Abstract
Background:
There are advantages and disadvantages to both immediate 1-stage and 2-stage autologous-breast reconstruction. The 2-stage procedure may suffer from a hitherto overlooked difficulty: the tissue expander may induce chest wall depression that may require using a heavier-than-expected flap to generate symmetrical breasts. We conducted a retrospective observational study to assess this phenomenon.
Methods:
Consecutive patients who underwent 1-stage or 2-stage unilateral autologous-breast reconstruction with a deep inferior epigastric perforator flap were included. The 2 groups were compared in terms of age, body mass index, mastectomized tissue weight, inset-flap weight, and percentage additional flap weight (defined as [inset-mastectomy]/mastectomy × 100). The latter reflects the amount of additional flap tissue relative to mastectomized tissue that was needed to generate symmetrical breasts. The chest wall deformity after tissue expansion in the 2-stage patients was quantitated with computed tomography.
Results:
Patients’ healthy and affected breasts were symmetrical before surgery (P > 0.05). Compared with the 1-stage patients (n = 37), the 2-stage patients (n = 31) only differed in terms of a significantly higher mean percentage additional flap weight (28% versus 12%, P = 0.0077). Relative to preoperative values, nearly all 2-stage patients had mild (74%) or moderate (19%) chest wall deformity before tissue expander removal.
Conclusions:
Due to tissue expander-induced chest wall deformity, 2-stage breast reconstruction may require a larger flap volume than is anticipated on the basis of preoperative volumetric measurements. Considering this phenomenon when choosing between immediate 1-stage and 2-stage reconstruction could potentially help improve patient outcomes.
Takeaways
Question: What are the differences between immediate 1-stage and 2-stage reconstruction in unilateral autologous-breast reconstruction with a DIEP flap?
Findings: We conducted a prospective study comparing immediate 1-stage and 2-stage reconstruction patients in terms of mastectomized tissue weight, harvested-flap weight, and the inset-flap weight. We also investigated the chest wall deformity in our 2-stage patients. The present study showed that 2-stage reconstruction requires more donor tissue to achieve breast symmetry. Besides, 94% did have mild-to-moderate deformity.
Meaning: Due to tissue expander–induced chest wall deformity, 2-stage breast reconstruction may require a larger flap volume than is anticipated on the basis of preoperative volumetric measurements.
INTRODUCTION
In recent years, many surgical methods have been developed for breast reconstruction, including prosthesis reconstruction and autologous-tissue reconstruction with various flaps or fat grafting. We mainly perform immediate 1-stage and 2-stage autologous-breast reconstruction with the deep inferior epigastric perforator (DIEP) flap. The DIEP flap was first proposed for autologous-breast reconstruction by Koshima et al1 in 1989 and has since become one of the most commonly used methods. This is largely because this flap provides abundant adipose tissue, which makes it easier to shape the breast and increases the range of indications. There are also relatively few donor-site complications.2–4 Immediate 1-stage reconstruction involves performing mastectomy followed immediately by reconstructive surgery. Immediate 2-stage reconstruction involves conducting mastectomy followed immediately by insertion of tissue expanders to expand the breast skin; the reconstructive surgery is performed when the breast skin is considered sufficiently expanded. These 2 approaches have various relative advantages and disadvantages. The advantages of immediate 1-stage reconstruction include its avoidance of multiple procedures and temporary loss of the breast. It also involves less pain for the patient5 and creates a softer breast6 because skin scarring is minimized. However, some cases may require reoperation because mastectomy carries a risk of skin necrosis.7 Moreover, the duration of surgery is long.8 Immediate 2-stage reconstruction involves 2 shorter procedures and gives the patients time to consider the reconstruction method. However, the tissue expanders can lead to complications such as infection and leakage that may require correction with additional procedures, thus increasing the burden on the patient.6 Moreover, the temporary loss of the breast can be highly psychologically burdensome for the patient.5
Another potential difficulty of immediate 2-stage breast-reconstructive surgery is that the tissue expanders can induce depression of the underlying rib cage.9–11 Makiguchi et al11 showed that tissue expansion causes the anterior-to-posterior depth of the rib cage at the fourth costal bone to decrease on average by 3.7%, whereas Kim et al10 showed that this depression was on average 4.2 mm. This change is also common, having been reported in 53%–100% of 2-stage patients.9,12,13 To obtain breast symmetry, the depression must be compensated for by using a flap that is larger than would be anticipated on the basis of measurements taken before mastectomy. However, this aspect of 2-stage breast reconstruction is currently not well understood and may therefore be overlooked, thereby requiring unexpected modifications during surgery. To assess this issue, we conducted a retrospective study comparing immediate 1-stage and 2-stage reconstruction patients in terms of (1) mastectomized tissue weight, (2) harvested-flap weight, and (3) the “inset-flap weight.” To investigate the frequency and extent of chest wall deformity in our 2-stage patients, we also measured the chest wall deformity index (CDI) by using the method described by Kim et al.10
METHODS
Study Design and Ethics
This retrospective observational study was conducted at Nippon Medical School Hospital, Tokyo, Japan. It was approved by the hospital institutional ethics review board (No. 2023-1491) and was conducted according to the principles of the Declaration of Helsinki. Informed consent in the form of opt-out was enforced in this study.
Patient Selection
Consecutive patients who underwent immediate 1-stage or 2-stage unilateral autologous-breast reconstruction using a DIEP flap in May 2019–March 2023 were eligible for inclusion. The choice of immediate 1-stage or immediate 2-stage reconstruction was based on patient preference, operating room and surgeon schedules, and other factors. Patients were excluded if their medical records lacked information about the target variables; they underwent combined fat injections and reconstruction; or they underwent reconstruction after total mastectomy due to recurrence after partial mastectomy.
Computed Tomography Scans, Surgical Techniques, and Postoperative Care
All patients, including those with ductal carcinoma in situ or BRCA mutations, underwent preoperative full-body positron-emission tomography/computed tomography (CT) scanning at the department of breast surgery to search for distant metastases. All patients also underwent separate thin-slice angiographic CT scanning to select a perforating branch of the DIEP flap and evaluate recipient vessels. In the 2-stage patients, this angiographic CT scan was conducted just before expander removal and reconstruction.
The day before reconstructive surgery, the surgeon marked the chest reference line, evaluated the transverse width, length, height, and ptosis of the affected breast and measured the thickness of the abdominal-subcutaneous tissue with palpation and the CT imaging data. The perforating branch of the DIEP flap was also selected on the basis of the CT imaging, and the perforating branch was confirmed and marked by using Doppler ultrasound. The morphology of the abdomen was assessed to ensure an aesthetic result after DIEP flap harvest. All of these factors were used to design the flap.
Surgery was performed under general anesthesia. For immediate 1-stage reconstruction, mastectomy was conducted; the pectoralis-major muscle was divided and the internal-thoracic artery was approached from the third intercostal or third costal cartilage; and the flap was elevated and set into the recipient site. For the latter step, the main DIEP flap perforating branch was identified; a vascular pattern dissection was performed; the flap was dissected after confirming its blood flow with indocyanine green angiography; and vascular anastomosis was performed. The areas with inadequate reflux on indocyanine green angiography were trimmed. The breast was mounded and the flap was further trimmed as appropriate to create a symmetrical breast. For immediate 2-stage reconstruction, mastectomy was conducted; a tissue expander was inserted and inflated; the skin-incision line used during the initial surgery was incised; the tissue expander was removed; and the flap was set in, anastomosed, and sculpted as described earlier.
The patients were monitored after surgery for total and partial flap loss; hematoma; infection; breast-skin necrosis; and donor-site healing complications, namely, hematoma, wound margin necrosis, seroma, and hypertrophic scarring. Revision surgery was performed if the postoperative breast morphology was deemed inadequate.
Recorded Variables
Patient age, body mass index, type of mastectomy, duration of tissue expansion, adjuvant radiotherapy, postoperative complications, and need for revision surgery were obtained retrospectively from medical records. The weights of the mastectomized tissue, the harvested flap, and the inset flap after trimming were determined. The harvested DIEP flap was placed in a sterile bag and weighed in the operation room within 1–2 minutes of harvest. The DIEP flap tissues that were trimmed off during breast mounding were also weighed and subtracted from the weight of the harvested flap to calculate the inset-flap weight. We used these values to calculate two percentages: (1) Percentage excess flap weight = (harvested-flap weight – inset-flap weight) / harvested-flap weight × 100. This reflects the amount of trimming needed to obtain a symmetrical breast; if it is large, it indicates that excessive flap was harvested. (2) Percentage additional flap weight = (inset-flap weight – the mastectomy weight) / the mastectomy weight × 100. This reflects the unexpected additional flap weight relative to the mastectomy weight that was needed to obtain symmetry. Because breast sculpting was conducted relative to the healthy breast and breast asymmetry could affect the percentage additional flap weight, we also determined the symmetry of the affected and healthy breasts before reconstruction by measuring their depths at the nipple position from preoperative CT images.
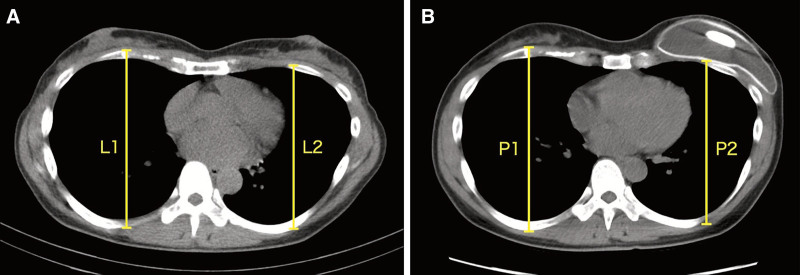
The CDI of the 2-stage patients was determined retrospectively from the transverse CT images obtained just before each of the 2 stages, as shown in Figure 1. Preoperative CT images were used to measure the distance from the top of the fourth rib to the bottom of the rib cage for both the healthy side (termed L1) and the affected side (L2). Before reconstructive surgery, the second CT scan was performed, and the same measurements were taken for the healthy side (termed P1) and the affected side (P2). The CDI was then calculated as (P2/P1) / (L2/L1) (Fig. 1). On the basis of the range of CDIs we observed, we categorized the CDIs as less than or equal to 0.899 (severe chest depression), 0.900–0.949 (moderate depression), 0.950–0.999 (mild depression), and greater than or equal to 1.000 (no depression). The degree to which the chest was depressed was also expressed as (1) percentage depression = (1 − CDI) × 100 and (2) depth of depression in millimeters.
Fig. 1.
Measurement of the CDI. Representative transverse CT images of a patient undergoing 2-stage autologous-breast reconstruction are shown. The distance from the top of the fourth rib to the bottom of the rib cage was measured for both the healthy (1) and affected (2) sides (A) before mastectomy (generating L1 and L2 measurements, respectively) and (B) just before tissue expander removal (generating P1 and P2 measurements, respectively). CDI was calculated as (P2/P1)/(L2/L1).
Statistical Analysis
The continuous and categorical variables were expressed as mean ± SD and n (%), respectively. The immediate 1-stage and 2-stage reconstruction groups were compared in terms of these variables with unpaired Student t test and chi-squared test. All statistical analyses were conducted with SPSS. P values of less than 0.05 were considered significant.
RESULTS
In total, 68 unilateral autologous-breast reconstructions using the DIEP flap were conducted: 37 and 31 were immediate 1-stage and 2-stage reconstructions, respectively (Table 1). Of the 1-stage patients, 24 and 13 underwent nipple-sparing and skin-sparing mastectomy, respectively. Of the 2-stage patients, 21 and 10 underwent nipple-sparing and skin-sparing mastectomy, respectively. Radiotherapy was administered in three 1-stage (8%) and three 2-stage (10%) cases, and only after completing reconstruction. The duration between mastectomy and radiation for the three 2-stage patients who underwent radiotherapy was based on clinical and other factors and was 10, 11, and 12 months, respectively.
Table 1.
The Data are Shown as Mean ± SD (Range)
| Variables | Immediate 1-stage Reconstruction | Immediate 2-stage Reconstruction | P |
|---|---|---|---|
| No. patients | 37 | 31 | |
| Type of mastectomy | |||
| Nipple-sparing mastectomy | 24 | 21 | |
| Skin-sparing mastectomy | 13 | 10 | |
| Adjuvant radiotherapy | 3 (8.1%) | 3 (9.7%) | 0.86 |
| Age (y) | 48.6 ± 7.4 (36–70) | 49.8 ± 7.2 (26–65) | 0.51 |
| BMI (kg/m2) | 22.3 ± 2.8 (17.1–28.3) | 22.9 ± 3.1 (16.6–31.6) | 0.44 |
| Duration of tissue expansion (mo) | 10.6 ± 4.9 (6–23) | ||
| CDI | 0.969 ± 0.027 (0.912–1.029) | ||
| Harvested-flap weight (g) | 489 ± 240 (119–960) | 529 ± 346 (180–2000) | 0.573 |
| Inset-flap weight (g) | 337 ± 169 (95–734) | 328 ± 166 (124–794) | 0.812 |
| Mastectomized tissue weight (g) | 318 ± 188 (70–820) | 271 ± 160 (74–636) | 0.28 |
| % Excess flap weight* | 28.4 ± 14.8 (2.5–62.4) | 34.2 ± 13.8 (3.1–63.1) | 0.105 |
| % Additional flap weight† | 11.5 ± 20.5 (−32.9 to 62.4) | 28.0 ± 28.7 (−14.6 to 90.5) | 0.0077 (<0.05) |
| Breast complication | |||
| Total flap loss | 0 (0%) | 1 (3.2%) | 0 |
| Partial flap loss (>1/3) | 1 (2.7%) | 1 (3.2%) | 0.204 |
| Partial flap loss (<1/3) | 4 (10.8%) | 3 (9.7%) | 0.915 |
| Hematoma | 1 (2.7%) | 1 (3.2%) | 0.204 |
| Infection | 0 (0%) | 0 (0%) | 0 |
| Breast skin necrosis | 3 (8.1%) | 0 (0%) | 0.09 |
| Hypertrophic scar | 4 (10.8%) | 9 (29.0%) | 0.068 |
| Donor complication | |||
| Hematoma | 0 (0%) | 0 (0%) | 0 |
| Wound margin necrosis | 1 (2.7%) | 0 (0%) | 0 |
| Seroma | 6 (16.2%) | 5 (16.1%) | 0.992 |
| Hypertrophic scar | 8 (21.6%) | 8 (25.8%) | 0.777 |
| Revision surgery for breast morphology | 1 (2.7%) | 3 (9.7%) | 0.243 |
All weights are in g.
BMI, body mass index.
% Excess flap weight was calculated as: % excess flap weight = (harvested-flap weight – inset-flap weight) / harvested-flap weight × 100. This reflects the amount of trimming needed to obtain a symmetrical breast.
% Additional flap weight was calculated as: % additional flap weight = (inset-flap weight – the mastectomy weight)/ the mastectomy weight × 100. This reflects the unexpected additional flap weight relative to the mastectomy weight that was needed to obtain symmetry.
On average, the 1-stage and 2-stage patients were, respectively, 49 and 50 years old; the body mass index was 22.3 and 22.9 kg/m2; the harvested-flap weight was 489 and 529 g; the inset-flap weight was 337 and 328 g; and the mastectomized tissue weight was 318 and 271 g. The 2 groups did not differ significantly in terms of these variables nor in percentage excess flap weight (28% versus 34%, P = 0.105). Importantly, however, the 2-stage group required significantly higher percentage additional flap weight (12% versus 28%, P = 0.0077): this indicates that although the 1-stage procedure required slightly more flap than the tissue that was mastectomized, this amount was more than doubled in the 2-stage procedure. Breast asymmetry played no role in percentage additional flap weight values because the mean preoperative affected/healthy breast depths were 31.4/30.6 mm for the 1-stage patients (P = 0.776) and 31.1/31.3 mm for the 2-stage patients (P = 0.911) (Table 2).
Table 2.
Data Are Shown as Mean ± SD (Range)
| Thickness of Affected Breast (mm) | Thickness of Healthy Breast (mm) | P * | |
|---|---|---|---|
| Immediate 1-stage reconstruction | 31.4 ± 11.9 (15–58) | 30.6 ± 10.8 (12–52) | 0.776 |
| Immediate 2-stage reconstruction | 31.1 ± 8.6 (19–49) | 31.3 ± 9.6 (16–52) | 0.911 |
P values were determined with the Student t test.
The 2 groups had similar frequencies of breast and abdominal complications. They also did not differ in terms of frequency of revision surgery to improve breast morphology (P = 0.243), which suggests there were similar levels of patient and surgeon satisfaction with the breast sculpting that was conducted during surgery (Table 1).
The mean indwelling duration of the expanders in the 2-stage group was 10.6 months. The mean ± SD CDI was 0.969 ± 0.027 (range, 0.912–1.029) (Table 1). This equated to 3.06% ± 2.74% depression (range, −2.94% to 8.82%) and 5.14 ± 4.77 mm depression (range, −4.00 to 16.44 mm). In total, 19%, 74%, and 6% of the patients had moderate (CDI = 0.999–0.949), mild (CDI =0.950–0.999), and no (CDI ≥ 1.000) chest depression, respectively. None had severe chest depression (CDI ≤ 0.899). The duration of expansion was 8.5, 11, and 10.2 months in the no-depression, mild-depression, and moderate-depression cases, respectively (P = 0.873) (Table 3).
Table 3.
The Data Are Shown as Mean ± SD (Range)
| Severe (≦0.899) | Moderate (0.900–0.949) | Mild (0.950–0.999) | None (≧1.000) | P | |
|---|---|---|---|---|---|
| No. patient | 0 | 6 | 23 | 2 | |
| CDI | — | 0.927 ± 0.013 (0.912–0.943) | 0.977 ± 0.015 (0.951–0.999) | 1.015 ± 0.021 (1.000–1.029) | |
| % Depression | — | 7.3 ± 1.3 (5.7–8.8) | 2.3 ± 1.5 (0.1–4.9) | −1.5 ± 2.1 (−2.9 to 0.0) | |
| Depth of depression (mm) | — | 13.0 ± 2.6 (9.9–16.4) | 3.7 ± 2.4 (0.1–8.1) | −2.0 ± 2.8 (−4.0 to 0.0) | |
| Duration of tissue expansion (mo) | — | 10.2 ± 4.8 (6–18) | 11.0 ± 5.2 (6-23) | 8.5 ± 0.7 (8–9) | 0.873 |
Representative Case 1
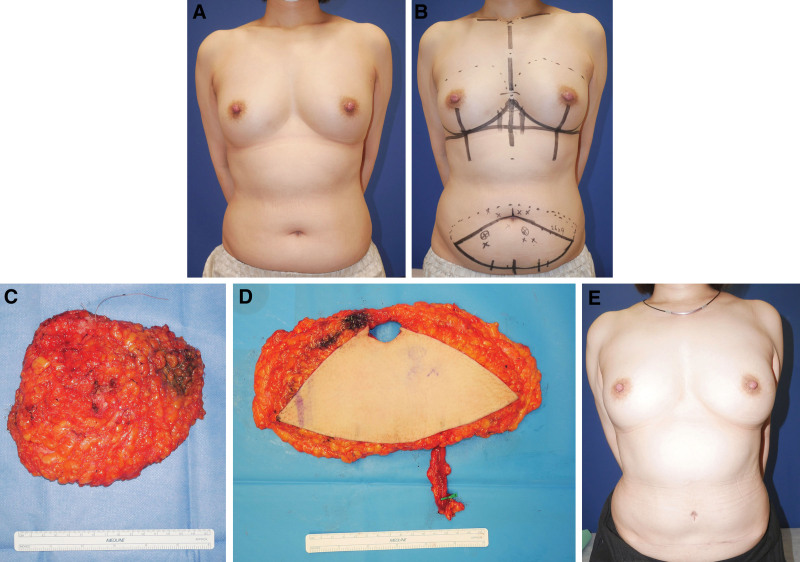
A 49-year-old woman with left-sided invasive ductal breast carcinoma (Fig. 2A) underwent nipple-sparing mastectomy and sentinel lymph node biopsy and was scheduled for immediate 1-stage DIEP flap reconstruction. The original breast was 13-cm wide, 12-cm long, and 4 cm in projection. A 9 × 26-cm flap was designed the day before surgery (Fig. 2B). The mastectomized tissue weight was 311 g (Fig. 2C). The harvested flap (Fig. 2D) weight was 534 g and inset flap weight was 350 g. The percentage excess flap weight was 35%. The percentage additional flap weight was 13%. The postoperative period passed without any major problems, and the breast morphology at 15 postoperative months was excellent (Fig. 2E).
Fig. 2.
Representative case of immediate 1-stage reconstruction with a DIEP flap. A, The patient was a 49-year-old woman with left-sided invasive ductal carcinoma of the breast. B, The flap was designed, after which nipple-sparing mastectomy, sentinel lymph node biopsy, and reconstruction were conducted in one operation. C, The mastectomy specimen. D, The DIEP flap. E, View of the patient 15 months after surgery.
Representative Case 2
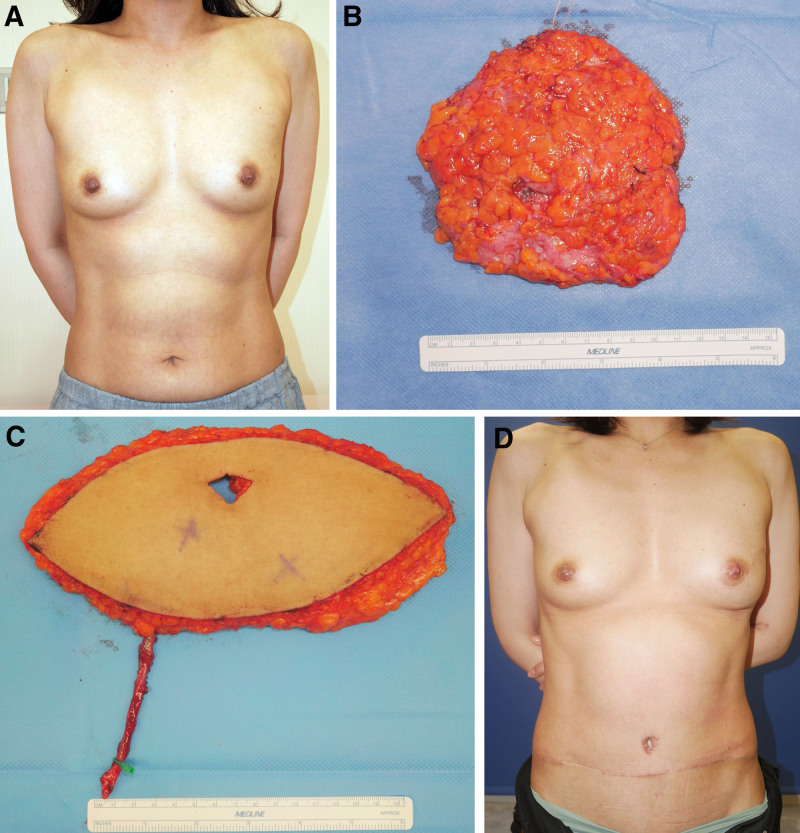
A 46-year-old woman with left-sided ductal carcinoma in situ of the breast (Fig. 3A) underwent nipple-sparing mastectomy and sentinel lymph node biopsy followed by tissue expander insertion. The mastectomized tissue weighed 124 g (Fig. 3B). The patient was scheduled for immediate 2-stage reconstruction with a DIEP flap 7 months after the initial surgery. The original breast was 12-cm wide, 13-cm long, and 5 cm in projection. A 9 × 22-cm flap was designed the day before the second surgery. The harvested flap (Fig. 3C) and inset flap weights were 311 and 215 g, respectively. The percentage of excess flap weight was 31%. The percentage additional flap weight was 73%. The CDI was 0.956. The postoperative course was good, and the breast morphology at 17 postoperative months was excellent (Fig. 3D).
Fig. 3.
Representative case of immediate 2-stage reconstruction with a DIEP flap. A, The patient was a 46-year-old woman with left-sided ductal carcinoma in situ of the breast who underwent nipple-sparing mastectomy, sentinel lymph node biopsy, and tissue expander insertion in the first operation. B, Mastectomy specimen. C, Seven months later, reconstruction with a DIEP flap was performed. D, View of the patient 17 months after reconstruction.
DISCUSSION
The present study showed that compared with 1-stage autologous-breast reconstruction, 2-stage reconstruction requires more donor tissue to achieve breast symmetry: the percentage additional flap weight that was needed was 12% for immediate 1-stage reconstruction and 28% for immediate 2-stage reconstruction. This difference was not due to breast asymmetry; rather, it appeared to reflect the chest concavity induced by the tissue expanders in the 2-stage patients. Notably, the greater flap tissue used in the 2-stage patients did not significantly increase the rate of revision surgery to improve breast morphology, meaning that the surgeons estimated how much flap tissue was needed with similar accuracy in both methods.
Thus, when designing the DIEP flap for the 2-stage procedure, it is necessary to consider in advance that more flap tissue may be needed to obtain breast symmetry than was originally expected on the basis of preoperative measurements. This is pertinent for most methods that are used to estimate the volume of flap needed. For example, we and others14 use not only preoperative assessments of the breast shape but also the mastectomy weight; the latter is useful for such volumetric estimations given that abdominal and thoracic subcutaneous tissues have a similar density (close to 1 g/mL).15,16 Similarly, the other methods of determining breast volume and estimating the required flap volume are generally based on preoperative assessments. These include the use of volumetric software solutions: all are based on ultrasound, CT, or magnetic resonance imagining data that are obtained before the first surgery.14,17–20
We observed that although none of the 2-stage patients demonstrated severe chest wall deformity, 94% did have mild-to-moderate deformity. Such high rates of deformity have also been observed in other studies (53%, 100%, and 100%),9,12,13 including in a cohort whose mean expansion time was similar to ours (9.6 versus 10.6 mo).9 The mean CDI that we observed in our study (0.969) was very similar to that reported by Kim et al in 57 two-stage patients (0.975).10 Moreover, we observed mean depression of 3.2% and 5.1 mm, which is similar to the values reported by Makiguchi et al (3.7%)11 and Kim et al (4.2 mm).10 Expansion duration may play a mild role in promoting deformity because our cases without deformity tended to have a shorter expansion time than the other cases (8.5 versus 10.2–11 mo, P = 0.567). Cherubino et al9 also noted that their 4 severe chest-deformity cases had longer expansion times than the remaining cases (3.3 versus 5.3 mo). However, expansion duration is likely to play at best a small role: although our mild- and moderate-deformity cases had similar expansion durations (11 and 10.2 mon, respectively), their average chest depression differed markedly (3.7 and 13 mm, respectively). It should be noted that the chest wall deformity can change over time after reconstructive surgery: Sinow et al12 and Cherubino et al9 showed with semiquantitative CT-based assessments that 11–12 months after surgery, 57% and 28% of patients demonstrated improved chest wall deformity scores. However, Cherubino et al also showed that although the deformity stayed the same in 61% of cases, 11% of cases worsened.9 Further longitudinal studies are needed to determine whether there are predictors of chest wall deformity. Two studies have already shown that capsular contracture and an older patient age may be associated with less chest wall deformity, possibly because the capsule acts as a buffer and older patients demonstrate less cartilage remodeling due to age-related calcification.10,11
Although it is important to ensure there is sufficient flap volume, large flaps can induce donor-site morbidity. For example, Park et al21 showed that larger harvested DIEP flap weights predict more postoperative pain. Thus, it is also important to minimize the harvested flap weight. This also leads to a more efficient procedure with fewer modifications during mounding. In our hospital, the mean percentage excess flap weight was 28% and 34% for immediate 1-stage and 2-stage reconstruction, respectively. This is similar to that reported for a series of 71 mostly immediate DIEP flap-based breast reconstruction cases (37%).17 This suggests that the amount of harvested flap was kept to a minimum in our patients.
It should be noted that our practice to wait ~6 months between expansion completion and reconstruction in the 2-stage patients largely reflects the greater proneness of Asian patients to develop hypertrophic scarring compared with White patients (odds ratio = 4.3): in our experience, the expanded skin in our patients takes ~6 months to stabilize. Even then, our 2-stage patients demonstrated a 2-fold higher rate of hypertrophic scarring than our 1-stage patients. Although this difference did not achieve statistical significance, it demonstrates the importance of considering the ethnic/familial risk of pathological scarring when offering immediate 1- or 2-stage breast reconstruction.
Study Limitations
This study has several limitations. First, the numbers of 1-stage and 2-stage patients were relatively low (37 and 31, respectively). However, previous studies examining 2-stage patients have similarly low sample sizes (n = 19–57).9–13 Second, we used the need for revision surgery to improve breast morphology as a surrogate measure of postoperative breast symmetry. Further studies should use a more objective measure. Third, the effect of chest wall deformity on the long-term outcomes of the 2-stage procedure was not assessed in our study. Further studies are needed to validate our findings and determine long-term outcomes. Fourth, our patients had little need for skin replacement, and radiation was only conducted after reconstruction. Therefore, our study findings should not be extrapolated to patients who require skin replacement and/or had preoperative radiotherapy. Fifth, our findings may be more pertinent for reconstructions of small breasts compared with large breasts.
CONCLUSIONS
When deciding between immediate 1-stage or 2-stage autologous-breast reconstruction, it is necessary to consider the advantages and disadvantages of each. The present study showed that immediate 2-stage reconstruction may require a heavier flap weight than anticipated from preoperative measurements because of chest wall concavity induced by the tissue expanders. This issue should be taken into consideration during the flap design phase and could help improve breast reconstruction outcomes and donor-site complications.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online 23 December 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645–648. [DOI] [PubMed] [Google Scholar]
- 2.Gill P, Hunt J, Guerra A, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153–1160. [DOI] [PubMed] [Google Scholar]
- 3.Garza R, Ochoa O, Chrysopoulo M. Post-mastectomy breast reconstruction with autologous tissue: current methods and techniques. Plast Reconstr Surg Glob Open. 2021;9:e3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahabedian M, Momen B, Galdino G, et al. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466–475. [DOI] [PubMed] [Google Scholar]
- 5.Zhong T, Hu J, Bagher S, et al. A comparison of psychological response, body image, sexuality, and quality of life between immediate and delayed autologous tissue breast reconstruction: a prospective long-term outcome study. Plast Reconstr Surg. 2016;138:772–780. [DOI] [PubMed] [Google Scholar]
- 6.Chevray P. Timing of breast reconstruction: immediate versus delayed. Cancer J. 2008;14:223–229. [DOI] [PubMed] [Google Scholar]
- 7.Barnes L, Patterson A, Lem M, et al. Immediate versus delayed-immediate autologous breast reconstruction after nipple-sparing mastectomy. Ann Plast Surg. 2023;90:432–436. [DOI] [PubMed] [Google Scholar]
- 8.Issa C, Lu S, Boudiab M, et al. Comparing plastic surgeon operative time for DIEP flap breast reconstruction: 2-stage more efficient than 1-stage? Plast Reconstr Surg Glob Open. 2021;9:e3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherubino M, Scamoni S, Maggiulli F, et al. Breast reconstruction by tissue expansion: What is the integrity of the chest wall? J Plast Reconstr Aesthet Surg. 2016;69:e48–e54. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Lee S, Najmiddinov B, et al. Risk factors for chest wall depression after implant insertion for breast reconstruction: a retrospective quantitative study. Gland Surg. 2022;11:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makiguchi T, Atomura D, Nakamura H, et al. Quantitative assessment and risk factors for chest wall deformity resulting from tissue expansion for breast reconstruction. Breast Cancer. 2019;26:446–451. [DOI] [PubMed] [Google Scholar]
- 12.Sinow J, Halvorsen R, Matts J, et al. Chest-wall deformity after tissue expansion for breast reconstruction. Plast Reconstr Surg. 1991;88:998–1004. [DOI] [PubMed] [Google Scholar]
- 13.Moor E, Wexler M, Bar-Ziv Y, et al. Chest wall deformity following maximal tissue expansion for breast reconstruction. Ann Plast Surg. 1996;36:129–132. [DOI] [PubMed] [Google Scholar]
- 14.Kono H, Ishii N, Takayama M, et al. A simple calculation for the preoperative estimation of transverse rectus abdominis myocutaneous free flap volume in 2-stage breast reconstruction using a tissue expander. Arch Plast Surg. 2018;45:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pot W, Kreulen M, Melis P, et al. Specific volume of female subcutaneous abdominal tissue as a reference in autologous breast reconstruction. J Reconstr Microsurg. 2010;26:185–188. [DOI] [PubMed] [Google Scholar]
- 16.Katch V, Campaigne B, Freedson P, et al. Contribution of breast volume and weight to body fat distribution in females. Am J Phys Anthropol. 1980;53:93–100. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Lim S, Pyon J, et al. Preoperative computed tomographic angiography of both donor and recipient sites for microsurgical breast reconstruction. Plast Reconstr Surg. 2012;130:11e–20e. [DOI] [PubMed] [Google Scholar]
- 18.Herold C, Reichelt A, Stieglitz L, et al. MRI-based breast volumetry-evaluation of three different software solutions. J Digit Imaging. 2010;23:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minn K, Hong K, Lee S. Preoperative TRAM free flap volume estimation for breast reconstruction in lean patients. Ann Plast Surg. 2010;64:397–401. [DOI] [PubMed] [Google Scholar]
- 20.Rosson G, Shridharani S, Magarakis M, et al. Three-dimensional computed tomographic angiography to predict weight and volume of deep inferior epigastric artery perforator flap for breast reconstruction. Microsurgery. 2011;31:510–516. [DOI] [PubMed] [Google Scholar]
- 21.Park J, Seong I, Woo K. Factors influencing postoperative abdominal pain in DIEP flap breast reconstruction. Gland Surg. 2021;10:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]