Abstract
Background
Male circumcision is defined as the surgical removal of all or part of the foreskin of the penis and may be practiced as part of a religious ritual, as a medical procedure, or as part of a traditional ritual performed as an initiation into manhood. Since the 1980s, over 30 observational studies have suggested a protective effect of male circumcision on HIV acquisition in heterosexual men. In 2002, three randomised controlled trials to assess the efficacy of male circumcision for preventing HIV acquisition in men commenced in Africa. This review evaluates the results of these trials, which analysed the effectiveness and safety of male circumcision for preventing acquisition of HIV in heterosexual men.
Objectives
To assess the evidence of an interventional effect of male circumcision for preventing acquisition of HIV‐1 and HIV‐2 by men through heterosexual intercourse
Search methods
We formulated a comprehensive and exhaustive search strategy in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). In June 2007 we searched the following electronic journal and trial databases: MEDLINE, EMBASE, and CENTRAL. We also searched the electronic conference databases NLM Gateway and AIDSearch and the trials registers ClinicalTrials.gov and Current Controlled Trials. We contacted researchers and relevant organizations and checked reference lists of all included studies.
Selection criteria
Randomised controlled trials of male circumcision versus no circumcision in HIV‐negative heterosexual men with HIV incidence as the primary outcome.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data, and graded methodological quality. Data extraction and methodological quality were checked by a third author who resolved differences when these arose. Data were considered clinically homogeneous and meta‐analyses and sensitivity analyses were performed.
Main results
Three large RCTs of men from the general population were conducted in South Africa (N = 3 274), Uganda (N = 4 996) and Kenya (N = 2 784) between 2002 and 2006. All three trials were stopped early due to significant findings at interim analyses. We combined the survival estimates for all three trials at 12 months and also at 21 or 24 months in a meta‐analysis using available case analyses using the random effects model. The resultant incidence risk ratio (IRR) was 0.50 at 12 months with a 95% confidence interval (CI) of 0.34 to 0.72; and 0.46 at 21 or 24 months (95% CI: 0.34 to 0.62). These IRRs can be interpreted as a relative risk reduction of acquiring HIV of 50% at 12 months and 54% at 21 or 24 months following circumcision. There was little statistical heterogeneity between the trial results (χ² = 0.60; df = 2; p = 0.74 and χ² = 0.31; df = 2; p = 0.86) with the degree of heterogeneity quantified by the I² at 0% in both analyses. We investigated the sensitivity of the calculated IRRs and conducted meta‐analyses of the reported IRRs, the reported per protocol IRRs, and reported full intention‐to‐treat analysis. The results obtained did not differ markedly from the available case meta‐analysis, with circumcision displaying significant protective effects across all analyses.
We conducted a meta‐analysis of the secondary outcomes measuring sexual behaviour for the Kenyan and Ugandan trials and found no significant differences between circumcised and uncircumcised men. For the South African trial the mean number of sexual contacts at the 12‐month visit was 5.9 in the circumcision group versus 5 in the control group, which was a statistically significant difference (p < 0.001). This difference remained statistically significant at the 21‐month visit (7.5 versus 6.4; p = 0.0015). No other significant differences were observed.
Incidence of adverse events following the surgical circumcision procedure was low in all three trials.
Reporting of methodological quality was variable across the three trials, but overall, the potential for significant biases affecting the trial results was judged to be low to moderate given the large sample sizes of the trials, the balance of possible confounding variables across randomised groups at baseline in all three trials, and the employment of acceptable statistical early stopping rules.
Authors' conclusions
There is strong evidence that medical male circumcision reduces the acquisition of HIV by heterosexual men by between 38% and 66% over 24 months. Incidence of adverse events is very low, indicating that male circumcision, when conducted under these conditions, is a safe procedure. Inclusion of male circumcision into current HIV prevention measures guidelines is warranted, with further research required to assess the feasibility, desirability, and cost‐effectiveness of implementing the procedure within local contexts.
Keywords: Humans; Male; Circumcision, Male; HIV‐1; HIV‐2; Heterosexuality; HIV Infections; HIV Infections/prevention & control; HIV Infections/transmission; Kenya; Randomized Controlled Trials as Topic; South Africa; Uganda
Plain language summary
Male circumcision for prevention of heterosexual acquisition of HIV in men
Results from three large randomised controlled trials conducted in Africa have shown strong evidence that male circumcision prevents men in the general population from acquiring HIV from heterosexual sex. At a local level, further research will be needed to assess whether implementing the intervention is feasible, appropriate, and cost‐effective in different settings.
Background
Male circumcision is defined as the surgical removal of all or part of the prepuce (foreskin) of the penis and may be practiced as part of a religious ritual usually conducted shortly after birth or in childhood; as a medical procedure to treat or prevent infections, injury, or anomalies of the foreskin; or as part of a traditional ritual performed as an initiation into manhood (Horizons 2000). Since the 1980s, observational studies have suggested an association between male circumcision and HIV infection in males. Most of these studies suggested a protective effect of male circumcision on HIV acquisition in men.
Theories to support the biological basis for a protective effect of circumcision on HIV exist. Researchers have noted that the inner aspect of the foreskin is well supplied with Langerhans cells (Szabo 2000) and that in vitro, HIV‐1 demonstrates a specific tropism (attraction) for these cells (Soto‐Ramirez 1996), in particular the CD4 receptors (Hussain 1995) on them. CD4 and other HIV co‐receptors have been shown to facilitate HIV entry into host cells. According to this theory, circumcision would remove the potential entry site for HIV; however, not all Langerhans cells are removed during circumcision, as after the procedure there may be residual penile mucosa of the glans; and also there are Langerhans cells in the penile shaft (Cold 1999). In direct contradiction to the above theory, the inner prepuce contains apocrine glans which secrete lysozyme (Fleiss 1998). Lysozyme reportedly kills HIV‐1 in vitro (Lee‐Huang 1999), suggesting a protective effect of the foreskin. In a 2002 study that used immunofluorescence and image analysis to quantify cells expressing HIV‐1 co‐receptors, adult foreskin mucosa had greater susceptibility to infection than cervical mucosa or the external surface of the foreskin tissue (Patterson 2002). More recently, in 2006, an immunohistochemical analysis of foreskin tissue from 49 Kenyan men confirmed that the inner mucosal surface of the human foreskin contains 2% of Langerhans cells and macrophages, making it highly susceptible to HIV infection (Donoval 2006).
Prior to 2003, six reviews (Moses 1994; De Vincenzi 1994; Moses 1998; Van Howe 1999; Weiss 2000; Bailey 2001) and one meta‐analysis (Weiss 2000) had been published which reached different conclusions on the association between male circumcision and HIV infection. In 2003 we published a Cochrane review of 35 observational studies and concluded that insufficient evidence existed to support an interventional effect of male circumcision on HIV acquisition in heterosexual men (Siegfried 2003). The review supported previous review findings that the results from existing observational studies showed a strong epidemiological association between male circumcision and prevention of HIV, especially among high‐risk groups. Unlike a previous review by Weiss 2000, however, no meta‐analysis was performed due to the high degree of statistical, clinical, and methodological heterogeneity between the included studies. In particular, we noted study quality to be highly variable, with no studies including the same set of potential confounding variables in their adjusted analyses. We identified three ongoing randomised controlled trials (RCTs) during the conduct of the review, and for this reason, we recommended that the results of these three trials be awaited before circumcision be implemented as a public health intervention for prevention of sexually transmitted HIV. The Cochrane review was updated in 2005 with two additional observational studies, but the inclusion of these studies did not alter the review conclusions (Siegfried 2005).
In 2005 the results of a South African RCT of male circumcision for preventing HIV acquisition in heterosexual men was published (ANRS 1265), followed in early 2007 by the publication of Kenyan (Bailey 2007) and Ugandan (Gray 2007) RCT findings. In the light of these RCT results, we now are able to assess the efficacy and safety of male circumcision as an intervention to reduce heterosexual acquisition of HIV infection by men in the update of our Cochrane review.
Objectives
To assess the evidence of an interventional effect of male circumcision for preventing acquisition of HIV‐1 and HIV‐2 by men through heterosexual intercourse
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials. Studies performed in general or specific populations and in hospitals or clinics were included. Studies performed in any country and published in any language were included.
Types of participants
Sexually active men from the general population who were HIV‐uninfected at time of enrolment into the trial. Men are defined as males 12 years of age or older.
Types of interventions
Male circumcision is defined as the surgical removal of the foreskin of the penis.
Types of outcome measures
PRIMARY OUTCOME: HIV‐1 or HIV‐2 infection in men (incidence), based on laboratory results. The outcome was assessed at time points common to all trials (12 months) and at end points of trials (21 or 24 months).
SECONDARY OUTCOMES: Behavioural outcomes, including sexual activity, two or more sexual partners, non‐marital partner, casual last contact, inconsistent or no condom use, any unprotected sex, alcohol with sex, and transactional sex. The outcomes were assessed at time points common to all trials (12 months) and at end points of trials (21 or 24 months).
ADVERSE EVENTS: Any adverse events associated with circumcision were recorded if reported in the studies.
Search methods for identification of studies
See: HIV/AIDS Collaborative Review Group search strategy
The original review conducted the searches in 2002 and the updated version published in The Lancet Infectious Diseases repeated the search in November 2004. For this update the search strategy was further refined with the assistance of the HIV/AIDS Review Group Trials Search Co‐ordinator. We formulated a comprehensive and exhaustive search strategy in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). Full details of the Cochrane HIV/AIDS Review Group methods and the journals handsearched are published in the section on Collaborative Review Groups in The Cochrane Library. We used the RCT strategy developed by The Cochrane Collaboration and detailed in the Cochrane Reviewers' Handbook in combination with terms specific to male circumcision. We searched the following databases:
1. Journal and trial databases
MEDLINE This search was conducted on 14 June 2007 using the strategy outlined in Table 1. This search yielded 126 records of which we identified 16 records for full article retrieval.
1. Search strategy for MEDLINE.
| Search number | Seach terms | Result |
| #5 | Search #1 AND #2 AND #3 Limits: Publication Date from 2004 to 2007 | 126 |
| #4 | Search #1 AND #2 AND #3 | 300 |
| #3 | Search MALE CIRCUMCISION OR MALE CIRCUMCISIONS OR CIRCUMCISION OR CIRCUMCIS* OR UNCIRCUMCIS* | 4008 |
| #2 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | 2941243 |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] | 218614 |
EMBASE This search was conducted on 15 June 2007 using the strategy outlined in Table 2. This search yielded 25 records of which we identified five records for full article retireval.
2. Search strategy for EMBASE.
| Search number | Search terms | Results |
| #5 | #1 AND #2 AND #3 AND [2004‐2007]/py | 25 |
| #4 | #1 AND #2 AND #3 | 32 |
| #3 | 'male circumcision' OR 'male circumcisions' OR ('circumcision'/exp OR 'circumcision') OR circumcis* OR uncircumcis* | 4,512 |
| #2 | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over:ab OR crossover*:ti AND orr AND crossover*:ab OR placebo*:ti OR placebo*:ab OR ((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab)) OR ((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab)) OR assign*:ti OR assign*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/de OR 'double‐blind procedure'/de OR 'single‐blind procedure'/de OR 'randomized controlled trial'/de OR allocat*:ti OR allocat*:ab | 499,109 |
| #1 | ('human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus'/exp) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) | 258,468 |
Cochrane Central Register of Controlled Trials (CENTRAL) This search of CENTRAL, published in Issue 2 of The Cochrane Library (2007), was conducted on 15 June 2007 using the strategy outlined in Table 3. The search yielded 9 records of which we identified two records for full article retrieval.
3. Search strategy for CENTRAL.
| Search number | Search term | Results |
| #1 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUN* DEFICIENCY VIRUS) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUN* DEFICIENCY SYNDROME) in All Fields in all products | 6892 |
| #2 | MeSH descriptor HIV Infections explode all trees in MeSH products | 4675 |
| #3 | MeSH descriptor HIV explode all trees in MeSH products | 1395 |
| #4 | ((ANIMAL OR ANIMALS) AND (NOT HUMANS)) in All Fields in all products | 460 |
| #5 | (MALE CIRCUMCISION) OR (MALE CIRCUMCISIONS) OR CIRCUMCISION OR CIRCUMCIS* OR UNCIRCUMCIS* | 198 |
| #6 | (#1 OR #2 OR #3) | 7008 |
| #7 | (#5 AND #6) | 20 |
| #8 | (#7 AND NOT #4) | 20 |
| #9 | (#8), from 2004 to 2007 | 9 |
2. Conference databases
We searched AIDSearch on 15 June 2007 using the strategy outlined in Table 4. AIDSearch covers abstracts from a number of relevant international conferences, including the International AIDS Conference, Conference on Retroviruses and Opportunistic Infections, the British HIV Association Conference, and the International Congress on Drug Therapy in HIV Infection. The search yielded 33 records and three records were identified for full article retrieval.
4. Search strategy for AIDSearch.
| Search number | Search terms |
| #1 | PT=RANDOMIZED CONTROLLED TRIAL |
| #2 | PT=CONTROLLED CLINICAL TRIAL |
| #3 | RANDOMIZED CONTROLLED TRIALS |
| #4 | RANDOM ALLOCATION |
| #5 | DOUBLE BLIND METHOD |
| #6 | SINGLE BLIND METHOD |
| #7 | PT=CLINICAL TRIAL |
| #8 | CLINICAL TRIALS OR CLINICAL TRIALS, PHASE 1 OR CLINICAL TRIALS, PHASE II OR CLINICAL TRIALS, PHASE III OR CLINICAL TRIALS, PHASE IV OR CONTROLLED CLINICAL TRIALS OR MULTICENTER STUDIES |
| #9 | (SINGL* OR DOUBL* OR TREBL* OR TRIPL*) NEAR6 (BLIND* OR MASK*) |
| #10 | CLIN* NEAR6 TRIAL* |
| #11 | PLACEBO* |
| #12 | PLACEBOS |
| #13 | RANDOM* |
| #14 | RESEARCH DESIGN |
| #15 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 |
| #16 | ANIMALS NOT (HUMAN AND ANIMALS) |
| #17 | #15 NOT #16 |
| #18 | MALE CIRCUMCISION OR MALE CIRCUMCISIONS OR CIRCUMCISION OR CIRCUMCIS* OR UNCIRCUMCIS* |
| #19 | #17 AND #18 AND PY=2004‐2007 |
3. Ongoing trials
We also searched ClinicalTrials.gov (http://clinicaltrials.gov/) (six records) and Current Controlled Trials (www.controlled‐trials.com/) (one record) to identify any ongoing trials in April 2008.
4. Researchers and relevant organizations
We were in close contact with individual researchers working in the field, and policymakers based in inter‐governmental organizations, including the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO).
5. Reference lists
We also checked the reference lists of all studies identified by the above methods and examined any systematic reviews, meta‐analyses, or prevention guidelines we identified during the search process.
There was some overlap between references retrieved from the above methods.
Data collection and analysis
The titles, abstracts, and descriptor terms of all downloaded material from the electronic searches were read by NS and MM and irrelevant reports were discarded. All citations identified then were independently inspected by NS and MM to establish relevance of the article according to the prespecified criteria. Where there was uncertainty as to the relevance of the study, the full article was obtained.
1. Selection of studies Two reviewers (NS and MM) independently applied the inclusion criteria, and differences were resolved by discussions with a third reviewer (JV). Studies were reviewed for relevance based on study design, types of participants, exposures, and outcome measures.
2. Data extraction Data were extracted independently by NS and MM. A standardised data extraction form was used, and the following characteristics were extracted from each included study.
Administrative details Identification; author(s); published or unpublished; year of publication; number of studies included in paper; year in which study was conducted; details of other relevant papers cited.
Details of study Study design; method(s) of recruitment; inclusion and exclusion criteria; number of participants assessed for eligibility, number excluded, number enrolled, and number analysed; country and location of the study; setting in which the study was performed (e.g., urban or rural; general population, occupational or hospital/clinic‐based); background HIV prevalence in the general population; background religion in the general population; background HIV prevalence of the selected study population (high‐risk or low‐risk); number of participants; dominant cultural practices regarding circumcision (age of circumcision and reason).
Characteristics of participants Age; location; education; occupation; religion; socio‐economic status; marital status; age at first intercourse; number of sexual partners; contact with sex workers; condom use; other identified risk factors (e.g., presence of sexually transmitted infections; injection; blood transfusion).
Details of intervention Circumcision surgical procedure used; whether an incentive was offered.
Details of outcomes Incidence of HIV infection: HIV‐1, HIV‐2, both, or unclear; number of HIV‐positive and HIV‐negative men in the circumcised and uncircumcised groups (n/N); types of tests used to determine and confirm HIV status; whether assessors of HIV status were blinded to circumcision status; method of surveillance of adverse effects associated with circumcision; number of men with specific adverse effects associated with circumcision (n/N);
Details of analysis Type of statistical analysis; time intervals included in analysis; type of effect measure (relative risks; hazard ratios); size of effect measure with confidence interval. When adjusted analysis was performed, we also recorded the factors adjusted for in the analyses.
Details of study ethics Informed consent obtained for participation; type of informed consent (oral or written); approving institutions' ethics board details.
3. Assessment of the risk of bias
The reviewers (NS and MM) independently examined the components of each included trial for risk of bias using a standard form. This form included information on the sequence generation, allocation concealment, blinding (i.e., participants, personnel, and outcome assessor), incomplete outcome data, selective outcome reporting, and other sources of bias. The methodological components of the trials were assessed and classified as adequate, inadequate, or unclear as per the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). Differences were resolved by discussions with a third reviewer, JD, who also independently rated the trials on the components below.
Sequence generation
Adequate: investigators described a random component in the sequence generation process, such as the use of random number table, coin tossing, card or envelope shuffling, etc.
Inadequate: investigators described a non‐random component in the sequence generation process, such as the use of odd or even date of birth, algorithm based on the day or date of birth, hospital, or clinic record number.
Unclear: insufficient information to permit judgement of the sequence generation process.
Allocation concealment
Adequate: participants and the investigators enrolling participants cannot foresee assignment (e.g., central allocation; or sequentially numbered, opaque, sealed envelopes).
Inadequate: participants and investigators enrolling participants can foresee upcoming assignment (e.g., an open random allocation schedule, a list of random numbers); or envelopes were unsealed or nonopaque or not sequentially numbered.
Unclear: insufficient information to permit judgement of the allocation concealment or the method not described.
Blinding
Adequate: blinding of the participants, key study personnel, and outcome assessor, and unlikely that the blinding could have been broken. Or lack of blinding unlikely to introduce bias. No blinding in the situation where non‐blinding is not likely to introduce bias.
Inadequate: no blinding, incomplete blinding and the outcome is likely to be influenced by lack of blinding.
Unclear: insufficient information to permit judgement of adequacy or otherwise of the blinding.
In the case of circumcision trials it was not possible to blind personnel delivering the intervention or the participants. It is possible, however, to blind the assessors, and we therefore only rated the blinding of assessors as adequate, inadequate, or unclear.
Incomplete outcome data
Adequate: no missing outcome data, reasons for missing outcome data unlikely to be related to true outcome, or missing outcome data balanced in number across groups.
Inadequate: reason for missing outcome data likely to be related to true outcome, with either imbalance in number across groups or reasons for missing data.
Unclear: insufficient reporting of attrition or exclusions.
Selective reporting
Adequate: a protocol is available which clearly states the primary outcome as the same as in the final trial report.
Inadequate: the primary outcome differs between the protocol and final trial report.
Unclear: no trial protocol is available or there is insufficient reporting to determine if selective reporting is present.
Other forms of bias
Adequate: there is no evidence of bias from other sources.
Inadequate: there is potential bias present from other sources (e.g., early stopping of trial, fraudulent activity, extreme baseline imbalance, or bias related to specific study design).
Unclear: insufficient information to permit judgement of adequacy or otherwise of other forms of bias.
4. Data analysis
The primary outcome, HIV incidence, was expressed using survival estimates which incorporate the time until men were diagnosed with HIV infection, or the time free from infection, or the time until they were lost to follow‐up, either because they did not return for testing or because the trial was stopped early. The effect measure of choice for these time‐to‐event data was the incidence risk ratio (hazard ratio) calculated from data extracted from the trial report (Analysis 1.1). We used an intention‐to‐treat analysis (as defined by receipt of intervention according to randomised group). For the secondary outcomes (i.e., sexual behaviour outcomes) we calculated the relative risk as the effect measure for dichotomous data (Analysis 1.2, Analysis 1.3).
1.1. Analysis.
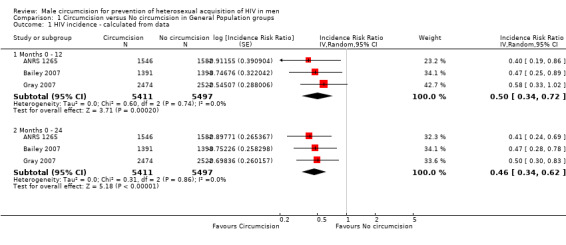
Comparison 1 Circumcision versus No circumcision in General Population groups, Outcome 1 HIV incidence ‐ calculated from data.
1.2. Analysis.
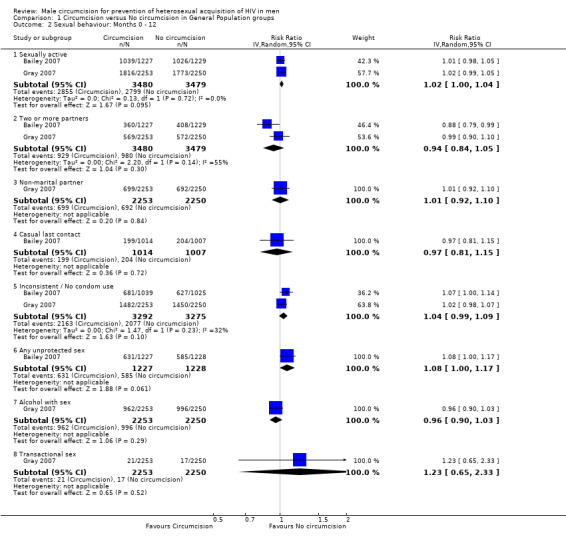
Comparison 1 Circumcision versus No circumcision in General Population groups, Outcome 2 Sexual behaviour: Months 0 ‐ 12.
1.3. Analysis.
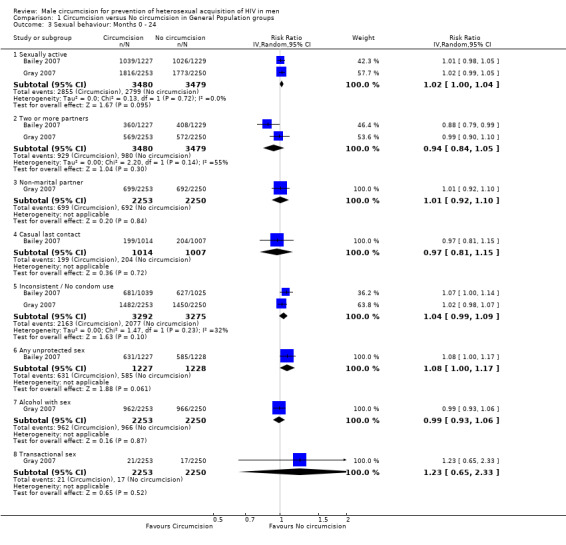
Comparison 1 Circumcision versus No circumcision in General Population groups, Outcome 3 Sexual behaviour: Months 0 ‐ 24.
We assessed homogeneity in the study results using the Chi‐square test for heterogeneity with a 10% level of significance as the cut‐off. The impact of statistical heterogeneity was quantified using the I² statistic (Higgins 2002).
A meta‐analysis was conducted because the identified studies were sufficiently homogenous. We combined incidence risk ratios (hazard ratios) using the generic inverse variance function available in RevMan (Version 5.0). Relative risks were combined using a random effects inverse variance method.
In the event of significant clinical heterogeneity, we had planned to explore possible causes by looking at the characteristics of the various studies critically. Possible sources of heterogeneity in this review may have included HIV1 versus HIV2, age at circumcision, religion, socio‐economic status, marital status, sexual behaviour, sexually transmitted diseases, and condom use.
We used sensitivity analysis to explore the difference in meta‐analysis results when using:
Reported estimates of the survival probabilities (Analysis 1.4) compared to calculated estimates of survival probabilites (from number of events and total number at risk) (Analysis 1.1).
Per protocol estimates of survival probabilities (Analysis 1.5) as reported in the studies compared to calculated intention‐to‐treat survival probabilities (Analysis 1.1).
Risk difference (between circumcised and uncircumcised men) in HIV infection at 12 months and at the end of the studies (Analysis 1.6) compared to hazard ratios (Analysis 1.1).
1.4. Analysis.
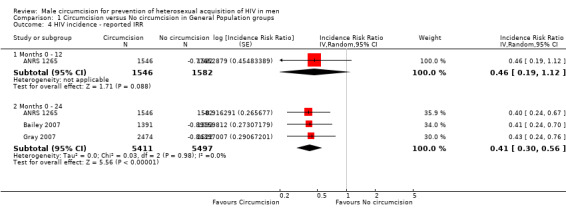
Comparison 1 Circumcision versus No circumcision in General Population groups, Outcome 4 HIV incidence ‐ reported IRR.
1.5. Analysis.
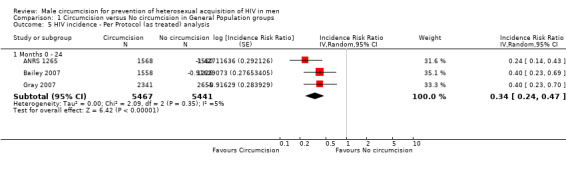
Comparison 1 Circumcision versus No circumcision in General Population groups, Outcome 5 HIV incidence ‐ Per Protocol (as treated) analysis.
1.6. Analysis.
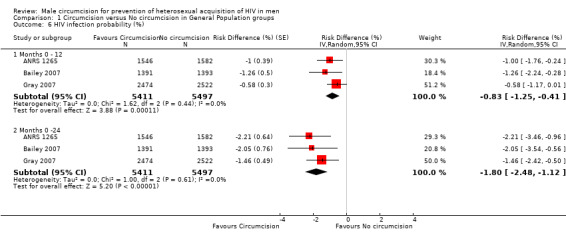
Comparison 1 Circumcision versus No circumcision in General Population groups, Outcome 6 HIV infection probability (%).
Results
Description of studies
See Characteristics of Included Studies for complete details.
Three large RCTs have been conducted in Orange Farm, South Africa (N = 3 274), Rakai, Uganda (N = 4 996), and Kisumu, Kenya (N = 2 784). All were conducted on men from the general population. In the South African and Kenyan trials, participants were 18 to 24 years old and in the Ugandan trial, men were 15 and 49 years old. At the commencement of the Kenyan and Ugandan trials, men who were HIV‐infected were excluded from participating, but in the South African trial, men who were HIV‐positive at trial enrolment were retained in the trial but excluded from the analysis.
Circumcision was performed using commonly used surgical techniques under local anaesthesia (ANRS 1265 and Bailey 2007 used the forceps‐guided technique and Gray 2007 used the sleeve procedure). Those randomised to the control arm were offered circumcision at the end of each trial. Total follow‐up was planned for 21 months in the South African trial and for 24 months in the Kenyan and Ugandan trials. All three trials commenced in 2002. The South African trial was stopped early in April 2005 by the Institutional Monitoring and Safety Board after interim results exceeded the limits of the early stopping rule. The Kenyan trial and the Ugandan trial were also stopped early in December 2006 for the same reason.
It is important to note that the estimates for the studies are based on different time scales, assumptions, and methods.
Each of the studies' assessed and reported HIV infection status at different time points (Table 5). We therefore combined the 21 month and 24 months end points from different trials.
Studies analysed and reported results for different time intervals (Table 5).
Studies used different criteria for excluding participants who were included, but who were later considered to be HIV‐positive at the start of the trial (Table 6).
Studies had different methods to assign the time point for an event (HIV infection) (Table 6).
A "missed visit" and "lost to follow‐up" were defined differently in the studies (Table 6).
The analysis methods (type of models) differed for the studies (Table 6).
Different studies conducted and reported different types of analyses (e.g., available case analysis, adjusted analysis, as treated (per protocol) analysis, full ITT analysis) (Data and analyses).
Adjusted analyses were reported in different ways and adjusted for different factors (Table 7).
5. Time points.
| Study | Time of follow‐up visits (month) | Analysis Time Intervals (months) | ||||||||||
| 1 | 3 | 6 | 12 | 18 | 21 | 24 | 1‐3 | 3‐12 | 12‐21 | 1‐21 | 1‐24 | |
| ANRS 1265 | x | x | x | x | x | x | x | x | ||||
| Bailey 2007 | x | x | x | x | x | x | x | |||||
| Gray 2007 | x (4‐6 weeks) |
x | x | x | x | |||||||
6. Methods of Analysis.
| Study | HIV+ after start of trial | Time points for event or censoring | Definitions: Missed visit and lost to follow‐up | Type of Analysis | Unadjusted IRR Notes |
| ANRS 1265 | Participants with an HIV‐positive test at 1 month excluded from analysis | Month 3, month 12, month 21. Time continuous, observed in a grouped form (at the end of each period). | Missing visit: a visit not completed prior to a completed visit. Lost to follow‐up: not completed a planned visit in the 2 months following the planned date of the visit | Piecewise exponential proportional hazards model with baseline hazard constant in each period. Implemented with a Poisson log‐linear model | Included Period number (periods: month 1 ‐ 3, month 4‐12, month 12‐21), logarithm of duration of exposure in each period in days as an offset. |
| Bailey 2007 | Secondary analysis excluded participants subsequently confirmed as HIV+ by PCR at baseline or at 1 month. | HIV+ status was credited to the follow‐up visit when HIV was first detected | Missing visit: 6 months late for 1 month visit, 2 months late for 3 month visit, 5 months late for 6,12,18, or 24 month visits. Lost to follow‐up: not followed to seroconversion and a follow‐up visit had been missed. | Kaplan‐Meier method. All hazard or RRs from Cox regression. An exact method for computing the likelihood was specified to handle ties. | |
| Gray 2007 | Excluded PCR‐positive but antibody‐negative at enrolment | Assumed HIV infection occured at the mid‐point between the last negative and first positive serological test, or at the time of the first positive RT‐PCR for those participants seen during the period before HIV antibody seroconversion. For positive PCR and negative HIV antibody, the date of the positive PCR was used as date of infection. Data from visits were ascribed to the time of scheduled follow‐up. | Kaplan‐Meier method for RRs. |
7. Adjusted Analysis ‐ factors.
| Study | |||
| ANRS 1265 | Bailey 2007 | Gray 2007 | |
| Adjusted IRR | 0.39 [0.23, 0.66] | "adjusted RR varied between 0.44 and 0.47" | "adjusted IRR of 0.49 (95% CI 0.29‐0.81) based on the unadjusted IRR of 0.49 (95% CI 0.28‐0.84)." |
| Method | Piecewise exponential proportional hazards model with baseline hazard constant in each period. Implemented with a Poisson log‐linear model | Kaplan‐Meier method. All hazard or risk ratios from Cox regression. An exact method for computing the likelihood was specified to handle ties. | IRR estimated with exact methods, with Possion multiple regression for adjusted analysis |
| Notes | See Footnote 1 | ||
| Factors adjusted for : | |||
| Time factors | Period number, with log of duration of exposure in each period in days as an offset. Calender period of recruiting (≤30/12/2002, >30/12/2002) |
||
| Ethnic group | Zulu, Sotho, Other | Luo, Other | |
| Religion | Catholic/Protestant, African Traditional, Other | ||
| Alcohol consumption | Past month | With sex in past 6 months | |
| Age group (years) | ≤21; >21 | 8‐20; 21‐24 | 15‐19; 20‐24; 25‐29; 30‐49 |
| Marital status | See Spousal partner column | Balanced | At enrolment: never, currently, previously |
| #Number of sexual partners | x | ||
| *Sexual contact (≥1) unprotected by condom | x | ||
| *Have a spousal partner | x | ||
| *No. of non‐spousal partners | x | Past year: No, Yes | |
| *Sexual partnership(≥1) with only 1 sexual contact | x | ||
| **Visit (≥1) to a clinic for a genital problem | x | ||
| #Condom use | x | ||
| Sexual intercourse for money/gifts | x | ||
| Educational level | Balanced | ||
| Employment status | Balanced | ||
| Occupation | x (NS) | ||
| Weight | Balanced | ||
| Haemoglobin | Balanced | ||
| Herpes simplex virus 2 | x (significant) | ||
| Syphilis | Balanced | ||
| Trichomonas vaginalis | Balanced | ||
| Neisseria gonorrhoeae | Balanced | ||
| Clamydia trachomatis | x (NS) | ||
| Haemophilus ducreyi | Balanced | ||
| Age at 1st sexual intercourse | Balanced | ||
| Sexual intercourse with any partner (previous 6 months) | Balanced | ||
1. Bailey 2007: "After adjustment for baseline variables for which there seemed to be differences between the two study groups at baseline, only infection with herpes simplex virus 2 at baseline was found to be associated with HIV incidence."
*In the past 3 month period before M3, in the past 9 month period before M12 and M21
**In the 12 month period prior to M3, M12 and M21
# At enrolment, past year
Risk of bias in included studies
See Risk of Bias Tables for each trial following the Tables of Characteristics of Included Studies for full details and Figure 1 and Figure 2 for an overall graphical summary of bias risk.
1.
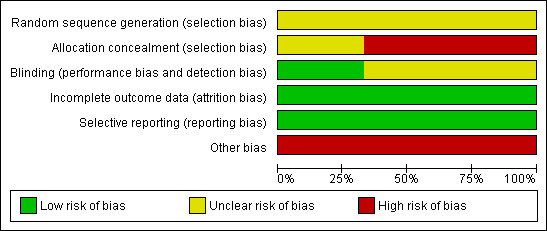
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
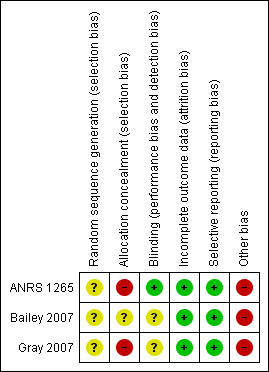
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Random generation
None of the trials clearly described the method used for generating the random sequence. ANRS 1265 described preparing sets of 10 envelopes, and both Bailey 2007 and Gray 2007 report using "blocking," so it is likely that the sequence was generated by a computer for all three trials. For this reason we assumed the risk of bias for this domain to be low in all three trials.
Allocation concealment
Concealment of allocation requires that the next assignment not be known until the next patient has been recruited. For envelope‐based methods it requires the envelopes being opaque, sealed, serially numbered on the outside, and used in the order which the numbers indicate. Although all three trials reported sequential envelopes, participants in ANRS 1265 and Gray 2007 were asked to select one envelope from a box of x (where x is the block size in the trial) after which the envelope was replaced by the next envelope from the next batch. This resulted in imbalances between study groups, thereby reducing the blocking effect. As the process allowed participants to pick envelopes, it would not ensure that the envelopes were opened one at a time and used in order. Therefore, the procedure must be graded as inadequate. As Bailey 2007 did not explicitly report using sealed envelopes, although the process was otherwise adequately reported, we have marked allocation concealment as unclear. There may be a moderate to high risk of bias introduced into the trials due to the methods of allocation concealment.
Blinding of participants, personnel, and outcome assessors
Participants and study personnel could not be blinded to the allocated interventions (circumcision or not). It is unclear whether this lack of blinding could influence the outcome (HIV status) via, for example, sexual risk behaviour or differentiated treatment by study personnel. The primary outcome was HIV incidence based on laboratory results. In ANRS 1265, the specimens were clearly reported as being identified only by a participant number, and so masking was adequate for assessors and the risk of bias was low. In the other two trials, blinding of outcome assessors was poorly described and was graded as unclear. The risk of bias is likely to be low, however.
Incomplete outcome data
Attrition was high in all three trials. We rated the risk of bias due to incomplete outcome reporting as moderate in all three trials, as acceptable statistical survival analysis techniques were used to estimate HIV event distribution over time by accumulating for staggered enrolment and incomplete discrete follow‐up.
Selective outcome reporting
All three trials clearly stated in their protocols that the primary outcome was HIV incidence. The risk of bias due to incomplete outcome reporting is therefore low in all three trials.
Other potential threats to validity
All three trials were stopped early due to data‐dependent processes (formal‐stopping rules), and this may have introduced a risk of bias to the studies.
Effects of interventions
Primary outcome
HIV incidence at 12 months and at 21 or 24 months
We combined the survival estimates for all three trials at 12 months and also at 21 or 24 months in a meta‐analysis using available case analyses using the random effects model (see Analysis 1.1). The resultant incidence risk ratio (IRR) was 0.50 at 12 months with a 95% confidence interval (CI) of 0.34 to 0.72, and 0.46 at 21 or 24 months (95% CI: 0.34 to 0.62). These IRRs can be interpreted as a relative risk reduction of acquiring HIV of respectively 50% (at 12 months) and 54% (at 21 or 24 months) following circumcision. There was little statistical heterogeneity between the trial results (χ² = 0.60; df = 2; p = 0.74 and χ² = 0.31; df = 2; p = 0.86) with the degree of heterogeneity quantified by the I² as 0% in both analyses. Thus the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is not important.
The background risk of HIV infection in the population should be considered in the decision to circumcise men, selecting the populations who stand to benefit most for this intervention. To calculate the number needed to treat (NNT) from the above figures (at 21 or 24 months), it is valuable to consider first the populations with a low assumed control risk (ACR) of HIV infection of 1% (10 per 1000) over two years. For this ACR, the NNT is 186 (95% CI 152 to 264); this would prevent 5 (95% CI: 3 to 6) HIV infections over two years per 1000 men. For a higher ACR of HIV infection of 3% (30 per 1000) over two years, the NNT is 62 (95%CI: 51;88); this would prevent 16 (95%CI: 11;19) HIV infections over two years per 1000 men. For an even higher ACR of 5% (50 per 1000) over two years, the NNT is 38 (95% CI: 31 to 53); this would prevent 26 (95% CI: 18 to 32) HIV infections over two years per 1000 men.
Sensitivity analysis
In order to investigate the sensitivity of the calculated IRRs, we conducted meta‐analyses of the reported IRRs, the reported per protocol IRRs, and a reported full ITT analysis.
We combined the reported results for all three trials at 21 to 24 months (see Analysis 1.4). This resulted in a slightly stronger effect than the calculated IRRs (IRR = 0.41 with 95% CI from 0.30 to 0.56). This can be interpreted as a relative reduction in risk of acquiring HIV of 59% following circumcision. There is, again, little statistical heterogeneity present (χ² = 2.09, df = 2; p = 0.35; I² = 5%). Thus the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is very small and not important. Only one trial (ANRS 1265) presented reported results from an analysis at 12 months (IRR=0.46, 95% CI: 0.19 to 1.12). This effect is somewhat weaker and is not significant compared to the significant effect from the calculated IRR for this specific study (see ANRS 1265 at 12 months in Analysis 1.1).
We also combined the reported per protocol results for all three trials at 21 to 24 months (see Analysis 1.5). This resulted in a stronger effect of IRR = 0.34 with 95% CI from 0.24 to 0.47. This result can be interpreted as a relative reduction in risk of acquiring HIV of 66% following circumcision. There is, again, little statistical heterogeneity present (χ² = 2.09, df = 2; p = 0.35; I² = 5%). Thus the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is very small and not important.
Only one trial (ANRS 1265) reported results from a full ITT analysis (IRR=0.38, 95% CI: 0.22 to 0.66) and an adjusted analysis (IRR=0.39, 95% CI: 0.23 to 0.66), both at 21 months. In each case, the effect of circumcision was protective and significant and in the same range as the calculated estimate (see ANRS 1265 in Analysis 1.1) and reported estimate (see ANRS 1265 in Analysis 1.4) for this study.
At 12 months the combined risk difference in HIV infection was ‐0.83% with 95% CI from ‐1.25% to ‐0.41% between circumcised and uncircumcised men with minimal statistical heterogeneity (χ² = 1.62, df = 2; p = 0.44; I² = 0%) (see Analysis 1.6). At the end of the studies (21 or 24 months), the risk difference dropped further to ‐1.80% with 95% CI from ‐2.48% to ‐1.12% with minimal statistical heterogeneity (χ² = 1.00, df = 2; p = 0.61; I² = 0%) (see Analysis 1.6). This can be interpreted as a significant absolute risk reduction of 0.83% at 12 months and of 1.80% at 21 or 24 months, following circumcision. The NNT calculated from the above figures (at 21 or 24 months) is 56 (95% CI: 41 to 90). Circumcision would prevent 17 HIV infections (95% CI: 11 to 24) over two years per 1000 men (for the combined observed control risk of the populations in the three trials).
Secondary outcomes
We combined sexual behaviour results at 12 months (see Analysis 1.2) and at 21 or 24 months (see Analysis 1.3). from the Bailey 2007 and Gray 2007 trials, where available. The ANRS 1265 trial reported only IRRs from adjusted analysis.
At 12 months (Analysis 1.2) as well as 24 months (Analysis 1.3), we obtained combined risk ratios (RR) with 95% confidence intervals for behavioural outcomes. At both time points the effects for two or more partners, non‐marital partner, casual last contact, inconsistent or no condom use, alcohol with sex ,and transactional sex were not significant. At 12 months, the combined RRs for sexually active (Bailey 2007 and Gray 2007) and any unprotected sex (only Bailey 2007) were not significant at, respectively, 1.02 (95%CI: 1.00 to 1.04) and 1.08 (95%CI: 1.00 to 1.17), but the direction of the effect indicates a relative increase in risky behaviour of 2% and 8%, respectively, and possible disinhibition among circumcised men. These two estimates remained exactly the same at 24 months. Only for two or more partners (at 12 as well as 24 months) was there statistical heterogeneity between the trial results (χ² = 2.20, df = 1; p = 0.14 in both analyses) with the degree of heterogeneity quantified by the I² at 55% in both analyses. Thus the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is moderate.
In the ANRS 1265 trial, number of sexual contacts greater than five was recorded in the three months preceeding the first three‐month visit and in the nine months preceeding the 12‐ and 21‐month visits. In an adjusted model, the IRR for men who were circumcised versus men in the control arm was 1.61 with 95% CI: 0.90 to 2.88 and was not statistically significant (p = 0.11). In the same model, the IRR for at least one sexual contact not protected by condom was not significant at 1.02 (95% CI: 0.57 to 1.83; p = 0.95). The mean number of sexual contacts at the 12‐month visit was 5.9 in the circumcision group versus 5 in the control group, which was a statistically significant difference (p < 0.001). This difference remained statistically significant at the 21‐month visit (7.5 versus 6.4; p = 0.0015).
Adverse effects
Each trial recorded adverse events including events considered related and not related to circumcision. In ANRS 1265, however, only adverse events for the circumcised group were reported.
In ANRS 1265, 60 (3.8%) adverse events were recorded during the first month post surgery for the 1 568 participants who were circumcised; this included the 73 HIV‐positive men who also were circumcised and not included in the final analysis. Adverse events were categorised according to event and not severity. No deaths were recorded and the most common adverse events were pain (31.7%), excessive bleeding (15%), swelling or haematoma (16.7%), and problems with appearance (15%). Other events recorded were infection (5%), damage to the penis (6.7%), insufficient skin removed (6.7%), delayed wound healing (3.3%), and other cause not defined (8.3%). At 21 months post‐circumcision, 11 adverse events were noted in the 1 131 men returning for their 21‐month visit. These included problems with urinating (3/1131), problems with appearance (4/1131), and mild or moderate erectile dysfunction (4/1131).
In Bailey 2007, 24 adverse events were recorded as possibly, probably, or definitely related to circumcision in 23 (1.7%; 95%CI: 1.2 to 2.6) of 1334 participants. None of these events were classified as serious and included post‐operative bleeding (5), infections (5), wound disruptions (4), delayed healing (3), swelling at the incision site (2), convulsion following anaesthetic (1), wound at base of penis (1), pubic abscess (1), folliculitis (1), and erectile dysfunction (1). All events resolved within hours or days except for the erectile dysfunction—the investigators note that erectile dysfunction post‐randomisation occurred with an incidence of 1.5% in the circumcised group and at 1.0% in the control group. This was not statistically significant (p = 0.24). A total of 10 154 unrelated adverse events were recorded among 71% of participants. Of those events classified as serious, 17 occurred in 16 men in the intervention group and 15 in 14 men in the control group. Marginally statistically significant differences in abdominal or gastrointestinal conditions were noted in the control group (p = 0.047), and the incidence of balanitis, phimosis, and paraphimosis was statistically significantly higher in the control group (p < 0.0001).
In Gray 2007 there were 178 adverse events in the 2 328 circumcisions that were carried out, with five events classified as severe, including wound infection (1), haematoma requiring re‐exploration and ligation of active bleeding vessels (2), wound disruption (1), and post‐operative herpetic ulceration not involving the surgical wound (1). An additional 79 moderate events and 94 mild events related to surgery were recorded. All severe and moderate events were managed and resolved. Overall, the number of all adverse events reported was 1 391 in the intervention group and 1 320 in the control group (56% versus 52%; p = 0.083). Of the events in the control group, none were considered to be related to the trial; 87% of events in the circumcision group were considered to be related to the trial.
Discussion
Three large African RCTs assessing the effectiveness of male circumcision in preventing HIV acquisition in sexually active men in the general population were conducted between 2002 and 2006. The results indicate compelling evidence that male circumcision, when conducted using a medical procedure, reduces the acquisition of HIV by heterosexual men by between 38% and 66% over 24 months. Incidence of adverse events is very low, indicating that male circumcision, when conducted under these conditions, is a safe procedure. In the light of the results from all three trials, UNAIDS and the WHO released a press release in March 2007 advocating the inclusion of male circumcision into current HIV prevention measures guidelines in countries with low circumcision rates and generalized epidemics.
Strengths and limitations of our review
We conducted comprehensive searches of both journal and conference databases to ensure that all relevant published and unpublished trials were identified. We did not limit the searches to a specific language. Our ongoing interaction with the investigators of all three trials from before the trials commenced until trial completion, and our regular communication with global policymakers at UNAIDS and the WHO, ensured that we were aware of all trial activity in this field. Furthermore, given the high‐profile nature of the intervention and the complexity of conducting circumcision trials, it is unlikely that our search strategy failed to detect existing current trial evidence. Potential bias in the conduct of our review also was minimised by having two independent researchers extracting data and assessing the methodological quality of each study. This detailed process allows for a thorough assessment of trial conduct and an exploration of the possible biases that may be present in each trial.
Each trial employed early stopping rules considered acceptable statistical practice (Kim 1987). Stopping each of the three trials early, however, resulted in high attrition within each trial, ranging from 30% to 46%. Use of survival analysis, which incorporates the results from all of those who completed the trial and who are censored due to loss to follow‐up or early stopping of the trial, will have reduced the potential attrition bias in each trial. This was done in each trial. It is important to note, however, that in a systematic review (Montori 2005) of RCTs stopped early for benefit, such RCTs were found to overestimate treatment effects. When trials with events fewer than the median number (n=66) were compared with those with event numbers above the median, the odds ratio for a magnitude of effect greater than the median was 28 (95% CI 11 to 73) (Montori 2005, Mills 2006, McCartney 2008). Each of the trials included in our review yielded fewer than 66 events and may thus overestimate the treatment effect; however, the magnitude of effect was consistent across all three trials, which strengthens the evidence in favour of male circumcision.
The review is of aggregate trial data. Published aggregate data from trials can be variable in the completeness of outcome reporting and in definitions of both the intervention and outcomes used between trials. This is particularly relevant where survival analysis has been used for outcome reporting and individuals have been censored at different end points during a trial, as occurred in all three included trials. Ideally we would have conducted a meta‐analysis of the individual participant data (IPD) from each trial. Obtaining IPD allows the outcome and time period for each participant to contribute to an overall analysis, ensuring that valuable information from each participant is incorporated into the analysis. In addition, use of only the aggregate data meant that we were not able to explore subgroup analysis for different risk factors of participants (e.g., marital status, number of sexual partners, age at time of randomisation). During the review process, we contacted each of the principal investigators of the three trials to ascertain their willingness to share their data. A proposal to conduct the IPD under the auspices of The Cochrane Collaboration but with full involvement and authorship of each principal investigator was submitted to UNAIDS. To date, two of the three investigators have agreed to share their data.
Adverse effects and behavioural disinhibition
All three trials assessed adverse effects of the circumcision procedure and found these to be consistently low. It is important to recognise that the circumcision was conducted as a medical procedure and within the trial environment. Policy‐makers wishing to implement circumcision as a preventive measure against acquisition of HIV will need to consider that in many countries, male circumcision is practiced as part of the rites of initiation into manhood, with the circumcision procedure conducted by traditional healers who are not trained in the aseptic surgical technique (Horizons 2000). Adverse effects following traditional circumcisions can be high (Peltzer 2008). In a 2004 study of both traditional and medical circumcisions performed in Kenya, rates of adverse events following traditional circumcisions was 35% (Bailey 2008). Notably the rate for medical circumcisions was also high at 18%. The investigators speculate that the high rate following medical circumcision is due to a lack of knowledge about wound care and identification of complications in this setting. Promotion of male circumcision thus must be explicit that the procedure be conducted by an appropriate and skilled practitioner in a sterile clinic environment and must include education about post‐operative care. Inclusion of medical procedures into traditional customs is a potential solution, but further exploration of this topic is beyond the scope of this review.
In the South African trial (ANRS 1265), men in the circumcised group practised more risky sexual behaviours following the procedure than did those in the control group. Circumcised men in the Kenyan (Bailey 2007) and Ugandan (Gray 2007) trials were consistently more sexually active than uncircumcised men, and Kenyan circumcised men (Bailey 2007) consistently engaged in more unprotected sex. These more risky behaviours could indicate possible disinhibition among circumcised men. Despite this increase in risky behaviour, however, circumcision remained protective for men within the 24‐month trial period, but we do not know whether this protective effect persists in the long term. In a partner trial to Gray 2007, 1 015 HIV‐infected men were randomised to immediate or delayed male circumcision for two years, and their partners were invited to participate in the study (Wawer 2008). Of 165 (1.5% of the initial 1 015 HIV‐infected men) married couples who were followed for two years, 13.8 percent of the women partners of men who were circumcised became infected with HIV compared with 9.6 percent of the women partners of men who were not circumcised. This trend toward higher infection rates among the male‐circumcised couples was too small to be considered statistically significant, but the investigators warn that male circumcision has no direct HIV benefits to women, and potentially, an increased risk of transmission with early resumption of sex. As recommended by UNAIDS and the WHO (UNAIDS and WHO 2007), promotion of male circumcision at a country level must clearly present circumcision as partly protective for the male partner and continue to advocate other preventive measures, including being faithful and using condoms consistently. Counselling during and after the procedure should be clear and consistent in this regard.
Authors' conclusions
Implications for practice.
Male circumcision can be considered as an effective measure to partly prevent HIV acquisition in heterosexual men. Current evidence is lacking for whether it also confers protection for women. Policy‐makers can consider implementation of male circumcision as part of prevention measures if considered feasible and socially and culturally acceptable for local conditions.
Implications for research.
Research on the effectiveness of male circumcision for preventing HIV acquisition in heterosexual men is complete. Future research must focus on the effects of male circumcision on the women partners of HIV‐infected circumcised men and whether it is protective, neutral, or harmful for women partners. This could be achieved in a cohort study. Other studies should focus on the feasibility of implementing the procedure into different contexts, the social and cultural issues regarding implementation, and the cost‐effectiveness of such implementation. The effects of male circumcision on HIV transmission during anal intercourse, both in men who have sex with men and between men and women, remains unclear and should also be a focus of future research. Inclusion of male circumcision into current HIV prevention measures guidelines is warranted, with further research required to assess the feasibility, desirability, and cost‐effectiveness of implementing the procedure within local contexts.
Feedback
Feedback from Dr. Jim Thornton, 30 April 2013
Summary
Comment: The conclusions; "There is strong evidence that medical male circumcision reduces the acquisition of HIV by heterosexual men"; "research on the effectiveness of male circumcision for preventing HIV acquisition in heterosexual men is complete. No further trials are required to establish this fact" seem too strong given the trial evidence.
The review authors noted a number of potential sources of bias, which probably don't matter much. But they also noted that two of the three trials had a high risk of inadequate allocation concealment and the third unclear allocation concealment. This matters because there is empirical evidence that inadequate allocation concealment is the most important source of bias in randomised trials (Schulz et al 1995).
The Cochrane Handbook states:
"An early example [.] (Schulz 1995) demonstrated that trials in which randomization was inadequately concealed or inadequately reported yielded exaggerated estimates of intervention effect compared with trials reporting adequate concealment [.]."
"one of the most important potential biases in randomized trials, namely allocation concealment".
"A pooled analysis of seven methodological studies found that effect estimates from trials with inadequate concealment of allocation or unclear reporting of the technique used for concealment of allocation were on average 18% more 'beneficial' than effect estimates from trials with adequate concealment of allocation (95% confidence interval 5 to 29%) (Pildal 2007)."
The effect size seen in the present review is the best estimate at present, and it is reasonable that policy makers act on it until further data become available. But it is surely not justified to state that the evidence is "strong", or that "research is complete and no further trials are required", when the only trials available were all at high or unclear risk of the empirically most important source of bias.
Schulz KF; Chalmers I; Hayes RJ; Altman DG. Dimensions of Methodological Quality Associated With Estimates of Treatment Effects in Controlled Trials JAMA. 1995; 273(5):408‐412
Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. Int J Epidemiol. 2007 Aug;36(4):847‐57.
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Thank you for your comment. We appreciate that you have read our review so carefully.
As you identify, allocation concealment is an important measure of methodological quality.
Recent empirical work has been published on the effects of allocation concealment on effect estimates [Savovic J et al. Influence of Reported Study Design Characteristics on Intervention Effect Estimates From Randomized, Controlled Trials. Ann Intern Med. 18 September 2012;157(6):429‐438]. Results from 1292 trials from 146 informative meta‐analyses, found that intervention effect estimates were exaggerated by on average 7% in trials with inadequate or unclear allocation concealment (ROR 0.93, 95% Credible Interval 0.87 to 0.99).
In our review it is likely that the magnitude of any bias caused by the limitation of inadequate allocation concealment is much smaller than the observed intervention effect seen in the three trials (Incidence risk ratio = 0.46 at 21 or 24 months [95% CI: 0.34 to 0.62]).
When making our interpretation of the results from the three trials in this review we took into consideration the totality of the evidence rather than only focusing on one dimension. These factors are:
Strength and direction of the overall effect estimate
The consistency of results across all three trials
The large sample size
The use of suitable early stopping rules and suitable survival analysis
Methodological quality of the trials including blinding, sequence generation and allocation concealment, and attrition
Although allocation concealment was inadequate in two trials, the third trial in which this was rated as unclear (Bailey 2007), used opaque envelopes with age stratum, envelope number and randomisation identity number printed on outside of envelope. The next envelope was used based on the next envelope number for the participant’s age stratum. The authors failed to report that the envelopes were sealed and so we rated this as unclear according to the Cochrane Handbook; however it is important to note it is rated as ‘unclear’ and not ‘inadequate’ and it is equally possible that allocation was adequately concealed. This trial also shows results consistent with the two other trials, supporting our decision to rate the evidence as ‘strong’.
We have amended the statement ‘no further trials are required’ and under Implications for Research, we now state: 'Inclusion of male circumcision into current HIV prevention measures guidelines is warranted, with further research required to assess the feasibility, desirability, and cost‐effectiveness of implementing the procedure within local contexts.'
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2013 | Feedback has been incorporated | Feedback, and author response |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 16 March 2011 | Review declared as stable | This review will no longer be updated. |
| 12 August 2009 | Amended | Feedback added. |
| 18 February 2009 | New search has been performed | Updated: Conclusions changed. |
| 18 February 2009 | New citation required and conclusions have changed | Update of previous review of observational studies; now contains data from three large RCTs. Evidence conclusive and no further updates required. |
| 3 March 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Each of the trial Principal Investigators for sharing information and progress Gail Kennedy, George Rutherford, and Tara Horvath of the HIV/AIDS Group for their ongoing support and advice Joy Oliver for research assistance and her encouragement on a daily basis Karishma Busgeeth for providing the search strategy and results Elizabeth Pienaar for assistance with RevMan 5 Dr Cate Hankins from UNAIDS for her strategic advice and assistance The staff of the South African Cochrane Centre for their interest and enthusiasm The Institute for Maritime Technology (IMT) for encouraging Martie in her involvement with The Cochrane Collaboration
Data and analyses
Comparison 1. Circumcision versus No circumcision in General Population groups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV incidence ‐ calculated from data | 3 | Incidence Risk Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Months 0 ‐ 12 | 3 | 10908 | Incidence Risk Ratio (Random, 95% CI) | 0.50 [0.34, 0.72] |
| 1.2 Months 0 ‐ 24 | 3 | 10908 | Incidence Risk Ratio (Random, 95% CI) | 0.46 [0.34, 0.62] |
| 2 Sexual behaviour: Months 0 ‐ 12 | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Sexually active | 2 | 6959 | Risk Ratio (IV, Random, 95% CI) | 1.02 [1.00, 1.04] |
| 2.2 Two or more partners | 2 | 6959 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 2.3 Non‐marital partner | 1 | 4503 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.92, 1.10] |
| 2.4 Casual last contact | 1 | 2021 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.81, 1.15] |
| 2.5 Inconsistent / No condom use | 2 | 6567 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.99, 1.09] |
| 2.6 Any unprotected sex | 1 | 2455 | Risk Ratio (IV, Random, 95% CI) | 1.08 [1.00, 1.17] |
| 2.7 Alcohol with sex | 1 | 4503 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.90, 1.03] |
| 2.8 Transactional sex | 1 | 4503 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.65, 2.33] |
| 3 Sexual behaviour: Months 0 ‐ 24 | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Sexually active | 2 | 6959 | Risk Ratio (IV, Random, 95% CI) | 1.02 [1.00, 1.04] |
| 3.2 Two or more partners | 2 | 6959 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 3.3 Non‐marital partner | 1 | 4503 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.92, 1.10] |
| 3.4 Casual last contact | 1 | 2021 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.81, 1.15] |
| 3.5 Inconsistent / No condom use | 2 | 6567 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.99, 1.09] |
| 3.6 Any unprotected sex | 1 | 2455 | Risk Ratio (IV, Random, 95% CI) | 1.08 [1.00, 1.17] |
| 3.7 Alcohol with sex | 1 | 4503 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.93, 1.06] |
| 3.8 Transactional sex | 1 | 4503 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.65, 2.33] |
| 4 HIV incidence ‐ reported IRR | 3 | Incidence Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Months 0 ‐ 12 | 1 | 3128 | Incidence Risk Ratio (Random, 95% CI) | 0.46 [0.19, 1.12] |
| 4.2 Months 0 ‐ 24 | 3 | 10908 | Incidence Risk Ratio (Random, 95% CI) | 0.41 [0.30, 0.56] |
| 5 HIV incidence ‐ Per Protocol (as treated) analysis | 3 | Incidence Risk Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Months 0 ‐ 24 | 3 | 10908 | Incidence Risk Ratio (Random, 95% CI) | 0.34 [0.24, 0.47] |
| 6 HIV infection probability (%) | 3 | Risk Difference (%) (Random, 95% CI) | Subtotals only | |
| 6.1 Months 0 ‐ 12 | 3 | 10908 | Risk Difference (%) (Random, 95% CI) | ‐0.83 [‐1.25, ‐0.41] |
| 6.2 Months 0 ‐24 | 3 | 10908 | Risk Difference (%) (Random, 95% CI) | ‐1.80 [‐2.48, ‐1.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ANRS 1265.
| Methods | Randomized controlled trial conducted in Orange Farm, a semi‐urban settlement close to the city of Johannesburg in South Africa. The trial was conducted between July 2002 and 30 April 2005, a total of 2 years and 10 months. 3,274 young men from the general population were enrolled into the trial after recruitment at community meetings and underwent a pre‐screening visit at the investigation centre which was established for the purposes of the study 200 m away from a local public clinic. The study was approved by the University of Witwatersrand Human Research Ethics Committee (Medical) and the Scientific Commission of the French National Agnecy for AIDS Research (ANRS). Participants signed written informed consent. | |
| Participants | Men aged 18 to 24 years wishing to be circumcised who resided in the Orange Farm area and who agreed to attend three follow‐up visits at the investigation centre and undergo a genital examination. Exclusion criteria included men with genital ulcerations who were referred for treatment and were able to enrol after successful treatment of the ulcerations; men who were circumcised; and men with contra‐indications to medical circumcision | |
| Interventions | INTERVENTION GROUP: Standardised forceps‐guided methods widely used in South Africa. Circumcision conducted by one of three local general practitioners experienced in male circumcision in their surgeries. CONTROL GROUP: Participants were asked to wait until the end of the trial before being circumcised. Follow‐up comprised attendance at the investigation centre where participants completed a questionnaire, underwent a genital examination, had blood taken, and received individual counseling. Three visits took place after the screening visit—at month three, month 12, and month 21. | |
| Outcomes | HIV‐1 established by an ELISA screen plus two confirmatory tests. All three tests were required to be positive in order to be classified as HIV‐positive. All other test combinations were classified as negative. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomisation method described. |
| Allocation concealment (selection bias) | High risk | Envelopes described as "sealed." Participants were asked to select one envelope from a basket of 10, after which the envelope was replaced by the next sequential envelope from a set of pre‐prepared envelopes that contained five for the control and five for the intervention arm, suggesting use of blocking, although this is not clearly stated. The final numbers between the two groups differ by 34, suggesting that imbalances occurred with this method of randomisation and allocation. As the process allowed participants to pick envelopes, this would not ensure that the envelopes were opened one at a time and used in order. Therefore, the procedure must be graded as inadequate. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The samples were sent to the laboratory identified only by the participant number. Participants were not blinded. The randomisation group was not available to study personnel responsible for counselling or collecting information during participant visits. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Randomisation included HIV‐positive men who were retained in the trial but excluded from the analysis (4.5% of the total men randomised). Loss to follow‐up, including those who were HIV‐positive, was 30.4% for the circumcised group and 33.4% for the uncircumcised group. Survival analysis was used to incorporate censored outcome data in the analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol clearly stated that HIV incidence was the primary outcome. |
| Other bias | High risk | Trial stopped early in April 2005. At final follow‐up, the total follow‐up analysis included an estimated 63% of the total number of anticipated person‐years. The Lan‐DeMets alpha spending function was used to determine whether to terminate the trial early. |
Bailey 2007.
| Methods | Randomized controlled trial conducted in Kisumu, the capital city of Nyanza Province, Kenya. Trial enrollment commenced on 4 February 2002 and went through to 6 September 2005. The trial was stopped on 12 December 2006 and ran for a total of 4 years and 9 months. 2 784 young men from the general population were enrolled into the trial after recruitment via local newspapers, radio, flyers, and street shows. Public and private clinics were enlisted to refer patients with sexually transmitted infections and recruitments from local youth organizations. The trial received ethics approval from Kenyatta National Hospital Ethics and Research Committee, the University of Illinois Institutional Review Board number 3, the Research Triangle Institutional Review Board number 1, and the University of Washington Institutional Review Board. Written informed consent was required. | |
| Participants | Men aged 18 to 24 years, who were HIV‐negative, sexually active, and uncircumcised and lived in Kisumu and had no plans for moving for at least two years, who were able to give consent and had Hb greater than 90 g/L. Exclusion criteria were men who were HIV‐positive, whose foreskin covered less than half of the glans, who suffered from bleeding disorders, who had a high prothrombin index, or medical contraindications to surgery or an absolute indication for circumcision. | |
| Interventions | INTERVENTION GROUP: Surgical Krieger forceps‐guided method under local anaesthesia conducted by a study clinician in the study clinic. Post‐circumcision visits comprised checking of the wound at 3, 8, and 30 days and participants were counseled to refrain from sexual activity for 30 days after the procedure. CONTROL GROUP: Participants were asked to wait until the end of the trial before being circumcised. Follow‐up comprised attendance at the investigation centre at 1, 3, 6, 12, and 24 months. Participants were asked questions about their sexual activity, underwent a genital examination, and had HIV counselling and testing |
|
| Outcomes | HIV‐1 and HIV‐2 established by using the Determine HIV 1/2 rapid testing on finger‐prick blood. If positive on two tests or if discordant, serum was sent for a double ELISA. Participants were deemed to be HIV‐positive if both ELISA tests were positive. Discordant tests were indeterminate and participants were asked to return for additional testing from one to six months later, depending on the visit. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not clearly reported. Reported as "randomly permuted blocks of size 10 and 20 within age group strata 18 to 20 and 21 to 24." |
| Allocation concealment (selection bias) | Unclear risk | Described as concealment by opaque envelopes with age stratum, envelope number and randomisation identity number printed on outside of envelope. The next envelope was used based on the next envelope number for the participant's age stratum selected. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | HIV testing conducted in a laboratory but unclear whether assessors were blinded. Participants and providers were not blinded, but nurses providing voluntary counselling and testing (VCT) and conducting the sampling for testing of participants were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 24 months the overall loss to follow‐up was 46% (1 283/2 784) of men who had not completed the trial. Loss to follow‐up not reported for circumcision and no circumcision groups. Survival analysis used to incorporate censored outcome data in the analysis. |
| Selective reporting (reporting bias) | Low risk | The protocol clearly stated that HIV incidence was the primary outcome. |
| Other bias | High risk | Trial stopped early in December 2006. At the third interim analysis done in October 2006, a reported 87% of the follow‐up experience had been accrued. Formal statistical monitoring used the Lan‐DeMets spending function to minimise the chance of inappropriate premature trial termination. |
Gray 2007.
| Methods | Randomised controlled trial conducted in Rakai, Uganda, in a rural setting. The trial was conducted starting August 2002 and stopped on the 12 December 2006, a total of 4 years and 3 months. 4,996 men from the general population, aged 15 to 49 years, were enrolled into the trial. No detail is provided regarding the method of recruitment into the trial. The communities were stratified but no details are provided regarding the stratification. The trial received ethical approval from the Science and Ethics Committee of the Uganda Virus Research Institute in Uganda and from the Committee for Human Research at the Johns Hopkins University and the Western Institutional Review Board in the United States, and participants provided written informed consent. | |
| Participants | Men aged 15 to 49 years, HIV‐negative, uncircumcised, and who agreed to receive their HIV results via VCT. Exclusion criteria included men who were HIV‐positive, had anatomical urological abnormalities or medical contra‐indications to surgical circumcision. Men who were HIV‐positive or declined to receive their HIV results were enroled in a complementary trial. | |
| Interventions | INTERVENTION GROUP: Circumcision "sleeve procedure" was conducted by trained and certified medical doctors in well‐equipped operating theatres in the trial surgical facility. Post‐operative follow‐up visits were scheduled at 24 to 48 hours (at the surgical facility), and at 5 to 9 days and 4 to 6 weeks at mobile clinics in the community. CONTROL GROUP: Participants were asked to wait 24 months before being offered circumcision. Follow‐up for both groups comprised attendance at mobile clinics in the community at 4 to 6 weeks and 6, 12, and 24 months. At each visit, the participants completed a questionnaire, underwent a genital examination, had blood, urine, and penile swabs taken and received HIV counselling and testing. | |
| Outcomes | HIV‐1 established by two ELISA tests and for discordant results, a confirmatory Western blot. For those who sero‐converted during follow‐up, the previous negative sample and in selected cases (not detailed) the first positive sample, were tested by polymerase chain reaction (PCR). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Blocks of 20 were used. |
| Allocation concealment (selection bias) | High risk | Envelopes described as "opaque.". Blocks of 20 envelopes were stratified on community, with no report of how many communities were involved. Participants were asked to select one envelope from a box of 20, after which the envelope was replaced by the next envelope from the next batch for that community. The trialists recognise that this procedure resulted in temporary imbalances between study groups, thereby reducing the blocking effect. As the process allowed participants to pick envelopes, this would not ensure that the envelopes were opened one at a time and used in order. Therefore the procedure must be graded as inadequate. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | HIV testing conducted in a laboratory, but unclear whether assessors were blinded. Participants and providers were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months overall, reported loss to follow‐up was 9%. At 24 months, the overall loss to follow‐up was 60.5% due to the early stopping of the trial. Loss to follow‐up was not reported according to circumcision and no circumcision groups. Survival analysis was used to incorporate censored outcome data in the analysis. |
| Selective reporting (reporting bias) | Low risk | The protocol clearly stated that HIV incidence was the primary outcome. |
| Other bias | High risk | Trial stopped early in December 2006 when reportedly about 73% of anticipated total person‐time had been accrued. Formal statistical monitoring used the Lan‐DeMets group sequential approach with a O'Brien‐Fleming type alpha spending function to minimise the chance of inappropriate premature trial termination. |
Differences between protocol and review
In the protocol to the initial review of observational studies we had a secondary objective to assess the feasability of conducting individual participant data analysis (IPD). We removed this as an objective from this updated review of randomised controlled trials and addressed this under the limitations section of the Discussion section.
Contributions of authors
NS and MM conducted data extraction and quality assessment, entered the data, and jointly wrote the first draft of the review. MM conducted the analysis. JD conducted data extraction, meta‐analyses, and quality assessment. JV provided feedback into the overall results and their interpretation.
In the previous review of non‐randomised studies:
JD and MM developed the eligibility form. NS, PW, ME and NL provided input to the final version.
NS and MM developed the first draft of the data extraction forms. JD, PW, ME and NL contributed to the finalising of the data extraction forms.
MM, JD and PW developed the first draft of the quality assessment instruments. ME, NL, NS and JV were involved in further developments and finalising of the quality assessment instruments.
HW provided copies of, and references to, many relevant published studies and contributed insight gained from previous experience in the field.
NL provided the EMBASE search as well as articles that were difficult to obtain.
NS and MM conducted study selection and data extraction and JV assisted when difficulties arose.
NS, MM and JD conducted the quality assessment and all reviewers assisted with issues arising.
NS contacted authors for additional information.
NS entered the data and MM conducted the REVMAN analysis. JD and MM conducted the meta‐regression and calculated the Higgins statistics.
SW contributed to the individual participant data (IPD) section
NS wrote the first draft of the text of the review. All reviewers contributed to the final text of the review.
NS co‐ordinated the writing and revision of the protocol and review.
Sources of support
Internal sources
South African Cochrane Centre, Medical Research Council, South Africa.
HIV/AIDS Mentoring Programme, South Africa.
Institute for Maritime Technology, South Africa.
External sources
No sources of support supplied
Declarations of interest
Nandi Siegfried, Martie Muller, Jimmy Volmink, and Jon Deeks were authors on the initial Cochrane review of observational studies (Siegfried 2003; Siegfried 2005). None have been involved in other previous research into the subject and are not currently involved in other related research.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
ANRS 1265 {published data only}
- Auvert B, Taljaard D, Lagarde E, Sobngwi‐Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2005;2(11):e298. [1549‐1676 (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2007 {published data only}
- Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya‐Achola JO. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007;369(9562):643‐56. [1474‐547X (Electronic)] [DOI] [PubMed] [Google Scholar]
Gray 2007 {published data only}
- Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire‐Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007;369(9562):657‐66. [1474‐547X (Electronic)] [DOI] [PubMed] [Google Scholar]
Additional references
Bailey 2001
- Bailey RC, Plummer FA, Moses S. Male circumcision and HIV prevention: current knowledge and future research directions. The Lancet Infectious Diseases 2001;1:223‐30. [DOI] [PubMed] [Google Scholar]
Bailey 2008
- Bailey RC, Egesah O, Rosenberg S. Male circumcision for HIV prevention: a prospective study of complications in clinical and traditional settings in Bungoma, Kenya. Bulletin of the World Health Organization 2008;86(9):669‐77. [PUBMED: 18797642] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cold 1999
- Cold CJ, Taylor JR. The prepuce. British Journal of Urology 1999;83:34‐44. [DOI] [PubMed] [Google Scholar]
De Vincenzi 1994
- Vincenzi ID, Mertens T. Male circumcision: a role in HIV prevention?. AIDS 1994;8:153‐60. [PubMed] [Google Scholar]
Donoval 2006
- Donoval BA, Landay AL, Moses S, Agot K, Ndinya‐Achola JO, Nyagaya EA, et al. HIV‐1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol 2006;125(3):386‐91. [PubMed] [Google Scholar]
Fleiss 1998
- Fleiss PM, Hodges FM, Howe RS. Immunological function of the human prepuce. Sexually Transmitted Infections 1998;74:364‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgens JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. [Available from www.cochrane‐handbook.org] [Google Scholar]
Horizons 2000
- Horizons. Report: Male circumcision and HIV prevention: Directions for future research. Washington DC: Office of Health and Nutrition, Global Bureau, U.S. Agency for International Development 2000.
Hussain 1995
- Hussain LA, Lehner T. Comparative investigation of Langerhans' cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology 1995;85:465‐84. [PMC free article] [PubMed] [Google Scholar]
Kim 1987
- Kim K, DeMets DL. Design and analysis of group sequential tests based on the type 1 error spending rate function. Biometrika 1987;74:149‐54. [Google Scholar]
Lee‐Huang 1999
- Lee‐Huang S, Huang PL, Sun Y, Kung HF, Blithe DL, Chen HC. Lysozyme and RNAses as anti‐HIV components in Beta‐core preparations of human chorionic gonadotropin. Proceedings of the National Academy of Science (USA). 1999;96:2678‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCartney 2008
- Margaret McCartney. Leaping to conclusions. BMJ 2008;336:1213‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2006
- Mills E, Siegfried N. Cautious optimism for new HIV/AIDS prevention strategies. The Lancet 2006;368:1236. [DOI] [PubMed] [Google Scholar]
Montori 2005
- Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: a systematic review. JAMA 2005;294:2203‐09.. [DOI] [PubMed] [Google Scholar]
Moses 1994
- Moses S, Plummer FA, Bradley JE, Ndiya‐Achola JO, Nagelkerke NJD, Ronald AR. [The association between lack of male circumcision and risk for HIV infection: a review of the epidemiological data]. Sexually Transmitted Diseases 1994;21:201‐10. [DOI] [PubMed] [Google Scholar]
Moses 1998
- Moses S, Bailey RC, Donald AR. Male circumcision: assessment of health benefits and risks. Sexually Transmitted Infections 1998;74:368‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patterson 2002
- Patterson. American Journal of Pathology 2002;161. [Google Scholar]
Peltzer 2008
- Peltzer K, Nqeketo A, Petros G, Kanta X. Traditional circumcision during manhood initiation rituals in the Eastern Cape, South Africa: a pre‐post intervention evaluation. BMC Public Health. 2008;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soto‐Ramirez 1996
- Soto‐Ramirez LE, Renjifo B, McLane MF, Marlink R, O'Hara C, Sutthent R, Wasi C, Vithayasi P, Vithayasai V, Apichartpiyakul C, Auewarakul P, Cruz VP, Chui D‐S, Osathanondh R, Mayer K, Lee T‐H, Essex M. HIV‐1 Langerhans' cell tropism with heterosexual transmission of HIV. Science 1996;271:1291‐3. [DOI] [PubMed] [Google Scholar]
Szabo 2000
- Szabo R, Short RV. How does male circumcision protect against HIV infection?. British Medical Journal 2000;320:1592‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS and WHO 2007
- UNAIDS, WHO. New data on male circumcision and HIVprevention: policy and programme implications; WHO/UNAIDStechnical consultation male circumcision and HIV prevention:research implications for policy and programming. Montreux, Geneva: Joint United Nations Programme on HIV/AIDS and WorldHealth Organization 2007.
Van Howe 1999
- Howe RS. Circumcision and HIV infection: review of the literature and meta‐analysis. International Journal of STD and AIDS 1999;10:8‐16. [DOI] [PubMed] [Google Scholar]
Wawer 2008
- Wawer M, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S, Buwembo D, Ssempijja V, Moulton L, Gray R. Trial of circumcision in HIV+ men in Rakai, Uganda: effects in HIV+ men and women partners. Fifteenth Conference on Retroviruses and Opportunistic Infections. Boston, 2008; Vol. Abstract 33LB.
Weiss 2000
- Weiss HA, Quigley MA, Hayes R. Male circumcision and risk of HIV infection in sub‐Saharan Africa: a systematic review and meta‐analysis. AIDS 2000;14:2361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Siegfried 2003
- Siegfried N, Muller M, Volmink J, Deeks J, Egger M, Low N, et al. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev 2003, (3):CD003362. [DOI] [PubMed] [Google Scholar]
Siegfried 2005
- Siegfried N, Muller M, Deeks J, Volmink J, Egger M, Low N, et al. HIV and male circumcision‐‐a systematic review with assessment of the quality of studies. Lancet Infect Dis 2005;5(3):165‐73. [DOI] [PubMed] [Google Scholar]


