Abstract
Current learning theories are based on the idea that learning is driven by the difference between expectations and experience (the delta rule). In extinction, one learns that certain expectations no longer apply. Here, we test the potential validity of the delta rule by manipulating memory retrieval (and thus expectations) during extinction learning. Adrenergic signaling is critical for the time-limited retrieval (but not acquisition or consolidation) of contextual fear. Using genetic and pharmacologic approaches to manipulate adrenergic signaling, we find that long-term extinction requires memory retrieval but not conditioned responding. Identical manipulations of the adrenergic system that do not affect memory retrieval do not alter extinction. The results provide substantial support for the delta rule of learning theory. In addition, the timing over which extinction is sensitive to adrenergic manipulation suggests a model whereby memory retrieval occurs during, and several hours after, extinction learning to consolidate long-term extinction memory.
Keywords: memory extinction, norepinephrine, hippocampus, contextual fear, β-adrenergic
Modern learning theories are based on the detection of a mismatch between expectations and experience (the delta rule) (1–5), with expectations depending on the retrieval of past experiences. When an organism is naïve, there are no relevant memories (expectations) to retrieve, and learning may occur because of the large difference (delta) between experience and the lack of any expectations. In extinction, however, one learns that a previously acquired association, outcome, or concept no longer applies, at least under some circumstances (6–9). According to learning theories based on the delta rule, the process of extinction begins when an expected event fails to occur. Thus, it is postulated that memory for the expected event must be retrieved during extinction learning. On the other hand, extinction learning could proceed in the absence of retrieval, similar to what may occur for naïve learning; however, this situation would be contrary to learning theories based on the delta rule. Thus, examining the requirement for memory retrieval in extinction learning provides an opportunity to test a fundamental tenet of modern learning theories. This hypothesis has been difficult to examine because it has not been clear how to specifically block memory retrieval under conditions that elicit extinction.
Pavlovian conditioning is a learning paradigm in which neutral stimuli become associated with a salient event (unconditioned stimulus, US) (10). Neutral stimuli become conditioned stimuli (CSs) that predict the US and elicit a conditioned response (CR). This simple form of associative learning has been widely used to study learning and memory in humans and animals (11). A paradigm for rapid Pavlovian learning, fear conditioning results in robust associative memory after a single experience. In one training session, rodents can learn to associate CSs such as the apparatus (context) and a cue (e.g., tone) with shock. Extinction of fear occurs when there is sufficient exposure to a CS in the absence of the US (shock) to reduce fear.
Extinction of conditioned fear could occur by various mechanisms. The simplest model is that CS–US associations degrade after their retrieval in the absence of reinforcement. This model is thought to be unlikely because much evidence suggests that CS–US associations remain intact after extinction (6–9, 12, 13). Instead, extinction is thought to result from the formation of new associations that lead to inhibition of the CR. These associations could form in a number of ways (3, 7, 10, 14). As an example of an extinction model that could be independent of memory retrieval, CS presentation in the absence of the US would result in the formation of excitatory CS–no US associations that ultimately lead to inhibition of the CR (14). Retrieval of the CS–US association upon presentation of the CS alone would normally occur but would not be necessary for extinction. For example, in fear conditioning, the CS-activated representation of shock elicits fear and freezing, whereas the CS-activated no-US representation might signal “safety” and replace freezing with another behavior. A similar process may occur to produce latent inhibition (15), in which pretraining exposure to the CS in the absence of the US reduces freezing to the CS after US association.
In contrast, in two other types of extinction models that are differentiated by whether performance of the CR is required for extinction, learning depends on memory retrieval. These models compare current experience to either expectation or CR performance. In one type of model, performance of the CR in the absence of the US is deemed inappropriate, resulting in the formation of new associations that lead to inhibition of the CR (7, 10). Experiments examining extinction of conditioned eyeblink provide support for this model (16). In the other type of model, expectation of the US in the absence of the US drives new learning regardless of CR performance (also resulting in the formation of new associations that lead to inhibition of the CR) (3, 14). In support of both of these models, presentation of a second conditioned excitor during excitatory CS presentation in the absence of the US results in both greater responding during CS presentation and greater subsequent extinction to the CS (17). Likewise, presentation of a conditioned inhibitor during excitatory CS presentation in the absence of the US results in both lesser responding during CS presentation and lesser subsequent extinction to the CS (18). The results are consistent with either performance of the CR or the magnitude of the difference between expectation and experience being an important factor for determining the subsequent level of extinction.
The aim of this study was to distinguish between these various types of models in part by examining whether extinction depends on memory retrieval. We recently described a specific but time-limited role for β1-adrenergic signaling in contextual memory retrieval by using fear conditioning (19). That finding was initially based on the study of mice (Dbh-/-) genetically altered to lack the endogenous ligands for the adrenergic receptors, norepinephrine and epinephrine (NE/E), through targeted disruption of the dopamine β-hydroxylase gene (Dbh) (20, 21). Experiments with the Dbh-/- mice indicated that NE/E are required for contextual fear memory retrieval but not its acquisition or consolidation. Further, NE/E are not required for all fear memories because tone fear conditioning is intact in the Dbh-/- mice. A role for adrenergic signaling in contextual, but not cued, fear suggested that NE may act to promote retrieval of hippocampus-dependent memory (22, 23). This idea was confirmed when intracerebral infusions demonstrated that β1- adrenergic receptor signaling in the dorsal hippocampus is necessary and sufficient for the retrieval of contextual fear mediated by NE (19). Here, we use fear conditioning in conjunction with pharmacologic and genetic approaches to demonstrate that extinction of contextual fear requires memory retrieval during and after extinction learning. The results provide strong experimental support for the delta rule of modern learning theories and favor mechanisms of extinction learning that do not depend on performance of the conditioned response.
Materials and Methods
Paradigms. Mice habituated to handling were placed in context S for 2 min, after which an 84 dB, 4.5 kHz tone was activated for 30 s. Two seconds before the end of the tone, a 2-s, 1-mA foot shock was delivered, and the mouse was returned to its home cage 30 s after the shock. Extinction and test sessions were for 5 min unless noted (minimal within-session extinction occurs during this time for contextual fear; ref. 19). Cued fear was tested 2 min after placement in a distinct context (T) by activating the training tone or a distinct tone (84 dB, 2.9 kHz). Freezing was scored as the presence or absence of nonrespiratory movement every 5 s.
Treatments. l-threo-3,4-dihydroxyphenylserine (L-DOPS) plus benserazide were administered s.c. 5 h before testing to restore central NE. (-)-Propranolol HCl, CGP 20712A (CGP), and xamoterol hemifumarate were administered s.c. 30 min (xamoterol 60 min) before testing unless noted. Bilateral hippocampal and intracerebroventricular (ICV) infusions were through chronically implanted cannulas one week after surgery. (-)- Atenolol (1 μg per side for hippocampus, 10 μg per side for ICV) or (±)-isoproterenol (2 μg per side) was delivered in 1 μl per side for hippocampus or 2 μl per side for ICV at a rate of 0.4 μl/min. Additional details are published as Supporting Materials and Methods, which are published as supporting information on the PNAS web site.
Analysis. Data were analyzed with statistica 6.0 (StatSoft, Tulsa, OK) with freezing as the dependent variable by using ANOVA as indicated in each figure legend. Post hoc comparisons were made by using Duncan's range test or Student's t test, the latter being adjusted for multiple comparisons by using Bonferroni's method.
Results
Blocking Memory Retrieval Prevents Extinction. Our initial goals were twofold: to determine whether there is any role for adrenergic signaling in extinction learning and, if there were, to determine whether that role correlated with the role of adrenergic signaling in memory retrieval. Adrenergic signaling is necessary for the retrieval of contextual fear memory from ≈2 h to 4 d after training (19). Therefore, the first set of experiments examined whether adrenergic signaling and memory retrieval are required for extinction learning by manipulating adrenergic signaling (and thus contextual fear memory retrieval) during the extinction session 1 d after training. Mice received a single tone-shock pairing in context S (for shock) on day 0 to induce fear of the training tone and context S. The mice were then exposed to context S for 5 min on day 1 without shock to initiate extinction and, again on day 2 for 5 min, to quantitate extinction of contextual fear (assessed by freezing behavior). Two methods were used to manipulate adrenergic signaling and retrieval of contextual fear. One used genetics: Dbh+/- control mice have normal tissue content of NE/E and are phenotypically indistinguishable from wild-type Dbh+/+ mice, whereas Dbh-/- mice have no detectable NE/E (21). The other method was pharmacologic: drugs were administered that activate or block signaling through β1-adrenergic receptors, the receptors through which NE acts to promote contextual fear memory retrieval. When testing intact retrieval of contextual fear (“Ret” in Figs. 1 and 2), Dbh+/- mice (filled symbols) were given saline (Sal); when testing impaired retrieval of contextual fear 1–2 d after training (Block), Dbh+/- mice were given the β1,2-adrenergic receptor antagonist (-)-propranolol (Prop), or the β1 antagonist CGP (19). When testing intact retrieval of contextual fear 1–2 d after training, Dbh-/- mice (open symbols) were given the β1 agonist xamoterol (Xam); when testing impaired retrieval of contextual fear 1–2 d after training, Dbh-/- mice were given Sal (19). Xam was not administered before training of the Dbh-/- mice because NE is not required for acquisition/consolidation of fear conditioning (19). Using both genotypes provided the opportunity to test whether β1 signaling was necessary and sufficient for any role NE/E might have in extinction.
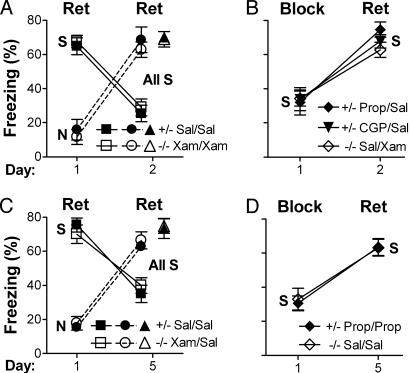
Fig. 1.
Manipulation of β1-adrenergic signaling affects both contextual memory retrieval and long-term extinction of contextual fear. Fear conditioning was performed on day 0 in the training apparatus (context S = shock). Context exposure was performed on day 1 for 5 min (without shock) either in context S (S, squares and diamonds) or a distinct context (N = neutral; circles). Some mice were not exposed on day 1 (triangles). Extinction was assessed on day 2 or 5 in context S. For A and B, control mice (+/- for Dbh+/-, filled symbols) were treated with Sal and NE/E-deficient mice (-/- for Dbh-/-, open symbols) were treated with the β1 agonist Xam (3 mg/kg) when testing intact retrieval (Ret) of contextual fear. Control mice were treated with the β1,2 antagonist Prop (1 mg/kg) or the β1 antagonist CGP (1 mg/kg), and Dbh-/- mice were treated with Sal when testing impaired retrieval (Block). Drug treatments (e.g., Prop/Sal) are for day 1/day 2, respectively. (A) When retrieval was intact during context exposure (day 1) and testing (day 2), extinction (low freezing) was apparent on day 2 for mice exposed to context S but not context N on day 1 (relative to mice not exposed on day 1). (B) When retrieval was impaired during context exposure, extinction was not observed on day 2. C and D demonstrate that contextual fear, its extinction, and the block of extinction last at least 5 d and that the latter is not due to state-dependent drug effects. State-dependent drug effects could be evaluated because contextual memory retrieval does not depend on adrenergic signaling 5 d or longer after conditioning. (C) When retrieval was intact during context exposure and testing, extinction was apparent on day 5 for mice exposed to context S on day 1. (D) When retrieval was impaired during context S exposure on day 1, extinction was not apparent on day 5. For all graphs, there were no significant differences by genotype under equivalent retrieval conditions. Data were analyzed by ANOVA with exposure context, genotype, treatment, and day as factors. For all figures, symbols indicate mean ± standard error, and there were 5–12 mice per group.
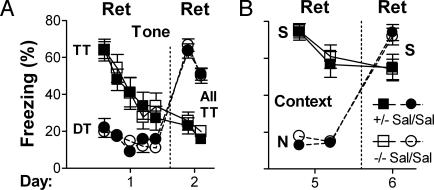
Fig. 2.
Correlation between the requirement for adrenergic signaling in memory retrieval and in extinction. (A) Retrieval and extinction of cued (tone) fear is independent of adrenergic signaling. One group of mice was exposed to the training tone (TT) and another group to a distinct tone (DT) in context T (for tone testing, distinct from context S) on day 1. All mice were exposed to the TT on day 2 in context T. Data points represent 2-min blocks. Within genotype, freezing on day 2 was significantly different depending on tone exposure on day 1 (P < 0.0001). (B) Retrieval and extinction of contextual fear on days 5 and 6 is independent of adrenergic signaling. Data points represent 5-min blocks. Within genotype, freezing on day 6 was significantly different depending on day 5 context exposure (P < 0.05). Importantly, for both graphs, there were no significant differences by genotype. Data were analyzed by repeated-measures ANOVA with exposure condition, genotype, and day as factors.
When mice of both genotypes were tested with intact retrieval in context S on days 1 and 2, freezing was high on day 1 and significantly lower on day 2 (P < 0.01, Fig. 1 A, squares). Freezing on day 2 was also significantly lower than that for mice either not tested on day 1 (P < 0.001, triangles) or exposed to a distinct context not associated with shock (context N for neutral) on day 1 (P < 0.01, circles). The results indicated that extinction of conditioned fear had occurred and that extinction learning depended on reexposure to the context that had been paired with shock. As expected, when retrieval was impaired on day 1, freezing was significantly lower during context exposure on day 1 than when retrieval was intact on day 1 (P < 0.01, Fig. 1 A and B). Importantly, when retrieval was impaired on day 1, freezing was significantly higher on day 2 versus day 1 (P < 0.01, Fig. 1B) and versus day 2 when retrieval was intact on days 1 and 2 (P < 0.001, Fig. 1 A and B). The results indicated that extinction was blocked when adrenergic signaling and memory retrieval were impaired on day 1.
State Dependency. Although the results above are consistent with a role for retrieval in extinction, they are also consistent with context-dependent learning based on the internal state of the organism as determined by drug treatment (6, 8). Extinction learning appeared to be blocked when control mice were treated with a β blocker during exposure and then tested without the drug, or when mutant mice were treated with Sal during context exposure and then tested with Xam. To examine potential state-dependent explanations and to determine whether spontaneous recovery of fear might occur after nonreinforced CS exposure, mice were exposed to context S on day 1 and extinction was assessed on day 5. Day 5 was chosen because adrenergic signaling is not required for retrieval of contextual fear memory 5 d or longer after training (19). Day 5 results were very similar to those for day 2 (Fig. 1 C and D versus A and B; P < 0.01 for comparisons on days 1 and 5 that were significant on days 1 and 2; P > 0.3 for comparing analogous data between days 2 and 5). These data indicated that there was no spontaneous recovery of fear 5 d after context S exposure. In addition, there was no delayed appearance of extinction for mice in which β1 signaling and extinction were blocked on day 1, in contrast to what has been observed after blockade of the NMDA subtype of glutamate receptor (24). Importantly, the results demonstrate that the block in extinction observed on days 1 and 2 was not due to state-dependent effects. Extinction was impaired when Dbh+/- mice were given Prop and when Dbh-/- mice were given Sal on days 1 and 5 (Fig. 1D).
Role for NE/E in Extinction Is Specific to Retrieval. Results from the experiments above are consistent with two hypotheses. In one, adrenergic signaling is required for extinction, because retrieval is required for extinction and NE is required for retrieval. In the other, adrenergic signaling is required for extinction independent of its role in retrieval. To distinguish between these hypotheses, additional experiments were performed by using paradigms in which NE/E are not required for retrieval of fear memory. Adrenergic signaling is not required for retrieval of cued (e.g., tone) fear memory at any time after training (19) or contextual fear memory beginning 5 d after training. When tone fear was extinguished and tested on days 1 and 2, respectively, short- and long-term extinction was equivalent between genotypes (P > 0.8, Fig. 2 A). When contextual fear was extinguished and tested on days 5 and 6, respectively, short- and long-term extinction was also equivalent between genotypes (P > 0.7, Fig. 2B).
One caveat of the above, however, is that longer sessions (10 min) were used to elicit extinction to the tone on day 1 and to the context on day 5 than to the context on day 1 (5 min). To determine whether duration of CS exposure was a factor, mice were exposed to context S or N for 10 min on day 1. Results were analogous to those for 5-min context exposure; blocking retrieval on day 1 impaired extinction on day 2 (P < 0.01; Fig. 5 A and B, which is published as supporting information on the PNAS web site). A second caveat was that less extinction occurred after exposure to context S on day 5 as compared with day 1. To test whether longer exposure leading to greater extinction might reveal a role for NE/E, mice were exposed to context S or N for 25 min on day 5. Considerable short- and long-term extinction was observed but was equivalent between genotypes (P > 0.8, Fig. 5C). In total, the results suggested that adrenergic signaling is only required for extinction when it is required for retrieval.
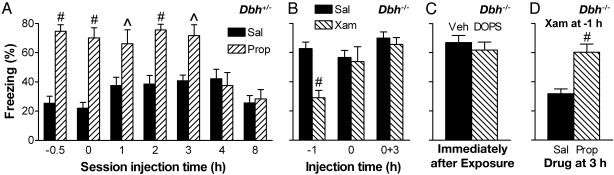
NE/E Are Required During and After Extinction Learning. In the experiments above, adrenergic signaling was either restored in Dbh-/- mice or disrupted in control mice before and up to several hours after context exposure because of the durations of the drug effects. As a result, the critical role for adrenergic signaling could occur during context exposure, as would be expected if adrenergic signaling were important for retrieval, or after exposure, as would be expected if adrenergic signaling were important for the consolidation of extinction. To test the latter, Prop was given to control mice at various times after context S exposure on day 1. Prop impaired extinction retention when administered 0–3 h after the extinction session on day 1 (Fig. 3A). Block of extinction was also observed when CGP was administered shortly after context S exposure, implicating β1 signaling specifically (day 2 freezing 71.4 ± 9.6%, n = 6, P < 0.001). These data indicated that adrenergic signaling is required for the consolidation of extinction.
Fig. 3.
β1-adrenergic signaling is required during and after context exposure for long-term extinction. All mice were conditioned on day 0 and exposed to context S for 5 min on day 1. Results shown are for testing in context S on day 2 with retrieval intact. (A) Effect of blocking β1,2 signaling in control Dbh+/- mice before (-0.5 h) or beginning 0–8 h after context S exposure. The main effect of treatment (P < 0.001) and interaction of treatment by time (P < 0.01) were significant. Prop blocked long-term extinction when given 0–3 h after context exposure, indicating a role for β1-adrenergic signaling in the consolidation of extinction (see text for analogous results with CGP). Sal given 1–4 h after context exposure slightly increased freezing on day 2, possibly due to a small nonspecific disruption of extinction consolidation. (B) Stimulation of β1 receptors in Dbh-/- mice beginning shortly after context exposure does not rescue extinction. Xam was given before (-1 h) or immediately after context exposure (0 h and 0 + 3 h, with a second injection at 3 h for the latter group). The main effect of treatment (P < 0.02) and interaction of treatment by time (P < 0.01) were significant. (C) CNS restoration of NE in Dbh-/- mice beginning shortly after context exposure does not rescue extinction (P > 0.5). Mice were given vehicle (Veh) or 1 g/kg L-DOPS plus 50 mg/kg benserazide (DOPS) immediately after context exposure. (D) The rescue of extinction by stimulation of β1 receptors before context exposure in Dbh-/- mice depends on stimulation of β1 receptors 3 h after exposure. Mice were treated with Xam 1 h before context exposure and either Sal or Prop 3 h after exposure. For all graphs, retrieval on day 2 was facilitated by giving Sal/Xam-treated mice Xam 1 h before testing and by giving Veh/DOPS-treated mice 1 g/kg L-DOPS plus 50 mg/kg benserazide 5 h before testing. Significance comparing treatments within time: ^, P < 0.01; #, P < 0.001. Data were analyzed by ANOVA with treatment and time as factors.
It remained unclear, however, as to whether retrieval during context exposure is required for extinction. To examine this possibility, β1 signaling was activated in Dbh-/- mice by giving Xam immediately after context exposure on day 1. In contrast to results from Xam treatment before context exposure, treatment immediately after exposure did not rescue extinction irrespective of whether a second injection of Xam was given 3 h aftercontext exposure to ensure stimulation at that time as well (P > 0.5, Fig. 3B). To test whether stimulation of adrenergic receptors in addition to β1 might also be required for extinction consolidation after context exposure, central NE was restored in the Dbh-/- mice by giving L-DOPS plus benserazide immediately after exposure (21). This treatment also did not rescue extinction (P > 0.5, Fig. 3C), suggesting that adrenergic signaling during (as well as after) context exposure is required for extinction. If β1 signaling is required at both times, then the results suggested that Xam given to the Dbh-/- mice 1 h before context exposure activated β1 receptors for at least 4 h (i.e., during the critical period 3–4 h after exposure). To test this possibility, Dbh-/- mice were treated with Xam 1 h before context exposure and, with Prop, 3 h after exposure. In this case, extinction was blocked (P < 0.05, Fig. 3D), supporting the existence of two critical periods for β1 signaling, one during context exposure and the other 3–4 h later. It is possible that β1 signaling is also required 0–3 h after exposure. However, due to the duration of drug actions (several hours), that cannot be concluded with certainty from the present data. Therefore, intracerebral infusion experiments (see below) were focused on the established critical periods that occur during exposure and 3–4 h after exposure.
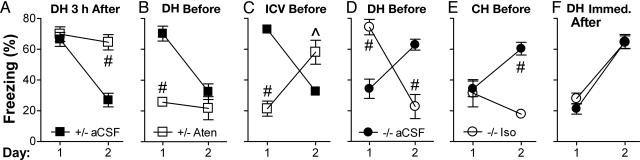
The Hippocampus, Retrieval, and Extinction. Because the role of adrenergic signaling in retrieval of contextual fear is mediated by β1 signaling in the dorsal hippocampus (DH) (19), we predicted that if the roles of adrenergic signaling in extinction of contextual fear were mediated by retrieval, then they would also be mediated by β1 signaling in the DH. To examine this hypothesis, adrenergic drugs were delivered bilaterally to the DH through chronically implanted cannulas. The requirement for β1 signaling 3–4 h after context exposure suggested that retrieval might be required for consolidation of extinction. To further test this idea, DH infusion was performed in control mice to determine whether β1 signaling in the DH was required at this time. (-)-Atenolol (Aten, a β1 antagonist used because it does not readily cross the blood–brain barrier) or artificial cerebrospinal fluid was infused 3 h after context exposure. Aten blocked extinction (P < 0.001, Fig. 4A), analogous to results with systemic Prop (Fig. 3A). Thus, similar to contextual fear memory retrieval, consolidation of extinction requires β1 signaling in the DH.
Fig. 4.
Conditioned responding (freezing) is not required for extinction, and the role of adrenergic signaling in extinction can be mediated by β1 receptors in the DH or CH. Mice were trained on day 0, exposed to context S for 5 min on day 1, and tested in context S on day 2. Infusions were given through chronically implanted cannulas on day 1 at the times indicated. On day 2, control mice were given Sal and Dbh-/- mice were given Xam (both systemically) before testing so that retrieval would be intact. (A) DH infusion of Aten (1 μg per side) in control mice 3 h after exposure blocks extinction on day 2. (B) DH infusion of Aten in control mice 15 min before exposure blocks freezing on day 1 but does not block extinction on day 2. (C) ICV infusion of Aten (10 μg per side) in control mice 45 min before exposure blocks freezing on day 1 and extinction on day 2. (D) DH infusion of Iso (2 μg per side) in Dbh-/- mice 30 min before context exposure on day 1 rescues freezing on day 1 and leads to extinction on day 2. (E) CH infusion of Iso (2 μg per side) in Dbh-/- mice 30 min before context exposure on day 1 does not rescue freezing but leads to extinction on day 2. (F) DH infusion of Iso in Dbh-/- mice immediately after exposure does not lead to extinction on day 2. For all data except F, the interaction of treatment and day was significant (P < 0.01). Significance of comparing treatments within day: ^, P < 0.01; #, P < 0.001. Data were analyzed by ANOVA with treatment and day as factors.
Next, we predicted that blocking retrieval by infusing Aten into the DH before context exposure would also prevent extinction. Surprisingly, it did not (P > 0.2, Fig. 4B). Similarly, infusion of Aten before and 45 min after context exposure failed to block extinction (data not shown). Because these results were unexpected, we examined whether non-retrieval-based effects of Aten infusion might explain the findings. Systemic β-blockers and DH Prop do not block freezing 1 week after training (19), indicating that they affect memory retrieval rather than freezing per se. Likewise, DH Aten did not block freezing 5 d after training (Fig. 6A, which is published as supporting information on the PNAS web site). Further, DH Aten did not cause extinction independent of its effect on retrieval because treatment before context exposure on day 5 did not elicit extinction on day 6. Importantly, the results with DH Aten indicate that a normal level of conditioned responding (freezing) is not necessary for extinction learning.
The results from DH Aten suggested that a site in addition to the DH is capable of mediating extinction of contextual fear and that β1 signaling in either the DH or the other site is sufficient for extinction. To test this possibility, a higher dose of Aten was infused ICV before context exposure to block β1 signaling throughout the CNS. This manipulation blocked freezing on day 1 and extinction on day 2 (P < 0.01, Fig. 4C), analogous to results with systemic β blockers. As expected, ICV Aten did not block freezing 5 d after training (Fig. 6B). Further, ICV Aten did not block extinction independent of its effect on retrieval because treatment before context exposure on day 5 did not prevent extinction on day 6. These results support the idea that the effects of ICV Aten on extinction are the result of its effects on retrieval.
Taken together, the data above provide strong support for the existence of a second brain region capable of mediating extinction through β1 signaling during context exposure. We hypothesized that the DH and the other brain region share some functionality and that more ventral regions of the hippocampus might serve this purpose. To test this hypothesis, (±)-isoproterenol (Iso, a β agonist used because it does not readily cross the blood–brain barrier) was infused into the DH or central hippocampus (CH) of Dbh-/- mice before context exposure. When Iso was infused into the DH, retrieval and extinction were rescued (P < 0.001, Fig. 4D). When Iso was infused into the CH, retrieval (as assessed by freezing) was not rescued but extinction was (P < 0.001, Fig. 4E). The rescue of extinction with CH infusion was not due to nonspecific effects because the same manipulation did not elicit extinction when performed on day 5 (Fig. 6C). The results indicate that β1 signaling in the CH is not sufficient during context exposure for retrieval that results in freezing (ruling out the spread of Iso into the DH at that time) but is sufficient for retrieval that results in long-term extinction.
For the rescue of extinction with DH Iso before context exposure to be consistent with the notion that β1 signaling during and 3–4 h after context exposure is required for extinction retention, β1 signaling and retrieval of contextual fear were predicted to be maintained for at least 4 h after DH Iso infusion. To examine this possibility, Dbh-/- mice were infused with Iso on day 1 and tested for contextual fear 4 h later. As predicted, freezing (72.6 ± 4.8%, n = 7, P > 0.7) was comparable with controls infused with artificial cerebrospinal fluid and to Dbh-/- mice infused with Iso 30 min before testing contextual fear. Thus, rescue of retrieval and extinction with DH Iso before exposure is compatible with the requirement for β1 signaling during and 3–4 h after exposure.
Finally, one concern in the systemic rescue experiments with the Dbh-/- mice (Fig. 3 B and C) was that Xam or L-DOPS given immediately after context exposure might not have rescued extinction because they require ≈1 h to be maximally effective. DH infusions provide a much more rapid delivery of drug to the site mediating retrieval. To determine whether the negative extinction rescue results from the systemic treatments were due to slow latency of onset, Iso was infused into the DH of Dbh-/- mice immediately after context exposure. Similar to results from the systemic treatments, extinction was not rescued (P > 0.9, Fig. 4F). The results provide strong evidence that memory retrieval during and after context exposure is required for extinction.
Discussion
This study has taken advantage of the requirement for β1 signaling in the retrieval of contextual fear to examine the requirement for memory retrieval in extinction learning. The results indicate that β1 signaling is required during and several hours after an exposure to the training context for retention of extinction. The most parsimonious explanation is that memory retrieval is required at both times. The data support the retrieval hypothesis because adrenergic signaling is not required for extinction at times when adrenergic signaling is not required for retrieval (of tone and longer-term contextual fear memory). These observations rule out a critical role for NE/E in the initiation and consolidation of extinction in general and in memory-independent processes that could affect extinction, such as alterations in sensory processing, attention, arousal, fear, or performance (freezing), consistent with prior data on the role of NE/E in memory retrieval (19). A role for memory retrieval in long-term extinction consolidation suggests an interesting potential role for reflection (offline retrieval of a recent experience and comparison of it to prior experience) in this process. This process may be somewhat analogous to the synaptic reentry reinforcement model (25) proposed to explain the requirement for NMDA receptor activation over a period of days to permit long-term memory consolidation (26).
Other studies have suggested a role for adrenergic signaling in extinction, although the nature of the role was not well defined (27–32). Somewhat similar to our results, blockade of NMDA receptors can diminish conditioned responding and prevent long-term extinction (24, 33–38). However, it is often not clear whether blockade interferes with retrieval, US representation, or consolidation of extinction. A role in consolidation is supported by the observation that long-term, but not short-term, extinction is impaired when NMDA receptors are blocked systemically (24, 38). Conversely, augmenting NMDA receptor signaling genetically or pharmacologically facilitates extinction (39, 40).
Of note, one other study has manipulated memory retrieval at the molecular/cellular level to examine its role in extinction (41). In that study, retrieval of short-term olfactory memory was blocked in Drosophila by temperature-dependent inactivation of specific neuronal populations. Interestingly, the results suggested that extinction is mediated by the same neurons in which memory formation occurs. That conclusion is inconsistent with numerous data indicating that extinction results from the formation of new associations rather than erasure of the original associations (6–9, 12, 13). It may be that extinction of short-term memory hastens the loss of that memory and, thus, affects the very changes that mediate the short-term memory. It is possible that such a mechanism may only apply to the extinction of short-term memories. In contrast, our study examines extinction of fear memory that lasts for weeks (unpublished observations).
With respect to the different types of models for extinction learning referred to in the introduction, our results provide strong support for models that depend on the detection of a difference between expectation and experience (the delta rule). In addition, our data argue against performance of the CR being required for extinction learning. The dissociation of CR performance and extinction is in contrast to results from a study examining extinction of conditioned eyeblink. In that study, extinction was blocked when performance of the CR during nonreinforced trials was prevented by temporary inactivation of the facial nucleus (16). However, results from an unpublished eyeblink study examining inactivation of the red nucleus during nonreinforced trials suggests that performance of the CR is not required for extinction.† Dissociation of CR performance and extinction was also described in Drosophila, where inactivation of the mushroom body Kenyon cells blocked conditioned responding for short-term olfactory memory but not extinction (41).
Interestingly, β1 signaling in the DH or CH is able to support retrieval that is sufficient for extinction, but only the DH is able to support retrieval that is sufficient for freezing. This observation suggests that there is functional differentiation along the longitudinal axis of the hippocampus. Such differentiation probably reflects common and distinct afferent and efferent pathways along this hippocampal axis (42).
In summary, our results provide strong support for the theory initially formalized by Rescorla and Wagner (4) (and extended by others to incorporate the idea that extinction learning does not undo original learning, refs. 1–3, 5) that learning is driven by the detection of differences between expectations (which require memory retrieval) and experience (the delta rule). In addition, our results are consistent with some psychotherapies for phobias, anxiety, and stress disorders that seek to extinguish undesirable feelings and responses through repeated retrieval of the CSs that elicit them (43, 44).
Supplementary Material
Acknowledgments
We thank H. Luo for technical assistance and Sumitomo Pharmaceuticals (Osaka) for their generous gift of L-DOPS. This work was supported by National Institutes of Health Grant MH063352 (to S.A.T.).
Author contributions: M.O. performed research; M.O. and S.A.T. analyzed data; S.A.T. designed research; and S.A.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Aten, (-)-atenolol; CGP, CGP 20712A; CH, central hippocampus; CR, conditioned response; CS, conditioned stimulus; DH, dorsal hippocampus; E, epinephrine; ICV, intracerebroventricular; Iso, (±)-isoproterenol; L-DOPS, l-threo-3,4-dihydroxyphenylserine; NE, norepinephrine; Prop, (-)-propranolol; Sal, saline; US, unconditioned stimulus; Xam, xamoterol.
See Commentary on page 9091.
Footnotes
Robleto, K. & Thompson, R. F. (2004) Soc. Neurosci. Abstr. 30, 325.327 (abstr.).
References
- 1.Schmajuk, N. A. & DiCarlo, J. J. (1992) Psychol. Rev. 99, 268-305. [DOI] [PubMed] [Google Scholar]
- 2.Dragoi, V. (1997) Neural Netw. 10, 201-229. [DOI] [PubMed] [Google Scholar]
- 3.Wagner, A. R. & Brandon, S. E. (1989) in Contemporary Learning Theories: Pavlovian Conditioning and the Status of Traditional Learning Theory, eds. Klein, S. B. & Mowrer, R. R. (Erlbaum, Hillsdale, NJ), pp. 149-189.
- 4.Rescorla, R. A. & Wagner, A. R. (1972) in Classical Conditioning II: Current Research and Theory, eds. Black, A. H. & Prokasky, W. F. (Appleton-Century-Crofts, New York), pp. 64-99.
- 5.Pearce, J. M. & Hall, G. (1980) Psychol. Rev. 87, 332-352. [PubMed] [Google Scholar]
- 6.Myers, K. M. & Davis, M. (2002) Neuron 36, 567-584. [DOI] [PubMed] [Google Scholar]
- 7.Rescorla, R. A. (2001) in Handbook of Contemporary Learning Theories, eds. Mowrer, R. R. & Klein, S. B. (Lawrence Erlbaum Associates, Mahwah, NJ), pp. 119-154.
- 8.Delamater, A. R. (2004) Q. J. Exp. Psychol. B 57, 97-132. [DOI] [PubMed] [Google Scholar]
- 9.Bouton, M. E. (2004) Learn. Mem. 11, 485-494. [DOI] [PubMed] [Google Scholar]
- 10.Pavlov, I. P. (1927) Conditioned Reflexes (Oxford Univ. Press, London).
- 11.Maren, S. (2001) Annu. Rev. Neurosci. 24, 897-931. [DOI] [PubMed] [Google Scholar]
- 12.Rescorla, R. A. (1996) Q. J. Exp. Psychol. B 49, 245-258. [Google Scholar]
- 13.Delamater, A. R. (1996) Anim. Learn Behav. 24, 437-449. [Google Scholar]
- 14.Konorski, J. (1967) Integrative Activity of the Brain (University of Chicago Press, Chicago).
- 15.Killcross, A. S., Kiernan, M. J., Dwyer, D. & Westbrook, R. F. (1998) Q. J. Exp. Psychol. B 51, 75-90. [DOI] [PubMed] [Google Scholar]
- 16.Krupa, D. J. & Thompson, R. F. (2003) J. Neurosci. 23, 10577-10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rescorla, R. A. (2000) J. Exp. Psychol. Anim. Behav. Processes 26, 251-260. [DOI] [PubMed] [Google Scholar]
- 18.Rescorla, R. A. (2003) Learn. Behav. 31, 124-132. [DOI] [PubMed] [Google Scholar]
- 19.Murchison, C. F., Zhang, X.-Y., Zhang, W.-P., Ouyang, M., Lee, A. & Thomas, S. A. (2004) Cell 117, 131-143. [DOI] [PubMed] [Google Scholar]
- 20.Thomas, S. A., Matsumoto, A. M. & Palmiter, R. D. (1995) Nature 374, 643-646. [DOI] [PubMed] [Google Scholar]
- 21.Thomas, S. A., Marck, B. T., Palmiter, R. D. & Matsumoto, A. M. (1998) J. Neurochem. 70, 2468-2476. [DOI] [PubMed] [Google Scholar]
- 22.Kim, J. J. & Fanselow, M. S. (1992) Science 256, 675-677. [DOI] [PubMed] [Google Scholar]
- 23.Phillips, R. G. & LeDoux, J. E. (1992) Behav. Neurosci. 106, 274-285. [DOI] [PubMed] [Google Scholar]
- 24.Santini, E., Muller, R. U. & Quirk, G. J. (2001) J. Neurosci. 21, 9009-9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittenberg, G. M., Sullivan, M. R. & Tsien, J. Z. (2002) Hippocampus 12, 637-647. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu, E., Tang, Y. P., Rampon, C. & Tsien, J. Z. (2000) Science 290, 1170-1174. [DOI] [PubMed] [Google Scholar]
- 27.Cain, C. K., Blouin, A. M. & Barad, M. (2004) Learn. Mem. 11, 179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick, D. A. & Thompson, R. F. (1982) Brain Res. 245, 239-249. [DOI] [PubMed] [Google Scholar]
- 29.Mason, S. T. (1983) Neurosci. Biobehav. Rev. 7, 325-347. [DOI] [PubMed] [Google Scholar]
- 30.Cole, B. J. & Robbins, T. W. (1987) Behav. Neurosci. 101, 476-488. [DOI] [PubMed] [Google Scholar]
- 31.Dunn, L. T. & Everitt, B. J. (1987) Behav. Neurosci. 101, 409-422. [DOI] [PubMed] [Google Scholar]
- 32.Jarbe, T. U., Callenholm, N. E., Mohammed, A. K. & Archer, T. (1986) Physiol. Behav. 38, 495-501. [DOI] [PubMed] [Google Scholar]
- 33.Falls, W. A., Miserendino, M. J. & Davis, M. (1992) J. Neurosci. 12, 854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, H. & Kim, J. J. (1998) J. Neurosci. 18, 8444-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox, J. & Westbrook, R. F. (1994) Q. J. Exp. Psychol. B 47, 187-210. [PubMed] [Google Scholar]
- 36.Kehoe, E. J., Macrae, M. & Hutchinson, C. L. (1996) Psychobiology 24, 127-135. [Google Scholar]
- 37.Baker, J. D. & Azorlosa, J. L. (1996) Behav. Neurosci. 110, 618-620. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, A., Josselyn, S. A., Frankland, P. W., Masushige, S., Silva, A. J. & Kida, S. (2004) J. Neurosci. 24, 4787-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker, D. L., Ressler, K. J., Lu, K. T. & Davis, M. (2002) J. Neurosci. 22, 2343-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang, Y. P., Shimizu, E., Dube, G. R., Rampon, C., Kerchner, G. A., Zhuo, M., Liu, G. & Tsien, J. Z. (1999) Nature 401, 63-69. [DOI] [PubMed] [Google Scholar]
- 41.Schwaerzel, M., Heisenberg, M. & Zars, T. (2002) Neuron 35, 951-960. [DOI] [PubMed] [Google Scholar]
- 42.Naber, P. A., Witter, M. P. & Lopes Silva, F. H. (2000) Ann. N.Y. Acad. Sci. 911, 392-403. [DOI] [PubMed] [Google Scholar]
- 43.Boudewyns, P. A. & Shipley, R. H. (1983) Flooding and Implosive Therapy (Plenum, New York).
- 44.Stampfl, T. G. & Levis, D. J. (1976) in Behavioral Approaches to Therapy, eds. Spence, J. T., Carson, R. C. & Thibaut, J. W. (General Learning, Morristown, NJ), pp. 86-110.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.