Abstract
The timing of amniotomy after the Foley balloon catheter removal is crucial for successful labor induction. This study aimed to assess the effects of the Bishop score on the timing of amniotomy in patients undergoing labor induction after the Foley balloon catheter removal. This was a retrospective cohort study based on electronic medical records. We performed a Chester sampling in patients with singleton-term pregnancies who initially underwent cervical ripening using a Foley balloon catheter at the Obstetrical Department of Taixing People’s Hospital from January 2023 to July 2023. A total of 889 patients were admitted to the study. After excluding 330 patients according to the exclusion criteria, 103 patients were included. Following the Foley balloon removal, an amniotomy with a Bishop score < 6 was defined as an amniotomy with an unfavorable Bishop score (n = 62), and an amniotomy with a Bishop score ≥ 6 was defined as an amniotomy with a favorable Bishop score (n = 41). The primary outcome was the incidence of cesarean delivery and the interval from induction to delivery. The secondary outcomes included the incidence of operative vaginal delivery, intrapartum hemorrhage, postpartum hemorrhage, infection, thrombosis, and neonatal outcomes. All statistical comparisons were analyzed by GraphPad Prism 9. All data were presented as the mean ± SD or percentage. Statistical analysis comparing both groups was performed using the t test, chi-square test, or Fisher exact test where appropriate. The baseline data, operative vaginal delivery rate, postpartum hemorrhage rate, infection rate, thrombosis rate, intrapartum and postpartum hemorrhage volume, and neonatal outcomes showed no significant differences between the 2 groups. However, the cesarean delivery rate, interval from induction to delivery, and hemoglobin postdelivery decline were significantly decreased in the favorable Bishop score group. Amniotomy with a favorable Bishop score after Foley balloon catheter removal is linked to lower cesarean delivery rates, shorter induction-to-delivery intervals, and less postdelivery hemoglobin decline without increasing adverse maternal or neonatal outcomes.
Keywords: amniotomy, Bishop score, cesarean delivery, Foley balloon catheter, induction of labor
1. Introduction
Induction of labor involves stimulating cervical ripening and uterine contractions artificially to accelerate labor. It is currently one of the most common obstetric procedures.[1] Using a Foley balloon catheter for cervical ripening along with oxytocin was considered the most effective approach in labor induction.[2,3] Amniotomy following Foley balloon catheter removal had been recommended for expediting labor based on published studies.[2,4] However, there is still a doubt regarding the timing of amniotomy following Foley balloon catheter removal. Previous studies have demonstrated that performing amniotomy following Foley balloon catheter removal before the active phase of labor could reduce the time from induction to delivery without increasing the risk of cesarean delivery.[2,5,6] A randomized controlled trial found that early amniotomy at the beginning of induction of labor was associated with a shorter mean time to birth but an increased rate of cesarean births compared to delayed amniotomy.[7] Supporting the above findings, another study indicated an increased cesarean rate associated with performing amniotomy when cervical dilatation is <4 cm in women with a BMI of 30 kg/m² or higher.[8] A recent study reported that delaying amniotomy >4 hours after Foley balloon catheter removal prolonged the time from Foley balloon catheter removal to active labor and delivery and raised the risk of postpartum hemorrhage.[9] Early amniotomy may accelerate labor and shorten the time to birth, although its effect on cesarean birth rates varies across studies. However, there are also potential complications associated with early amniotomy, such as cord prolapse, ascending infections, chorioamnionitis, and fetal hypoxia, which may require an emergency cesarean section in some cases.[10,11] While there are risks associated with early amniotomy, there are also complications related to prolonged labor from late amniotomy, including cesarean delivery, chorioamnionitis, endometritis, and postpartum hemorrhage.[12] Hence, performing an amniotomy at the appropriate time after removing a Foley balloon catheter is crucial for positive pregnancy outcomes.
The Bishop score was developed to assess the likelihood of successful vaginal delivery. A Bishop score of 6 or higher is considered to indicate a favorable cervical condition and predict a successful vaginal delivery.[13,14] Initiating labor induction with oxytocin in patients with an unfavorable Bishop score has been associated with a higher likelihood of failed induction and an increased risk of cesarean delivery compared to patients with a favorable Bishop score.[15,16] There are limited studies examining the use of the cervical Bishop score to determine when to perform an amniotomy after Foley balloon catheter removal in patients undergoing labor induction. This study aimed to assess the effects of the Bishop score on the timing of amniotomy in patients undergoing labor induction after the Foley balloon catheter removal, providing evidence for the ongoing discussion regarding the optimal timing for amniotomy after a Foley balloon catheter removal during labor induction.
2. Methods
2.1. Patients
This was a retrospective cohort study. The patients were obtained from the Obstetrical Department of Taixing People’s Hospital from January 2023 to July 2023. In this study, we only included patients with singleton-term pregnancies who initially underwent cervical ripening only using a Foley balloon catheter. Patients with elective cesarean section, preterm, premature rupture of membranes, infection, lack of cervical ripening with Foley balloon catheter, or did not administration of oxytocin were excluded. This study assessed the Bishop score for all patients through cervical examination. Two senior obstetricians performed cervical examinations. The first cervical exam was performed on admission. After removing the Foley balloon catheter, another cervical exam was conducted to assess the Bishop score. A Bishop score of <6 was assigned to the unfavorable Bishop score group (UBS), while a Bishop score of 6 or higher was assigned to the favorable Bishop score group (FBS).[1,13,14] Data for each variable of interest were collected. The sample size was determined using the formulae for comparative studies proposed by Charan and Biswas.[17] All procedures followed relevant laws and institutional guidelines and were approved by the appropriate institutional committee(s). Written informed consent was obtained for all the procedures in this study. The datasets generated and (or) analyzed during the current study are available from the corresponding author upon reasonable request. Sample size calculation was conducted by EPIINFO.
2.2. Placement and removal of Foley balloon catheter
According to the women’s choice, the final decision to perform catheterization at our institution was made by 2 senior obstetricians. All women who underwent cervical ripening received a single-size 18 Foley balloon catheter inflated with 80 mL saline solution at 18:00 following admission evaluation. The Foley balloon catheter would be removed if not expelled within 12 hours after placement. The Foley balloon catheter would not be continued if there was a spontaneous balloon rupture, spontaneous expulsion, rupture of the membrane, regular uterine contractions, severe pain perception, unexplained vaginal bleeding, placenta abruption, or suspected fetal intrauterine hypoxia.
2.3. Amniotomy and oxytocin administration
After the Foley balloon catheter was removed, the obstetrician reassessed the Bishop score and then artificially ruptured the membranes. After an amniotomy for 30 minutes, the hospital-standardized oxytocin administration protocol was performed. The same protocol of oxytocin administration was used in both groups. One-to-one care was carried out during all processes. Oxytocin was administered intravenously via a syringe infusion pump. The infusion rate commenced at 2 milliunits/minute and escalated by 2 milliunits/minute every 15 to 20 minutes until regular uterine contractions (2–3 times/minute) occurred. Then the infusion rate was maintained at a constant level, the maximum infusion rate was 42 milliunits/minute. Constant fetal heart rate and uterine contractions were monitored throughout the oxytocin infusion. The oxytocin infusion would be stopped or reduced in case of uterine hyperstimulation or abnormal fetal heart rate.[18,19] Intravertebral anesthesia was offered when the contractions became regular in both groups. Cervical exams were performed every 4 hours in the latent phase and every hour during the active phase.
2.4. Primary and secondary outcomes
The primary outcome was the cesarean delivery rate and interval from induction to delivery. The secondary outcomes were the incidence of operative vaginal delivery, intrapartum hemorrhage, postpartum hemorrhage, infection, thrombosis, and neonatal outcomes. Blood loss during vaginal delivery was measured using a calibrated under-buttocks drape. Postpartum hemorrhage was assessed by calculating the cumulative blood loss after delivery.[20] Maternal infection is defined as clinical chorioamnionitis, which is characterized by the presence of fever before or during labor (≥38 °C) along with one or more of the following symptoms: elevated maternal leukocytosis (>15,000/m³), maternal tachycardia (>100 beats per minute), fetal tachycardia (baseline heart rate >160 beats per minute for 10 minutes or longer), uterine tenderness, or foul-smelling amniotic fluid. It can also be indicated by postpartum fever (≥38 °C) or leukocytosis more than 15,000/mm³.
2.5. Statistical analysis
All statistical comparisons were analyzed by GraphPad Prism 9 (La Jolla, CA). All data were presented as the mean ± SD or percentage. Statistical analysis comparing both groups was performed using the t test, chi-square test, or Fisher exact test where appropriate. P < .05 represented statistical significance. Kolmogorov–Smirnov test was used to assess data distribution.
3. Results
During the study period, 889 patients were admitted to our institution. After excluding 330 patients who had elective cesarean section due to a history of cesarean, malpresentation, multiple gestations, etc, as well as 34 patients with preterm (<37 weeks), 43 patients with premature rupture of membrane, and 379 patients who did not receive Foley balloon catheter or oxytocin, 103 patients remained in the analysis (Fig. 1). According to the Bishop score after Foley balloon catheter removal, 62 patients were in the UBS group, and 41 were in the FBS group.
Figure 1.
Patients’ eligibility and enrollment information for the current study. Of the 889 patients admitted to our institution during the study period, 103 were included in the analysis. We excluded patients with elective cesarean section, preterm (<37 weeks), premature rupture of membrane, without Foley balloon catheter, or oxytocin.
3.1. Baseline characteristics of patients in this study
Patient baseline characteristics, including maternal age, body mass index, gestational age at delivery, gravida, parity, and the percentage of nulliparous and multiparous did not differ between groups. The cervical Bishop score on admission was similar for the 2 groups. However, the Bishop score after Foley balloon catheter removal in the UBS group was significantly lower than that in the FBS group (FBS vs UBS, 6.73 ± 0.63 vs 4.92 ± 0.45, P < .0001) (Table 1). The birth weight in the FBS group was significantly higher than in the UBS group (FBS vs UBS, 3645 ± 369.7 g vs 3459 ± 444.5 g, P = .039). However, the incidence of macrosomia was also more significant in the FBS group than in the UBS group (Table 3).
Table 1.
Characteristics of the included patients (mean ± SD or percentage).
| UBS | FBS | P-value | |
|---|---|---|---|
| Age (years) | 28.97 ± 4.43 | 28.56 ± 4.20 | .642 |
| BMI (kg/m2) | 25.56 ± 2.67 | 25.61 ± 2.73 | .934 |
| Gestational age at delivery (weeks) | 39.94 ± 0.92 | 39.90 ± 0.89 | .857 |
| Gravida | 1.47 ± 0.94 | 1.63 ± 0.83 | .358 |
| Parity | 1.19 ± 0.47 | 1.27 ± 0.50 | .445 |
| Nulliparous, n/N (%) | 53/62 (85.48%) | 31/41 (75.61%) | |
| Multiparous, n/N (%) | 9/62 (14.52%) | 10/41 (24.39%) | .299 |
| Bishop score on admission | 2.67 ± 0.48 | 2.73 ± 0.45 | .455 |
| Bishop score after Foley removal | 4.92 ± 0.45 | 6.73 ± 0.63 | <.0001* |
P < .05 was considered a statistically significant difference.
FBS = amniotomy with favorable Bishop score, UBS = amniotomy with unfavorable Bishop score.
P < .0001.
Table 3.
Neonatal outcomes (mean ± SD or percentage).
| UBS | FBS | P-value | |
|---|---|---|---|
| Gender (man), n/N (%) | 23/49 (46.94%) | 18/39 (46.15%) | |
| Gender (woman), n/N (%) | 26/49 (53.06%) | 21/39 (53.85%) | >.9999 |
| Birthweight (g) | 3459 ± 444.5 | 3645 ± 369.7 | .039* |
| Birthweight < 4000 g, n/N (%) | 42/49 (85.71%) | 33/39 (84.62%) | |
| Birthweight ≥ 4000 g, n/N (%) | 7/49 (14.29%) | 6/39 (15.38%) | >.9999 |
| Apgar ≤ 7 at 1 minute, n/N (%) | 2/49 (4.08%) | 0/39 (0%) | |
| Apgar 8–10 at 1 minute, n/N (%) | 47/49 (95.92%) | 39/39 (100%) | .501 |
| Apgar ≤ 7 at 5 minutes, n/N (%) | 0/49 (0%) | 0/39 (0%) | |
| Apgar 8–10 at 5 minutes, n/N (%) | 49/49 (100%) | 39/39 (100%) | >.999 |
| Lactic acid (mmol/L) | 4.55 ± 1.20 | 3.74 ± 1.49 | .138 |
| Lactic acid < 6 mmol/L, n/N (%) | 18/23 (78.26%) | 19/21 (90.48%) | |
| Lactic acid ≥ 6 mmol/L, n/N (%) | 5/23 (21.74%) | 2/21 (9.52%) | .416 |
| Base excess ‐5.6 to 2.7 mmol/L, n/N (%) | 9/23 (39.13%) | 9/21 (42.86%) | |
| Base excess < ‐5.6 mmol/L, n/N (%) | 14/23 (60.87%) | 12/21 (57.14%) | >.999 |
| Base excess ≥ ‐12 mmol/L, n/N (%) | 23/23 (100%) | 21/21 (100%) | |
| Base excess < ‐12 mmol/L, n/N (%) | 0/23 (0%) | 0/21 (0%) | >.999 |
| pH value | 7.24 ± 0.09 | 7.25 ± 0.08 | .867 |
| pH ≥ 7.24, n/N (%) | 14/23 (60.87%) | 11/21 (52.38%) | |
| pH < 7.24, n/N (%) | 9/23 (39.13%) | 10/21 (47.62%) | .761 |
| pH ≥ 7.0, n/N (%) | 23/23 (100%) | 21/21 (100%) | |
| pH < 7.0, n/N (%) | 0/23 (0%) | 0/21 (0%) | >.999 |
| Admission to the neonatal intensive care unit, n/N (%) | 7/49 (14.29%) | 7/39 (17.95%) | |
| Rooming-in care, n/N (%) | 42/49 (85.71%) | 32/39 (82.05%) | .771 |
P < .05 was considered a statistically significant difference.
FBS = amniotomy with favorable Bishop score, UBS = amniotomy with unfavorable Bishop score.
P < .05.
3.2. Comparison of cesarean rate and induction-to-delivery duration between 2 groups
There was a significantly lower rate of cesarean delivery in the FBS group compared to the UBS group (FBS vs UBS, 4.88 % vs 20.97%, P = .025). Additionally, the time from induction to delivery was shorter in the FBS group (FBS vs UBS, 385.2 ± 191.5 minutes vs 483.1 ± 215.0 minutes, P = .03). However, no significant difference between both groups in the term of the rate of operative vaginal delivery and postpartum hemorrhage (Table 2).
Table 2.
Maternal outcomes (mean ± SD or percentage).
| UBS | FBS | P-value | |
|---|---|---|---|
| Vaginal delivery, n/N (%) | 49/62 (79.03%) | 39/41 (95.12%) | |
| Cesarean delivery, n/N (%) | 13/62 (20.97%) | 2/41 (4.88%) | .025* |
| Spontaneous vaginal delivery, n/N (%) | 47/62 (75.81%) | 30/41 (73.17%) | |
| Operative vaginal delivery, n/N (%) | 15/62 (24.19%) | 11/41 (26.83%) | .819 |
| The interval from induction to delivery (minutes) | 483.1 ± 215.0 | 385.2 ± 191.5 | .03* |
| Postpartum hemorrhage < 1000 mL (24 hours), n/N (%) | 47/49 (95.92%) | 39/39 (100%) | |
| Postpartum hemorrhage ≥ 1000 mL (24 hours), n/N (%) | 2/49 (4.08%) | 0/39 (0%) | .5 |
| Postpartum hemorrhage < 500 mL (24 hours), n/N (%) | 42/49 (85.71%) | 33/39 (84.62%) | |
| Postpartum hemorrhage ≥ 500 mL (24 hours), n/N (%) | 7/49 (14.29%) | 6/39 (15.38%) | >.999 |
| Postpartum hemorrhage < 400 mL (24 hours), n/N (%) | 26/49 (53.06%) | 20/39 (51.28%) | |
| Postpartum hemorrhage ≥ 400 mL (24 hours), n/N (%) | 23/49 (46.94%) | 19/39 (48.72%) | >.999 |
| Infection, n/N (%) | 7/49 (14.29%) | 1/39 (2.56%) | .072 |
P < .05 was considered a statistically significant difference.
FBS = amniotomy with favorable Bishop score, UBS = amniotomy with unfavorable Bishop score.
P < .05.
3.3. Comparison of hemoglobin postdelivery decline between 2 groups
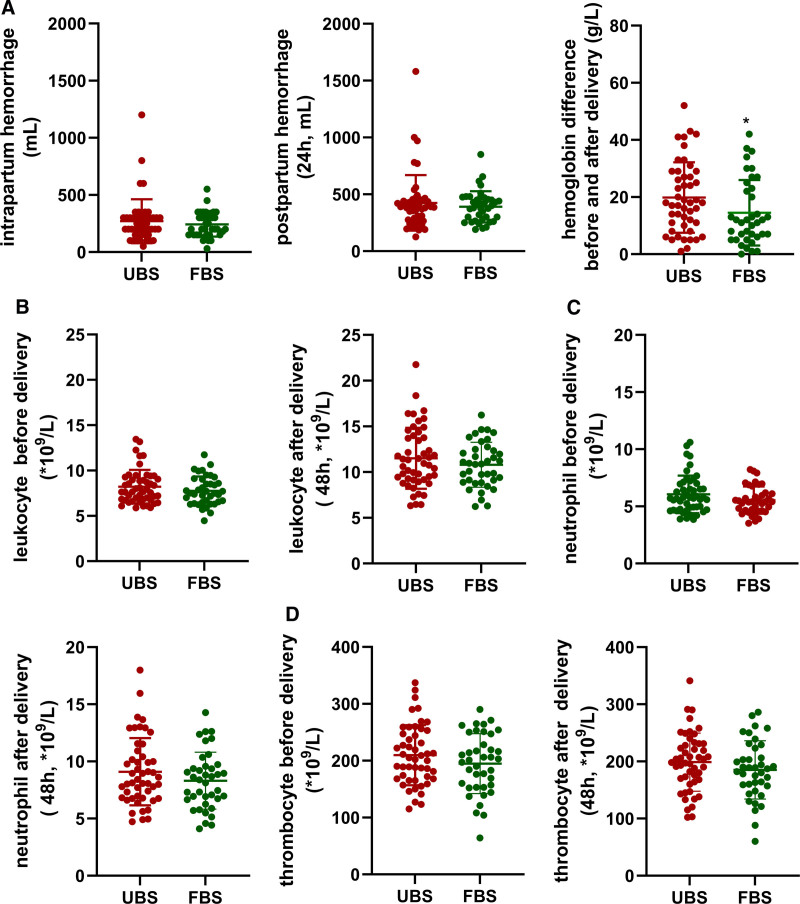
The amount of intrapartum hemorrhage (FBS vs UBS, 243.1 ± 107.9 mL vs 270.4 ± 192.8 mL, P = .431) and postpartum hemorrhage (24h, FBS vs UBS, 390.4 ± 137.8 mL vs 424.9 ± 243.7 mL, P = .434) was higher in the UBS group, but the difference was not statistically significant. However, the FBS group showed a smaller decrease in hemoglobin levels after delivery compared to admission (FBS vs UBS, 14.47 ± 11.46 g vs 19.83 ± 12.33 g, P = .042) (Fig. 2A).
Figure 2.
Comparison of intrapartum and postpartum hemorrhage volume, hemoglobin decline after delivery, infection markers, and thrombosis markers between the 2 groups. A showed changes in intrapartum hemorrhage volume, postpartum hemorrhage volume, and hemoglobin decline after delivery in both groups. B–D showed changes in infection and thrombosis markers including leukocyte, neutrophil, and thrombocyte before after delivery in 2 groups. FBS = amniotomy with favorable Bishop score, UBS = amniotomy with unfavorable Bishop score. *P < .05.
3.4. Comparison of infection and thrombosis markers between 2 groups
While the maternal infection rate was 14.29%, it was higher in the UBS group than in the FBS group, which had a rate of 2.56%. However, this difference was not statistically significant (Table 2). The levels of infection markers, including leukocytes and neutrophils, did not show significant differences between the 2 groups before and after delivery. There was also no significant difference in thrombocyte levels before and after delivery (Fig. 2B–D).
3.5. Comparison of neonatal outcomes between 2 groups
As presented in Table 3, there were no significant differences in neonatal outcomes, including the rate of Apgar Score at 1 to 5 minutes <7, arterial cord pH <7, lactic acid >6, Base excess <‐12 mmol/L, and admission to the neonatal intensive care unit.
4. Discussion
In this study, we demonstrated that amniotomy with a favorable Bishop score after Foley balloon catheter removal is associated with a lower incidence of cesarean delivery, a shorter duration from induction to delivery, less postdelivery hemoglobin decline, and no escalation in adverse maternal or neonatal outcomes.
Induction of labor is a common practice in obstetrics when the risks associated with continuing the pregnancy outweigh the risk of induction.[4,21] Labor induction involves cervical ripening and stimulating contractions. Amniotomy and oxytocin infusion followed by Foley balloon catheter removal are the main methods used for labor induction.[2,3] Many factors could affect the success of induced labor, such as age, BMI index, abortion history, number of previous births, cervical Bishop score at the time of induction, timing of amniotomy, methods for cervical ripening, and the protocol for oxytocin administration.[1,22] The baseline data from this study revealed that, except for Bishop score after Foley balloon catheter removal, there were no statistically significant variances in patient’s characteristics, including age, BMI index, gestational age, gravida, parity, percentage of nulliparous and multiparous, and Bishop score on admission between the 2 groups. Consequently, the findings from this study have the potential to offer a more precise and detailed analysis of the optimal timing of amniotomy after Foley balloon catheter removal in relation to the success of labor induction.
Previous studies demonstrated that early amniotomy after Foley balloon catheter removal was linked to a shorter duration of labor induction without any adverse maternal or neonatal outcomes, including cesarean delivery.[2,23,24] However, these studies defined early amniotomy as amniotomy at <1 to 2 hours after Foley balloon catheter removal without taking Bishop score under consideration. Other studies showed that amniotomy with cervical dilation <4 cm after Foley balloon catheter removal increased the risk of cesarean delivery among obese women,[8] this might be related to unfavorable Bishop score. These studies defined early or late amniotomy based on the duration between the removal of the Foley balloon catheter and the amniotomy or the cervical dilation at the time of the amniotomy. However, they did not consider the cervical Bishop score at the time of the amniotomy. Different from these studies, this study takes the Bishop score under consideration at the time of amniotomy after Foley balloon catheter removal and found that lower incidence of cesarean and shorter duration from induction to delivery in the patients who had amniotomy with favorable Bishop score, but no difference in the rate of operative vaginal delivery, demonstrated that the Bishop score assessed before amniotomy following removal of the Foley balloon catheter is a crucial factor in achieving successful vaginal delivery and shortening the process of labor. These results provide valuable insights into the potential benefits of considering the Bishop score in clinical decision-making regarding the timing of amniotomy.
A recent study found that delaying amniotomy for >4 hours after Foley balloon catheter removal extended the time from Foley balloon catheter removal to active labor and delivery and increased the postpartum hemorrhage rate.[9] The increased postpartum hemorrhage rate might be due to prolonged labor. In our study, the difference in volume of intrapartum hemorrhage and postpartum hemorrhage was not significant between the 2 groups, but amniotomy with favorable Bishop score after Foley balloon catheter removal exhibited a lower postdelivery hemoglobin decline, this may be associated with less blood loss. Moreover, there was no difference in the risk of chorioamnionitis and thrombosis between the 2 groups, these findings were consistent with previously published literatures.[9,25]
There have been no reported adverse neonatal outcomes associated with amniotomy,[23] we also did not find a significant difference in the rates of Apgar score at 1 to 5 minutes <7, arterial cord pH <7, lactic acid >6, base excess <‐12 mmol/L, and admission to the neonatal intensive care unit between 2 groups.
This study had some limitations. Only 103 clinical cases were included, and the limited data collected may affect the results. To improve the research quality, future studies will require a multicenter, large sample, and randomized controlled observations.
In conclusion, amniotomy with a favorable Bishop score after Foley balloon catheter removal has been shown to decrease the cesarean delivery rate, the time from induction to delivery, and hemoglobin decline after delivery. These benefits were observed without any adverse maternal or neonatal outcomes.
Author contributions
Conceptualization: Yun He, Yu Tao, Lanhua Liu.
Data curation: Yun He, Lanhua Liu.
Formal analysis: Zhuoyue Li.
Funding acquisition: Yun He, Lanhua Liu.
Investigation: Yu Tao, Ying Huang.
Methodology: Qin Ni, Zhuoyue Li, Ying Huang.
Project administration: Yun He, Yu Tao, Lanhua Liu.
Resources: Qin Ni.
Software: Ying Huang.
Supervision: Yun He, Yu Tao, Lanhua Liu.
Validation: Qin Ni.
Writing – original draft: Yun He.
Writing – review & editing: Lanhua Liu.
Abbreviations:
- FBS
- favorable Bishop score
- UBS
- unfavorable Bishop score
This work was supported by The Maternal and Child Health Research Project of Jiangsu Province (F202203), Taixing People’s Hospital doctoral foundation (trybs2022002), and The Research project of Jiangsu Association for Science and Technology (JSKXKT2023034).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: He Y, Tao Y, Ni Q, Li Z, Huang Y, Liu L. Assessing the timing of amniotomy after Foley balloon catheter removal in women with labor induction: The role of Bishop score: An observational study. Medicine 2024;103:51(e41068).
YH and YT contributed equally to this work.
Contributor Information
Yu Tao, Email: 804413071@qq.com.
Qin Ni, Email: niqin0316@163.com.
Zhuoyue Li, Email: 563069393@qq.com.
Ying Huang, Email: hy18761039358@163.com.
Lanhua Liu, Email: 13812492159@163.com.
References
- [1].Carlson N, Ellis J, Page K, Dunn Amore A, Phillippi J. Review of evidence-based methods for successful labor induction. J Midwifery Womens Health. 2021;66:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jamaluddin A, Azhary JMK, Hong JGS, Hamdan M, Tan PC. Early versus delayed amniotomy with immediate oxytocin after Foley catheter cervical ripening in multiparous women with labor induction: a randomized controlled trial. Int J Gynaecol Obstet. 2023;160:661–9. [DOI] [PubMed] [Google Scholar]
- [3].Appadurai U, Gan F, Hong J, Hamdan M, Tan PC. Six compared with 12 hours of Foley balloon placement for labor induction in nulliparous women with unripe cervices: a randomized controlled trial. Am J Obstet Gynecol MFM. 2023;5:101157. [DOI] [PubMed] [Google Scholar]
- [4].Wheeler V, Hoffman A, Bybel M. Cervical ripening and induction of labor. Am Fam Physician. 2022;105:177–86. [PubMed] [Google Scholar]
- [5].Stolyar S, Marx CJ. Align to define: ecologically meaningful populations from genomes. Cell. 2019;178:767–8. [DOI] [PubMed] [Google Scholar]
- [6].Hasan NA, Hong JGS, Teo IH, Zaidi SN, Hamdan M, Tan PC. Early versus delayed amniotomy with immediate oxytocin after Foley catheter cervical ripening in nulliparous labor induction: a randomized trial. Int J Gynaecol Obstet. 2022;159:951–60. [DOI] [PubMed] [Google Scholar]
- [7].Bala A, Bagga R, Kalra J, Dutta S. Early versus delayed amniotomy during labor induction with oxytocin in women with Bishop’s score of ≥6: a randomized trial. J Matern Fetal Neonatal Med. 2018;31:2994–3001. [DOI] [PubMed] [Google Scholar]
- [8].Battarbee AN, Glover AV, Stamilio DM. Association between early amniotomy in labour induction and severe maternal and neonatal morbidity. Aust N Z J Obstet Gynaecol. 2020;60:108–14. [DOI] [PubMed] [Google Scholar]
- [9].Berry M, Lamiman K, Slan MN, et al. Early vs delayed amniotomy following transcervical Foley balloon in the induction of labor: a randomized clinical trial. Am J Obstet Gynecol. 2024;230:567.e1–567.e11. [DOI] [PubMed] [Google Scholar]
- [10].Afrose R, Mirza TT, Sarker K, et al. Effect of amniotomy on outcome of spontaneous labour. Mymensingh Med J. 2021;30:6–12. [PubMed] [Google Scholar]
- [11].Smyth RM, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;2013:CD006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gimovsky AC, Pham A, Ahmadzia HK, Sparks AD, Petersen SM. Risks associated with cesarean delivery during prolonged second stage of labor. Am J Obstet Gynecol MFM. 2021;3:100276. [DOI] [PubMed] [Google Scholar]
- [13].Ivars J, Garabedian C, Devos P, et al. Simplified Bishop score including parity predicts successful induction of labor. Eur J Obstet Gynecol Reprod Biol. 2016;203:309–14. [DOI] [PubMed] [Google Scholar]
- [14].Kolkman DG, Verhoeven CJ, Brinkhorst SJ, et al. The Bishop score as a predictor of labor induction success: a systematic review. Am J Perinatol. 2013;30:625–30. [DOI] [PubMed] [Google Scholar]
- [15].Lee DS, Tandel MD, Kwan L, Francoeur AA, Duong HL, Negi M. Favorable simplified Bishop score after cervical ripening associated with decreased cesarean birth rate. Am J Obstet Gynecol MFM. 2022;4:100534. [DOI] [PubMed] [Google Scholar]
- [16].Melkie A, Addisu D, Mekie M, Dagnew E. Failed induction of labor and its associated factors in Ethiopia: a systematic review and meta-analysis. Heliyon. 2021;7:e06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simpson KR, James DC. Effects of oxytocin-induced uterine hyperstimulation during labor on fetal oxygen status and fetal heart rate patterns. Am J Obstet Gynecol. 2008;199:34.e1–5. [DOI] [PubMed] [Google Scholar]
- [19].Tsipoura A, Giaxi P, Sarantaki A, Gourounti K. Conventional cardiotocography versus computerized CTG analysis and perinatal outcomes: a systematic review. Maedica (Bucur). 2023;18:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Turkoglu O, Friedman P. Evaluation during postpartum hemorrhage. Clin Obstet Gynecol. 2023;66:357–66. [DOI] [PubMed] [Google Scholar]
- [21].Berghella V, Bellussi F, Schoen CN. Evidence-based labor management: induction of labor (part 2). Am J Obstet Gynecol MFM. 2020;2:100136. [DOI] [PubMed] [Google Scholar]
- [22].D’Souza R, Ashraf R, Foroutan F. Prediction models for determining the success of labour induction: a systematic review and critical analysis. Best Pract Res Clin Obstet Gynaecol. 2022;79:42–54. [DOI] [PubMed] [Google Scholar]
- [23].Gomez Slagle HB, Fonge YN, Caplan R, Pfeuti CK, Sciscione AC, Hoffman MK. Early vs expectant artificial rupture of membranes following Foley catheter ripening: a randomized controlled trial. Am J Obstet Gynecol. 2022;226:724.e1–9. [DOI] [PubMed] [Google Scholar]
- [24].Battarbee AN, Palatnik A, Peress DA, Grobman WA. Association of early amniotomy after Foley balloon catheter ripening and duration of nulliparous labor induction. Obstet Gynecol. 2016;128:592–7. [DOI] [PubMed] [Google Scholar]
- [25].Varvoutis MS, Sayres LC, Dotters-Katz SK. Is early amniotomy associated with higher likelihood of vaginal birth after cesarean? AJP Rep. 2020;10:e37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]