Abstract
The absence of a robust cell culture model of hepatitis C virus (HCV) infection has severely limited analysis of the HCV life cycle and the development of effective antivirals and vaccines. Here we report the establishment of a simple yet robust HCV cell culture infection system based on the HCV JFH-1 molecular clone and Huh-7-derived cell lines that allows the production of virus that can be efficiently propagated in tissue culture. This system provides a powerful tool for the analysis of host-virus interactions that should facilitate the discovery of antiviral drugs and vaccines for this important human pathogen.
Keywords: CD81, Huh-7, viral entry, viral spread, interferon
Hepatitis C virus (HCV) is a noncytopathic positive-stranded RNA virus that causes acute and chronic hepatitis and hepatocellular carcinoma (1). The hepatocyte is the primary target cell, although various lymphoid populations, especially B cells and dendritic cells, may also be infected at lower levels (2-4). A striking feature of HCV infection is its tendency toward chronicity, with at least 70% of acute infections progressing to persistence (1), which is often associated with significant liver disease, including chronic active hepatitis, cirrhosis, and hepatocellular carcinoma (5). Thus, with >170 million people currently infected (5), HCV represents a growing public health burden.
The HCV life cycle and host-virus interactions that determine the outcome of infection have been difficult to study, because cell culture and small animal models of HCV infection are not available. Thus, HCV infection studies to date have involved infected patients (6-8) and chimpanzees (9-12). The recent development of HCV replicon systems has also permitted the study of HCV translation and RNA replication in human hepatoma-derived Huh-7 cells in vitro (13, 14), revealing some of the host-virus interactions that regulate these processes (15-19). Nonetheless, these replicons do not replicate efficiently without adaptive mutations (20, 21), nor do they produce infectious virions. Thus, the relevance of replicons to HCV infection is unclear, and they do not permit analysis of the complete viral life cycle.
Wakita and colleagues (22, 23), however, have developed an HCV genotype 2a replicon (JFH-1) that replicates efficiently in Huh-7 cells, other human hepatocyte-derived cells (e.g., HepG2 and IMY-N9) (24), and nonhepatic cells (e.g., HeLa and HEK293) (25) without adaptive mutations. This group also recently reported that Huh-7 cells transfected with in vitro transcribed JFH-1 genomic RNA can secrete infectious viral particles.∥ Unfortunately, the infection efficiency observed was low, and infectious particles could not be propagated in naïve Huh-7 cells (26, ∥).
In contrast, we now report the establishment of a robust highly efficient in vitro infection system based on Huh-7-derived cell lines and the JFH-1 consensus clone. This system yields viral titers of 104-105 infectious units per ml of culture supernatant; infection spreads throughout the culture within a few days after inoculation at low multiplicities of infection (moi), and the virus can be serially passaged without loss in infectivity.
Materials and Methods
HCV Constructs and Transcription. The HCV consensus clone used was derived from a Japanese patient with fulminant hepatitis and has been designated JFH-1 (23). Wakita et al. (22) cloned this HCV cDNA behind a T7 promoter to create the plasmid pJFH-1, as well as a replication-defective NS5B negative control construct pJFH-1/GND (22). To generate genomic JFH-1 and JFH-1/GND RNA, the pJFH-1 and pJFH-1/GND plasmids were linearized at the 3′ end of the HCV cDNA by XbaI digestion. The linearized DNA was then purified and used as a template for in vitro transcription (MEGAscript; Ambion, Austin, TX).
Cell Culture. The hepatic (Huh-7 and Huh-7.5.1) and nonhepatic HEK293 (27) and HeLa (28) cells were maintained in complete DMEM supplemented with 10% FCS/10 mM Hepes/100 units/ml penicillin/100 mg/ml streptomycin/2 mM l-glutamine (Invitrogen) at 5% CO2. The human promyeloblastic HL-60 and monoblastoid U-937 cell lines were obtained from American Type Culture Collection and cultivated as recommended. The human hepatocarcinoma cell line HepG2 (American Type Culture Collection) (29) and Epstein-Barr virus-transformed B cells were maintained in RPMI medium 1640 with the same supplements described above (Invitrogen).
Huh-7.5.1 cells were derived from the Huh-7.5 GFP-HCV replicon cell line I/5A-GFP-6 (30), kindly provided by Charles Rice (Rockefeller University, New York). The I/5A-GFP-6 replicon cells were cultured 3 weeks in the presence of 100 units/ml human IFN-γ to eradicate the I/5A-GFP-6 replicon. Clearance of the HCV replicon bearing the neomycin resistance gene was confirmed by G418 sensitivity and HCV-specific reverse transcription real-time quantitative PCR (RT-QPCR) analysis.
HCV RNA Transfection. In vitro transcribed genomic JFH-1 RNA was delivered to cells by electroporation or liposome-mediated transfection. Electroporation was performed as described by Krieger et al. (31). Briefly, trypsinized cells were washed twice with and then resuspended in serum-free Opti-MEM (Invitrogen) at 1 × 107 cells per ml. Ten micrograms of JFH-1 RNA was mixed with 0.4 ml of the cells in a 4-mm cuvette, and a Bio-Rad Gene Pulser system was used to deliver a single pulse at 0.27 kV, 100 ohms, and 960 μF and the cells were plated in a T162 Costar flask (Corning). Liposome-mediated transfection was performed with Lipofectamin 2000 (Invitrogen) at an RNA/lipofectamin ratio of 1:2 by using 5 μg of JFH-1 RNA in cell suspensions containing 104 cells. Cells were then plated in DMEM with 20% FCS for overnight incubation. In both cases, transfected cells were transferred to complete DMEM and cultured for the indicated period. Cells were passaged every 3-5 days; the presence of HCV in these cells and corresponding supernatants were determined at the indicated time points.
RNA Analysis. Total cellular RNA was isolated by the guanidine thiocyanate method by using standard protocols (32). RT-QPCR analysis (for primer sequences, see Fig. 7, which is published as supporting information on the PNAS web site) was performed as described (19, 33), and HCV and GAPDH transcript levels were determined relative to a standard curve comprised of serial dilutions of plasmid containing the HCV JFH-1 cDNA or human GAPDH gene.
Indirect Immunofluorescence. Intracellular staining was performed as described (33). Polyclonal anti-NS5A rabbit antibody MS5 [a gift from Michael Houghton (Chiron)] was used at a dilution of 1:1,000 followed by incubation with a 1:1,000 dilution of Alexa555-conjugated goat anti-rabbit IgG (Molecular Probes) for 1 h at room temperature. Cell nuclei were stained by Hoechst dye.
Titration of Infectious HCV. Cell supernatants were serially diluted 10-fold in complete DMEM and used to infect 104 naïve Huh-7.5.1 cells per well in 96-well plates (Corning). The inoculum was incubated with cells for 1 h at 37°C and then supplemented with fresh complete DMEM. The level of HCV infection was determined 3 days postinfection by immunofluorescence staining for HCV NS5A. The viral titer is expressed as focus-forming units per milliliter of supernatant (ffu/ml), determined by the average number of NS5A-positive foci detected at the highest dilutions.
Amplification of HCV Viral Stocks. To generate viral stocks, infectious supernatants were diluted in complete DMEM and used to inoculate naïve 10-15% confluent Huh-7.5.1 cells at an moi of 0.01 in a T75 flask (Corning). Infected cells were trypsinized and replated before confluence at day 4-5 postinfection (p.i.). Supernatant from infected cells was then harvested 8-9 days p.i. and aliquoted for storage at -80°C. The titer of viral stock was determined as described above.
Concentration and Purification of HCV. Sucrose density-gradient ultracentrifugation analysis was performed as described (34). Pooled supernatant from two mock or two HCV-infected T162 cm2 Costar flasks (Corning) were centrifuged at 4,000 rpm for 5 min to remove cellular debris and then pelleted through a 20% sucrose cushion at 28,000 rpm for 4 h by using a SW28 rotor in an L8-80M ultracentrifuge (Beckman). The pellet was resuspended in 1 ml of TNE buffer (50 mM Tris·HCl, pH 8/100 mM NaCl/1 mM EDTA) containing protease inhibitors (Roche Applied Science, Indianapolis), loaded onto a 20-60% sucrose gradient (12.5-ml total volume), and centrifuged at 120,000 × g for 16 h at 4°C in a SW41Ti rotor (Beckman). Fractions of 1.3 ml were collected from the top of the gradient. The fractions were analyzed by RT-QPCR to detect HCV RNA. To determine the infectivity titer of each fraction, fractions were titrated on Huh7.5.1 cells as described above.
Blocking Infection with CD81- and E2-Specific Antibodies. Recombinant human monoclonal (IgG1) anti-E2 antibody was derived from a cDNA expression library (prepared from mononuclear cells of a HCV patient) that was screened against recombinant HCV genotype 1a E2 protein (GenBank accession no. M62321) by phage display. The antibody was serially diluted and preincubated with 15,000 ffu of JFH-1 virus in a volume of 250 μl for 1 h at 37°C. The virus antibody mixture was then used to infect 45,000 Huh-7.5.1 cells in a 24-well plate (Corning) for 3 h at 37°C. Mouse monoclonal anti-human CD81 antibody 5A6 (35) at 1 mg/ml (a gift from Shoshana Levy, Stanford University, Stanford, CA) was serially diluted (1:2,000, 1:200, and 1:20) and preincubated in a volume of 50 μl with 104 Huh-7.5.1 cells seeded in a 96-well plate for 1 h at 37°C. Cells were subsequently inoculated with infectious JFH-1 supernatant at an moi of 0.3 for 3 h at 37°C. The efficiency of the infection in the presence of antibodies was monitored 3 days p.i. by RT-QPCR and immunofluorescence.
Results
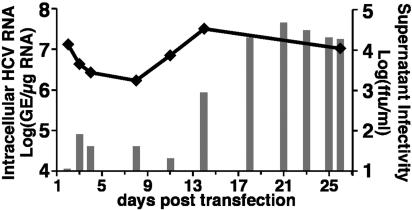
Production of Infectious HCV Particles in HCV RNA-Transfected Hepatoma Cells. Blight et al. (36) have established an Huh-7-derived cell line, termed Huh-7.5, that is highly permissive for replication of HCV replicons, including the I/5A-GFP-6 replicon (30) that expresses an NS5A-GFP fusion protein. For the current study, we cured I/5A-GFP-6 replicon cells with IFN-γ (see Materials and Methods) establishing an HCV-negative Huh-7.5-derived cell line, which we refer to as Huh-7.5.1. In a first set of experiments, 10 μg of in vitro transcribed genomic JFH-1 RNA was delivered into Huh-7.5.1 cells by electroporation. Transfected cells were then passaged when necessary (usually every 3-4 days) to maintain subconfluent cultures throughout the experiment. At the indicated times, total RNA was isolated from the transfected Huh-7.5.1 cells, and the level of HCV RNA was determined by HCV-specific RT-QPCR. Two days posttransfection, 1.3 × 107 copies of HCV RNA per μg of cellular RNA were detected (Fig. 1), probably reflecting a combination of input RNA and RNA produced by intracellular HCV replication. HCV RNA levels subsequently decreased, reaching a minimum level of 1.6 × 106 copies per μg of cellular RNA at day 8 posttransfection (Fig. 1). Importantly, however, intracellular HCV RNA levels began to increase thereafter, reaching maximal levels of >107 copies per μg of total RNA by day 14 posttransfection, and these levels were maintained until the experiment was terminated on day 26 (Fig. 1). These results suggested that HCV was actively replicating in transfected Huh-7.5.1 cells. This notion is supported by a rapid disappearance of a replication-incompetent JFH-1 RNA genome after transfection (Fig. 7).
Fig. 1.
Production of infectious HCV after transfection of genomic JFH-1 RNA. Ten micrograms of in vitro transcribed JFH-1 RNA was electroporated into 4 × 106 Huh-7.5.1 cells. Transfected cells and supernatant were harvested at the indicated time points posttransfection. Intracellular HCV RNA was analyzed by RT-QPCR and displayed as genome equivalents (GE)/μg total RNA (line). Supernatant infectivity titers were determined in naïve Huh-7.5.1 cells and are expressed as ffu/ml (bars).
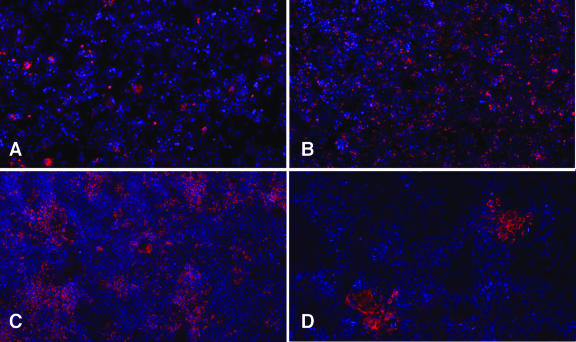
Interestingly, immunofluorescence staining for NS5A indicated that the percentage of NS5A-positive cells in the transfected cell cultures increased from 2% on day 5 (Fig. 2A) to almost 100% on day 24 (Fig. 2B). These results were consistent with the amplification of HCV RNA and further suggested either that HCV-transfected cells had acquired a selective growth advantage or that HCV was spreading to untransfected cells within the culture.
Fig. 2.
Detection of infected cells by NS5A immunofluorescence. (Upper) Immunofluorescent detection of NS5A in transfected cells: (A) day 5 and (B) day 24 posttransfection. (Lower) Infectivity titration of transfected cell supernatant on naïve Huh-7.5.1 cells; (C) undiluted supernatant; (D) 10-fold diluted supernatant. NS5A staining in red; nuclei stained with Hoechst (blue) (×50).
To determine whether the JFH-1-transfected Huh-7.5.1 cells were releasing infectious virus, we inoculated naïve Huh-7.5.1 cells with supernatants collected at different time points during the transfection experiment. Not only did immunofluorescence staining 3 days postinoculation reveal NS5A positive cells in the culture (Fig. 2C) but also, when the supernatants were serially diluted, the infection resulted in discrete foci of NS5A-positive cells (Fig. 2D), which allowed us to determine the ffu/ml in the supernatants collected at different times posttransfection. This type of supernatant titration was performed for the transfection experiment described in Fig. 1 and is indicated by vertical bars (Fig. 1). Infectious virus was detected in the culture medium 3 days after transfection (80 ffu/ml) and then increased, reaching a maximum of 4.6 × 104 ffu/ml by day 21 posttransfection, concomitant with the amplification of intracellular JFH-1 RNA.
Taken together, these results strongly suggest that Huh-7.5.1 cells transfected with genomic JFH-1 RNA were able not only to support HCV replication but also to produce infectious HCV particles. Notably, similar results were obtained when JFH-1 RNA was delivered to Huh-7.5.1 cells by an alternative transfection method (i.e., liposomes; Fig. 8, which is published as supporting information on the PNAS web site).
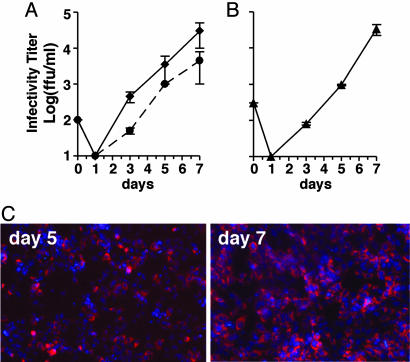
Propagation of HCV Virus Generated by Transfection. Next, we determined whether cells infected with JFH-1-transfected cell supernatant produced progeny virus that could be serially passaged to naïve Huh-7.5.1 cells. To do so, we infected naive Huh-7.5.1 cells at low multiplicity (moi = 0.01) with infectious supernatants collected from two independent transfection experiments and monitored infectious virus production by titrating the infected cell supernatants at the indicated time points. On day 1 after inoculation, no infectious particles were detectable in the supernatant of cells infected with either transfection cell inoculate (Fig. 3A). However, infectious particles exponentially accumulated in the supernatant thereafter, reaching a maximal titer of at least 104 ffu/ml on day 7 after both infections (Fig. 3A). Thus, within 7 days p.i., HCV produced in two independent transfection experiments was amplified in naïve Huh-7.5.1 cells >100-fold with very similar kinetics. Of note, we also performed additional experiments in which we monitored the intracellular levels of HCV RNA and proteins. This analysis confirmed that the appearance of infectious virus in the cell culture supernatant directly correlated with the amplification and subsequent translation of the input HCV RNA (Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 3.
HCV infection kinetics and passage in tissue culture cells. Naïve Huh 7.5.1 cells were inoculated with culture supernatants at an moi of 0.01. Supernatants from the inoculated cells were collected at the indicated times p.i. and evaluated for infectivity (ffu/ml). Data represent the average of two or more experiments with error bars. (A) Huh-7.5.1 cells inoculated with supernatant harvested at day 19 after transfection of Huh-7.5.1 cells with JFH-1 genomic RNA by electroporation (dashed line) or day 24 after lipofection (solid line). (B) Huh-7.5.1 cells inoculated with supernatant collected at day 5 from the infection shown as a solid line in A; (C) Increasing NS5A immunostaining in Huh-7.5.1 cells between days 5 and 7 p.i. in the experiment shown in B.
To determine whether the progeny virus produced by infection could be further passaged, we infected naive Huh-7.5.1 cells with the virus collected from one of the experiments shown in Fig. 3A (lipofection). As shown in Fig. 3B, this secondary infection progressed with kinetics similar to the primary infection (Fig. 3A), again reaching maximal levels on day 7. This was reflected by increasing numbers of NS5A-positive cells over the time course of the infection, with almost all of the cells being positive for NS5A at day 7 (Fig. 3C). These results indicate that the JFH-1 virus generated by transfection can be passaged in Huh-7.5.1 cells without a detectable loss in infectivity, and that it infects a high proportion of the cells in a relatively short period.
HCV Infection Is Inhibited by Anti-E2 Antibodies. Previous studies using HCV surface glycoprotein (E1/E2) pseudotyped viruses (37, 38) have suggested that E1 and/or E2 mediate the interaction with cellular receptors that are required for viral adsorption. To verify whether such an interaction is required for HCV infection in vitro, we performed neutralization experiments using anti-E2 antibodies in which the JFH-1 virus was preincubated with serial dilutions of a recombinant human monoclonal antibody specific for HCV E2 or an isotype-negative control antibody for 3 h at 37°C before infection.
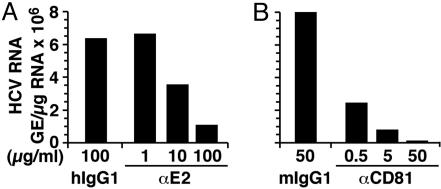
Huh-7.5.1 cells infected with JFH-1 virus (moi = 0.3) in the presence of 100 μg/ml of anti-E2 antibody were found to have 5-fold lower intracellular HCV RNA levels compared with cells infected in the presence of the same amount of an isotype control antibody (Fig. 4A), and this was reflected by a reduction in NS5A-positive cells as determined by immunofluorescence (data not shown). Titration of the anti-E2 antibody indicated that 10 μg/ml of antibody was required for a 50% reduction in intracellular HCV RNA 3 days p.i. (Fig. 4A). These results are therefore consistent with the conclusion that in vitro HCV infection in this system is at least in part mediated by the viral envelope E2 protein.
Fig. 4.
Inhibition of HCV infection by anti-E2 and anti-CD81 antibodies. (A) JFH-1 virus was preincubated with the indicated concentrations of anti-E2 antibody or irrelevant human IgG1 antibody for 1 h at 37°C before inoculating Huh-7.5.1 cells. Total cellular RNA was analyzed by RT-QPCR at day 3 p.i. (B) Huh-7.5.1 cells were preincubated with the indicated concentrations of anti-human CD81 or control mouse IgG1 antibody for 1 h at 37°C before inoculation with JFH-1 virus at an moi of 0.3. Total cellular RNA was analyzed by RT-QPCR at day 3 p.i.
HCV Infection Is Inhibited by Anti-CD81 Antibodies. Previous studies using pseudotyped viruses that express HCV E1/E2 have also suggested that the interaction between HCV E2 and CD81 is crucial for viral entry (39). To determine whether CD81 is required in this HCV infection system, anti-CD81 antibody-pretreated naïve Huh-7.5.1 were infected with JFH-1 virus at an moi of 0.3 and analyzed 3 days p.i. Intracellular HCV RNA levels were reduced in a dose-dependent manner with a 50-fold reduction at 50 μg/ml anti-CD81 antibody compared with the control antibody-treated cells (Fig. 4B).
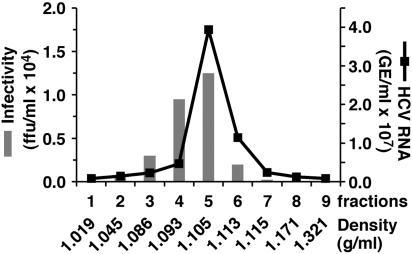
Biophysical Properties of Infectious HCV JFH-1 Particles. To examine the density of the secreted infectious HCV virions, supernatants collected from uninfected and HCV-infected Huh7.5.1 cells were subjected to sucrose gradient centrifugation. Gradient fractions were collected after centrifugation and analyzed for the presence of HCV RNA and infectivity (Fig. 5). Maximal infectivity titers (1.25 × 104 ffu/ml) were present in fraction 5 and coincided with the peak of HCV RNA. The ≈1.105 g/ml apparent density of the peak infectivity fraction is consistent with that previously reported for HCV virions isolated from patient sera (40, 41), indicating that the density of the recombinant JFH-1 virus is similar to that of human isolates.
Fig. 5.
Sucrose gradient sedimentation of infectious HCV. Supernatant from infected Huh-7.5.1 cells was fractionated as described in Materials and Methods. Fractions (1-9) were collected from the top of the gradient and analyzed by RT-QPCR for HCV RNA (line). The infectivity of each fraction was determined (bars) by titration. Fraction densities are expressed as g/ml.
In Vitro Tropism of JFH-1 HCV. To determine whether infection with the JFH-1 virus was restricted to Huh-7.5.1 cells, we attempted to infect a panel of hepatic (Huh-7 and HepG2) and nonhepatic cell lines (HeLa, HEK293, HL-60, U-937, and EBV-transformed B cells). Besides the Huh-7.5.1 cells, only the Huh-7 cells were permissive for HCV infection, as determined by immunofluorescent staining for the viral NS5A protein at day 3 p.i. (data not shown).
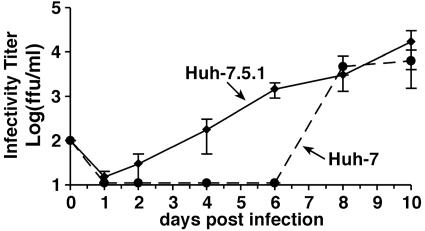
To determine whether there are quantitative differences in infection efficiency between the Huh-7.5.1 and Huh-7 cells, we infected both cell lines in parallel. As shown in Fig. 6, infectious particle release into the supernatant of infected Huh-7 cells appears to be delayed when compared with the particle production by Huh-7.5.1 cells. Nevertheless, Huh-7 cells produced similar amounts of infectious particles by days 8 and 10. Similar delayed kinetics in the amplification of intracellular HCV RNA was also observed in the Huh-7 cells (Fig. 10, which is published as supporting information on the PNAS web site). Taken together, these results demonstrate that Huh-7 cells can produce similar amounts of progeny virus as Huh-7.5.1 cells but with delayed kinetics.
Fig. 6.
Kinetics of JFH-1 HCV infection in Huh-7.5.1 and Huh-7 cells. A virus stock generated in Huh-7.5.1 was diluted to infect Huh-7.5.1 and Huh-7 cells at an moi of 0.01. Culture supernatant was collected at the indicated times and titrated. Infectious titers in Huh-7.5.1 (solid lines) and Huh-7 cells (dashed lines) are expressed as ffu/ml. Average values of two independent infection experiments are shown.
Discussion
The absence of a robust cell culture model of HCV infection has limited the analysis of the HCV life cycle and the development of effective antivirals and vaccines. Despite intensive efforts by many groups and the finding that in vitro transcribed HCV RNA is infectious when transfected into the liver of chimpanzees (42, 43), production of infectious HCV in vitro has remained problematic. Although a cell line capable of producing HCV-like virions was recently reported, the infectivity of those particles was not demonstrated (34). Furthermore, until recently, the replication efficiency of HCV replicons has depended on adaptive mutations (reviewed in ref. 44) that are attenuating in chimpanzees (45).
Interestingly, Kato et al. (22) produced an HCV genotype 2a replicon (JFH-1) that replicates efficiently in Huh-7 cells without adaptive mutations. Importantly, Wakita et al. (26, ∥) have also reported that JFH-1 RNA generated from the genomic JFH-1 viral clone can produce virus when transfected into Huh-7 cells. The limitation of this discovery has been the low efficiency of infection achieved and the resulting inability to perform controlled infection experiments.
Here, we report the establishment of a simple yet robust cell culture HCV infection system based on the reverse genetics system designed by Wakita et al. (26, ∥), which allows the rescue of infectious virus from the JFH-1 consensus cDNA clone. Our results demonstrate that transfection of JFH-1 RNA into the Huh-7-derived cells allows for the recovery of a viable JFH virus that can then be serially passaged and used for infection-based experimentation. Impressively, infection with serial dilutions of the virus resulted in the formation of infected cell foci that allowed us to quantitatively titrate the HCV being produced. Although the molecular basis of this viral spread pattern requires additional investigation, one can speculate that after infection at low multiplicity, HCV may spread by multiple mechanisms such as by attaching preferentially to adjacent cells after secretion and/or by spreading via cell-to-cell transmission.
The ability to monitor the complete HCV infection process in vitro has already provided some insight into the HCV life cycle. Specifically, the disappearance of input virus from the supernatant within 24 h p.i. suggests that particles were able to enter the cells within this time frame. As infectious viral titers rose from these undetected levels to 104-105 ffu/ml, the number of NS5A-positive cells also increased, suggesting that the virus was spreading to new cells (Fig. 3C). Importantly, when passaged to naïve Huh-7.5.1 cells, the virus produced by both transfected and infected cells exhibited the same infection kinetics with an HCV doubling time of ≈22 h. This doubling time is longer than the 6-8 h previously reported in infected patients (46) and chimpanzees (47); however, technical and biological factors may be responsible for this discrepancy. For example, earlier estimates were based on the number of HCV genome equivalents detected in the serum of infected individuals, not the infectivity titer as in the current study. The different doubling times may also reflect genotypic differences between the infecting viruses in the various studies and/or biological differences between Huh-7.5.1 cells and primary hepatocytes in the context of the liver.
The tropism of the JFH-1 virus thus far appears to be limited to Huh-7-derived cell lines. Even HepG2, HeLa, and HEK293 cells, which have been shown to support replication of the subgenomic JFH-1 replicon (22, 24, 25), failed to become infected. This suggests that the block to HCV infection in these cells may exist before RNA replication and translation. Importantly, that an antibody directed against the viral surface glycoprotein E2 reduced the infectivity of the JFH-1 virus suggests the process of viral adsorption and entry can be studied in this system. Consistent with this claim, HCV infection of Huh-7-derived cells was inhibited by an antibody against CD81 (Fig. 4), an extensively characterized putative HCV receptor (48).
Our initial efforts focused on the use of Huh7.5.1 cells, a cell line derived from Huh7.5 cells that has been shown to be highly permissive for HCV genotype 1 replicon replication (36). Importantly, however, non-HCV-adapted Huh-7 cells were also found to be susceptible to infection with the JFH-1 virus (Fig. 6). Virus amplification in Huh-7 cells did tend to display slower kinetics, but the Huh-7 cells eventually produced viral titers comparable to those attained in Huh-7.5.1 cells. The specific characteristics of the Huh-7.5.1 cells might be crucial in the future, because we expect mutants with reduced viability to be produced during reverse genetics studies investigating HCV biology.
Although the different kinetics of virus production in these two Huh-7-based cell lines is still under continued investigation, at this point, the differences observed are compatible with the idea that HCV infection may induce an innate antiviral response in the Huh-7 cells that is compromised in the Huh-7.5.1 cells. Specifically, it has been demonstrated that Huh-7.5 cells contain an inactivating mutation in RIG-I (49), a key component of the cellular double-stranded RNA-sensing machinery (50). Hence, our results suggest that HCV infection may induce a double-stranded RNA antiviral defense pathway in the Huh-7 cells, which transiently delays viral replication and/or spread. That HCV eventually overcomes the limitations present in Huh-7 cells and reaches titers similar to those produced by Huh-7.5.1 cells further suggests that expression of one or more viral encoded functions (e.g., NS3 or NS5A) may block or negate the intracellular antiviral defense(s). HCV infection, however, remained sensitive to the effects of exogenously added IFN, because both IFN-α and -γ prevented JFH-1 virus infection of Huh-7.5.1 cells (Fig. 11, which is published as supporting information on the PNAS web site). Interestingly, these in vitro observations appear to parallel those seen clinically (5, 46, 51), in which IFN therapy is able to reduce viral titers in some patients regardless of the mechanisms the virus has evolved that allow it to persist in the presence of the IFN it induces. Ongoing comparison of HCV infection in these different Huh-7-based cell lines should provide a unique opportunity to dissect and understand these critical virus-host interactions.
Conclusion
We have established a robust cell culture model of HCV infection in which infectious HCV can be produced and serially passaged to naïve cells. The availability of this system creates the opportunity to address aspects of the virus life cycle, including entry, trafficking, viral assembly, and egress, that have not been previously approachable. Likewise, many aspects of the host-virus relationship can now be explored, particularly the proviral factors that are required for viral production and the antiviral factors that control HCV infection. In addition to the deeper understanding of basic HCV infection biology that should be forthcoming from this system, it creates new investigative opportunities that could greatly accelerate the discovery of anti-viral drugs and contribute to the development of an HCV vaccine.
Supplementary Material
Acknowledgments
We thank Dr. Charles Rice (Rockefeller University, New York) and Dr. Darius Moradpour (University of Lausanne, Lausanne, Switzer-land) for providing the I/5A-GFP-6 replicon cell line, Dr. Rice for information about the susceptibility of Huh-7.5 cells to HCV infection before publication, and Dr. Shoshana Levy (Stanford University, Stanford, CA) for providing anti-CD81 antibodies. This study was supported by Grants CA108304 (to F.V.C.) and AI060391 (to S.L.U.) from the National Institutes of Health and by the Sam and Rose Stein Charitable Trust. S.K. was supported by the American Cancer Society-Gloria Rosen Postdoctoral Research Fellowship. T.W. was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Pharmaceuticals and Medical Devices Agency (PMDA) and by the Research on Health Sciences focusing on Drug Innovation from the Japan Health Sciences Foundation. This is manuscript number 17387-MEM from The Scripps Research Institute.
Author contributions: F.V.C. designed research; J.Z., P.G., G.C., and S.K. performed research; J.Z., P.G., G.C., S.K., S.L.U., S.F.W., and F.V.C. analyzed data; T.K., D.R.B., and T.W. contributed new reagents/analytic tools; and P.G., S.F.W., S.L.U., and F.V.C. wrote the paper.
Abbreviations: HCV, hepatitis C virus; moi, multiplicity of infection; RT-QPCR, reverse transcription real-time quantitative PCR; ffu/ml, focus-forming units per milliliter; p.i., postinfection.
Footnotes
Wakita, T., Kato, T., Date, T. & Miyamoto, M. 11th International Symposium on Hepatitis C and Related Viruses, 2004, Oct. 3-7, 2004, Heidelberg, Germany.
References
- 1.Hoofnagle, J. H. (2002) Hepatology 36, S21-S29. [DOI] [PubMed] [Google Scholar]
- 2.Kanto, T., Hayashi, N., Takehara, T., Tatsumi, T., Kuzushita, N., Ito, A., Sasaki, Y., Kasahara, A. & Hori, M. (1999) J. Immunol. 162, 5584-5591. [PubMed] [Google Scholar]
- 3.Auffermann-Gretzinger, S., Keeffe, E. B. & Levy, S. (2001) Blood 97, 3171-3176. [DOI] [PubMed] [Google Scholar]
- 4.Hiasa, Y., Horiike, N., Akbar, S. M., Saito, I., Miyamura, T., Matsuura, Y. & Onji, M. (1998) Biochem. Biophys. Res. Commun. 249, 90-95. [DOI] [PubMed] [Google Scholar]
- 5.Alter, H. J. & Seeff, L. B. (2000) Semin. Liver Dis. 20, 17-35. [DOI] [PubMed] [Google Scholar]
- 6.Thimme, R., Oldach, D., Chang, K. M., Steiger, C., Ray, S. C. & Chisari, F. V. (2001) J. Exp. Med. 194, 1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaki, A., Wiese, M., Maertens, G., Depla, E., Seifert, U., Liebetrau, A., Miller, J. L., Manns, M. P. & Rehermann, B. (2000) Nat. Med. 6, 578-582. [DOI] [PubMed] [Google Scholar]
- 8.Lechner, F., Wong, D. K., Dunbar, P. R., Chapman, R., Chung, R. T., Dohrenwend, P., Robbins, G., Phillips, R., Klenerman, P. & Walker, B. D. (2000) J. Exp. Med. 191, 1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logvinoff, C., Major, M. E., Oldach, D., Heyward, S., Talal, A., Balfe, P., Feinstone, S. M., Alter, H., Rice, C. M. & McKeating, J. A. (2004) Proc. Natl. Acad. Sci. USA 101, 10149-10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoukry, N. H., Sidney, J., Sette, A. & Walker, C. M. (2004) J. Immunol. 172, 483-492. [DOI] [PubMed] [Google Scholar]
- 11.Thimme, R., Bukh, J., Spangenberg, H. C., Wieland, S., Pemberton, J., Steiger, C., Govindarajan, S., Purcell, R. H. & Chisari, F. V. (2002) Proc. Natl. Acad. Sci. USA 99, 15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukh, J. (2004) Hepatology 39, 1469-1475. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, M., Yi, M., Li, K. & Lemon, S. M. (2002) J. Virol. 76, 2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmann, V., Korner, F., Koch, J., Herian, U., Theilmann, L. & Bartenschlager, R. (1999) Science 285, 110-113. [DOI] [PubMed] [Google Scholar]
- 15.Gale, M., Jr. (2003) Hepatology 37, 975-978. [DOI] [PubMed] [Google Scholar]
- 16.Katze, M. G., He, Y. & Gale, M., Jr. (2002) Nat. Rev. Immunol. 2, 675-687. [DOI] [PubMed] [Google Scholar]
- 17.Dubuisson, J. & Rice, C. M. (1996) J. Virol. 70, 778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye, J., Wang, C., Sumpter, R., Jr., Brown, M. S., Goldstein, J. L. & Gale, M., Jr. (2003) Proc. Natl. Acad. Sci. USA 100, 15865-15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapadia, S. B. & Chisari, F. V. (2005) Proc. Natl. Acad. Sci. USA 102, 2561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohmann, V., Korner, F., Dobierzewska, A. & Bartenschlager, R. (2001) J. Virol. 75, 1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blight, K. J., Kolykhalov, A. A. & Rice, C. M. (2000) Science 290, 1972-1975. [DOI] [PubMed] [Google Scholar]
- 22.Kato, T., Date, T., Miyamoto, M., Furusaka, A., Tokushige, K., Mizokami, M. & Wakita, T. (2003) Gastroenterology 125, 1808-1817. [DOI] [PubMed] [Google Scholar]
- 23.Kato, T., Furusaka, A., Miyamoto, M., Date, T., Yasui, K., Hiramoto, J., Nagayama, K., Tanaka, T. & Wakita, T. (2001) J. Med. Virol. 64, 334-339. [DOI] [PubMed] [Google Scholar]
- 24.Date, T., Kato, T., Miyamoto, M., Zhao, Z., Yasui, K., Mizokami, M. & Wakita, T. (2004) J. Biol. Chem. 279, 22371-22376. [DOI] [PubMed] [Google Scholar]
- 25.Kato, T., Date, T., Miyamoto, M., Zhao, Z., Mizokami, M. & Wakita, T. (2005) J. Virol. 79, 592-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakita, T., Pietschmann, T., Kato, T., Date, T., Miyamoto, M., Zhao, Z., Murthy, K., Habermann, A., Krausslich, H. G., Mizokami, M., Bartenschlager, R. & Liang, T. J. (2005) Nat. Med., in press. [DOI] [PMC free article] [PubMed]
- 27.Graham, F. L., Smiley, J., Russell, W. C. & Nairn, R. (1977) J. Gen. Virol. 36, 59-74. [DOI] [PubMed] [Google Scholar]
- 28.Gey, G. O., Coffman, W. D. & Kubicek, M. T. (1952) Cancer Res. 12, 264-265. [Google Scholar]
- 29.Knowles, B. B., Howe, C. C. & Aden, D. P. (1980) Science 209, 497-499. [DOI] [PubMed] [Google Scholar]
- 30.Moradpour, D., Evans, M. J., Gosert, R., Yuan, Z., Blum, H. E., Goff, S. P., Lindenbach, B. D. & Rice, C. M. (2004) J. Virol. 78, 7400-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger, N., Lohmann, V. & Bartenschlager, R. (2001) J. Virol. 75, 4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chomczynski, P. & Sacchi, N. (1987) Anal. Biochem. 162, 156-159. [DOI] [PubMed] [Google Scholar]
- 33.Kapadia, S. B., Brideau-Andersen, A. & Chisari, F. V. (2003) Proc. Natl. Acad. Sci. USA 100, 2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heller, T., Saito, S., Auerbach, J., Williams, T., Moreen, T. R., Jazwinski, A., Cruz, B., Jeurkar, N., Sapp, R., Luo, G. & Liang, T. J. (2005) Proc. Natl. Acad. Sci. USA 102, 2579-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy, S., Todd, S. C. & Maecker, H. T. (1998) Annu. Rev. Immunol. 16, 89-109. [DOI] [PubMed] [Google Scholar]
- 36.Blight, K. J., McKeating, J. A. & Rice, C. M. (2002) J. Virol. 76, 13001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartosch, B., Dubuisson, J. & Cosset, F. L. (2003) J. Exp. Med. 197, 633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu, M., Zhang, J., Flint, M., Logvinoff, C., Cheng-Mayer, C., Rice, C. M. & McKeating, J. A. (2003) Proc. Natl. Acad. Sci. USA 100, 7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, J., Randall, G., Higginbottom, A., Monk, P., Rice, C. M. & McKeating, J. A. (2004) J. Virol. 78, 1448-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hijikata, M., Shimizu, Y. K., Kato, H., Iwamoto, A., Shih, J. W., Alter, H. J., Purcell, R. H. & Yoshikura, H. (1993) J. Virol. 67, 1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trestard, A., Bacq, Y., Buzelay, L., Dubois, F., Barin, F., Goudeau, A. & Roingeard, P. (1998) Arch. Virol. 143, 2241-2245. [DOI] [PubMed] [Google Scholar]
- 42.Yanagi, M., Purcell, R. H., Emerson, S. U. & Bukh, J. (1997) Proc. Natl. Acad. Sci. USA 94, 8738-8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolykhalov, A. A., Agapov, E. V., Blight, K. J., Mihalik, K., Feinstone, S. M. & Rice, C. M. (1997) Science 277, 570-574. [DOI] [PubMed] [Google Scholar]
- 44.Bartenschlager, R., Kaul, A. & Sparacio, S. (2003) Antiviral Res. 60, 91-102. [DOI] [PubMed] [Google Scholar]
- 45.Bukh, J., Pietschmann, T., Lohmann, V., Krieger, N., Faulk, K., Engle, R. E., Govindarajan, S., Shapiro, M., St Claire, M. & Bartenschlager, R. (2002) Proc. Natl. Acad. Sci. USA 99, 14416-14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann, A. U., Lam, N. P., Dahari, H., Gretch, D. R., Wiley, T. E., Layden, T. J. & Perelson, A. S. (1998) Science 282, 103-107. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka, J., Katayama, K., Kumagai, J., Komiya, Y., Yugi, H., Kishimoto, S., Mizui, M., Tomoguri, T., Miyakawa, Y. & Yoshizawa, H. (2005) Intervirology 48, 120-123. [DOI] [PubMed] [Google Scholar]
- 48.Cormier, E. G., Tsamis, F., Kajumo, F., Durso, R. J., Gardner, J. P. & Dragic, T. (2004) Proc. Natl. Acad. Sci. USA 101, 7270-7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumpter, R., Jr., Loo, Y. M., Foy, E., Li, K., Yoneyama, M., Fujita, T., Lemon, S. M. & Gale, M., Jr. (2005) J. Virol. 79, 2689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoneyama, M., Kikuchi, M., Natsukawa, T., Shinobu, N., Imaizumi, T., Miyagishi, M., Taira, K., Akira, S. & Fujita, T. (2004) Nat. Immunol. 5, 730-737. [DOI] [PubMed] [Google Scholar]
- 51.Mbow, M. L. & Sarisky, R. T. (2004) Trends Biotechnol. 22, 395-399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.