Abstract
Background and Objectives
Nonfocal transient neurologic attacks (TNA) have been suggested to increase the risk of stroke, yet the optimal clinical approach of these attacks remains uncertain. We determined whether people who have a nonfocal TNA are at an increased risk of subsequent cardiovascular disease (CVD), akin to the known increased risk of stroke following transient ischemic attack (TIA).
Methods
Within a population-based cohort study among Dutch participants aged 45 years or older, we selected participants who had first-ever TNA, defined as an attack of sudden neurologic symptoms resolving within 24 hours without clear evidence for an alternative diagnosis, during follow-up between 1990 and 2020. Nonfocal TNAs were attacks with nonfocal symptoms only, whereas attacks with focal symptoms were regarded as TIA. Each participant with TNA was matched to 2 participants who did not experience TNA, with similar age and sex at the date of TNA diagnosis. Participants were then followed up for any incident CVD, defined by any stroke or any coronary heart disease (CHD), and follow-up was complete for 98.6% of potential person-years. The association between TNA and risk of subsequent CVD was analyzed using Cox proportional hazard models adjusting for demographic and cardiovascular risk factors at baseline.
Results
During follow-up, 1,208 participants (mean [SD] age 76.9 [9.3] years, 65.4% female) experienced a first-ever TNA and were matched to 2,416 participants without TNA. After 27,833 person-years of follow-up, 230 (19.0%) participants suffered stroke and 94 (7.8%) suffered CHD. For those without TNA, these numbers were 250 (10.4%) and 176 (7.3%). Incident nonfocal TNA was not associated with the risk of any stroke (hazard ratio 1.25%, 95% CI [0.89–1.77]), only ischemic stroke (1.26 [0.76–2.08]), any CHD (0.80 [0.49–1.31]), and only acute myocardial infarction (0.89 [0.51–1.56]). By comparison, participants with TIA had an increased risk of all stroke (2.55 [2.04–3.19]) and ischemic stroke (2.51[1.88–3.35]).
Discussion
In this study, participants with nonfocal TNA did not have a subsequently elevated risk of CVD when compared with their matched counterparts, which contrasts with the elevated risk of stroke following a TIA. In the absence of other indications, initiating secondary prevention specifically after nonfocal TNA seems unwarranted.
Introduction
A transient neurologic attack (TNA) is a temporary attack of neurologic symptoms, lasting <24 hours and can be subtyped into focal, nonfocal, and mixed depending on the symptoms.1 Focal and mixed TNAs, sometimes coined typical and atypical transient ischemic attacks (TIAs), are known harbingers of ischemic stroke.2,3 Furthermore, mixed TNA has also been associated with an elevated risk of coronary heart disease (CHD).3,4
In contrast to the well-established prognosis following focal and mixed TNA, there is a notable scarcity of knowledge on the clinical relevance of nonfocal TNA. The limited available evidence on the clinical consequences following nonfocal TNAs suggests that the risk of ischemic stroke is elevated afterward.3,5,6 The risk of nonfocal TNA for those aged older than 55 years is estimated at 3.8%,3 potentially leaving a considerable portion of the elderly population at an elevated risk of future stroke.
However, this evidence is limited by biases because these studies are hospital-based,6 are retrospective in nature,5 or have short follow-up periods.3 Owing to these limitations, uncertainty persists among clinicians regarding the optimal choice of management of nonfocal TNA.7 In particular, the question remains as to whether these attacks require secondary prevention measures, in line with the management of a TIA.
Therefore, we investigated the risk of cardiovascular disease (CVD) following a nonfocal TNA and assessed whether this risk was different when compared with TIA.
Methods
Study Setting
This study was embedded in the Rotterdam Study, a population-based observational cohort study among residents aged 45 years or older in the Ommoord district of Rotterdam, the Netherlands. The Rotterdam Study started in 1990 with 7,983 participants and expanded in 2000 and 2006 with 3,011, and 3,932 participants, respectively. Further details of the study are described elsewhere.8 Participants are invited for follow-up examinations every 3 to 6 years and are continuously monitored through electronic linkage of the study database with their general practitioner's (GP) medical records. General practitioners are the essential component in the Dutch health care system, acting as gatekeepers to further specialized medical care. Consequently, they are the overseers of all medical information of their patients, receiving all discharge notes and letters from medical and paramedical staff. All relevant medical documents undergo scrutinization by a trained research personnel and are then submitted to consensus with experienced vascular specialists to finalize the event diagnosis. This monitoring of medical records, and subsequent scrutinization of documents from medical specialists, enabled the identification of incident clinical events.
Assessment of TNA
For this study, we assessed the occurrence of incident TNA as part of the larger stroke follow-up using medical records found in the study database. These records originate from GP visit notes, emergency department or hospital discharge letters, or letters from nursing home physicians.1,9 We defined TNA as an attack of sudden neurologic symptoms completely resolving within 24 hours, without clear evidence for migraine, epilepsy, Menière disease, hyperventilation, cardiac syncope, hypoglycemia, or orthostatic hypotension. If only focal neurologic deficit was reported, that is, weakness, loss of sensation, aphasia, anopsia, dysarthria, dysphagia, ataxia, diplopia, or vertigo, then the event was classified as a focal TNA. If only nonfocal symptoms were reported, that is, decreased consciousness, unconsciousness, amnesia, confusion, incontinence, positive visual phenomena, unsteadiness, nonrotatory dizziness, paresthesias, or bilateral weakness, then the event was classified as a nonfocal TNA. When both focal and nonfocal symptoms were reported, the event was classified as a mixed TNA.1 In line with current clinical practice, both focal and mixed TNAs were regarded as TIA.
Furthermore, baseline interviews were conducted to establish the prevalence of these conditions, which were subsequently verified with general practitioner's records. In addition to the identification using these records, participants are interviewed during each visit to the research center for the occurrence of any neurologic symptoms and the approximate date of this occurrence to facilitate targeted screening of medical records.
Assessment of Cardiovascular Outcomes
We included incident stroke and CHD in our definition of CVD. Stroke events were identified using the definition of stroke from the World Health Organization: “a syndrome of rapidly developing clinical signs of focal or global disturbance of cerebral function, where symptoms last 24 hours or longer or lead to death, with no apparent cause other than of vascular origin.”9 When sufficient imaging information was available in medical records (e.g., clinical notes and neuroimaging reports), we subtyped each stroke as ischemic or hemorrhagic. If this information was insufficient to determine the subtype, then the stroke subtype was classified as unspecified. Subarachnoid hemorrhages due to ruptured aneurysms were not considered stroke events. Due to limited case numbers for hemorrhagic and undetermined stroke, we only report associations with ischemic stroke only or all stroke.
CHD was defined by the occurrence of any acute myocardial infarction, and the acute or elective myocardial reperfusion interventions of percutaneous coronary intervention and coronary artery bypass grafting. Whether death occurred due to possible CVD was not included in this outcome due to incomplete follow-up assessment for cause-specific mortality. These incidences were identified in medical records by trained research physicians, with a cardiovascular expert deciding on the final diagnosis. A more detailed description of the CVD follow-up is available elsewhere.10 We report associations with acute myocardial infarction only and all CHD.
Confounder Assessment
Relevant demographic, lifestyle, and clinical information was assessed by personal interview and physical examination with a trained physician, and by blood sampling during visits to the research center.
In this study, we selected confounder data from each participant's initial visit. The alternative of selecting the most recent confounder data from the visit preceding the TNA would risk introducing bias from inconsistent data usage. Specifically, there was a considerable time gap (range 0.0–23.1 years) between the most recent date of covariate assessment and the date of TNA for some participants, with 34.4% of participants not having any information from within 5 years before their TNA. By selecting baseline data, we ensured uniformity in baseline characteristics across all participants.
The highest attained level of education and smoking behavior was assessed in personal interview. The body mass index was calculated using the measured weight in kilograms, divided by the measured height in meters squared. Information on medication use was assessed in personal interview and included an inventorization of the use of blood pressure lowering, lipid lowering, antithrombotic (vitamin K antagonists, antiplatelet agents, and carbasalate calcium), and any diabetes medication. Blood pressure was measured in a seating position after a resting period of 5 minutes on the right upper arm using a random-zero sphygmomanometer. Hypertension was defined as the use of antihypertensive medication or otherwise as the average of 2 measurements performed with a 2-minute interval, ≥140/90 mm Hg. Dyslipidemia was defined as having either a serum total cholesterol ≥6.2 mmol/L, a non-HDL cholesterol level ≥5.6 mmol/L, or the use of lipid-lowering medication. Diabetes mellitus was defined as having fasting serum glucose levels ≥7.0 mmol/L, or nonfasting serum glucose levels ≥11.1 mmol/L if fasting samples were unavailable, or the use of diabetes medication. The presence of atrial fibrillation at baseline was based on clinical information taken from medical records, and a resting ECG obtained at the first examination.10
Population for Analysis
For this study, we used a matched cohort design. Using this approach, participants who developed a TNA during follow-up were considered as the exposed group. Those participants who did not develop a TNA during follow-up were the unexposed group. Participants with TNA were matched on the date of their TNA to 2 randomly selected unexposed participants without a history of stroke or CHD at the date of matching. Matching was performed by birthyear and sex, resulting in a maximum possible age difference of 1 year between the exposed and unexposed groups relative to the date of matching. For each participant, we used follow-up information from the date of matching until a censoring point consisting of a combined outcome of either date of death, stroke or CHD, last health status update when they were known to be free of any outcome, or the end of the study period on 1st of January 2020, whichever came first. Follow-up in this manner was complete for 98.6% of potential person-years.
Statistical Methods
Summary characteristics of the matched cohort are first presented using frequencies and percentages, or mean and SD for continuous variables. We explored differences in characteristics between the exposed and unexposed groups using 2-tailed independent t tests for continuous variables and the χ2 test for categorical variables.
Next, we estimated the absolute risk of all stroke, ischemic stroke only, all CHD, and acute myocardial infarction by calculating cumulative incidences and stratified these estimates per exposure status. We then analyzed these estimates separately through stratification for TNA subtype to explore differences in cardiovascular prognosis after nonfocal TNA and TIA. We tested for differences in cumulative incidences between groups and subgroups using the log-rank test.
Finally, we used multivariable, cause-specific Cox proportional hazard regression models to assess the association between the different TNA subtypes and the cardiovascular outcomes of all stroke, ischemic stroke only, all CHD, and acute myocardial infarction only. In this manner, participants were also censored on the date of all-cause mortality to account for the competing risk of death. Subsequently, we applied multivariable adjustment using the following confounders: age at matching, study cohort number, highest attained education status, obesity, smoking, hypertension, diabetes mellitus, dyslipidemia, prevalent atrial fibrillation, and the use of antithrombotic medication. The proportional hazards assumption was checked through inspection of the Schoenfeld residuals plotted against follow-up time, and no major violations were detected.
All analyses were performed in R, version 4.2.2, using the “survival” 3.5.7, “survminer” 0.4.9, “cmprsk” 2.2.11, packages. Matching was performed using the “MatchIt” 4.5.5 package. Missing data for confounders (≤10%, except for diabetes mellitus with 13.8%) were addressed through multiple imputation, generating 20 iterations and imputations using the R package “mice” 3.16.0. Results from analyses using pooled imputed data sets are presented.
Standard Protocol Approvals, Registrations, and Patient Consents
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC1712001. The Rotterdam Study has been entered into the Netherlands National Trial Register and into the WHO International Clinical Trials Registry Platform under shared catalogue number NTR6831. All participants provided written informed consent for participation in the study and for researchers to access medical information from their personal physicians.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author. All requests are directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, the data underlying this article cannot be made freely available in a public repository.
Results
Sample Selection and Characteristics
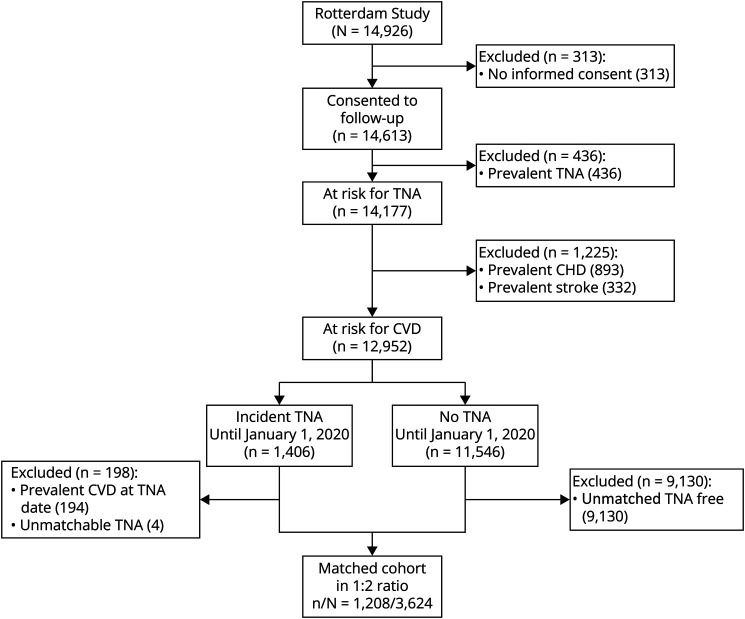
After excluding participants without informed consent for follow-up procedures (n = 313) and those participants with prevalent TNA (n = 436), prevalent CHD (n = 893), and stroke (n = 332) at study start, 12,952 participants were followed up over a total of 177,008 person-years. During this period, 1,406 participants experienced a first-ever TNA. Of these, 194 developed either CHD or stroke before the TNA and were subsequently excluded from the study. Another 4 people who developed TNA over time were unable to be exact matched because there were no longer any participants of the same sex and birth year remaining. Thus, 1,208 participants exposed to TNA were matched with 2,416 participants unexposed to TNA, with a median 7.4 (IQR 3.5–12.4) years between baseline assessment and the TNA diagnosis. Figure 1 illustrates this selection process.
Figure 1. Flowchart Illustrating the Selection and Matching Processes of Eligible Participants Illustrating Selection.
Table 1 displays baseline characteristics of the exposed and nonexposed groups. Participants were a mean 76.9 (SD 9.3) years old at the time of matching, and the majority was female (65.4%). In 372 (30.8%) of participants, only nonfocal symptoms were reported, while 836 (69.2%) experienced a TIA. Only 1 TNA occurred in 910 (75.3%) participants, while 298 (24.7%) experienced multiple TNAs during follow-up. For those who reported nonfocal symptoms only during their first-ever TNA, 92 (24.7%) would experience recurrent TNAs during follow-up and 40 (10.8%) of whom would also report TIA. For those with TIA as their first-ever TNA, 206 (24.6%) would experience multiple TNAs during follow-up and 45 (5.4%) of whom would also report a nonfocal TNA.
Table 1.
Baseline Characteristics by Exposure Status
| Characteristics | Mean/No. (SD%) | p Value | |
| Exposed (n = 1,208) | Unexposed (n = 2,416) | ||
| TNA type | |||
| Focal | 679 (56.21) | — | |
| Mixed | 157 (13.00) | — | |
| Nonfocal | 372 (30.79) | — | |
| Age at matching, y | 76.94 (9.25) | 76.92 (9.26) | 0.96 |
| Sex, female | 790 (65.40) | 1,580 (65.40) | 1.00 |
| Study cohort | 0.01 | ||
| RS-I | 875 (72.43) | 1,627 (67.34) | |
| RS-II | 208 (17.22) | 511 (21.15) | |
| RS-III | 125 (10.35) | 278 (11.51) | |
| Education | 0.72 | ||
| Primary | 245 (20.28) | 511 (21.15) | |
| Low | 534 (44.21) | 1,028 (42.55) | |
| Medium | 303 (25.08) | 604 (25.00) | |
| High | 126 (10.43) | 273 (11.30) | |
| Smoking status | 0.21 | ||
| Never | 469 (38.82) | 961 (39.78) | |
| Former | 519 (42.96) | 970 (40.15) | |
| Current | 220 (18.21) | 485 (20.07) | |
| BMI, kg/m2 | 26.94 (3.99) | 26.79 (4.01) | 0.30 |
| Obesity, ≥30 BMI | 232 (19.21) | 459 (19.00) | 0.92 |
| Systolic, mm Hg | 141.06 (21.21) | 139.84 (22.26) | 0.11 |
| Diastolic, mm Hg | 76.19 (11.20) | 75.49 (11.73) | 0.08 |
| Anti-hypertensive medication use | 361 (29.88) | 680 (28.15) | 0.29 |
| Hypertension | 736 (60.93) | 1,370 (56.71) | 0.02 |
| Total cholesterol, mmol/L | 6.48 (1.17) | 6.37 (1.26) | 0.01 |
| HDL cholesterol, mmol/L | 1.37 (0.36) | 1.39 (0.37) | 0.09 |
| Statin medication use | 66 (5.46) | 132 (5.46) | 1.00 |
| Dyslipidaemia | 645 (53.39) | 1,204 (49.83) | 0.05 |
| Diabetic medication use | 44 (3.64) | 110 (4.55) | 0.23 |
| Diabetes mellitus | 86 (7.12) | 193 (7.99) | 0.39 |
| Atrial fibrillation | 40 (3.31) | 108 (4.47) | 0.11 |
| Anti-thrombotic medication use | 46 (3.81) | 141 (5.84) | 0.01 |
| Follow-up time after matching, y | 7.35 (5.97) | 7.85 (6.00) | 0.02 |
Abbreviations: BMI = body mass index; HDL = high-density lipoprotein; RS = Rotterdam Study; TNA = transient neurologic attack.
Data were missing for medication use (0.4%), smoking (2.1%), education (2.2%), blood pressure (9.5%), BMI (10.1%), total cholesterol (10.2%), and HDL cholesterol (10.4%).
Risk of CVD After TNA
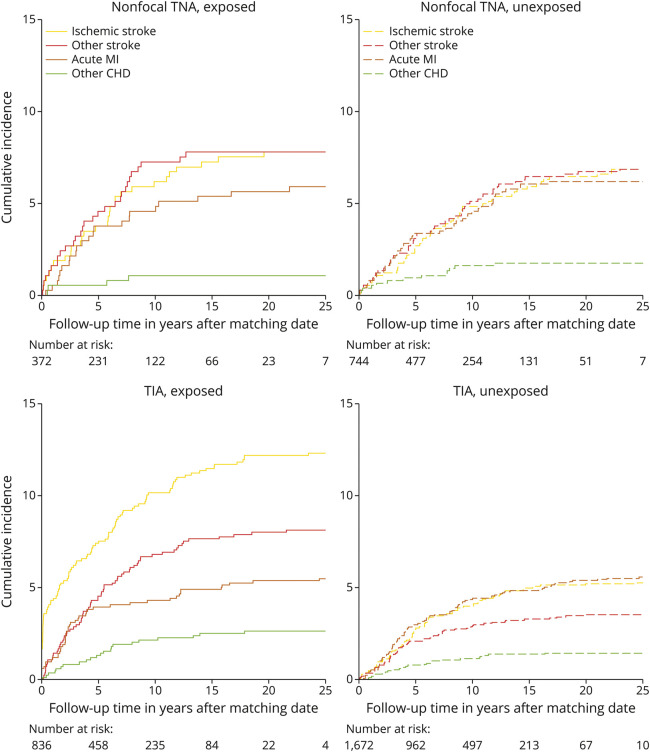
Figure 2 shows the cumulative incidence plots depicting the absolute risk of the first cardiovascular event, stratified by TNA status. After 27,833 person-years of follow-up, 324 (26.8%) participants with TNA and 426 (17.6%) without TNA developed an outcome. Stroke occurred in 229 (19.0%) participants with TNA, including 132 (57.6%) of ischemic type, 20 (8.7%) of hemorrhagic type, and 77 (33.6%) of undetermined type. In participants without TNA, stroke occurred in 249 (10.3%), with 139 (55.8%) of ischemic type, 27 (10.8%) of hemorrhagic type, and 83 (33.3%) of undetermined type. CHD occurred in 94 (7.8%) participants with TNA and 176 (7.3%) in participants without TNA.
Figure 2. Cumulative Incidence Curves Depicting the Risk of Stroke and Coronary Heart Disease in the Years Following Matching Risk Stroke.
No difference in the incidence of any of the CVD outcomes was found between participants with nonfocal TNA compared with their matched counterparts, with log-rank p values ranging between 0.33 and 0.98. For those who developed a stroke after a nonfocal TNA, the median time between nonfocal TNA diagnosis and subsequent stroke was 4.6 years (IQR 1.5–7.5 years). Whereas, after TIA, a statistically significant higher incidence of all stroke (p < 0.001) and ischemic stroke (p < 0.001) was found for those with TIA compared with their matched counterparts, but the incidence of all CHD (p = 0.10) and acute myocardial infarction only (p = 0.61) was not elevated after a TIA. For those who developed a stroke after a TIA, the median time between TIA diagnosis and subsequent stroke was 3.8 years (IQR 0.7–8.0 years).
Table 2 summarizes the results of Cox proportional hazard regression analyses on the association between the TNA subtypes and the incidence of CVD. Patients who experienced a nonfocal TNA did not have a higher risk of any of the outcomes afterward, and this did not change after adjustment. Participants who experienced a TIA had an elevated risk of all stroke, hazard ratio (HR) 2.56, 95% CI (2.05–3.20), and ischemic stroke, HR 2.57 (1.93–3.42). These associations did not change after adjustment for cardiovascular risk factors and the use of anti-thrombotic medication, HR: 2.55 (2.04–3.19) and HR: 2.51 (1.88–3.35), respectively. No elevated risk of CHD was found after a TIA.
Table 2.
Results of Cox Regression for the Association Between TNA Status and Incident Cardiovascular Disease, Stratified by TNA Subtype
| TNA type | Nonfocal | TIA | ||
| Exposure groups, n | Exposed | Nonexposed | Exposed | Nonexposed |
| 372 | 744 | 836 | 1,672 | |
| Outcomes, n | ||||
| All stroke | 58 | 102 | 171 | 147 |
| Ischemic stroke | 29 | 51 | 103 | 88 |
| All CHD | 26 | 59 | 68 | 117 |
| Acute MI | 22 | 46 | 46 | 93 |
| Effect estimates | HR (95% CI) | |||
| Model 1 | ||||
| All stroke | 1.26 (0.90–1.77) | 2.56 (2.05–3.20) | ||
| Ischemic stroke | 1.29 (0.78–2.12) | 2.57 (1.93–3.42) | ||
| All CHD | 0.83 (0.51–1.35) | 1.24 (0.92–1.68) | ||
| Acute MI | 0.90 (0.52–1.54) | 1.05 (0.73–1.50) | ||
| Model 2 | ||||
| All stroke | 1.25 (0.89–1.77) | 2.55 (2.04–3.19) | ||
| Ischemic stroke | 1.26 (0.76–2.08) | 2.51 (1.88–3.35) | ||
| All CHD | 0.80 (0.49–1.31) | 1.22 (0.90–1.65) | ||
| Acute MI | 0.89 (0.51–1.56) | 1.04 (0.73–1.50) | ||
Abbreviations: CHD = coronary heart disease; HR = hazard ratio; MI = myocardial infarction; n = count per group; TNA = transient neurologic attack.
Model 1 adjusted for age at match date, study cohort (RS-I as reference), and maximal level of education (primary education as a reference). Model 2 in addition adjusted for obesity, smoking behavior (never smoking as reference), hypertension, diabetes mellitus, dyslipidemia, prevalent atrial fibrillation, and use of anti-thrombotic medication.
Discussion
In this population-based, matched-cohort study, participants who experienced a nonfocal TNA did not have an increased risk of subsequent cardiovascular diseases when compared with their age-matched and sex-matched counterparts. This implies that patients experiencing transient nonfocal symptoms without an alternative neurologic diagnosis may be reassured by the fact that these attacks do not lead to an elevated risk of CVD, in contrast to the elevated risk of stroke following a TIA.
Existing literature on TNAs with solely nonfocal symptoms is scarce7 because most research on this domain focusses either on focal or mixed TNAs. As such, little is known about the consequences of these nonfocal TNAs. A previous study conducted within this population-based cohort in 2007 investigated the risk of stroke and dementia following TNA. This study followed 6,062 participants for 60,535 person-years and reported an elevated risk of all stroke and especially vascular dementia among 228 participants who had a nonfocal TNA during follow-up, which resembled the prognosis of 320 participants who had a TIA.3 However, these findings have been questioned by other subsequent studies6,11 investigating nonfocal symptoms, suggesting that the conclusions drawn from the 2007 study were perhaps based on chance results due to sample size limitations. Indeed, our results, which are based on almost thrice the duration of follow-up and over twice the number of first-ever TNA observations compared with the earlier study from 2007,3 align with these suggestions from other studies because we did not observe an elevated risk of cardiovascular diseases following nonfocal TNA.
Beyond acute clinical outcomes, some literature is available on neuroimaging and cognitive changes following nonfocal TNAs. The CONNECT study highlighted that both nonfocal TNA and TIA patients demonstrated increased diffusion-weighted imaging signs of acute ischemia,12 reduced white matter integrity,13 subjective cognitive complaints,14 and reduced executive functioning,15 to a similar extent. However, the authors of CONNECT suggest a considerable diagnostic overlap between nonfocal TNA and TIAs as an explanation for their findings,12 in that a subset of patients diagnosed with nonfocal TNA may in fact be misdiagnosed TIA cases. Consequently, this subset of misdiagnosed patients may thus have comparable clinical prognosis, neuroimaging changes, and cognitive functioning with patients with TIA. In studies with a small number of TNA cases, such as the previous 2007 publication,3 these misdiagnosed TIAs among nonfocal TNAs may therefore drive results toward a statistically significant increased risk of cardiovascular diseases following a TNA. Whereas our results, based on a large sample of individuals with nonfocal TNA, imply that experiencing nonfocal symptoms does not lead to an increased risk of CVD afterward.11,16
Furthermore, we found an increased risk of stroke following a TIA, which is well-established in the literature.2,17 However, in contrast to previous literature,3,4,18 we found that the risk of CHD was not increased after a TIA. Not including fatal CHD in our outcome definition may explain these findings, although this seems unlikely considering the risk of fatal myocardial infarction was not elevated following a TIA in a recent large meta-analysis on the subject.18 Rather, the authors of this meta-analysis propose an alternative explanation that aligns with our findings regarding the risk of CHD following a TIA. They suggest that advancements in secondary prevention strategies over the past 30 years have considerably reduced the heightened risk of CVD post-TIA. This trend mirrors the observed reduction in the risk of recurrent stroke following an initial stroke in the past 15 years19 Therefore, the nonstatistically significant increase in the risk of CHD after a TIA observed in our study may reflect the attenuating effects of improvements in secondary prevention strategies.
This study has several important limitations. First, propensity score matching could have improved the distribution of all variables between the comparison groups; however, previous literature suggests that this method may fail to address the complexity between the covariates used in the matching procedure, potentially increasing confounding bias rather than minimizing it.20 Our approach, which involves matching on key confounders of age and sex, and subsequently applying multivariable regression models, offers a more appropriate strategy for addressing confounding bias to the greatest extent achievable in observational cohort studies. However, residual confounding may still be an issue in this study, particularly due to our choice of using confounder data measured at the first visit of each participant. This approach ensures consistency in measuring baseline characteristics, yet may not fully capture changes in participants' health status over time or immediately before a TNA. Second, misclassification bias in distinguishing the different TNA types might have biased our results toward the null. Notably, previous reports suggest that approximately half of individuals experiencing temporary focal or nonfocal neurologic deficit never seek medical attention for these symptoms,21,22 meaning that unexposed participants in our study could have experienced a TNA. For those who do seek medical assistance, the subsequent diagnostic process of TNA is challenging, demonstrated by reports where 1 in 3 experienced neurologists disagreed on a TIA diagnosis.11,23,24 As TIA is a more familiar clinical entity compared with nonfocal TNA, there may be even more disagreement in diagnosing nonfocal TNA. Our reliance on medical records in identifying events could further exacerbate this problem. However, we tried to mitigate this potential information bias by closely collaborating with general practitioners, who serve as gatekeepers to the Dutch health care system and whose records therefore contain all medical information of their patients. This collaboration ensured a comprehensive overview of all clinical events among participants. Furthermore, each possible event underwent a consensus-based adjudication process, which is known to maximize diagnostic accuracy.25 Third, the variety of symptoms that are covered by the methods described in this study could have led to the inclusion of a heterogeneous group of diagnoses. Finally, some participants with nonfocal TNA may have started secondary prevention on a pragmatic basis, which could have influenced their cardiovascular prognosis. Subtle increases in the risk of cardiovascular diseases compared with matched participants may have been mitigated by this secondary prevention, but we have no detailed information on this medication use.
To conclude, patients presenting with TNA featuring solely nonfocal symptoms do not have a higher risk of subsequent CVD. Our findings suggest that these nonfocal attacks by themselves are not a reason to initiate secondary prevention strategies in patients who experience them, akin to those required after a TIA. Further research is necessary to establish the origin of these nonfocal TNAs to provide patients with a complete understanding of these apparent benign symptoms.
Acknowledgment
The authors are grateful to the study participants, the staff from the Rotterdam Study, and the participating general practitioners and pharmacists.
Glossary
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HR
hazard ratio
- TNA
transient neurologic attack
Author Contributions
B.P. Berghout: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. A. Heshmatollah: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. D. Bos: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. M. Kavousi: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. M.K. Ikram: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data; additional contributions (in addition to 1 or more of the above criteria).
Study Funding
B.P. Berghout, D. Bos, and M.K. Ikram were supported by the Erasmus Medical Center MRACE grant (grant number 386070).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Bots ML, van der Wilk EC, Koudstaal PJ, Hofman A, Grobbee DE. Transient neurological attacks in the general population. Prevalence, risk factors, and clinical relevance. Stroke. 1997;28(4):768-773. doi: 10.1161/01.str.28.4.768 [DOI] [PubMed] [Google Scholar]
- 2.Lioutas VA, Ivan CS, Himali JJ, et al. Incidence of transient ischemic attack and association with long-term risk of stroke. JAMA. 2021;325(4):373-381. doi: 10.1001/jama.2020.25071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298(24):2877-2885. doi: 10.1001/jama.298.24.2877 [DOI] [PubMed] [Google Scholar]
- 4.Ishihara T, Sato S, Uehara T, et al. Significance of nonfocal symptoms in patients with transient ischemic attack. Stroke. 2018;49(8):1893-1898. doi: 10.1161/STROKEAHA.118.022009 [DOI] [PubMed] [Google Scholar]
- 5.Caplan LR. Funny turns: they do mean something. Arch Neurol. 2008;65(5):601-602. doi: 10.1001/archneur.65.5.601 [DOI] [PubMed] [Google Scholar]
- 6.Parikh NS, Merkler AE, Kummer BR, Kamel H. Ischemic stroke after emergency department discharge for symptoms of transient neurological attack. Neurohospitalist. 2018;8(3):135-140. doi: 10.1177/1941874417750996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minhas JS. Non-Focal Transient Neurological Attacks (TNAs): A Form of Haemodynamic Disturbance? [Internet]. Stroke Research. European Stroke Organisation, 2019. [Google Scholar]
- 8.Ikram MA, Kieboom BCT, Brouwer WP, et al. The Rotterdam Study. design update and major findings between 2020 and 2024. Eur J Epidemiol. 2024;39(2):183-206. doi: 10.1007/s10654-023-01094-1 [DOI] [PubMed] [Google Scholar]
- 9.Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27(4):287-295. doi: 10.1007/s10654-012-9673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leening MJ, Kavousi M, Heeringa J, et al. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27(3):173-185. doi: 10.1007/s10654-012-9668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos M, Canhao P. One-year prognosis of transient ischemic attacks with nonfocal symptoms. Clin Neurol Neurosurg. 2020;196:105977. doi: 10.1016/j.clineuro.2020.105977 [DOI] [PubMed] [Google Scholar]
- 12.van Rooij FG, Vermeer SE, Goraj BM, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol. 2015;78(6):1005-1010. doi: 10.1002/ana.24539 [DOI] [PubMed] [Google Scholar]
- 13.van Rooij FG. Transient Neurological Attacks: Neuroimaging, Etiology, and Cognitive Consequences. White matter integrity after a recent TIA or transient neurological attack: association with diffusion-weighted imaging. Radboud University Nijmegen; 2018. [Google Scholar]
- 14.van Rooij FG, Plaizier NO, Vermeer SE, et al. Subjective cognitive impairment, depressive symptoms, and fatigue after a TIA or transient neurological attack: a prospective study. Behav Neurol. 2017;2017:5181024. doi: 10.1155/2017/5181024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Rooij FG, Plaizier NO, Vermeer SE, et al. Executive function declines in the first 6 Months after a transient ischemic attack or transient neurological attack. Stroke. 2017;48(12):3323-3328. doi: 10.1161/STROKEAHA.117.018298 [DOI] [PubMed] [Google Scholar]
- 16.Alpert JN. Funny turns. Arch Neurol. 2008;65(11):1548. doi: 10.1001/archneur.65.11.1548-b [DOI] [PubMed] [Google Scholar]
- 17.Ducrocq G, Amarenco P, Labreuche J, et al. A history of stroke/transient ischemic attack indicates high risks of cardiovascular event and hemorrhagic stroke in patients with coronary artery disease. Circulation. 2013;127(6):730-738. doi: 10.1161/CIRCULATIONAHA.112.141572 [DOI] [PubMed] [Google Scholar]
- 18.Boulanger M, Bejot Y, Rothwell PM, Touze E. Long-Term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: systematic review and meta-analysis. J Am Heart Assoc. 2018;7(2):e007267. doi: 10.1161/JAHA.117.007267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berghout BP, Bos D, Koudstaal PJ, Ikram MA, Ikram MK. Risk of recurrent stroke in Rotterdam between 1990 and 2020: a population-based cohort study. Lancet Reg Health Eur. 2023;30:100651. doi: 10.1016/j.lanepe.2023.100651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King G, Nielsen R. Why propensity scores should not Be used for matching. Polit Anal. 2019;27(4):435-454. doi: 10.1017/pan.2019.11 [DOI] [Google Scholar]
- 21.Wolters FJ, Li L, Gutnikov SA, Mehta Z, Rothwell PM. Medical attention seeking after transient ischemic attack and minor stroke before and after the UK face, arm, speech, time (FAST) public education campaign: results from the Oxford Vascular Study. JAMA Neurol. 2018;75(10):1225-1233. doi: 10.1001/jamaneurol.2018.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard VJ, Lackland DT, Lichtman JH, et al. Care seeking after stroke symptoms. Ann Neurol. 2008;63(4):466-472. doi: 10.1002/ana.21357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves MJ, Mullard AJ, Wehner S. Inter-rater reliability of data elements from a prototype of the Paul Coverdell National Acute Stroke Registry. BMC Neurol. 2008;8:19. doi: 10.1186/1471-2377-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonseca AC, Canhao P. Diagnostic difficulties in the classification of transient neurological attacks. Eur J Neurol. 2011;18(4):644-648. doi: 10.1111/j.1468-1331.2010.03241.x [DOI] [PubMed] [Google Scholar]
- 25.Koudstaal PJ, van Gijn J, Staal A, Duivenvoorden HJ, Gerritsma JG, Kraaijeveld CL. Diagnosis of transient ischemic attacks: improvement of interobserver agreement by a check-list in ordinary language. Stroke. 1986;17(4):723-728. doi: 10.1161/01.str.17.4.723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. All requests are directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, the data underlying this article cannot be made freely available in a public repository.