Abstract
Patient: Male, 24-year-old
Final Diagnosis: Acute myocardial infarction • coronary artery ectasia
Symptoms: Chest pain
Clinical Procedure: —
Specialty: Cardiology
Objective:
Unusual clinical course
Background:
Coronary artery ectasia (CAE) represents not only an anatomical variant but also a clinical constellation of coronary artery disease associated with acute coronary syndrome (ACS). There is no consensus on the antithrombotic treatment for patients with CEA.
Case Report:
A 34-year-old man with severe diffuse dilatation of the left main artery and left circumflex (LCX) proximal segment confirmed by coronary angiography (CAG) developed 2 acute myocardial infarctions (AMIs), due to occlusion of a dilated LCX segment with thrombus shadows, within a 9-year interval. Emergency percutaneous coronary intervention with aspiration thrombectomy failed to restore adequate blood flow at the first presentation of AMI, and the patient was eventually discharged with warfarin. After 15 months, however, he discontinued the medication on his own. After a 9-year interval, the patient experienced the second AMI, and CAG revealed occlusion of the LCX opening with thrombus shadows and collateral circulation visible from the distal segment of the right coronary artery to the circumflex branch. Thereafter, the patient began to take rivaroxaban 20 mg once daily. No chest pain or ACS events occurred during 1 year of follow-up.
Conclusions:
This case of 2 AMI events at the CAE segment caused by local thrombus formation suggests that lifetime systemic anticoagulation therapy for secondary prevention should be considered in such cases, and a novel oral anticoagulant may be a better choice for effectively preventing thrombosis.
Key words: Anticoagulants, Thrombosis, Ischemia, Warfarin, Heart Diseases, Coronary Artery Disease, Coronary Angiography
Introduction
Coronary artery ectasia (CAE) is defined as diffuse dilatation exceeding more than a third of the coronary artery length, with the diameter of the ectatic segment 1.5 times greater than that of the adjacent normal segment [1]. This is distinct from coronary artery aneurysm, defined as a coronary artery segment that exceeds the diameter of the normal adjacent coronary segment by 1.5 times and involves less than a third of the total length of the vessel [2]. CAE sometimes induces acute coronary syndrome (ACS) regardless of the presence or absence of coronary stenosis or atrial fibrillation [3]. A study by Papadakis et al revealed the apparent slow-flow phenomenon in the dilated segments of the coronary artery in CAE [4]. This slow-flow phenomenon may lead to ischemia and thrombosis [5]. There are few reports in the literature about patients with AMI coexisting with CAE, and the standard therapy for this condition has not been clearly established. Here we report a case of recurrent AMI caused by local thrombus formation at the same CAE lesion at an interval of 9 years, and we discussion the potential for long-term effectivity of anticoagulant therapy.
Case Report
Written informed consent was obtained, and the institutional Ethics Committee of the Tianjin Medical University General Hospital approved this case report.
This case report describes a 34-year-old man with no hypertension, diabetes, family history of cardiovascular disease or genetic history, but with 1 cardiovascular risk factor: smoking.
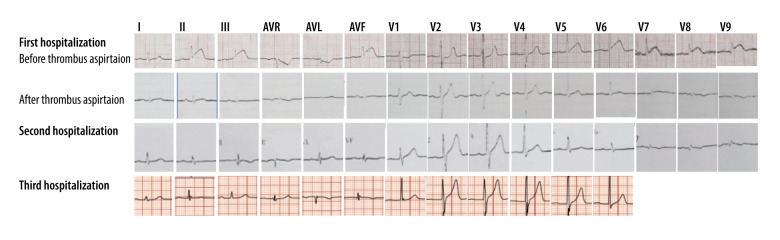
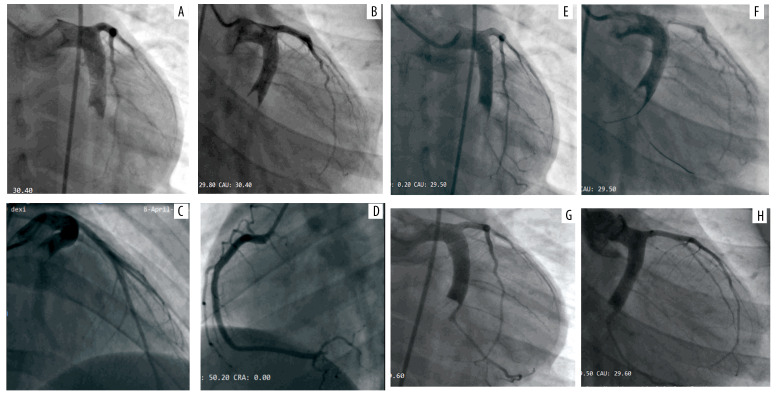
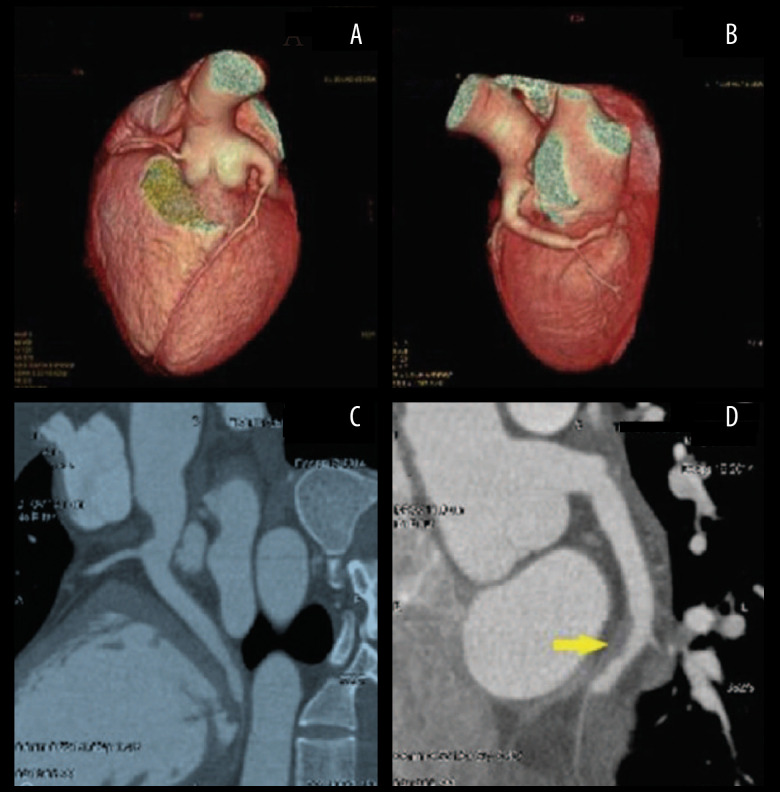
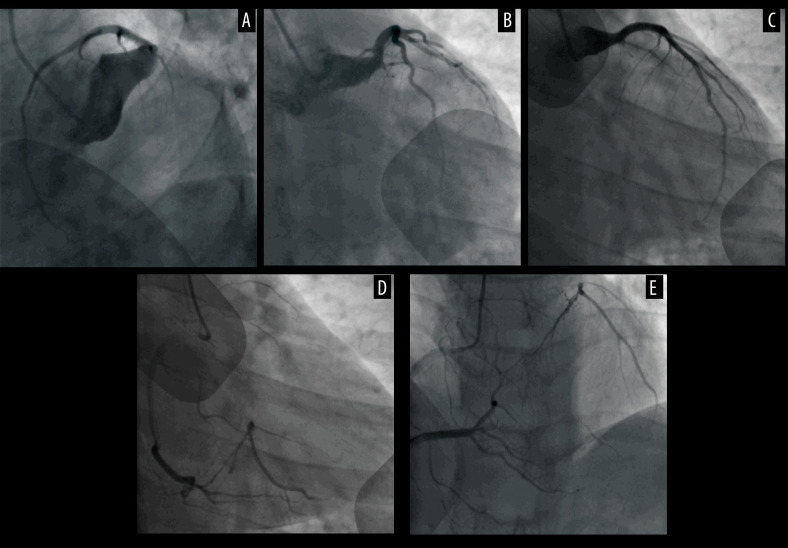
His first hospitalization was 10 years prior to the writing of this report. He presented in the hospital 4 hours after the onset of severe, crushing chest pain. The initial electrocardiogram (ECG) demonstrated sinus rhythm with ST-elevation at leads II, III, AVF, V5, V6, and V7-V9, as shown in Figure 1, and echocardiography displayed a left ventricular ejection fraction of 52% with reduced movement in the middle basal segment of the left ventricular inferior and posterior wall. The troponin T level was elevated (0.567 ng/mL). The patient underwent emergency coronary angiography (CAG) which showed severe diffuse dilatation of the left main artery and left circumflex (LCX) proximal segment, the occlusion of the LCX segment with a huge thrombus shadow, a normal left anterior descending artery (Figure 2A–2C) and a normal right coronary artery (Figure 2D). We attempted percutaneous coronary intervention using repetitive coronary aspirations with a 6-French Export Catheter (Medtronic, Minneapolis, MN) for thrombus extraction, and a small amount of red thrombus was extracted after several aspirations. However, the lesion was resistant to repetitive aspirations and the blood flow was only partially restored in the second obtuse marginal branch (OM2) with Thrombolysis in Myocardial Infarction (TIMI) grade 2 flow (Figure 2E, 2F). Consequently, the patient received tirofiban (10 mg/kg body weight) through a guiding catheter after thrombus aspiration, and following 36 hours of intravenous infusion (0.15 mg/kg/min), and subcutaneous anticoagulation with enoxaparin and oral dual antiplatelet drugs with aspirin and clopidogrel. His chest pain was completely relieved following medical therapy and his ECG showed ST-segment resolution (Figure 1). Coronary computed tomographic angiography (CTA) 10 days after admission showed CAE involving the left main artery and LCX and confirmed the presence of an occlusive intraluminal thrombus in the distal portion of the LCX, with no evidence of atherosclerotic plaques (Figure 3A–3D). On the 15th day of admission, CAG was again performed, revealing that the ectasia remained and that there was an obstructive filling defect in the distal portion of the LCX, with TIMI 3 flow restored in OM2 (Figure 2G, 2H). Meanwhile, left ventricular angiography revealed reduced movement of the left ventricular inferior wall. After discharge, the patient took aspirin and warfarin for 1 month, and then took warfarin alone for 14 months, during which the international normalized ratio (INR) was monitored to maintain a range of 2.0–2.5.
Figure 1.
Evolution of the electrocardiogram over 3 hospitalizations.
Figure 2.
Coronary angiography for first hospitalizations. (A–C) Ectasia of LM, and ectasia and total thrombotic occlusion of the LCX, with normal left anterior descending artery. (D) Normal right coronary artery. (E, F) Angiographic images after several attempted thrombus aspirations. (G, H) The second coronary angiography images, taken 15 days after admission, showing an obstructive filling defect in the distal portion of the LCX. LCX – left circumflex artery; LM – left main artery.
Figure 3.
Sixty-four-slice coronary computed tomographic angiography images of the LCX, 10 days after admission. (A, B) Three-dimensional computed tomography reconstruction showing coronary artery ectasia involving the LM and LCX. (C, D) A filling defect is shown in the distal portion of the LCX (arrow). LCX – left circumflex artery; LM – left main artery.
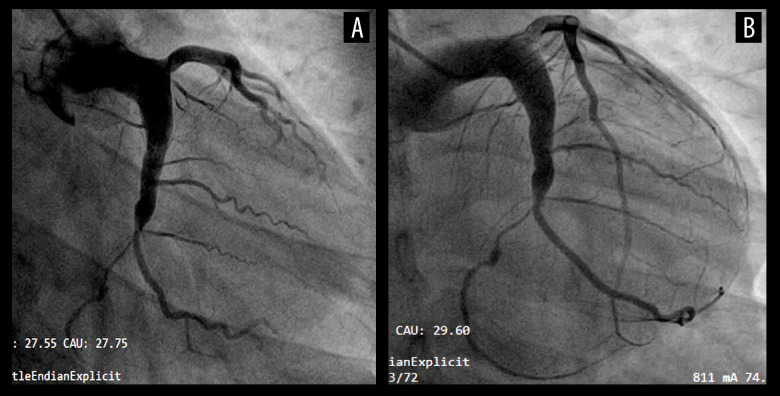
The patient’s second hospitalization took place 15 months after the first discharge. He underwent a follow-up CAG which displayed an almost complete resolution of the thrombus in the LCX, in which TIMI 3 flow was observed (Figure 4A, 4B). After this discharge, the patient continued to receive anticoagulants with warfarin, but subsequently discontinued the anticoagulant warfarin by himself without the consent of the physician, due to fear of affecting sperm quality during pregnancy preparation.
Figure 4.
The third coronary angiography images taken during the second hospitalization of the LCX, 15 months after the first discharge. TIMI 3 flow was restored in the LCX and almost complete resolution of the thrombus was noted. LCX – left circumflex artery; TIMI – Thrombolysis in Myocardial Infarction.
In the patient’s third hospitalization, he was admitted due to 4 days of intermittent chest pain associated with activity. Biomarkers of myocardial injury indicated an elevated cardiac troponin T at 0.694 ng/ml (normal range: <0.1 ng/ml). His echocardiography demonstrated global normality of the left ventricular wall with an ejection fraction of 62%. An electrocardiogram on admission suggested sinus rhythm without ST-segment elevation (Figure 1). CAG conducted on admission revealed occlusion of the LCX opening with thrombus shadows (Figure 5A–5C), and collateral circulation visible from the distal segment of the right coronary artery to the circumflex branch (Figure 5D, 5E). Coronary intervention was not performed during the surgery due to the formation of collateral circulation supplying the culprit vessel. After this discharge, the patient switched to the novel oral anticoagulant (NOAC) rivaroxaban 20 mg once daily.
Figure 5.
The fourth coronary angiography images at the third hospitalization. (A–C) Ectasia and total thrombotic occlusion of the LCX opening. (D, E) Collateral circulation from the distal segment of the right coronary artery to the LCX. LCX – left circumflex artery.
The patient insisted on taking oral rivaroxaban 20 mg once daily, and no chest pain or ACS event occurred during the 1-year follow-up.
Discussion
The patient experienced 2 episodes of AMI within a 9-year interval, which were caused by acute thrombosis in the dilated segment of the LCX confirmed by CAG. He took warfarin for only 15 months after the first AMI event due to fear of affecting sperm quality during pregnancy preparation, and began the NOAC rivaroxaban after the second AMI event and ACS event. He remained free of symptoms during 1 year of follow-up, which highlights the importance of anticoagulant therapy in a patient with CAE combined with AMI without obstructive lesions.
CAE can present with symptoms caused by isolated or concomitant atherosclerosis. ACS in patients with CAE has varying pathophysiology and may be divided into pathology that is detectable by CAG, such as visible thrombosis of the epicardial coronary artery, or not detectable by CAG, such as slow flow, vasospasm, and thrombosis in the microvasculature. The dilated coronary segments in CAE exhibit a pronounced coronary slow-flow phenomenon [4], which may result in ischemia and thrombosis [5], especially during stress. Tomioka [6] found sluggish blood flow only in the CAE lesion in this patient but not in the left atrium by Doppler velocimetry in a 78-year-old woman who developed AMI twice caused by thrombosis in the same CAE segment of the right coronary artery within a 1-month interval. In our case, during the first AMI event, thrombus aspiration removed only a small amount of thrombus, and subsequent coronary CTA also identified an intraluminal occlusive thrombus in the LCX without atherosclerosis or stenosis, and the patient had no history of atrial fibrillation.
Taken together, both AMI episodes at the same CAE segment in this patient were caused by local thrombus formation due to stagnant nonlaminar flow and not plaque rupture or thromboembolism originating from the left atrium or left ventricle, which is also supported in other case reports [7,8]. Therefore, the goal of therapy is to prevent further myocardial ischemia and minimize the risk of thrombosis due to slow flow.
So far, there is no consensus approach in the management of CAE due to a lack of studies and guidelines, and most of the published recommendations have been based on the personal experience of authors, including medical, percutaneous, and surgical approaches. In our report, this CAE patient was treated with warfarin only for more than 15 months after the first AMI event, with an INR maintained at 2–2.5. However, after discontinuing warfarin for about 8 years, this patient experienced the second AMI event due to thrombosis in the dilated left circumflex artery segment and subsequently began adhering to the NOAC drug rivaroxaban, with no ACS events occurring during 1 year of follow-up. This case report suggests that for severe CAE complicated with thrombosis, lifetime systemic anticoagulation therapy for secondary prevention should be considered if there are no contraindications after careful assessment of the bleeding risk. To date, only a single retrospective study has addressed the finding that CAE patients with AMI treated with vitamin K antagonists (warfarin), who achieved an adequate percentage of time in the target therapeutic range (%TTR ≤60%), had a lower occurrence of major adverse cardiac events than those with%TTR <60% or without anticoagulation therapy [9]. Unfortunately, regular monitoring of INR may reduce patient compliance with warfarin, and there have not been any studies on NOACs for therapy of CAE. A recently published report also used rivaroxaban to treat CAE combined with AMI, but no follow-up after discharge was available [10].
In our case, the patient started taking rivaroxaban 20 mg once per day after the second AMI and was followed up for 1 year without any ACS or bleeding events. The ongoing OVER-TIME trial [11] is the first randomized controlled trial to compare dual antiplatelet therapy (acetyl-salicylic acid plus a P2Y12 inhibitor) with the combination of an antiplatelet monotherapy (a P2Y12 inhibitor) plus a low dose anticoagulant (rivaroxaban, 15 mg oral dose) for the prevention of recurrent ischemic events among patients with CAE after an ACS. This trial will offer preliminary data for the prevention of major cardiovascular and bleeding events in this group of patients at higher risk of recurrency.
Interestingly, in the present case report, the patient took warfarin for only 15 months and subsequently stopped taking it due to concerns about the impact of anticoagulant drugs on sperm quality. Therefore, a question that needs to be addressed is, do anticoagulants have an impact on male reproduction? Vitamin K is present in the testes, although its function in male reproduction is poorly understood. In Sprague-Dawley rats, warfarin-induced vitamin K deficiency has a negative impact on spermatogenesis, including delayed spermiation, presence of multinucleated giant cells in the seminiferous tubules, germ cell degeneration, asthenozoospermia, oligozoospermia, and an increase in the percentage of abnormal sperm morphology [12]. Conversely, another rat trial suggests that rivaroxaban increased serum testosterone and sperm count and counteracted testicular damage caused by cisplatin via suppressing oxidative stress, inflammation, and coagulation [13]. Therefore, rivaroxaban may be a better choice than warfarin in terms of reproductive issues for young men. And more importantly, NOACs are preferred over warfarin mainly due to lower hemorrhagic risk. Studies and meta-analyses have shown that NOACs appear to be superior to warfarin for major bleeding in patients with nonvalvular atrial fibrillation [14,15]. Therefore, NOACs may be a better choice than warfarin in terms of safety.
Besides medical treatment, the data are more limited when it comes to percutaneous and surgical approaches. In the setting of ACS with an ectatic infarct-related artery, the dilated coronary segments provide more space for a much larger thrombus burden compared with nondilated coronary vessels. They also confer a high thrombus burden with distal embolization and microvascular damage. These features substantially increase the technical complexity of percutaneous coronary intervention, with a high risk of procedural failure and adverse events in the long term [16]. In our case, this patient underwent incomplete thrombus aspiration during emergency CAG procedure for his first AMI, with TIMI flow improvement. Lee [17] reported a young man who suffered from anterior ST-elevation myocardial infarction caused by thrombotic occlusion of the proximal left anterior descending (LAD) artery with prominent vascular ectasia of all 3 coronary arteries. This patient was managed with surgical thrombectomy and coronary artery bypass graft of his LAD after failed thrombolysis. Unfortunately, the ideal surgical approach is unclear.
Conclusions
In patients with AMI comorbid with severe CAE, local thrombus formation due to stagnant local blood flow may be the cause of coronary thrombosis. For such patients, lifetime systemic anticoagulation therapy for secondary prevention should be considered, and NOACs may be a better choice for effectively preventing thrombosis.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
Department of Cardiology, Tianjin Medical University General Hospital, Tianjin, PR China.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Aboeata AS, Sontineni SP, Alla VM, Esterbrooks DJ. Coronary artery ectasia: Current concepts and interventions. Front Biosci (Elite Ed) 2012;4(1):300–10. doi: 10.2741/377. [DOI] [PubMed] [Google Scholar]
- 2.Krüger D, Stierle U, Herrmann G, et al. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms (‘dilated coronaropathy’) J Am Coll Cardiol. 1999;34:1461–70. doi: 10.1016/s0735-1097(99)00375-7. [DOI] [PubMed] [Google Scholar]
- 3.Mavrogeni S. Coronary artery ectasia: From diagnosis to treatment. Hellenic J Cardiol. 2010;51:158–63. [PubMed] [Google Scholar]
- 4.Papadakis MC, Manginas A, Cotileas P, et al. Documentation of slow coronary flow by the TIMI frame count in patients with coronary ectasia. Am J Cardiol. 2001;88:1030–32. doi: 10.1016/s0002-9149(01)01984-1. [DOI] [PubMed] [Google Scholar]
- 5.Sayin T, Döven O, Berkalp B, et al. Exercise-induced myocardial ischemia in patients with coronary artery ectasia without obstructive coronary artery disease. Int J Cardiol. 2001;78(2):143–49. doi: 10.1016/s0167-5273(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 6.Tomioka T, Takeuchi S, Ito Y, et al. Recurrent acute myocardial infarction in a patient with severe coronary artery ectasia: Implication of antithrombotic therapy. Am J Case Rep. 2016;17:939–43. doi: 10.12659/AJCR.900474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheikhly KA, Kaki A. Medical management of STEMI in massive coronary artery ectasia. J Am Coll Cardiol. 2022;79:2991. [Google Scholar]
- 8.Madgula AS, Condit D, Robinson P. Coronary artery ectasia presenting as acute coronary syndrome. J Am Coll Cardiol. 2020;75(11):2423. [Google Scholar]
- 9.Doi T, Kataoka Y, Noguchi T, et al. Coronary artery ectasia predicts future cardiac events in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2017;37(12):2350–55. doi: 10.1161/ATVBAHA.117.309683. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Zhang H, Ren P, et al. Sudden cardiac death caused by a right coronary artery aneurysm complicated with acute myocardial infarction: A case report. J Int Med Res. 2023;51:3000605231175635. doi: 10.1177/03000605231175635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araiza-Garaygordobil D, Gopar-Nieto R, Sierra-Lara Martínez D, et al. Dual antiplatelet therapy versus antiplatelet monotherapy plus oral anticoagulation in patients with acute coronary syndrome and coronary artery ectasia: Design and rationale of OVER-TIME randomized clinical trial. High Blood Press Cardiovasc Prev. 2022;29:463–68. doi: 10.1007/s40292-022-00535-4. [DOI] [PubMed] [Google Scholar]
- 12.Sanyaolu AO, Oremosu AA, Osinubi AA, et al. Warfarin-induced vitamin K deficiency affects spermatogenesis in Sprague-Dawley rats. Andrologia. 2019;51:e13416. doi: 10.1111/and.13416. [DOI] [PubMed] [Google Scholar]
- 13.Shafiey SI, Abo-Saif AA, Abo-Youssef AM, et al. Protective effects of rivaroxaban against cisplatin-induced testicular damage in rats: Impact on oxidative stress, coagulation, and p-NF-κB/VCAM-1 signaling. Food Chem Toxicol. 2022;169:113419. doi: 10.1016/j.fct.2022.113419. [DOI] [PubMed] [Google Scholar]
- 14.Li WJ, Archontakis-Barakakis P, Palaiodimos L, et al. Dabigatran, rivaroxaban, and apixaban are superior to warfarin in Asian patients with non-valvular atrial fibrillation: An updated meta-analysis. World J Cardiol. 2021;13:82–94. doi: 10.4330/wjc.v13.i4.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu VCC, Wang CL, Lee CH, et al. Novel oral anticoagulant vs. warfarin in elderly atrial fibrillation patients with normal, mid-range, and reduced left ventricular ejection fraction. ESC Heart Failure. 2020;7:2862–70. doi: 10.1002/ehf2.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogana Shanmugam V, Psaltis PJ, D TLW, et al. Outcomes after primary percutaneous coronary intervention for st-elevation myocardial infarction caused by ectatic infarct related arteries. Heart Lung Circ. 2017;26:1059–68. doi: 10.1016/j.hlc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Ramkumar S, Khav N, Dundon BK. Coronary artery ectasia presenting with ST-elevation myocardial infarction in a young indigenous man: A case report. Eur Heart J Case Rep. 2020;4(5):1–5. doi: 10.1093/ehjcr/ytaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]