Abstract
Background
It is unknown if glucocorticoid malabsorption contributes to the approximate 50% treatment failure rate in dogs with protein‐losing enteropathy (PLE).
Objective
To compare pharmacokinetics (PK) of orally administered prednisolone in dogs with PLE vs healthy controls.
Animals
Fourteen dogs with well‐characterized PLE and 7 control dogs.
Methods
Prospective case‐controlled study. Dogs were treated with 1 mg/kg prednisolone PO once daily for approximately 3 weeks. Venous blood samples were collected at set timepoints before and after prednisolone administration on the first (T1) and final (T2) study days. Total and non‐protein bound serum prednisolone concentrations were determined using liquid chromatography tandem‐mass spectrometry, and pharmacokinetics variables were derived from the drug concentration data. Pharmacokinetics variables were compared between PLE and control dogs and between PLE short‐term responders and non‐responders.
Results
The PLE dogs had a shorter half‐life of the terminal slope than control dogs (harmonic mean of 1.3 vs 1.8 hours; P = .05) whereas the percentage of serum prednisolone that was non‐protein bound was higher in PLE dogs than in control dogs (median of 15.7% vs 6.7%; P = .02) at T1. Total prednisolone drug exposures and maximum total serum drug concentrations did not differ between PLE and control dogs at T1 or T2, nor did they differ between short‐term responders and non‐responders within the PLE population (P > .05 for all comparisons).
Conclusions and Clinical Importance
Overall drug exposures are similar between PLE dogs and healthy controls. Glucocorticoid malabsorption is unlikely to be a common cause of treatment failure in dogs with PLE.
Keywords: chronic inflammatory enteropathy, inflammatory bowel disease, lymphangiectasia, treatment
Abbreviations
- CCECAI
canine chronic enteropathy clinical activity index
- CIE
chronic inflammatory enteropathy
- IBD
inflammatory bowel disease
- IL
intestinal lymphangiectasia
- PLE
protein‐losing enteropathy
- T1
Day 1 of therapy
- T2
Day 21 of therapy
1. INTRODUCTION
Protein‐losing enteropathy (PLE) is a life‐threatening syndrome of uncompensated intestinal protein loss caused by diseased or dysfunctional enteric mucosa. There are many potential causes of PLE in dogs, but chronic enteropathies (CE) characterized by mucosal inflammation (CIE) and intestinal lymphangiectasia (IL) are most common, and often observed together. 1 , 2 Dogs with PLE caused by CIE or IL frequently are treated with anti‐inflammatory or immunosuppressive doses of orally administered glucocorticoids. 1 Approximately 50% of dogs with PLE have a positive clinicopathologic response, 1 , 3 but the reasons that 50% of dogs fail to respond to PO glucocorticoid administration are unknown. Failure of adequate absorption of glucocorticoids from the diseased intestine has been proposed as an explanation for therapeutic failure. 1 For this reason, some veterinary practitioners use parenterally administered glucocorticoids in the initial management of PLE. Others may switch to parenterally administered glucocorticoids if the dog fails to respond to orally administered glucocorticoids at appropriate doses or may even consider escalation of the PO dose. 1
Most prospective clinical trials evaluating serum prednisolone concentrations and pharmacokinetics in humans with Crohn's disease, ulcerative colitis, and Celiac disease have concluded that glucocorticoid absorption generally is similar whether the disease is active or in remission and also similar when diseased humans are compared to healthy controls. 4 , 5 , 6 However, peak plasma concentrations of prednisolone were lower in patients with extensive small bowel disease when compared to patients with ulcerative colitis, colonic or ileocolic Crohn's disease, terminal ileal Crohn's disease and healthy controls in 1 study. 7 These findings suggest that absorption of orally administered prednisolone might be impaired in patients with disease affecting larger portions of the small intestine. Overall drug exposures also were lower in a study of Crohn's disease patients when compared with normal subjects. 8
Similar studies of glucocorticoid pharmacokinetics have not been performed in dogs with PLE secondary to CIE or IL. Therefore, our primary objectives were to determine and compare the pharmacokinetics of orally administered prednisolone in dogs with PLE and healthy controls. We further sought to determine if differences in pharmacokinetics existed between short‐term responders and short‐term non‐responders within the PLE population.
2. MATERIALS AND METHODS
2.1. Sample size
A prospective study was conducted to determine the pharmacokinetics of orally administered prednisolone in dogs with PLE caused by IL, CIE, or both as well as in healthy controls. The study was designed in a 2 : 1 test to control ratio to account for presumed greater pharmacokinetics variability within the PLE population because of disease heterogeneity. This strategy also increased the likelihood that both treatment‐responsive and treatment‐non‐responsive PLE dogs would be captured. A sample size calculation utilizing previously published data 9 suggested 12 dogs with PLE and 6 controls would be needed to detect a 35% difference in total prednisolone drug exposure (estimated to be 350 ng × h/mL) between study populations with power of 0.8 and α of 0.05. 9 This number of dogs also would result in adequate power (>0.8) to detect a 40%‐50% difference in drug exposures between responders and non‐responders within the PLE population assuming an approximate 50% treatment failure rate. 1
2.2. Animals
The PLE population consisted of dogs with clinical signs of gastrointestinal disease (eg, vomiting, diarrhea, weight loss, hyporexia) for at least 3 weeks, concurrent hypoalbuminemia (serum albumin concentration <2.5 g/dL; reference interval [RI], 2.8‐3.6 g/dL), and a canine chronic enteropathy clinical activity index (CCECAI) score >3. 10 Non‐gastrointestinal disease was excluded with routine hematologic and serum biochemical assessments and abdominal ultrasound examination. In addition, dogs were required to have normal serum bile acid concentrations, normal trypsin‐like immunoreactivity concentrations, and either a negative urine dipstick for protein detection or a urine protein‐to‐creatinine ratio <0.5. 11 Hypoadrenocorticism was excluded by documenting baseline cortisol concentrations >55 nmol/L (2 μg/dL) or post ACTH‐stimulated cortisol concentrations >138 nmol/L (5 μg/dL). 12 Intestinal parasitism was excluded using fecal flotation, empirical anthelmintic treatment, or both. All PLE dogs meeting these criteria underwent gastrointestinal endoscopic examination or laparotomy with collection of duodenal and, when possible, ileal mucosal biopsies. Histopathologic assessments were required to obtain evidence of inflammatory enteritis, intestinal lymphangiectasia, or both, with no evidence of an infectious or neoplastic enteropathy. 13 If lacteal dilatation was present and occupied 0%‐25% of the villus width, the degree of lacteal dilatation was considered mild, 25%‐50% was considered moderate, and 50%‐75% was considered severe. The scores were based on the most severely affected villus in each case. 14
The control population consisted of clinically healthy dogs that were matched to PLE dogs to ensure similar ages, weights, and sex distribution. Control dogs were excluded if they had historical gastrointestinal disease. Serum biochemical profiles were assessed to ensure normal serum albumin concentrations and to exclude metabolic diseases such as kidney or liver disease. Control dogs also were required to have CCECAI scores of 0 to further support absence of clinically relevant gastrointestinal disease. All control dogs had a negative fecal flotation within 1 year of study participation. Both PLE and control dogs were excluded if they had received exogenous glucocorticoids in the preceding 21 days.
2.3. Experimental protocol
Dogs meeting the above criteria were enrolled in the study after informed consent was obtained. The CCECAI score was determined at the time of enrollment and T2 through the use of an owner questionnaire. Dogs were treated with a target dosage of 1 mg/kg prednisolone PO once daily for 3 weeks (allowable range between first and second visit, 15‐22 days) using commercially available prednisolone tablets (Lloyd Inc., Shenandoah, Iowa). Owners or caretakers maintained drug administration logs to ensure compliance (File S1). Dogs in the PLE population could be treated with dietary modification, cobalamin, vitamin D products, anti‐emetics, or acid‐reducing medications at the discretion of the attending clinicians because withholding such treatments was considered unethical for client‐owned pets. Control dogs did not undergo any dietary modifications, nor were they treated with additional medications during the study period.
Evaluations were conducted at the Michigan State University Veterinary Medical Center at T1 and T2. Dogs were fasted for 8 hours before the scheduled evaluations, and study investigators administered prednisolone PO in‐hospital on these days. Although food affects absorption and elimination of enteric‐coated prednisolone caplets, 15 there appears to be minimal effect on plain tablets 16 , 17 similar to those used in our study. A tablespoon‐sized amount of their current diet (canned or dry) was used to aid in drug administration because doing so is likely to mimic drug administration practices of dog owners while minimally affecting pharmacokinetics. Three milliliters of venous blood were collected immediately before (0 hour) and 1, 2, 3, 4, 6, and 8 hours after prednisolone administration. Blood samples for prednisolone measurements were immediately transferred into serum collection tubes, and after clot formation, centrifuged for 10 minutes at 1200g. Serum then was transferred into plastic cryovials for storage at −80°C until analysis. A portion of the 0‐hour blood sample also was used for determination of serum biochemistry analytes, which were assessed in real‐time. Diagnostic and treatment recommendations after T2 were at the discretion of the attending clinician and independent of study participation, but additional follow‐up data were available for all PLE dogs.
2.4. Laboratory assessments
All analyses were performed at the Michigan State University Veterinary Diagnostic Laboratory, which is accredited by the American Association of Veterinary Laboratory Diagnosticians. Serum biochemical profiles were assessed using a clinical chemistry analyzer (AU680; Beckman Coulter, Inc) that is routinely used for clinical testing and research purposes. Serum prednisolone concentrations were determined using a liquid chromatography tandem‐mass spectrometry assay that has been described elsewhere, 18 with the addition of an unbound compound protocol. An assay description is available in File S2.
2.5. Pharmacokinetics analysis
Pharmacokinetics variables, which included maximum serum drug concentrations (C max), time to maximum serum drug concentrations (T max), area under the serum drug concentration vs time curve (AUC0‐8h), and half‐life from the terminal slope (t½) were derived from the drug concentration data for each dog. Analyses were performed for total serum prednisolone concentration as well as non‐protein bound (eg, free) serum prednisolone concentration. Both C max and T max were obtained directly from the experimental data. The AUC0‐8h was calculated using the linear trapezoidal method. The slope of the terminal phase (k) was derived from the linear regression of the terminal 2‐3 observations plotted as the log mean concentrations against time, and the corresponding t½ was calculated as t½ = ln2/k. Serum prednisolone concentrations below the assay limit of quantification of 1 ng/mL were recorded as 0.1 ng/mL for statistical purposes. Doing so affected <4% of post‐treatment samples, all but 1 of which occurred at the 8‐hour time point.
2.6. Statistical analyses
Data were assessed for normality using boxplot analyses and Shapiro‐Wilk testing and reported as mean ± SD or median and range, with the exception of t½, which was reported as harmonic mean and coefficient of variation (CV%). Characteristics of the PLE and control populations were compered using Student's t‐tests, Mann‐Whitney U tests, or Fisher exact tests. Pharmacokinetics variables (C max, T max, AUC0‐8h, and t½) were compared between and within populations using paired and unpaired Student's t‐tests. In addition, pharmacokinetics variables were further compared between short‐term complete responders vs non‐responders within the PLE population. Dogs considered to be short‐term complete responders were those in which CCECAI scores were ≤3 and serum albumin concentrations were ≥2.5 g/dL at the second study evaluation timepoint (T2). Dogs considered to be short‐term non‐responders were those that had CCECAI score >3 and serum albumin concentrations <2.0 g/dL at the second study timepoint (T2). Dogs not meeting the above criteria were considered short‐term partial responders and not included in the analysis of responders vs non‐responders. Statistical analyses were performed using commercially available software (GraphPad Prism Version 6.0; GraphPad Software Inc, La Jolla, California or NCSS 2019 Statistical Software, Version 19, Kaysville, Utah), and P values ≤.05 were considered significant.
3. RESULTS
3.1. Dogs
Twenty‐one dogs, including 14 dogs with PLE and 7 controls, were included in the study. The PLE population consisted of various breeds including German Shepherd dog (2), Labrador Retriever (2), mixed breed dog (2) and 1 each of American Staffordshire Terrier, French Bulldog, Jack Russell Terrier, King Charles Cavalier Spaniel, Miniature Australian Shepherd, Newfoundland, Pitbull, and Yorkshire Terrier. Reported clinical signs at the time of diagnosis included diarrhea (13/14; 93%), weight loss (12/14; 86%), decreased appetite (9/14; 64%), vomiting (6/14; 43%), and clinical signs (eg, abdominal distention, difficulty breathing) associated with cavity effusions (2/14; 14%). Median CCECAI score 10 was 10.5 (range, 5‐19) and median Purina fecal score 19 at the time of initial presentation was 6/7 (range, 4‐7/7). Diets the dogs were currently eating at the time of presentation were recorded (Table S1). Median duration of clinical signs before initial presentation was 9 weeks (range, 3‐20 weeks). Median body condition score and muscle condition score were 5/9 (range, 3‐6/9) and 2/3 (range, 1‐3), respectively. 20 , 21 Relevant biochemical data from the time of initial evaluation is reported in Table 1. Individual clinical and biochemical data are available in Table S2.
TABLE 1.
Selected biochemical data from 14 dogs with PLE at time of study enrollment.
| Variable | Median (range) | % Below RI | % Above RI | % Within RI |
|---|---|---|---|---|
| Albumin (g/dL) | 1.3 (1.0‐2.3) | 100 | 0 | 0 |
| Globulin (g/dL) | 1.6 (2.2‐2.6) | 93 | 0 | 7 |
| Cholesterol (mg/dL) | 100 (53‐187) | 79 | 0 | 21 |
| Total calcium (mg/dL) | 7.7 (5.2‐9.5) | 86 | 0 | 14 |
| Ionized calcium (mg/dL) | 1.3 (1.07‐1.43) | 14 | 0 | 86 |
| 25(OH)D (nmol/L) | 21 (12‐154) | 86 | 0 | 14 |
| Cobalamin (ng/L) | 219 (150‐632) | 79 | 0 | 21 |
| Folate (μg/L) | 12 (4.2‐44.4) | 29 | 7 | 64 |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; PLE, protein‐losing enteropathy; RI, reference interval.
Abdominal ultrasound examination was performed in all dogs. Esophagogastroduodenoscopy was performed in 13/14 (93%) dogs and ileocolonoscopy was attempted in 13/14 (93%) dogs and successfully completed in 12/13 (92%). Laparotomy was performed in 1 dog. Histopathology of the small intestine was available in all cases, with duodenal tissue available in all cases (13 endoscopic, 1 full thickness) and ileal tissue available in 12/14 (86%) cases. Individual ultrasonographic and histological data are presented in Table S3. Small intestinal inflammatory infiltrates of various types and severity were described in all dogs. Eleven dogs (11/14; 79%) also had histologic evidence of lymphangiectasia, with the severity of lacteal dilatation ranging from mild to marked based on published guidelines. 14 Nine of these 11 dogs had more severe inflammatory infiltrates than lacteal dilatation, and thus CIE was considered to be the primary underlying disorder. In the other 2 dogs, lacteal dilatation was severe and accompanied by only mild to moderate inflammatory infiltrates, and thus IL was considered to be the primary underlying disorder.
The control dogs were clinically healthy, had normal serum albumin concentrations, and were not being treated with other medications. All control dogs had CCECAI scores of 0. Breeds included in the control population were beagle (n = 3), mixed breed (n = 2), German Shepherd (n = 1), and Labrador Retriever cross (n = 1). No significant differences in age, sex, and weight were identified between the PLE and control populations (Table 2).
TABLE 2.
Characteristics of study populations.
| Controls (n = 7) | PLE (n = 14) | P‐value | |
|---|---|---|---|
| Age (years) | 5.7 (1.7‐8.0) | 6.4 (2.4‐12.1) | .32 |
| Sex (male/female) | 4/3 | 8/6 | >.99 |
| Weight (kg) | 17.8 ± 9.4 | 21.9 ± 12.7 | .46 |
| Prednisolone dosage (mg/kg) | 0.98 ± 0.05 | 1.02 ± 0.10 | .30 |
| CCECAI (severity score) | 0 (0‐0) | 10.5 (5‐19) | <.001 |
| Albumin (g/dL) | 3.1 ± 0.3 | 1.4 ± 0.4 | <.001 |
| Study duration (days) | 19.6 ± 2.3 | 18.9 ± 3.2 | .61 |
Note: Data are reported as median and range (age, CCECAI), absolute numbers (sex), or mean ± SD (weight, prednisolone dosage, albumin, study duration).
Abbreviation: CCECAI, canine chronic enteropathy clinical activity index.
Additional medications that were prescribed to PLE dogs after the first study visit or at the time of diagnosis included clopidogrel (n = 13; median dose, 2.4 mg/kg PO q24h, range, 1.9‐3.1 mg/kg PO daily), cobalamin (n = 13; 1000 μg PO q24h in 11 dogs and 250 μg PO q24h in 2 dogs), omeprazole (n = 2; 1.1 and 1.2 mg/kg PO q12h), ondansetron (n = 2; 0.5 and 0.8 mg/kg PO q12h), calcitriol (n = 1; 16 ng/kg PO q12h), and maropitant (n = 1; 2.2 mg/kg PO q24h). All dogs prescribed omeprazole, ondansetron and maropitant received these medications for ≤7 days. Ten PLE dogs were concurrently enrolled in a placebo‐controlled clinical trial and randomized to receive either cholecalciferol (400 IU/kg PO q24h) or placebo (microcrystalline cellulose). Thirteen dogs were prescribed a veterinary therapeutic diet (n = 9, canned low‐fat diet; n = 4, dry hydrolyzed diet), and 1 dog was prescribed a home‐cooked diet with commercially available vitamin and mineral supplementation (Table S2). At T2, 13 dogs were still being treated with clopidogrel and cobalamin as previously prescribed and 10 dogs were still being treated with study medication (cholecalciferol [400 IU/kg] vs placebo). All PLE dogs still were receiving the initially prescribed diet (Table S1).
The CCECAI scores (median, range) in the PLE population decreased from 10.5 (range, 5‐19) at T1 to 3 (range, 1‐5) at T2 (P < .001) whereas serum albumin concentrations (mean ± SD) increased from 1.4 ± 0.4 to 2.4 ± 0.6 g/dL (P < .001). Serum albumin concentrations also increased in control dogs from 3.1 ± 0.3 g/dL at T1 to 3.4 ± 0.2 g/dL at T2 (P < .001). Changes over time in serum albumin concentrations, serum alkaline phosphatase (ALP) activities, CCECAI scores, and body weights for the PLE and control populations are summarized in Table 3.
TABLE 3.
Changes in selected clinical and biochemical variables over time within the study populations.
| Variable | T1 | T2 | P‐value | |
|---|---|---|---|---|
|
PLE n = 14 |
Albumin (2.8‐3.6 g/dL) | 1.4 ± 0.4 | 2.4 ± 0.6 | <.001 |
| ALP (10‐92 U/L) | 34 (13‐153) | 99 (24‐493) | .005 | |
| CCECAI | 10.5 (8.5‐17.3) | 3 (1‐4) | <.001 | |
| Weight (kg) | 21.9 ± 12.7 | 21.1 ± 12.2 | .20 | |
|
Controls n = 7 |
Albumin (2.8‐3.6 g/dL) | 3.1 ± 0.3 | 3.4 ± 0.2 | <.001 |
| ALP (10‐92 U/L) | 53 (34‐161) | 69 (45‐149) | .28 | |
| CCECAI | 0 (0‐0) | 0 (0‐0) | >.99 | |
| Weight (kg) | 17.8 ± 9.4 | 17.5 ± 8.9 | .37 |
Note: Data are reported as mean ± SD (albumin, weight) or median and range (ALP, CCECAI). Reference intervals and units of measure are provided in parentheses.
Abbreviations: CCECAI, canine chronic enteropathy clinical activity index; PLE, protein‐losing enteropathy.
3.2. Pharmacokinetics: PLE vs controls
The total prednisolone pharmacokinetics in PLE and control population dogs are summarized in Table 4. Maximum total serum drug concentrations decreased over time in both the PLE and control populations (P = .02 for both comparisons). The t½ (harmonic mean) of 1.3 hours in the PLE population was less than the t½ of 1.8 hours in the control population (P = .05) at T1, but no differences were observed between study populations at T2 (P = .78). No other significant differences were identified in the total serum prednisolone pharmacokinetics variables between or within the PLE and control populations. Serum prednisolone concentrations during the 8‐hour sampling period at T1 and T2 are depicted in Figure 1.
TABLE 4.
Summary of total and non‐protein bound prednisolone pharmacokinetics in dogs with PLE and healthy controls.
| Controls (n = 7) | PLE (n = 14) | ||||
|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||
| C max (ng/mL) | Tot | 395.7 ± 66.3 a | 264.7 ± 52.2 a | 313.1 ± 137.7 b | 210.8 ± 93.0 b |
| NPB | 26.1 ± 8.5 c | 19.6 ± 7.4 | 57.1 ± 41.5 c , d | 24.5 ± 15.4 d | |
| T max (hour) | Tot | 1.4 ± 0.5 | 1.7 ± 0.8 | 1.5 ± 0.9 | 1.9 ± 1.0 |
| NPB | 1.4 ± 0.5 | 1.7 ± 0.8 | 1.6 ± 1.3 | 2.1 ± 1.0 | |
| t½ (hour) | Tot | 1.8 (16.1%) e , f | 1.6 (14.1%) e | 1.3 (32.4%) f | 1.5 (21.8%) |
| NPB | 1.9 (15.0%) g | 1.8 (34.7%) h | 1.4 (21.7%) g | 1.4 ± (28.2%) h | |
| AUC0‐8h (ng × mL/h) | Tot | 1137.7 ± 336.1 i | 799.5 ± 127.3 i , k | 879.3 ± 529.7 j | 621.2 ± 208.6 j , k |
| NPB | 81. 0 ± 27.9 l | 60.4 ± 17.4 l | 154.7 ± 127.2 m | 67.7 ± 38.8 m | |
Note: Data are reported as mean ± SD, with the exception of t½, which is reported as harmonic mean and CV%. Both Total (Tot) and non‐protein bound (NPB) data are shown. Comparisons between and within study populations in which P values were ≤.1 are indicated by superscripted lower‐case letters. Bolded superscripted lower‐case letters indicate statistically significant differences.
P = .02;
P = .02;
P = .08;
P = .002;
P = .1;
P = .05;
P = .004;
P = .07;
P = .06;
P = .08;
P = .06;
P = .09;
P = .007.
FIGURE 1.
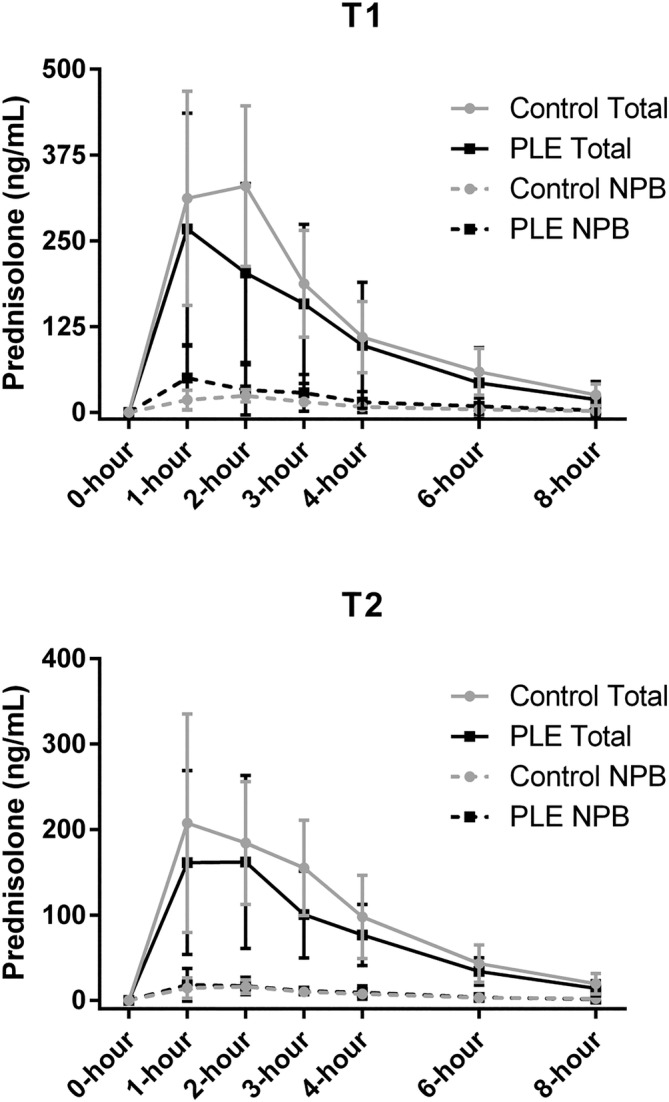
Serum prednisolone concentrations (mean ± SD) in 14 dogs with PLE and 7 healthy controls (A) after an initial dosage of 1 mg/kg prednisolone PO and (B) after approximately 3 weeks of daily administration of 1 mg/kg prednisolone PO. Straight lines represent total prednisolone concentrations whereas dashed lines represent non‐protein bound (NPB) prednisolone concentrations.
The non‐protein bound prednisolone pharmacokinetics also are summarized in Table 4. Although non‐protein bound C max was not significantly different between PLE and controls at T1 (P = .08), the non‐protein bound C max of the 10 PLE dogs with severe hypoalbuminemia (serum albumin ≤1.5 g/dL) was significantly higher than that of controls at T1 (71.1 ± 40.0 ng/mL vs 26.1 ± 8.5 ng/mL; P = .01). Non‐protein bound C max and AUC0‐8h decreased over time in the PLE population from 57.1 ± 41.5 to 24.5 ± 15.4 ng/mL (P = .002) and from 154.7 ± 127.2 to 67.7 ± 38.8 ng × mL/h (P = .007), respectively. The non‐protein bound t½ (harmonic mean) of 1.4 hours in the PLE population was less than the t½ of 1.9 hours in the control population (P = .004) at T1.
The percentage of the total AUC0‐8h that was non‐protein bound (ie, non‐protein bound AUC0‐8h/total AUC0‐8h) was evaluated to further explore the effects of protein‐binding. The non‐protein bound fraction (median, range) of 15.7% (range, 2.4%‐74.2%) in the PLE population was higher than the non‐protein bound fraction of 6.7% (range, 4.9%‐10.0%) in the control population at T1 (P = .02). The non‐protein bound fraction also decreased over time within the PLE population to 9.2% (range, 5.6%‐25.3%) at T2 (P = .02). The non‐protein bound fraction of 9.2% (range, 5.6%‐25.3%) in the PLE population at T2 was not different from the non‐protein bound fraction in the control population of 6.9% (range, 5.1%‐9.7%) at T2 (P = .26).
3.3. Pharmacokinetics: Short‐term responders vs non‐responders
Eight of the 14 (57%) dogs in the PLE population had CCECAI scores ≤3 and serum albumin concentrations ≥2.5 g/dL at T2 and were considered short‐term complete responders. The median CCECAI score in these dogs at T2 was 1.5 (range, 1‐3). Four PLE dogs (29%) had CCECAI scores >3 and serum albumin concentrations <2.0 g/dL at T2 and were considered short‐term non‐responders. The 4 PLE dogs considered non‐responders at T2 were an American Pitbull, mixed breed dog (Rottweiler mix), Labrador Retriever, and Yorkshire Terrier. Of these 4 dogs, 2 were considered to have IL as the primary disorder, 1 dog had moderate CIE and moderate IL, and the last dog had moderate CIE and no evidence of IL. Lymphatic hypoplasia was considered in the dog with no evidence of IL, but not confirmed. The median CCECAI score in these dogs at T2 was 4 (range, 4‐5). None of the total or non‐protein bound serum prednisolone pharmacokinetics variables were different between short‐term treatment responders and short‐term treatment non‐responders (Table 5). Serum prednisolone concentrations at T1 and T2 for the PLE dogs when classified based on treatment responses are depicted in Figure 2.
TABLE 5.
Summary of prednisolone pharmacokinetics in PLE dogs based on treatment response.
| Short‐term responders (n = 8) | Short‐term non‐responders (n = 4) | ||||
|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||
| C max (ng/mL) | Tot | 333.5 ± 176.7 | 202.1 ± 83.8 | 325.4 ± 81.7 | 205.9 ± 101.5 |
| NPB | 52.6 ± 46.9 | 20.4 ± 12.4 | 48.6 ± 26.7 | 22.9 ± 9.4 | |
| T max (hour) | Tot | 1.6 ± 1.1 | 1.6 ± 0.7 | 1.5 ± 1.0 | 2.3 ± 1.3 |
| NPB | 1.9 ± 1.7 | 1.8 ± 0.7 a | 1.5 ± 1.0 | 2.8 ± 1.0 a | |
| t½ (hour) | Tot | 1.3 (36.5%) | 1.5 (27.5%) | 1.3 (22.8%) | 1.5 (10.4%) |
| NPB | 1.4 (20.7%) | 1.4 (30.7%) | 1.5 (19.2%) | 1.3 (29.9%) | |
| AUC0‐8h (ng × mL/h) | Tot | 994.1 ± 666.6 | 596.2 ± 208.6 | 862.0 ± 338.5 | 632.2 ± 130.5 |
| NPB | 150.8 ± 149.1 | 58.0 ± 40.1 | 118.7 ± 60.7 | 75.4 ± 37.0 | |
Note: Data are reported as mean ± SD, with the exception of t½, which is reported as harmonic mean and CV%. Both Total (Tot) and non‐protein bound (NPB) data are shown. Comparisons were made only between study populations, and P values ≤.1 are indicated by superscripted lower‐case letters.
P = .07.
FIGURE 2.
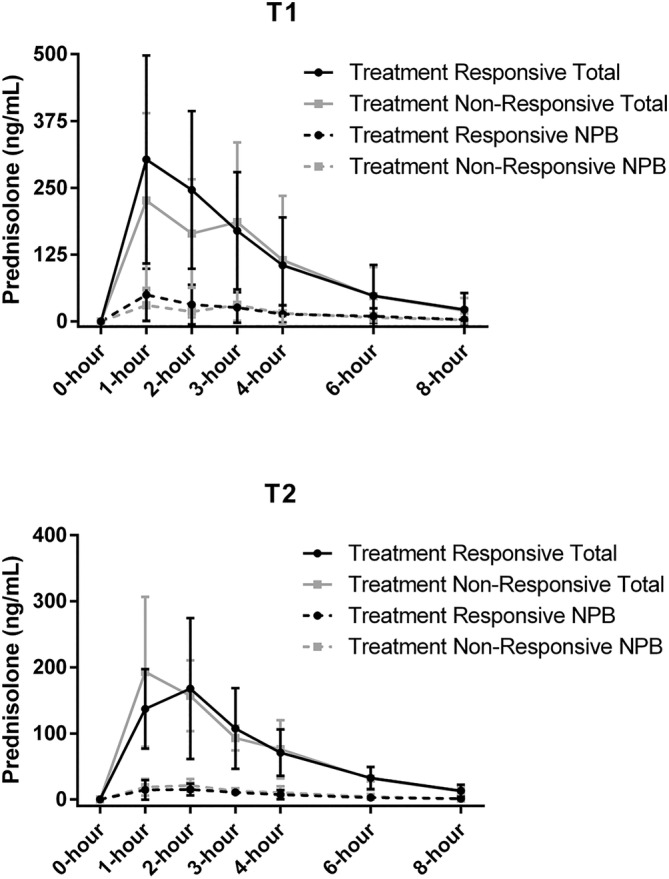
Serum prednisolone concentrations (mean ± SD) at (A) Day 1 and (B) approximately Day 21 in prednisolone‐treated dogs with PLE based on response to therapy. The short‐term treatment responders (n = 8) had serum albumin concentrations ≥2.5 g/dL at study conclusion, and the short‐term treatment non‐responders (n = 4) had serum albumin concentrations <2.0 g/dL. The straight lines represent total prednisolone concentrations whereas dashed lines represent non‐protein bound (NPB) prednisolone concentrations.
3.4. Long‐term outcomes
Long‐term follow‐up information was available for all PLE dogs. Seven of 8 of the short‐term responders had sustained control of their disease at a median of 9 months (range, 6‐11 months) after the original study visit. Two of the 7 dogs maintained remissions with veterinary therapeutic diets alone 3 and 6 months after glucocorticoid discontinuation. The other 5 dogs still were receiving a veterinary therapeutic diet and either low‐dose prednisolone (<0.5 mg/kg PO every 24 or 48 hours) or budesonide. One dog relapsed at 11 months post‐diagnosis despite ongoing treatment with budesonide and a veterinary therapeutic hydrolyzed diet, and euthanasia was elected by the owner. The 2 dogs that were considered partial responders had normal serum albumin concentrations 2 weeks after the second study visit and had sustained control of disease (1 on veterinary therapeutic diet alone and the other on a veterinary therapeutic diet and budesonide). Of the 4 dogs that were considered non‐responders, 2 are deceased. One was euthanized because of PLE 2 months after T2. Additional attempted treatments included a change to a home‐cooked diet formulated by a veterinary nutritionist and cyclosporine (5 mg/kg PO q12h). The other deceased dog died from vehicular trauma shortly after T2. The 2 other dogs from the short‐term non‐responders group were alive with acceptable control of clinical signs (CCECAI score <3) and stable serum albumin concentrations of 1.9 and 2.2 g/dL, 9 and 11 months post‐diagnosis, respectively. Both currently are receiving home‐cooked diets formulated by a veterinary nutritionist. One dog remains on prednisolone (0.25 mg/kg PO q24h) whereas glucocorticoids were discontinued in the other dog. The T1 mean ± SD AUC0‐8h for the 3 dogs that never had a convincing response to glucocorticoids (excluding the dog that died from vehicular trauma) was 953.9 ± 348.1 ng × mL/h, which compares to the T1 AUC0‐8h of 965.9 ± 714.8 ng × mL/h in the 7 dogs that were short‐term responders and still had long‐term control of disease. The T2 mean ± SD AUC0‐8h in the 3 long‐term non‐responding dogs described above was 638.1 ± 159.2 ng × mL/h, which compares to the T2 AUC0‐8h of 537.6 ± 200.8 ng × mL/h in the 7 dogs that were short‐term responders and still had long‐term control of disease.
4. DISCUSSION
Minor differences in pharmacokinetics variables were observed between PLE dogs and healthy controls. The t½ of both total and non‐protein bound prednisolone was shorter in dogs with PLE when compared with control dogs at baseline. The proportion of total prednisolone that was non‐protein bound also was higher in PLE dogs compared with controls at baseline. However, most other pharmacokinetics variables, including overall drug exposures (AUC0‐8h), did not differ between dogs with PLE and controls at T1 or T2 for either total or non‐protein bound prednisolone. Furthermore, pharmacokinetics variables in dogs with PLE that were considered short‐term treatment non‐responders were nearly identical to the pharmacokinetics variables in dogs with PLE that were considered short‐term treatment responders. Interestingly, C max decreased over time in both the PLE and control populations.
Our results share many similarities with pharmacokinetics studies of glucocorticoids in humans with chronic gastrointestinal disorders. 4 , 5 , 6 , 7 , 22 Serum prednisolone concentrations in patients with Crohn's disease or ulcerative colitis are similar to those in healthy controls after administration of 20 mg prednisolone PO. 5 Orally administered prednisone also is effectively absorbed and converted to prednisolone in humans with inflammatory bowel disease (IBD). 6 Prednisolone pharmacokinetics in humans with active IBD are similar to those in patients with inactive (ie, in remission) IBD across several studies, findings that were true for both total and non‐protein bound prednisolone. 6 , 22 Drug exposures in children with IBD that were treated with equivalent doses of prednisolone IV and PO in crossover design were not different. 4 In another study, adults with Crohn's disease and children with IBD that did not experience clinical improvement in response to medical management still achieved serum prednisolone concentrations similar to patients who did respond to medical management. 7 , 22 Study results are not uniformly in agreement, however, because decreased drug absorption might occur in some Crohn's disease patients, especially those with “extensive small bowel” involvement. 7 In addition, humans with Crohn's disease given a tracer dose of PO prednisolone were found to excrete less labeled drug in the urine, excrete more labeled drug in the feces, and have lower overall drug exposures when compared with normal subjects. 8 Still, the aggregate of existing evidence does not suggest that increased glucocorticoid doses are indicated for most humans with inflammatory bowel disease. 4 , 5 , 6 Our findings are largely in agreement when considering that no statistical differences were found in total or non‐protein bound AUC0‐8h between PLE and control populations at either timepoint.
Prednisolone pharmacokinetics were similar in dogs with PLE that were defined as short‐term responders compared to dogs defined as short‐term non‐responders. These results provide even more evidence that dogs with PLE considered non‐responsive to PO glucocorticoids are unlikely to be experiencing difficulty with drug absorption. Rather, their disease is probably not primarily or solely glucocorticoid‐responsive. Some dogs with PLE can be responsive to therapeutic diets as monotherapy 23 , 24 , 25 and some dogs with PLE considered refractory to PO glucocorticoid treatment improve in response to specific dietary changes. 26 Considering these findings in conjunction with our study results, it would seem more prudent to try a different therapeutic approach as opposed to dose escalation or changing to an injectable glucocorticoid in most dogs with PLE that initially fail PO glucocorticoid treatment. Another consideration is that glucocorticoid resistance could be occurring for other reasons. Mechanisms of glucocorticoid resistance that have been described in humans include alterations in the glucocorticoid receptor heterocomplex or proteins involved in nuclear translocation and transcription, and overexpression of P‐glycoprotein in lymphocytes and epithelial cells, among others. 27 Alternative therapeutic approaches are recommended in patients with glucocorticoid‐resistant Crohn's disease, including other immunomodulators. 28 In support of this premise, some dogs with glucocorticoid refractory chronic enteropathy, including dogs with hypoalbuminemia, can achieve clinical remission or rescue with the addition of cyclosporine to their treatment regimens. 10 , 29 There is no evidence of an autoimmune cause of IL in dogs, 30 however, and a recent retrospective review of 31 dogs with PLE secondary to CIE found that the addition of a secondary immunosuppressive agent to glucocorticoid treatment did not result in shorter time to improvement in albumin concentrations or improved outcome when compared to glucocorticoids alone. 31 However, glucocorticoids may have positive effects in this patient population when acting as anti‐inflammatory agents, or because of their potential ability to improve enterocyte function. 32 Determining the most appropriate course of action in dogs with glucocorticoid‐refractory PLE warrants further study and was beyond the scope of our investigation.
The complex and non‐linear pharmacokinetics of prednisolone are influenced by plasma protein binding. 17 , 33 , 34 Prednisolone binds to transcortin (ie, corticosteroid‐binding globulin) with high affinity but low capacity whereas albumin‐binding is with low affinity but high capacity. 35 , 36 Transcortin is readily saturated with supraphysiologic prednisolone doses, and most protein‐binding is with albumin at pharmacologic doses. 17 As such, decreased protein binding of prednisolone is not surprising in conditions causing hypoalbuminemia. 37 Several pharmacokinetics studies in hypoalbuminemic children and adults with nephrotic syndrome identified an increase in the non‐protein bound fraction of prednisone or prednisolone. 38 , 39 , 40 , 41 The non‐protein bound drug has a larger volume of distribution and also is eliminated more rapidly than protein‐bound drug. However, steady state concentrations of unbound drug in hypoalbuminemic patients with nephrotic syndrome and normal subjects are similar. 38 , 39 , 40 , 41 The non‐protein bound fraction of prednisolone was higher in dogs with PLE compared with the control population at T1, and the non‐protein bound C max in PLE dogs with severe hypoalbuminemia also was 2.5× higher than the non‐protein bound C max of controls. Perhaps any possible decreases in drug absorption in PLE dogs would be offset by higher non‐protein bound drug concentrations. Higher non‐protein drug concentrations might even increase the risk of adverse effects as has been described in humans. 42 However, the differences in non‐protein bound prednisolone in dogs with PLE appear to normalize over time. This finding might be related to improvements in serum albumin concentrations or the overall high‐capacity binding of prednisolone with albumin. 35 , 36 The reasons for and clinical relevance of these changes in dogs with PLE warrant additional evaluation.
Our study had several other notable findings such as decreasing total C max over time in both the PLE and control populations. Reasons for this change are not clear but might be related to development of some form of increased or adaptive drug metabolism. Some anti‐epileptic drugs are known to stimulate production of higher amounts of hepatic microsomal enzymes that cause more rapid drug elimination over time. 43 This phenomenon has not been studied extensively with chronic glucocorticoid use, but changes in methylprednisolone pharmacokinetics over time have been described in human renal transplant recipients. 44 The t½ was shorter in PLE dogs (1.3 hours vs 1.8 hours). It is unknown if the shorter t½ is actually because of faster elimination or if this effect was influenced by protein‐binding and ongoing tissue distribution in some dogs. Regardless, the small difference in t½ is unlikely to have any implications on dosing frequency in clinical practice. Another interesting finding was the minimal change in ALP activity over time. Only 7 of 14 (50%) PLE dogs had increases above the reference interval at T2. No significant change occurred in the control population. Drug concentration data clearly established that prednisolone absorption occurred. These findings emphasize the fact that increases in ALP activity in response to exogenous glucocorticoids are variable and age‐dependent, and should not be used as a surrogate for drug exposure. 45 , 46 , 47
One limitation of our study is that additional sampling times would have permitted a more detailed evaluation of prednisolone pharmacokinetics. Sampling times before 1 hour, which was the observed T max for many dogs, would have aided in characterizing absorption features. Prednisolone still might have been in its distribution phase in some dogs at 8 hours, and sampling times between 8 and 24 hours would have aided in determining terminal elimination half‐lives. 48 Despite this, our results are similar to those of other pharmacokinetics studies of prednisolone in dogs. 9 , 48 , 49 We designed our study to detect an approximate 35% difference in total drug exposure between PLE and controls, and another limitation of our study is that we cannot exclude the possibility that some differences exist. A post‐study analysis suggests that at least 28 dogs with PLE and 14 controls would be needed to detect an approximate 20% difference in total prednisolone AUC0‐8h between populations. Several hundred PLE dogs would be needed to detect the potentially small numerical difference in AUC0‐8h between short‐term treatment responders and non‐responders. The clinical relevance of these potential differences is questionable, especially when considering the similarities between short‐term treatment responders and non‐responders. Finally, dogs with PLE were treated with other medications and therapeutic diet changes, whereas dogs in the control group were given only the study drug. Most of these confounders were deemed unavoidable. We could not advocate for feeding all dogs with PLE the same diet for ethical reasons. Because dietary heterogeneity would be present in the PLE population, we elected not to change the diet of the dogs in the control population to avoid any additional disruption to their usual routine. Similarly, most other treatments such as antacids, anti‐emetics, cobalamin, and anti‐platelet drugs were prescribed for PLE dogs as clinically indicated on a case‐by‐cases basis. Various gastroprotectants have not been shown to affect prednisolone pharmacokinetics in humans, 50 , 51 and clopidogrel is routinely administered in conjunction with glucocorticoids to treat immune‐mediated diseases in dogs without any apparent detrimental effects on efficacy. 52 Although these additional treatments seem unlikely to affect prednisolone pharmacokinetics, 53 the possibility of drug interactions cannot be excluded. This limitation is somewhat offset by the fact that our results reflect what occurs in clinical practice.
In conclusion, although some variation in glucocorticoid absorption is present in dogs with PLE, overall prednisolone drug exposures did not differ between dogs with PLE and healthy controls nor did they differ between short‐term responders and non‐responders within the PLE population. These results suggest that poor absorption is unlikely to be a major cause of treatment failure in dogs with glucocorticoid‐refractory PLE. Thus, rather than escalate doses of PO glucocorticoids or change to parenterally administered glucocorticoids in these dogs, clinicians should consider that the underlying condition may not be primarily or solely glucocorticoid‐responsive.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Author's declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC of Michigan State University, IACUC ID# PROTO202000326.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
File S1: Prednisone PK study medication log.
File S2: Prednisolone assay description.
Table S1: Pre‐ and post‐T1 diets in dogs with protein‐losing enteropathy.
Table S2: Baseline demographic, clinical, and biochemical data in 14 dogs with protein‐losing enteropathy.
Table S3: Baseline demographic, clinical, ultrasonographic, and histologic data in 14 dogs with protein‐losing enteropathy.
ACKNOWLEDGMENTS
Funding provided by the Michigan State University College of Veterinary Medicine Endowed Research Funds, specifically the Della K. Swander Fund. Funding also was provided by the Michigan State University College of Veterinary Medicine Trinket Fund. The authors thank Dr. Joe Hauptman for statistical support. Portions of this work were presented as a research report at the 2024 American College of Veterinary Medicine Forum, Minneapolis, Minnesota.
Jablonski SA, Strohmeyer JL, Buchweitz JP, Lehner AF, Langlois DK. Prednisolone pharmacokinetics in dogs with protein‐losing enteropathy. J Vet Intern Med. 2025;39(1):e17277. doi: 10.1111/jvim.17277
Sara A. Jablonski and Daniel K. Langlois contributed equally to this work.
REFERENCES
- 1. Craven MD, Washabau RJ. Comparative pathophysiology and management of protein‐losing enteropathy. J Vet Intern Med. 2019;33(2):383‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jablonski Wennogle SA, Priestnall SL, Webb CB. Histopathologic characteristics of intestinal biopsy samples from dogs with chronic inflammatory enteropathy with and without hypoalbuminemia. J Vet Intern Med. 2017;31(2):371‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simmerson SM, Armstrong PJ, Wünschmann A, Jessen CR, Crews LJ, Washabau RJ. Clinical features, intestinal histopathology, and outcome in protein‐losing enteropathy in Yorkshire Terrier dogs. J Vet Intern Med. 2014;28(2):331‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olivesi A. Normal absorption of oral prednisolone in children with active inflammatory bowel disease, including cases with proximal to distal small bowel involvement. Gastroenterol Clin Biol. 1985;9(8–9):564‐571. [PubMed] [Google Scholar]
- 5. Tanner AR, Halliday JW, Powell LW. Serum prednisolone levels in Crohn's disease and coeliac disease following oral prednisolone administration. Digestion. 1981;21(6):310‐315. [DOI] [PubMed] [Google Scholar]
- 6. Milsap RL, George DE, Szefler SJ, Murray KA, Lebenthal E, Jusko WJ. Effect of inflammatory bowel disease on absorption and disposition of prednisolone. Dig Dis Sci. 1983;28(2):161‐168. [DOI] [PubMed] [Google Scholar]
- 7. Rodrigues CA, Nabi EM, Spiliadis C, et al. Prednisolone absorption in inflammatory bowel disease: correlation with anatomical site and extent. Aliment Pharmacol Ther. 1987;1(5):391‐399. [DOI] [PubMed] [Google Scholar]
- 8. Shaffer JA, Williams SE, Turnberg LA, Houston JB, Rowland M. Absorption of prednisolone in patients with Crohn's disease. Gut. 1983;24(3):182‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van der Heyden S, Croubels S, Gadeyne C, et al. Influence of P‐glycoprotein modulation on plasma concentrations and pharmacokinetics of orally administered prednisolone in dogs. Am J Vet Res. 2012;73(6):900‐907. [DOI] [PubMed] [Google Scholar]
- 10. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21(4):700‐708. [DOI] [PubMed] [Google Scholar]
- 11. Mansfield C. Practical interpretation and application of exocrine pancreatic testing in small animals. Vet Clin North Am Small Anim Pract. 2013;43(6):1241‐1260. v–vi. [DOI] [PubMed] [Google Scholar]
- 12. Gold AJ, Langlois DK, Refsal KR. Evaluation of basal serum or plasma cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs. J Vet Intern Med. 2016;30(6):1798‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jergens AE, Willard MD, Allenspach K. Maximizing the diagnostic utility of endoscopic biopsy in dogs and cats with gastrointestinal disease. Vet J. 2016;214:50‐60. [DOI] [PubMed] [Google Scholar]
- 14. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24(1):10‐26. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Habet SMH, Rogers HJ. Effect of food on the absorption and pharmacokinetics of prednisolone from enteric‐coated tablets. Eur J Clin Pharmacol. 1989;37(4):423‐426. [DOI] [PubMed] [Google Scholar]
- 16. Tembo AV, Sakmar E, Hallmark MR, Weidler DJ, Wagner JG. Effect of food on the bioavailability of prednisone. J Clin Pharmacol. 1976;16(11–12):620‐624. [DOI] [PubMed] [Google Scholar]
- 17. Gambertoglio JG, Amend WJC, Benet LZ. Pharmacokinetics and bioavailability of prednisone and prednisolone in healthy volunteers and patients: a review. J Pharmacokinet Biopharm. 1980;8(1):1‐52. [DOI] [PubMed] [Google Scholar]
- 18. Heine LK, Benninghoff AD, Ross EA, et al. Comparative effects of human‐equivalent low, moderate, and high dose oral prednisone intake on autoimmunity and glucocorticoid‐related toxicity in a murine model of environmental‐triggered lupus. Front Immunol. 2022;13:972108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Purina Fecal Score Chart [Online]. https://www.proplanveterinarydiets.ca/wp‐content/uploads/2016/04/PPPVD‐Fecal‐Scoring‐Chart‐EN‐FINAL.pdf. Accessed March 22, 2024.
- 20. Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract. 1997;22(4):10‐15. [Google Scholar]
- 21. Freeman LM, Michel KE, Zanghi BM, Vester Boler BM, Fages J. Evaluation of the use of muscle condition score and ultrasonographic measurements for assessment of muscle mass in dogs. Am J Vet Res. 2019;80(6):595‐600. [DOI] [PubMed] [Google Scholar]
- 22. Faure C, André J, Pelatan C, et al. Pharmacokinetics of intravenous methylprednisolone and oral prednisone in paediatric patients with inflammatory bowel disease during the acute phase and in remission. Eur J Clin Pharmacol. 1998;54(7):555‐560. [DOI] [PubMed] [Google Scholar]
- 23. Rudinsky AJ, Howard JP, Bishop MA, Sherding RG, Parker VJ, Gilor C. Dietary management of presumptive protein‐losing enteropathy in Yorkshire Terriers. J Small Anim Pract. 2017;58(2):103‐108. [DOI] [PubMed] [Google Scholar]
- 24. Myers M, Martinez SA, Shiroma JT, Watson AT, Hostutler RA. Prospective evaluation of low‐fat diet monotherapy in dogs with presumptive protein‐losing enteropathy. J Am Anim Hosp Assoc. 2023;59(2):74‐84. [DOI] [PubMed] [Google Scholar]
- 25. Nagata N, Ohta H, Yokoyama N, et al. Clinical characteristics of dogs with food‐responsive protein‐losing enteropathy. J Vet Intern Med. 2020;34(2):659‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jablonski Wennogle SA, Stockman J, Webb CB. Prospective evaluation of a change in dietary therapy in dogs with steroid‐resistant protein‐losing enteropathy. J Small Anim Pract. 2021;62(9):756‐764. [DOI] [PubMed] [Google Scholar]
- 27. De Iudicibus S, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol. 2011;17(9):1095‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manz M, Vavricka SR, Wanner R, et al. Therapy of steroid‐resistant inflammatory bowel disease. Digestion. 2012;86(Suppl 1):11‐15. [DOI] [PubMed] [Google Scholar]
- 29. Allenspach K, Rüfenacht S, Sauter S, et al. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid‐refractory inflammatory bowel disease. J Vet Intern Med. 2006;20(2):239‐244. [DOI] [PubMed] [Google Scholar]
- 30. Jablonski SA. Pathophysiology, diagnosis, and management of canine intestinal lymphangiectasia: a comparative review. Animals (Basel). 2022;12(20):2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salavati Schmitz S, Gow A, Bommer N, Morrison L, Mellanby R. Diagnostic features, treatment, and outcome of dogs with inflammatory protein‐losing enteropathy. J Vet Intern Med. 2019;33(5):2005‐2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batt RM, Scott J. Response of the small intestinal mucosa to oral glucocorticoids. Scand J Gastroenterol Suppl. 1982;74:75‐88. [PubMed] [Google Scholar]
- 33. Ferry JSJ, Wagner JG. The nonlinear pharmacokinetics of prednisone and prednisolone. II. Plasma protein binding of prednisone and prednisolone in rabbit and human plasma. Biopharm Drug Disp. 1987;8(3):261‐272. [DOI] [PubMed] [Google Scholar]
- 34. Li X, DuBois DC, Almon RR, Jusko WJ. Physiologically based pharmacokinetic modeling involving nonlinear plasma and tissue binding: application to prednisolone and prednisone in rats. J Pharmacol Exp Ther. 2020;375(2):385‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rocci ML, Johnson NF, Jusko WJ. Serum protein binding of prednisolone in four species. J Pharm Sci. 1980;69(8):977‐978. [DOI] [PubMed] [Google Scholar]
- 36. Alvinerie M, Houin G, Toutain PL. Prednisolone binding to plasma proteins in domestic species. J Pharm Sci. 1988;77(11):937‐938. [DOI] [PubMed] [Google Scholar]
- 37. Gandia P, Decheiver S, Picard M, Guilhaumou R, Baklouti S, Concordet D. Hypoalbuminemia and pharmacokinetics: when the misunderstanding of a fundamental concept leads to repeated errors over decades. Antibiotics (Basel). 2023;12(3):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teeninga N, Guan Z, Stevens J, et al. Population pharmacokinetics of prednisolone in relation to clinical outcome in children with nephrotic syndrome. Ther Drug Monit. 2016;38(4):534‐545. [DOI] [PubMed] [Google Scholar]
- 39. Miller PF, Bowmer CJ, Wheeldon J, Brocklebank JT. Pharmacokinetics of prednisolone in children with nephrosis. Arch Dis Child. 1990;65(2):196‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frey FJ, Frey BM. Altered plasma protein‐binding of prednisolone in patients with the nephrotic syndrome. Am J Kidney Dis. 1984;3(5):339‐348. [DOI] [PubMed] [Google Scholar]
- 41. Rocci ML, Assael BM, Appiani AC, Edefonti A, Jusko WJ. Effect on nephrotic syndrome on absorption and disposition of prednisolone in children. Int J Pediatr Nephrol. 1982;3(3):159‐166. [PubMed] [Google Scholar]
- 42. Lewis GP, Jusko WJ, Graves L, Burke CW. Prednisone side‐effects and serum‐protein levels. A collaborative study. Lancet. 1971;2(7728):778‐780. [DOI] [PubMed] [Google Scholar]
- 43. Löscher W, Schmidt D. Experimental and clinical evidence for loss of effect (tolerance) during prolonged treatment with antiepileptic drugs. Epilepsia. 2006;47(8):1253‐1284. [DOI] [PubMed] [Google Scholar]
- 44. Tornatore KM, Reed KA, Venuto RC. Repeated assessment of methylprednisolone pharmacokinetics during chronic immunosuppression in renal transplant recipients. Ann Pharmacother. 1995;29(2):120‐124. [DOI] [PubMed] [Google Scholar]
- 45. Pettersson H, Ekstrand C, Hillström A, Lilliehöök I. Effect of 1 mg/kg oral prednisolone on biochemical analytes in ten dogs: a cross‐over study. Comp Clin Pathol. 2021;30(3):519‐528. [Google Scholar]
- 46. Moore GE, Mahaffey EA, Hoenig M. Hematologic and serum biochemical effects of long‐term administration of anti‐inflammatory doses of prednisone in dogs. Am J Vet Res. 1992;53(6):1033‐1037. [PubMed] [Google Scholar]
- 47. Syakalima M, Takiguchi M, Yasuda J, Hashimoto A. The age dependent levels of serum ALP isoenzymes and the diagnostic significance of corticosteroid‐induced ALP during long‐term glucocorticoid treatment. J Vet Med Sci. 1997;59(10):905‐909. [DOI] [PubMed] [Google Scholar]
- 48. Nam A, Kim SM, Jeong JW, Song KH, Koo TS, Seo KW. Comparison of body surface area‐based and weight‐based dosing format for oral prednisolone administration in small and large‐breed dogs. Pol J Vet Sci. 2017;20(3):611‐613. [DOI] [PubMed] [Google Scholar]
- 49. Sebbag L, Mochel JP. Pharmacokinetics of oral prednisone at various doses in dogs: preliminary findings using a Naïve pooled‐data approach. Front Vet Sci. 2020;7:571457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cavanaugh JH, Karol MD. Lack of pharmacokinetic interaction after administration of lansoprazole or omeprazole with prednisone. J Clin Pharm. 1996;36(11):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 51. Gambertoglio JG, Romac DR, Yong CL, Birnbaum J, Lizak P, Amend WJ. Lack of effect of sucralfate on prednisone bioavailability. Am J Gastroenterol. 1987;82(1):42‐45. [PubMed] [Google Scholar]
- 52. Mellett AM, Nakamura RK, Bianco D. A prospective study of clopidogrel therapy in dogs with primary immune‐mediated hemolytic anemia. Vet Intern Med. 2011;25(1):71‐75. [DOI] [PubMed] [Google Scholar]
- 53. Kyler KE, Szefler SJ. Fifty years of unraveling the clinical pharmacology of corticosteroids. J Pharm Sci. 2024;113(1):47‐54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1: Prednisone PK study medication log.
File S2: Prednisolone assay description.
Table S1: Pre‐ and post‐T1 diets in dogs with protein‐losing enteropathy.
Table S2: Baseline demographic, clinical, and biochemical data in 14 dogs with protein‐losing enteropathy.
Table S3: Baseline demographic, clinical, ultrasonographic, and histologic data in 14 dogs with protein‐losing enteropathy.


