main category | Specific factors |
|---|---|
| Clinical factors | Presence of bleeding, risk of bleeding, thromboembolism, and infection |
| Access to medications | Availability of medication/expertise, regulatory restrictions, label, and price |
| Patient profile | Age, concurrent medications (eg, anticoagulants), comorbidities, desire for pregnancy in women of childbearing age |
| Drug properties | Onset of action, efficacy, safety profiles, effect on HRQoL, and induction of SROT |
| Patient preferences | Maintenance or remission-inducing therapies, compliance, lifestyle, and hobbies |
HRQoL, health-related quality of life; SROT, sustained response off-treatment.
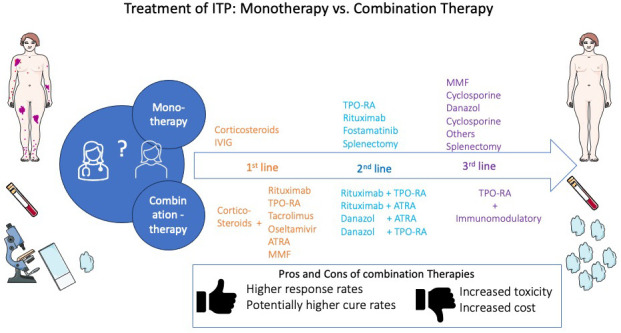
Mono vs combination therapies
The main approaches for treating ITP involve immunomodulation, stimulation of thrombopoiesis, or splenectomy. In ITP, monotherapy represents standard practice, and apart from certain situations (eg, the need for rescue therapy or refractory disease), combination therapy is rarely used. Table 2 summarizes the characteristics of the main drugs used as initial and subsequent therapy.
Table 2.
Characteristics of first- and second-line drugs
| Agent | Indication | Administration | Response rates | Main side effects |
|---|---|---|---|---|
| Predniso(lo)ne | Initial therapy | Oral, short course of up to 8 weeks | Durable response rates 30%-35% | Weight gain, insomnia, glucose intolerance, mood changes, hypertension, osteoporosis |
| Dexamethasone | Initial therapy | Oral, pulse therapy over 4 days, up to 3 courses | Responses at 6 months of 35%-40% | |
| IVIg | Initial therapy and later phases as rescue | Intravenous, 1 to 5 days, can be repeated if needed | 80% achieve short-term transient response | Headache, aseptic meningitis, thromboembolism |
| Romiplostim (TPO-RA) |
Subsequent therapy | Weekly subcutaneous administration of 1-10 ug/kg,* maintenance therapy | Durable response rates of 38% in splenectomized patients and 56% in non-splenectomized17§ |
Key special warnings and precautions for use for all
TPO-RAs include: increased thromboembolic complications; increased bone marrow reticulin; eltrombopag can cause hepatotoxicity |
| Eltrombopag (TPO-RA) |
Subsequent therapy | Oral, daily administration of 25-75 mg,* maintenance therapy | Durable response rates on treatment of 60%-70%15§ | |
| Avatrombopag (TPO-RA) |
Subsequent therapy | Oral, daily administration of 20-40 mg,* maintenance therapy | Durable response rates of 34%16§ | |
| Rituximab (B-cell depleting agent) |
Subsequent therapy | 2 IV infusions of 1000 mg 2 weeks apart or 4 weekly IV infusions of 375 mg/m2, with lower doses showing effect in studies; remission-inducing therapy |
Durable response rate of 39% at 6 months1 and 29% at 5 years28 | Infusion-related reactions, immunosuppression, and slight increase in the risk of bacterial infections; reactivation of hepatitis B virus; serum sickness disease; secondary hypogammaglobulinemia |
| Fostamatinib Spleen tyrosine kinase inhibitor |
Subsequent therapy | Oral, daily administration of 100-150 mg × 2, maintenance therapy | Durable response rates of 18%27§ | Hypertension, diarrhea, hepatotoxicity, neutropenia |
Occasionally lower doses are needed. §In pivotal phase 3 trials, all agents demonstrated significantly greater durable platelet response rates vs placebo at 6 months; however, definitions of durable platelet response rate differed slightly, making direct cross-study comparisons difficult.
IVIg, IV immunoglobulin; TRO-RA, thrombopoietin receptor agonist.
Many studies have been conducted during the last 2 decades investigating the efficacy and safety of combining drugs with different mechanisms of actions. Investigated approaches involve combinations of drugs targeting autoreactive B or T cells (eg, rituximab or mycophenolate mofetil [MMF]) with corticosteroids or combinations targeting autoreactive cells and megakaryocytes (eg, corticosteroids or fostamatinib and thrombopoietin receptor agonists [TPO-RAs]). The rationale for combining drugs is mainly to improve response and/or remission rates, particularly if there is synergy between treatments with different mechanisms. The disadvantages include higher cost and the potential for increased toxicity.
We discuss the current treatment algorithm covering evidence for mono and combination therapies for initial and for subsequent therapies (Figure 1). Of note, varying efficacy endpoints and definitions have been used in clinical trials, including initial response, overall response, and/or sustained, stable, or durable response. The latter category requires multiple platelet counts >30 or 50 × 109/l within a certain period of time, which indicate a sustained response that is desired to achieve in clinical practice.
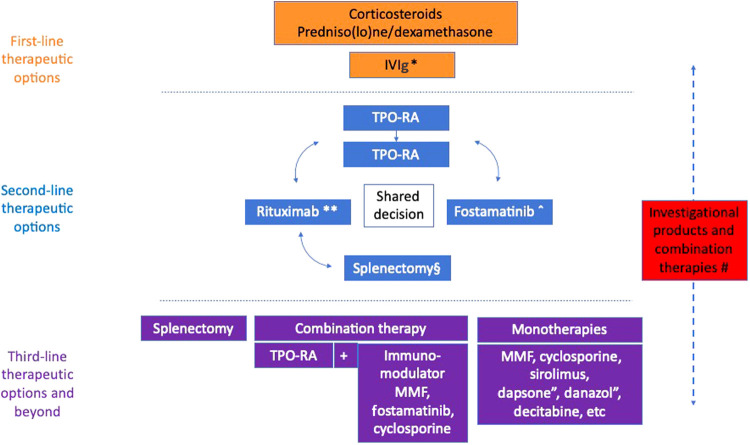
Figure 1.
Current therapeutic approach for adults with ITP: a multistep algorithm. The therapies included in the 3 categories may vary according to availability, clinician experience, and cost. Switching to the next line can be carried out before exhausting all options. *Used for patients with active serious bleeding or high risk of bleeding. **Rituximab can be used prior to TPO-RA if the patient places a high value on limiting the duration of therapy or if TPO-RA is not advised because of the risk of thromboembolism. ˆFostamatinib can be used off-label prior to TPO-RA if TPO-RA is not advised because of the risk of thromboembolism. ″Dapsone and danazol can be used in countries where alternative treatment is not available. #Investigational products should be considered in all stages, not only for refractory ITP; many combination therapies have shown promising results in the first- and second-line but are currently not considered as standard treatment. §Splenectomy is still an option that can be considered among second-line treatments, although it is recommended to delay it until at least 1 year after ITP onset. IVIg, IV immunoglobuli; MMF, mycophenolate mofetil; TOP-RA, thrombopoietin receptor agonist.
First-line therapeutic options
For initial treatment, current guidelines recommend a short course of predniso(lo)ne not exceeding 6 to 8 weeks or up to 3 courses of dexamethasone (Table 2).1,2,4 The usual platelet count threshold for starting therapy is 20-30 × 109/l. Occasionally, treatment at higher platelet levels (50 × 109/l) is indicated in patients at a high risk of bleeding (eg, those on anticoagulation). A combination of corticosteroids and IVIg is recommended in patients with severe bleeding or a high risk of bleeding, where a rapid increase in the platelet count is warranted. High dose TPO-RA can be used as an add-on therapy in patients with life-threatening bleeding not responding well to standard treatment.4
Several studies have evaluated the role of combining immunomodulatory agents with corticosteroids to improve durable response rates that persist after discontinuation of therapy (Table 3).
Table 3.
Summary of large RCTs assessing various combination therapies for treating newly diagnosed ITP (first line)
| Combination arm | Monotherapy arm | Study design | Efficacy | Remarks |
|---|---|---|---|---|
| Rituximab (375 mg/m2 weekly for 4 weeks) + dexamethasone (1-6, 4-day cycle)5 | Dexamethasone (1-6, 4-day cycle) |
Open-label RCT with 1:1 randomization, (n = 133) | Response rates at 6 months were 58% in the combination vs 37% in the dexamethasone groups, p = 0.02, with significantly longer time to relapse (p = 0.03) | Increased incidence of grade 3-4 adverse events in the combination group |
| Rituximab (375 mg/m2 weekly for 4 weeks) + dexamethasone (4-day cycle)6 | Dexamethasone (4-day cycle) |
Open-label RCT with 1:1 randomization (n = 101) | Response rate at 6 months 63% vs 36%, p = 0.004 | Increased incidence of grade 3-4 adverse events in the combination group |
| Mycophenolate mofetil (MMF) (1-2 g daily) + prednisolone or dexamethasone.7 | Prednisolone or dexamethasone | Open-label RCT with 1:1 randomization (n = 120) | Rate of treatment failure was 22% in the MMF group vs 44% in the control group; HR = 0.41 (95% CI: 0.21-0.80; p = 0.008 | Patients in the MMF group reported worse HRQoL |
| Tacrolimus (initial dose 0.03 mg/kg/day for 12 weeks) + dexamethasone8 | Dexamethasone (1-2, 4-day cycles) |
Phase 2, open-label RCT with 1:1 randomization (n = 140) | Sustained response in the combination group was 65% vs 43% in the monotherapy (p = 0.007), rates of treatment failure (19.4% vs 38.2%, p = 0.0014) | Published only in abstract form |
| rhTPO (300 ug/kg sc for up to 14 days) + dexamethasone (1-2, 4-day cycle)10 | Dexamethasone (1-2, 4-day cycle) |
Open-label RCT with 1:1 randomization (n = 206) | The combination resulted in higher initial (89% vs 67%; p < 0.001) and durable response rates at 6 months (51% vs 36%; p = 0.02) compared with dexamethasone | Well-tolerated study drugs; only one thromboembolic event occurred in the combination; rhTPO is only available in certain countries in Asia |
| Oseltamivir (75 mg × 2/day for 10 days) + dexamethasone (1-2 4-day cycle)11 | Dexamethasone (1-2, 4-day cycle) |
Phase 2 open-label, RCT with 1:1 randomization (n = 96) | Response at day 14 (86% vs 66%; p = 0.030) and at 6 month (53% vs 30%; p = 0.032) in the combination vs monotherapy groups | 19% suffered from gastrointestinal side effects in the combination group |
| ATRA (10 mg × 2/day for 12 weeks) + dexamethasone (14-day cycle)12 | Dexamethasone (1-2, 4-day cycle) |
Phase 2 open-label, RCT with 1:1 randomization (n = 132) | Sustained* response rate at 6 months in the combination arm was 68% vs 41% in the monotherapy arm ( = R = 3, p = 0.0017) | Dry skin was reported in 48% of the patients treated with ATRA |
ATRA, all-trans retinoic acid; CI, confidence interval; HR, hazard ratios; HRQoL, health related qualty of life; MMF, mycophenolate mofetil; RCT, randomized controlled trial; rhTPO, recombinant thrombopoietin receptor agonist.
Two large randomized controlled trials (RCTs) investigated the efficacy and safety of dual immunosuppression with rituximab in combination with dexamethasone as up-front therapy vs dexamethasone alone. Both studies showed significantly higher response rates at 6 months in the combination compared to the control arms.5,6 Despite the superiority of the combination, this approach has not gained wide acceptance and is not recommended as standard practice.1 The main reasons are that (a) 56% of those who did not respond to dexamethasone initially responded to salvage therapy with rituximab-dexamethasone,6 (b) relatively high rates of adverse events were encountered in the combination arm, although the majority were not considered treatment-related, and (c) there is a lack of data on HRQoL.
The combination of MMF and prednisolone vs prednisolone monotherapy was investigated in a RCT. MMF inhibits T-cell function through its actions on the purine synthesis pathway. The combination showed superiority compared to prednisolone with regard to time-to-treatment failure. At mean follow-up of 18 months, patients receiving MMF plus prednisolone had fewer treatment failures than prednisolone alone. There was no difference between the groups in term of treatment-related side effects, including infection. However, patients in the MMF group reported worse HRQoL. This finding, in addition to potential teratogenicity with MMF, has limited use of this combination as a standard up-front therapy.7
Another immune suppressive agent that was investigated in the first line is tacrolimus. Preliminary results showed that 12 weeks of treatment with tacrolimus plus dexamethasone was superior to dexamethasone alone in early response, sustained response, and rate of treatment failure.8
Combining TPO-RA and dexamethasone is a different though attractive approach aimed at inducing sustained response off-treatment (SROT). In a single-arm study of eltrombopag plus 1-3 cycles of dexamethasone, 56% of evaluable patients maintained platelet counts >50 × 109/l for more than 6 months without further ITP therapy. Most responders maintained response at last follow-up, the longest over 3 years.9 The efficacy of up-front TPO-RA plus dexamethasone in inducing SROT is currently being evaluated in ongoing RCTs (NCT04346654, NCT05325593).
Recombinant TPO (rhTPO) was investigated as up-front medication administered subcutaneously for 14 days in combination with dexamethasone vs dexamethasone alone. The combination resulted in higher initial and durable response rates. Ischemic stroke occurred in one patient receiving the combination.10
Oseltamivir is a sialidase inhibitor that is believed to reduce platelet clearance and increase proplatelet production by inhibiting platelet desialylation. The combination of dexamethasone plus a short course of oseltamivir was investigated in a RCT. Patients in the dexamethasone plus oseltamivir group had a significantly higher initial response rate compared to dexamethasone alone at day 14 and at 6 months. The combination was well-tolerated and induced improvement in symptoms related to ITP and overall HRQoL.11
All-trans retinoic acid (ATRA) has been shown to exert immunomodulatory effects and promote thrombopoiesis. ATRA given for 12 weeks in combination with dexamethasone was compared to dexamethasone alone in a RCT, showing a significantly higher sustained response rate in the combination arm. Dry skin was reported in half of patients treated with ATRA.12
A recent meta-analysis suggested that compared with corticosteroids alone, combination regimens demonstrated better early (80% vs 69%; odds ratio [OR] = 1.8, 95% confidence interval [CI]: 1.1–3.0) and sustained responses (60% vs 37%; OR = 2.6, 95% CI: 1.9–3.4).13
However, despite higher durable response rates shown by various combinations, guidelines still recommend corticosteroid monotherapy as first-line treatment. Attaining early remission fulfills many patients' goals and expectations in addition to potentially reducing societal costs. The reluctance toward adopting combination therapies in the first line may be attributable to concerns related to toxicities and the lack of experience with use and/or availability of some agents and combinations. In some jurisdictions, challenges obtaining insurance authorization for combination therapy may also pose a barrier.
Subsequent treatment
Around 70% of adult ITP patients fail to respond, relapse after, or become dependent on large doses of corticosteroids to maintain a response and thus require subsequent therapy. We divide subsequent therapy into second- and third-line therapies.
Second-line therapeutic options
Most guidelines recommend starting with a TPO-RA, rituximab, or fostamatinib, since these agents have been investigated in RCTs (Figure 1).1,2,4 Romiplostim, eltrombopag, avatrombopag, hetrombopag, and rhTPO are thrombopoietic agents licensed for ITP in different parts of the world. TPO-RAs are effective and well-tolerated,14 providing durable response rates in 34% to 60% of treated patients.15-18 Meta-analyses have failed to show a significant increase in the rate of thromboembolic events (TEE) associated with TPO-RAs. The most recent meta-analysis found an OR of 1.76, with a 95% CI: 0.78-4.00, p = 0.18 (mostly explained by lack of precision),19 which is in line with real-world studies suggesting an increased risk of thrombosis with these drugs.20,21 Elderly patients with multiple risk factors for thromboembolism, patients with previous venous or arterial thrombosis who are not on antithrombotic therapy, and patients with systemic lupus erythematosus, antiphospholipid syndrome, or strong antiphospholipid antibody positivity may be at increased risk of developing TEE on TPO-RA and should thus be considered for other treatments.22 Those who do not respond initially to a TPO-RA or lose response usually benefit from switching to either another agent or to a combination with predniso(lo)ne.23,24 The latter approach, although potentially effective at limiting platelet count fluctuation, is not recommended because of prolonged corticosteroid exposure. Tapering of TPO-RAs is advised after 2 to 12 months of stable response since about 30% of patients (and up to 50% of patients achieving a stable complete response on treatment) may achieve SROT.25,26 Patients who fail to respond/lose response to 2 TPO-RAs or do not tolerate TPO-RAs may switch to rituximab or fostamatinib.24
The SYK-inhibitor, fostamatinib, which is licensed for treatment of chronic ITP, provides a modest durable response rate and could be a good alternative in case of contraindication to TPO-RA or lack of response/relapse after TPO-RA/rituximab.27 Effect is expected within 2 weeks in most responding patients.27 This might be an advantage over rituximab in the choice of treatment since response to rituximab may take 6 to 12 weeks. Besides, unlike rituximab, there are no indications that fostamatinib causes prolonged immunosuppression after discontinuation.
Rituximab provides response in 45% to 60% of patients at 6 months. However, half of responding patients will lose response within 2 to 5 years.28 Rituximab may be preferred in patients with high thrombotic risk and those who prefer a limited treatment period. Although not confirmed in prospective trials, rituximab might be an appropriate choice for younger patients.29
There are no trials comparing the efficacy and safety of second- line agents directly. Patient involvement is therefore essential for making a shared decision on which treatment the patient should start, since these treatments have different modes of administration, properties, response, and safety profiles (Table 2).
Several studies have been conducted to assess the efficacy of combination therapies at this stage, too (Table 4). The effect of rituximab has been investigated in combination with other therapies in patients who did not respond to or relapsed after corticosteroids. A study comparing the efficacy and safety of rituximab plus rhTPO vs rituximab monotherapy showed no significant difference in overall and long-term responses.30
Table 4.
Summary of large RCTs assessing various combination therapies for subsequent treatment lines for ITP
| Combination arm | Monotherapy arm | Study design | Efficacy | Remarks |
|---|---|---|---|---|
| rhTPO (300 ug/kg sc for up to 14 days) + rituximab (100 mg weekly x4)30 | Rituximab (100 mg weekly × 4) | Open-label RCT with 2:1 randomization (n = 123) | Complete response was achieved 45% vs 24% (p = 0.02) and overall response was achieved in 79% vs 71% (p = 0.36) of patients in the combination monotherapy groups, with the combination having significantly shorter median response time (7 vs 28 days; p < 0.01) | There was no difference in the duration of response between the two groups; side effects were generally mild |
| ATRA (20 mg/m2 for 12 weeks) + low dose rituximab (100 mg weekly for 6 weeks)31 | Rituximab (100 mg weekly for 6 weeks) |
Open-label RCT with 2:1 randomization (N = 168) |
Overall response was achieved in 80% vs 59% (between-group difference, 0.22; 95% CI, 0.07-0.36), and sustained response was achieved in 61% vs 41% (between-group difference, 0.20; 95% CI, 0.04-0.35) in combination vs monotherapy groups | Most common adverse events for the combination group were dry skin and headache/dizziness |
| ATRA (10 mg × 2/day) + danazol (200 mg × 2/day) for 16 weeks32 |
Danazol (200 mg × 2/day) for 16 weeks | Phase 2 open-label RCT; 1:1 randomization (N = 96) |
Sustained response at 12 months was achieved in 62% of patients receiving ATRA plus danazol vs 25% in patients receiving danazol monotherapy (OR 4.94, p = 0.00037) | Skin desquamation was reported in 62% of patients in the combination arm |
| rhTPO (100 ug/kg sc for up to 14 days) + danazol (200 mg × 3 daily)33 | Danazol (200 mg × 3 daily) | 2 phase (14 days each), open-label RCT with 1:1 randomization (N = 140) | Total response rate in the combination group was 60% vs 36%, p = 0.01 | Well-tolerated treatments with mild side effects |
Another study compared the efficacy and safety of ATRA plus low-dose rituximab with low-dose rituximab alone. Overall response at 1 year and sustained response, defined as a platelet count >30 × 109/l for 6 consecutive months after achieving response, were significantly higher in the combination arm.31 ATRA has also been investigated in combination with danazol compared to danazol monotherapy for 16 weeks. Sustained response at 12 months was achieved more frequently in patients receiving ATRA plus danazol than in those receiving danazol monotherapy.32
Finally, rhTPO in combination with danazol was compared to danazol alone in a RCT. The combination was more effective in increasing mean maximal platelet count and achieved response in a shorter time compared to danazol alone.33
CLINICAL CASE (continued)
Although response to rituximab may take several months, an intervention was required because of the patient's persistent severe thrombocytopenia. She responded to the addition of avatrombopag with an increase in platelet count to 115 × 109/L within a week. However, the platelet count dropped to 12 × 109/L upon tapering prednisolone. Fostamatinib 100 mg BID was added to avatrombopag, resulting in a rise in platelet count to 165 × 109/L. Because the patient experienced headache as a side effect, avatrombopag was discontinued. The patient lost response shortly after discontinuation, leading to the reintroduction of avatrombopag but at a weekly dose of 2 tablets.
A minority of patients (<10%) fail to respond to several second-line options including TPO-RAs, one or more immunomodulatory agents, and/or splenectomy administered sequentially. These patients are usually labeled as refractory and require next line of therapy.34-36
Third-line therapeutic options
The main approaches to treating refractory ITP involve either a combination approach; continuation with sequential monotherapies, preferably with drugs having different mechanisms of action/targets than previous ones; or splenectomy. The field lacks randomized trials, and therefore evidence is based mainly on small observational studies.
Combination therapies
Available reports indicate that the combination of TPO-RA and an immunomodulatory agent is more effective than monotherapy.35,37 In one study on “multirefractory” ITP, only 1 of 14 patients who received an immunosuppressive drug as monotherapy achieved a response, while 7 out of 10 treated with TPO-RA plus an immunosuppressive drug achieved a response.35 This trend was confirmed by a subsequent study on 39 refractory patients, with a 77% response rate to such combinations.37 The combination seems to be effective whatever immunomodulatory or thrombopoietic agent is used. MMF, cyclosporin, and fostamatinib have been tried. A recently reported series of 18 patients receiving avatrombopag and fostamatinib showed a 78% response rate.38 Fostamatinib in combination with TPO-RA might be an attractive combination because of its relatively rapid response compared to MMF or cyclosporin. Following the achievement of a stable response, one can start tapering one of the two agents with the goal of minimizing the treatment needed to maintain the lowest possible adequate platelet count.
Monotherapy
Several agents have been investigated in refractory ITP. These include MMF, cyclosporine A, azathioprine, dapsone, danazol, daratumumab, sirolimus, and decitabine. We believe that using agents directed toward previously untargeted mechanisms, such as T-cell-directed therapy with MMF or DNA hypomethylating agent like decitabine,39 may be a good option in patients who have received standard second-line options. Consideration of investigational products is particularly pertinent to this group, although inclusion in clinical trials should be considered whenever appropriate.
Splenectomy
As a treatment, splenectomy remains an option in this population. Its main advantage is that if response is achieved, no other ITP therapy is needed. However, if there is no response, the patient will have to bear the consequences of splenectomy with an increased risk of infection and thrombosis, in addition to the side effects of medical therapy. Of note, one study performed in the era of rituximab and TPO-RA reported comparable response rates to studies performed before this era (60%-90%).40
In conclusion, the therapeutic landscape for ITP is rapidly evolving. Several new agents are currently under investigation. Combination therapies may improve both short- and long-term disease outcomes and may change the natural course of the disease when administered early. Despite encouraging results, combination therapies are still not recommended under current guidelines. More studies are needed to confirm the effectiveness and safety of these approaches, whether as up-front or subsequent therapies.
CLINICAL CASE (continued)
Our patient is dependent on a combination of fostamatinib and avatrombopag since attempts to discontinue either of the two agents resulted in relapse. She is tolerating the combination well with no side effects.
Conflict-of-interest disclosure
Waleed Ghanima: membership on advisory board: Amgen, Novartis, Pfizer, Principia Biopharma Inc. (a Sanofi Company), Sanofi, SOBI, Grifols, UCB, Argenx, Cellphire, Alpine, Kedrion, HiBio, Hutchmed, and Takeda; honoraria: Amgen, Novartis, Pfizer, Bristol Myers Squibb, SOBI, Grifols, Sanofi, Bayer; research grants: Bayer, BMS/Pfizer, UCB.
Adam Cuker: consultancy: MingSight, Pfizer, Sanofi, Synergy; royalties: UpToDate.
Marc Michel: consultancy: Novartis, Sanofi, Sobi, Alexion, Amgen, Griffols.
Off-label drug use
Waleed Ghanima: rituximab, MMF, cyclosporine, sirolimus, ATRA, dapsone, danazol, decitabine, oseltamivir.
Adam Cuker: rituximab, MMF, cyclosporine, sirolimus, ATRA, dapsone, danazol, decitabine, oseltamivir.
Marc Michel: rituximab, MMF, cyclosporine, sirolimus, ATRA, dapsone, danazol, decitabine, oseltamivir.
References
- 1.Neunert C, Terrell DR, Arnold DM, et al.. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provan D, Arnold DM, Bussel JB, et al.. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper N, Kruse A, Kruse C, et al.. Immune thrombocytopenia (ITP) world impact survey (iWISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol. 2021;96(2):188-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matzdorff A, Alesci SR, Gebhart J, et al.. Expert report on immune thrombocytopenia: current diagnostics and treatment—recommendations from an expert group from Austria, Germany, and Switzerland. Oncol Res Treat. 2023;46 (suppl 2):5-44. [DOI] [PubMed] [Google Scholar]
- 5.Gudbrandsdottir S, Birgens HS, Frederiksen H, et al.. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121(11):1976-1981. [DOI] [PubMed] [Google Scholar]
- 6.Zaja F, Baccarani M, Mazza P, et al.. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115(14):2755-2762. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury CA, Pell J, Hill Q, et al.. Mycophenolate mofetil for first-line treatment of immune thrombocytopenia. N Engl J Med. 2021;385(10): 885-895. [DOI] [PubMed] [Google Scholar]
- 8.An Z-Y, Wu Y-J, He Y, et al.. Tacrolimus plus high-dose dexamethasone versus high-dose dexamethasone alone as first-line treatment for adult immune thrombocytopenia: the phase 2, open label, randomized trial (TARGET 020). Blood. 2021;138(suppl 1):13. [Google Scholar]
- 9.Zhang L, Zhang M, Du X, Cheng Y, Cheng G.. Safety and efficacy of eltrombopag plus pulsed dexamethasone as first-line therapy for immune thrombocytopenia. Br J Haematol. 2020;189(2):369-378. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Wang M, Hou Y, et al.. High-dose dexamethasone plus recombinant human thrombopoietin vs high-dose dexamethasone alone as frontline treatment for newly diagnosed adult primary immune thrombocytopenia: a prospective, multicenter, randomized trial. Am J Hematol. 2020;95(12):1542-1552. [DOI] [PubMed] [Google Scholar]
- 11.Sun L, Wang J, Shao L, et al.. Dexamethasone plus oseltamivir versus dexamethasone in treatment-naive primary immune thrombocytopenia: a multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2021;8(4):e289-e298. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q-S, Liu Y, Wang J-B, et al.. All-trans retinoic acid plus high-dose dexamethasone as first-line treatment for patients with newly diagnosed immune thrombocytopenia: a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Haematol. 2021;8(10):e688-e699. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Sheng L, Han F, et al.. Efficacy and safety of treatments in newly diagnosed adult primary immune thrombocytopenia: a systematic review and network meta-analysis. EClinicalMedicine. 2023;56:101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104(6): 1112-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng G, Saleh MN, Marcher C, et al.. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393-402. [DOI] [PubMed] [Google Scholar]
- 16.Jurczak W, Chojnowski K, Mayer J, et al.. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuter DJ, Bussel JB, Lyons RM, et al.. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395-403. [DOI] [PubMed] [Google Scholar]
- 18.Mei H, Liu X, Li Y, et al.. A multicenter, randomized phase III trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. J Hematol Oncol. 2021;14(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen N, Qiao J, Jiang Y, et al.. Thrombopoietin receptor agonists use and risk of thrombotic events in patients with immune thrombocytopenic purpura: a systematic review and meta‑analysis of randomized controlled trials. Biomed Rep. 2024;20(3):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang C, Chen Q, Zhang Y.. Association of thrombopoietin-related drugs with thromboembolic events: mendelian randomization and a real-world study. Ther Adv Drug Saf. 2024;15:20420986231224236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goncalves I, Lewis C, Grainger B, et al.. Thrombosis in patients with immune thrombocytopenia: incidence, risk, and clinical outcomes. Res Pract Thromb Haemost. 2024;8(1):102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moulis G, Audemard-Verger A, Arnaud L, et al.. Risk of thrombosis in patients with primary immune thrombocytopenia and antiphospholipid antibodies: a systematic review and meta-analysis. Autoimmun Rev. 2016;15(3):203-209. [DOI] [PubMed] [Google Scholar]
- 23.Al-Samkari H, Jiang D, Gernsheimer T, et al.. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: a multicentre US study. Br J Haematol. 2022;197(3):359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghanima W, Gernsheimer T, Kuter DJ. How I treat primary ITP in adult patients who are unresponsive to or dependent on corticosteroid treatment. Blood. 2021;137(20):2736-2744. [DOI] [PubMed] [Google Scholar]
- 25.Cooper N, Ghanima W, Vianelli N, et al.. Sustained response off-treatment in eltrombopag-treated adult patients with ITP who are refractory or relapsed after first-line steroids: primary, final, and ad-hoc analyses of the Phase II TAPER trial. Am J Hematol. 2024;99(1):57-67. [DOI] [PubMed] [Google Scholar]
- 26.Guillet S, Crickx E, Azzaoui I, et al.. Prolonged response after TPO-RA discontinuation in primary ITP: results of a prospective multicenter study. Blood. 2023;141(23):2867-2877. [DOI] [PubMed] [Google Scholar]
- 27.Bussel J, Arnold DM, Grossbard E, et al.. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshayes S, Khellaf M, Zarour A, et al.. Long-term safety and efficacy of rituximab in 248 adults with immune thrombocytopenia: results at 5 years from the French prospective registry ITP-ritux. Am J Hematol. 2019;94(12):1314-1324. [DOI] [PubMed] [Google Scholar]
- 29.Moulis G, Garabet L.. Markers of refractory primary immune thrombocytopenia. Br J Haematol. 2023;203(1):112-118. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Xu M, Qin P, et al.. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125(10):1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y-J, Liu H, Zeng Q-Z, et al.. All-trans retinoic acid plus low-dose rituximab vs low-dose rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2022;139(3):333-342. [DOI] [PubMed] [Google Scholar]
- 32.Feng F-E, Feng R, Wang M, et al.. Oral all-trans retinoic acid plus danazol versus danazol as second-line treatment in adults with primary immune thrombocytopenia: a multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2017;4(10):e487-e496. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Yang R, Zou P, et al.. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol. 2012;96(2):222-228. [DOI] [PubMed] [Google Scholar]
- 34.Moulis G, Rueter M, Duvivier A, et al.; CARMEN-France Investigators Group. Difficult-to-treat primary immune thrombocytopenia in adults: prevalence and burden. Results from the CARMEN-France registry. Br J Haematol. 2024;204(4):1476-1482. [DOI] [PubMed] [Google Scholar]
- 35.Mahévas M, Gerfaud-Valentin M, Moulis G, et al.. Characteristics, outcome, and response to therapy of multirefractory chronic immune thrombocytopenia. Blood. 2016;128(12):1625-1630. [DOI] [PubMed] [Google Scholar]
- 36.Arnold DM, Clerici B, Ilicheva E, Ghanima W.. Refractory immune thrombocytopenia in adults: towards a new definition. Br J Haematol. 2023;203(1):23-27. [DOI] [PubMed] [Google Scholar]
- 37.Crickx E, Ebbo M, Rivière E, et al.. Combining thrombopoietin receptor agonists with immunosuppressive drugs in adult patients with multirefractory immune thrombocytopenia, an update on the French experience. Br J Haematol. 2023;202(4):883-889. [DOI] [PubMed] [Google Scholar]
- 38.Mingot-Castellano ME, Bastida JM, Ghanima W, et al.. Avatrombopag plus fostamatinib combination as treatment in patients with multirefractory immune thrombocytopenia. Br J Haematol. 2024; 10.1111/bjh.19602. [DOI] [PubMed] [Google Scholar]
- 39.Zhou H, Qin P, Liu Q, et al.. A prospective, multicenter study of low dose decitabine in adult patients with refractory immune thrombocytopenia. Am J Hematol. 2019;94(12):1374-1381. [DOI] [PubMed] [Google Scholar]
- 40.Godeau B. Is splenectomy a good strategy for refractory immune thrombocytopenia in adults? Br J Haematol. 2023;203(1):86-95. [DOI] [PubMed] [Google Scholar]