Abstract
Toll-like receptor 5 (TLR5) recognizes an evolutionarily conserved site on bacterial flagellin that is required for flagellar filament assembly and motility. The α and ε Proteobacteria, including the important human pathogens Campylobacter jejuni, Helicobacter pylori, and Bartonella bacilliformis, require flagellar motility to efficiently infect mammalian hosts. In this study, we demonstrate that these bacteria make flagellin molecules that are not recognized by TLR5. We map the site responsible for TLR5 evasion to amino acids 89-96 of the N-terminal D1 domain, which is centrally positioned within the previously defined TLR5 recognition site. Salmonella flagellin is strongly recognized by TLR5, but mutating residues 89-96 to the corresponding H. pylori flaA sequence abolishes TLR5 recognition and also destroys bacterial motility. To preserve bacterial motility, α and ε Proteobacteria possess compensatory amino acid changes in other regions of the flagellin molecule, and we engineer a mutant form of Salmonella flagellin that evades TLR5 but retains motility. These results suggest that TLR5 evasion is critical for the survival of this subset of bacteria at mucosal sites in animals and raise the intriguing possibility that flagellin receptors provided the selective force to drive the evolution of these unique subclasses of bacterial flagellins.
Keywords: flagellin, innate immunity, motility, Helicobacter pylori, Campylobacter jejuni
Toll-like receptors (TLRs) are an important family of innate immune receptors that recognize pathogen-associated molecular patterns, evolutionarily conserved structures that are required for microbial fitness and are not present in the host (1, 2). We have previously defined the amino acids on bacterial flagellin that are recognized by TLR5 (3). These amino acids are located in the highly conserved D1 domain of the flagellin protein and cluster on the convex surface that contacts adjacent flagellin monomers in the flagellar protofilament. Mutating individual residues in the TLR5 recognition site significantly reduced or completely abolished bacterial motility, suggesting that evolving a functional flagellin that evades TLR5 would require a complex series of mutations.
Recent reports conflict on the ability of TLR5 to recognize flagellin from a highly motile bacterium, Helicobacter pylori. Two studies (4, 5), using HEK293 cell reconstitution systems, reported that H. pylori is recognized by TLR5. Two other groups (6, 7) demonstrated that flagellin-responsive epithelial cell lines do not detect native or recombinant H. pylori flagellin, suggesting that H. pylori flagellin evades TLR5 recognition.
H. pylori infects the gastric mucosa of approximately two-thirds of the world's population (www.cdc.gov/ulcer/md.htm#howcommon) and is the primary cause of gastritis, peptic ulcer disease, gastric cancer, and mucosa-associated lymphatic tissue (MALT) lymphomas (8). It belongs to the ε clade of the Proteobacteria, which includes commensals that inhabit the gut of ruminants (e.g., Wolinella succinogenes), and another extremely important human pathogen, Campylobacter jejuni (9, 10). C. jejuni infects the small and large intestine and is one of the most frequent causes of diarrhea worldwide (9). The ε Proteobacteria are flagellated, and their motility is necessary for efficient colonization and infection of the gut (10-16). The best studied organisms in this clade (Helicobacter spp., Campylobacter spp., and Wolinella spp.) live on mucosal surfaces where they are in persistent contact with the epithelial cell barrier.
Several studies have demonstrated that bacterial flagellin is a major stimulus of human epithelial cells (17-19) where it is recognized by TLR5 (20). We demonstrate here that the ε, as well as the α, Proteobacteria, although highly motile, are not recognized by TLR5. They possess specific changes in the TLR5 recognition site on flagellin that destroy TLR5 recognition, and compensatory amino acid changes in their flagellin molecules that preserve motility. These changes are conserved among all flagellated members of the α and ε Proteobacteria that infect mammals, suggesting that evasion of TLR5 may contribute to persistence of these bacteria at mucosal surfaces.
Materials and Methods
Cell Lines and Bacterial Strains. CHO K1 cells (American Type Culture Collection) were grown as described (3). The following bacteria were grown overnight, shaking in LB: Salmonella typhimurium strain TH4778 (FljB-/FliC+; K. Hughes, University of Washington, Seattle), Salmonella typhi (S. I. Miller, University of Washington, Seattle), Escherichia coli, clinical isolate H9049 (S. Swanzy, University of Washington, Seattle), Listeria monocytogenes strain 10403 (D. Portnoy, University of California, San Francisco), Legionella pneumophila, serogroup 1, Corby strain (K. Heuner, Universität Würzburg, Würzburg, Germany), Shigella flexneri (B. Cookson, University of Washington, Seattle), Pseudomonas aeruginosa strain PAK (D. Speert, University of British Columbia, Vancouver, BC, Canada), Proteus mirabilis (S. Swanzy, University of Washington, Seattle), Bacillus subtilis (S. Swanzy, University of Washington, Seattle), and Staphylococcus aureus [American Type Culture Collection (ATCC) 12599]. Serratia marcescens (clinical isolate, University of Washington, Seattle) was grown on LB agar plates. Vibrio anguillarum strain 775 was grown at 15°C in tryptic soy broth (TSB) supplemented with 1.5% wt/vol sodium chloride, and Edwardsiella tarda was cultured in TSB at 25°C (Maureen Purcell, University of Washington, Seattle). Bartonella bacilliformis (ATCC 35686) and Rhizobium (Ensifer) meliloti (ATCC 10310) were grown according to ATCC recommendations. The following bacteria were grown under microaerophilic conditions according to ATCC recommendations: C. jejuni (ATCC 700819), H. pylori strain G27 (N. Salama, University of Washington, Seattle), H. pylori clinical isolates (S. Swanzy, University of Washington, Seattle), Helicobacter hepaticus (ATCC 51449), and Helicobacter felis (ATCC 49179). W. succinogens (ATCC 29543) was grown under anaerobic conditions according to ATCC recommendations.
NF-κB Luciferase Reporter Assays. CHO K1 cells were transfected with human TLR5 cDNA cloned into the pEF6 V5/His TOPO vector (Invitrogen) and ELAM-LUC (Promega) plasmids, and luciferase assays were performed as described (3).
Immunoblots. Flagellin from Bartonella bacilliformis was detected by using a rabbit polyclonal antiserum to the protein (M. Minnick, University of Montana, Missoula, MT). Flagellin from Rhizobium meliloti was detected by using a rabbit polyclonal antiserum (B. Scharf, Universität Regensburg, Regensburg, Germany). Flagellin from ε Proteobacteria was detected by using mouse monoclonal anti-C. jejuni flagellin antibody (NovoCastra, Newcastle, U.K.). Flagellin from Salmonella typhimurium was detected by using rabbit polyclonal anti-FliCi (Difco). Horseradish peroxidase conjugate secondary antibodies (Zymed) were used for immunoblots.
Purification of Native Bacterial Flagellin. Bacteria were grown as described above and flagellin was purified as described (3). Protein concentration was determined by using the BCA assay (Pierce), and purity was confirmed by SDS/PAGE and Coomassie blue staining. C. jejuni 81-176 flagellin was from S.M.L.
Flagellin Sequence Alignments. Flagellin sequences were aligned by using clustalw (www.ch.embnet.org/software/ClustalW.html) and displayed with boxshade (www.ch.embnet.org/software/BOX_form.html). A molecular tree was displayed with phylodendron.
Creation of Flagellin Chimeras. Salmonella typhimurium fliC (GenBank accession no. D13689) and H. pylori 26695 flaA (GenBank accession no. NC_000915) were amplified and cloned into the NcoI and XbaI sites of the ptrc99a plasmid containing an N-terminal 6xHIS tag. Flagellin D0-D1 domain chimeras and 89-96 aa substitutions were made by using a standard PCR mutagenesis strategy (21). All mutations were verified by DNA sequencing.
Purification of Flagellin Chimeras. Escherichia coli BL-21RIL cells (Stratagene) were transformed with the flagellin chimera plasmids, and flagellin expression was induced by 3-4 h of culture in the presence of 1 mM isopropyl β-d-thiogalactoside (IPTG). The 6xHIS FliC was purified under native conditions by using B-PER protein extraction reagent (Pierce) and a TALON metal affinity column (BD Biosciences). The 6xHIS FlaA and all chimeras were purified from inclusion bodies under denaturing conditions and were then refolded. Insoluble proteins were spun out of the solution at 16,000 × g in a microcentrifuge for 10 min. Flagellins were heated to 70°C for 20 min to depolymerize any filaments into flagellin monomers. Protein concentrations were determined by using the BCA assay (Pierce), and purity was confirmed by SDS/PAGE and Coomassie blue staining.
Motility Assays. Salmonella typhimurium BC696 [SL1344 fliC-fljB-, (3)], transformed with flagellin constructs, were stabinoculated into motility plates [LB media containing 0.3% agar, with 50 μg/ml ampicillin and 1 mM isopropyl β-d-thiogalactoside (IPTG) for flagellin constructs]. Cultures were incubated upright at 37°C for 8 h (Salmonella typhimurium) or at 26°C for 5 days (Rhizobium meliloti), and then photographed.
Results
Flagellins from α and ε Proteobacteria Are Not Detected by TLR5. We screened flagellated bacteria for their ability to be recognized by human TLR5. As predicted, a wide variety of heat-killed flagellated bacteria, including Salmonella typhimurium, Salmonella typhi, Shigella flexneri, Pseudomonas aeruginosa, Listeria monocytogenes, Serratia marcescens, Legionella pneumophila, and Escherichia coli stimulated TLR5-dependent NFκB activation (Fig. 1A). Similar results were obtained with Proteus mirabilis, Bacillus subtilis, Edwardsiella tarda, and V. anguillarum (data not shown). As expected, nonflagellated Staphylococcus aureus did not trigger TLR5 (Fig. 1A). Interestingly, Bartonella bacilliformis, Rhizobium (Ensifer) meliloti, C. jejuni, H. pylori (strain 26695 and two clinical isolates), H. hepaticus, H. felis, and W. succinogenes were not recognized by TLR5 (Fig. 1B), despite vigorous motility (data not shown) and flagellin expression (Fig. 1C).
Fig. 1.
Flagellin from α and ε Proteobacteria is not detected by TLR5. (A and B) Approximately 1 × 105 CHO cells stably expressing human TLR5 and an NF-κB luciferase reporter were stimulated for 4 h with heat-killed samples of stationary phase bacterial cultures diluted 1:100 in media. Data represent % fold induction of luciferase activity relative to maximal stimulation achieved with Salmonella typhimurium (≈10- to 12-fold increase in luciferase activity over stimulation with LB alone) for at least three independent experiments, each run in triplicate. Error bars represent 1 SD. Control CHO cells stably transfected with empty expression vector and NF-κB luciferase reporter did not respond to flagellated bacteria (data not shown). (C) Immunoblots of sonicated samples of stationary phase bacterial cultures diluted 1:10. (C Left) Bartonella bacilliformis flagellin, as detected with anti-flagellin antiserum. (Center) Rhizobium (Ensifer) meliloti flagellin, as detected with anti-flagellin antiserum (bands at ≈23 and 26 kDa likely represent degraded flagellin). (Right) ε Proteobacteria flagellins as detected with anti-C. jejuni flagellin antiserum (additional band in H. pylori lanes likely represents crossreacting hook protein ≈75 kDa). The left margins show molecular size in kDa. (D) TLR5 dose-response curve to purified flagellins. Flagella were sheared from bacteria and purified by ultracentrifugation, and purity was confirmed by SDS/PAGE and Coomassie blue staining. Purified flagellin was incubated with CHO-hTLR5 cells for 4 h. ST, Salmonella typhimurium; EC, Escherichia coli; PA, Pseudomonas aeruginosa; LM, Listeria monocytogenes; HP, H. pylori; CJ, C. jejuni. Error bars represent 1 SD. (E) Molecular tree of flagellin sequences from flagellated bacteria, constructed by using clustalw and displayed with phylodendron. -, flagellated bacteria tested that did not activate TLR5; *, flagellated bacteria tested that activated TLR5.
The data obtained with heat-killed bacteria (Fig. 1 A and B) were confirmed by using purified flagellin from nonstimulatory C. jejuni and H. pylori, as well as from stimulatory Salmonella typhimurium, Escherichia coli, Pseudomonas aeruginosa, Listeria monocytogenes, and Serratia marcescens (Fig. 1D). Purified flagellin from C. jejuni and H. pylori did not stimulate human TLR5 over a wide concentration range, whereas the other flagellins did (Fig. 1D). Because natural variants of LPS can act as antagonists (22), we determined whether nonstimulatory C. jejuni and H. pylori flagellins antagonize TLR5 activation by Salmonella typhimurium FliC. Incubation of cells with 100-fold excess of either C. jejuni or H. pylori flagellin failed to inhibit TLR5 activation by FliC, indicating that these flagellins do not act as TLR5 antagonists (data not shown).
We aligned the flagellin sequences of the bacteria tested with those from other flagellated bacteria, and constructed a molecular tree (Fig. 1E). Flagellated bacteria that were recognized by TLR5 were spread among many branches of the tree and were members of either the Proteobacteria (β and γ clades) or of the Spirochetes and Firmicutes. Flagellated bacteria that were not recognized by TLR5 clustered in only two clades, the α and ε Proteobacteria.
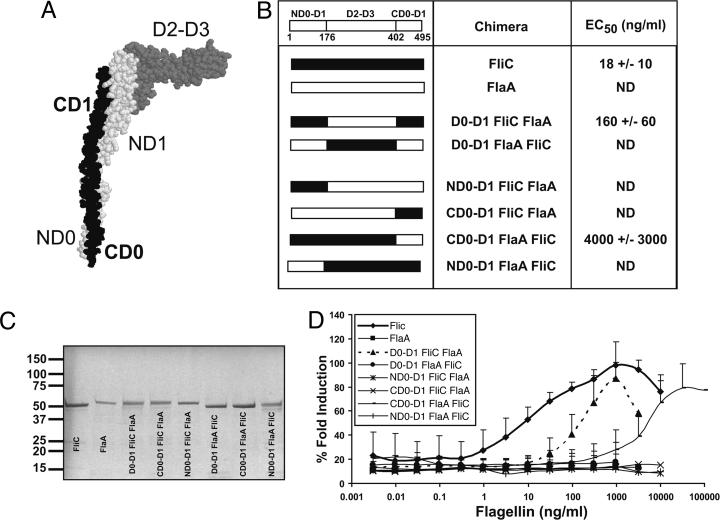
The N-Terminal D0-D1 Domain of Flagellin Is Required but Not Sufficient for TLR5 Recognition. The most likely explanation for our observations was that α and ε Proteobacteria possess specific amino acid changes that prevent TLR5 recognition. The crystal structure of flagellin from Salmonella typhimurium (FliC) has been determined (23, 24) and is shown in Fig. 2A. FliC is divided into four major domains, D0, D1, D2, and D3, comprised of regions from both the N- and C-terminal ends of the protein. FlaA, flagellin from H. pylori, has not been crystallized, but its amino acid sequence is highly similar to FliC in the D0-D1 domains. Fig. 2B shows a linear representation of both molecules.
Fig. 2.
The N-terminal D0-D1 domain of flagellin is required but not sufficient for TLR5 recognition. (A) Structure of FliC from Salmonella typhimurium with major domains labeled. The switch region (S) connects D0 and D1, but is not labeled. The protein Database (PDB) identification code 1ucu, is displayed in protein explorer. (B) Table listing flagellin chimeras made and the effective concentration required for 50% maximal TLR5 activation in ng/ml (EC50) ± SD. A linear schematic of FliC shows the amino acid numbers of the domain boundaries. ND, not detected. (C) Coomassie-stained SDS/PAGE of chimeric flagellins purified from Escherichia coli BL-21RIL (1 μg of protein loaded per lane). The left margin shows molecular size in kDa. (D) Dose-response curve of CHO-hTLR5 to purified flagellin chimeras as in 1 A.
We constructed a panel of flagellin chimeras by combining domains from stimulatory Salmonella typhimurium FliC and nonstimulatory H. pylori FlaA; in particular, we wanted to determine what portions of FliC would convert FlaA into a TLR5 agonist. We expressed and purified the chimeric proteins from flagellin-deficient Escherichia coli and demonstrated their purity by using SDS/PAGE (Fig. 2C). A chimera containing both the N- and C-terminal D0-D1 domains of FliC with the D2-D3 domain of FlaA activated human TLR5 at higher doses than wild-type FliC (EC50 160 ng/ml) (Fig. 2 B and D), in agreement with another published study (25). The converse, a chimera containing the N- and C-terminal DO-D1 domains of FlaA with the D2-D3 domain of FliC was inactive (Fig. 2 B and D). Molecules containing only the N-terminal or C-terminal D0-D1 domain of FliC were also inactive (Fig. 2 B and D). A FliC molecule containing the C-terminal D0-D1 domain of FlaA retained weak agonist activity, whereas a FliC molecule containing the N-terminal D0-D1 domain of FlaA was completely inactive (Fig. 2 B and D). The data demonstrate that TLR5 agonist activity is contributed by both the N- and C-terminal D0-D1 domains of FliC; however, the N-terminal D0-D1 domain of FliC is more critical for this activity.
Definition of Amino Acids Within the N-Terminal D0-D1 Domain of Flagellin Required for TLR5 Activation. Having demonstrated the importance of the N-terminal D0-D1 domain of flagellin in TLR5 recognition, we wanted to define the specific amino acids responsible for TLR5 activation in the β and γ Proteobacteria and to determine whether the loss of these amino acids was responsible for the lack of activity of flagellins from the α and ε Proteobacteria. We compared the flagellin N-terminal D0-D1 domain amino acid sequences from TLR5-stimulatory bacteria to bacteria that do not activate TLR5. Interestingly, the highest proportion of amino acid differences between TLR5-stimulatory and nonstimulatory bacteria was within a specific region of the D1 domain (amino acids 89-96) (Fig. 3A). Furthermore, this block contained three of the thirteen amino acids in FliC that we had previously defined as important for TLR5 activation (residues 89, 90, and 94) (3). These amino acids were highly conserved within all bacteria that activate TLR5, but were different in the α and ε Proteobacteria (Fig. 3A). Replacement of amino acids 89-96 of FliC with the corresponding amino acids from FlaA abolished the TLR5-agonist activity of FliC, emphasizing the importance of these amino acids (Fig. 3 B and C).
Fig. 3.
Amino acids 89-96 are required for TLR5 activation. (A) Sequence alignment (clustalw) of flagellin proteins from bacteria that activate TLR5 with those of the α and ε Proteobacteria. Asterisks indicate residues in this region previously determined to be important for TLR5 recognition (3). (B) Table listing flagellin chimeras made and their corresponding EC50 ± SD. ND, not detected. (C) Dose-response curve of CHO-hTLR5 to purified flagellin chimeras. (D) Salmonella typhimurium BC696 (SL1344 fliC-fljB-) expressing wild-type or the 89-96 FlaA FliC flagellin chimera was stab-inoculated into motility agar and photographed. Data are representative of three independent experiments.
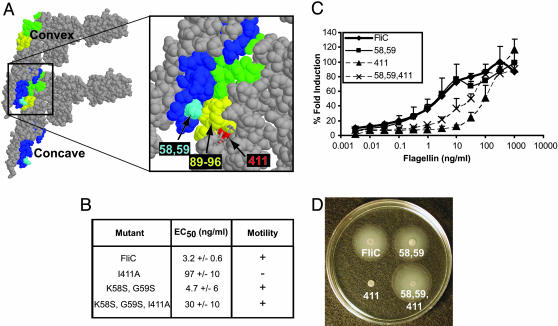
The question arises as to why this stretch of 8 aa that are crucial for TLR5 recognition of flagellin has remained highly conserved among β and γ Proteobacteria, because their mutation would allow pathogen evasion of host surveillance. The answer lies in the observation that these 8 aa are also crucial for flagellar filament formation (data not shown) and motility (Fig. 3 B and D). Crystallographic analysis of flagellar filaments has demonstrated that contact between monomers is mediated by the association of complementary convex (green and yellow) and concave (dark blue) surfaces (Fig. 4A Left) (23). The amino acids required for TLR5 recognition are found within the convex surface, explaining their crucial contribution to filament assembly and motility (3).
Fig. 4.
ε Proteobacteria possess compensatory changes that allow filament formation and motility. (A) Stacking of FliC monomers using PDB file 1io1 was performed according to Samatey et al. (24) and is represented in protein explorer to show contact surfaces. (A Left) View of two monomers: dark blue, concave contact surface; green and yellow, convex contact surface; yellow, amino acids 89-96. (A Right) Close-up of contact surface showing the location of 89-96 (yellow), I411 (red), and K58, G59 (light blue). Amino acids N87, L88, and R118 are represented as sticks to show the buried residue, I411. (B) Table listing mutant flagellins made and their corresponding EC50 ± SD. (C) Dose-response curves of CHO-hTLR5 stimulated with purified mutant flagellins. (D) Salmonella typhimurium BC696 (SL1344 fliC-fljB-) expressing wild-type or FliC mutant constructs were stab-inoculated into motility agar and photographed. Data are representative of three independent experiments.
Compensatory Mutations in ε Proteobacteria Flagellin Preserve Bacterial Motility. In contrast to the β and γ Proteobacteria, the α and ε Proteobacteria are not recognized by TLR5, yet are motile. We hypothesized that preservation of motility was due to compensatory amino acid changes within the contact surfaces between flagellin monomers that retain the ability to form filaments that propel the bacterium, yet destroy TLR5 recognition.
To test this hypothesis, we chose a simple model system using a single amino acid mutation in FliC from Salmonella typhimurium that we previously determined also plays a role in TLR5 recognition. Amino acid 411 is an isoleucine in Salmonella typhimurium FliC, and, in our previous alanine-scanning mutagenesis study, mutation of this residue in FliC to alanine caused the greatest reduction in TLR5 recognition and completely abolished bacterial motility (3). All of the ε Proteobacteria flagellins have an alanine at position 411. Significantly, FliC isoleucine 411 is buried just below amino acids 89-96 in the convex surface (Fig. 4A, red). Our data suggest that the FliC I411A mutation affects TLR5 recognition and motility by changing the conformation of the exposed residues in the 89-96 region (Fig. 4A, yellow) (3). As expected, the FliC I411A mutation reduced TLR5 activation and abrogated bacterial motility (Figs. 4 B-D).
Comparison of the concave surface revealed that Salmonella typhimurium and H. pylori flagellin differ in amino acid residues 58 and 59 (Fig. 4A, light blue), which in FliC interact directly with the convex surface amino acids 89-96 (yellow, Fig. 4A). We therefore mutated residues 58 and 59 of Salmonella typhimurium FliC to the H. pylori FlaA sequence (K58S and G59S). The 58/59 mutations in FliC did not affect motility or TLR5 recognition (Figs. 4 B-D). However, mutations 58/59 completely restored motility to FliC I411A, while preserving reduced TLR5 activity (Figs. 4 B-D).
This series of mutations suggests a mechanism by which a TLR5-stimulatory flagellin could mutate to reduce its TLR5 activity, while retaining motility. The first mutations would alter the contact surface but not change motility or TLR5 activity, as did the K58S/G59S mutation, and would permit a subsequent mutation that would reduce TLR5 activity without affecting motility. Several cycles of this process could result in the complex series of changes and the loss of TLR5 recognition that we observe in the α and ε Proteobacteria flagellin molecules.
Discussion
In this study, we demonstrate that members of the α and ε Proteobacteria, including three important human pathogens, C. jejuni, H. pylori, and Bartonella bacilliformis, possess flagellin molecules that cannot be recognized by TLR5. Their unique flagellin sequences contain amino acid differences in the TLR5 recognition site that permit TLR5 evasion, as well as compensatory mutations that preserve bacterial motility.
This study independently confirms the location of the TLR5 recognition site on flagellin. Flagellin's TLR5-stimulatory activity lies predominantly in the N-terminal D1 domain, centered around amino acids 89-96, but requires additional contribution from the D2-D3 and the C-terminal D1 domain. Flagellin is a good adjuvant (e.g. see ref. 26), and this study and our previous report (3) clearly demonstrate that flagellin's adjuvant activity is contained entirely within the amino acid sequence and is easily amenable to manipulation. Our studies indicate that proper folding and three-dimensional structure are critical for TLR5 recognition of flagellin, and must be taken into account when designing flagellin-based adjuvants for vaccines. Identification of sequences that disrupt proper stacking of flagellin monomers in the filament also points to potential sites for drug targets.
Intriguingly, the bacteria that have flagellin amino acid changes that evade TLR5 recognition also require flagellar-based motility for infection or colonization. The ε Proteobacteria C. jejuni, H. pylori, and Helicobacter mustelae all require motility for colonization of their mammalian hosts (10-16). Once inside, they inhabit a variety of mucosal surfaces, including the stomach (H. pylori) and the small and large intestine (C. jejuni and H. hepaticus) where they are in close proximity to the epithelial cell layer (27-29). Another ε Proteobacterium, W. succinogenes, is a commensal that lives on mucosal surfaces of the bovine rumen (30). We predict that evasion of TLR5 recognition confers a selective advantage to the ε Proteobacteria, because epithelial cells recognize bacterial flagellin and activate proinflammatory responses that may prevent colonization of mucosal surfaces (18, 19). In addition to causing disease, many individuals are asymptomatic carriers of H. pylori and C. jejuni (8, 31). TLR5 evasion may be critical for establishing this asymptomatic carrier state, which facilitates fecal-oral spread of the bacteria and the persistence of the bacteria within human populations. H. pylori also has hypostimulatory LPS (32, 33), suggesting that structural modification of other pathogen-associated molecular patterns may be important for its pathogenicity.
The α Proteobacterium Bartonella bacilliformis is transmitted by sandflies, but humans are its natural reservoir (34). It is a highly motile pathogen that invades and multiplies within red blood cells, causing the diseases Oroya fever and verruga peruana (34). Its flagella are required for efficient binding and invasion of human red blood cells (35), suggesting that TLR5 evasion may also be relevant to its pathogenesis.
Some pathogenic bacteria of the β and γ clades seem to have developed strategies to down-regulate flagellin expression, rather than mutate their flagellin molecules to a form that is not detected by TLR5. Some strains of Listeria monocytogenes down-regulate flagellin at 37°C (36), whereas Salmonella typhimurium turns off flagellin production only after entering host phagocytes (37) after breaching the epithelial barrier during invasion of the gastrointestinal tract. Escherichia coli and Pseudomonas species form biofilms in vivo and during this process down-regulate flagellin expression (38, 39). This mechanism averts TLR5 recognition, but also eliminates flagellar-based motility. The retention of flagellar-based motility in the α and ε clades of Protobacteria presumably gives them a selective advantage for their ecological niche and allows them to compete efficiently in hosts with flagellin receptors.
The other member of the α Proteobacteria that we found to evade TLR5, Rhizobium (Ensifer) meliloti, is a nitrogen-fixing plant symbiont that forms root nodules, but is not known to infect animals (40). Motility in this bacterium confers a competitive advantage for root colonization (40). This symbiont, as well as Agrobacterium tumefaciens, a plant pathogen whose flagella also increase virulence (41), both evade recognition by FLS2, the plant defense receptor for flagellin (42). FLS2 recognizes a linear site distinct from the TLR5 recognition site that is present in plant pathogens such as Pseudomonas syringae, a member of the γ Proteobacteria (42, 43). The FLS2 recognition site is a highly conserved 15-amino acid peptide that spans portions of the N-terminal S and D1 domains (42, 43), and is located adjacent to the concave contact surface of flagellin (23). Like the TLR5 recognition site, this peptide also contacts adjacent monomers in the protofilament (24), and thus is also predicted to be important for flagellar protofilament formation and bacterial motility. Both Rhizobium meliloti and A. tumefaciens flagellin sequences contain changes that we also predict preserve bacterial motility.
An examination of published flagellin sequences in the 89-96 region reveals that not all α Proteobacteria are predicted to evade TLR5. Rhodobacter sphaeroides and Rhodospirillum rubrum, both of which live in aquatic environments, possess conserved amino acids of the β and γ clades in the 89-96 region (Fig. 5). Because the α clade comprises bacteria that make TLR5-stimulatory and nonstimulatory flagellin, it is likely that the TLR5-nonstimulatory flagellin arose within the α clade and that the primordial flagellin most closely resembles the β/γ sequence. The conserved ε Proteobacteria flagellin 89-96 sequence is unique from the α clade, suggesting that it arose independently during evolution. Flagellin sequence data for the diverse array of ε Proteobacteria is currently limited to animal pathogens and commensals that live at mucosal sites, but this clade is actually comprised of a diverse array of bacteria, including species that live in hydrothermal vents (44). As more ε clade flagellin sequences become available, we predict that other members will possess flagellin molecules that share the 89-96 sequence with the β and γ clades, indicating that the TLR5-nonstimulatory ε Proteobacterial flagellin also arose within this clade after the divergence of the Proteobacteria.
Fig. 5.
Some members of the α Proteobacteria are predicted to be recognized by TLR5. Sequence alignment (clustalw) of flagellin proteins from bacteria that activate TLR5 with those of the α and ε Proteobacteria in the 89-96 region. Asterisks indicate residues in this region previously determined to be important for TLR5 recognition (3).
An intriguing question that remains is which selective force drove the independent selection of TLR5-nonstimulatory flagellin in subsets of the α and ε Proteobacteria. TLR5 is evolutionarily conserved within vertebrates, and has been demonstrated to recognize flagellin in species as diverse as man (20) and rainbow trout (45). Genetic studies indicate that the specificity of vertebrate TLR5 arose before the Cambrian period (ref. 46 and J. Roach, personal communication). Thus, TLR5 and its functional recognition of flagellin has had sufficient time to influence the evolution of the α and ε Proteobacterial flagellins. Alternatively, other forces in nature may have selected for these flagellin variants, which serendipitously provided these bacteria with selective growth advantages at mucosal sites. Regardless of the evolutionary history, identification of flagellin molecules present in human pathogenic bacteria and in a commensal of the bovine rumen that are not recognized by TLR5 suggests that TLR5 recognition at mucosal surfaces is an important host defense mechanism against some pathogens.
Acknowledgments
We thank D. Underhill, T. Hawn, E. Gold, A. Diercks, N. Yudkovsky, C. Rosenberger, K. Kennedy, A. Ozinsky, and the A.A. laboratory. We also thank R. Bonneau, J. Roach, N. Salama, S. Swanzy, and C. Verlinde. This work was supported by a predoctoral training grant from the Cancer Research Institute (to E.A.-N.), National Institutes of Health (NIH) Grant RO1 AIO52286 (to A.A.), and NIH Grant RO1 AI47242 (to B.T.C.).
Author contributions: E.A.-N. and K.D.S. designed research; E.A.-N., K.D.S., K.L.S., and S.L.R.B. performed research; E.A.-N. and K.D.S. analyzed data; B.T.C. and S.M.L. contributed new reagents/analytic tools; and E.A.-N., K.D.S., and A.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: TLR, Toll-like receptor.
References
- 1.Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197-216. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov, R. & Janeway, C. A., Jr. (1997) Cell 91, 295-298. [DOI] [PubMed] [Google Scholar]
- 3.Smith, K. D., Andersen-Nissen, E., Hayashi, F., Strobe, K., Bergman, M. A., Barrett, S. L., Cookson, B. T. & Aderem, A. (2003) Nat. Immunol. 4, 1247-1253. [DOI] [PubMed] [Google Scholar]
- 4.Smith, M. F., Jr., Mitchell, A., Li, G., Ding, S., Fitzmaurice, A. M., Ryan, K., Crowe, S. & Goldberg, J. B. (2003) J. Biol. Chem. 278, 32552-32560. [DOI] [PubMed] [Google Scholar]
- 5.Torok, A. M., Bouton, A. H. & Goldberg, J. B. (2005) Infect. Immun. 73, 1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, S. K., Stack, A., Katzowitsch, E., Aizawa, S. I., Suerbaum, S. & Josenhans, C. (2003) Microbes Infect. 5, 1345-1356. [DOI] [PubMed] [Google Scholar]
- 7.Gewirtz, A. T., Yu, Y., Krishna, U. S., Israel, D. A., Lyons, S. L. & Peek, R. M., Jr. (2004) J. Infect. Dis. 189, 1914-1920. [DOI] [PubMed] [Google Scholar]
- 8.Peek, R. M., Jr., & Blaser, M. J. (2002) Nat. Rev. Cancer 2, 28-37. [DOI] [PubMed] [Google Scholar]
- 9.Allos, B. M. (2001) Clin. Infect. Dis. 32, 1201-1206. [DOI] [PubMed] [Google Scholar]
- 10.Diker, K. S., Hascelik, G. & Diker, S. (1992) Acta Microbiol. Hung. 39, 133-136. [PubMed] [Google Scholar]
- 11.Aguero-Rosenfeld, M. E., Yang, X. H. & Nachamkin, I. (1990) Infect. Immun. 58, 2214-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlovskis, O. R., Rollins, D. M., Haberberger, R. L., Jr., Green, A. E., Habash, L., Strocko, S. & Walker, R. I. (1991) Infect. Immun. 59, 2259-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavermann, H., Burns, B. P., Angermuller, K., Odenbreit, S., Fischer, W., Melchers, K. & Haas, R. (2003) J. Exp. Med. 197, 813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton, K. A., Morgan, D. R. & Krakowka, S. (1989) Infect. Immun. 57, 1119-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton, K. A., Suerbaum, S., Josenhans, C. & Krakowka, S. (1996) Infect. Immun. 64, 2445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrutis, K. A., Fox, J. G., Schauer, D. B., Marini, R. P., Li, X., Yan, L., Josenhans, C. & Suerbaum, S. (1997) Infect. Immun. 65, 1962-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng, H., Carlson, A. Q., Guo, Y., Yu, Y., Collier-Hyams, L. S., Madara, J. L., Gewirtz, A. T. & Neish, A. S. (2003) J. Immunol. 171, 3668-3674. [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J. & Madara, J. L. (2001) J. Immunol. 167, 1882-1885. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz, A. T., Simon, P. O., Jr., Schmitt, C. K., Taylor, L. J., Hagedorn, C. H., O'Brien, A. D., Neish, A. S. & Madara, J. L. (2001) J. Clin. Invest. 107, 99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., Eng, J. K., Akira, S., Underhill, D. M. & Aderem, A. (2001) Nature 410, 1099-1103. [DOI] [PubMed] [Google Scholar]
- 21.Smith, K. D., Valenzuela, A., Vigna, J. L., Aalbers, K. & Lutz, C. T. (1993) PCR Methods Appl. 2, 253-257. [DOI] [PubMed] [Google Scholar]
- 22.Golenbock, D. T., Hampton, R. Y., Qureshi, N., Takayama, K. & Raetz, C. R. (1991) J. Biol. Chem. 266, 19490-19498. [PubMed] [Google Scholar]
- 23.Samatey, F. A., Imada, K., Nagashima, S., Vonderviszt, F., Kumasaka, T., Yamamoto, M. & Namba, K. (2001) Nature 410, 331-337. [DOI] [PubMed] [Google Scholar]
- 24.Yonekura, K., Maki-Yonekura, S. & Namba, K. (2003) Nature 424, 643-650. [DOI] [PubMed] [Google Scholar]
- 25.Murthy, K. G., Deb, A., Goonesekera, S., Szabo, C. & Salzman, A. L. (2004) J. Biol. Chem. 279, 5667-5675. [DOI] [PubMed] [Google Scholar]
- 26.McSorley, S. J., Ehst, B. D., Yu, Y. & Gewirtz, A. T. (2002) J. Immunol. 169, 3914-3919. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber, S., Stuben, M., Josenhans, C., Scheid, P. & Suerbaum, S. (1999) Infect. Immun. 67, 5151-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber, S., Konradt, M., Groll, C., Scheid, P., Hanauer, G., Werling, H. O., Josenhans, C. & Suerbaum, S. (2004) Proc. Natl. Acad. Sci. USA 101, 5024-5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, A., O'Rourke, J. L., Barrington, P. J. & Trust, T. J. (1986) Infect. Immun. 51, 536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolin, M. J., Wolin, E. A. & Jacobs, N. J. (1961) J. Bacteriol. 81, 911-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wooldridge, K. G. & Ketley, J. M. (1997) Trends Microbiol. 5, 96-102. [DOI] [PubMed] [Google Scholar]
- 32.Muotiala, A., Helander, I. M., Pyhala, L., Kosunen, T. U. & Moran, A. P. (1992) Infect. Immun. 60, 1714-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darveau, R. P. (1998) Curr. Opin. Microbiol. 1, 36-42. [DOI] [PubMed] [Google Scholar]
- 34.Dehio, C. (2004) Annu. Rev. Microbiol. 58, 365-390. [DOI] [PubMed] [Google Scholar]
- 35.Scherer, D. C., DeBuron-Connors, I. & Minnick, M. F. (1993) Infect. Immun. 61, 4962-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kathariou, S., Kanenaka, R., Allen, R. D., Fok, A. K. & Mizumoto, C. (1995) Can. J. Microbiol. 41, 572-577. [DOI] [PubMed] [Google Scholar]
- 37.Bergman, M. A., Cummings, L. A., Barrett, S. L. Smith, K. D., Lara, J. C., Aderem, A. & Cookson, B. T. (2005) Infect. Immun. 73, 1350-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prigent-Combaret, C., Vidal, O., Dorel, C. & Lejeune, P. (1999) J. Bacteriol. 181, 5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiteley, M., Bangera, M. G., Bumgarner, R. E., Parsek, M. R., Teitzel, G. M., Lory, S. & Greenberg, E. P. (2001) Nature 413, 860-864. [DOI] [PubMed] [Google Scholar]
- 40.Ames, P. & Bergman, K. (1981) J. Bacteriol. 148, 728-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chesnokova, O., Coutinho, J. B., Khan, I. H., Mikhail, M. S. & Kado, C. I. (1997) Mol. Microbiol. 23, 579-590. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Gomez, L., Felix, G. & Boller, T. (1999) Plant J. 18, 277-284. [DOI] [PubMed] [Google Scholar]
- 43.Felix, G., Duran, J. D., Volko, S. & Boller, T. (1999) Plant J. 18, 265-276. [DOI] [PubMed] [Google Scholar]
- 44.Corre, E., Reysenbach, A. L. & Prieur, D. (2001) FEMS Microbiol. Lett. 205, 329-335. [DOI] [PubMed] [Google Scholar]
- 45.Tsujita, T., Tsukada, H., Nakao, M., Oshiumi, H., Matsumoto, M. & Seya, T. (2004) J. Biol. Chem. 279, 48588-48597. [DOI] [PubMed] [Google Scholar]
- 46.Beutler, B. & Rehli, M. (2002) Curr. Top. Microbiol. Immunol. 270, 1-21. [DOI] [PubMed] [Google Scholar]