Abstract
The synaptic vesicle protein Rab3A is a small GTP-binding protein that interacts with rabphilin and RIM1α, two presynaptic substrates of protein kinase A (PKA). Mice lacking RIM1α and Rab3A have a defect in PKA-dependent and NMDA receptor (NMDAR)-independent presynaptic long-term potentiation (LTP) at hippocampal mossy-fiber and cerebellar parallel-fiber synapses. In contrast, the NMDAR-dependent and PKA-independent early phase of LTP at hippocampal CA3–CA1 synapses does not require these presynaptic proteins. Here, we ask whether Rab3A and RIM1α participate in forms of LTP that require both PKA and NMDAR activation. We find that Rab3A is necessary for corticoamygdala LTP and late-phase LTP at CA3–CA1 synapses, two forms of LTP that require NMDAR and PKA activation. The latter form of LTP also requires RIM1α. These results provide genetic evidence that presynaptic proteins are required in LTP induced through the postsynaptic activation of NMDARs. Thus Rab3A and its effectors are general modules for four distinct types of PKA-dependent LTP in the brain.
Keywords: long-term potentiation, protein kinase A, RIM, Rab3A
The molecular analysis of long-term plasticity in the mammalian brain has to confront the existence of multiple forms of plasticity, both at a single synapse and across distinct synapses (1). Thus, whereas long-term potentiation (LTP) in its early phase (E-LTP) at the Schaffer collateral synapses between hippocampal CA3 and CA1 pyramidal neurons depends on postsynaptic NMDA receptor (NMDAR) activation and is independent of protein kinase A (PKA), LTP at hippocampal mossy-fiber synapses and cerebellar parallel-fiber synapses requires presynaptic PKA activation but not postsynaptic activation of NMDARs (2, 3). Moreover, whereas expression of Schaffer collateral E-LTP has a prominent postsynaptic component, both mossy-fiber and parallel-fiber LTP are expressed presynaptically. These distinctions have been confirmed at the molecular level. Although the presynaptic vesicle proteins Rab3A, a small GTP-binding protein, and its effector, RIM1α, are required for both mossy-fiber and parallel-fiber LTP, they are not necessary for NMDAR-dependent, Schaffer collateral LTP (4–7). These results suggest that NMDAR-dependent/PKA-independent LTP and NMDAR-independent/PKA-dependent LTP may constitute discrete cellular modules that are differentially expressed at different synapses. Here we investigate whether these modules can be combined to yield more complex forms of synaptic plasticity.
We address this question by examining whether Rab3A and RIM1α can also participate in forms of LTP that require both PKA and NMDA receptors and that have a presynaptic component to their expression. Rab3A and RIM1α are attractive targets for long-term PKA-dependent presynaptic plasticity because they both act to regulate the efficiency of transmitter release (7, 8), and RIM1α (but not Rab3A) is phosphorylated by PKA (9). Thus, we were interested in knowing whether Rab3A/RIM constitutes a module that is generally required for PKA-mediated forms of presynaptic long-term plasticity or is required for only those forms of plasticity that do not recruit NMDAR-dependent postsynaptic induction mechanisms.
Here, we examined knockout mice deficient in Rab3A or RIM1α to study the role of these proteins in the induction of corticoamygdala LTP and late LTP (L-LTP) at CA3–CA1 hippocampal synapses, two forms of PKA-mediated long-term presynaptic plasticity that require NMDAR-dependent postsynaptic Ca2+ influx (10–14). Our results show that Rab3A is required for the expression of both corticoamygdala LTP and CA3–CA1 L-LTP; RIM1α is also required for CA3–CA1 L-LTP. These results demonstrate a surprising unity in the molecular pathway that mediates four forms of PKA-dependent presynaptic plasticity at four distinct types of synapses in the mammalian brain.
Materials and Methods
The generation of the Rab3A and RIM1α mutant mouse lines (6, 8) and mouse breeding and care (15, 16) have been described previously. All analyses were carried out with homozygous wild-type and mutant littermates from matings of heterozygotes.
Transverse slices (400 μm) were prepared from either wild-type or mutant 6- to 12-week-old mice and maintained in an interface chamber (Fine Science Tools, Foster City, CA). Extracellular recordings were made in the CA1 region of hippocampus and the lateral amygdala as reported in refs. 10–12.
Calcium imaging in hippocampal CA1 neurons was performed by using whole-cell recordings obtained with an EPC-9 amplifier. Patch pipettes (3–5 MΩ) were filled with the following solution: 115 mM KMeSO4, 20 mM KCl, 10 mM Hepes, 4 mM MgCl2, 4 mM Na2ATP, 10 mM sodium phosphocreatine, 0.4 mM Na2GTP, 0.04 mM Alexa Fluor 594, and 0.6 mM Fluo-5F, titrated to pH 7.3 with KOH. Access resistance was 10–25 MΩ. Imaging was performed as described in ref. 17. Neurons were loaded with dye for 20–30 min before imaging. Red fluorescence (Alexa Fluor 594) was used to locate spines on apical dendrites within 50–150 μm of the soma. To measure Ca2+ concentration ([Ca2+]) changes, green fluorescence (Fluo-5F) was collected by using a 500-Hz line scan that intersected both the spine head and its parent dendrite. [Ca2+] changes were measured as ΔF/F0. Synaptic [Ca2+] transients were evoked with a tungsten bipolar electrode (FHC, Bowdoinham, ME) positioned 50–100 μm from the neuron at a stimulus intensity 50% of that needed to evoke a maximal field excitatory postsynaptic potential (fEPSP).
Results
LTP in the Corticoamygdala Pathway. We first investigated whether the PKA-dependent form of LTP at corticoamygdala synapses induced through postsynaptic NMDAR activation and expressed, at least in part, presynaptically also requires Rab3A. We previously characterized LTP at these synapses in rats (10, 11). Here, we first show that a similar form of LTP is present in wild-type mice. Orthodromic stimuli applied to the external capsule, a fiber bundle that carries axons from the auditory cortex to the amygdala, elicit a negative field potential in the lateral nucleus of the amygdala. In wild-type mice, delivery of 3 × 2 trains of stimulation (100 Hz for 1s at 10-s intervals) 3 min apart produced a significant enhancement of the fEPSP to 132 ± 7% (n = 6) of its initial level (Fig. 1A). A somewhat weaker LTP was observed with 20-Hz stimulation, (45 s) which enhanced the fEPSP to 116 ± 6% (n = 5) of its initial level, 30 min after the tetanus (Fig. 1B).
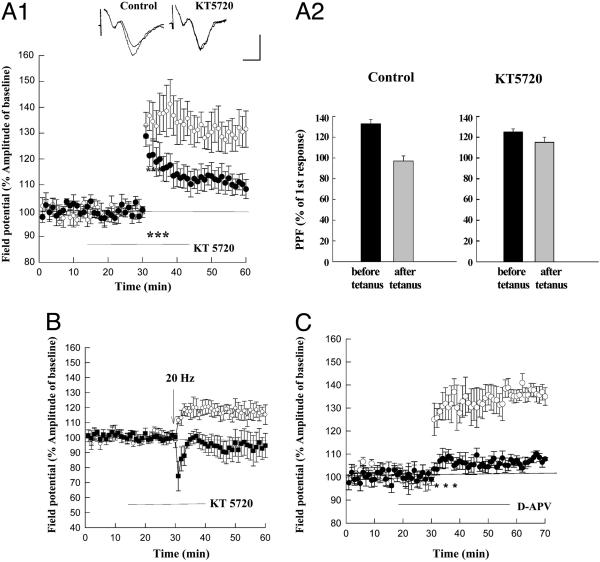
Fig. 1.
PKA-dependent synaptic plasticity at corticoamygdala synapses. (A1) LTP elicited by 3 × 2 trains (*, 100 Hz, 1 s) was significantly reduced in the presence of the PKA inhibitor KT5720 (filled circles). (Insets) Sample traces 10 min before and 30 min after the tetanus. (Scale bars: 5 ms, 1 mV.) (A2) The PPF ratio (fEPSP2/fEPSP1) is significantly reduced 15 min after the induction of LTP in wild-type mice (Left). There is no significant change in the PPF ratio after the delivery of the LTP-induction protocol in the presence of KT5720 (Right). (B) LTP induced by a 20-Hz train (45 s) was significantly depressed in the presence of KT5720 (filled circles). (C) Amygdala LTP induced by 3 × 2 trains of tetanic stimulation (*) was significantly depressed when D-APV was present during the tetanic stimulation (filled circles). Open circles in A1, B, and C are control LTP.
Both forms of LTP depended on PKA activation. Thus, in the presence of the PKA inhibitor KT5720 (1 μM), the 100-Hz tetanic stimulation enhanced the fEPSP only to 108 ± 3% of its initial value (n = 8), significantly less than that observed in the absence of the inhibitor (P < 0.01; Fig. 1 A1). In the presence of the PKA inhibitor, 20-Hz stimulation induced a small synaptic depression (94 ± 7%, n = 6), rather than the synaptic potentiation seen normally (P < 0.05, Student's t test; Fig. 1B). The amygdala LTP in wild-type mice depended on NMDAR activation, similar to amygdala LTP in the rat (11). Thus, when the tetanic stimulation was delivered in the presence of the NMDAR inhibitor d-2-amino-5-phosphonovaleric acid (D-APV) (50 μM), the fEPSP was enhanced only to 107 ± 1% (n = 6) of its initial value 40 min after the tetanus, significantly less than the 135 ± 3.8% enhancement seen under control conditions (n = 6, P < 0.01; Fig. 1C).
In agreement with previous studies in rats, we found that corticoamygdala LTP in wild-type mice also has a presynaptic component of expression, mediated by PKA. Thus, the magnitude of paired-pulse facilitation (PPF) (50-ms inter-pulse interval), a marker of presynaptic function, was reduced from its initial value of 133 ± 4% to only 97 ± 5%, 15 min after the induction of LTP (n = 6, P < 0.01; Fig. 1 A2 Right). By contrast, in the presence of the PKA inhibitor KT5720, the change in PPF after tetanic stimulation was blocked [126 ± 2% (before) and 115 ± 6% (after) LTP; n = 6, P > 0.5; Fig. 1 A2 Left].
The above results confirm that corticoamygdala LTP has properties of both Schaffer collateral LTP, because it requires NMDARs, and mossy-fiber/parallel-fiber LTP, because it requires PKA and has a presynaptic expression component. We next examined the importance of Rab3A for corticoamygdala synaptic function.
We first examined the PPF of basal synaptic transmission because it is enhanced in Rab3A-/- mice at Schaffer collateral synapses but not at mossy-fiber synapses (6, 7). In this respect, we find that the corticoamygdala synapses resemble Schaffer collateral synapses because the PPF is significantly enhanced in the Rab3A-/- mice (Fig. 2A). The PPF at a 40-ms interval is 124 ± 2% in wild-type mice (n = 14 slices, five mice) and increases to 141 ± 5% in Rab3A-/- mice (n = 11, six mice; P < 0.01). These results confirm the finding at CA3–CA1 synapses that Rab3A regulates transmitter-release efficiency.
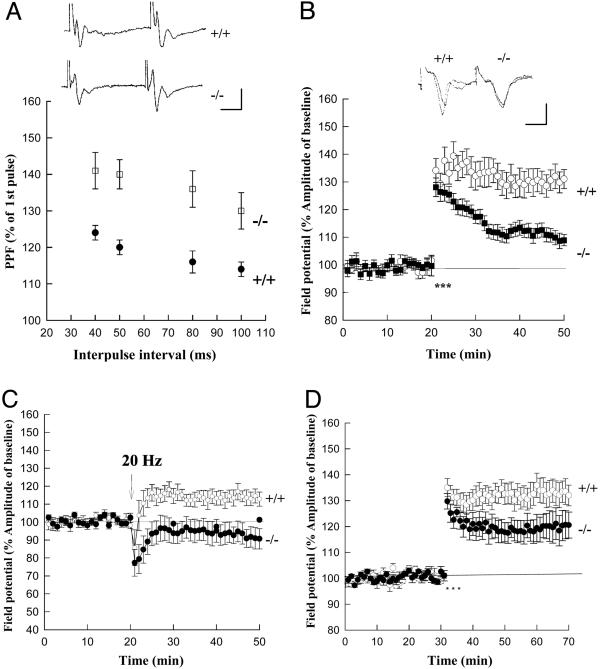
Fig. 2.
Short- and long-term synaptic plasticity in the corticoamygdala pathway of Rab3A-knockout mice. (A) The PPF ratio (fEPSP2/fEPSP1 amplitude) at intervals of 40, 50, 80, and 100 ms was significantly enhanced in the Rab3A-knockout (open squares) compared with the wild-type (filled circles) mice. (Insets) Sample traces from wild-type (+/+, upper trace) and knockout (-/-, lower trace) mice. (B) LTP elicited by 3 × 2 trains (*, 100 Hz, 1s) was significantly reduced in Rab3A-/- (filled squares) compared with wild-type (open circles) mice. (Insets) Sample traces 10 min before and 30 min after tetanus. (C) LTP induced by a 20-Hz train (45 s) was significantly depressed in Rab3A-/- (filed circles) compared with wild-type (open circles) mice. (D) LTP elicited by 3 × 2 trains of tetanus (*, 100 Hz, 1 s) in the RIM1α-/- (filled circles) mice was not significantly different from that in wild-type (open circles) mice. (Scale bars: 10 ms, 1 mV.)
Does deletion of Rab3A alter corticoamygdala LTP? We find that in the Rab3A-/- mice, the magnitude of LTP elicited by either 100-Hz or 20-Hz tetanic stimulation is greatly diminished. Thus, 100-Hz tetanic stimulation in the Rab3A-/- mice increased the fEPSP to only 108 ± 2% of its initial value, as determined 30 min posttetanus (n = 16, nine mice), significantly less than the enhancement of 131 ± 3% seen in wild-type mice (n = 14, nine mice; P < 0.01; Fig. 2B). Similarly, although the 20-Hz train induced a modest potentiation of 113 ± 4% (n = 10, five mice) in wild-type mice, it caused a small depression of synaptic transmission in the Rab3A-/- mice to 90 ± 5% of the initial fEPSP (n = 14, six mice; P < 0.05; Fig. 2C). The impairment of LTP with both 100-Hz and 20-Hz induction protocols indicates that Rab3A-deficient mice have a general deficit in LTP in the corticoamygdala pathway.
What are the downstream targets of Rab3A? How does Rab3A, which is not a PKA substrate, participate in PKA-dependent LTP? To address these questions, we examined the role of RIM1α, an active zone protein that binds to Rab3A, is a substrate of PKA, and is required for mossy-fiber LTP (5, 6). Forty minutes after the tetanus, the size of amygdala LTP was 120 ± 5% in RIM1α-/- mice (n = 7), less than that in wild-type mice (132 ± 4%) (n = 7; Fig. 2D), although the difference is not statistically significant (P = 0.18). The weaker deficit of amygdala LTP in RIM1α-/- mice, as compared with that in Rab3A-/- mice, could be due to a compensating effect of RIM2 expression.
L-LTP in the Hippocampal Schaffer Collateral Pathway Requires Rab3A. We next examined the role of Rab3A and RIM1α in another form of LTP that depends on both PKA and NMDAR activation, the translation- and transcription-dependent L-LTP at Schaffer collateral synapses (12, 13). L-LTP, presumably, has an important postsynaptic induction component, based on its requirement for NMDAR activation. However, quantalanalysis indicates that the expression of L-LTP induced by SP-adenosine 3′,5′-cyclic monophosphorothioate (SP-cAMPS) has a presynaptic component that involves an increase in the number of functional sites of transmitter release (14), thus suggesting a role for presynaptic proteins.
Does the deletion of Rab3A alter the magnitude of L-LTP at CA3–CA1 synapses, as would be predicted if this molecule were generally required for PKA-dependent forms of presynaptic plasticity? We first confirmed that deletion of Rab3A enhances PPF at CA3–CA1 synapses (Fig. 3A) (6, 7). At a 50-ms interpulse interval, PPF increased from 137 ± 3% in wild-type mice (n = 18, eight mice) to 189 ± 8% in Rab3A-/- mice (n = 16, eight mice; P < 0.01). Despite the enhancement in PPF, we observed no change in the fEPSP input–output curves. The maximal fEPSP slope was 5.2 ± 1.0 mV/ms (n = 8) in wild-type and 5.0 ± 0.7 mV/ms (n = 7) in Rab3A-/- mice (P > 0.5).
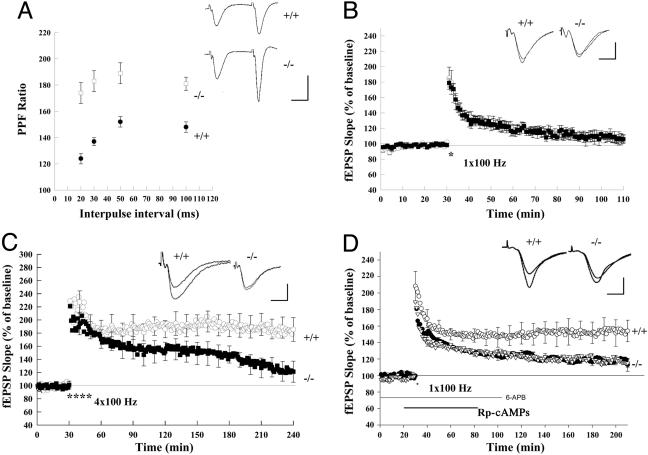
Fig. 3.
Short- and long-term plasticity is altered at Schaffer collateral synapses in Rab3A-/- mice. (A) The PPF ratio (fEPSP2/fEPSP1 slope) was significantly enhanced in knockout (open squares) compared with wild-type (filled circles) mice. (Insets) Sample traces of PPF in wild-type and Rab3A-/- mice. (Scale bars: 20 ms, 2 mV.) (B) E-LTP induced by a single 100-Hz tetanus (*) in Rab3A-knockout (filled squares) mice is not significantly different from that in wild-type (open circles) mice. (C) L-LTP induced by four trains of tetanic stimulation (*, 100 Hz, 1 s) was significantly reduced in Rab3A-knockout (filled squares) mice. (D) Pairing a single tetanus (*, 100 Hz, 1 s) with the application of a D1 receptor agonist (6-APB, 6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide) induced L-LTP in the wild-type (open circles) mice. This L-LTP was depressed in the presence of 100 μM RP-cAMPS (open triangles) and in Rab3A-/- (filled circles) mice. (C and D, Insets) Sample traces 10 min before and 3 h after the tetanus. (Scale bars in B, C, and D: 10 ms, 2 mV.)
We next confirmed previous findings (7) that E-LTP induced by a single train of 1-s, 100-Hz tetanic stimulation is normal in Rab3A-/- mice (116 ± 4% enhancement in knockout versus 120 ± 5% in wild-type mice measured at 30 min) (n = 5, P > 0.5; Fig. 3B). In contrast, L-LTP induced by four trains of 100-Hz tetanic stimulation was significantly impaired in the knockout mice (Fig. 3C). Although L-LTP in wild-type mice was sustained for at least 3.5 h after the tetani (185 ± 18% enhancement), in Rab3A-/- mice, the LTP started to decay back to baseline levels 2 h after tetanic stimulation, with only a 121 ± 16% enhancement at 3.5 h, significantly less than that observed in wild-type mice (n = 5, five mice; P < 0.05; Fig. 3C).
In contrast to the reduction in L-LTP induced by tetanic stimulation, Rab3A deletion had no effect on the potentiating response to direct application of SP-cAMPS, a membrane-permeant analog of cAMP. Thus, a 15-min application of SP-cAMPS (100 μM) induced a slow, persistent potentiation of the fEPSP that was similar in wild-type mice, with a 146 ± 15% increase 3 h after the application (n = 6), and Rab3A-/- mice, with a 142 ± 13% increase (n = 6, P > 0.5; data not shown). The enhancement of synaptic transmission by SP-cAMPS could indicate that Rab3A is required at a step that lies upstream of cAMP production. Alternatively, because there are parallel pathways by which cAMP can modulate release (18, 19), the direct application of SP-cAMPS might recruit a Rab3A-independent pathway that is not recruited by physiological stimuli.
To distinguish between these possibilities, we tested whether the elevation of cAMP through a more physiological mechanism involving dopamine-receptor stimulation might specifically target the Rab3A-dependent presynaptic facilitatory process. Previous studies have shown that pairing the application of the D1/D5 agonist 6-APB, which enhances endogenous cAMP levels, with a single tetanic stimulation that normally induces only E-LTP can induce PKA-dependent L-LTP (20). We confirmed that, in wild-type mice, delivery of a single 1-s train of 100-Hz stimulation in the presence of 6-APB did, indeed, induce a stable L-LTP; there was a 154 ± 15%, enhancement of the EPSP 3 h after the induction protocol (n = 7; Fig. 3D). The L-LTP was mediated by PKA, because it was blocked by application of the PKA inhibitor RP-cAMPS (100 μM) during the induction protocol, with the EPSP enhanced to only 112 ± 6% (n = 6; Fig. 3D). In contrast to our finding that the potentiating response to SP-cAMPS was normal in the Rab3A-/- mice, the L-LTP induced by pairing a tetanus with 6-APB was largely blocked in the mutant mice (Fig. 3D). Thus, 3 h after the pairing protocol, the EPSP was enhanced to only 115 ± 5% (n = 6), significantly smaller than the L-LTP in wild-type mice (F = 6.86 ANOVA test, P < 0.05). The finding that Rab3A deletion blocks L-LTP when cAMP is elevated through direct stimulation of D1/D5 receptors indicates that Rab3A acts at a stage downstream of cAMP elevation.
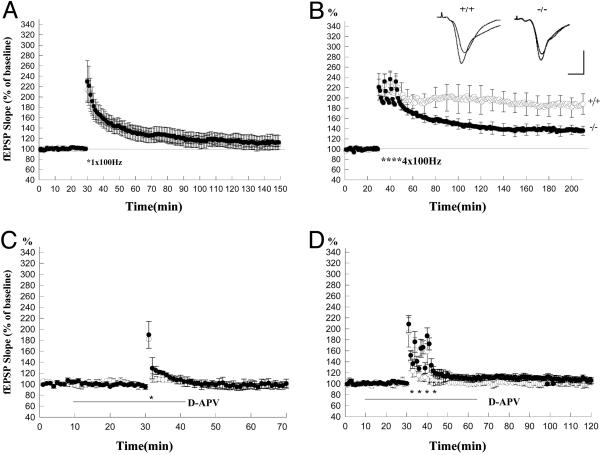
L-LTP in the Hippocampal Schaffer Collateral Pathway Requires RIM1α. Because the Rab3A effector protein RIM1α is necessary for mossy- and parallel-fiber LTP (4), we next examined whether Schaffer collateral L-LTP also requires RIM1α. Although the deletion of RIM1α did not alter Schaffer collateral E-LTP induced by a single 1-s train of 100-Hz tetanic stimulation (Fig. 4A), as reported in ref. 6, L-LTP induced by four trains of tetanic stimulation was significantly impaired in the RIM1α-/- mice (Fig. 4B). Three hours after tetanic stimulation, the EPSP was enhanced to only 136 ± 10% (n = 7) of its initial value, significantly smaller than L-LTP in wild-type mice of 188 ± 20% (n = 6, P < 0.05). Both E- and L-LTP required NMDAR activation, because they were completely blocked when the tetanic stimulation was delivered in the presence of D-APV (50 μM) (Fig. 4 C and D). These results provide evidence that the presynaptic active zone protein RIM1α is required for certain forms of NMDAR-dependent synaptic plasticity.
Fig. 4.
L-LTP is reduced in the Schaffer collateral pathway of RIM1α-knockout mice. (A) E-LTP induced by a single 100-Hz tetanus in the RIM1α-knockout (filled circles) mice is not significantly different from that in wild-type (open circles) mice. (B) L-LTP induced by four trains of tetanic stimulation (*, 100 Hz, 1 s) was significantly reduced in the RIM1α-knockout (filled circles) mice. (Insets) Sample traces 10 min before and 3 h after the tetanus. (Scale bars: 10 ms, 2 mV.) (C) E-LTP induced by a single 100-Hz tetanus (*) was blocked by D-APV in both wild-type (open circles) and RIM1α-/- (filled circles) mice (n = 4). (D) L-LTP induced by four trains of tetanic stimulation (*) was also blocked by D-APV in both wild-type (open circles) and RIM1α-/- (filled circles) mice (n = 4).
The Defect in L-LTP in Rab3A- and RIM1α-Deficient Mice Lies Downstream of Postsynaptic Activation. Because deletion of either Rab3A or RIM1α can alter glutamate release (5, 6, 8, 21), we asked whether the defect in L-LTP is due to a reduction in glutamate release during the LTP-induction protocol, resulting in diminished postsynaptic activation, or reflects a more direct participation of the presynaptic proteins in the induction or expression of L-LTP itself. Our finding that, in Rab3A-/- mice, E-LTP induced by a single train of 100-Hz stimulation is normal, whereas L-LTP induced by pairing an identical train of stimulation with dopamine-receptor activation is deficient (Fig. 3D), suggests that transmitter release is adequate to induce long-term plasticity and that the defect may be specific to L-LTP.
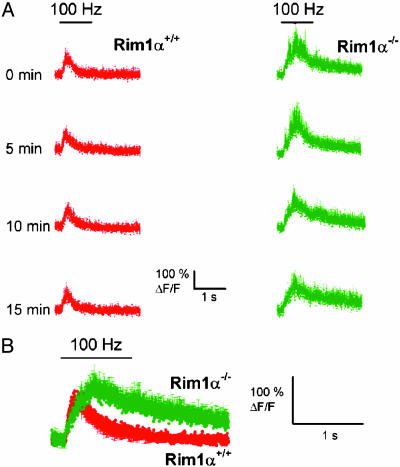
To directly evaluate the extent of postsynaptic activation during the induction of L-LTP, we measured calcium levels in single dendritic spines of CA1 pyramidal neurons during the four 1-s, 100-Hz trains (Fig. 5A). Despite the alteration in presynaptic function in the RIM1α-/- mice, we found a large increase in spine Ca2+ in response to each of the four trains used to induce L-LTP. Surprisingly, the average peak calcium transient during the four 100-Hz trains was, if anything, slightly larger in the RIM1α-/- mice compared with wild-type mice [maximal ΔF/F0 was 134 ± 31% for RIM1α-/- (n = 16) versus 108 ± 22% for wild-type littermates (n = 16), not statistically significant; P = 0.066, Mann–Whitney rank sum test]. Thus, there appears to be near-normal postsynaptic activation during the tetanic stimulation.
Fig. 5.
Calcium response in the dendritic spines of postsynaptic CA1 neurons upon LTP induction in RIM1α+/+ (red) and RIM1α-/- (green) mice. (A) Averaged CA1 spine Ca2+ signals, measured as ΔF/F0 (change in fluorescence intensity divided by initial baseline fluorescence intensity ×100%), in dendritic spines in response to each of four trains of 1-s, 100-Hz Schaffer collateral stimulation given every 5 min (times shown on the left) in RIM1α+/+ (Left) mice (n = 4) and RIM1α-/- (Right) mice (n = 4). (B) Averaged Ca2+ signal from all four trains shown in A. (Error bars show SE.)
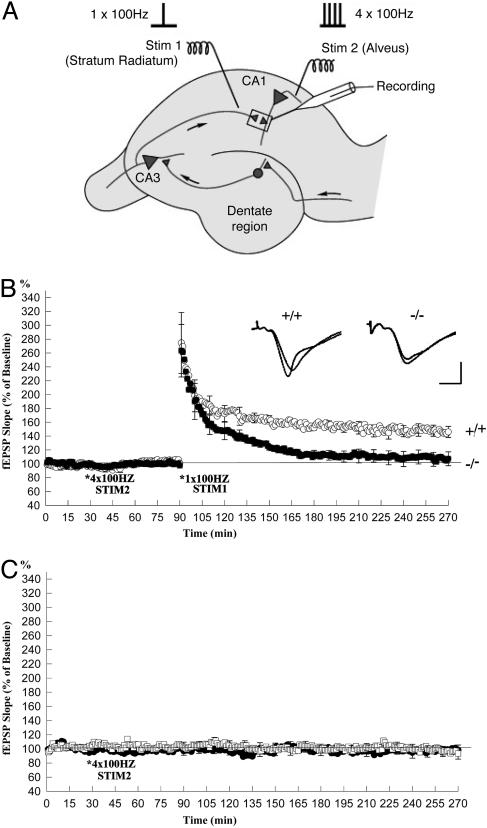
As a final test of whether the defect in L-LTP in the RIM1α-/- mice was due to a deficit in the extent of synaptic activation during the induction protocol, we performed an experiment in which we induced L-LTP through a synaptic-capture experiment (22). In this protocol, a single train of 1-s, 100-Hz Schaffer collateral stimulation is applied after four trains of antidromic stimulation of the axons of the CA1 pyramidal neurons, delivered by a stimulating electrode placed in the alveus (Fig. 6A). Because the induction of E-LTP with the same single train of Schaffer collateral stimulation is normal in the RIM1α-/- mice, a defect in L-LTP with this capture protocol would suggest a defect that lies downstream of glutamate release during the tetanus.
Fig. 6.
RIM1α is required for L-LTP induced by synaptic capture. (A) Diagram illustrating the placement of stimulating and recording electrodes for synaptic-capture experiment shown in B and C. Stim 1 was placed in the stratum radiatum to stimulate the Schaffer collateral fiber input from CA3 neurons. Stim 2 was placed in the alveus to stimulate antidromically the axons of the CA1 pyramidal neurons. (B) The role of RIM1α in synaptic capture. Four trains of 100-Hz antidromic stimulation (1 s, *) were applied to Stim 2, located in the alveus, 1 h before the application of a single 100-Hz tetanus to Stim 1, located in the stratum radiatum. In wild-type mice, the single train (*) applied to Stim 1, primed with four trains of tetanus to Stim 2, induced a long-lasting synaptic potentiation (open circles). In RIM1α-knockout mice, the synaptic potentiation was reduced to baseline in 1.5–2 h (filled circles). (Insets) Sample traces 10 min before and 3 h after the LTP. (Scale bars: 10 ms, 2 mV.) (C) The baseline EPSPs elicited by Stim 1 were not significantly changed in wild-type or RIM1α-/- mice for up to 3 h when the four trains of 100-Hz tetanic stimulation to Stim 2 (alveus) were delivered in the absence of the single 100-Hz tetanus to the Schaffer collaterals (Stim 1) (n = 4). Open squares, wild type; filled squares, RIM1α-/-.
We found that the four trains of antidromic stimulation, by themselves, did not induce any significant change of the fEPSP elicited by synaptic stimulation in either wild-type or RIM1α-knockout mice (Fig. 6C). However, in the wild-type mice, when the antidromic stimuli were paired with the single train of synaptic stimulation (capture protocol), there was a stable L-LTP of 146 ± 8% (at 3 h, n = 5). By contrast, the capture protocol produced only a very small synaptic enhancement in the RIM1α-knockout mice (107 ± 10% at 3 h, n = 7) that was significantly smaller than the L-LTP in wild-type mice (P < 0.05).
Discussion
Our results indicate that Rab3A is required for LTP in the cortical pathway to the amygdala as well as for L-LTP in the Schaffer collateral pathway of the hippocampus. Moreover, we found that RIM1α is required for L-LTP in the Schaffer collateral pathway. Previous studies found that Rab3A and RIM1α are required for hippocampal mossy-fiber LTP and cerebellar parallel-fiber LTP, two related forms of plasticity that (i) require PKA, (ii) are thought to be induced largely in the presynaptic terminal, and (iii) have a prominent presynaptic expression mechanism (2, 3). Because both corticoamygdala LTP and Schaffer collateral L-LTP also require PKA, our studies demonstrate a unity of presynaptic mechanism among four types of PKA-dependent long-term synaptic plasticity at four distinct synapses. However, corticoamygdala LTP and Schaffer collateral L-LTP also require the activation of NMDARs, whereas mossy-fiber and parallel-fiber LTP do not. Thus, our results extend previous findings by providing genetic evidence that presynaptic Rab3A and RIM1α proteins are required for forms of plasticity that clearly depend on postsynaptic activity for their induction.
Because the elimination of Rab3A and RIM1α affects the basic properties of transmitter release at CA3–CA1 synapses (6, 8, 21), a key question is whether the defects we observe in L-LTP are secondary to a reduction in transmitter release during the four trains of 1-s, 100-Hz tetanic stimulation used to induce LTP. Such an effect would lead to a reduced level of postsynaptic activation and, thus, a weaker induction of LTP. However, four independent lines of experiments suggest that the defects we observe in L-LTP are likely to be downstream of the initial postsynaptic activation, because (i) E-LTP induced by the 1-s, 100-Hz tetanus was normal in both Rab3A- and RIM1α-knockouts, (ii) L-LTP induced by pairing bath application of dopamine with a single 1-s, 100-Hz tetanus identical to that used to induce E-LTP was reduced in the knockout mice, (iii) L-LTP induced by pairing antidromic stimulation of CA1 neurons with a single 1-s, 100-Hz tetanus was also reduced in the knockout mice, and (iv) the peak rise in Ca2+ in dendritic spines during the four 100-Hz tetanic trains used to induce L-LTP was not reduced in the RIM1α-knockout mice. These results thus support the argument that the defect in L-LTP is likely to reflect a specific role of Rab3A and RIM1α in the induction or expression of L-LTP and is not simply a consequence of a failure to achieve sufficient postsynaptic activation to adequately induce L-LTP.
Previous studies using pharmacological inhibitors suggested that the site of action of PKA for mossy-fiber, parallel-fiber, and corticoamygdala LTP was likely to be presynaptic (2, 3, 10). In contrast, the site of PKA action for L-LTP at CA3–CA1 synapses has been less well defined but has been presumed to be, at least in part, postsynaptic because of the requirement for transcription (13). Moreover, Duffy and Nguyen (23) found that PKA activation in the CA1 neuron is necessary and sufficient to induce L-LTP at Schaffer collateral synapses, although a role for presynaptic PKA was not directly addressed. Based on our finding that RIM1α, a prominent PKA substrate, is required for L-LTP, we suggest that this form of plasticity is likely to also have a presynaptic requirement for PKA activation in addition to any postsynaptic role for this enzyme.
One puzzling result found by us and others (4, 18) is that the deletion of Rab3A inhibits PKA-dependent forms of LTP evoked by synaptic stimulation but does not affect synaptic potentiation in response to direct pharmacological elevation of cAMP, suggesting that cAMP may act through parallel pathways, including mechanisms that are independent of PKA, to enhance transmitter release (19). Cell-wide elevation of cAMP by pharmacological manipulations might lead to the activation of all cAMP-dependent pathways. In contrast, recruitment of cAMP by synaptic activity might be restricted in its action to those pathways that require Rab3A/RIM1α to mediate their facilitation.
A remarkable insight into long-term synaptic potentiation is the finding of synaptic capture. Frey and Morris (24) originally reported the existence of synaptic capture in the hippocampus. They found that a single 100-Hz tetanus that normally induces only E-LTP when applied to a set of Schaffer collateral inputs, could induce L-LTP if primed by the application of a repeated tetanus to an independent set of Schaffer collateral inputs to the same neuron. An alternative way to achieve synaptic capture, by using a priming protocol of repeated tetanic bursts of antidromic stimulation to the CA1 pyramidal neuron soma, has been reported by Dudek and Fields (22). Thus, a single train of 100-Hz synaptic stimulation not only serves to produce a transient potentiation (E-LTP) but also can mark and stabilize synaptic potentiation to produce L-LTP at that synapse by capture. We now show that the potentiation produced during capture recruits the same presynaptic machinery as is necessary for the directly induced expression of L-LTP. Thus, the process of synaptic capture resembles L-LTP in both its pre- and postsynaptic PKA-mediated mechanisms. Finally, our results on PKA-dependent LTP reveal a surprising unity and generality of mechanisms involving, in each case, a PKA- and Rab3A-mediated presynaptic module for the long-term enhancement of transmitter release.
Acknowledgments
We thank Paul Pavlidis for participation in an early phase of these experiments and Robert D. Hawkins for comments on the manuscript. This work was partially supported by the Howard Hughes Medical Institute and by a National Institute of Mental Health grant (to E.R.K. and S.A.S.). P.S.K. was supported by a postdoctoral fellowship from the Swiss National Science Foundation.
Author contributions: Y.-Y.H., T.C.S., S.A.S., and E.R.K. designed research; Y.-Y.H., S.S.Z., S.S., P.S.K., and R.J. performed research; Y.-Y.H. and S.S.Z. analyzed data; and Y.-Y.H., S.S.Z., T.C.S., S.A.S., and E.R.K. wrote the paper.
Abbreviations: 6-APB, 6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide; D-APV, d-2-amino-5-phosphonovaleric acid; SP-cAMPS, SP-adenosine 3′,5′-cyclic monophosphorothioate; EPSP, excitatory postsynaptic potential; fEPSP, field EPSP; LTP, long-term potentiation; E-LTP, early-phase LTP; L-LTP, late-phase LTP; NDMAR, NDMA receptor; PKA, protein kinase A; PPF, paired-pulse facilitation.
References
- 1.Sanes, J. R. & Lichtman, J. W. (1999) Nat. Neurosci. 7, 597-604. [DOI] [PubMed] [Google Scholar]
- 2.Nicoll, R. A. & Malenka, R. C. (1995) Nature 377, 115-118. [DOI] [PubMed] [Google Scholar]
- 3.Malenka, R. C. & Nicoll, R. A. (1999) Science 285, 1870-1874. [DOI] [PubMed] [Google Scholar]
- 4.Castillo, P. E., Janz, R., Südhof, T. C., Tzounnopoulos, T., Malenka, R. C. & Nicoll, R. A. (1997) Nature 388, 590-593. [DOI] [PubMed] [Google Scholar]
- 5.Castillo, P. E., Schoch, S., Schmitz, F., Südhof, T. C. & Malenka, R. C. (2002) Nature 415, 590-593. [DOI] [PubMed] [Google Scholar]
- 6.Schoch, S., Castillo, P. E., Jo, T., Mukherjee, K. & Südhof, T. C. (2002) Nature 415, 321-326. [DOI] [PubMed] [Google Scholar]
- 7.Geppert, M., Bolshakov, V. Y., Siegelbaum, S. A., Takel, K., Camili, P. D., Hammer, R. E. & Südhof, T. C. (1994) Nature 369, 493-497. [DOI] [PubMed] [Google Scholar]
- 8.Gepert, M., Goda, Y., Stevens, C. F. & Südhof, T. C. (1997) Nature 387, 810-814. [DOI] [PubMed] [Google Scholar]
- 9.Wang, Y., Okamoto, M., Schmitz, F., Hofman, K. & Südhof, T. C. (1997) Nature 388, 593-598. [DOI] [PubMed] [Google Scholar]
- 10.Huang, Y.-Y. & Kandel, E. R. (1998) Neuron 21, 169-178. [DOI] [PubMed] [Google Scholar]
- 11.Huang, Y.-Y., Martin, K. C. & Kandel, E. R. (2000) J. Neurosci. 20, 6317-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, Y.-Y. & Kandel, E. R. (1994) Learn. Mem. 1, 74-82. [PubMed] [Google Scholar]
- 13.Abel, T., Nguyen, P. V., Barad, M., Denel, T. A. & Kandel, E. R. (1997) Cell 88, 615-626. [DOI] [PubMed] [Google Scholar]
- 14.Bolshakov, V. Y., Golan, H., Kandel, E. R. & Siegelbaum, S. A. (1997) Neuron 19, 635-651. [DOI] [PubMed] [Google Scholar]
- 15.Rosahl, T. W., Spillane, D., Missler, M., Herz, J., Selig, D. K., Wolff, J. R., Hammer, R. E., Malenka, R. C. & Südhof, T. C. (1995) Nature 375, 488-493. [DOI] [PubMed] [Google Scholar]
- 16.Schlüter, O. M., Schnell, E., Verhage, M., Tzonopoulos, T., Nicoll, R. A., Janz, R., Malenka, R. C., Geppert, M. & Südhof, T. C. (1999) J. Neurosci. 19, 5834-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang, C., Barco, A., Zablow, L., Kandel, E. R., Siegelbaum, S. A. & Zakharenko, S. S. (2004) Proc. Natl. Acad. Sci. USA 101, 16665-16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonart, G., Janz, R., Johnson, K. M. & Südhof, T. C. (1998) Neuron 21, 1141-1150. [DOI] [PubMed] [Google Scholar]
- 19.Zhong, N. & Zucker, R. S. (2005) J. Neurosci. 25, 208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bach, M. E., Barad, M., Son, H., Zhuo, M., Lu, Y.-F., Shih, R., Mansuy, I., Hawkins, R. D. & Kandel, E. R. (1999) Proc. Natl. Acad. Sci. USA 96, 5280-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calakos, N., Schoch, S., Südhof, T. C. & Malenka, R. C. (2004) Neuron 42, 889-896. [DOI] [PubMed] [Google Scholar]
- 22.Dudek, S. M. & Fields, D. (2002) Proc. Natl. Acad. Sci. USA 99, 3962-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy, S. N. & Nguyen, P. V. (2003) J. Neurosci. 23, 1142-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey, U. & Morris, G. M. (1997) Nature 385, 533-536. [DOI] [PubMed] [Google Scholar]