Children with severe cerebral palsy are vulnerable to orthopedic complications, particularly in resource-limited settings, which can compound disability. A focus on home-based programs may help to improve their quality of life and participation.
Key Messages
Children with cerebral palsy in resource-limited settings are at increased risk of developing musculoskeletal deformities that diminish their quality of life.
Program managers should equip caregivers to implement basic prevention strategies to help ensure social participation and inclusion.
Policymakers should prioritize scalable and sustainable home-based training and the development of locally produced, low-cost technologies for program implementation.
INTRODUCTION
Cerebral palsy (CP) is a leading cause of childhood motor disability worldwide. Secondary musculoskeletal (MSK) complications of CP are common and can further incapacitate children functionally and restrict their participation in ordinary life. Conventional, comprehensive management approaches for preventing complications of CP require regular access to specialized and coordinated medical interventions, rehabilitation, and equipment, all of which may be scarce or unavailable in resource-limited settings (RLSs).1 In this article, we present the case for a standardized home-based program aimed at preventing secondary MSK complications in children with severe CP living in RLSs.
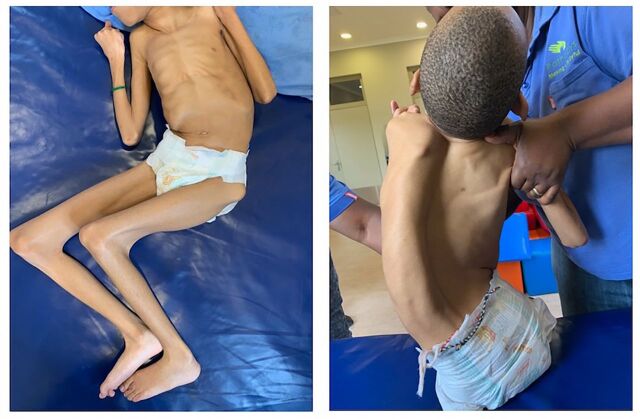
The current birth prevalence of CP in low- and middle-income countries (LMICs) is estimated to be as high as 3.4 per 1000 births, compared to 1.6 per 1000 live births in high-income countries (HICs).2 Due to differences in maternal and perinatal health and the particular risk factors for CP in LMICs, these countries also tend to have higher proportions of children with more severe subtypes of CP,3 including bilateral spastic and dyskinetic presentations4–7 (i.e., more children functioning at the non- or partially ambulant Gross Motor Function Classification System levels [GMFCS III to V]).8 Although their MSK systems usually appear to be radiologically normal at birth, children with such severe neurological impairments are at increased risk of developing MSK complications, including hip displacement, muscle contractures, and scoliosis,9 which often present before the age of 5 years.10 Figure 1 shows the extent of MSK complications in a child aged 11 years visiting an outpatient clinic in an RLS, a presentation that is commonly seen.
FIGURE 1.
Common Musculoskeletal Complications of Severe Cerebral Palsy, as Seen in a Child Aged 11 Years With Severe Cerebral Palsy, Pathways Stimulation Centre, Durban, South Africa. © 2020 Shayne R. van Aswegen
The Functional Impact of Secondary Musculoskeletal Complications in Cerebral Palsy
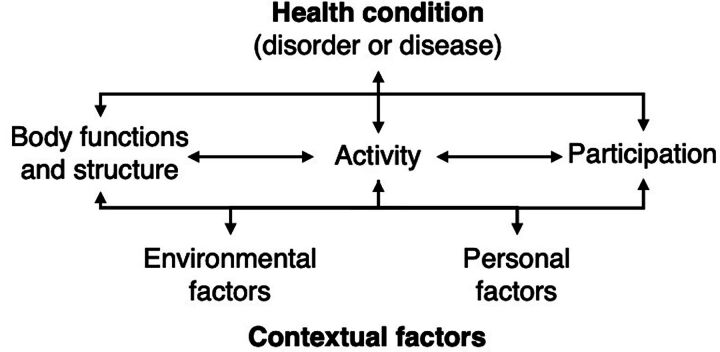
The many ways that MSK complications affect the quality of life of the child and their family are best considered through the holistic lens of the International Classification of Functioning, Disability and Health framework (ICF)11 (Figure 2). Multiple intrinsic and extrinsic factors or domains converge to determine the overall functional status of an individual, notwithstanding the primary diagnosis or disorder, in this case, CP.
FIGURE 2.
International Classification of Functioning, Disability and Health Framework
Source: World Health Organization, 2002.11
For example, as shown in the child in Figure 1, the child has developed secondary MSK deformities (ICF domain of body functions and structure) that profoundly restrict opportunities for age-appropriate activity and participation. Chronic, nociceptive, MSK pain, thought to be at least partially due to joint contractures, has been reported in more than 50% of children with severe spastic and dyskinetic CP.12 Pain and joint range limitations prevent the child from assuming sitting and standing positions in which most functional daily activities occur (ICF domain of activity) (e.g., sharing a family meal around a dining table), even where appropriate assistive devices are available. Therefore, the child will likely default to the lying position for extended periods, which, in turn, predisposes to further spinal and joint asymmetry.13 Daily activities, such as bathing, dressing, and cleaning the perineal area, become challenging for the caregiver and painful and distressing for the child. Safe feeding and drinking can be particularly difficult and may be a prolonged exercise due to the child’s oro-motor impairment and poor head control. Participation in other social spheres (e.g., enrollment in an educational institution) is likely very limited (ICF domain of participation) because the child cannot sit either for transportation or for the duration of the school day. Therefore, an increased focus on early prevention of MSK complications in this population is mandatory to provide activity and participatory opportunities for these children and their families.
THE CHALLENGES OF CEREBRAL PALSY MANAGEMENT IN RESOURCE-LIMITED SETTINGS
The Inadequacy of Current Guidelines
Current practice guidelines for children with CP all originate in HICs, where the disease burden of CP differs from LMICs. Thus, the guidelines neither focus on the severe presentations of CP nor make provision for an RLS, mainly due to a lack of research from these settings. The recommendations they do make presuppose access to regular therapy oversight, pharmacological and surgical management, and the availability of assistive technologies.14,15 Additionally, best therapy guidelines strongly promote early, child-active, task-oriented interventions, according to motor learning theory.16 If a child with CP has already developed serious MSK complications, opportunities for child-active functional gains are limited, even in places where there is access to rehabilitation services. Although surgical correction may be considered in some instances and where funds allow, it is often unfeasible, owing to the scale of remediation required, the poor general health of the child, and lack of access to adequate post-operative support.
Current practice guidelines for children with CP all originate in HICs and neither focus on the severe presentations of CP nor make provision for an RLS.
The Contextual Challenges in a Resource-Limited Setting
Minimizing MSK complications relies on a timely, continuous, integrated approach, which is traditionally coordinated by a team of rehabilitation therapists and orthotists, a scarce commodity in RLSs. For example, in South Africa, a middle-income country with extremely high levels of inequality (Gini Coefficient of >60 in 202417), up to one-third of therapists working in poorly-resourced, rural settings are brand new graduates performing their obligatory “community service” year.18 In addition to the multiple cultural and social barriers they often face, therapists are unlikely to have the training, experience, and tools to manage children with complex long-term health conditions in an RLS, where they are required to “do more with less.”19
In South Africa, CP surveillance is not yet standardized and is frequently either inconsistent or absent in peri-urban and rural areas. We have come across many children in these settings that are simply missed or lost to follow-up. In these cases, the onus is on caregivers to bring their children to health facilities for rehabilitation and checkups, which requires both a mode of appropriate and affordable transport and a level of health awareness and literacy most would not possess.20 Caregivers frequently report that they struggle to cope with the care of their child with CP, and the perceived burden of care often relates to difficulties with activities of daily living (e.g., feeding), lack of knowledge about the condition, and general feelings of inadequacy.21,22 In RLSs, the inexperience and knowledge gaps of primary health workers in identifying the families in need and not feeling equipped to support them compound these issues.23
IMPROVING CARE FOR CHILDREN WITH CEREBRAL PALSY IN RESOURCE-LIMITED SETTINGS
The Benefits of Caregiver-Implemented Programs
Instead of trying to increase coverage of formal rehabilitative health services in these areas, we propose that existing health delivery structures in RLSs be strengthened to deliver a standardized, community-led program that is built around the prevention of MSK sequelae in CP. Recruiting primary caregivers to perform simple, cost-effective interventions in the child’s natural environment is pragmatic because it reduces access barriers (e.g., transport costs) and reliance on the availability of specialized rehabilitative services. Importantly, it is also in line with the concept of family-centered care.24 Supported by trained community health workers (CHWs) already based in RLSs who are, in turn, supported by rehabilitation therapists, caregivers would be empowered to take an active role in decision-making, setting functional goals, and delivering effective interventions. There is already an increasing recognition of the value of home-based intervention programs (HBIPs), even in HICs, where primary caregivers are coached by health professionals to implement essential parts of the therapy program.25,26 Community-based initiatives usually require the establishment of local support networks for implementation and/or sustainability. These groups would benefit the families involved and help to increase knowledge of the condition and address cultural barriers, such as stigma, which are significant in African RLSs.27 This would increase the likelihood of acceptability and program adherence.
We propose that existing health delivery structures in RLSs be strengthened to deliver a standardized, community-led program that is built around the prevention of MSK sequelae in CP.
An example of a successful intervention that demonstrates this type of model is the study undertaken by Zuurmond et al. on the impact of an 11-month community-based caregiver training program on the quality of life of caregivers living in an RLS in Ghana.28 The study found significant improvements in caregiver knowledge and confidence in caring for their child, which included feeding practices, and caregivers reported that their children exhibited improvements in their physical and psychological health.
Preventing Musculoskeletal Complications in Cerebral Palsy
The development of an HBIP should be informed by existing evidence of effective interventions that prevent MSK complications in CP and feasibility for a low-resource setting must be considered. A few comprehensive clinical guidelines exist that specifically focus on preventing MSK complications,29–31 and a few systematic reviews of therapeutic interventions aimed to guide clinical practice.14,15,32 The following nonsurgical, non-pharmacological, and low-tech physical modalities are included in these guidelines and reviews.
24-hour postural management, including lying, adapted seating, and supported standing using appropriate assistive devices.14,15,29–32
Orthotics or splints for upper and lower limbs to prevent loss of joint range of motion and to assist with standing and walking (lower limbs).14,15,29–32
These interventions ideally require continuity and integration into everyday functional routines.33–35 Caregivers are required to move, feed, bathe, position, and dress a child with severe impairment many times a day, and we have observed them struggling to cope. Helping them modify their caregiving practices to promote MSK symmetry and flexibility using these simple techniques should reduce their burden of care and offer greater participation opportunities for the child.
A potential challenge would be the current cost and availability of the required equipment for postural management and splinting, which is significant for children in GMFCS level IV and V.36 Currently, in South Africa, local production of assistive technology is limited, and it is our experience that assistive devices are often imported, rendering them too costly for patients in the public sector.
Lessons Learned From Existing Home-Based Initiatives for Cerebral Palsy in Resource-Limited Settings
Although no empirical studies have specifically evaluated the effectiveness of HBIPs in preventing MSK complications in severe CP, there is evidence that parent-implemented programs are acceptable and effective in improving other child and caregiver outcomes37 when accompanied by adequate professional training and support.38 Qualitative studies from RLSs suggest that, despite poverty and low caregiver education levels, parent-implemented intervention programs positively influence posture, positioning, self-care, feeding, and social function in children with severe CP. The intervention programs also significantly reduce parental stress because parents understand more about the condition and have increased confidence in their handling skills.28,39
From the caregivers’ perspectives, factors affecting their adherence to HBIPs include the presence of ongoing trusting and supportive relationships between the child, caregiver, and health care professionals and the ability to establish context-specific coping strategies.38,40 The program should have an element of fun, be able to be integrated into the family’s daily routines, and provide clear instructions and demonstrations for techniques and activities.41 Health care professionals value effective communication with families, regular joint goal-setting to maximize compliance, and specific training in clinical skills and using resources for CP management in an RLS.42
POLICY RECOMMENDATIONS AND IMPLICATIONS
Principles of Implementation
The success of an HBIP depends on developing a context-specific application; therefore, any initiative should be informed by an a priori analysis of the target population, including all stakeholders (e.g., caregivers, CHWs, and other health care professionals that work with families with children who have CP).
The program should be developed by a team of expert clinicians with appropriate experience in CP management as part of a standardized, comprehensive early intervention care package for this population.
An important prerequisite for the program would be the provision of affordable, appropriate assistive technologies. Thus, the development and/or local production of low-tech, low-cost assistive devices should be prioritized. Appropriate paper-based technology devices have been effective options in RLSs.43 Some devices may even be made or adapted from readily available household equipment, such as PVC piping and recycled material. Regardless of the materials used, equipment should be as simple as possible, durable, and easy to apply in the correct manner.
For effective prevention of deformities, the HBIP should be commenced early in the child’s life, even before an official diagnosis of CP is made because, in LMICs, this process can be delayed by several years. The local health service delivery platform for a particular setting will dictate how the program is initiated, supported, and maintained, but we recommend that infants at high risk of CP or with neurodevelopmental delay or a firm diagnosis of CP be added to an “at risk” register that is kept at the district level or wherever therapist trainers are located and that these caregivers receive the training as early as possible. Considering that the main beneficiaries of this HBIP are children with severe impairment, we would recommend a reevaluation of children at age 2 years when GMFCS level has stabilized;45 however, in most children, GMFCS will not change, especially in more severely affected infants.46
The success of an HBIP depends on developing a context-specific application.
Recommendations for a Home-Based Intervention Program for Severe Cerebral Palsy in Resource-Limited Settings
The Elements of the Program
The daily program should include the following components.
24-hour postural management regimes using variations of lying, sitting, and standing positions according to current clinical guidelines. This should be commenced from infancy or when diagnosis of CP or risk is established. Recommended low-cost assistive devices would include a side-lyer or weighted positioning cushions for lying, a positioning chair for sitting activities, and a simple wooden stander.
Stretching and splinting regimes according to current clinical guidelines. For resting (sustained) stretching of both lower and upper limbs, thermoplastic splints or fabric gaiters can be used, if available, or firm foam can be covered with breathable materials and wrapped around the joints. To maintain a child’s ankle alignment during standing weight-bearing, ankle boots may suffice for a young child. For older children, thermoplastic splints or a caliper may be required.
Handling skills and positioning options for daily activities, such as feeding, bathing, and dressing. A plastic bathing chair and a feeding and/or activity chair should be provided to facilitate care and promote activity. The child would also need a wheelchair or posture support buggy for mobility and to allow social engagement.
The Training and Post-Training Support of the Program
The training should be delivered by rehabilitation therapists who can demonstrate the basic techniques but who would be able to adapt techniques and application of basic equipment for individual children as needed.
If therapists are not available on-site, and if sufficient mobile networks are in place, guided video instruction may be feasible via telehealth platforms.
Along with practical demonstrations of techniques and equipment use, a theoretical module on CP as a condition, the life course, management options, and prognosis should be mandatory.
Low-tech training and reference materials, such as printed booklets with predominantly graphic illustrations of techniques, would be preferable in these settings where literacy may be low. Artificial intelligence and other mobile applications may also be used for post-training support, especially if available in local languages.
Training should occur in a small group setting to encourage the formation of caregiver support groups. A CHW based in the area should attend the training with the caregivers they will be supporting.
The Community Support Network
A support network should include therapists, where available, and nursing staff who should provide home visits at intervals to ensure that caregivers are managing the program effectively and oversee the work of the CHWs who provide primary-level support for families. Zuurmond et al. found that monthly telephone or in-person check-ins with caregivers and a group chat via social media for program coordinators were effective support mechanisms for their community-based program in Ghana.28
Community engagement with local religious bodies, nongovernmental organizations, educational institutions, and other community players should be facilitated by social workers and/or via established community processes, which may be culturally and socio-geographically variable.
Measures of program adherence and process (e.g., CHW visits, evidence of intervention adherence, such as postural management), clinical outcome measures (e.g., range of joint motion, pain, and incidence of other MSK complications), and participation indicators (e.g., frequency of attendance at school and other social activities) should be collected by CHWs and evaluated by therapists using a quality improvement process that allows for continuous and responsive feedback, adaptation, and implementation processes as new evidence emerges.
CONCLUSION
Policymakers and program managers in RLSs should prioritize home-based packages of care for children with severe CP in these settings to mitigate the MSK consequences of CP, relieve caregiver burden, and ultimately improve the quality of life of those with and affected by CP. We have demonstrated that building capacity at a community level is feasible by prioritizing training and the development of low-cost technologies. We believe this is achievable through key partnerships with international health entities and nongovernmental organizations. As stated in the Sustainable Development Goals, we have an ethical obligation to ensure that no one with health needs is left behind and “ensure healthy lives and promote well-being for all at all ages” in all sociogeographic contexts by 2030.46
Author contributions
Shayne van Aswegen: conceptualization, data curation, funding acquisition, investigation, methodology, resources, visualization, writing–original draft, and writing–review and editing. Mark Richards: conceptualization, supervision, validation, and writing– review and editing. Brenda Morrow: conceptualization, methodology, supervision, validation, writing – original draft, and writing–review and editing.
Funding
The student researcher was supported by a research grant from the Department of Paediatrics and Child Health Research Committee at the University of Cape Town.
Competing interests
None declared.
Peer Reviewed
First Published Online: November 11, 2024.
Cite this article as: van Aswegen SR, Richards MT, Morrow BM. The case for parent-implemented programs to mitigate musculoskeletal complications in children with severe cerebral palsy in resource-limited settings. Glob Health Sci Pract. 2024;12(6):e2300463. https://doi.org/10.9745/GHSP-D-23-00463
REFERENCES
- 1.Donald KA, Kakooza AM, Wammanda RD, et al. Pediatric cerebral palsy in Africa. J Child Neurol. 2015;30(8):963–971. 10.1177/0883073814549245. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre S, Goldsmith S, Webb A, et al. Global prevalence of cerebral palsy: a systematic analysis. Dev Med Child Neurol. 2022;64(12):1494–1506. 10.1111/dmcn.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shevell M, Dagenais L, Hall N, Repacq C. The relationship of cerebral palsy subtype and functional motor impairment: a population‐based study. Dev Med Child Neurol. 2009;51(11):872–877. 10.1111/j.1469-8749.2009.03269.x. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone M. A review of the incidence and prevalence, types and aetiology of childhood cerebral palsy in resource-poor settings. Ann Trop Paediatr. 2010;30(3):181–196. 10.1179/146532810X12786388978481. [DOI] [PubMed] [Google Scholar]
- 5.Van Toorn R, Laughton B, Van Zyl N, Doets L, Elsinger F. Aetiology of cerebral palsy in children presenting at Tygerberg Hospital. South Afr J Child Health. 2007;1(2):275–277. [Google Scholar]
- 6.Kakooza-Mwesige A, Forssberg H, Eliasson AC, Tumwine JK. Cerebral palsy in children in Kampala, Uganda: clinical subtypes, motor function and co-morbidities. BMC Res Notes. 2015;8:166. 10.1186/s13104-015-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearden DR, Monokwane B, Khurana E, et al. Pediatric cerebral palsy in Botswana: etiology, outcomes, and comorbidities. Pediatr Neurol. 2016;59:23–29. 10.1016/j.pediatrneurol.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palisano R, Rosenbaum P, Bartlett D, Livingston M. Gross Motor Functional Classification System - Expanded & Revised. CanChild Centre for Childhood Disability Research Institute for Applied Health Sciences; 2007. Accessed October 22, 2024. https://www.canchild.ca/system/tenon/assets/attachments/000/000/058/original/GMFCS-ER_English.pdf [Google Scholar]
- 9.Hollung SJ, Bakken IJ, Vik T, et al. Comorbidities in cerebral palsy: a patient registry study. Dev Med Child Neurol. 2020;62(1):97–103. 10.1111/dmcn.14307. [DOI] [PubMed] [Google Scholar]
- 10.Hägglund G, Pettersson K, Czuba T, Persson-Bunke M, Rodby-Bousquet E. Incidence of scoliosis in cerebral palsy. Acta Orthop. 2018;89(4):443–447. 10.1080/17453674.2018.1450091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). Towards a Common Language for Functioning, Disability and Health. WHO; 2002. Accessed October 22, 2024. https://cdn.who.int/media/docs/default-source/classification/icf/icfbeginnersguide.pdf [Google Scholar]
- 12.Westbom L, Rimstedt A, Nordmark E. Assessments of pain in children and adolescents with cerebral palsy: a retrospective population‐based registry study. Dev Med Child Neurol. 2017;59(8):858–863. 10.1111/dmcn.13459. [DOI] [PubMed] [Google Scholar]
- 13.Porter D, Michael S, Kirkwood C. Patterns of postural deformity in non-ambulant people with cerebral palsy: what is the relationship between the direction of scoliosis, direction of pelvic obliquity, direction of windswept hip deformity and side of hip dislocation? Clin Rehabil. 2007;21(12):1087–1096. 10.1177/0269215507080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan C, Fetters L, Adde L, et al. Early intervention for children aged 0 to 2 years with or at high risk of cerebral palsy. JAMA Pediatr. 2021;175(8):846–858. 10.1001/jamapediatrics.2021.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novak I, Morgan C, Fahey M, et al. State of the Evidence Traffic Lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. 2020;20(2):3. 10.1007/s11910-020-1022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackman M, Sakzewski L, Morgan C, et al. Interventions to improve physical function for children and young people with cerebral palsy: international clinical practice guideline. Dev Med Child Neurol. 202;64(5):536–549. 10.1111/dmcn.1505. [DOI] [PubMed] [Google Scholar]
- 17.Metreau E, Young K, Eapen S. World Bank country classifications by income level for 2024-2025. World Bank Blogs; 2024. July 1, 2024. Accessed October 22, 2024. https://blogs.worldbank.org/en/opendata/world-bank-country-classifications-by-income-level-for-2024-2025 [Google Scholar]
- 18.Conradie T, Berner K, Louw Q. Describing the rehabilitation workforce capacity in the public sector of three rural provinces in South Africa: a cross-sectional study. Int J Environ Res Public Health. 2022;19(19):12176. 10.3390/ijerph191912176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naidoo D, Van Wyk J, Waggie F. Occupational therapy graduates’ reflections on their ability to cope with primary healthcare and rural practice during community service. S Afr J Occup Ther. 2017;47(3). 10.17159/2310-3833/2017/v47n3a7 [DOI] [Google Scholar]
- 20.Krüger D, Sello TM. Educating rural parents in South Africa about their children’s cerebral palsy: why wait for full-service schools or resource centres? Int J Diversity Org Communities Nations Ann Rev. 2008;8(2):245–250. 10.18848/1447-9532/CGP/v08i02/39569 [DOI] [Google Scholar]
- 21.Manyuma D, Maluleke M, Raliphaswa NS, et al. Title: caring for children with cerebral palsy: a challenge to caregivers in rural areas of South Africa. Children (Basel). 2023;10(3):440. 10.3390/children10030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwinbam MM, Suglo JN, Agyeman YN, Kukeba MW. Family caregivers’ experience of care with a child with cerebral palsy: the lived experiences and challenges of caregivers in a resource-limited setting in northern Ghana. BMJ Paediatr Open. 2023;7(1):e001807. 10.1136/bmjpo-2022-001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naidoo S, Naidoo D, Govender P. Community healthcare worker response to childhood disorders: inadequacies and needs. Afr J Prim Health Care Fam Med. 2019;11(1):a1871. 10.4102/phcfm.v11i1.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo DZ, Houtrow AJ, Arango P, Kuhlthau KA, Simmons JM, Neff JM. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J. 2012;16(2):297–305. 10.1007/s10995-011-0751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akhbari Ziegler S, Hadders-Algra M. Coaching approaches in early intervention and paediatric rehabilitation. Dev Med Child Neurol. 2020;62(5):569–574. 10.1111/dmcn.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckers LWME, Geijen MME, Kleijnen J, et al. Feasibility and effectiveness of home-based therapy programmes for children with cerebral palsy: a systematic review. BMJ Open. 2020;10(10):e035454. 10.1136/bmjopen-2019-035454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trani JF, Moodley J, Anand P, Graham L, Thu Maw MT. Stigma of persons with disabilities in South Africa: Uncovering pathways from discrimination to depression and low self-esteem. Soc Sci Med. 2020;265:113449. 10.1016/j.socscimed.2020.113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuurmond M, O’Banion D, Gladstone M, et al. Evaluating the impact of a community-based parent training programme for children with cerebral palsy in Ghana. PLoS One. 2018;13(9):e0202096. 10.1371/journal.pone.0202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence (NICE). Spasticity in Under 19s: Management. NICE; 2012. Accessed October 22, 2024. https://www.nice.org.uk/guidance/cg145 [Google Scholar]
- 30.Waikato District Health Board (WDHB). Cerebral Palsy Clinical Practice Guideline CP-CPG. WDHB; 2014. Accessed October 22, 2024. https://efisiopediatric.com/wp-content/uploads/2017/08/Cerebral-Palsy-Clinical-Practice-Guideline.pdf [Google Scholar]
- 31.Paleg G, Livingstone R, Rodby-Bousquet E, Story M, Maitre N. Care Pathways: Central Hypotonia. American Academy for Cerebral Palsy and Developmental Medicine; 2019. Accessed October 22, 2024. https://www.aacpdm.org/UserFiles/file/care-pathways-central-hypotonia-print.pdf [Google Scholar]
- 32.Damiano DL, Longo E, Carolina de Campos A, Forssberg H, Rauch A. Systematic review of clinical guidelines related to care of individuals with cerebral palsy as part of the World Health Organization efforts to develop a global package of interventions for rehabilitation. Arch Phys Med Rehabil. 2021;102(9):1764–1774. 10.1016/j.apmr.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinsson C, Himmelmann K. Abducted standing in children with cerebral palsy: effects on hip development after 7 Years. Pediatr Phys Ther. 2021;33(2):101–107. 10.1097/PEP.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 34.Tornberg ÅB, Lauruschkus K. Non-ambulatory children with cerebral palsy: effects of four months of static and dynamic standing exercise on passive range of motion and spasticity in the hip. PeerJ. 2020;8:e8561. 10.7717/peerj.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groppe M, Mattern-Baxter K, Davenport T. Passive stretching and its effect on spasticity and range of motion in children with cerebral palsy: a systematic review. J Student Physical Ther Res. 2012;5. Accessed October 22, 2024. https://www.researchgate.net/publication/260390219_Passive_Stretching_and_its_Effect_on_Spasticity_and_Range_of_Motion_in_Children_with_Cerebral_Palsy_A_Systematic_Review [Google Scholar]
- 36.Novak I, Smithers-Sheedy H, Morgan C. Predicting equipment needs of children with cerebral palsy using the Gross Motor Function Classification System: a cross-sectional study. Disabil Rehabil Assist Technol. 2012;7(1):30–36. 10.3109/17483107.2011.556210. [DOI] [PubMed] [Google Scholar]
- 37.Colver A, Thyen U, Arnaud C, et al. Association between participation in life situations of children with cerebral palsy and their physical, social, and attitudinal environment: a cross-sectional multicenter European study. Arch Phys Med Rehabil. 2012;93(12):2154–2164. 10.1016/j.apmr.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lord C, Rapley T, Marcroft C, Pearse J, Basu A. Determinants of parent-delivered therapy interventions in children with cerebral palsy: a qualitative synthesis and checklist. Child Care Health Dev. 2018. Sep;44(5):659–669. 10.1111/cch.12592. [DOI] [PubMed] [Google Scholar]
- 39.Branjerdporn N, Benfer K, Crawford E, Ziviani J, Boyd R, Sakzewski L. Efficacy of early interventions with active parent implementation in low-and-Middle income countries for young children with cerebral palsy to improve child development and parent mental health outcomes: a systematic review. Disabil Rehabil. 2022;44(23):6969–6983. 10.1080/09638288.2021.1989063. [DOI] [PubMed] [Google Scholar]
- 40.Halvarsson S, Asplund R, Fjellman-Wiklund A. From authority to coach – parents’ experiences of stretching as a home programme for children with cerebral palsy. Adv Physiother. 2010;12(4):208–216. 10.3109/14038196.2010.528023 [DOI] [Google Scholar]
- 41.Lillo-Navarro C, Medina-Mirapeix F, Escolar-Reina P, Montilla-Herrador J, Gomez-Arnaldos F, Oliveira-Sousa SL. Parents of children with physical disabilities perceive that characteristics of home exercise programs and physiotherapists’ teaching styles influence adherence: a qualitative study. J Physiother. 2015;61(2):81–86. 10.1016/j.jphys.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Rezaie L, Kendi S. Exploration of the influential factors on adherence to occupational therapy in parents of children with cerebral palsy: a qualitative study. Patient Prefer Adherence. 2020;14:63–72. 10.2147/PPA.S229535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barton C, Buckley J, Samia P, Williams F, Taylor SR, Lindoewood R. The efficacy of appropriate paper-based technology for Kenyan children with cerebral palsy. Disabil Rehabil Assist Technol. 2022;17(8):927–937. 10.1080/17483107.2020.1830442. [DOI] [PubMed] [Google Scholar]
- 44.Gorter JW, Ketelaar M, Rosenbaum P, Helders PJ, Palisano R. Use of the GMFCS in infants with CP: the need for reclassification at age 2 years or older. Dev Med Child Neurol. 2009;51(1):46–52. 10.1111/j.1469-8749.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 45.Nylén E, Grooten WJA. The stability of the gross motor function classification system in children with cerebral palsy living in Stockholm and factors associated with change. Phys Occup Ther Pediatr. 2021;41(2):138–149. 10.1080/01942638.2020.1830915. [DOI] [PubMed] [Google Scholar]
- 46.Sustainable Development Goals. United Nations. Accessed October 22, 2024. https://sdgs.un.org/goals [Google Scholar]