Abstract
Cone photoreceptors show briefer photoresponses than rod photoreceptors. Our previous study showed that visual pigment phosphorylation, a quenching mechanism of light-activated visual pigment, is much more rapid in cones than in rods. Here, we measured the early time course of this rapid phosphorylation with good time resolution and directly compared it with the photoresponse time course in cones. At the time of photoresponse recovery, almost two phosphates were incorporated into a bleached cone pigment molecule, which indicated that the visual pigment phosphorylation coincides with the photoresponse recovery. The rapid phosphorylation in cones is attributed to very high activity of visual pigment kinase [G protein-coupled receptor kinase (GRK) 7] in cones. Because of this high activity, cone pigment is readily phosphorylated at very high bleach levels, which probably explains why cone photoresponses recover quickly even after a very bright light and do not saturate under intense background light. The high GRK7 activity is brought about by high content of a highly potent enzyme. The expression level of GRK7 was 10 times higher than that of rod kinase (GRK1), and the specific activity of a single GRK7 molecule was ≈10 times higher than that of GRK1. The specific activity of GRK7 is the highest among the GRKs so far known. Our result seems to explain the response characteristics of cone photoreceptors in many aspects, including the nonsaturation of the cone responses during daylight vision.
Keywords: rod, photoreceptors, retina, phototransduction
Our visual system consists of two components: rods and cones (1, 2). These photoreceptors differ in their light sensitivity so that rods mediate twilight vision, and cones mediate daylight vision. Rods and cones are distinguished not only in their light sensitivity, but also in other response characteristics. The photoresponse time course is much briefer in cones, which improves the time resolution of our daylight vision greatly. Rods are saturated with bright background light and do not respond to more intense light (3). In contrast, cones are not saturated and respond to very bright light (4, 5). The molecular mechanisms underlying in the differences of these response characteristics are not yet known. In previous biochemical studies on the cone phototransduction mechanism, only slight quantitative differences were known in the transduction components between rods and cones (6-8).
In our previous study, we obtained a large quantity of isolated cones (enough to perform biochemistry) and showed that transducin activation and cGMP phosphodiesterase activation, the reactions involved in the generation of a photoresponse, are less efficient in cones (9). These findings reasonably explained the lower light sensitivity in cones. Another remarkable difference was found in the phosphorylation of light-activated visual pigment. When light-activated, visual pigment is phosphorylated by a class of kinase known as rhodopsin kinase (rod kinase or G protein-coupled receptor kinase (GRK) 1) in rods. Visual pigment phosphorylation (10) and subsequent binding of arrestin (11) to the phosphorylated pigment are the mechanisms necessary for the quench of light-activated visual pigment (for review, see ref. 12). In cones, a distinct visual pigment kinase (cone kinase or GRK7) has been reported in some classes of vertebrates, including teleosts (13-15), which raised the possibility that the rod pigment/rod kinase system and the cone pigment/cone kinase system contribute to termination of a photoresponse in a cell type-specific manner.
In the study by Tachibanaki et al. (9), visual pigment phosphorylation was at least 20 times faster in cones than in rods and completed within 1 sec after a light flash in a membrane preparation of purified carp cones. Similar rapid phosphorylation in blue cones was observed in a living zebrafish (16). This rapid phosphorylation is probably one of the underlying mechanisms of quick termination of the cone photoresponse. However, because the reaction was too rapid, the early phase of the phosphorylation reaction could not be measured precisely in these studies. In the present study, therefore, we first measured the rapid phosphorylation time course in the cone membranes. In addition, we show here that the kinase in cones is highly effective as a result of high content of a highly active enzyme.
Materials and Methods
Preparation of Rod and Cone Membranes. Carp (Cyprinus carpio) rods and cones were isolated as described in ref. 9. Cones were washed additionally with a K-gluconate (K-gluc) buffer (115 mM K-gluc/2.5 mM KCl/2 mM MgCl2/0.2 mM EGTA/0.1 mM CaCl2/1 mM DTT/10 mM Hepes, pH 7.5) by centrifugation (600 × g for 12 sec and then 3,000 × g for 4 sec). With this manipulation, the contaminated erythrocytes were disrupted and removed in the supernatant. The resultant purified rods and cones were frozen, freeze-thawed, and washed twice with the K-gluc buffer. An aliquot of the washed membranes was used to quantify the amount of each type of cone visual pigment in the cone membranes. All of the membranes were then stored at -80°C until use.
Membrane Fusion Assay. We prepared a membrane preparation containing intact visual pigments but lacking the visual pigment kinase activity (pigment membranes) and a membrane preparation retaining the kinase activity but lacking the pigment activity (kinase membranes). Rod pigment membranes were prepared by denaturing rod kinase with 5 M urea according to the method described in ref. 17. Cone pigment membranes were prepared similarly, but we used 3.7 M urea and 100 mM NaCl (Fig. 6, which is published as supporting information on the PNAS web site).
Kinase membranes were prepared by bleaching the pigment in rod or cone membranes extensively in the presence of 1 mM GTP and 20 mM hydroxylamine with a 100-W tungsten-halogen lamp for 20 min at 4°C. To complete thermal bleach processes, the bleached membranes were incubated for an additional 12-18 hr at 4°C and then washed twice with the K-gluc buffer.
Pigment membranes and kinase membranes were mixed at various ratios depending on the type of the study. A mixture was frozen at -80°C and then thawed to fuse the membranes (18). This manipulation was repeated one more time to fuse the membranes completely. A control experiment showed that without freeze-thawing, kinases in the kinase membranes did not phosphorylate the visual pigment in the pigment membranes significantly (10% of the phosphorylation level in native membranes). When both membranes were fused twice, the phosphorylation level increased to ≈85%. Additional freeze-thawing increased the phosphorylation slightly. The mixing ratio is expressed as the ratio of the content of the visual pigment in the membranes: The 1:1 mixing ratio indicates that the content of the pigment originally present in the kinase membranes is equal to that in the pigment membranes.
Phosphorylation Assays. Phosphorylation assays were performed as described in ref. 9. Rod or cone membranes (15 μl) were mixed with 10 μl of the K-gluc buffer containing [γ-32P]ATP, GTP, and EGTA. The ATP concentration used was 1 mM in the measurements in the cone membranes, and it was either 1 mM or 100 μM in the measurements in the rod membranes. After preincubation for 30 sec, the sample was irradiated with a light flash. The reaction was terminated by adding 150 μl of 10% (wt/vol) trichloroacetic acid. After centrifugation (20,000 × g for 10 min), the precipitate was washed with the K-gluc buffer and subjected to SDS/PAGE. We quantified the amount of 32P incorporated into the visual pigment band, which was visualized with Coomassie brilliant blue staining and identified immunochemically. Dark activities, which were almost negligible, were always subtracted to determine the light-dependent incorporation of 32P. Phosphorylation of visual pigment is expressed in units of the number of phosphates incorporated per visual pigment bleached (Pi/R*) or total amount of phosphate (pmol of Pi).
For the measurement of the early time course of phosphorylation with good time resolution, we used a calibrated rapidquench apparatus in which the timing of the addition of trichloroacetic acid after/before a light flash was controlled (Fig. 7, which is published as supporting information on the PNAS web site).
Electrophysiological Measurements. The outer segment of a mechanically dissociated carp red cone or frog rod was sucked into an electrode, and photoresponses were recorded as reported in ref. 19. The responses were low-pass filtered at 40 Hz. The peak amplitude of the response was 1.4-5.3 pA in carp red cones and 12-28 pA in frog rods.
Light Source. The flash light source used was an Auto 25SR (Sunpak, Tokyo), and the light intensity was attenuated by neutral density filters (9). In biochemical studies, flash intensities were calibrated by measuring the amount of visual pigment bleached in detergent-solubilized rod or cone pigment (9). In electrophysiological studies, a light flash was given through a quartz light guide, one end of which was positioned at ≈2 cm from the cell. Bleach levels were measured in detergent-solubilized rod or cone pigment at the approximate position of the cell. The amount of bleach of red cone pigment was determined by a differential bleaching method (9). Photoresponses were recorded by giving a light flash to the cell from the side, and this geometrical arrangement was taken into account (20) for estimation of the bleach level in intact rods and cones.
Preparation of Polyclonal Sera. Anti-rod kinase (GRK1) anti-sera were raised against an N terminus GST-fused peptide of the C terminus (409-563 aa) of carp GRK1 [BAB32497, National Center for Biotechnology Information (NCBI), Bethesda] in a mouse. Anti-cone kinase (GRK7) anti-sera were raised against a partial peptide of carp GRK7 (BAB32498, NCBI) (TGLFDELNDPNRKE, corresponding to the sequence at 519-532 aa) in a rabbit. Anti-cone kinase-specific antibodies were affinity-purified with the cone kinase peptide. In fish genome, there are a few evolutionary sisters of GRK1 and GRK7 (unpublished data). The peptide sequence used in this study was that from a major one and is well conserved (>90%) among the major sisters.
Expression and Purification of Recombinant Rod Kinase and Cone Kinase. Recombinant carp rod kinase and cone kinase were expressed in Sf9 cells and purified based on the method described in ref. 21. Briefly, harvested cells were suspended and sonicated in a Hepes buffer (10 mM Hepes/115 mM K-gluc/2.5 mM KCl/2 mM Mg Cl2/1 mM DTT, pH 7.5) supplemented with 1 mM CaCl2. The lysate was centrifuged (27,000 × g for 20 min), and the supernatant containing the kinase was collected. The kinases were affinity-purified with nonacylated S-modulin as described in ref. 22 and concentrated in the K-gluc buffer. The amount of the expressed protein was quantified with Coomassie brilliant blue staining after SDS/PAGE, using BSA as a standard.
Results and Discussion
Km Values for ATP of Rod Kinase and Cone Kinase. Because the Km value for ATP of the visual pigment kinase in rods (rod kinase GRK1) has been estimated to be 2-8 μM (17, 23), we initially used 100 μM ATP in the phosphorylation measurement. During the course of the subsequent measurements, however, we realized that the Km value for ATP of the kinase in carp cones (cone kinase GRK7) is much higher than that of rod kinase. In the membrane preparations, it was 30 ± 7 μM in rod kinase and 160 ± 58 μM in cone kinase. In the following studies, therefore, we used 1 mM ATP in the measurements of the activities of cone kinase. In the measurement of rod kinase, we used either 100 μM or 1 mM ATP, but the results were indistinguishable at these two different ATP concentrations.
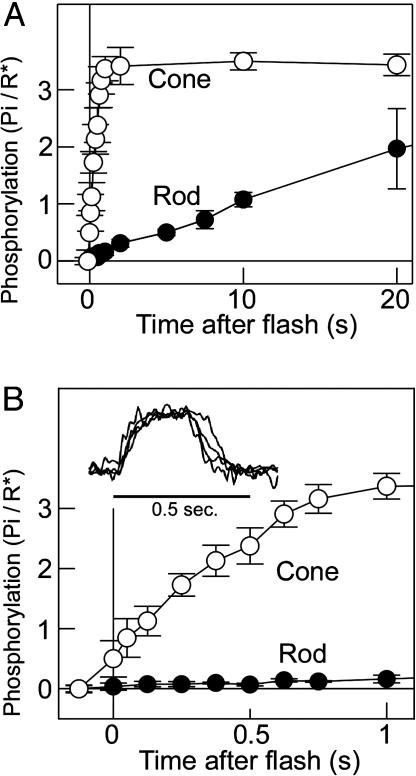
Early Time Course of Visual Pigment Phosphorylation in Cones. Fig. 1A shows the time courses of phosphorylation measured with a rapid-quench apparatus (Fig. 7) in the membrane preparations of purified carp rods and cones at a slow time scale, and Fig. 1B shows the same at an expanded time scale. As seen, cone visual pigment phosphorylation (open circles) is rapid and completes after a light flash at 1 sec with half maximum phosphorylation at ≈250 msec. A light flash used in this study bleached 1.3% of the rod visual pigment and 2.5% of the cone pigment. The phosphorylation rate was 0.09 Pi incorporated into a visual pigment bleached per second (Pi/R*·sec-1) in rods and 4.8 Pi/R*·sec-1 in cones. The phosphorylation rate was 50 times faster in cones. However, this ratio depends on the bleach level (see Fig. 3 D and E). In Fig. 1, the phosphorylation level in cones at time 0 was slightly higher than 0, which is probably due to a 50-70 msec time delay necessary for the quench of the reaction (see the legend of Fig. 7). The phosphorylation level at the steady state in cones in the present study was approximately three phosphates per pigment (Fig. 1 A), which is similar to the number of phosphates necessary for the complete suppression of the light-activated visual pigment in rods (24).
Fig. 1.
Early phosphorylation time course measurement in rod and cone membranes. (A) Phosphorylation time course in rod (filled circles) and cone (open circles) membranes at a slow time scale (n = 3). The bleach level was 1.3% in the rod membranes and 2.5% in the cone membranes. (B) Same phosphorylation time course as in A at an expanded time scale. (Inset) Normalized carp red cone photoresponses (four cells) elicited by a light flash bleaching 2.3% of the red pigment.
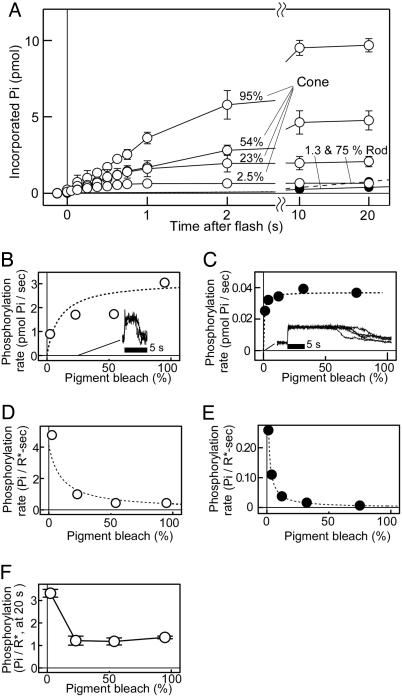
Fig. 3.
Visual pigment phosphorylation at high bleach levels. (A) Phosphorylation time courses at high bleach levels in rod and cone membranes. Phosphorylation time courses were measured at indicated bleach levels in rod and cone membranes containing 7.5 pmol of visual pigment (n = 3 in both membranes). (B and C) Initial phosphorylation rates determined in A were plotted as a function of bleach level for the cone (B) and rod (C) membranes. (Insets) The normalized photoresponses elicited by a brightest light flash (28% bleach) in carp red cones (four cells, B) and a bright flash (0.3% bleach) in frog rods (five cells, C). The dotted curves were drawn based on a Michaelis-Menten equation, V = Vmax S/(S + Km), with Km = 10% bleach and Vmax = 3.1 Pi/sec for the cone membranes (B), and with Km = 0.61% bleach and Vmax = 0.037 Pi/sec for the rod membranes (C) (see Supporting Text). (D and E) The initial rate of phosphorylation per bleached pigment (Pi/R*·sec-1) was plotted against bleach level for the cone (D) and rod (E) membranes. The dotted curves were drawn based on an equation of V/S = Vmax/(S + Km), with the same Km and Vmax values in B and C (see Supporting Text). (F) Final phosphorylation levels per bleached cone pigment were determined at 20 sec after a light flash and were plotted against bleach level (n = 3).
In our purified cone preparation, all cell types of cones are present (9). Because our purified cone preparation contained mostly red cones (red:green:blue = 3:1:1; ref. 9), the measured phosphorylation time course reflects mostly that of red cones.
Comparison of Time Courses Between Pigment Phosphorylation and Photoresponse Recovery. The study of the phototransduction mechanism has been performed mainly in rods, and it has been generally known that rod visual pigment phosphorylation measured biochemically is much slower than a rod photoresponse recovery. One of the problems present in this inconsistency is that the light intensity usually used in the phosphorylation measurement is >103 times higher than that used in the photoresponse measurement so that both reactions cannot be compared directly. Because the light intensity required for generation of a photoresponse is much higher in cones, it may be possible to compare the time courses directly between pigment phosphorylation and photoresponse recovery in cones.
Fig. 1B Inset shows carp red cone saturated photoresponses elicited by a light flash of similar light intensity (2.3% bleach) used in the phosphorylation measurement (2.5% bleach) in Fig. 1. The photoresponse started to recover at ≈0.3 sec after the light flash. At the time of this recovery, 1.5-2 phosphates were incorporated into a bleached cone pigment. The result, therefore, showed that the time course of visual pigment phosphorylation coincides with the recovery of a photoresponse.
In intact photoreceptors, Ca2+ concentration decreases in the light (1, 2), and this decrease would cause disinhibition of the visual pigment kinase activity through inactivation of S-modulin/recoverin (25-27). Because our biochemical measurement was done at a constant low Ca2+ concentration without the inhibition, the time course measured in the present study could be a little bit faster than that observed in intact cells. However, because the Ca2+ concentration decrease in cones is rapid (τ = 43 msec) (28), its effect on the phosphorylation would not be so large in the case of cones.
Molecular Determinant of Rapid Phosphorylation in Cones. There are potentially two possible mechanisms that account for the rapid phosphorylation in cones. One is that cone visual pigment is much more readily phosphorylated by kinases, and the other is that cone kinase shows much higher activity than rod kinase. To distinguish which is the molecular determinant of the rapid phosphorylation in cones, cone pigment or cone kinase, we measured both the phosphorylation of rod pigment by cone kinase and the phosphorylation of cone pigment by rod kinase. In this study, we used fused membrane preparations and measured the phosphorylation time course at a slow time scale.
We prepared rod pigment membranes containing rod visual pigment but lacking the rod kinase activity. We also prepared cone kinase membranes containing cone kinase but lacking cone visual pigment. Both membranes were fused by freeze-thawing. The resultant membranes contained active rod visual pigment and active cone kinase. Similarly, we prepared cone pigment membranes lacking the cone kinase activity. Therefore, we prepared four types of fused membranes, each of which contained either rod or cone pigment and rod or cone kinase.
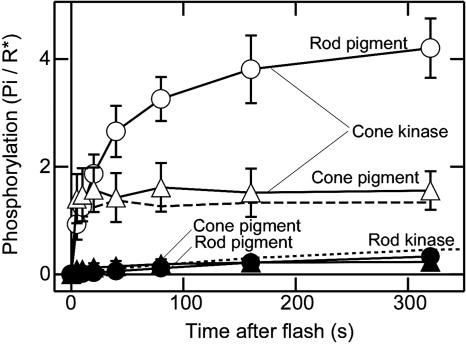
Fig. 2 shows the measurements of the phosphorylation time courses in the four types of the membranes. In these measurements, the amount of visual pigment in the pigment membranes was equal to that of visual pigment originally present in the kinase membranes (1:1 mixing ratio).
Fig. 2.
Rod/cone visual pigment phosphorylation by rod/cone kinase. Visual pigment phosphorylation in the fused membranes prepared at a 1:1 mixing ratio. Phosphorylation was measured in the membranes consisting of rod pigment and cone kinase (open circles), cone pigment and cone kinase (open triangles), rod pigment and rod kinase (filled circles), and cone pigment and rod kinase (filled triangles). Results are the mean ± the range of deviations in three independent sets of measurement. The bleach level was 75% in the rod pigment membranes and 95% in the cone pigment membranes. The phosphorylation time courses in the native membranes without fusion are shown by the dashed line (cone) and the dotted line (rod).
The phosphorylation was rapid and enormous when cone kinase was present (open symbols) no matter whether the pigment was rod type (open circles) or cone type (open triangles). In contrast, when rod kinase was present (filled symbols), the phosphorylation was small and slow. The result showed that the rapid phosphorylation in cones (Fig. 1) is due to high cone kinase (total) activity. Our result supports a similar conclusion drawn from an electrophysiological study by Kefalov et al. (29) that showed that rod and cone pigments expressed in the same cell produce photoresponses with identical amplification and kinetics.
One interesting feature of the result in Fig. 2 was that the initial rate of phosphorylation by cone kinase (open symbols) was similar independent of the type of visual pigment but that the final level of the phosphorylation was smaller, and the phosphorylation terminates more quickly in the case of cone pigment (compare open circles with open triangles). It is probably because the lifetime of meta II intermediate is shorter in cone pigment (30).
Phosphorylation at High Bleach Levels. It has been well known that rod responses remain saturated for >10 sec when the light stimulus is intense (31), whereas cone responses recover rather quickly even after exposure to very bright light (4, 5) (see Fig. 3 B and C Insets). As shown above, the cone kinase activity is very high so that cone kinase may be able to phosphorylate cone visual pigment even at very high bleach levels to result in quick photoresponse recovery. In Fig. 3A, we measured the time courses of cone visual pigment phosphorylation and rod visual pigment phosphorylation at various levels of bleach in the native cone membranes and rod membranes. In this figure, note that the total phosphorylation is plotted against time after a light flash. From the initial slope, the rate of total phosphorylation (total rate) was calculated and was plotted against bleach level. The result showed that in the cone membranes (Fig. 3B), the total rate increased as the bleach level increased. In contrast, in the rod membranes, the rate stayed at almost a constant level when the bleach level was >3.9% (Fig. 3C).
The result in Fig. 3C suggested that in the rod membranes, rod kinase fully expresses its activity at 3.9% bleach and that the total phosphorylation rate does not increase even when more rod pigment is bleached. In contrast, cone kinase seems to be able to phosphorylate bleached cone pigment at much higher bleach levels (Fig. 3B). These results probably explain the long saturation in rod responses and quick recovery in cone responses at high bleach levels. For comparison, saturated photoresponses of carp red cones at the bleach level of 28% (Fig. 3B Inset) and those of frog rods at the bleach level of 0.3% (Fig. 3C Inset) are shown. The photoresponses of carp rods were rather small in amplitude (<2 pA), and reliable measurements were not possible.
Quick recovery in cone responses seems to have important roles during light adaptation. When a background light is given, both rods and cones are initially hyperpolarized and then stabilized at a certain level. When an increment stimulus is given at this point, both rods and cones are hyperpolarized. When a momentary decrease in background illumination is given instead, rods do not respond (3, 4), but cones depolarize quickly (4, 5). This difference in the response to a decrement stimulus is another notable difference between rods and cones and is attributable to the difference in the speed of the recovery of a photoresponse. In addition, rods are saturated with bright background light (3), but cones are hardly saturated even under extremely bright background light (5). It is highly possible that very effective phosphorylation of visual pigment in cones is one of the important underlying mechanisms of the above kinds of behavior of cones.
In Fig. 3 A-C, total phosphorylation increased as the bleach level increased. However, when the phosphorylation was determined in units of number of phosphates incorporated per visual pigment bleached (Pi/R*) as in Figs. 1 and 2, the rate Pi/R*·sec-1 decreased as the bleach level increased (Fig. 3 D and E). Therefore, the rate of phosphorylation on bleached pigment depends on the bleach level, and the phosphorylation on each bleached pigment is faster at low bleach levels. The decrease in the rate Pi/R*·sec-1 at high bleach levels (Fig. 3 D and E) is due to an intrinsic nature of an enzyme reaction (see Supporting Text, which is published as supporting information on the PNAS web site).
The final phosphorylation level per cone visual pigment bleached (Pi/R*) at 20 sec after the flash was plotted against bleach level (Fig. 3F). The result showed that the number of the phosphates incorporated into a single bleached cone pigment molecule decreases as the bleach level increases.
The number of phosphates incorporated into a single bleached cone pigment at >23% bleach was approximately one, which suggests that only a single site is phosphorylated. This result seems to indicate that the phosphorylation reaction in cones competes with the thermal decay of meta II intermediate, the major bleaching intermediate thought to be the substrate of the phosphorylation. The competition could be very significant at high bleach levels. Because the phosphorylation takes much longer especially at high bleach levels, similar determinations were not possible in the rod membranes.
Maximum Kinase Activity. As shown in Fig. 3C, the rod kinase activity stayed at a constant level at >3.9% bleach, which indicated that the substrate/enzyme ratio is already high at 3.9% bleach in the rod membranes. In the cone membranes, however, the total phosphorylation increased to the level up to 95% bleach (Fig. 3B). This result suggested that the enzyme, cone kinase, is not saturated with the substrate R* in the native cone membranes even at 95% bleach. Based on this idea, we tried to increase the R*/cone kinase ratio to measure the maximum cone kinase activity.
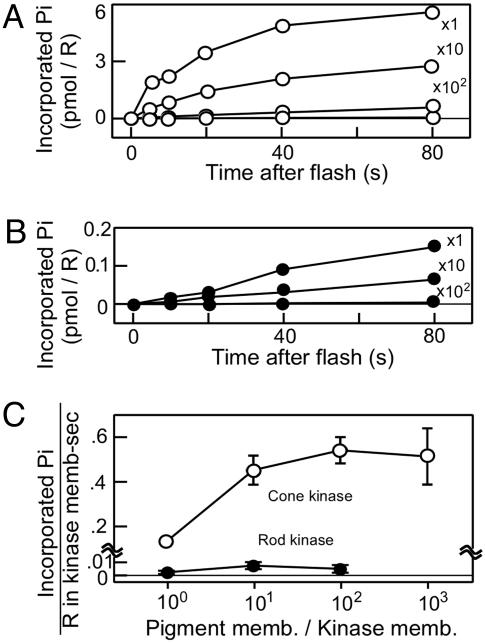
In this measurement, fused membrane preparations were used similarly as in Fig. 2, but the pigment membranes were mixed with reduced amounts of the kinase membranes to increase the R*/cone kinase ratio. In addition, we used rod pigment membranes as the phosphorylation substrate because rod pigment and cone pigment are equally good substrates of cone kinase (Fig. 2), but the lifetimes of the intermediates are longer in rod pigment (30) so that we can measure the phosphorylation easily. We mixed rod pigment membranes with cone kinase membranes at various ratios (labels in Fig. 4A). After fusion, the phosphorylation time course was measured (Fig. 4A), and similar measurements were done by using rod kinase membranes (Fig. 4B). In both measurements, the initial rate of the phosphorylation was determined and expressed as the kinase activity per visual pigment originally present in the kinase membranes (Fig. 4C). Because the pigment concentration in rods and cones is similar (9, 32), this unit represents the kinase activity per unit area of outer segment membranes in both rods and cones.
Fig. 4.
Determination of maximum activity of rod kinase and cone kinase. (A) Time course of phosphorylation of rod pigment by cone kinase. Rod visual pigment membranes were mixed with cone kinase membranes at the indicated ratios (pigment membrane/kinase membrane) and fused with freeze-thawing. A light flash bleaching 75% of the rod pigment was given, and the phosphorylation time course was measured. (B) Time course of phosphorylation of rod pigment by rod kinase. A light flash bleaching 75% of the rod pigment was given. (C) The initial phosphorylation rates were determined in A and B and expressed in units of Pi incorporated per visual pigment in the kinase membranes per second. They are plotted as a function of mixing ratio (n = 3).
Fig. 4C shows that the cone kinase activity increased when the substrate/enzyme ratio increased. In contrast, the rod kinase activity did not change significantly, which is consistent with the above-mentioned idea that rod kinase is saturated with the substrate even at a low substrate concentration (i.e., low bleach level). Fig. 4C shows that under extreme conditions at full bleach levels, the cone kinase activity is almost 100 times higher than that of rod kinase (0.54 Pi/R*·sec-1 in cone membranes and 0.006 Pi/R*·sec-1 in rod membranes).
Molecular Bases of High Cone Kinase Activity. The large difference in the kinase activity between rods and cones can be explained by the notion that the expression level of cone kinase is higher than that of rod kinase and/or that the specific activity of a single cone kinase molecule is higher than that of a single rod kinase molecule. To know which is the case, we measured the content of rod and cone kinase in their native membranes (Fig. 5).
Fig. 5.
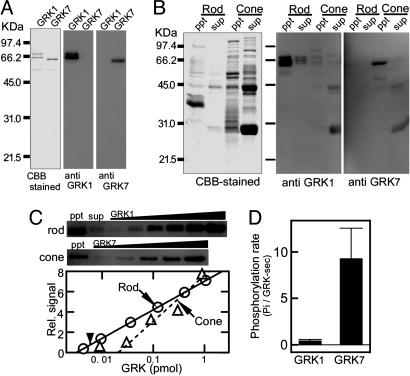
Contents of rod and cone kinase and their specific activities. (A) Specificity of antibody. Antibody against a rod or a cone kinase peptide was raised, and its specificity was checked by recombinant rod (GRK1) or cone (GRK7) kinase. (B) Protein content (Left) and immunoblot (Center and Right) of purified rods and cones. Rods containing 25 pmol of visual pigment and cones containing 5 pmol of cone pigment were homogenized and centrifuged. Both the precipitated membrane fraction (ppt) and the supernatant soluble fraction (sup) were probed with the antibodies. (C) Estimation of the content of rod and cone kinase in the membranes. The immunoblot signals were obtained in the samples of rod ppt, rod sup, and cone ppt (Top and Middle). In addition, we obtained signals of known amount of recombinant rod kinase or cone kinase to plot a calibration curve (Bottom). Arrows indicate the signals in the rod ppt fraction and the cone ppt fraction, and an arrowhead indicates the signal in the rod sup fraction. (D) Specific activities of the recombinant rod kinase and cone kinase (n = 3).
We obtained recombinant rod kinase and cone kinase in Sf9 cells (Fig. 5A Left) and also obtained specific antibodies against them (Fig. 5A Center and Right). Purified rods and cones were homogenated and centrifuged. The resultant precipitate membrane (ppt) fraction and the supernatant soluble (sup) fraction were both electrophoresed (Fig. 5B Left) and immunoblotted. Based on the density of the bands at ≈66 kDa, ≈95% of rod kinase was found in the membrane fraction, and 5% was found in the soluble fraction (Fig. 5B Center). In the cone preparation, the cone kinase signals were exclusively found in the membrane fraction (Fig. 5B Right). In the cone soluble fraction, nonspecific signals were detected at the positions of 45 kDa and 31 kDa, which is probably due to high content of these proteins in the cone preparation (see Fig. 5B Left). Using the recombinant kinases as standards, we obtained calibration curves (Fig. 5C Bottom) and quantified (arrows) the amount of the kinases in the rod membrane fraction and the rod soluble fraction (two leftmost lanes in Fig. 5C Top) and the amount in the cone membrane fraction (leftmost lane in Fig. 5C Middle).
The result showed that the content of rod kinase was 0.004 ± 0.002 per rod pigment (or 12 μM, assuming that the visual pigment concentration is 3 mM; n = 9) in agreement with a previous study (33), and that of cone kinase was 0.04 ± 0.02 per cone pigment (120 μM; n = 4). Although cone kinase was found to be 10 times more abundant than rod kinase, this difference does not account for the 100 times difference in the total activity (Fig. 4C). Because we knew both the maximum kinase activity expressed in units of number of phosphates incorporated into a visual pigment per sec (Fig. 4C) and the content of the kinase expressed in the same units (Fig. 5 B and C), we can calculate the specific activity of a single kinase molecule in the native membranes. The calculation indicated that the specific activity of cone kinase (14 Pi per cone kinase per sec) is ≈10 times higher than that of rod kinase (1.5 Pi per rod kinase per sec), which, together with the 10 times greater abundance of cone kinase, explains the 100 times difference in the total activity. In recombinant kinases, we determined their specific activities. They were 9.3 ± 3.3 Pi/sec in cone kinase and 0.41 ± 0.16 Pi/sec in rod kinase (Fig. 5D) and are similar to the calculated specific activities in the native membranes. This result suggested that the recombinant kinase and/or similar kinases are the major kinases present in native membranes in both rods and cones.
Rod pigment is thought to have evolved from cone pigment (34), and rods are known to be more sensitive to light than cones. Our present study suggested that rods have evolved by reducing both the expression level and the specific activity of a visual pigment kinase, increasing the lifetime of light-activated visual pigment to result in the increase in the transduction efficiency.
Rod and cone visual pigment kinases, GRK1 and GRK7, respectively, are members of the GRK family of proteins. Our estimation of the specific activity of rod kinase was 1.5 Pi per GRK1 per sec (Figs. 4 and 5), and it is similar to those reported for GRK1 (0.7 Pi per GRK1 per sec) (23), GRK2 (0.08 Pi per GRK2 per sec) (36), GRK5 (1.1 Pi per GRK5 per sec) (37), and GRK6 (0.06 Pi per GRK6 per sec) (38). (We calculated the values from the results shown in the references cited.) However, our estimation of the native cone kinase activity (14 Pi per GRK7 per sec; Figs. 4 and 5) is 13 times higher than the highest activity so far reported in other GRKs (1.1 Pi per GRK5 per sec). Therefore, cone kinase is the most powerful member among known GRKs.
In the present study, we showed that GRK7 has much higher activity than GRK1 and suggested a significant contribution of GRK7 in the turn-off mechanism of a photoresponse in cones. In some mammal cones, however, GRK7 is not present (15, 38), or its contribution is minor (39), although the cones [for example, mouse cones (40)] show briefer photoresponses than rods. It would be interesting to see the time course of visual pigment phosphorylation in mammal cones to evaluate the contribution of this reaction in these cells.
Supplementary Material
Acknowledgments
We thank Prof. Kanazawa (Osaka University) and Mr. T. Kimura for help in establishing the GRK expression system. This work was supported by grants from the Japan Society for the Promotion of Science (JSPS) (to S.K. and S. Tachibanaki), the Human Frontier Science Program (to S.K.), and the Senri Life Science Foundation (to S. Tachibanaki). Y.S.-M. is a Postdoctoral Fellow of the JSPS, and D.A. is a Research Fellow of the JSPS.
Author contributions: S. Tachibanaki, D.A., and S.K. designed research; S. Tachibanaki, D.A., Y.S.-M., S. Tsushima, and S.K. performed research; Y.S.-M. contributed new reagents/analytic tools; S. Tachibanaki, D.A., Y.S.-M., and S.K. analyzed data; and S. Tachibanaki and S.K. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GRK, G protein-coupled receptor kinase; K-gluc, K-gluconate.
References
- 1.Yau, K.-W. (1994) Invest. Ophthalmol. Visual Sci. 35, 9-32. [PubMed] [Google Scholar]
- 2.Pugh, E. N., Jr., & Lamb, T. D. (2000) Handb. Biol. Phys. 3, 184-255. [Google Scholar]
- 3.Fain, G. L. (1976) J. Physiol. 261, 71-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Normann, R. A. & Werblin, F. S. (1974) J. Gen. Physiol. 63, 37-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkhardt, D. A. (1994) J. Neurosci. 14, 1091-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie, P. G. & Beavo, J. A. (1988) J. Biol. Chem. 263, 8133-8141. [PubMed] [Google Scholar]
- 7.Fukada, Y., Kokame, K., Okano, T., Shichida, Y., Yoshizawa, T., McDowell, J. H., Hargrave, P. A. & Palczewski, K. (1990) Biochemistry 29, 10102-10106. [DOI] [PubMed] [Google Scholar]
- 8.Starace, D. M. & Knox, B. E. (1997) J. Biol. Chem. 272, 1095-1100. [DOI] [PubMed] [Google Scholar]
- 9.Tachibanaki, S., Tsushima, S. & Kawamura, S. (2001) Proc. Natl. Acad. Sci. USA 98, 14044-14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, C. K., Burns, M. E., Spencer, M., Niemi, G. A., Chen, J., Hurley, J. B., Baylor, D. A. & Simon, M. I. (1999) Proc. Natl. Acad. Sci. USA 96, 3718-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu, J., Dodd, R. L., Makino, C. L., Simon, M. I., Baylor, D. A. & Chen, J. (1997) Nature 389, 505-509. [DOI] [PubMed] [Google Scholar]
- 12.Maeda, T., Imanishi, Y. & Palczewski, K. (2003) Prog. Retin. Eye Res. 22, 417-434. [DOI] [PubMed] [Google Scholar]
- 13.Hisatomi, O., Matsuda, S., Satoh, T., Kotaka, S., Imanishi, Y. & Tokunaga, F. (1998) FEBS Lett. 424, 159-164. [DOI] [PubMed] [Google Scholar]
- 14.Weiss, E. R., Raman, D., Shirakawa, S., Ducceschi, M. H., Bertram, P. T., Wong, F., Kraft, T. W. & Osawa, S. (1998) Mol. Vis. 4, 27. [PubMed] [Google Scholar]
- 15.Weiss, E. R., Ducceschi, M. H., Horner, T. J., Li, A., Craft, C. M. & Osawa, S. (2001) J. Neurosci. 21, 9175-9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy, M. J., Dunn, F. A. & Hurley, J. B. (2004) Neuron 25, 915-928. [DOI] [PubMed] [Google Scholar]
- 17.Shichi, H. & Somers, R. L. (1978) J. Biol. Chem. 253, 7040-7046. [PubMed] [Google Scholar]
- 18.MacDonald, R. I. & MacDonald, R. C. (1983) Biochim. Biophys. Acta 735, 243-251. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura, S. & Murakami, M. (1991) Nature 349, 420-423. [DOI] [PubMed] [Google Scholar]
- 20.Liebman, P. A. (1962) Biophys. J. 2, 161-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen, C.-K., Inglese, J., Lefkowitz, R. J. & Hurley, J. B. (1995) J. Biol. Chem. 270, 18060-18066. [DOI] [PubMed] [Google Scholar]
- 22.Tachibanaki, S., Nanda, K., Sasaki, K., Ozaki, K. & Kawamura, S. (2000) J. Biol. Chem. 275, 3313-3319. [DOI] [PubMed] [Google Scholar]
- 23.Palczewski, K., McDowell, J. H. & Hargrave, P. A. (1988) J. Biol. Chem. 263, 14067-14073. [PubMed] [Google Scholar]
- 24.Mendez, A., Burns, M. E., Roca, A., Lem, J., Wu, L. W., Simon, M. I., Baylor, D. A. & Chen, J. (2000) Neuron 28, 153-164. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura, S. (1993) Nature 362, 855-857. [DOI] [PubMed] [Google Scholar]
- 26.Dizhoor, A. M., Ray, S., Kumar, S., Niemi, G. Spencer, M., Brolley, D., Walsh, K.A., Philipov, P. P., Hurley, J. B. & Stryer, L. (1991) Science 251, 915-918. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura, S., Kuwata, O., Yamada, M., Matsuda, S., Hisatomi, O. & Tokunaga, F. (1996) J. Biol. Chem. 271, 21359-21364. [DOI] [PubMed] [Google Scholar]
- 28.Sampath, A. P., Matthews, H. R., Cornwall, M. C., Bandarchi, J. & Fain, G. L. (1999) J. Gen. Physiol. 113, 267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kefalov, V., Fu, Y., Marsh-Armstrong, N. & Yau, K.-W. (2003) Nature 425, 526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shichida, Y. & Imai, H. (1998) Cell. Mol. Life Sci. 54, 1299-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepperberg, D. R., Cornwall, M. C., Kahlert, M., Hofmann, K. P., Jin, J., Jones, G. J. & Ripps, H. (1992) Visual Neurosci. 8, 9-18. [DOI] [PubMed] [Google Scholar]
- 32.Harosi, F. I. (1975) J. Gen. Physiol. 66, 357-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sitaramayya, A. (1986) Biochemistry 25, 5460-5468. [DOI] [PubMed] [Google Scholar]
- 34.Okano, T., Kojima, D., Fukada, Y., Shichida, Y. & Yoshizawa, T. (1992) Proc. Natl. Acad. Sci. USA 89, 5932-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Premont, R. T., Koch, W. J., Inglese, J. & Lefkowitz, R. J. (1994) J. Biol. Chem. 269, 6832-6841. [PubMed] [Google Scholar]
- 36.Kunapuli, P., Onorato, J. J., Hosey, M. M. & Benovic, J. L. (1994) J. Biol. Chem. 269, 1099-1105. [PubMed] [Google Scholar]
- 37.Loudon, R. P. & Benovic, J. L. (1994) J. Biol. Chem. 269, 22691-22697. [PubMed] [Google Scholar]
- 38.Lyubarsky, A. L., Chen, C., Simon, M. I. & Pugh, E. N., Jr. (2000) J. Neurosci. 20, 2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cideciyan, A. V., Jacobson, S. G., Gupta, N., Osawa, S., Locke, K. G., Weiss, E. R., Wright, A. F., Birch, D. G. & Milam, A. H. (2003) Invest. Ophthalmol. Visual Sci. 44, 1268-1274. [DOI] [PubMed] [Google Scholar]
- 40.Nikonov, S. S., Daniele, L. L., Zhu, X., Craft, C. M., Swaroop, A. & Pugh, E. N., Jr. (2005) J. Gen. Physiol., 125, 287-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.