Abstract
In adult cortical tissue, recruitment of GABAergic inhibition prevents the progression of synchronous population discharges to epileptic activity. However, at early developmental stages, GABA is excitatory and thus unable to fulfill this role. Here, we report that retrograde signaling involving endocannabinoids is responsible for the homeostatic control of synaptic transmission and the resulting network patterns in the immature hippocampus. Blockade of cannabinoid type 1 (CB1) receptor led to epileptic discharges, whereas overactivation of CB1 reduced network activity in vivo. Endocannabinoid signaling thus is able to keep population discharge patterns within a narrow physiological time window, balancing between epilepsy on one side and sparse activity on the other, which may result in impaired developmental plasticity. Disturbing this delicate balance during pregnancy in either direction, e.g., with marijuana as a CB1 agonist or with an antagonist marketed as an antiobesity drug, can have profound consequences for brain maturation even in human embryos.
Keywords: endocannabinoid, epilepsy, interneurons, neonates, drug abuse
Marijuana (cannabis) abuse during pregnancy represents a major health problem because of its consequences to embryonic/fetal development. Enhancing (or silencing) endocannabinoid signaling impairs oviductal transport of embryos (1). Furthermore, children of cannabis users display cognitive deficits, suggesting that maternal consumption has interfered with the proper maturation of the brain (2, 3). The action of cannabis in the adult brain includes the activation of presynaptic G protein-coupled cannabinoid type 1 (CB1) receptors and consequent reduction of the release probability of neurotransmitters, in particular GABA (4, 5). Modifying GABA release in the developing brain may have important functional consequences because GABA is excitatory in most brain structures and acts in synergy with glutamate to produce early life network activity (6, 7). Decreasing or increasing GABA release should alter network patterns. Indeed, activation or blockade of presynaptic GABAB receptors, which is another system controlling GABA release, respectively abolishes network activity and generates epileptiform discharges in the immature hippocampus (8). The present experiments were conducted to determine whether altering endocannabinoid signaling (with exogenous agonists or marketed antagonists of CB1 receptors) during the first postnatal week disrupted network activity in the rat hippocampus. This time window has been suggested to correspond, in terms of brain development and physiological activity, to the last third of gestation in human (9) and infra-human primates (10).
The endocannabinoid signaling system has been extensively characterized in adult tissue (5). When appropriately depolarized, some classes of neurons are able to synthesize and release endocannabinoids (4). Endocannabinoids then diffuse to presynaptic terminals to activate G protein-coupled CB1 receptors and decrease the release probability of GABA or glutamate (4, 5). CB1 receptors are exclusively located on GABAergic presynaptic terminals originating from cholecystokinin (CCK)-containing interneurons (INs). Although CB1 receptors constitute the most abundant class of G protein-coupled receptors in the brain, the physiological role of this retrograde signaling system remains to be established (5). CB1 receptors and CCK INs are already present at birth (11, 12). Whether this system is functional at early developmental stages remains to be established.
Here, we show that endocannabinoids are released by both INs and pyramidal cells (PCs) in the CA1 region of the hippocampus, activate CB1 receptors, and decrease GABA release. Overactivation of CB1 receptors decreases network activity, whereas their blockade leads to epileptiform activity in vivo. These findings provide evidence of the physiological role of endocannabinoids in the brain. They further suggest that interfering with this signaling system at early developmental stages in humans (e.g., during pregnancy) may alter cortical activity, potentially leading to cognitive deficits later in life.
Materials and Methods
Electron Microscopy. Perfusion and preembedding CB1 immunostaining of 4-day-old (P4) male Wistar rats (n = 5) were performed by using the protocols and antisera described in detail in ref. 13. Postembedding anti-GABA labeling procedures followed those described in ref. 14.
Slice Preparation and Electrophysiology. Transverse hippocampal slices (400 μm) were prepared from male Wistar rat pups (P0–P5) with a tissue slicer in modified artificial cerebrospinal fluid equilibrated with 5% CO2 in 95% O2 at 4°C. Slices were transferred in a recording chamber, maintained at 33–35°C, perfused with artificial cerebrospinal fluid containing 124 mM NaCl, 26 mM NaHCO3, 10 mM d-glucose, 3 mM KCl, 2 mM CaCl2, 1.3 mM MgSO4, and 1.25 mM NaH2PO4 equilibrated with 5% CO2 in 95% O2. Pharmacologically isolated GABAergic postsynaptic currents (PSCs) were measured in the presence of 10 μM 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline and 40 μM d-2-amino-5-phosphonovaleric acid to block AMPA/kainate and NMDA receptors, respectively. Experiments involving depolarization-induced suppression of GABA PSCs (DSG) were performed at room temperature (20–22°C), as described in ref. 4. Neurons were visualized by using differential interference contrast microscopy and were recorded in whole-cell voltage-clamp mode with a solution of 100 mM CsCH3SO4, 60 mM CsCl, 5 mM QX-314 chloride, 10 mM Hepes, 0.2 mM EGTA, 1 mM MgCl2, 1 mM Mg·ATP, 0.3 mM Na3GTP, and 0.5% biocytin (pH 7.3, 275 milliosmolar). Synaptic currents were recorded at a holding potential of -60 mV (Figs. 1, 2, 3, 4), filtered at 2 kHz and collected at 10 kHz. When series resistance exceeded 40 MΩ or varied by >20%, experiments were terminated. Evoked GABA PSCs were elicited by using bipolar tungsten electrodes near the soma of the recorded cell. DSG tests, performed every 120 s, consisted of 34 stimuli at 0.33 Hz, with depolarization from -60 to 0 mV for 5 s after the 17th stimulus (15). DSG was calculated by using the mean of the five evoked GABA PSCs just before the depolarization (ampbaseline) and the three evoked GABA PSCs just after the depolarization (amptest) as follows: DSG (%) = 100[1 - (amptest/ampbaseline)]. Drugs were from Tocris Cookson (Bristol, U.K.) except for SR141716A, which was kindly provided by Sanofi–Aventis (Montpellier, France). minianalysis software (Synaptosoft, Decatur, GA) was used to detect and calculate GABA PSC frequency and amplitude. We did not find obvious differences in DSG and drug effects between P0 and P5. Results thus were pooled together. Similar results were obtained with SR141716A and AM251, a more selective CB1 receptor antagonist. Error bars represent SEM. Significance (P < 0.05) was determined by two-sample paired t tests.
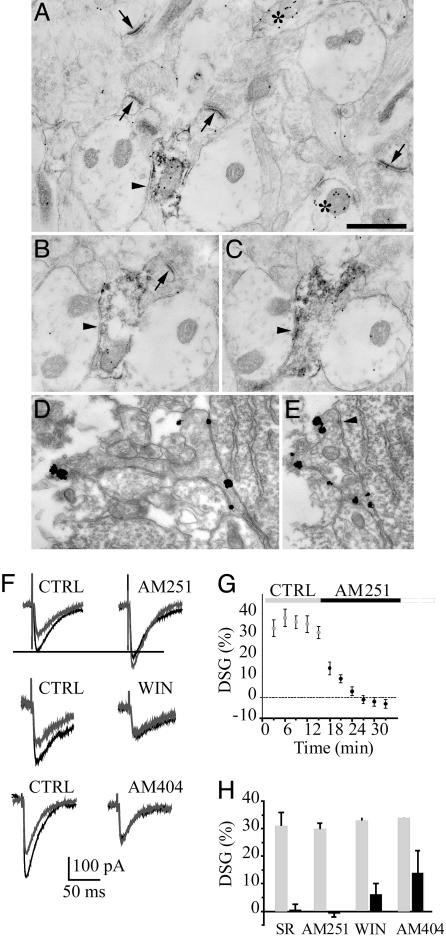
Fig. 1.
CB1 receptors are present and can be activated by endocannabinoids at IN–PC connections. (A–E) Subcellular distribution of CB1 in the hippocampus. Electron micrographs of the CA1 strata radiatum (A–C) and pyramidale (D and E) at P4. (A–C) CB1 receptors and GABA are labeled with diffuse immunoperoxidase–diaminobenzidine staining and immunogold particles (15 nm), respectively. B and C are serial sections of the same bouton. Double-immunolabeled (CB1 and GABA) synaptic boutons form a symmetric synapse (arrowheads in A–C) on the GABA-negative dendrite. Two other GABA-positive profiles (but CB1-negative) are marked with asterisks. Several axonal profiles producing asymmetric (presumed glutamatergic) synapses (arrows) are GABA-negative. (D and E) CB1 receptors are labeled with preembedding ultra-small immunogold particles with silver amplification. CB1 receptors are expressed only on the plasma membrane of the axon terminal that forms a symmetric synapse on a PC body (arrowhead in E). (Scale bar: 0.5 μm.) (F)Ina P3 PC, a 5-s depolarizing step resulted in a robust decrease of evoked GABA PSCs (gray traces). DSG was blocked 15 min after application of the CB1 receptor antagonists AM251. Note that control GABA PSCs were increased after AM251 (black traces) suggesting that CB1 receptors are tonically activated and decrease GABA release at IN–PC connections. Application of the CB1 receptor agonist WIN55212-2 resulted in a decrease of evoked GABA PSC and blocked DSG by occlusion. Endocannabinoid accumulation in the presence of AM404 also reduced DSG by occlusion. (G) Summary of all experiments showing DSG blockade by AM251. (H) Effect of WIN55212-2, AM251, SR141716A, and AM404 on DSG measured 15 min after drug application.
Fig. 2.
CB1 receptors are present and can be activated by endocannabinoids at IN–IN connections. (A) Electron micrograph of the CA1 stratum radiatum at P4. CB1 receptors and GABA are labeled with diffuse immunoperoxidase–diaminobenzidine staining and immunogold particles (15 nm), respectively. Double-immunolabeled (CB1 and GABA) synaptic bouton forms a symmetric synapse (arrowhead) on the GABA-positive dendrite. One GABA-positive profile (but CB1-negative) is marked with an asterisk. (Scale bar: 0.5 μm.) (B) In a P2 stratum radiatum IN, a 5-s depolarizing step resulted in a decrease of GABA PSCs, which was blocked after 15-min application of AM251. Note that control GABA PSCs were increased after AM251 (black traces) suggesting that CB1 receptors are tonically activated and decrease GABA release at IN–IN connections. (Upper) Example of 17 superimposed traces before and after the depolarizing step in control conditions. (Lower) The black trace represents the average of the five GABA PSCs evoked before the depolarizing step. The gray trace represents the average of the three GABA PSCs evoked after the depolarizing step. (C) Summary of seven experiments showing the time course of DSG blockade in INs by AM251.
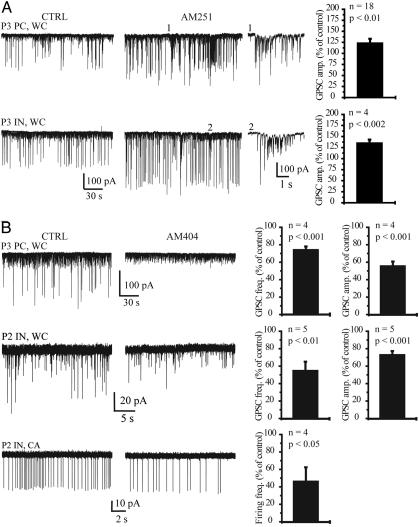
Fig. 3.
Ongoing GABAA receptor-dependent release of endocannabinoids in vitro.(A) The amplitude of pharmacologically isolated GABA PSCs (WC, whole-cell recording) was increased after application of AM251 (3 μM) in a PC (P3, Upper, first image from left) and in a stratum radiatum IN (P3, Lower, first image from left). Note the appearance of large-amplitude GABA PSCs in the presence of AM251 and of spontaneous pure GABAA receptor-mediated bursts of activity (traces 1 and 2, shown on a faster time scale). On the far right are summaries of the effect of AM251 on GABA PSC amplitude in PCs and INs. (B) The frequency and amplitude of GABA PSCs was decreased after AM404 (10 μM) in a P3 PC (Top) and in a P2 IN (Middle). The graphs on the far right show a summary of the effects of AM404 on GABA PSC frequency and amplitude in PCs and INs. The spontaneous firing frequency of INs was decreased the presence of AM404 (P2; CA, cell-attached recording; Bottom). On the right is a summary of the effect of AM404 on IN spontaneous firing frequency.
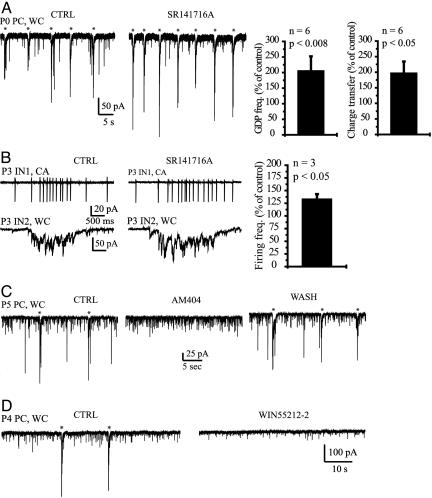
Fig. 4.
Resting activation of cannabinoid receptors limits network excitability in vitro. (A) Application of SR141716A (3 μM) resulted in an increase in GDP (*) frequency and amplitude in a PC (P0). On the right are histograms showing the effects of SR141716 on GDP frequency and charge transfer. (B) (Left and Center) Simultaneous recording of two stratum oriens INs (P3) showing IN1 firing during a GDP and the intracellular compound glutamate/GABA synaptic drive received by IN2. Firing (IN1) and GDP (IN2) charge transfer were increased in the presence of SR141716A (3 μM). (Right) Histogram summarizing the effect of SR141716A on IN firing frequency during GDPs. (C) Application of AM404 (10 μM) abolished GDP activity in a PC (P5), demonstrating that endocannabinoids are released in these conditions. (D) Direct activation of CB1 receptors with WIN55212-2 (3 μM) also abolished GDP activity in a PC (P4).
Morphology. Slices were processed for the detection of biocytin-filled neurons according to established procedures (16). Based on their characteristic morphological features, neurons were morphologically identified post hoc as CA1 PCs or INs.
In Vivo Recordings. Twenty-six Wistar rats (P5–6, 14 ± 3 g) from 12 litters were used. On the day of implantation, pups with a visible milk band were anesthetized, and four tungsten single-ended electrodes (100 μm) were implanted bilaterally at 1.5-mm depth from the surface of the cerebral cortex, approximately in frontal (1 mm anterior to bregma, 2 mm from the midline) and occipital (4 mm anterior to bregma, 2 mm from the midline) cortex areas. Muscular activity was monitored with a bipolar copper electrode implanted in the neck muscle. A ground wire was attached to the skin of the back. All electrodes were secured to the skull by using epoxy glue. Rats were allowed to recover from surgery for at least 30 min at 34°C. To minimize movement artifacts, we used a noninvasive, nonpainful retention system to keep the head in place, whereas the rats were allowed to move the rest of the body. Epileptic discharges contaminated by movement artifacts were not taken into account. Rats were first recorded (depth EEG) for a control period of 60 min (coherence, Deltamed, Paris). They then received a single s.c. injection (50 μl) of either DMSO alone or a drug (SR141716A, AM404, or WIN55212-2) dissolved in DMSO for a final concentration of 10 mg/kg. SR141716A was used instead of AM251 for the in vivo study because it is the active constituent of Rimonabant, a drug introduced to control obesity. Rats then were recorded for an additional period of 30–60 min. Control activity was not significantly different before and after vehicle injection in sham-treated animals (n = 6; data not shown).
Results
Endocannabinoid System Is Functional During the First Postnatal Week. CB1 receptors are present on presynaptic terminals of CCK INs in the CA1 region of the hippocampus at birth (11, 12). In the present study, anatomical data indicate that CB1-positive synaptic boutons were also immunoreactive for GABA at both IN–PC (Fig. 1 A–E) and IN–IN connections (Fig. 2A). CB1 receptors were expressed only on the membrane of axon terminals and preterminal axons but not on the somato-dendritic membrane. CB1 receptors were never observed at asymmetrical, presumably glutamatergic, synapses. CB1 receptors therefore are positioned to control GABA release, hence GABA-driven network activity.
We then tested whether endocannabinoid signaling was functional after birth. In the mature hippocampus, a transient depolarization of CA1 PCs leads to depolarization-induced suppression of inhibition (17), i.e., a decrease of the amplitude of pharmacologically isolated GABAergic PSCs. To prevent confusion, we used DSG instead of depolarization-induced suppression of inhibition, because GABA is excitatory during the first postnatal week (6). A transient depolarization of CA1 PCs led to DSG in neonates (Fig. 1F), as reported at later stages of development (5, 17, 18). Average DSG magnitude in PCs was 32 ± 2% (n = 32, P < 0.0001). In 8 of 40 cells (20%), DSG did not reach significant levels, suggesting that some GABAergic terminals lack CB1 receptors, that endocannabinoids fail to reach their receptors, or that some CB1 receptors are continuously activated (see below). DSG was blocked after the application of AM251, an antagonist of CB1 receptors (Fig. 1 G and H; -1 ± 1%, n = 11, P = 0.4) compared with 30 ± 2% before AM251 application (n = 11, P < 0.0001). DSG also was fully prevented by the CB1 antagonist SR141716A (Fig. 1H,0 ± 2%, n = 9, P = 0.9) compared with 31 ± 5% before SR141716A application (n = 9, P < 0.0001). DSG during the first postnatal week was similar to that found in older tissues (4, 19). We then verified that DSG and the CB1 receptor agonist WIN55212-2 acted on the same locus. Application of WIN55212-2 resulted in a decrease of evoked GABA PSCs (Fig. 1F; 62 ± 5% of control, n = 7, P < 0.004), showing that the direct activation of CB1 receptors by an exogenous agonist decreases GABA release. DSG was nearly blocked by occlusion after WIN55212-2 application (6 ± 4%, n = 7, P < 0.03) compared with before WIN55212-2 application (33 ± 3%, n = 7, P < 0.002) (Fig. 1 F and H). In the presence of AM404, a drug that allows endocannabinoid accumulation and CB1 receptor activation (see below), DSG also was reduced by occlusion (average DSG was 14 ± 8%, n = 5, P = 0.3) compared with before AM404 application (34 ± 3%, n = 5, P < 0.0001; Fig. 1 F and H).
Although endocannabinoids are not released by hippocampal INs in older animals (20), CB1 receptor-dependent DSG of evoked GABA PSCs also was found at IN–IN connections in neonates (Fig. 2B). Average DSG magnitude in INs was 41 ± 3% (n = 12, P < 0.0001). In 5 of 17 INs (29%), DSG did not reach significant levels. DSG was blocked by AM251 (Fig. 2 B and C; -5 ± 3%, n = 7).
Therefore, during the first postnatal week, endocannabinoid signaling is functional. Endocannabinoids are released by both INs and PCs, activate presynaptic CB1 receptors, and decrease GABA release probability.
CB1 Receptors Control GABA-Driven Activity in Vitro. The activation of GABAergic synapses can trigger action potentials and a rise in intracellular Ca2+ in the postsynaptic cell (6, 21), providing optimal conditions for the synthesis and release of endocannabinoids. We therefore examined whether GABA-driven network activity (in the presence of glutamatergic antagonists) could release endocannabinoids and activate CB1 receptors. After application of AM251, evoked GABA PSC amplitude was increased to 126 ± 5% of control in PCs (Fig. 1F; n = 20, P < 0.004) and to 125 ± 10% of control in INs (Fig. 2B; n = 7, P < 0.01). This result demonstrates the presence of tonic CB1 receptor activation, which results in a decreased probability of GABA release. If such activation depends on the production of endocannabinoids, we reasoned that drugs that allow their accumulation (such as AM404) would result in further activation of CB1 receptors. In the presence of AM404, evoked GABA PSC amplitudes were decreased to 76 ± 7% of control in PCs (Fig. 1F; n = 5, P < 0.03), a reduction similar to that found after the direct activation of CB1 receptors with WIN55212-2. This result demonstrates that endocannabinoids are released when GABA drives network activity. This release may be responsible for the tonic activation of CB1 receptors. When endocannabinoids accumulate, CB1 receptor activation becomes maximal, mimicking the effect of the direct application of the agonist. These effects of AM404 and AM251 on evoked GABA PSCs are specific to this developmental stage because they are not seen in older tissue (4).
We then determined whether endocannabinoids also controlled spontaneous ongoing network activity. We used PCs and INs, which can be targeted by various IN circuits (22), as sensors of overall GABAergic network activity. Blockade of CB1 receptors with AM251 resulted in an increase in spontaneous GABA PSC amplitude in hippocampal neurons (Fig. 3A; 136 ± 7% of control, n = 4 INs, and 124 ± 8%, n = 18 PCs). Although the increase in GABA PSC frequency was not significant (122 ± 12%, n = 22, P = 0.06), the application of CB1 antagonists induced the appearance of spontaneous epileptiform discharges never observed in control conditions (Fig. 3A). Such network control was specific to the postnatal hippocampus, because it was not observed in adult animals (4). CB1 receptor activation most likely results from endocannabinoid release, because their accumulation after application of AM404 decreased spontaneous GABA PSC frequency (Fig. 3B; 54 ± 11% of control, n = 5 INs, and 74 ± 4% of control, n = 4 PCs) and amplitude (Fig. 3B;74 ± 4% of control, n = 5 INs, and 55 ± 5% of control, n = 4 PCs) as well as the spontaneous firing frequency of INs (Fig. 3B;45 ± 17% of control, n = 4). Collectively, these results demonstrate that GABA-driven activity controls GABA release in an endocannabinoid- and CB1 receptor-dependent manner. Enhancing or decreasing this control system modifies network activity.
Physiological Role of Endocannabinoids in Vitro. The previous results suggest that endocannabinoid signaling may be recruited to limit the release of excitatory GABA and prevent network hyperexcitability. We tested this hypothesis in more physiological conditions (in the absence of glutamatergic antagonists). During the early postnatal period, giant depolarizing potentials (GDPs) provide most of in vitro ongoing activity (6). GDPs are generated by the synergistic excitatory actions of GABA and glutamate (6, 7, 23). Intracellularly, GDPs appear as bursts of mixed GABA and glutamate PSCs (Fig. 4). Application of the CB1 receptor antagonists SR141716A increased GDP frequency (Fig. 4A; 206 ± 46% of control, n = 6 PCs) and amplitude (the charge transfer was increased to 195 ± 38% of control, n = 6 PCs). Consistent with the increase in charge transfer, the firing frequency of INs during GDPs also was increased (Fig. 4B; 132 ± 9% of control, n = 3). Therefore, GDP activity is controlled by a tonic activation of CB1 receptors. We then determined whether this mechanism involved endocannabinoids. Application of AM404 significantly decreased GDP frequency in 66% of the slices (19 ± 8% of control, n = 6, P < 0.002) and totally abolished GDPs in 33% of the slices (Fig. 4C; n = 3), suggesting that endocannabinoids are released during GDP activity. To confirm that the endocannabinoid effects were mediated by the activation of CB1 receptors, we tested whether CB1 receptor agonists altered ongoing activity. Acute application of the synthetic cannabinoid agonist WIN55212-2, decreased GDP frequency (40 ± 12% of control, n = 5, P < 0.005) and amplitude (the charge transfer associated with each GDP was reduced to 46 ± 13% of control, n = 5, P < 0.003), nearly abolishing network activity (Fig. 4D).
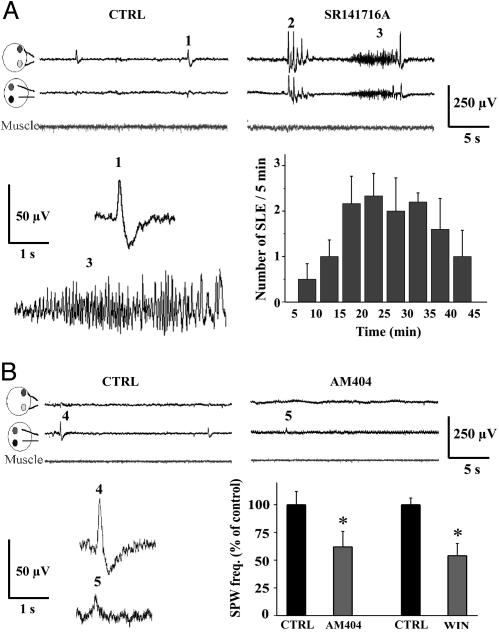
Interfering with Endocannabinoid Signaling Results in Pathological Activity in Vivo. Because endocannabinoids control network activity in vitro, we investigated this issue in vivo. Fast, short oscillations [sharp wave bursts (SPWs)] constitute the hallmark of neuronal network activity in the postnatal hippocampus in vivo (7). GDPs are believed to represent the in vitro image of SPWs (7). Cortical electrophysiological activity recorded in vivo in P5 rat pups was characterized by the occurrence of spontaneous biphasic SPWs (Fig. 5A). Injection of SR141716A resulted in the appearance of epileptic activity composed of large amplitude spikes (n = 9; Fig. 5A). In six of nine animals, such spikes often were followed by more complex and organized ictal-like events (Fig. 5A), consistent with the synchronized recruitment of large populations of neurons. In contrast, SR141716A did not trigger epileptic discharges in adult rats (24). These results strongly suggest that when GABA is excitatory, CB1 receptor activation is necessary to stabilize early network activity in vivo and that silencing this control system switches normal brain activity into a pathological epileptic state.
Fig. 5.
Ongoing activation of CB1 receptors prevents the occurrence of epileptic activity in vivo. (A) (Left Upper) In control conditions, most of the spontaneous cortical activity was composed of biphasic sharp waves (SPWs). The frequency and amplitude of SPWs ranged from 1.9 to 4 events per min and from 23 to 90 μV, respectively (n = 26 animals). Average duration was 468 ± 98 ms (event 1). (Right Upper) Injection of SR141716A (10 mg/kg) induced two patterns of hyperactivity. SPWs of larger amplitude (100–400 μV) occurred in bursts of three to five events (event 2) and lasting 5 ± 2 s, n = 9. In six of nine animals, this epileptic activity was followed (onset 13 ± 2.5 min) by ictal-like events (ILEs, event 3) lasting 6 ± 2 s. (Left Lower) SPW (event 1) and ILE (event 3) shown on a faster time scale. (Right Lower) Summary of all experiments in which ILEs were recorded (n = 6). (B) (Upper) Injection of AM404 (10 mg/kg) reduced both the frequency and amplitude of SPWs. (Lower) Summary of the effect of AM404 and WIN55212-2. The frequency and amplitude of SPWs were measured over a 5-min period 15–20 min after drug administration. AM404 (n = 6) reduces significantly these parameters to 62 ± 12% (*, P < 0.002) and 36 ± 3% (P < 0.006) of control, respectively. Injection of WIN55212-2 (10 mg/kg, n = 5) also significantly decreased the frequency and amplitude of SPWs to 54 ± 10% (*, P < 0.001) and 38 ± 8% (P < 0.007) of control, respectively.
We then examined the impact on network activity of CB1 receptor overactivation. s.c. injection of AM404 or WIN55212-2 in vivo considerably decreased spontaneous activity (n = 6; Fig. 5B). Overactivation of CB1 receptors by exogenous cannabinoid agonists is thus as disruptive for network activity as their pharmacological blockade, although it has opposite effects: shutdown of neuronal network activity instead of epileptic activity.
Discussion
We provide evidence that the abnormal enhancement or impairment of endocannabinoid signaling in the immature brain leads to shutdown of activity or epileptic activity, respectively. These results are clinically relevant because they show the potential dangers of cannabis abuse and marketed CB1 antagonist treatment during pregnancy on cortical activity in the fetus. Interference with cannabinoid signaling has such dramatic effects because it occurs at a period of development when tonic CB1 activation is required to prevent runaway excitation. This work thus has uncovered one physiological function for endocannabinoids in the central nervous system.
The underlying mechanism involves the activation of CB1 receptors on GABAergic presynaptic terminals at IN–PC and/or IN–IN connections. At all stages of development, GABAergic synapses carrying CB1 receptors originate from CCK-containing INs (5, 25, 26). CCK-containing INs appear very early during development, have a dense axonal network contacting PCs (11), and constitute the main class of GABAergic INs in the hippocampus. There is thus a morphological substrate for an endocannabinoid control system because CB1 receptors are widely expressed during the first postnatal week (12). Endocannabinoid signaling is functional. However, in contrast to the adult, endocannabinoid release is not limited to PCs but also occurs in INs. The lack of endocannabinoid release in mature hippocampal INs (20) may be due to the reduced density of CCK-containing INs in the adult (11). However, some classes of neocortical INs release endocannabinoids (26). In physiological conditions, GDPs are the most likely triggers of endocannabinoid synthesis because they can provide the necessary rise in intracellular Ca2+ (5, 21). Interestingly, endocannabinoid release can occur in the absence of GDP activity when glutamatergic neurotransmission is blocked. This finding shows that GABA-driven network activity controls GABA release. Although our observations are consistent with an activity-dependent release of endocannabinoids (see Fig. 6 and Supporting Text, which are published as supporting information on the PNAS web site), we can rule out neither a constitutive release of endocannabinoids nor a constitutive activity of CB1 receptors. This issue could not be addressed due to lack of a drug preventing endocannabinoid synthesis.
Blocking CB1 receptors or their overactivation has a strong impact on network activity, and our results show how the underlying mechanisms may involve GABAergic neurotransmission. It may not be the only one because endocannabinoids also control glutamate release in mature structures (5). Depolarization-induced suppression of excitation could not be tested here because repetitive activation of glutamatergic synapses makes them silent in immature structures (27). If endocannabinoids also control glutamate release during the first postnatal week, the functional impact would be the same because such a system would act as an additional brake on network activity.
Early life hippocampal activity is driven by two excitatory neurotransmitters, GABA and glutamate. Because GABA cannot exert the inhibitory action it has in mature tissue, other control systems are needed to stabilize network activity. One system involves presynaptic GABAB receptors (8). GABAB receptors act as sensors of the activity of presynaptic INs and switch off GABA release when IN firing exceeds a certain level (8). A complementary system involves endocannabinoids. They act as sensors of the activity of postsynaptic neurons to switch off excitatory GABA (and possibly glutamate) release onto them. The strong control of GABA-driven activity by endocannabinoids is unique to developing networks because it occurs in the specific context of depolarizing GABA. In the adult, CB1 receptor antagonists and agonists have little effects on network activity in physiological conditions (5), and endocannabinoids appear to act on-demand in pathological conditions (28) and in epileptic tissue (24, 29). Our observations provide a demonstration of a role of endocannabinoids in physiological conditions in vivo. Because the depolarizing actions of GABA and abundance of CB1 receptors during development are preserved across species and brain structures (5, 6), endocannabinoid-mediated control of network activity may constitute a universal mechanism.
The physiological action of endocannabinoid occurs within a narrow equilibrium window. Any deviation has important consequences because a strong activation of CB1 receptors results in network activity shutdown and their blockade leads to epileptic discharges in vivo. Altering SPW activity may have a strong impact on the maturation of the hippocampus because SPWs directly participate in the refinement of the circuitry via synaptic plasticity mechanisms (30, 31), some of which may be endocannabinoid-dependent (32). Suppressing correlated network activities during critical periods of development results in an inappropriate wiring of neuronal networks (33). The decrease of correlated SPW activity after overactivation of CB1 receptors may alter maturational processes, potentially leading to cognitive deficits found later in life (34). The first postnatal days in the rat have been suggested to correspond, in terms of brain development and physiological activity, to the last period of the gestation in human (9) and infra-human primates (10), respectively. Extrapolation of our data to humans thus suggests that interfering with endocannabinoid signaling during pregnancy may alter cortical activity in the fetus, either favoring epileptic activity when using marketed drugs acting as CB1 receptor antagonists (such as SR141716A) or decreasing it after overactivation of CB1 receptors (by cannabis, for example). Pregnancy appears to be a period critically sensitive to cannabis and CB1 receptor antagonists because of their strong peripheral (1) and central (present study) actions.
Supplementary Material
Acknowledgments
We thank Sanofi–Aventis for the gift of SR141716A; M. Esclapez for morphological analysis; and Drs. H. Beck, J. Jefferys, D. Kullmann, J. Noebles, and P. Tosetti for their helpful comments on the manuscript. This work was supported by Institut National de la Santé et de la Recherche Médicale, FRC, and Action Concertée Incitative “Temps et Cerveau” and “Biologie du Développement et Physiologie Intégrative.” T.F.F. was supported by the Howard Hughes Medical Institute, Országos Tudományos Kutatási Alapprogramok Grant T46820, and the Philip Morris External Research Program. M.M. was supported by an Institut National de la Santé et de la Recherche Médicale M.D.-Ph.D. fellowship.
Author contributions: C.B., H.G., T.F.F., and Y.B.-A. designed research; C.B., H.G., M.M., and Y.M.M. performed research; C.B., H.G., M.M., and Y.M.M. analyzed data; and C.B. and H.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CB1, cannabinoid type 1; PSC, postsynaptic current; DSG, depolarization-induced suppression of GABA PSCs; GDP, giant depolarizing potential; SPW, sharp wave burst; PC, pyramidal cell; IN, interneuron; CCK, cholecystokinin; Pn, n-day-old.
References
- 1.Wang, H., Guo, Y., Wang, D., Kingsley, P. J., Marnett, L. J., Das, S. K., DuBois, R. N. & Dey, S. K. (2004) Nat. Med. 10, 1074-1080. [DOI] [PubMed] [Google Scholar]
- 2.Fergusson, D. M., Horwood, L. J. & Northstone, K. (2002) Br. J. Obstet. Gynaecol. 109, 21-27. [DOI] [PubMed] [Google Scholar]
- 3.Fried, P. A., Watkinson, B. & Gray, R. (2003) Neurotoxicol. Teratol. 25, 427-436. [DOI] [PubMed] [Google Scholar]
- 4.Wilson, R. I. & Nicoll, R. A. (2001) Nature 410, 588-592. [DOI] [PubMed] [Google Scholar]
- 5.Freund, T. F., Katona, I. & Piomelli, D. (2003) Physiol Rev. 83, 1017-1066. [DOI] [PubMed] [Google Scholar]
- 6.Ben Ari, Y. (2002) Nat. Rev. Neurosci. 3, 728-739. [DOI] [PubMed] [Google Scholar]
- 7.Leinekugel, X., Khazipov, R., Cannon, R., Hirase, H., Ben Ari, Y. & Buzsaki, G. (2002) Science 296, 2049-2052. [DOI] [PubMed] [Google Scholar]
- 8.McLean, H. A., Caillard, O., Khazipov, R., Ben Ari, Y. & Gaiarsa, J. L. (1996) J. Neurophysiol. 76, 1036-1046. [DOI] [PubMed] [Google Scholar]
- 9.Clancy, B., Darlington, R. B. & Finlay, B. L. (2001) Neuroscience 105, 7-17. [DOI] [PubMed] [Google Scholar]
- 10.Khazipov, R., Esclapez, M., Caillard, O., Bernard, C., Khalilov, I., Tyzio, R., Hirsch, J., Dzhala, V., Berger, B. & Ben Ari, Y. (2001) J. Neurosci. 21, 9770-9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morozov, Y. M. & Freund, T. F. (2003) Neuroscience 120, 923-939. [DOI] [PubMed] [Google Scholar]
- 12.Morozov, Y. M. & Freund, F. (2003) Eur. J. Neurosci. 18, 1213-1222. [DOI] [PubMed] [Google Scholar]
- 13.Katona, I., Sperlagh, B., Sik, A., Kafalvi, A., Vizi, E. S., Mackie, K. & Freund, T. F. (1999) J. Neurosci. 19, 4544-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somogyi, P. & Hodgson, A. J. (1985) J. Histochem. Cytochem. 33, 249-257. [DOI] [PubMed] [Google Scholar]
- 15.Wilson, R. I., Kunos, G. & Nicoll, R. A. (2001) Neuron 31, 453-462. [DOI] [PubMed] [Google Scholar]
- 16.Esclapez, M., Hirsch, J. C., Ben-Ari, Y. & Bernard, C. (1999) J. Comp. Neurol. 408, 449-460. [DOI] [PubMed] [Google Scholar]
- 17.Pitler, T. A. & Alger, B. E. (1992) J. Neurosci. 12, 4122-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampson, R. E., Zhuang, S. Y., Weiner, J. L. & Deadwyler, S. A. (2003) J. Neurophysiol. 90, 55-64. [DOI] [PubMed] [Google Scholar]
- 19.Ohno-Shosaku, T., Tsubokawa, H., Mizushima, I., Yoneda, N., Zimmer, A. & Kano, M. (2002) J. Neurosci. 22, 3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman, A. F., Riegel, A. C. & Lupica, C. R. (2003) Eur. J. Neurosci. 18, 524-534. [DOI] [PubMed] [Google Scholar]
- 21.Leinekugel, X., Medina, I., Khalilov, I., Ben Ari, Y. & Khazipov, R. (1997) Neuron 18, 243-255. [DOI] [PubMed] [Google Scholar]
- 22.Freund, T. F. & Buzsáki, G. (1996) Hippocampus 6, 347-470. [DOI] [PubMed] [Google Scholar]
- 23.Ben Ari, Y., Cherubini, E., Corradetti, R. & Gaiarsa, J. L. (1989) J. Physiol. 416, 303-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace, M. J., Blair, R. E., Falenski, K. W., Martin, B. R. & DeLorenzo, R. J. (2003) J. Pharmacol. Exp. Ther. 307, 129-137. [DOI] [PubMed] [Google Scholar]
- 25.Losonczy, A., Biro, A. A. & Nusser, Z. (2004) Proc. Natl. Acad. Sci. USA 101, 1362-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacci, A., Huguenard, J. R. & Prince, D. A. (2004) Nature 431, 312-316. [DOI] [PubMed] [Google Scholar]
- 27.Xiao, M. Y., Wasling, P., Hanse, E. & Gustafsson, B. (2004) Nat. Neurosci. 7, 236-243. [DOI] [PubMed] [Google Scholar]
- 28.Marsicano, G., Goodenough, S., Monory, K., Hermann, H., Eder, M., Cannich, A., Azad, S. C., Cascio, M. G., Gutierrez, S. O., van der Stelt, M., et al. (2003) Science 302, 84-88. [DOI] [PubMed] [Google Scholar]
- 29.Chen, K., Ratzliff, A., Hilgenberg, L., Gulyas, A., Freund, T. F., Smith, M., Dinh, T. P., Piomelli, D., Mackie, K. & Soltesz, I. (2003) Neuron 39, 599-611. [DOI] [PubMed] [Google Scholar]
- 30.Caillard, O., Ben Ari, Y. & Gaiarsa, J. L. (1999) J. Neurosci. 19, 7568-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasyanov, A. M., Safiulina, V. F., Voronin, L. L. & Cherubini, E. (2004) Proc. Natl. Acad. Sci. USA 101, 3967-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevaleyre, V. & Castillo, P. E. (2003) Neuron 38, 461-472. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin, T., Torborg, C. L., Feller, M. B. & O'Leary, D. D. (2003) Neuron 40, 1147-1160. [DOI] [PubMed] [Google Scholar]
- 34.Mereu, G., Fa, M., Ferraro, L., Cagiano, R., Antonelli, T., Tattoli, M., Ghiglieri, V., Tanganelli, S., Gessa, G. L. & Cuomo, V. (2003) Proc. Natl. Acad. Sci. USA 100, 4915-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.