Abstract
Since the discovery of catalase, it has been postulated that aerobic organisms generate enough oxidants to threaten their own fitness and, in particular, their genetic stability. An alternative is that these enzymes exist to defend the cell against more-abundant oxidants imposed by external sources. These hypotheses were tested directly through study of Hpx- (katG katE ahpCF) mutants of Escherichia coli, which lack enzymes to scavenge hydrogen peroxide (H2O2). These strains grew well in anaerobic medium but poorly when they were aerated. The Hpx- bacteria formed filaments and exhibited high rates of mutagenesis, both indicators of DNA damage. An additional recA mutation caused Hpx- cells to die rapidly upon aeration, even though the intracellular H2O2 was <1 μM. Spin-trap experiments detected substantial hydroxyl radicals, and cell-permeable iron chelators eliminated both the phenotypic defects and hydroxyl-radical formation, confirming that the Fenton reaction was responsible. An Hpx- oxyR strain exhibited even more DNA lesions than did the Hpx- mutant, indicating that the OxyR stress response induced protein(s) that suppressed DNA damage. One critical protein was Dps, an iron-sequestration protein, because Hpx- dps mutants exhibited sensitivity similar to that of the Hpx- oxyR mutant. These results reveal that aerobic E. coli generates sufficient H2O2 to create toxic levels of DNA damage. Scavenging enzymes and controls on free iron are required to avoid that fate. The rate constant of the Fenton reaction measured at physiological pH was much higher than under the acidic conditions that were used to determine the commonly cited value.
Keywords: Fenton, oxidative DNA damage, Dps
In 1900, Oscar Loew reported the existence of an enzyme that catalyzes the disproportionation of H2O2 (1). In the same article, he speculated that the physiological role of the enzyme, which he named catalase, might be to protect cells from H2O2 that is generated as a by-product of aerobic metabolism. McCord and Fridovich (2) made an analogous suggestion after their discovery in 1969 of superoxide dismutase. In the years since, much effort has been expended to test these hypotheses.
The toxicity of endogenous superoxide was first demonstrated with mutants of Escherichia coli that lack superoxide dismutase (3). Several of their growth defects were traced to the fact that superoxide rapidly destroys the [4Fe-4S] clusters of dehydratases (4-8). Similar injuries were subsequently discovered to occur during superoxide stress in higher organisms (9, 10).
However, analogous experiments with E. coli catalase mutants (11, 12) did not reveal any growth defect. This result suggested that these cells do not generate enough H2O2 to toxify themselves. If so, the purpose of catalase must be to protect cells against the far higher concentrations that it encounters when competing organisms release H2O2 as a toxin (13). Local H2O2 concentrations may rise to as high as 100 μM inside phagocytes and >1 mM near H2O2-generating lactic acid bacteria.
The dosimetry of H2O2 toxicity is of particular interest because acute exposure damages DNA. The mechanism involves the reduction of H2O2 to hydroxyl radical by transition metals such as iron:
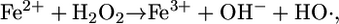 |
[1] |
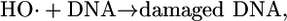 |
[2] |
 |
[3] |
In vivo experiments in E. coli supported this scheme: When H2O2 was added to growing cultures, DNA damage was detected, and DNA-repair mutants were killed rapidly (14-16). Cell-permeable iron chelators blocked the damage, thereby confirming the catalytic role of iron (17). High intracellular levels of either cysteine or reduced free flavins facilitated damage, indicating that either compound can act as the reductant in Eq. 3 (18, 19).
However, these experiments typically used millimolar concentrations of H2O2. Smaller doses could not be tested because of the high scavenging activity of cultures, which cause H2O2 to disappear rapidly during the experiment. The dosage issue is important, because the rate constant of the Fenton reaction (Eq. 3), generally cited to be 76 M-1s-1 (20), is relatively low. Because the intracellular concentration of H2O2 is expected to be submicromolar, workers have questioned whether the Fenton reaction is significant in vivo.
More recently, however, it was found that the catalase mutants that were used in those experiments retained the ability to scavenge endogenous H2O2 through the action of alkyl hydroperoxide reductase, an NADH peroxidase (21). In contrast, Hpx- mutants, which lack Ahp and both catalases, have very little scavenging activity, and their endogenous H2O2 equilibrates across the membrane and gradually accumulates up to ≈1 μM. These mutants offer an opportunity to identify injuries that occur in E. coli when intracellular H2O2 is maintained at a low level for an extended period.
In this work, we determine that these mutants suffer high levels of DNA damage. The cells would not be viable at all in aerobic media were it not for the induction of stress responses that sequester iron and facilitate repair.
Materials and Methods
Strains and Culture Conditions. Luria broth (LB) was prepared as described in ref. 22. Minimal medium contained minimal A salts (22), 0.2% glucose, 1 mM MgCl2, 0.2% casamino acids, and 5 mg/liter thiamine. Strains and plasmids used in this study are listed in Table 1, which is published as supporting information on the PNAS web site. All experiments compare congenic strains derived from the MG1655 background. Mutations were introduced by P1 transduction (22) anaerobically to avoid the possible outgrowth of suppressed mutants. Mutations in dps or oxyR were confirmed by PCR. The recA mutation was screened by UV sensitivity. Anaerobic cultures were grown in a Coy chamber (85% N2/10% H2/5% CO2), and aerobic cultures were grown in a shaking water bath.
Aerobic Cell Growth and Viability. Anaerobic overnight cultures were diluted into anaerobic medium to OD600 of ≈0.005, grown to OD600 of 0.2, and then diluted into fresh aerobic medium to OD600 of 0.001-0.005. Aerobic medium was filter sterilized and prepared immediately before use. To induce the synthesis of Dps or Dpr from expression plasmids, 10-30 μM isopropyl β-d-thiogalactoside was added 15 min before aeration and was present throughout the period of aerobic growth. Biomass was tracked by optical density, and filamentation was visualized with an Axiocam digital camera on a Zeiss Axioscop 2 by using a ×40 Plan-Neofluar objective. To determine cell viability, at intervals cells were diluted, mixed with LB top agar, and spread on anaerobic LB plates. Colonies were counted the next day.
Other Methods. H2O2 concentrations were measured by the amplex red/horseradish peroxidase method (23). H2O2 diffuses so rapidly across the cell membrane that in these experiments the intracellular concentration is at most 0.1 μM higher than the extracellular concentration (24). The detection of hydroxyl radicals by spin trapping electron paramagnetic resonance (EPR) followed our published method in ref. 25 except that LB medium was used. Quantitative PCR (qPCR) assays (with primers 10 kb apart) and mutation frequencies were carried out as described in ref. 19. qPCR data can be influenced by growth rate: when primers are positioned near the replication terminus, as in this study, the template DNA is slightly underrepresented relative to total DNA in faster-growing cells. Therefore, wild-type cells generate less PCR product aerobically, when they grow more quickly, and the oxygen-sensitive Hpx- mutants generate less product anaerobically. This bias slightly overestimates aerobic DNA damage in wild-type cells and underestimates it in Hpx- mutants. Statistical significance was determined by the two-tailed Student t test.
Overexpression of Dps and Dpr Protein. The dps ORF was PCR-amplified from E. coli MG1655 by using the forward primer 5′-CGGAATTCGGGACATAACATCAAGAG and the reverse primer 5′-GCTCTAGAGCGATGGATTTATTCG. The EcoRI and XbaI sites are underlined. PCR products were digested and cloned into pCKR101 vector to generate the pDps plasmid. The insert was verified by sequencing, and isopropyl-β-d-thiogalactoside-dependent overproduction of a 19-kDa band was verified by SDS/PAGE. The dpr ORF was PCR-amplified from Streptococcus mutans American Type Culture Collection 25175 chromosomal DNA by using the forward primer 5′-CGGGTACCTGCTTTTTATTTAGAATCGTT and the reverse primer 5′-GCTCTAGACGTCTGGGATATATCAAAAG. The KpnI and XbaI sites are underlined. The digested PCR products were cloned into pCKR101 vector to generate the pDpr plasmid. Overproduction of active Dpr protein was confirmed by native PAGE stained with Ferene S (26).
DNA Damage by Alkylating Agents. SP74/pDps cells were grown in LBamp aerobically ± isopropyl-β-d-thiogalactoside. When the OD600 reached 0.1≈0.2, various concentrations of methylmethanesulfonate or N′-methyl-N′-nitro-N-nitrosoguanidine were added. After 30 min, cultures were diluted (1:100) with 4% sodium thiosulfate to inactivate residual alkylating agents. Cells were further diluted, spread on LBamp plates, and incubated overnight to determine survival.
Measurement of Fenton Reaction Rates. Iron oxidation by H2O2 was performed in an anaerobic Coy chamber at either 13°C or 37°C. Reagents and stock solutions were prepared in anaerobic H2O. To avoid interactions between iron and conventional buffering compounds, none were used. Instead, solutions were adjusted with dilute NaOH to the desired pH, and the pH of the reaction solution was measured at the conclusion of the reaction to verify that it had not changed significantly (<0.2 pH units) (14). In a cuvette, 2 μM ferrous ammonium sulfate and 10 μM of H2O2 were mixed. When Mg·ATP and herring sperm DNA (Promega) were included as iron-binding molecules, saturating amounts were used (1 mM and 12.5 μg/ml, respectively, determined by saturation of their effects on the reaction rate). At each time point, aliquots were added to 0.01% catalase and 400 μM ferrozine in a 50 mM sodium phosphate buffer (pH 3.0) to stop the reaction and to displace and chelate the remaining ferrous iron. Absorbance was immediately determined at 561 nm (Beckman model DU640).
Results
Hpx- Cells Have an Aerobic Growth Defect. Hpx- mutants (katG katE ahpCF) lack the three major enzymes that scavenge H2O2 in E. coli. It was reported previously that these strains grew well anaerobically but that growth progressively slowed after aeration, particularly when they were repeatedly subcultured (21). The nature of the injury was not determined. To further investigate this toxic effect of H2O2, we monitored growth as Hpx- cells were precultured in LB anaerobically and then diluted into aerobic medium. The aerobic cells initially grew, but then their growth stalled for ≈3-4 h (Fig. 1A). Eventually, cells escaped from the lag and resumed growing. This phenotype is oxygen-dependent, because anaerobic cultures did not show any lag (data not shown). Further, single mutants that retain either catalase or peroxidase (Ahp) activity did not exhibit this phenotype (data not shown), confirming that it was due to an inability to scavenge H2O2. The growth lag was observed in complex LB medium, ruling out the possibility that it resulted from any biosynthetic auxotrophy.
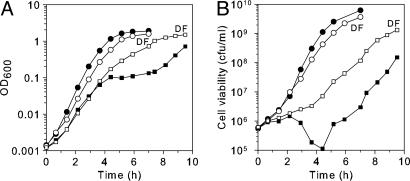
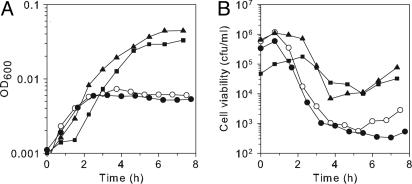
Fig. 1.
Hpx- mutants exhibit growth and viability defects. Aerobic growth (A) and cell viability (B) of wild-type (SP63, circles) and Hpx- (SP64, squares) strains were grown in anaerobic LB medium until early log phase and then diluted into aerobic LB medium. At intervals, the cells were removed to an anaerobic chamber and plated to determine cell viability. Filled symbols, no chelator. Open symbols, 2 mM of the cell-permeable iron chelator desferrioxamine (DF) was included in the aerobic medium.
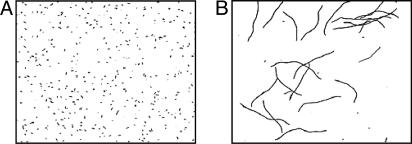
During the initial period of aeration, viable counts increased slowly but then dropped from ≈106 to ≈105 colony-forming unit/ml (Fig. 1B). Because OD600 continuously increased ≈100-fold while the viability diminished, we suspected that cells were filamenting with an arrest of cell septation. E. coli cells filament as a consequence of the SOS response to DNA damage (27, 28). Fig. 2 confirms that Hpx- cells form long filaments. These results indicate that Hpx- cells make sufficient enough H2O2 to toxify themselves to the point of death, and they also imply that DNA damage is occurring.
Fig. 2.
Filamentation of aerobically grown Hpx- cells. Cells were grown in LB anaerobically (A) or aerobically (B) until OD600 ≈ 0.1. (Magnification: ×400.)
The Aerobic Growth Defect Results from Fenton-Mediated DNA Damage. H2O2 damages DNA through Fenton chemistry, which requires an iron catalyst (Eqs. 1-3). Desferrioxamine is a cell-permeable iron chelator that blocks intracellular Fenton chemistry (17). Fig. 1 shows that when desferrioxamine was added, the growth lag disappeared, and the number of viable cells increased continuously. Similarly, when LB medium was supplemented with catalase, Hpx- cells were able to grow almost as quickly as wild-type cells (data not shown). Thus, the growth defect of Hpx- cells results from the combined action of H2O2 and intracellular iron.
PCR analysis was conducted to assess the damage more quantitatively. Although Hpx- cells typically exhibited more lesions than did wild-type cells, the difference was moderate and difficult to quantify by this relatively insensitive assay. The quantitation was further confounded by artifacts that arose from differences in growth rate (Materials and Methods). To detect DNA damage in a more-sensitive way, mutation frequency was measured by using Thy+ to Thy- phenotype conversion. After 4 h of aeration, the frequency of Thy- mutations was 90-fold higher in Hpx- cells than in wild-type cells.
E. coli relies on recombination to repair two-strand DNA lesions, which can arise when oxidized DNA is replicated; thus recA mutants are rapidly killed by exogenous H2O2 (15). When a recA mutation was introduced into Hpx- cells, the strain grew very poorly in air. Only 9% were still viable after 30 min of aeration. We concluded from these results, filamentation, loss of cell viability, enhanced mutation frequency, and the sensitivity of the Hpx- recA mutant, that Hpx- cells experience substantial DNA damage resulting from the iron-mediated Fenton reaction.
When Hpx- mutants are cultured aerobically, endogenous H2O2 rapidly equilibrates across the membranes so that it accumulates in both the cells and the culture medium. By measuring the concentration of H2O2 in the medium, one can determine the concentration inside the Hpx- cells (24). However, components of LB medium interfere with horseradish peroxidase-based assays; therefore, experiments were repeated in defined medium. Filamentation was less severe, possibly because the Hpx- cells grew more slowly. We also suspect that the powder from which LB medium is prepared may contain trace peroxides that exacerbate the growth defects of the Hpx- strain. However, even in defined medium, the Hpx- recA mutant died rapidly (data not shown). In a typical experiment, the H2O2 concentration had reached only 0.25 μM by the point at which 90% of the cells were dead.
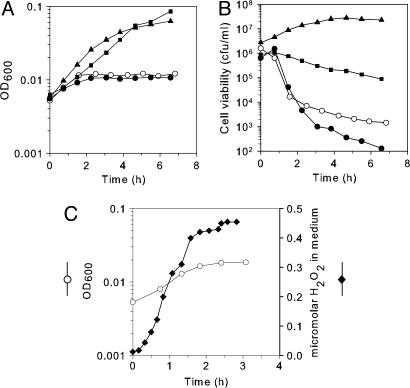
OxyR Is Required for the Aerobic Growth of Hpx- Cells. The eventual recovery and outgrowth of the Hpx- mutants (Fig. 1) suggested that the cells adapt so that they can tolerate the H2O2. Supranormal levels of H2O2 activate OxyR, a transcriptional regulator that, in turn, stimulates the transcription of ≈20 genes that defend E. coli against oxidative stress, including katG, ahpCF, and fur (29). The OxyR regulon is activated by the H2O2 that accumulates when Hpx- cells are grown aerobically (21); therefore, the oxyR mutation was introduced into the Hpx- background. The Hpx- oxyR mutant grew well anaerobically (data not shown), but upon aeration, its growth was worse than that of its Hpx- oxyR+ parent (Fig. 3A). The cell biomass increased twice, and then growth ceased completely and permanently. Viable counts began to decline soon after aeration (Fig. 3B). The experiment was repeated in defined medium so that H2O2 levels could be monitored (Fig. 3C). Growth ceased when the H2O2 concentration was <0.5 μM.
Fig. 3.
Growth and survival defect of the Hpx- oxyR strain. Cells were grown anaerobically until log phase and then diluted into the same aerobic medium. (A and B) Growth and viability in LB medium. ○, Hpx- oxyR (SP67) strain. Filled symbols, complementation by pDps plasmid (squares), pDpr plasmid (triangles), and empty vector (circles). (C) Growth (○) and H2O2 concentration (♦) of the Hpx- oxyR strain in minimal medium.
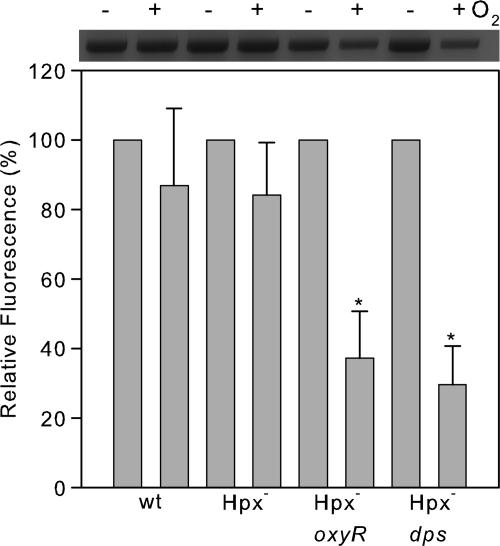
Desferrioxamine blocked the aerobic death of the Hpx- oxyR strain, suggesting that killing is due to the Fenton reaction. In parallel with the poor aerobic growth, these cells suffered from greatly increased DNA damage (Fig. 4). The DNA lesion frequency per total genomic DNA was predicted by Poisson analysis, and the results indicated that after 3 h of aerobic growth, the genome had accumulated ≈950 aeration-dependent lesions. Thus, an OxyR-inducible gene plays an critical role in protecting DNA during exposure to submicromolar H2O2.
Fig. 4.
Quantitative PCR measurement of DNA damage. Cells were grown in LB either anaerobically or for 4 h aerobically, and total genomic DNA was isolated. Quantitative PCR was performed by using equivalent amounts of template DNA for each reaction. PCR products were scanned, and the fluorescence was normalized to the anaerobic culture. Sample data are shown in Upper. Error bars indicate the SD of three independent culture experiments. *, P < 0.05 (Student's t test) compared with the sample from anaerobic cultures.
The Aerobic Growth of Hpx- Cells Depends on the dps Gene. The OxyR regulon apparently does not include any genes involved in DNA repair. Therefore, we anticipated that some member of the regulon must highly diminish the rate at which DNA lesions are produced. The dps gene drew our attention not only because dps is the most highly induced gene of the regulon (29), but also because it was observed to lessen the rate at which 40 mM H2O2 damaged DNA (30). The dps single mutant grew as well as did the wild-type strain (data not shown). However, like the Hpx- oxyR mutant, the Hpx- dps cells grew very poorly in aerobic medium (Fig. 5A). Viability initially diminished rapidly from ≈106 to ≈103 colony-forming units/ml and then increased slowly (Fig. 5B), and the cells suffered extensive DNA damage (Fig. 4). Thus, dps plays a pivotal role in allowing E. coli to survive H2O2 stress.
Fig. 5.
Growth and survival defect of the Hpx- dps strain. Cells were grown in LB anaerobically until log phase and then diluted into aerobic medium. ○, Hpx- dps (SP66) strain. Filled symbols, complementation by pDps plasmid (squares), pDpr plasmid (triangles), and empty vector (circles). Aerobic growth (A) and cell viability (B).
Overproduced Dpr Can Complement Hpx- dps Cells. Dps exhibits two biochemical activities in vitro: it coats DNA (30), and it sequesters iron in the manner of ferritin (31), its structural homologue (32). In principle, either of these two activities might protect DNA from H2O2. To identify the activity that is important in vivo, we attempted to complement Hpx- dps mutants by over-producing Dpr, a Dps homologue from Streptococcus mutans. Dpr shares with Dps its iron-sequestering activity, but it lacks the acidic terminus that allows Dps to oligomerize and coat DNA (33). Fig. 5 shows that the Dpr substantially complemented the mutant. The dps phenotype was not fully corrected, which we suspect is due to our inability to control Dpr levels. We also failed to get full complementation when we expressed bona fide dps from the same promoter. In fact, induction of dps from a plasmid in wild-type cells caused growth to slow and, in some experiments, viability to drop, perhaps from aggregation or disruption of iron metabolism (data not shown).
If Dps binds and shields DNA from chemical oxidants, then DNA alkylation should be similarly hindered. However, induction of Dps did not lessen the sensitivity of an alkA tagA mutant (34) to MMS or MNNG (data not shown). We conclude that it is the iron-binding activity of Dps that protects Hpx- cells from H2O2.
The Dpr-expressing plasmid enabled Hpx- oxyR cells, which otherwise could not grow in air, to grow with a doubling time of ≈50 min and with higher viability (Fig. 3). This finding implies that Dps is probably the protein whose absence was the primary reason for the oxyR phenotype. Dps protein also partially complemented the Hpx- oxyR cells.
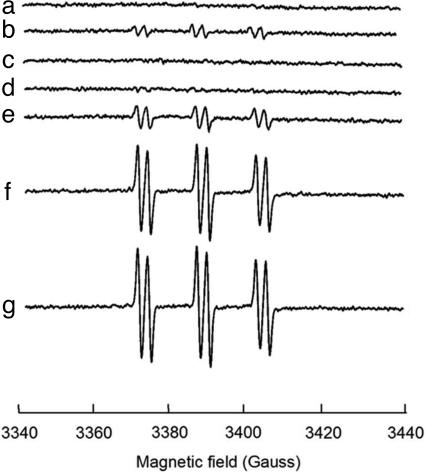
Intracellular Hydroxyl Radicals Can Be Detected by EPR Analysis. In the presence of ethanol, hydroxyl radicals can be trapped in vivo as a hydroxyethyl radical spin adduct to α-(4-pyridyl-1-oxide)-N-tert-butylnitrone (4-POBN) (35). Fig. 6 shows a marked increase in adduct formation in aerobic Hpx- cells compared with wild-type cells. The signal was absent when cells were treated with catalase or with the intracellular iron chelator dipyridyl, indicating that the radicals were produced through the Fenton reaction. The magnitude of the signals increased when 2 μM exogenous H2O2 was added (Fig. 6, trace e), but proportionality was lost at more than ≈10 μM. This result directly confirmed that hydroxyl radicals are generated in vivo by very low concentrations of H2O2. The loss of proportionality suggests that the rate-limiting step switches from the Fenton reaction (Eq. 1) to the recycling of oxidized iron (Eq. 3).
Fig. 6.
Detection of hydroxyl radical in Hpx- cells by α-(4-pyridyl-1-oxide)-N-tert-butylnitrone-ethanol spin trapping. The cell densities were equivalent in all samples. The signals are the average of 10 scans. Where indicated, 1 mM dipyridyl was preincubated with cells for 5 min, and catalase was added at 300 units/ml. (a) Wild-type cells plus 100 μM exogenous H2O2. (b) Hpx- (no exogenous H2O2), (c) Hpx- plus 10 μM H2O2 and catalase. (d) Hpx- plus 10 μM H2O2 and dipyridyl. (e) Hpx- plus 2 μM H2O2. (f) Hpx- plus 10 μM H2O2. (g) Hpx- plus 100 μM H2O2.
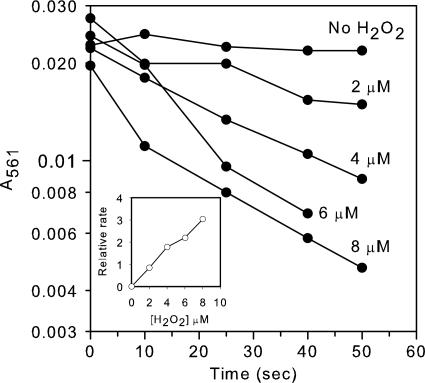
The Fenton Reaction Is Fast Enough to Produce Substantial DNA Damage in Vivo. The commonly cited second-order rate constant of the Fenton reaction is 76 M-1s-1 (20). This constant predicts a half-time for ferrous iron oxidation by 0.5 μM H2O2 of several hours, which seemed inconsistent with the rapid pace of DNA damage that we observed in our strains. However, that constant was determined (at room temperature) at pH 3 and in the absence of potential ligands. We obtained a similar value, 88 M-1s-1, at pH 4.1. Because iron chemistry is strongly influenced by pH and its ligand field, we remeasured the rate constant under more physiological conditions. At neutral pH, the rate constant was 6,500 M-1s-1 at 13°C; the reaction was too rapid to measure by our technique at 37°C. When ATP and DNA were included in the reaction as iron ligands, the rate constants at 13°C fell slightly, to 4,500 M-1s-1 and 890 M-1s-1, respectively. At 37°C, the rate constant for DNA-bound iron was 4400 M-1s-1 (Fig. 7).
Fig. 7.
Iron oxidation by H2O2 in the presence of DNA. Reactions were performed in anaerobic solutions at 37°C. Two micromolar Fe(NH4)2(SO2)4 was mixed with 12.5 μg/ml herring sperm DNA (37.5 μM nucleotides). Reactions were started by addition of the indicated concentrations of H2O2. (Inset) Reaction rate as a function of H2O2 concentration.
Discussion
Very Low Concentrations of Intracellular H2O2 Generate Substantial DNA Damage. Hpx- (katG katE ahpCF) strains of E. coli lack catalase and peroxidase and cannot scavenge the H2O2 that they form as a by-product of aerobic metabolism. During culture, this H2O2 gradually accumulates and equilibrates between the cell interior and the external medium, where it can be precisely measured. In the experiments reported here, toxic amounts of DNA damage were generated when submicromolar doses of H2O2 were present. Cells remained viable only through the induced synthesis of Dps, which suppressed the Fenton reaction, and the recombinational DNA repair system, which helped to repair DNA damage. Given that 0.5 μM H2O2 was lethal in the absence of these responses, it is fitting that OxyR protein (36), the transcription factor that controls the oxidative stress regulon, is so sensitive to H2O2. OxyR is activated when the H2O2 concentration is >0.1 μM in vivo (24).
These results confirm the long-held suspicion that aerobes generate enough H2O2 to damage their own DNA. The synthesis of scavenging enzymes is therefore a requirement for a robust aerobic lifestyle. An analogous result pertains to superoxide, as aerated superoxide dismutase mutants exhibit both growth defects and an elevated rate of DNA damage (3, 37).
The high rate of DNA damage that we observed was unexpected for two reasons. First, the widely accepted rate constant of the Fenton reaction was misleading, because it was measured at acidic pH. At physiological pH, ferrous iron is complexed by hydroxide anion. Electrostatic interaction with anions reduces the reduction potential of iron and facilitates its oxidation by H2O2. EDTA, citrate, nucleotides, and DNA have similar effects (38). Although the predominant ligands of unincorporated iron inside the cell are unknown, they are presumably anionic, and so the pertinent rate constants are probably two orders of magnitude higher than the 76 M-1s-1 value that is widely quoted.
Second, cells are more damaged when they are exposed to low micromolar concentrations of H2O2 for a long time than when they are exposed to millimolar doses for a short time. The latter protocol has been favored in experimental systems, because the scavenging activity of cells has typically precluded the low-concentration design. One reason for the disproportionate effect of low doses is that the production of hydroxyl radicals saturates as H2O2 concentrations increase, because the rate-limiting step becomes rereduction of oxidized iron by cellular reductants. A second reason is that millimolar concentrations of H2O2 quickly block metabolism. H2O2 does not substantially damage the DNA of nonmetabolizing cells (16), apparently because reductants become unavailable to drive the Fenton reaction.
How Much Oxidative DNA Damage Occurs in Wild-Type Cells? Exponentially growing E. coli generate intracellular H2O2 at a rate of 10-15 μM s-1 (21, 39), but the robust peroxidase activity of Ahp keeps the steady-state concentration at least as low as 20 nM (24). Nevertheless, this value is only an order of magnitude lower than the concentration that killed recA mutants. We infer that evolution has calibrated the basal synthesis of scavenging enzymes so that endogenous oxidants generate little DNA damage. It seems likely, however, that some amount of subtoxic DNA damage must nevertheless occur. Indeed, mutants that are multiply deficient in DNA-repair enzymes grow poorly in aerobic media (16, 40).
If the titer of scavenging enzymes is just sufficient to minimize damage from endogenous H2O2, then, without further adaptation, cells will be vulnerable to circumstances that either increase the rate at which H2O2 is formed within the cell or that allow H2O2 to enter from outside. For this reason, many organisms use H2O2 as a weapon to suppress the growth of their competitors. Both bacteria and plants excrete redox-cycling compounds which, when ingested by competitors, toxify them by accelerating the rate of intracellular H2O2 formation. Lactic acid bacteria, which are themselves unusually resistant to H2O2, use a pyruvate oxidase to excrete H2O2 into their habitat and, thereby, kill microbes that would otherwise compete for resources. More familiarly, mammalian phagocytes engulf microbial pathogens and dowse them with H2O2.
The oxidative-response regulons, which are widespread among both microbes and higher organisms, apparently exist to defend cells against such circumstances. As with the OxyR regulon of E. coli, these responses are not activated during routine aerobiosis. Basal defenses are sufficient to cope with that amount of stress (24). Instead, they are activated whenever cells encounter environments containing exogenous H2O2. One can calculate (24) that the steady-state concentration of H2O2 inside E. coli will rise to toxic levels, 1 μM H2O2, whenever cells are exposed to >5 μM H2O2. Thus, even at low doses, H2O2 can be a very effective toxin.
Low doses of H2O2 are toxic to mammalian cells as well. By using glucose oxidase to apply a near-constant exposure of H2O2 to mammalian cells, Antunes and Cadenas (41) estimated that ≈1 μM activated apoptotic pathways in Jurkat T cells. It seems plausible that Fenton chemistry either directly triggered the response, or else that signaling pathways have evolved, like OxyR, to respond to concentrations of H2O2 that would otherwise cause Fenton-mediated mutagenesis. Glutathione peroxidase averts toxicity from endogenous H2O2 (42), so even catalase-deficient individuals are asymptomatic.
The Importance of Iron Sequestration. Dps was initially identified as a protein that coats DNA in vitro, and subsequent electron microscopy studies have indicated that it does so in vivo in stationary-phase bacteria (43, 44). This behavior was rationalized on the grounds that by coating the DNA, the protein would protect it from chemical damage, including oxidants. However, Dps induction appears to allow continuous growth (Fig. 1), which would not be possible if the DNA locked into a crystalline array that precluded transcription. One possibility is that the DNA-coating behavior is artifactual and is not an important activity in vivo. It is more attractive to believe that Dps may have evolved to exhibit two different activities under different circumstances, and that in stationary phase cells, the DNA-binding activity may be important. At present, the ability of Dpr to complement dps mutants suggests that iron sequestration is sufficient to protect the DNA under the log-phase conditions of our experiments. A similar result was recently reported for a mutant form of Dps that could not form oligomers but nevertheless could sequester iron and protect DNA from oxidants (45).
The experiments reported here have shown that Dps synthesis provides a way for the OxyR regulon to protect DNA, but it may not be the only one. Hpx- dps mutants did not die as rapidly as did Hpx- oxyR mutants (Fig. 5), suggesting that at least one other OxyR-controlled gene contributes to protection. A candidate is fur, the repressor of iron import. Previous studies showed that fur mutants accumulate excessive amounts of iron and are therefore vulnerable to DNA damage by H2O2 (46). Zheng et al. (47) have suggested that the OxyR-directed induction of Fur protein may serve to further inhibit iron uptake during oxidative stress. Because Fur represses the synthesis of TCA-cycle enzymes, including several that have oxidant-sensitive iron-sulfur clusters, its induction may also indirectly diminish the amount of iron that they release into the cytosol (48). Indeed, in preliminary EPR experiments, we have observed that the amount of unincorporated intracellular iron is far higher in the Hpx- oxyR mutants than in the Hpx- dps mutants. These ideas require further testing.
Cell-permeable iron chelators prevent H2O2-mediated DNA damage in bacterial and mammalian cells (17, 49). Still, because those chelators have significant affinity for metals other than iron, including copper, it has been argued that other metals might nevertheless be responsible for damage (50). However, because Dps/Dpr specifically assembles ferric oxide crystals, its protective action seems to firmly establish iron as the source of DNA damage. A similar argument can be made in mammalian cells, where defects in frataxin cause elevated levels of unincorporated iron and DNA instability in mitochondria. Recent evidence indicates that a role of frataxin is to sequester loose iron, after the fashion of Dps (51).
Supplementary Material
Acknowledgments
We thank Mark Nilges of the Illinois EPR Research Center for assistance with EPR experiments and Michael Volkert (University of Massachusetts Medical School, Worcester, MA), Gigi Storz (National Institutes of Health, Bethesda), Steven Finkel (University of Southern California, Los Angeles), and Jeff Gardner (University of Illinois) for generously providing strains used in this study (see Table 1).
Author contributions: J.I. designed research; S.P. and X.Y. performed research; S.P., X.Y., and J.I. analyzed data; and S.P. and J.I. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LB, Luria broth; EPR, electron paramagnetic resonance.
References
- 1.Loew, O. (1900) Science 11, 701-702. [DOI] [PubMed] [Google Scholar]
- 2.McCord, J. M. & Fridovich, I. (1969) J. Biol. Chem. 244, 6049-6055. [PubMed] [Google Scholar]
- 3.Carlioz, A. & Touati, D. (1986) EMBO J. 5, 623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo, C. F., Mashino, T. & Fridovich, I. (1987) J. Biol. Chem. 262, 4724-4727. [PubMed] [Google Scholar]
- 5.Gardner, P. R. & Fridovich, I. (1991) J. Biol. Chem. 266, 1478-1483. [PubMed] [Google Scholar]
- 6.Gardner, P. R. & Fridovich, I. (1991) J. Biol. Chem. 266, 19328-19333. [PubMed] [Google Scholar]
- 7.Liochev, S. I. & Fridovich, I. (1992) Proc. Natl. Acad. Sci. USA 89, 5892-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flint, D. H., Tuminello, J. F. & Emptage, M. H. (1993) J. Biol. Chem. 268, 22369-22376. [PubMed] [Google Scholar]
- 9.Gardner, P. R., Raineri, I., Epstein, L. B. & White, C. W. (1995) J. Biol. Chem. 270, 13399-13405. [DOI] [PubMed] [Google Scholar]
- 10.Wallace, M. A., Liou, L.-L., Martins, J., Clement, M. H. S., Bailey, S., Longo, V. D., Valentine, J. S. & Gralla, E. B. (2004) J. Biol. Chem. 279, 32055-32062. [DOI] [PubMed] [Google Scholar]
- 11.Loewen, P. C. (1984) J. Bacteriol. 157, 622-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schellhorn, H. E. & Hassan, H. M. (1988) Can. J. Microbiol. 34, 1171-1176. [DOI] [PubMed] [Google Scholar]
- 13.Ma, M. & Eaton, J. W. (1992) Proc. Natl. Acad. Sci. USA 89, 7924-7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demple, B., Halbrook, J. & Linn, S. (1983) J. Bacteriol. 153, 1079-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsson, J. & Carpenter, V. S. (1980) J. Bacteriol. 142, 319-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imlay, J. A. & Linn, S. (1986) J. Bacteriol. 166, 519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imlay, J. A., Chin, S. M. & Linn, S. (1988) Science 240, 640-642. [DOI] [PubMed] [Google Scholar]
- 18.Woodmansee, A. N. & Imlay, J. A. (2002) J. Biol. Chem. 277, 34055-34066. [DOI] [PubMed] [Google Scholar]
- 19.Park, S. & Imlay, J. A. (2003) J. Bacteriol. 185, 1942-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walling, C. (1975) Acc. Chem. Res. 8, 125-131. [Google Scholar]
- 21.Seaver, L. C. & Imlay, J. A. (2001) J. Bacteriol. 183, 7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, J. H. (1972) Experiments in Molecular Genetics (Cold Spring Harbor Lab. Press, Plainview, NY)
- 23.Messner, K. R. & Imlay, J. A. (2002) Methods Enzymol. 349, 354-361. [DOI] [PubMed] [Google Scholar]
- 24.Seaver, L. C. & Imlay, J. A. (2001) J. Bacteriol. 183, 7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pericone, C. D., Park, S., Imlay, J. A. & Weiser, J. N. (2003) J. Bacteriol. 185, 6815-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto, Y., Higuchi, M., Poole, L. B. & Kamio, Y. (2000) J. Bacteriol. 182, 3740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huisman, O., D'Ari, R. & Gottesman, S. (1984) Proc. Natl. Acad. Sci. USA 81, 4490-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imlay, J. A. & Linn, S. (1987) J. Bacteriol. 169, 2967-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng, M., Wang, X., Templeton, L. J., Smulski, D. R., LaRossa, R. A. & Storz, G. (2001) J. Bacteriol. 183, 4562-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almiron, M., Link, A. J., Furlong, D. & Kolter, R. (1992) Genes Dev. 6, 2646-2654. [DOI] [PubMed] [Google Scholar]
- 31.Zhao, G., Ceci, P., Ilari, A., Giangiacomo, L., Laue, T. M., Chiancone, E. & Chasteen, N. D. (2002) J. Biol. Chem. 277, 27689-27696. [DOI] [PubMed] [Google Scholar]
- 32.Grant, R. A., Filman, D. J., Finkel, S. E., Kolter, R. & Hogle, J. M. (1998) Nat. Struct. Biol. 5, 294-303. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto, Y., Poole, L. B., Hantgan, R. R. & Kamio, Y. (2002) J. Bacteriol. 184, 2931-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonanno, K., Wyrzykowski, J., Chong, W., Matijasevic, Z. & Volkert, M. R. (2002) DNA Repair (Amsterdam) 1, 507-516. [DOI] [PubMed] [Google Scholar]
- 35.McCormick, M. L., Buettner, G. R. & Britigan, B. E. (1998) J. Bacteriol. 180, 622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aslund, F., Zheng, M., Beckwith, J. & Storz, G. (1999) Proc. Natl. Acad. Sci. USA 96, 6161-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farr, S. B., D'Ari, R. & Touati, D. (1986) Proc. Natl. Acad. Sci. USA 83, 8268-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rush, J. D., Maskas, Z. & Koppenol, W. H. (1990) FEBS Lett. 261, 121-123. [Google Scholar]
- 39.Seaver, L. C. & Imlay, J. A. (2004) J. Biol. Chem. 279, 48742-48750. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham, R. P., Saporito, S. M., Spitzer, S. G. & Weiss, B. (1986) J. Bacteriol. 168, 1120-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antunes, F. & Cadenas, E. (2001) Free Radical Biol. Med. 30, 1008-1018. [DOI] [PubMed] [Google Scholar]
- 42.Antunes, F., Han, D. & Cadenas, E. (2002) Free Radical Biol. Med. 33, 1260-1267. [DOI] [PubMed] [Google Scholar]
- 43.Nair, S. & Finkel, S. E. (2004) J. Bacteriol. 186, 4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf, S. G., Frenkiel, D., Arad, T., Finkel, S. E., Kolter, R. & Minsky, A. (1999) Nature 400, 83-85. [DOI] [PubMed] [Google Scholar]
- 45.Ceci, P., Cellai, S., Falvo, E., Rivette, C., Rossi, G. L. & Chiancone, E. (2004) Nucleic Acids Res. 32, 5935-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Touati, D., Jacques, M., Tardat, B., Bouchard, L. & Despied, S. (1995) J. Bacteriol. 177, 2305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng, M., Doan, B., Schneider, T. D. & Storz, G. (1999) J. Bacteriol. 181, 4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keyer, K. & Imlay, J. A. (1996) Proc. Natl. Acad. Sci. USA 93, 13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Filho, A. C. M., Hoffmann, M. E. & Meneghini, R. (1984) Biochem. J. 218, 273-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bar-Or, D. & Winkler, J. V. (2002) Free Radical Biol. Med. 32, 197-199. [DOI] [PubMed] [Google Scholar]
- 51.O'Neill, H. A., Gakh, O., Park, S., Cui, J., Mooney, S. M., Sampson, M., Ferreira, G. C. & Isaya, G. (2005) Biochemistry 44, 537-545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.