Abstract
Optimal development of fertilized eggs into preimplantation embryos is essential for reproduction. Although mammalian oocytes ovulated after luteinizing hormone (LH) stimulation can be fertilized and promoted into early embryos in vitro, little is known about ovarian factors important for the conditioning of eggs for early embryo development. Because LH interacts only with ovarian somatic cells, its potential regulation of oocyte functions is presumably mediated by local paracrine factors. We performed DNA microarray analyses of ovarian transcripts and identified brain-derived neurotrophic factor (BDNF) secreted by granulosa and cumulus cells as an ovarian factor stimulated by the preovulatory LH surge. Ovarian BDNF acts on TrkB receptors expressed exclusively in oocytes to enhance first polar body extrusion of oocytes and to promote the in vitro development of zygotes into preimplantation embryos. Furthermore, in vivo treatment with a Trk receptor inhibitor suppressed first polar body extrusion and the progression of zygotes into blastocysts. Thus, ovarian BDNF is important to nuclear and cytoplasmic maturation of the oocyte, which is essential for successful oocyte development into preimplantation embryos. Treatment with BDNF could condition the cultured oocytes for optimal progression into the totipotent blastocysts.
Keywords: early embryo development, gonadotropins, ovulation
In vertebrates, rupture of ovarian follicles and final maturation of oocytes occur in response to stimulation by pituitary-derived luteinizing hormones (LH) that act on the somatic granulosa and theca cells surrounding the oocyte. Shortly after stimulation by the preovulatory surge of LH, oocytes arrested at the late prophase resume meiosis characterized by germinal vesicle (nuclear envelope) breakdown (GVBD), chromosome condensation, and extrusion of the first polar body in preparation for fertilization and early embryonic development. Recent studies demonstrated that the endocrine hormone LH stimulates ovarian production of EGF-like factors from granulosa cells and insulin-like 3 from theca cells to promote GVBD (1, 2). In addition to nuclear maturation exemplified by GVBD and extrusion of the first polar body to complete the first meiotic division, oocytes also undergo cytoplasmic maturation characterized by cytoplasmic changes essential for monospermic fertilization, processing of the sperm, and preparation for development to preimplantation embryos (3, 4). Although the spermatozoon provides an essential element for embryo generation, the developmental fate of the embryo is principally dictated by the oocyte. However, few studies have explored ovarian factors that may be important for the conditioning of the oocyte in preparation for fertilization and preimplantation development.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of proteins known to activate the high-affinity TrkB receptor and the pan-neurotrophin low-affinity receptor p75 (5). Although neurotrophins are widely expressed in the central nervous system and are important for neuronal survival and differentiation (6), they also play important roles in nonneuronal tissues (7). In the ovary, BNDF was found to be essential for the development of early follicles (8, 9); however, its potential role during final stages of follicle maturation and ovulation has not been investigated. Based on DNA microarray analyses of ovarian genes during the preovulatory period, we have found major increases in BDNF expression after LH/human chorionic gonadotropin (hCG) stimulation. We demonstrated the preovulatory increases of BDNF in ovarian granulosa and cumulus cells and the exclusive expression of its receptor, TrkB, in the oocyte. Treatment of cultured oocytes with BDNF enhanced first polar body exclusion and increased the competence of oocytes to complete preimplantation development.
Materials and Methods
Animals. Immature female B6D2F1 mice were from Charles River Laboratories. Animal care was consistent with institutional and National Institutes of Health guidelines. Mice at 22 days of age were treated with 7.5 units of pregnant mare serum gonadotropin (PMSG; Calbiochem), followed by 10 units of hCG (Sigma) 48 h later to simulate follicle maturation and ovulation, respectively.
DNA Microarray Analyses. Mice (n = 108) were injected at 21 days of age with Humegon (7.5 units per animal, Organon) containing follicle-stimulating hormone and LH activities to stimulate follicular growth. Forty-eight hours later, some animals were treated i.p. with Pregnyl (5 units per animal) containing LH activity to induce ovulation. Ovaries were dissected from animals killed bi-hourly after Humegon treatment (three mice per group) and hourly after Pregnyl treatment (one mouse per group) for RNA extraction (TRIzol, Invitrogen). Aliquots of 6 μg of total RNA at 1 μg/μl for one-chipset hybridization were stored at -80°C. Samples were hybridized to the Affymetrix mouse MGU74v2 arrays A, B, and C according to standard Affymetrix protocols. The pooled follicular phase samples were hybridized in duplicate, and the postPregnyl samples were single determinations.
Follicle Cultures. Preovulatory follicles were excised from mouse ovaries 48 h after PMSG treatment and cultured to examine nuclear maturation of oocytes (2). Follicles (20-30 per vial) were cultured with or without recombinant human BDNF (Pepro-Tech, Rocky Hill, NJ) or 5 μg/ml LH (Organon) in Leibovitz's L-15 medium (Invitrogen). The vials were flushed at the start of the culture with O2/N2 (at a 1:1 ratio), sealed, and cultured at 37°C with gentle shaking for 6 h. After culture, cumulus oocyte complexes (COCs) were isolated, and, after cumulus cells were removed, oocytes were examined for the occurrence of GVBD.
Evaluation of First Polar Body Extrusion. For evaluating the transition from metaphase I stage to metaphase II (MII) stage oocytes, COCs were obtained from mouse ovaries 48 h after PMSG treatment by puncturing the largest follicles in M2 medium (Specialty Media, Phillipsburg, NJ). COCs were washed twice, transferred to modified M16 medium (Specialty Media) without FBS, and cultured with or without different doses of BDNF, mouse nerve growth factor (NGF; R & D Systems), or human neurotrophin-3 (NT-3) (R & D Systems) for 20 h at 37°C in 5% CO2/95% air. Some COCs were also cultured with BDNF with or without the TrkB ectodomain (R & D Systems), pan-specific Trk receptor inhibitor K252a (Calbiochem) (10), or K252b (11). The occurrence of first polar body extrusion in the oocyte was examined after removing cumulus cells by using a small-bore pipette under Hoffman contrast microscopy (Nikon).
In Vitro Maturation, Fertilization, and Early Embryonic Development. We performed in vitro maturation of oocytes followed by in vitro fertilization as described in ref. 12 with slight modifications. COCs from PMSG-primed mice were obtained in the M2 medium supplemented with 5% FBS before culturing in minimum essential media-α (Invitrogen) supplemented with Earle's salts, 10 μg/ml streptomycin sulfate, 75 μg/ml penicillin G, and 5% FBS in the presence or absence of 3 ng/ml BDNF at 37°C in 5% CO2/95% air. After 16 h of treatment, cumulus cells were removed and the oocytes were examined and classified according to their developmental stage (germinal vesicle, metaphase I, or MII). MII-stage oocytes were inseminated with sperm from B6D2F1 males and incubated for 4 h at 37°C in 5% CO2/95% air. After in vitro fertilization, fertilized oocytes were recovered, washed three times, and cultured in human tubal fluid medium (Specialty Media). The following morning, two-cell stage embryos were collected and cultured in 2 ml of modified M16 medium for 5 days more up to the blastocyst stage at 37°C in 5% CO2/95% air. Embryonic development was monitored daily by Hoffman modulation contrast microscopy, and the progression of fertilized eggs to preimplantation embryos was assessed.
In Vivo Analysis. To evaluate first polar body extrusion, immature mice were treated with 7.5 units of PMSG followed by 10 units of hCG 48 h later with or without an i.p. injection of K252a (dissolved in 10% dimethyl sulfoxide/0.9% saline). For negative controls, the plasma membrane nonsoluble K252b was used. Twelve hours after hCG injection, ovulated COCs were obtained from the oviducts. After treatment with hyaluronidase (Specialty Media) for 1-2 min, oocytes were separated from cumulus cells and the proportion of oocytes showing first polar body extrusion was evaluated. To evaluate the role of BDNF in the conditioning of oocytes for early embryo development, immature mice were treated with PMSG, followed by hCG as described above. Immediately after hCG injection, animals were allowed to mate before collection of fertilized oocytes 22 h later. To ensure the suppression of Trk receptor activity, 10 μg of K252a or K252b was administrated i.p. at 0, 4, and 8 h after hCG injection. Fertilized oocytes were separated from cumulus cells, washed three times in M2 medium, and cultured for 5 days in modified M16 medium. Embryonic development was monitored daily, and the progression of fertilized eggs into preimplantation embryos was assessed.
Real time RT-PCR, BDNF ELISA, immunohistochemistry, glutathione assay, blastocyst cell number determination, and statistical analyses are described in Supporting Materials and Methods and Fig. 5, which are published as supporting information on the PNAS web site.
Results
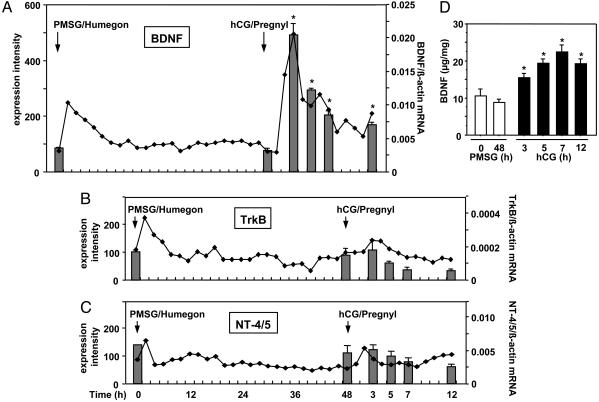
We used DNA microarray analyses to identify ovarian paracrine ligands induced by LH during the preovulatory period. Immature mice were treated with Humegon (containing follicle-stimulating hormone and LH activities) and Pregnyl (containing LH/hCG activity) to stimulate follicular maturation and ovulation, respectively. As shown in Fig. 1A (line graph), the expression of BDNF mRNA was stimulated after treatment with Humegon or Pregnyl. In contrast, transcript levels for the TrkB receptor and neurotrophin-4/5 (NT-4/5) showed minor changes (Fig. 1 B and C, line graphs). To confirm DNA microarray results, we performed real-time RT-PCR of ovarian transcripts for these genes in mice treated with PMSG followed by an injection of an ovulatory dose of hCG 48 h later. In addition to demonstrating preovulatory increases in BDNF transcripts (Fig. 1, bar graph), the stimulatory effect of hCG/Pregnyl on ovarian BDNF proteins was detected by using ELISA (Fig. 1D). Treatment with hCG increased ovarian BDNF antigen levels within 3 h, followed by a peak at 7 h.
Fig. 1.
Gonadotropin stimulation of BDNF expression. (A-C) Gonadotropin regulation of BDNF (A), TrkB (B), and NT-4/5(C) transcripts and the hCG stimulation of BDNF proteins in mouse ovaries. Line graphs represent DNA microarray data depicting the expression intensity of each transcript (left y axis), whereas bar graphs depict quantitative real-time RT-PCR results (right y axis). Values for expression intensity were derived from integration of hybridization signals from multiple probe sets for individual genes. (D) Ovarian content of BDNF (μg/mg ovarian wet weight) was measured by ELISA (mean ± SEM, n = 3). *, P < 0.05 vs. 0 h of PMSG treatment.
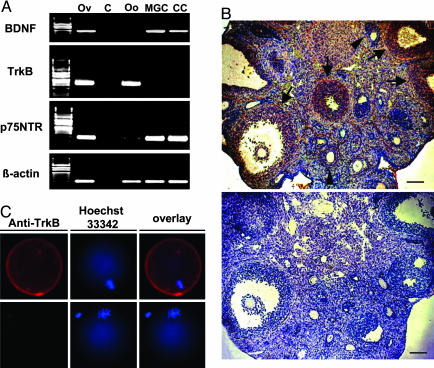
By using isolated ovarian cells, RT-PCR was performed to examine cells expressing BDNF and TrkB in the mouse ovary. We also examined the expression of the low-affinity receptor p75NTR for neurotrophins. Expression of the BDNF transcript was detected in mural granulosa and cumulus cells but not in oocytes from mice primed with PMSG for 48 h (Fig. 2A). In contrast, the TrkB mRNA was expressed exclusively in oocytes, although the p75NTR transcript was found in granulosa cells, oocytes (Fig. 2 A), and the theca shell (data not shown). We further confirmed the localization of BDNF and TrkB proteins by using immunohistochemistry. As shown in Fig. 2B and Fig. 5, strong BDNF staining was observed in mural and cumulus granulosa cells of preovulatory follicles at 7 h after hCG treatment, and a weaker signal was found in mural granulosa cells in small antral follicles. In addition, TrkB expression in the plasma membrane of the oocyte was confirmed by using immunofluorescence staining (Fig. 2C).
Fig. 2.
Localization of BDNF, TrkB, and p75 NTR transcripts as well as BDNF and TrkB antigens in the mouse ovary. (A) Expression of BDNF, TrkB, and p75 NTR mRNAs in isolated ovarian cells obtained from mice 48 h after PMSG treatment was detected by using nested RT-PCR. Levels of β-actin serve as loading controls. Total ovarian cDNA was used in positive control tests, whereas no template DNA was included for negative controls. Ov, ovary; C, negative control; Oo, oocyte; MGC, mural granulosa cells; CC, cumulus cells. (B) Immunohistochemical detection of BDNF in ovaries of PMSG-primed mice 7 h after hCG injection. BDNF was found in cumulus and mural granulosa cells of preovulatory follicles (arrows), whereas weaker staining was found in mural granulosa cells of small antral follicles (arrowheads). (Upper) BDNF staining. (Lower) Negative control. (Scale bars, 40 μm.) (C) Immunofluorescence staining of TrkB in an oocyte at the metaphase I stage. (Upper) TrkB staining. (Lower) Negative control. Overlay pictures showed combined staining of plasma membrane-bound TrkB and nuclear DNA (Hoechst staining).
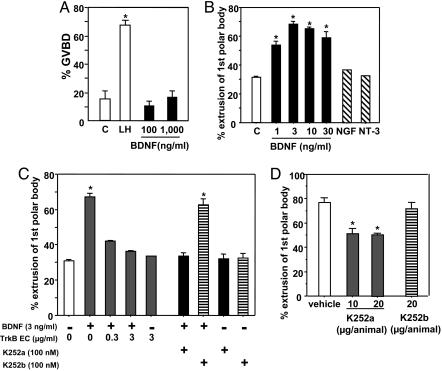
Based on hCG stimulation of BDNF expression in ovarian somatic cells and the exclusive expression of TrkB in oocytes, we hypothesized that BDNF acts as a paracrine factor to regulate oocyte function. In cultured preovulatory follicles, treatment with LH, but not BDNF, for 6 h induced GVBD in oocytes (Fig. 3A). Furthermore, treatment with BDNF, unlike LH, did not induce cumulus cell expansion (data not shown). Because oocytes obtained from preovulatory follicles underwent spontaneous GVBD when cultured as COC, we used the COC model to test the effect of BDNF to promote further oocyte development. As shown in Fig. 3B, treatment with BDNF, but not the related NGF or NT-3, increased first polar body extrusion in cultured oocytes in a dose-dependent manner. In addition, the stimulatory effect of BDNF was blocked by cotreatment with the TrkB ectodomain (Fig. 3C), whereas treatment with the TrkB ectodomain alone was ineffective. We further used the Trk receptor inhibitor K252a to block TrkB function in the oocyte. As shown in Fig. 3C, concomitant treatment with K252a, but not the membrane nonsoluble K252b, suppressed the stimulatory effect of BDNF on first polar body extrusion. Because BDNF, but not related neurotrophins, are increased during the preovulatory period (data not shown), we further used K252a to examine the role of endogenous BDNF on oocyte function in vivo. Although treatment with K252a or K252b did not affect the number of ovulated oocytes per animal (vehicle, 40.2 ± 7.5; K252a-treated, 37.8 ± 5.3; K252b-treated, 38.4 ± 8.7), treatment with K252a inhibited by 30% the first polar body extrusion by ovulated oocytes (Fig. 3D). In contrast, similar treatment with K252b was ineffective. Of interest, >95% of ovulated oocytes from all groups showed GVBD accompanied by expansion of the cumulus cells.
Fig. 3.
BDNF stimulation of first polar body extrusion by cultured oocytes. (A) Lack of effects of BDNF on GVBD of oocytes. Preovulatory follicles were cultured without (control, C) or with 5 μg/ml LH or BDNF for 6 h before evaluation of oocytes undergoing GVBD (n = 3, 39-68 oocytes per experiment). (B) Effects of BDNF treatment on first polar body extrusion by oocytes in vitro. COCs from preovulatory follicles were cultured without (controls, C) or with BDNF for 20 h. Some COCs were treated with 10 ng/ml NGF or 10 ng/ml NT-3. After culture, the percentage of oocytes showing first polar body extrusion was determined (n = 3, 30-148 oocytes per experiment). (C) Antagonistic effects of the TrkB ectodomain (TrkB EC) and the Trk receptor inhibitor K252a on BDNF stimulation of first polar body extrusion. COCs were cultured with BDNF with or without TrkB ectodomain, K252a, or K252b (n = 3, 11-82 oocytes per experiment). (D) Effects of the Trk receptor inhibitor on the extrusion of first polar body of oocytes in vivo. PMSG-primed mice were treated with hCG with or without K252a or the related K252b (i.p.). After 12 h of treatment, the percentage of ovulated oocytes showing first polar body extrusion was evaluated (n = 4). *, P < 0.05 vs. control or vehicle group.
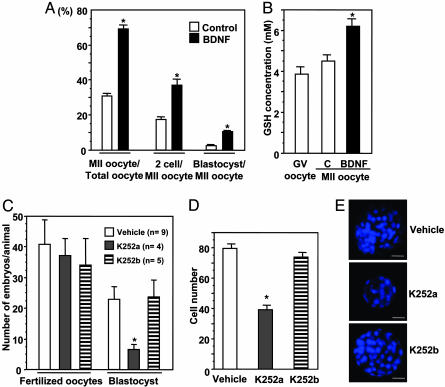
The role of BDNF in conditioning the oocytes for subsequent fertilization and progression to blastocysts was evaluated in vitro (Fig. 4). We cultured COCs obtained from mice primed for 48 h with PMSG and treated them with or without BDNF. To avoid hardening of the zona pellucida that is unfavorable for in vitro fertilization, 5% FBS was included for all cultures. Similar to serum-free cultures (Fig. 3B), treatment with BDNF increased more than 2-fold the proportion of oocytes showing first polar body extrusion (Fig. 4A, MII-stage oocytes). These MII oocytes were then fertilized in vitro without further treatment with BDNF. As shown in Fig. 4A, pretreatment with BDNF increased by 2- and 5-fold the proportions of MII oocytes that developed into the two-cell and blastocyst-stage embryos, respectively. Because the oocyte content of glutathione is important to sperm nuclear decondensation (13), the glutathione concentration in oocytes also was evaluated. As shown in Fig. 4B, glutathione levels were low in germinal vesicle-stage oocytes and in oocytes that spontaneously completed first polar body extrusion in vitro. However, treatment with BDNF increased glutathione content in MII oocytes (Fig. 4B).
Fig. 4.
BDNF conditioning of oocytes for development into preimplantation embryos. (A) COCs obtained from preovulatory follicles were cultured without (control) or with BDNF (3 ng/ml) for 16 h. After progression to the MII stage, oocytes were inseminated and cultured for 5 days more without hormones. The percentage of MII oocytes that developed to two-cell or blastocyst-stage embryos were evaluated (n = 5, 34-95 oocytes per experiment). (B) Effects of BDNF treatment on glutathione (GSH) content in oocytes. After treatment of COCs for 16 h without (control, C) or with BDNF, glutathione levels in oocytes at MII oocytes were evaluated (mean ± SEM, n = 6). (C) Effects of K252a treatment in vivo on the progression of zygotes into blastocysts in vitro. PMSG-primed mice were treated with hCG with or without K252a or K252b (10 μg, injections at 0, 4, and 8 h after hCG). After initial injection, mice were allowed to mate with fertile males. Fertilized oocytes were cultured for 5 days, and the number of blastocyst embryos was evaluated. (D) Effects of K252a treatment on the number of cells in the blastocysts. (E) Epifluorescence images of blastocysts stained with Hoechst 33342. (Scale bar, 20 μm.) *, P < 0.05 vs. control or vehicle group.
By using the Trk receptor inhibitor, we further examined the role of endogenous BDNF in conditioning the preovulatory oocytes for optimal development into preimplantation embryos. PMSG-primed animals were treated with hCG with or without K252a and mated with fertile males. Twenty-two hours after hCG treatment, fertilized oocytes were obtained from the oviduct and cultured in vitro. Although the number of fertilized oocytes did not differ between the control and K252a-treated groups, treatment with K252a suppressed by 72% the development of zygotes into blastocyst stage embryos (Fig. 4C). In contrast, treatment with K252b was ineffective. Because many blastocysts are smaller in the K252a-treated group (Fig. 4E), the number of cells in the blastocysts were counted and found to be decreased by 51% after K252a, but not K252b, treatment (Fig. 4D).
Discussion
The present findings demonstrate the essential role of the ovarian paracrine factor, BDNF, in promoting first polar body extrusion and in conditioning the eggs for optimal fertilization and development into preimplantation embryos. Mammalian oocytes undergo spontaneous GVBD after removal from surrounding somatic cells, but only a fraction of them reach the first polar stage. Although earlier studies demonstrated the important roles of ovarian EGF-like factors and insulin-like 3 in GVBD during the preovulatory period (1, 2), these factors are not needed for nuclear envelope breakdown of isolated oocytes during in vitro culture. In contrast, the present in vitro and in vivo findings demonstrated that BDNF is essential for first polar body extrusion as well as for cytoplasmic maturation of the oocyte, which is important for early embryo development.
Four of the five known neurotrophins, including NGF, BDNF, NT-3, and NT-4/5, and their receptors (TrkA, TrkB, Trk C, and p75 NFR) are expressed in early ovarian follicles (14). Mice defective in the expression of TrkB or its ligands (BDNF and NT-4/5) exhibited arrest in follicle development at the primary follicle stage (8, 9). Furthermore, treatment with the Trk receptor inhibitor, K252a, or the combined addition of antibodies against BDNF and NT-4/5 decreased primordial follicle survival in vitro (9). Although the expression of TrkA and its ligand, NGF, are increased in the thecal cells of preovulatory follicles and immunoneutralization of NGF actions inhibit follicle rupture (15, 16), the role of BDNF and its receptor, TrkB, in preovulatory follicles is unknown.
DNA microarray analyses of the ovarian transcriptome during the preovulatory period allowed us to identify major stimulation of BDNF expression after the LH/hCG induction of ovulation. Our findings of gonadotropin stimulation of ovarian BDNF secretion in mice in vivo are consistent with an earlier study using cultured human cumulus cells (17). BDNF acts on the TrkB receptors. Although kinase domain-containing TrkB receptors are expressed only at low levels in the oocytes and granulosa cells of primordial and growing follicles in mice (8), our data demonstrating TrkB expression in oocytes of preovulatory mouse follicles are consistent with an earlier work with humans (18). Ovarian localization of these genes indicated that BDNF produced by mural and cumulus granulosa cells is a paracrine factor acting on the TrkB receptor that is expressed exclusively in the oocyte.
Completion of nuclear maturation involves GVBD and extrusion of the first polar body. Although treatment of cultured COCs with BDNF did not affect GVBD, it facilitated first polar body extrusion, consistent with an earlier finding (18). We further demonstrate that the stimulatory effect of BDNF was blocked by the soluble ectodomain of TrkB or the Trk receptor inhibitor, K252a, suggesting mediation by the TrkB receptor. The important role of endogenous BDNF in promoting first polar body extrusion is underscored by the suppressive effects of the Trk receptor inhibitor, but not the related K252b, in ovulating animals in vivo. Because treatment with the Trk inhibitor did not affect GVBD of ovulated oocytes in these animals, it is apparent that sequential steps of nuclear maturation of the oocyte are controlled by different paracrine factors. In addition to improving the in vitro maturation of oocytes for successful in vitro fertilization, elucidation of the molecular mechanisms underlying ovarian BDNF actions could also allow the formulation of novel contraceptive strategies.
Some oocytes competent to complete nuclear maturation are unable to develop into the blastocyst stage, which is indicative of deficient or defective cytoplasmic maturation of the oocyte (3). During maturation of preovulatory oocytes, cytoplasmic changes in oocytes are necessary to allow for the acquisition of the maternal components required for optimal development of fertilized oocytes into preimplantation embryos. We demonstrated that in vitro treatment of COCs with BDNF not only augmented first polar body extrusion but also enhanced the subsequent development of MII oocytes to two-cell and blastocyst embryos. Furthermore, in vivo treatment with the Trk receptor inhibitor indicated the essential role of preovulatory increases of endogenous BDNF in the cytoplasmic maturation of the oocyte, thereby showing a major suppression of embryos capable of developing into the blastocyst stage. Even for embryos developed to the blastocyst stage, pretreatment with the Trk receptor inhibitor decreased their cell numbers by half. Furthermore, oocytes that spontaneously reached the first polar body stage had lower levels of glutathione (Fig. 4B), which is believed to be important in the fertilization of MII oocytes by facilitating sperm nuclear decondensing activity (13). Because BDNF treatment enhanced oocyte glutathione content, these findings suggest that BDNF could play a role in successful fertilization. Our data are consistent with earlier studies showing a correlation between glutathione levels in mature pig oocytes and their ability to form male pronuclei after fertilization (19). Thus, ovarian BDNF could activate separate downstream pathways in the oocyte to facilitate nuclear maturation (first polar body extrusion) as well as fertilization (glutathione content) and early embryo development.
In addition to the promotion of GVBD, treatment with EGF also increases first polar body extrusion (20, 21) and the number of cells in the blastocyst (22). Because the expression of three EGF-like factors, in a manner similar to BDNF, is increased after the preovulatory LH surge (1), these ovarian paracrine factors could act synergistically to promote first polar body extrusion. In addition, meiosis-activating sterol, activin, and a heparin-binding growth factor, midkine, also were found to promote the developmental competence of cultured mouse and bovine oocytes (12, 23, 24). Although the physiological significance of these three factors during the preovulatory period is unclear, it is becoming apparent that redundant intraovarian pathways in oocytes (TrkB and LGR8) and cumulus cells (EGF receptors) are activated during the preovulatory period to ensure successful oocyte maturation, fertilization, and early embryo development. Gene expression during oocyte maturation, fertilization, and early embryo development is regulated mainly by translational activation of maternally derived mRNAs, and the proper conditioning of the oocyte cytoplasm enables the development of totipotent blastocysts. Future studies on the translation of oocyte mRNAs regulated by BDNF and other oocyte-conditioning factors could provide clues regarding the molecular mechanisms underlying the unique developmental potential of fertilized oocytes. These studies could also shed light on the capacity of the oocyte cytoplasm to reprogram substituted nuclei from somatic cells after nuclear replacement or cloning (25, 26).
Note Added in Proof. A recent study (27) reported that the proportion of parthenogenetically activated bovine oocytes forming blastocyst was increasted after in vitro culture with BDNF.
Supplementary Material
Acknowledgments
We thank C. Spencer for editorial assistance. This work was supported by National Institutes of Health Grant HD31398 as part of the Specialized Cooperative Centers Program in Reproduction Research. K.K. was supported in part by the Akita University School of Medicine.
Author contributions: A.J.W.H. designed research; K.K., N.K., and S.M.M. performed research; K.K., N.K., M.D.S.G., and A.J.W.H. analyzed data; S.M.M. and M.D.S.G. contributed new reagents/analytic tools; and K.K. and A.J.W.H. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BDNF, brain-derived neurotrophic factor; COCs, cumulus oocyte complexes; GVBD, germinal vesicle breakdown; hCG, human chorionic gonadotropin; LH, luteinizing hormone; MII, metaphase II; NGF, nerve growth factor; NT-3, neurotrophin-3; NT-4/5, neurotrophin-4/5; PMSG, pregnant mare serum gonadotropin.
References
- 1.Park, J. Y., Su, Y. Q., Ariga, M., Law, E., Jin, S. L. & Conti, M. (2004) Science 303, 682-684. [DOI] [PubMed] [Google Scholar]
- 2.Kawamura, K., Kumagai, J., Sudo, S., Chun, S. Y., Pisarska, M., Morita, H., Toppari, J., Fu, P., Wade, J. D., Bathgate, R. A., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 7323-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eppig, J. J. (1996) Reprod. Fertil. Dev. 8, 485-489. [DOI] [PubMed] [Google Scholar]
- 4.Fulka, J., Jr., First, N. L. & Moor, R. M. (1998) Mol. Hum. Reprod. 4, 41-49. [DOI] [PubMed] [Google Scholar]
- 5.Barbacid, M. (1994) J. Neurobiol. 25, 1386-1403. [DOI] [PubMed] [Google Scholar]
- 6.Jones, K. R., Farinas, I., Backus, C. & Reichardt, L. F. (1994) Cell 76, 989-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ip, N. Y., Stitt, T. N., Tapley, P., Klein, R., Glass, D. J., Fandl, J., Greene, L. A., Barbacid, M. & Yancopoulos, G. D. (1993) Neuron 10, 137-149. [DOI] [PubMed] [Google Scholar]
- 8.Paredes, A., Romero, C., Dissen, G. A., DeChiara, T. M., Reichardt, L., Cornea, A., Ojeda, S. R. & Xu, B. (2004) Dev. Biol. 267, 430-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spears, N., Molinek, M. D., Robinson, L. L., Fulton, N., Cameron, H., Shimoda, K., Telfer, E. E., Anderson, R. A. & Price, D. J. (2003) Development (Cambridge, U.K.) 130, 5481-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapley, P., Lamballe, F. & Barbacid, M. (1992) Oncogene 7, 371-381. [PubMed] [Google Scholar]
- 11.Ross, A. H., McKinnon, C. A., Daou, M. C., Ratliff, K. & Wolf, D. E. (1995) J. Neurochem. 65, 2748-2756. [DOI] [PubMed] [Google Scholar]
- 12.Marin Bivens, C. L., Grondahl, C., Murray, A., Blume, T., Su, Y. Q. & Eppig, J. J. (2004) Biol. Reprod. 70, 1458-1464. [DOI] [PubMed] [Google Scholar]
- 13.Perreault, S. D., Barbee, R. R. & Slott, V. L. (1988) Dev. Biol. 125, 181-186. [DOI] [PubMed] [Google Scholar]
- 14.Ojeda, S. R., Romero, C., Tapia, V. & Dissen, G. A. (2000) Mol. Cell. Endocrinol. 163, 67-71. [DOI] [PubMed] [Google Scholar]
- 15.Dissen, G. A., Hill, D. F., Costa, M. E., Les Dees, C. W., Lara, H. E. & Ojeda, S. R. (1996) Endocrinology 137, 198-209. [DOI] [PubMed] [Google Scholar]
- 16.Mayerhofer, A., Dissen, G. A., Parrott, J. A., Hill, D. F., Mayerhofer, D., Garfield, R. E., Costa, M. E., Skinner, M. K. & Ojeda, S. R. (1996) Endocrinology 137, 5662-5670. [DOI] [PubMed] [Google Scholar]
- 17.Feng, B., Chen, S., Shelden, R. M. & Seifer, D. B. (2003) Fertil. Steril. 80, 658-659. [DOI] [PubMed] [Google Scholar]
- 18.Seifer, D. B., Feng, B., Shelden, R. M., Chen, S. & Dreyfus, C. F. (2002) J. Clin. Endocrinol. Metab. 87, 655-659. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida, M., Ishigaki, K., Nagai, T., Chikyu, M. & Pursel, V. G. (1993) Biol. Reprod. 49, 89-94. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi, M., Dominko, T., Yamauchi, N., Leibfried-Rutledge, M. L., Nagai, T. & First, N. L. (2002) Reproduction 123, 135-142. [PubMed] [Google Scholar]
- 21.Das, K., Stout, L. E., Hensleigh, H. C., Tagatz, G. E., Phipps, W. R. & Leung, B. S. (1991) Fertil. Steril. 55, 1000-1004. [DOI] [PubMed] [Google Scholar]
- 22.De La Fuente, R., O'Brien, M. J. & Eppig, J. J. (1999) Hum. Reprod. 14, 3060-3068. [DOI] [PubMed] [Google Scholar]
- 23.Silva, C. C. & Knight, P. G. (1998) Biol. Reprod. 58, 558-565. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda, S., Ichihara-Tanaka, K., Azuma, T., Muramatsu, T. & Yamada, M. (2000) Biol. Reprod. 63, 1067-1074. [DOI] [PubMed] [Google Scholar]
- 25.Mermillod, P., Oussaid, B. & Cognie, Y. (1999) J. Reprod. Fertil. Suppl. 54, 449-460. [PubMed] [Google Scholar]
- 26.Fulka, J., Jr., First, N. L., Loi, P. & Moor, R. M. (1998) BioEssays 20, 847-851. [DOI] [PubMed] [Google Scholar]
- 27.Martins da Silva, S. J., Gardner, J. O., Taylor, J. E., Springbett, A., De Sousa, P. A. & Anderson, R. A. (2005) Reproduction (Cambridge, U.K.) 129, 423-434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.