Abstract
After specific activation, CD8+ cytotoxic T lymphocytes (CTLs) enter a refractory state termed activation-induced nonresponsiveness (AINR) that is characterized by the inability of T cells to respond to a secondary stimulus. Here, we show that T cell receptor triggering results in rapid degradation of the src-family protein kinase lck through a mechanism that is proteasome- and lysosome-independent, sensitive to cysteine protease inhibitors, and distinct from the pathways involved in degradation of ZAP-70 kinase or ζ-chain of the CD3 complex. Pharmacologic blockade of lck degradation, as well as transfection of refractory cells with an lck expression vector, increased responsiveness of CTLs to repeated antigenic challenge. The development or maintenance of AINR was not affected by exogenously added IL-2, whereas IL-15 or IFN-α restored both lck expression and responsiveness of preactivated CTLs. Our results suggest that lck degradation plays an important role in the development of AINR in human CTLs and that this condition can be reverted by pharmacologic agents or lymphokines that prevent lck degradation or induce its expression.
Keywords: activation, kinase
CD4+ T cells are driven into an anergic state by T cell receptor (TCR) triggering in the absence of costimulation. In contrast, CD8+ cytotoxic T lymphocytes (CTLs) become unable to proliferate or produce IL-2 in response to specific stimulation subsequent to the primary TCR triggering combined with the engagement of costimulatory molecules, such as CD28 (1, 2). This functional condition of CTLs is often referred to as activation-induced nonresponsiveness (AINR) (3). In anergic CD4+ T cells, expression of phospholipase C-γ1 (PLC-γ), protein kinase C (PKC)-Θ and RasGTPase-activating protein (RASGAP) is down-regulated (4). Alterations in signal transduction were also reported in refractory CTLs (5) but the mechanisms responsible for the development and maintenance of AINR remain poorly defined. In mouse CTLs, AINR may be reverted by exogenous IL-2 (6) but it is not known whether the lymphokine acts in a similar way on human CTLs.
One of the first molecules to be activated downstream of the TCR is lck, an src-family protein kinase, which is essential for normal development of CD4 and CD8 single positive thymocytes, proliferation of naïve mature T cells as well as functional activity of effector and memory T-lymphocytes (reviewed in ref. 7). During the process of T cell activation, lck acts both as a tyrosine kinase and adaptor molecule, which binds and phosphorylates a number of cell-surface and intracellular substrates. The activity and intracellular distribution of lck is regulated by several posttranslational modifications (for reviews, see refs. 7-9). In addition, the level of lck expression affects its net activity. Lck expression is often significantly decreased in lymphocytes infiltrating tumors or circulating in the blood of patients with chronic infections or inflammation (10-14) that may play a role in the functional incapacity of T cells observed during cancer progression or chronic infections.
The mechanisms and regulation of lck degradation have not been extensively studied. The activation-induced degradation (AID) of the two components of the TCR heterodimer and ζ-chain have been shown to occur in the endosomal/lysosomal compartment (15, 16) while the ZAP-70 kinase can be degraded by calcium dependent calpain(s) (17). AID of lck has been reported in CD4+ human T cells (18). Rao and colleagues have shown that Ab-mediated co-ligation of TCR and CD4 molecules promotes association of lck with the Cbl ubiquitin ligase which induces ubiquitination and inhibits lck activity (19). However, the mechanisms of lck degradation induced by physiological stimuli or functional consequences of this process have not been analyzed.
Materials and Methods
Abs and Reagents. The lck (3A5) and CD3-ζ (6B110.2) mAbs were purchased from Santa Cruz Biotechnology, and the polyclonal rabbit anti-lck Ab was purchased from BD Biosciences. The rabbit polyclonal anti-ZAP-70 Ab was from Calbiochem, and actin-specific Ab was from Sigma. The FITC-labeled anti-CD3 (Leu-4), PE-anti-CD8 (M055725), PE-anti-CD94 (HP-3D9), anti 4-1BB (4B4-1), and APC-labeled anti-human IFN-γ Abs were from BD Biosciences. The RPE-conjugated donkey anti-rabbit (NA93NV) and sheep anti-mouse (NXA931) Abs were from Amersham Pharmacia Biosciences, and the FITC-anti-mouse Ab, p53-specific mAb DO-7, and antiubiquitin rabbit polyclonal Ab were from DAKO. The anti-CD3 Ab OKT3 was purified from cell-culture supernatants of the relevant hybridoma (CRL-8001, American Type Culture Collection). Ionomycin, TPA, and NH4Cl were from Sigma, and CD3/28 beads were from Dynal (Oslo). The protease inhibitors Z-LL-H (Peptides International), MG132, lactacystin, epoxomycin (Affinity Research Products, Devon, U.K.), and leupeptin (Calbiochem) were stored as stock solutions in DMSO. Recombinant IL-15 was from R & D Systems, recombinant IL-2 was from PeproTech (Rocky Hill, NJ), and human IFN-α (trademark Wellferon) was from Wellcome (London). Preparation, purification, storage, and handling of synthetic peptides were as described in ref. 20.
Cell Lines, CTL Cultures, and Clones. The generation of the HLA A11-transfected subline of the HLA class I-negative mutant cell line C1R and the Epstein-Barr virus-transformed HLA A11+ lymphoblastoid cell line (LCL) JAC-B2 is described in refs. 21 and 22. The L5 LCL is transformed by an Epstein-Barr virus strain that carries mutations in the anchoring residues of the IVT and AVF epitopes that prevent their presentation (20). All cell lines were maintained in RPMI medium 1640 with 100 μg/ml streptomycin, 100 units/ml penicillin, and 10% FCS (complete medium). The generation and analysis of specificity and TCR structure of the IVT-peptide-specific HLA A11-restricted CTL clones BK289 and CAR13, as well as polyclonal CTL cultures from HLA A11+ healthy Epstein-Barr virus carriers BK, EA, and CAR, are described in refs. 23 and 24. To generate PHA-activated T-blasts, peripheral blood mononuclear cells were isolated from the blood of healthy donors by Ficoll (Amersham Pharmacia) density gradient centrifugation, stimulated for 3 days in complete medium with 1 μg/ml PHA, and cultured in the presence of 10 units/ml IL-2 (IL-2 medium).
Analysis of Protein Expression by Immunoblotting. CTLs were lysed in electrophoresis sample buffer (1 × 105 cells in 10 μl), and lysates were separated by SDS/PAGE. The gels were blotted onto nitrocellulose filters, which were incubated with the indicated specific Ab diluted in PBS containing 5% milk. After incubation with secondary, horseradish peroxidase-conjugated Abs, the blots were visualized by using SuperECL (Amersham Pharmacia). Images were acquired on a Fuji Intelligent Dark Box II by using las-100pro imagereader v.2.1 software and were analyzed by using science lab 98 image gauge v.3.4x image analysis software (Fuji). Relative intensities of actin-specific bands were used, if necessary, for loading irregularities. Statistical analysis of data was performed by using the t test.
Inhibition of the Proteasome and Other Proteases. The effector cells were pretreated either with lactacystin (10 μM) or epoxomycin (300 nM) for 3 h or with leupeptin (100 μM), MG132 (50-100 μM), or Z-LL-H (5-20 μM) for 1 h at 37°C at a density of 1 million cells per ml before addition of pulsed or unpulsed APCs. The cells were then kept in the presence of the indicated inhibitor throughout the experiment.
FACS Analysis. The CTL cultures or clones were collected at the indicated time points, washed, and incubated for 30 min on ice with the indicated specific Ab diluted in PBS with 1% FCS. Cells were then washed twice in ice-cold PBS/1% FCS before FACS analysis or another 30-min incubation with a secondary FITC-labeled Ab. Intracellular IFN-γ-specific staining was performed by using the Cytofix/Cytoperm kit (Pharmingen) as described in ref. 25. The data were acquired and analyzed on a FACS analyzer by using cellquest software (Becton Dickinson).
Plasmid Preparation and Transfection of CTLs. The pcDNA3.1(-)B-lck plasmid encoding for wild-type lck was kindly provided by Jougnwa Won (Mogam Biotechnology Research Institute, Gynuggido, Korea). The integrity of the construct was confirmed by sequencing and analysis of lck expression in transfected HeLa cells. EndoFree Plasmid Maxi kits (Qiagen) were used to isolate pcDNA3.1 (vector), pcDNA3.1-lck, and pcDNA3.1-EGFP plasmids. Ten micrograms of each plasmid was used to transfect 5-10 million CTLs with the Human T Cell Nucleofector kit (Amaxa, Cologne, Germany) according to the manufacturer's instructions.
Analysis of T Cell Activation and Death. C1R/A11, JAC-B2, and L5 cells unpulsed or pulsed with the indicated concentrations of synthetic peptides were incubated for 1 h at 37°C, irradiated at 4,000 rad, extensively washed, and mixed with CTLs at the indicated effector-to-target (E:T) ratios. The mixed cells were centrifuged (5 min at 200 × g) to help the formation of conjugates and incubated at 37°C for 1 h. IL-2 (10 units/ml), IL-15 (5 ng/ml), CD3/CD28 beads (T cell-to-bead ratio of 2:1) were added where indicated. In all long-culture experiments (>4 h), the cells were cultured in six-well plates at a cell density of 1 × 106 per ml. In reactivation experiments with Z-LL-H, the first stimulation was performed in six-well plates coated overnight at +4°C with 10 μg/ml anti-CD3 Ab or with mouse serum in PBS. The cells were cultured in Ab-coated plates for 2 h and transferred to Ab-free plates to prevent further activation. The recovery of living cells was evaluated by trypan blue exclusion or FACS analysis as described in ref. 26. To measure CTL proliferation, peptide-pulsed, irradiated C1R/A11 cells were mixed with effectors in complete medium at an E:T ratio of 10:1, and [3H]thymidine incorporation was analyzed as described in ref. 26. The cytotoxic activity of CTLs was measured in standard 4-h 51Cr release assays (26).
The concentrations of IFN-γ and IL-2 in the culture supernatants were detected by ELISA using OPTEIA human IFN-γ and IL-2 set according the manufacturer's instructions (BD Biosciences).
Results
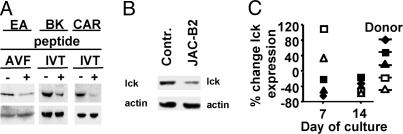
TCR Triggering Induces Lck Down-Regulation in Activated Human T Cells. As shown in Fig. 1A, stimulation with C1R/A11 cells pulsed with either of the two Epstein-Barr virus nuclear antigen-4-derived HLA A11-restricted peptide epitopes, IVTDFSVIK or AVFDRKSDAK (referred to as IVT or AVF), significantly decreased the levels of lck expression in three polyclonal CTL cultures that contained between 50% and 80% of peptide-specific T cells as revealed by MHC class I tetramer staining (27). A similar decrease of lck expression was observed in these CTLs after stimulation with the HLA A11+ LCL JAC-B2 (Fig. 1B), which processes and presents the two peptides endogenously (28). Therefore, down-regulation of lck in response to TCR triggering is not clone- or donor-dependent and can be induced by physiological amounts of specific antigen. We also observed down-regulation of lck in TCR transgenic CD8+ T cells stimulated in vivo by immunization with the specific peptide and rechallenged in vitro by addition of the same peptide into splenocyte culture (see supporting information, which is published on the PNAS web site).
Fig. 1.
TCR triggering induces lck down-regulation in activated CD8+ T cells. (A) C1R/A11 cells were pulsed with the AVF or IVT peptide (1 × 10-7 M), irradiated, and used to stimulate the indicated polyclonal CTL cultures. Expression of lck in CTL lysates was determined by immunoblotting with polyclonal lck-specific Abs. Shown is one representative of five experiments. (B) Expression of lck in CAR CTLs stimulated with the JAC-B2 LCL or L5 LCL (control). The latter carries mutations in the AVF and IVT epitopes that prevent their presentation at the cell surface (42). Shown is one representative of three experiments. (C) T cell blasts were generated from peripheral blood mononuclear cells of five healthy blood donors by PHA activation. On day 7 of culturing in IL-2 medium, >95% of cells in every culture were CD3+/CD8+ as determined by FACS analyses. On the indicated day of culture, cells were stimulated with beads conjugated with anti-CD3 Abs for 24 h and lysed in sample buffer, and lck expression was tested by immunoblotting and quantified as described in Materials and Methods.
To analyze whether the activation/differentiation status of T cells affects activation-induced down-regulation of lck, T cell blasts were generated from peripheral blood mononuclear cells of five healthy blood donors by activation with PHA and culturing in IL-2 medium. Activation of T cell blasts with anti-CD3/CD28 beads on day 7 of culture caused lck down-regulation in T-blasts of three donors and its up-regulation in T-blasts from the other two individuals. In contrast, lck was down-regulated in response to TCR triggering in T cell blasts of all of the five donors at day 14 of culture (Fig. 1C).
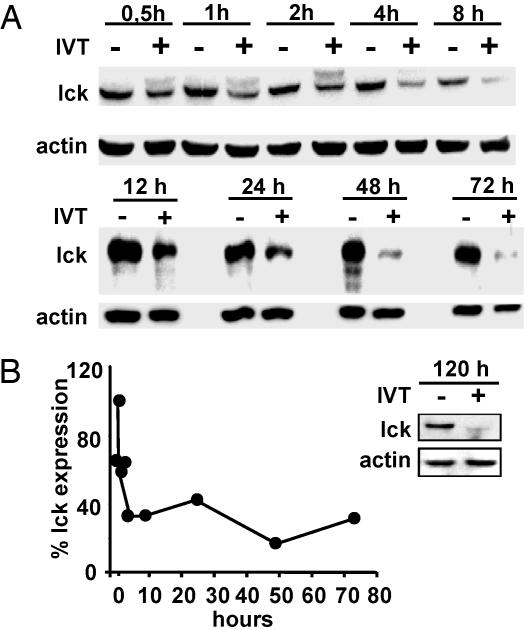
Specific Activation of CTLs Induces Rapid and Persistent Down-Regulation of Lck Expression. To understand the mechanism of activation induced down-regulation of lck, we first analyzed the kinetics of this process in the IVT-specific CTL clone BK289 after triggering with IVT-pulsed APCs. In addition to the characteristic polypeptide of 56 kDa expressed in resting CTLs, a second band of ≈59 kDa corresponding to the phosphorylated form of lck was revealed by immunoblotting in CTLs collected between 30 min and 4 h after activation. This form of lck was not detectable from 8 h postactivation (Fig. 2A), consistent with its relatively rapid dephosphorylation (29). Densitometry performed on all of the lck-specific bands revealed an ≈50% decrease in lck levels within 30 min of activation. Further rapid decline was observed during 8-12 h postactivation, and there-after lck expression stabilized at ≈20-30% of control levels (Fig. 2B). Culturing for an additional 4-5 days in IL-2 medium did not restore lck expression in CTLs (Fig. 2B Inset).
Fig. 2.
Kinetics of lck down-regulation in IVT-specific CTL. BK289 CTLs were activated with IVT-pulsed C1R/A11 cells for the indicated periods of time. Viable cells were counted, harvested, and lysed in SDS sample buffer. (A) Results of one representative immunoblotting experiment with lck-specific Ab. (B) Densitometry of lck-specific bands revealed in lysates of activated CTLs was performed as described above, and band intensities were expressed as the percentage relative to that of controls. Shown is one representative of five experiments.
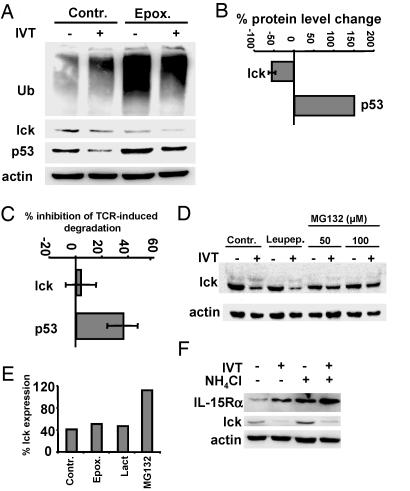
Degradation of Lck Is Blocked by Cysteine Protease Inhibitors. The kinetics of lck down-regulation in activated CTLs was consistent with degradation of the enzyme. Down-regulation of lck was not affected by the presence of epoxomycin (Fig. 3A) or lactacystin (Fig. 3E) during T cell triggering, while the inhibitors caused accumulation of ubiquitinated proteins in both activated and control CTLs (Fig. 3A), induced accumulation of p53 in unstimulated CTLs (Fig. 3 A and B), and blocked AID of p53 (Fig. 3 A and C). Interestingly, the steady-state levels of lck were decreased in the presence of proteasome inhibitors, whereas activation-induced degradation of lck was not affected (Fig. 3 A-C). In contrast, MG132, a less selective inhibitor of the proteasome, blocked down-regulation of lck in a dose-dependent manner (Fig. 3 D and E). The presence of NH4Cl induced accumulation of the IL-15 receptor α-chain in nonactivated CTLs and further increased its up-regulation after CTL activation (Fig. 3F). However, NH4Cl did not affect AID of lck, indicating that lysosomal enzymes are not involved in this process.
Fig. 3.
AID of lck is proteasome- and lysosome-independent but sensitive to inhibition by MG132. BK bulk CTLs were activated by IVT-pulsed APCs for 4 h in the absence (control) or presence of the indicated protease inhibitors. (A) Immunoblotting of lysates of BK289 cells with ubiquitin-, lck-, p53-, or actin-specific Abs. (B) Effect of epoxomycin on the steady-state levels of lck and p53. Intensities of lck- and p53-specific bands in lysates of cells incubated in the presence of epoxomycin were quantified and expressed as the percentage relative to control. Shown are the means ± SD of three experiments. (C) Effects of epoxomycin on AID of lck or p53 were analyzed and expressed as described in B. Shown are the means ± SD of three experiments. (D) Effect of MG132 on lck degradation assessed at the indicated concentrations. Cells activated by peptide-pulsed APCs in the presence of leupeptin were used as an additional control. (E) Quantification of the effect of MG132 at a concentration of 100 μM. Shown is one representative of three experiments in which samples of cells treated with lactacystin were also included. (F) Control or activated CTLs were cultured in the presence or absence of NH4Cl. Immunoblotting of total cell lysates with lck-, IL-15Rα-, or actin-specific Abs. Shown is one representative of five experiments.
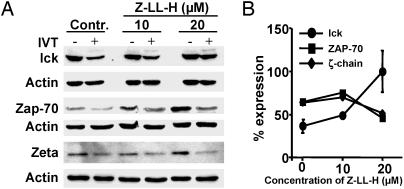
The calpain inhibitor Z-LL-H structurally resembles MG132 but exhibits a much higher selectivity for cysteine proteases (30). The AID of lck in CTLs preincubated with and activated in the presence of Z-LL-H was strongly inhibited (Fig. 4 A and B). The steady-state levels of ZAP-70 and ζ-chain increased in T cells cultured in the presence of Z-LL-H; however, the AID of these molecules (Fig. 4), as well as degradation of p53 (data not shown), was not affected by the inhibitor. The calpain inhibitor calpeptin, which has been shown to block degradation of ZAP-70 induced by T cell activation, did not affect the levels of lck (see supporting information).
Fig. 4.
The calpain inhibitor Z-LL-H blocks AID of lck. BK bulk CTLs were activated by IVT-pulsed APCs for 4 h in the absence (control) or presence of Z-LL-H at the indicated concentrations. (A) Effect Z-LL-H on the AID of lck, ZAP-70, or ζ-chain tested by immunoblotting with the relevant Abs. (B) Intensities of lck, ZAP-70, or ζ-chain-specific bands expressed as percentage expression relative to band intensities in samples of unstimulated CTLs. Shown are the means ± SD of five experiments performed with lck-specific Abs and one representative of two experiments performed with ZAP-70- and ζ-chain-specific Abs.
Lck Degradation Is Not a Prerequisite for Efficient T Cell Activation. Lck down-regulation in activated CD4+ T cells is enhanced by the engagement of costimulatory molecules such as CD28 (18). One possible interpretation of these data are that lck degradation is required for efficient TCR signal transduction and T cell activation. To address this issue, we analyzed the effect of Z-LL-H on the capacity of BK CTLs to kill C1R/A11 cells pulsed with the indicated concentrations of the IVT peptide and produce IL-2 or IFN-γ in response to stimulation with IVT-pulsed APCs. None of these parameters of T cell activation was negatively affected by Z-LL-H, whereas the release of IFN-γ was slightly increased in the presence of the inhibitor (see supporting information). Triggering of CTLs is accompanied by activation-induced cell death, which has been previously shown to be Fas-mediated in IVT-specific BK CTLs (26). A comparable decrease of cell recovery was observed in BK bulk CTL cultures activated by IVT-pulsed APCs in the presence or absence of Z-LL-H (see supporting information). Thus, lck degradation is not required for efficient T cell activation.
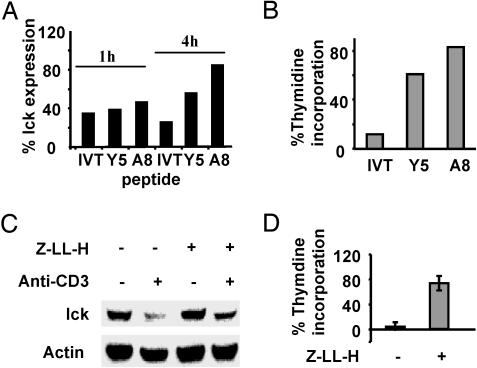
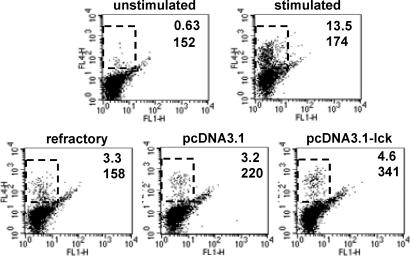
Down-Regulation of Lck Through Degradation Plays a Role in the Development of AINR in CTLs. To analyze whether the level of lck down-regulation correlates with the extent of nonresponsiveness in CTLs, IVT-specific CTLs were activated by using the wild-type peptide or its partially agonistic variants Y5 or A8, which contain an F-to-Y substitution in position 5 or an I-to-A substitution in position 8, respectively, and act as partial agonists inducing only some activation events in IVT-specific CTL clones and lines (25-27, 31). As shown in Fig. 5A, similar levels of lck down-regulation were observed in IVT-, Y5-, or A8-activated CTLs after 1 h of triggering. After 4 h of activation, the level of lck expression further decreased from 34% to 25% in IVT-stimulated cells, whereas a significant reconstitution of lck expression was observed in Y5- and A8-stimulated CTLs. The CTLs were then restimulated with peptide-pulsed APCs, and their capacity to proliferate in response to this second challenge was evaluated by thymidine incorporation assays 72 h after retriggering. A strong correlation was observed between the level of lck down-regulation induced by a given peptide and the extent to which responsiveness of the CTLs was inhibited (Fig. 5B). To investigate whether blocking of lck degradation can inhibit the development of AINR, polyclonal CTL cultures or clones were stimulated by immobilized CD3-specific Abs in the absence or presence of Z-LL-H and transferred to new plates to prevent continuous CTL activation. Their responsiveness was assessed after 72 h as described above. The presence of Z-LL-H during the first CTL triggering inhibited lck degradation (Fig. 5C) as well as the development of AINR in these cells (Fig. 5D). To directly assess the role of lck down-regulation in AINR, CAR bulk CTLs were transfected with pcDNA3.1-lck or control pcDNA3.1 plasmid 24 h after the first triggering, cultured overnight, and restimulated. The capacity of CTLs to produce INF-γ was measured by intracellular staining as a parameter of CTL responsiveness. Proliferation assays could not be performed because of a strong negative effect of the transfection procedure on CTL viability in long-term cultures. In preactivated cultures, the percentage of CTLs producing INF-γ in response to TCR triggering was strongly decreased. Transfection with pcDNA3.1 increased the percentage and mean fluorescence intensity of INF-γ-positive cells, whereas transfection with control plasmid or pcDNA3.1-EGFP had no effect (Fig. 6).
Fig. 5.
Responsiveness of CTLs correlates with lck expression, and treatment with Z-LL-H prevents the development of AINR in specific CTLs. (A) BK bulk CTLs were stimulated during the indicated periods of time with C1R/A11 cells preincubated with the indicated synthetic peptides. The expression of lck was accessed by immunoblotting and quantified as described above. (B) CTLs preactivated by using the indicated peptides were restimulated with IVT-pulsed APCs after 48 h, and their proliferation was evaluated by a [3H]thymidine incorporation assay. Shown are the results of one representative of two comparable experiments. (C and D) BK bulk CTLs were activated for 2 h on plastic plates with absorbed CD3-specific Ab, transferred to a clean plate, and cultured for 48 h either in the absence or presence of 20 μM Z-LL-H. Lck expression in these cells was evaluated by immunoblotting. Shown are data of one representative experiment. (D) Cells were then activated by IVT-peptide-pulsed APCs, and their proliferation was evaluated by a [3H]thymidine incorporation assay. The results are expressed as the percentage relative to thymidine incorporation in control cultures not stimulated by anti-CD3 and cultured either with or without Z-LL-H. Shown are the means ± SD of three experiments.
Fig. 6.
Transfection of pcDNA3.1-lck into refractory CTLs enhances their capacity to produce IFN-γ in response to specific stimulation. Refractory CAR bulk CTLs were transfected with pcDNA3.1, pcDNA3.1-lck, or pcDNA3.1-EGFP plasmids and restimulated 18 h after transfection with IVT-pulsed APCs. The secretion of IFN-γ in control and transfected cells was assessed by intracellular staining with the specific APC-conjugated Ab and FACS analysis. Monitoring of green fluorescence (FL1) was used to determine the general efficiency of transfection based on the percentage of green cells in pcDNA3.1-EGFP-transfected culture that varied between 7% and 10% in different experiments (data not shown). The increase of IFN-γ-positive cells observed after pcDNA3.1-lck transfection corresponded with the expected values calculated from the percentage of positive cells in nonrefractory CTL cultures and general transfection efficiency.
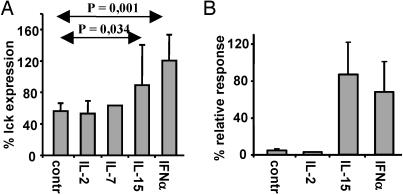
IFN-α and IL-15 Reconstitute Lck Expression and Abrogate the Development of AINR in Specific CTLs. In our search for physiological signals that can reconstitute lck expression after TCR triggering, we analyzed a panel of lymphokines for their capacity to affect lck expression in activated IVT-specific CTLs. None of the tested cytokines, including IL-2, IL-4, IL-7, IL-10, IL-12, IL-15, IFN-α, IFN-β, IFN-γ, and TNF-α, interfered with AID of lck measured 4 h after triggering (data not shown). However, CTLs cultured in the presence of IL-15 or IFN-α for 48 h after activation expressed levels of lck comparable to those of unstimulated cells. The effect of the lymphokines varied in different experiments, probably reflecting variations in the conditions of CTLs in vitro, but was statistically significant (Fig. 7A). IL-2, IL-7, or the other tested lymphokines did not affect lck expression under the same conditions (Fig. 7 and data not shown). IL-15 or IFN-α did not affect the expression levels of CD28 or 4-1BB costimulatory molecules, down-regulation of TCR or CD8, or up-regulation of CD94 molecule (data not shown), which can form activating or inhibitory heterodimers on the surface of CTLs to antigenic stimuli (32). Nevertheless, IVT-specific CTLs cultured in the presence of exogenously added IL-15 or IFN-α did not develop AINR in response to TCR triggering and proliferated after the secondary challenge almost as efficiently as control cells (Fig. 7B). Importantly, the lymphokines did not affect the extent of activation-induced cell death in IVT-stimulated CTL cultures (data not shown).
Fig. 7.
IL-15 and IFN-α increase lck expression after specific activation and restore responsiveness in preactivated CTLs. (A) The BK bulk CTLs were activated with peptide-pulsed APCs and cultured for 48 h in standard medium alone (control) or with the addition of exogenous IL-2, IL-7, IL-15, or IFN-α. The expression of lck was analyzed as described above. (B) Capacity of CTLs to proliferate in response to the secondary challenge as evaluated by a [3H]thymidine incorporation assay. The results are expressed as the percentage relative to thymidine incorporation of control CTLs, which were not preactivated with peptide-pulsed APCs and cultured in the absence (control) or presence of the indicated lymphokine. Shown are the means ± SD of four experiments.
Discussion
In this study, we provide evidence implicating activation-induced degradation of lck in the development of AINR in CTLs. Increased ubiquitination of lck has been observed in CD4-positive T cells and transformed human T cell lines only after CD4 ligation or its coligation with CD3, whereas CD3 triggering alone did not stimulate this process (19, 33). Although the electrophoretic mobility of ubiquitinated lck was consistent with its polyubiquitination, monoubiquitination at multiple sites, described for other receptor-associated kinases (34, 35), has not been excluded, and involvement of the proteasome in lck degradation has been supported only by the effect of MG132 while more specific proteasome inhibitors have not been tested (19). In our study, inhibition of the proteasome activity did not prevent AID of lck in CTLs. On the contrary, proteasome inhibition induced a decrease of steady-state lck levels in CTLs (Fig. 3). The blocking effect of MG132 and Z-LL-H on lck degradation is most likely attributed to their capacity of interfering with the activity of cellular proteases other than the proteasome. Both MG132 and Z-LL-H block the activity of calpains, a ubiquitously expressed family of cysteine proteases (36, 37). T cell activation induces redistribution of calpains into lipid rafts (38) where they co-localize with lck. However, inhibition of lck degradation by Z-LL-H did not affect calpain-mediated degradation of ZAP-70 (17), treatment with ionomycin triggered degradation of ZAP70 but not of lck, and calpeptin, a well characterized calpain inhibitor, did not block lck degradation (supporting information and data not shown). Furthermore, degradation of ζ-chain was still observed in Z-LL-H-treated cells, and agents that block lysosomal acidification did not interfere with the degradation of lck (Fig. 3). This finding indicates that the Ca2+-dependent calpains and the endosomal/lysosomal compartment, responsible for degradation of ZAP-70 and ζ-chain, respectively, are not involved in activation-induced lck degradation. Recently, degradation of signaling molecules, other than lck, through a proteasome-independent, ubiquitination-dependent pathway has been shown to be involved in the development of anergy in CD4+ cells (4). Initiation of this degradation pathway requires Ca2+-influx, is blocked by cyclosporine A, and has been suggested to take place in the endosomal/lysosomal compartment. In human CTLs, lck degradation and the development of AINR was not induced by ionomycin and was insensitive to cyclosporine A (M.U., unpublished results). Therefore, the precise targeting mechanism and proteases involved in degradation of signaling molecules in refractory CD8+ cells remain to be identified.
Identification of Z-LL-H as an inhibitor of activation-induced lck degradation allowed us to investigate the role of this event in the process of T cell activation. Lck degradation was not required for specific lysis of target cells, release of cytokines, or AICD of specific CTLs (see supporting information), suggesting that this process may be involved in the negative regulation of CTL function. In fact, low levels of lck expression were associated with AINR in specific CTLs. Although Z-LL-H completely blocks lck degradation observed during the first 4 h after TCR triggering, activated CTLs cultured in the presence of Z-LL-H for prolonged periods of time express lck levels intermediate to that of nonactivated and activated control cells. This finding suggests the existence of another, degradation-independent mechanism, of lck down-regulation operating at the level of lck transcription/translation. Nevertheless, partial reconstitution of lck expression observed in the presence of Z-LL-H was sufficient to reconstitute responsiveness of CTLs as demonstrated by their capacity to proliferate and produce lymphokines in response to the secondary antigenic challenge (Fig. 5). Transfection of specific CTLs with an lck-encoding plasmid specifically enhanced their ability to produce IFN-γ in response to a secondary challenge, suggesting that the level of lck expression directly regulates at least some functional components of CTL responsiveness (Fig. 6). The enhancing effect of lck transfection on IL-2 production by CTLs was not significant, which may be explained by the limitations of available gene delivery techniques for CTLs. In our hands, the cells were highly resistant to transduction with a variety of viral vectors.
Activated CTLs cultured for 5-7 days in standard medium alone or in the presence of exogenous IL-2 remained refractory to stimulation. This finding is consistent with our previously published data demonstrating that the addition of exogenous IL-2 has a marginal effect on the capacity of refractory CTLs to produce IL-2 mRNA (25). We screened a panel of lymphokines and demonstrated that IL-15 and IFN-α are capable of reconstituting the expression of lck in activated CTLs. The lymphokines did not interfere with AID of lck but reconstituted its expression in CTLs, probably enhancing lck gene transcription or mRNA translation (Fig. 6). This effect correlated with the capacity of the lymphokines to relieve AINR in CTLs not affecting the levels of expression of several molecules that modulate CTL responsiveness such as CD3, CD8, CD94, CD28, and 4-1BB (data not shown). Therefore, our data strongly suggest that modulation of lck expression is one of the key molecular events responsible for the well documented capacity of IL-15 and INF-α to promote both primary and memory CTL responses. IL-15 has been recently shown to augment CTL responses to HIV antigens both in experimental vaccination models and in peripheral blood mononuclear cells of AIDS patients where functional incapacity of HIV-specific CTLs represents a critical factor in the pathogenesis of the disease (39-41). Notably, IL-2 and IL-7 were significantly less potent (40). Because IL-15 and IFN-α are produced primarily by monocytes and different subsets of dendritic cells, the up-regulation of lck by these lymphokines may represent one of the mechanisms responsible for the enhancing effect of innate immune activation on CTL responses.
Down-regulation of lck is observed in T cells isolated from patients with a number of pathologic conditions including tumors, chronic infections, or chronic systemic inflammation. Our demonstration that low levels of lck correlate with AINR in CTLs and that reconstitution of lck expression and reversion of AINR can be achieved by pharmacologic agents and lymphokines paves the way to the development of new approaches aiming to improve the immunologic control of tumors and infections.
Supplementary Material
Acknowledgments
We thank Håkan Hall and Petter Höglund for technical assistance. This work was supported by grants from the Swedish Cancer Society, the Swedish Paediatric Cancer Foundation, the Swedish Foundation of Strategic Research, and the Karolinska Institutet.
Author contributions: V.L. designed research; M.U. and V.L. performed research; M.U., M.G.M., and V.L. analyzed data; and V.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AID, activation-induced degradation; AINR, activation induced nonresponsiveness; CTL, cytotoxic T lymphocyte; LCL, lymphoblastoid cell line; TCR, T cell receptor.
References
- 1.Höllsberg, P., Batra, V., Dressel, A. & Hafler, D. A. (1996) J. Immunol. 157, 5269-5276. [PubMed] [Google Scholar]
- 2.Deeths, M. J., Kedl, R. M. & Mescher, M. F. (1999) J. Immunol. 163, 102-110. [PubMed] [Google Scholar]
- 3.Tham, E. L. & Mescher, M. F. (2002) J. Immunol. 169, 1822-1828. [DOI] [PubMed] [Google Scholar]
- 4.Heissmeyer, V., Macian, F., Im, S. H., Varma, R., Feske, S., Venuprasad, K., Gu, H., Liu, Y. C., Dustin, M. L. & Rao, A. (2004) Nat. Immunol. 5, 255-265. [DOI] [PubMed] [Google Scholar]
- 5.Tham, E. L. & Mescher, M. F. (2001) J. Immunol. 167, 2040-2048. [DOI] [PubMed] [Google Scholar]
- 6.Tham, E. L., Shrikant, P. & Mescher, M. F. (2002) J. Immunol. 168, 1190-1197. [DOI] [PubMed] [Google Scholar]
- 7.Zamoyska, R., Basson, A., Filby, A., Legname, G., Lovatt, M. & Seddon, B. (2003) Immunol. Rev. 191, 107-118. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, S. J., Levin, S. D. & Perlmutter, R. M. (1994) Adv. Immunol. 56, 151-178. [DOI] [PubMed] [Google Scholar]
- 9.Weil, R. & Veillette, A. (1996) Curr. Top. Microbiol. Immunol. 205, 63-87. [DOI] [PubMed] [Google Scholar]
- 10.Desbarats, J., You-Ten, K. E. & Lapp, W. S. (1995) Cell. Immunol. 163, 10-18. [DOI] [PubMed] [Google Scholar]
- 11.Maccalli, C., Pisarra, P., Vegetti, C., Sensi, M., Parmiani, G. & Anichini, A. (1999) J. Immunol. 163, 6912-6923. [PubMed] [Google Scholar]
- 12.Matache, C., Onu, A., Stefanescu, M., Tanaseanu, S., Dragomir, C., Dolganiuc, A. & Szegli, G. (2001) Autoimmunity 34, 27-38. [DOI] [PubMed] [Google Scholar]
- 13.Romagnoli, P., Strahan, D., Pelosi, M., Cantagrel, A. & van Meerwijk, J. P. (2001) Int. Immunol. 13, 305-312. [DOI] [PubMed] [Google Scholar]
- 14.Stefanova, I., Saville, M. W., Peters, C., Cleghorn, F. R., Schwartz, D., Venzon, D. J., Weinhold, K. J., Jack, N., Bartholomew, C., Blattner, W. A., et al. (1996) J. Clin. Invest. 98, 1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naramura, M., Jang, I. K., Kole, H., Huang, F., Haines, D. & Gu, H. (2002) Nat. Immunol. 3, 1192-1199. [DOI] [PubMed] [Google Scholar]
- 16.Valitutti, S., Muller, S., Salio, M. & Lanzavecchia, A. (1997) J. Exp. Med. 185, 1859-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penna, D., Muller, S., Martinon, F., Demotz, S., Iwashima, M. & Valitutti, S. (1999) J. Immunol. 163, 50-56. [PubMed] [Google Scholar]
- 18.Viola, A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. (1999) Science 283, 680-682. [DOI] [PubMed] [Google Scholar]
- 19.Rao, N., Miyake, S., Reddi, A. L., Douillard, P., Ghosh, A. K., Dodge, I. L., Zhou, P., Fernandes, N. D. & Band, H. (2002) Proc. Natl. Acad. Sci. USA 99, 3794-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitsky, V., Zhang, Q.-J., Levitskaya, J., Kurilla, M. G. & Masucci, M. G. (1997) J. Immunol. 159, 5383-5390. [PubMed] [Google Scholar]
- 21.Zemmour, J., Little, A. M., Schendel, D. J. & Parham, P. (1992) J. Immunol. 148, 1941-1948. [PubMed] [Google Scholar]
- 22.Frisan, T., Levitsky, V. & Masucci, M. (2001) Methods Mol. Biol. 174, 125-127. [DOI] [PubMed] [Google Scholar]
- 23.de Campos-Lima, P.-O., Levitsky, V., Imreh, M., Gavioli, R. & Masucci, M. G. (1997) J. Exp. Med. 186, 83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitsky, V., de Campos-Lima, P.-O., Frisan, T. & Masucci, M. G. (1998) J. Immunol. 161, 594-601. [PubMed] [Google Scholar]
- 25.Wei, C.-H., Beeson, C., Masucci, M. G. & Levitsky, V. (1999) J. Immunol. 163, 2601-2609. [PubMed] [Google Scholar]
- 26.Wei, C.-H., Yagita, H., Masucci, M. G. & Levitsky, V. (2001) J. Immunol. 166, 989-995. [DOI] [PubMed] [Google Scholar]
- 27.Wei, C.-H., Uhlin, M., Masucci, M. G. & Levitsky, V. (2002) Hum. Immunol. 63, 821-833. [DOI] [PubMed] [Google Scholar]
- 28.Levitsky, V., Zhang, Q.-J., Levitskaja, J. & Masucci, M. G. (1996) J. Exp. Med. 183, 915-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, J. E., Cossoy, M. B., Chau, L. A., Singh, B. & Madrenas, J. (1997) J. Immunol. 159, 61-69. [PubMed] [Google Scholar]
- 30.Donkor, I. O. (2000) Curr. Med. Chem. 7, 1171-1188. [DOI] [PubMed] [Google Scholar]
- 31.Sandalova, E., Wei, C. H., Masucci, M. G. & Levitsky, V. (2004) Proc. Natl. Acad. Sci. USA 101, 3011-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Drean, E., Vely, F., Olcese, L., Cambiaggi, A., Guia, S., Krystal, G., Gervois, N., Moretta, A., Jotereau, F. & Vivier, E. (1998) Eur. J. Immunol. 28, 264-276. [DOI] [PubMed] [Google Scholar]
- 33.Hawash, I. Y., Kesavan, K. P., Magee, A. I., Geahlen, R. L. & Harrison, M. L. (2002) J. Biol. Chem. 277, 5683-5691. [DOI] [PubMed] [Google Scholar]
- 34.Haglund, K., Shimokawa, N., Szymkiewicz, I. & Dikic, I. (2002) Proc. Natl. Acad. Sci. USA 99, 12191-12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haglund, K., Sigismund, S., Polo, S., Szymkiewicz, I., Di Fiore, P. P. & Dikic, I. (2003) Nat. Cell Biol. 5, 461-466. [DOI] [PubMed] [Google Scholar]
- 36.Glading, A., Lauffenburger, D. A. & Wells, A. (2002) J. Immunol. 12, 46-54. [DOI] [PubMed] [Google Scholar]
- 37.Perrin, B. J. & Huttenlocher, A. (2002) Int. J. Biochem. Cell Biol. 34, 722-725. [DOI] [PubMed] [Google Scholar]
- 38.Morford, L. A., Forrest, K., Logan, B., Overstreet, L. K., Goebel, J., Brooks, W. H. & Roszman, T. L. (2002) Biochem. Biophys. Res. Commun. 295, 540-546. [DOI] [PubMed] [Google Scholar]
- 39.Seder, R. A., Grabstein, K. H., Berzofsky, J. A. & McDyer, J. F. (1995) J. Exp. Med. 182, 1067-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chitnis, V., Pahwa, R. & Pahwa, S. (2003) Clin. Immunol. 107, 36-45. [DOI] [PubMed] [Google Scholar]
- 41.Oh, S., Berzofsky, J. A., Burke, D. S., Waldmann, T. A. & Perera, L. P. (2003) Proc. Natl. Acad. Sci. USA 100, 3392-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Campos-Lima, P. O., Levitsky, V., Brooks, J., Lee, S. P., Hu, L. F., Rickinson, A. B. & Masucci, M. G. (1994) J. Exp. Med. 179, 1297-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.