Abstract
Introduction
Recent trials have demonstrated the remarkable benefit of endovascular treatment (EVT) up to 24 h in patients with large vessel occlusion (LVO) and target mismatch profiles; however, benefits of late-window EVT in Vietnamese population remain poorly understood. This study aims to evaluate the real-world outcomes of EVT in acute ischemic stroke (AIS) patients selected using perfusion imaging within the 6–24-h window.
Methods
This is a prospective study of consecutive patients with anterior circulation LVO stroke who underwent EVT within 6–24 h after last known well between August 2022 and March 2024. Patients were selected based on the DAWN/DEFUSE-3 criteria (Perfusion-RAPID, iSchemaView). The primary outcome was the proportion of patients with functional independence at 90 days (modified Rankin Scale score of 0–2). The secondary outcomes were successful reperfusion defined by thrombolysis in cerebral infarction (TICI) ≥2b on the final procedure and subgroup analysis between good (hypoperfusion intensity ratio [HIR] <0.4) and poor collaterals (HIR ≥0.4) groups. Safety outcomes were mortality rate and symptomatic intracranial hemorrhage (sICH).
Results
Of 122 enrolled patients, 68% met inclusion criteria of DEFUSE-3 trial, 61% met DAWN trial criteria. Mean age was 66 years, median baseline NIHSS was 13, median time from stroke onset to hospital arrival was 12.55 h (9.50–16.48), and median infarct volume was 11.5 mL. The rate of functional independence at 90 days was 45.9%. Successful reperfusion (TICI score of ≥2b) was achieved by 83.6% of cases. The 90-day mortality rate was 10.7%; sICH was reported in 8 patients (6.6%). Patients with good collaterals had better functional outcome.
Conclusions
This real-world observational study suggests that late-window EVT may be safe and effective in eligible Vietnamese patients selected based on perfusion imaging, thus supporting its practical use in this patient population. HIR is a robust indicator of collateral status and could made it a valuable addition to stroke imaging workup in clinical setting.
Keywords: Acute ischemic stroke, Endovascular treatment of acute stroke, 6–24 h, Late window, Perfusion imaging, Hypoperfusion intensity ratio, Collaterals
Plain Language Summary
Recent trials have demonstrated the benefit of endovascular treatment (EVT) up to 24 h in patients with large vessel occlusion (LVO) and target mismatch profiles; however, benefits of late-window EVT in Vietnamese population remain poorly understood. This study aimed to evaluate the real-world outcomes of EVT in acute ischemic stroke (AIS) patients selected using perfusion imaging within the 6–24-h window. This is a prospective study of 122 consecutive patients with anterior circulation LVO stroke who underwent EVT within 6–24 h after last known well between August 2022 and March 2024. Patients were selected based on the DAWN/DEFUSE-3 criteria (Perfusion-RAPID). The primary outcome was the proportion of patients with functional independence at 90 days (modified Rankin Scale score of 0–2). The secondary outcomes were successful reperfusion defined by thrombolysis in cerebral infarction (TICI) ≥2b and subgroup analysis between good (hypoperfusion intensity ratio (HIR) <0.4) and poor collaterals (HIR ≥0.4) groups. Safety outcomes were mortality rate and symptomatic intracranial hemorrhage (sICH). The rate of functional independence at 90 days was 45.9%. Successful reperfusion was achieved by 83.6% of cases. The mortality rate was 10.7%; sICH rate was 6.6%. Patients with good collaterals had better functional outcome. This real-world observational study suggests that late-window EVT may be safe and effective in eligible Vietnamese patients selected based on perfusion imaging. Hypoperfusion intensity ratio is a robust indicator of collateral status and could made it a valuable addition to stroke imaging workup in clinical setting.
Introduction
Large vessel occlusion (LVO) is the major cause of acute ischemic stroke (AIS), accounts for up to 30–38%, and significantly impacts disability and mortality [1, 2]. In 2015, five landmarks randomized controlled clinical trials had proven the benefits and safety of endovascular treatment (EVT) in acute anterior circulation stroke presenting within 6 h after last known well (LKW) [3]. Time is the most crucial factor for selecting patients in the conventional window, with the neurologic improvement highly dependent on time [4]. Recently, the DAWN (the DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) and DEFUSE-3 (the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) clinical trials, using RAPID-perfusion imaging protocols, had proven the benefits of EVT in selected patients presenting in the 6–24 h after stroke onset with a relatively small infarct core and a large ischemic penumbra [5, 6]. Outcomes of patients presenting within 6–24 h are highly dependent on the rate of infarct growth and the extent of the ischemic core and penumbra. In fact, the DAWN and DEFUSE-3 trials effectively identified patients with slow infarct growth progression in the late time window who were still eligible to benefit from EVT [5, 6]. Therefore, the current recommendation for stroke treatment supports the “tissue-clock” concept instead of the “time-clock” approach for patients in the late window [7]. Several factors affect the progression of ischemic core, among which collateral status has been shown to be an important predictor of infarct growth [8], the risk of hemorrhagic transformation, and clinical outcomes for patients with AIS due to LVO [9, 10]. Perfusion imaging provides noninvasive quantification, enabling the estimation of the infarct core and identification of salvageable tissue. Hypoperfusion intensity ratio (HIR) is derived from perfusion imaging and is defined as the ratio of tissue with a time to maximum (Tmax) >10 s and Tmax >6 s. Recent studies suggest that the HIR is associated with collateral status, infarct growth, and functional outcomes after EVT in patients with anterior circulation LVO [11].
In Vietnam, the EVT treatment time window has been extended up to 24 h after stroke onset in eligible patients selected based on perfusion imaging; however, there is still a lack of data on the safety and effectiveness of late-window EVT reperfusion in this population. Moreover, differences in epidemiology, ethnicity, clinical characteristics, and cerebral vasculature of the Vietnamese population compared to those in previous studies may pose challenges for thrombectomy and affect its clinical outcomes. Therefore, our study aimed to evaluate the real-world outcomes of EVT treatment in AIS patients with anterior LVO selected using RAPID-perfusion imaging within the 6–24-h window at the Tertiary Stroke Hospital in Ho Chi Minh City, Vietnam.
Methods
Study Design and Patient Enrollment
We conducted a prospective, observational single-center study of EVT patients who were treated 6–24 h after onset or LKW between August 2022 and March 2024 at the Department of Cerebrovascular Diseases at People’s Hospital 115 in Ho Chi Minh City. The following inclusion criteria were applied, which are consistent with routine treatment criteria for EVT in the late window: (1) an interval between stroke onset and groin puncture of 6–24 h; (2) age ≥18 years; (3) pre-stroke mRS score of 0–2; (4) had CTA (computed tomographic angiography)/magnetic resonance angiography evidence of an LVO (occlusion of internal carotid artery, M1 or proximal M2 segments of middle cerebral artery [MCA], or tandem); (5) met DAWN or DEFUSE-3 criteria. Patients with intracranial hemorrhage (ICH) on CT/magnetic resonance imaging (MRI) or those who refused to participate in this study were excluded. Perfusion imaging, including computed tomography perfusion (CTP) or magnetic resonance perfusion, was used to select patients presenting in the late window, following the current American Heart Association (AHA) guidelines, which recommend EVT for patients who meet the inclusion criteria of the DAWN or DEFUSE-3 trials [12]. The RAPID AI (iSchemaView, Menlo Park, CA, USA) software was used to measure the infarct volume and penumbral regions. The HIR, defined as the ratio of the volume of tissue with a time to maximum (Tmax) of >10 s divided by the volume of tissue with a Tmax of >6 s, can be quantitatively derived from MR perfusion datasets. Good collaterals were defined as HIR <0.4, and poor collaterals were defined as HIR ≥0.4 [11]. EVT was performed using stent retriever or aspiration device. The decision for EVT was made by a treating physician according to the hospital protocol. The primary outcome was the proportion of patients with functional independence at 90 days, defined as a modified Rankin Scale (mRS) score of 0–2. Secondary outcome included rates of successful reperfusion (TICI score ≥2b) on the final procedure and subgroup analysis between good and poor collateral groups. Safety outcomes were defined as ICH according to the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST) criteria [13] and the mortality rate. Any hemorrhage was defined as the presence of any ICH including intracerebral, subarachnoid, subdural, epidural, or intraventricular hemorrhage on the 24-h follow-up CT/MRI. The follow-up assessment with mRS at 90 days was evaluated via telephone or during outpatient visits. The study was approved by the People’s Hospital 115 Research Ethics Committee and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Statistical Analysis
Continuous variables were reported as median ± interquartile range (IQR). Categorical variables were reported as proportions. We also analyzed and compared the baseline characteristics before EVT, including age, sex, NIHSS score at admission, type of onset, history of atrial fibrillation, hypertension, diabetes, artery occlusion site, infarct core; treatment data; and functional outcomes between good collaterals (HIR <0.4) and poor collaterals (HIR ≥0.4) groups using descriptive statistics, including the χ2 test, Fisher’s exact test (for categorical variables), and Mann-Whitney U test or t test (for continuous variables) where appropriate. Data were analyzed using R (version 4.0.3 R Core Team [2020]), with a p value of 0.05 considered statistically significant.
Results
Study Population
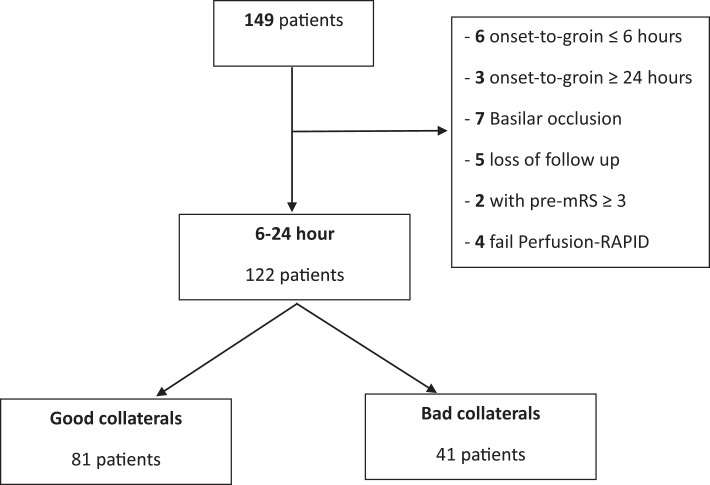
Between August 2022 and March 2024, of the 149 patients screened in People’s Hospital 115, a total of 122 patients were included in this study. Flowchart of study selection was shown in Figure 1. Eighty-three patients (68%) met inclusion criteria of DEFUSE-3 trial, and 74 patients (61%) met DAWN trial criteria. Baseline characteristics of patients are summarized in Table 1. The median age was 66 (IQR: 59–76) years, 68.9% were males, and median (IQR) baseline NIHSS was 13 (9.0–17.0). The rate of witness onset in our study is 54.9%. LVO was found in 43 (35.2%) patients in the intracranial internal carotid artery, 60 (49.2%) in the MCA-M1, 6 (4.9%) in the MCA-M2, and 13 (10.7%) in tandem. The median infarct volume was 11.5 mL. More than two-thirds of the patients had good collaterals (77.0%). The median of stroke onset to perfusion imaging and onset to groin puncture time was 15.95 h (IQR: 11.35–19.15) and 16.63 h (IQR: 13.25–21.30), respectively. On MR perfusion parameter maps, the median lesion volume of the ischemic core, hypoperfusion volume defined by Tmax >6 and Tmax >10 s was 11.5 mL (IQR: 4–26), 113 mL (IQR: 79–156), and 28 mL (IQR: 10–63), respectively. The median HIR was 0.3 (IQR: 0.1–0.4).
Fig. 1.
Study flowchart.
Table 1.
Baseline characteristics for all patients
| Patients and characteristics, n | All (n = 122) | Good collaterals (HIR <0.4), n = 81 | Poor collaterals (HIR ≥0.4), n = 41 | p value |
|---|---|---|---|---|
| Age, median (IQR), years | 66 (59–76) | 65 (56–72) | 71 (61–82) | 0.02 |
| Male sex, n (%) | 84 (68.9) | 56 (69.1) | 28 (68.3) | 1.0 |
| Medical history, n (%) | ||||
| Hypertension | 116 (95.1) | 77 (95.1) | 39 (95.1) | 1.0 |
| Diabetes mellitus | 30 (24.6) | 18 (22.2) | 39 (95.1) | 0.53 |
| Atrial fibrillation | 22 (18.2) | 11 (13.8) | 11 (26.8) | 0.13 |
| Type of onset, n (%) | ||||
| Witnessed onset | 67 (54.9) | 47 (58) | 20 (48.8) | 0.44 |
| Unwitnessed onset | 55 (45.1) | 34 (42) | 21 (51.2) | |
| NIHSS score (IQR) | 13 (9–17) | 12 (8–14) | 17 (14–21) | 0.00 |
| ASPECTS, median (IQR) | 7 (6–8) | 7 (6–8) | 6 (6–7) | 0.00 |
| Occlusion site, n (%) | ||||
| ICA | 43 (35.2) | 29 (35.8) | 14 (34.1) | 0.08 |
| MCA | 66 (54.1) | 42 (51.9) | 18 (43.9) | 0.9 |
| Tandem | 13 (10.7) | 9 (11.1) | 4 (9.8) | 1.0 |
| IV tPA given, n (%) | 6 (4.9) | 2 (2.5) | 4 (9.8) | 0.19 |
| TOAST, n (%) | ||||
| Cardioembolism | 24 (19.7) | 10 (12.3) | 14 (34.1) | 0.00 |
| Large artery atherosclerosis | 96 (78.7) | 69 (85.2) | 27 (65.9) | 0.03 |
| Other and undetermined | 2 (1.6) | 2 (2.5) | 0 (0) | 0.8 |
| Time metrics (median) (IQR), h | ||||
| Onset to arrival | 12.55 (9.50–16.48) | 13.68 (9.50–16.48) | 11.53 (9.80–15.15) | 0.47 |
| Door-to-perfusion imaging | 2.02 (1.37–3.02) | 2.03 (1.37–3.02) | 2.00 (1.40–3.02) | 0.67 |
| Perfusion imaging to groin | 1.48 (1.05–2.2) | 1.45 (1.05–2.18) | 1.58 (1.22–2.07) | 0.87 |
| Door to groin | 3.67 (2.72–5.1) | 3.63 (2.73–5.15) | 3.70 (2.68–4.80) | 0.69 |
| Groin to revascularization | 1.00 (0.70– 1.28) | 0.93 (0.65–1.23) | 1.02 (0.78–1.38) | 0.31 |
| Onset-to-perfusion imaging | 15.95 (11.35–19.15) | 16.38 (11.55–19.28) | 13.72 (11.10–18.75) | 0.34 |
| Onset to groin | 16.63 (13.25–21.30) | 17.68 (13.27–21.55) | 15.55 (13.25–20.35) | 0.36 |
| Onset to revascularization | 17.47 (14.38–21.92) | 18.02 (14.38–22.33) | 17.22 (14.58–21.18) | 0.49 |
| Endovascular devices | ||||
| Stentriever | 63 | 42 (51.9) | 21 (51.2) | 0.947 |
| Aspiration alone | 12 | 9 (11.1) | 3 (7.3) | 0.509 |
| Aspiration plus stentriever | 28 | 14 (17.3) | 14 (34.2) | 0.04 |
NIHSS, National Institutes of Health Stroke Scale; IQR, interquartile range; ASPECTS, Alberta Stroke Program Early CT Score; ICA, internal carotid artery; tPA, tissue plasminogen activator; TOAST, Trial of ORG 10172 in acute stroke treatment.
Clinical Outcomes
Of the 122 included patients, 56 (45.9%) were functionally independent at 90 days, while 30 (24.5%) had moderate to severe disability (mRS 4–5). The rate of successful reperfusion (TICI score ≥2b) on the final procedure was 83.6% of cases.
Mortality at 90 days was 10.7% of patients. Of these, 84.6% (11 of 13 patients) had an NIHSS score of 10 and above, and more than half (7 of 13 patients) had an NIHSS score above 15. Symptomatic ICHs were reported in 8 patients (6.6%). No thrombectomy-related complications were observed in our study (Table 2).
Table 2.
Primary and secondary outcomes between the good collaterals and poor collaterals
| Patients and characteristics, n | All (n = 122) | Good collaterals (HIR <0.4), n = 81 | Poor collaterals (HIR ≥0.4), n = 41 | p value |
|---|---|---|---|---|
| Efficacy outcome, n (%) | ||||
| mRS 0–2 at 90 days | 56 (45.9) | 43 (53.1) | 13 (31.7) | 0.04 |
| TICI ≥2b | 102 (83.6) | 66 (64.7) | 36 (35.3) | 0.53 |
| Safety outcomes, n (%) | ||||
| Symptomatic ICHa | 8 (6.6) | 3 (3.7) | 5 (12.2) | 0.16 |
| Any hemorrhageb | 41 (33.6) | 26 (32.1) | 24 (58.5) | 0.01 |
| Mortality | 13 (10.7) | 8 (9.9) | 5 (12.2) | 0.94 |
TICI, thrombolysis in cerebral infarction; ICH, intracranial hemorrhage.
aSymptomatic ICH was defined according to the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST) criteria as any parenchymal hemorrhage type 2 (PH-2) with a 4-point or more increase in NIHSS.
bAny hemorrhage was defined as the presence of any ICH including intracerebral, subarachnoid, subdural, epidural, or intraventricular hemorrhage on the 24-h follow-up CT/MRI.
Subgroup Analyses: Comparison between Good Collateral and Poor Collateral Groups
We performed a subgroup analysis of EVT patients, who were separated into good (HIR <0.4) and poor collateral (HIR ≥0.4) groups based on evaluation of perfusion datasets. Representative cases were shown in Figure 2. There were no significant differences between the two groups regarding sex, medical comorbidities, vessel occlusion site, time intervals (Table 1). The age was significantly lower in patients with good (median age 65; IQR: 56–72) compared with poor (median age 71; IQR: 61–82; p = 0.02) collaterals. The median NIHSS at admission was lower in those with good collaterals (12; IQR: 8–14) versus poor collaterals (17; IQR: 14–21; p < 0.05). The median ASPECTS was 7 (IQR: 6–8) for all patients, with a higher baseline ASPECTS seen in patients with good collaterals (p < 0.05). There were significant differences in the distribution of TOAST classifications between the two groups in which there was higher prevalence of large artery atherosclerosis in good collateral group, while poor collateral group had a higher prevalence of cardioembolism.
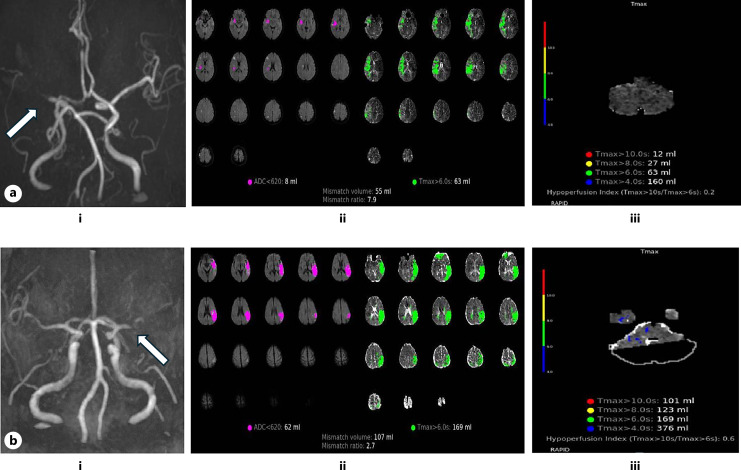
Fig. 2.
a Patient with favorable outcome (stroke onset to groin puncture time, 18.15 h; 90-day mRS score, 1). (i) Right MCA-M1 segment occlusion is observed on MRA image. (ii, iii) MRP parameter maps before thrombectomy indicate the ischemic core (ADC <620 and hypoperfusion volume [Tmax >6 s] of this patient are 8 and 63 mL, respectively). A small HIR (0.2) (good collaterals) was found. b Patient with unfavorable outcome (stroke onset to groin puncture time, 16.58 h; 90 day mRS score, 4). (i) Left MCA-M1 segment occlusion is shown on MRA image. (ii, iii) MRP parameter maps before thrombectomy indicate the ischemic core (ADC <620 and hypoperfusion volume [Tmax >6 s] of this patient are 62 and 169 mL, respectively). A large HIR (0.6) (bad collaterals) was found.
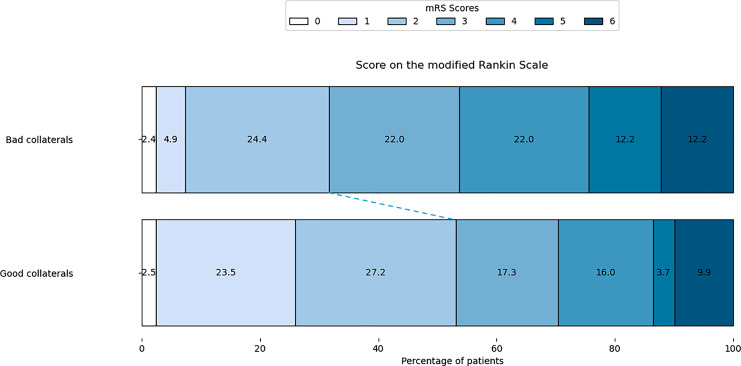
Ischemic core volume was lower in the good and poor collateral groups (8.0 mL [IQR: 3–17] vs. 26 mL [IQR: 14–45], respectively; p < 0.05). Patients with good collaterals had statistically smaller mismatch volumes, smaller Tmax >6 s and Tmax >10 s (Table 3). Clinical outcomes differed significantly between patients with HIR <0.4 and those with HIR ≥0.4, particularly in the rate of functional independence at 90 days, which was 53.1% in HIR <0.4 group compared to 31.7% in the HIR ≥0.4 group (p = 0.04). The result was shown in Figure 3.
Table 3.
HIR, core and perfusion volumes (technique: MRI)
| Patients and characteristics, n | All (n = 122) | Good collaterals (HIR <0.4), n = 81 | Poor collaterals (HIR ≥0.4), n = 41 | p value |
|---|---|---|---|---|
| Core and penumbra | ||||
| Core volume, mL | 11.5 (4–26) | 8 (3–17) | 26 (14–45) | 0.00 |
| Mismatch volume, mL | 94.5 (57–137) | 87 (53–128) | 110 (77–176) | 0.01 |
| Penumbra Tmax >6 volume, mL | 113 (79–156) | 94 (65–139) | 140 (115–194) | 0.00 |
| Penumbra Tmax >10 volume, mL | 28 (10–63) | 14 (4.0–30) | 74 (51–105) | 0.00 |
| Mismatch ratio | 8.6 (3.5–28.8) | 11.8 (4.9–49.3) | 4.2 (3.0–10.8) | 0.00 |
Data are given as n (%) and median (IQR).
MRI, magnetic resonance imaging; Tmax, time to maximum.
Fig. 3.
Score on the modified Rankin Scale at 90 days. Shown is the distribution of scores for disability on the modified Rankin scale (which ranges from 0 to 6, with higher scores indicating more severe disability) among patients in the bad collaterals group and the good collateral group. There was significant difference between groups (p = 0.04).
Discussion
According to current guidelines, EVT is strongly recommended for patients fulfilling the DAWN or DEFUSE-3 criteria within 24 h of stroke onset. This study provides a comprehensive analysis of EVT outcomes in AIS patients with anterior LVO within the 6–24-h window in a Vietnamese population, focusing on the impact of collateral circulation measured by the HIR. Our findings highlight the role of collateral circulation in determining stroke severity and patient outcomes following EVT in late window.
Our study demonstrated that 45.9% of patients achieved functional independence at 90 days. These outcomes were consistent with results from the DAWN trial (49%) and DEFUSE-3 trial (45.1%), which highlighted the benefits of EVT in carefully selected patients presenting beyond the conventional 6-h window. Regarding safety outcomes, the proportion of patients with sICH (6.6%) in our study was similar to the proportions of patients with sICH in the endovascular groups of the DAWN trial (6%) and DEFUSE-3 trial (7%). In contrast, mortality rate at 3 months in our study (10.7%) was lower compared to that in the endovascular groups of the DAWN trial (19%) and DEFUSE-3 trial (14%). Difference in mortality might be attributed to factors such as lower baseline age (66 vs. 69.4 [EVT arm in DAWN] and 70 [EVT arm in DEFUSE-3]), NIHSS score (13 vs. 16 [DAWN] and 17 [DEFUSE-3]). Access to MRI or CTP is not always available at many stroke centers globally. There are very little data on thrombectomy interventions using advanced imaging in Asian countries, especially in Southeast Asia. Katano et al. [14] investigate the efficacy and safety of mechanical thrombectomy for AIS patients selected through perfusion imaging when performed more than 16 h after the LKW. The results from this study are similar to those in our study. In the group of patients who underwent intervention within the 6–24-h window, the rate of favorable outcome (mRS 0–2) was 44.3%, while the rates of sICH and mortality were 6.25% and 9.9%, respectively. However, compared to PROSPR-SEA [15], a registry-based study conducted in Southeast Asian countries (Vietnam, Thailand, Singapore) that included EVT patients treated within 8 h of LKW, the rate of functional independence in PROSPR-SEA was higher (62.2%) and the mortality rate lower (7.8%) than in our study. The shorter treatment window and earlier intervention in PROSPR-SEA likely contributed to better outcomes. As a developing country, Vietnam struggles with the lack of resources, personnel, and apparatus, as well as a heavy workload, which cause prehospital and inhospital delays. As a result, our study had a longer median time from stroke onset to perfusion imaging (15.95 h) compared to the DEFUSE-3 trial (10.48 h). Despite this delay, patients in our study showed a small core (11.5 mL) and 77% of patients had favorable collateral circulation (HIR <0.4), resulting in slow progression and minimal harm caused by the delay.
Our subgroup analysis based on HIR <0.4 (good collaterals) and HIR ≥0.4 (poor collaterals) revealed critical differences. Patients with good collaterals were younger and presented with lower NIHSS scores at admission compared to those with poor collaterals. The lower ischemic core volumes, smaller mismatch volumes, lower Tmax >6 and >10 s, and higher ASPECT scores in the good collateral group suggest that robust collateral circulation helps preserve brain tissue, leading to better clinical outcomes. Functional independence at 90 days was significantly higher in patients with good collaterals (53.1%) compared to those with poor collaterals (31.7%), indicating that collateral status is a crucial determinant of long-term recovery. In addition, the good collateral group had a significantly higher prevalence of large artery atherosclerosis, whereas the poor collateral group had a higher prevalence of cardioembolism. AIS patients with underlying cardioembolism etiology are less likely to benefit from good collateral supply. This is consistent with findings by Rebello et al. [16], which indicate that AIS patients with cardioembolism do not typically present with favorable collateral status.
The quality of collateral blood supply to the affected hemisphere in LVO AIS is a significant predictor of infarct size and growth, functional outcome [17, 18], the rate of hemorrhagic transformation [9, 19, 20] and then has an impact on the clinical decision. Collateral assessment on digital subtraction angiography was considered the gold-standard method for evaluating the cerebral arteries, yet this assessment could be only derived during EVT, which limits its application on LVO AIS decision-making. Currently, imaging triage of collateral status is most commonly performed using CTA. Nevertheless, in our comprehensive stroke center, MRI and MR perfusion was the method used mostly (more than two-thirds of patients) in late-time window treatment for both patients direct triaged at our center or transferred from a primary stroke center. Time-of-flight magnetic resonance angiography in the setting of an LVO AIS does not allow evaluation of the distal vascular bed, preventing collateral assessment. As a simply derived perfusion parameter, HIR not only required experience from physicians in assessing collateral status as on CTA but also demonstrated a more detailed picture of collateral blood flow. A low HIR has been reported as a reliable marker of favorable collaterals [21] and was found to be associated with stroke severity and infarct growth in previous studies [11, 22, 23]. Moreover, RAPID AI software platform that automates the derivation and reporting of the HIR may provide valuable time savings and rapidly transfer results via messaging platforms to the entire stroke team, facilitating the decision-making process. Because the HIR can be derived from MR perfusion, centers that favor MR imaging for late-window EVT triage may also benefit from the HIR as a measure of collateral status.
Limitations
Our study has limitations. First, the study was conducted at a single thrombectomy-capable stroke center, which may lead to potential selection bias. Second, the study is limited by its prospective observational design and small sample size. However, our sample size was larger than that of the EVT group in the DAWN and DEFUSE-3 trials. Third, the only use of MR perfusion for patient selection in late-window treatment delayed time intervals and thus prolonged onset-to-revascularization window, which could impact on clinical outcomes. Fourth, the study involves an indirect comparison that suggests the safety and efficacy of thrombectomy within the 6–24-h window by comparing it to the EVT group treated in that timeframe across other studies, without a direct comparison to optimal medical treatment. This interpretation should be approached with caution. Future studies conducted at other stroke centers in Vietnam are needed to strengthen the reliability of our findings in this patient population.
Conclusion
The outcomes of extended window thrombectomy in our study was comparable with the results of DEFUSE-3 and DAWN trial. This real-world observational study suggests that late time-window EVT may be safe and effective in eligible Vietnamese patients selected based on perfusion imaging, thus supporting its practical use in this patient population. As an automated and quantitative tool, HIR is a robust indicator of collateral status and is easier to interpret than subjective CTA collateral scoring methods. This could made it a valuable addition to stroke imaging workup in clinical setting.
Acknowledgments
We would like to express our gratitude to all the clinicians, neurologists, interventionists, imaging and laboratory technicians, and statisticians for their contributions to the data collection and analysis for this study.
Statement of Ethics
This study protocol was reviewed and approved by the Ethics Committee of the People’s Hospital 115 and University of Medicine and Pharmacy at HCMC, Approval No. 566/HDDD-DHYD. We obtained written informed consent from all adult participants, and all participants’ next of kin if they were unable to provide consent, included in the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Dr. Binh Nguyen Pham, Dr. Hang T. Minh Tran, and Dr. An Thai Thanh Nguyen contributed to design the study, data acquisition, analysis, and interpretation and wrote the manuscript. Ryan Anh-Quang Nguyen and Huy Nguyen: literature review supervision. Dr. Anh Tuan Le Truong, Huong Bich Thi Nguyen, Trung Quoc Nguyen, Huy Quoc Do, Tri Quang Nguyen, Tra Vu Son Le, and Vu Thanh Tran collected the data. Dr. Huan Nguyen Pham did statistical analysis. Dr. Thang Ba Nguyen and Dr. Thang Huy Nguyen critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work, ensuring its integrity and accuracy.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
All data generated or analyzed during this study have been included in this article. For further inquiries, contact the corresponding author.
References
- 1. Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017;8:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lakomkin N, Dhamoon M, Carroll K, Singh IP, Tuhrim S, Lee J, et al. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. J Neurointerv Surg. 2019;11(3):241–5. [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31. [DOI] [PubMed] [Google Scholar]
- 4. Fransen PS, Berkhemer OA, Lingsma HF, Beumer D, van den Berg LA, Yoo AJ, et al. Time to reperfusion and treatment effect for acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2016;73(2):190–6. [DOI] [PubMed] [Google Scholar]
- 5. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. [DOI] [PubMed] [Google Scholar]
- 7. Vagal A, Aviv R, Sucharew H, Reddy M, Hou Q, Michel P, et al. Collateral clock is more important than time clock for tissue fate. Stroke. 2018;49(9):2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin L, Yang J, Chen C, Tian H, Bivard A, Spratt NJ, et al. Association of collateral status and ischemic core growth in patients with acute ischemic stroke. Neurology. 2021;96(2):e161–70. [DOI] [PubMed] [Google Scholar]
- 9. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42(3):693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qian J, Fan L, Zhang W, Wang J, Qiu J, Wang Y. A meta-analysis of collateral status and outcomes of mechanical thrombectomy. Acta Neurol Scand. 2020;142(3):191–9. [DOI] [PubMed] [Google Scholar]
- 11. Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. 2014;45(4):1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart association/American stroke association. Stroke. 2019;50(12):e344–418. [DOI] [PubMed] [Google Scholar]
- 13. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–82. [DOI] [PubMed] [Google Scholar]
- 14. Katano T, Suzuki K, Kimura R, Saito T, Nishiyama Y, Kimura K. Mechanical thrombectomy treatment more than 16 h after last known well for patients with large vessel occlusion. Cerebrovasc Dis Extra. 2023;13(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen HT, Ton MD, Vu DL, Churojana A, Nilanont Y, Ayudya SPSN, et al. Post-market registry of stroke patients treated with medtronic neurothrombectomy devices in Southeast Asia: PROSPR-SEA. American Heart Association. 2023;3(6):e000318. [Google Scholar]
- 16. Rebello LC, Bouslama M, Haussen DC, Grossberg JA, Dehkharghani S, Anderson A, et al. Stroke etiology and collaterals: atheroembolic strokes have greater collateral recruitment than cardioembolic strokes. Eur J Neurol. 2017;24(6):762–7. [DOI] [PubMed] [Google Scholar]
- 17. Menon BK, Qazi E, Nambiar V, Foster LD, Yeatts SD, Liebeskind D, et al. Differential effect of baseline computed tomographic angiography collaterals on clinical outcome in patients enrolled in the interventional management of stroke III trial. Stroke. 2015;46(5):1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47(3):768–76. [DOI] [PubMed] [Google Scholar]
- 19. Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79(6):625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42(8):2235–9. [DOI] [PubMed] [Google Scholar]
- 21. Guenego A, Fahed R, Albers GW, Kuraitis G, Sussman ES, Martin BW, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol. 2020;27(5):864–70. [DOI] [PubMed] [Google Scholar]
- 22. Christensen S, Mlynash M, Kemp S, Yennu A, Heit JJ, Marks MP, et al. Persistent target mismatch profile >24 hours after stroke onset in DEFUSE 3. Stroke. 2019;50(3):754–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guenego A, Marcellus DG, Martin BW, Christensen S, Albers GW, Lansberg MG, et al. Hypoperfusion intensity ratio is correlated with patient eligibility for thrombectomy. Stroke. 2019;50(4):917–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study have been included in this article. For further inquiries, contact the corresponding author.