Abstract
Background and Objectives
To compare the diagnostic performance of an immunoassay for plasma concentrations of phosphorylated tau (p-tau) 217 with visual assessments of fluorine-18 fluorodeoxyglucose [18F]FDG-PET in individuals who meet appropriate use criteria for Alzheimer dementia (AD) biomarker assessments.
Methods
We performed a retrospective analysis of individuals with early-onset (age <65 years at onset) and/or atypical dementia (features other than memory at onset), who were evaluated at a tertiary care memory clinic. All participants underwent measurements of CSF biomarkers (Aβ42, p-tau181, and total tau levels), as well as [18F]FDG-PET scans, amyloid-PET scans, and plasma p-tau217 quantifications. To determine whether the [18F]FDG-PET images were compatible with AD, images were visually rated by 2 nuclear medicine experts. Using a contingency analysis, we evaluated the accuracy of [18F]FDG-PET scan interpretation and plasma p-tau217 for an AD biomarker profile in CSF and for amyloid-PET positivity.
Results
A total of 81 individuals with early onset and/or atypical dementia were included in this study (mean age = 65 years; 48/81 female (59%). Both [18F]FDG-PET and plasma p-tau217 showed high levels of agreement with reference standard AD biomarkers ([18F]FDG-PET area under the curve [AUC]: 71%; plasma p-tau217 AUC: 81%). Although both biomarkers had similar specificity for AD [18F]FDG-PET: 70%, CI: 0.56–0.81; plasma p-tau217: 70%, CI: 0.56–0.81), plasma p-tau217 had higher sensitivity for AD (plasma p-tau217: 97%, CI: 0.85–0.99 vs [18F]FDG-PET: 73%, CI: 0.57–0.85) (p = 0.01). Overall accuracy was also higher for plasma p-tau217 (AUC = 84%, CI: 0.75–0.93 vs 72%, CI: 0.60–0.83 of [18F]FDG-PET) (p = 0.02). The same pattern of results was observed when using amyloid-PET as the reference standard.
Discussion
Our study provides evidence that plasma p-tau217 has strong discriminative accuracy for AD among patients with early-onset and/or atypical dementia assessed in specialized settings. Future work should replicate these findings in secondary care settings.
Introduction
Alzheimer dementia (AD) clinical heterogeneity makes it difficult to arrive at a diagnosis based solely on clinical symptoms.1 Indeed, around 15%–30% of individuals clinically diagnosed with Alzheimer dementia are found to lack the underlying AD pathology at autopsy.2,3 AD pathology might be linked with nonamnestic focal cortical syndromes, especially in early-onset cases.4-7 In addition, the presence of other coexisting diseases and pathologies in older adults can further complicate the process of diagnosing and treating AD.8 Particularly in these circumstances, AD biomarkers became a valuable tool in the clinical diagnosis of individuals with cognitive impairment.9 Furthermore, evidence of amyloid-β pathology is required for determining the eligibility of antiamyloid disease-modifying therapies for AD, emphasizing the potential importance of biomarkers in clinical practice.10
Appropriate use recommendations for AD biomarkers state that their clinical application should be limited to specific cases, including those with early-onset, atypical, or otherwise unclear clinical symptoms; rapidly or slowly progressing dementia; or coexisting neuropsychiatric conditions.11-13 Patients with these features may have a higher probability of suffering from non-AD causes of their symptoms,14 which is why AD biomarker testing is performed.
The use of biomarkers, such as amyloid-PET, CSF assessments of Aβ42 and p-tau181, and [18F]FDG-PET, is all associated with changes in diagnosis, management plan, and diagnostic confidence.9,11,15 However, different biomarkers indicate different aspects of AD pathophysiology and are not interchangeable: amyloid-PET and CSF measurements of Aβ42 and p-tau181 change early in the natural history of AD16-19 and are specific for AD pathology.20 The most widely used biomarker in specialized clinics, [18F]FDG-PET, in contrast, measures cortical metabolic dysfunction perceived in all neurodegenerative conditions and is closely related to the types of symptoms observed.21,22 Posterior polymodal cortical hypometabolism often indicates AD pathology.23 Blood-based biomarkers, particularly of p-tau217, demonstrate strong associations with AD pathology measured with PET or CSF.24-27 Plasma biomarkers, which are more cost-effective than PET and CSF and are less invasive, promise to provide accessible assessments of AD pathology. The high discriminative accuracy of p-tau217 for biological AD24,27,28 has raised the possibility of p-tau217 being used in the diagnosis of AD, although more work is needed to compare the diagnostic performance of plasma p-tau217 with existing tools available in clinical practice.29 Here, our study compared the performance of a plasma p-tau217 assay with [18F]FDG-PET visual ratings from expert nuclear medicine physicians for detecting biological AD in individuals evaluated by dementia specialists who met appropriate use criteria (early-onset and/or atypical dementia) for AD biomarker testing.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Participants signed consent forms before samples/data were collected. McGill's ethics committee approved the study.
Study Participants
We conducted a retrospective analysis of individuals with atypical dementia and/or early-onset dementia (features other than memory at onset), who were assessed at a specialized memory clinic, at McGill University Research Center for Studies in Aging in Canada between 2018 and 2023. Inclusion criteria were individuals with younger onset dementia (65 years and younger), regardless of their clinical presentation, and/or patients with dementia aged 65 or older who have behavioral or cognitive symptoms other than memory (i.e., language, visuospatial, and executive) as the predominant clinical symptom. Patients with clinically diagnosed probable AD30 and patients with suspected neurodegenerative diseases other than AD were excluded from this study. Every eligible patient received a [18F]FDG-PET scan at Centre Hospitalier de l'Université de Montréal (CHUM) and a lumbar puncture at the McGill University Research Centre for Studies in Aging. The maximum time difference between biomarker assessments was 12 months. Before AD biomarker assessments, each participant underwent a medical and neurologic evaluation, neuropsychological testing, Mini Mental State Examination (MMSE), and Montreal Cognitive Assessment (MoCA). Furthermore, complete blood count test and MRI were performed for all patients in the McGill Centre for Studies in Aging. All patients also underwent assessments of plasma p-tau217 and amyloid-PET as part of their participation in the Translational Biomarkers in Aging and Dementia (TRIAD) cohort. The TRIAD is a large-scale longitudinal cohort and was launched in 2017 as part of the McGill Centre for Studies in Aging. This cohort recruits individuals who are cognitively unimpaired and have mild cognitive impairment or dementia. Full information regarding the TRIAD inclusion/exclusion criteria is described elsewhere.31
CSF Data Collection and Analysis
A standard protocol elaborated by McGill University Research Center for Studies was used to collect CSF sample during clinical evaluations. Written informed consent was provided by all participants. Using an atraumatic 24 G Sprotte needle, the CSF was obtained from the L3/L4 or L4/L5 interspaces. The CSF samples were aliquoted into polypropylene test tubes of 2 mL (0.5 mL minimum), centrifuged at 20°C, and frozen within an hour of the collection. CSF samples were sent to a commercial laboratory (Athena Diagnostics, Worcester MA) for analyses of amyloid (Aβ1-42), total tau (t-tau), and phosphorylated tau (p-tau181) using the Admark ELISA kit. Moreover, an Aβ42 to t-tau index [Amyloid-Tau Index (ATI), calculated as Aβ42/(240 + 1.18t-tau)], was provided. Athena Diagnostics Laboratory considers results to be positive for AD if they show an ATI <1.0 and a p-tau181 > 61 pg/mL. Using these CSF biomarker thresholds, 34 patients were identified as having biological AD. The remaining 47 were negative for biological AD biomarker evidence.
Plasma Data Collection and Analysis
The collection of blood samples followed the protocols previously described.25 Plasma levels of p-tau217 were measured by researchers at Janssen R&D blinded to clinical and other biomarker data, as detailed previously.28 An abnormal result for plasma p-tau217 corresponded to a value above the threshold of 0.083 pg/mL defined for the Janssen plasma p-tau217 Simoa assay.28 That threshold was used in analyses assessing the individual degree of agreement between plasma p-tau217 and [18F]FDG-PET.
PET Imaging Data Acquisition and Analyses
[18F]FDG-PET
Commercial PET/CT scanners were used to perform [18F]FDG-PET brain imaging in all cases according to the protocol described before.23 During each [18F]FDG-PET session, the patient underwent a 20-minute PET scan, followed by a 10-minute CT transmission scan (to correct for attenuation). In this study, 2 certified nuclear medicine physicians (J.P.S. and K.P.) visually assessed the [18F]FDG-PET brain scans and 3D-SSP (3D-stereotaxic surface projection) maps of patients.32 CSF and plasma biomarker results were blinded to them, although clinical data were available. The interpretation made by these professionals is approved by the European Association of Nuclear Medicine and the European Academy of Neurology.
According to [18F]FDG-PET hypometabolic patterns, patients were divided into 2 groups: the AD-associated metabolic pattern (n = 39) and non–AD-associated metabolic pattern (n = 42). Briefly, the AD-associated pattern included [18F]FDG-PET scans with hypometabolism in the posterior cingulate gyrus, temporal lobes, and/or posterior parietal cortices, as well as [18F]FDG-PET scans that showed atypical cases of AD such as posterior cortical atrophy, logopenic progressive aphasia, and behavioral/dysexecutive AD. The non–AD-associated metabolic patterns included [18F]FDG-PET scans that do not fit the AD distribution but are still abnormal. For example, non-AD metabolic patterns include the [18F]FDG-PET metabolic distribution pattern linked with Lewy bodies (LBD), those from the frontal temporal lobar degeneration (FTLD) family, and mixed dementia. Patients with a nonneurodegenerative [18F]FDG-PET pattern (i.e., the scan is not indicative of AD or other neurodegenerative diseases) are also included in the non–AD-associated metabolic pattern group. Discrimination between behavioral/dysexecutive variant of AD and FTLD is based on a comparison of premotor frontal anomalies and posterior parietal anomalies. In FTLD, anterior anomalies dominate, while in behavioral/dysexecutive AD, posterior anomalies dominate.33 Posterior portions of the cingulate gyri are also evaluated. Finally, frontal areas are also examined for atrophy on MRI; if so, FTLD is suspected.33
Amyloid-PET
The acquisition and processing of amyloid-PET data have been described previously.34 Briefly, [18F]AZD4694 PET scans were obtained using a brain-dedicated Siemens High-Resolution Research Tomograph. In all participants, an overall composite amyloid-PET SUVR was calculated by averaging the SUVR from the precuneus, prefrontal, orbitofrontal, parietal, temporal, and cingulate cortices. Individuals with an AZD4694 PET SUVR above 1.5534 were considered amyloid-PET–positive. According to this amyloid-PET threshold, 34 individuals had 43 positives for amyloid-PET, while 38 were considered negative for amyloid-PET. We also investigated the correspondence between visually determined amyloid-PET positivity and quantitatively determined amyloid-PET positivity, which showed 100% agreement, likely because the quantitative threshold is based partially on visual assessments.34
Outcomes
The primary outcomes of our study are sensitivity, specificity, and area under the receiver operating characteristics (ROC) curve of 2 index tests (plasma p-tau217 and [18F]FDG-PET visual readings) for an AD biomarker status in CSF, as determined by the Aβ42 to t-tau index [ATI, calculated as Aβ42/(240 + 1.18t-tau)] plus p-tau181 > 61 pg/mL.21 In addition, a second reference standard was used (amyloid-PET status).
Statistical Analysis
The analyses of all data were performed using Prism-Graph 7.0 software (Graph-Pad Software, San Diego, CA) and R statistical software package, version 4.1.2. A descriptive analysis was first performed to compare demographic variables and clinical characteristics between CSF non-AD profile and CSF AD profile groups, using χ2 for categorical variables and Mann-Whitney U for continuous variables. Owing to the nonnormal distribution of biomarker data, participant groups were characterized using medians and interquartile ranges (IQRs). The second step was to determine the degree of agreement between plasma p-tau217 and [18F]FDG-PET biomarkers according to CSF biomarkers and amyloid-PET positivity using contingency analysis and Cohen kappa coefficient with 95% confidence intervals (CI). To evaluate if plasma p-tau217 and [18F]FDG-PET had significantly different sensitivities and specificities as well as likelihood ratio, we computed the McNemar test using the R package DTComPair.35 Third, ROC curves were computed to evaluate the accuracy of plasma p-tau217 and [18F]FDG-PET in identifying AD biomarker status according to reference standards (CSF and amyloid-PET). Statistical assessment of whether plasma p-tau217 and [18F]FDG-PET diagnostic accuracies were significantly different was determined by a bootstrap-based test implemented in the pROC package in R, a statistical framework for comparing 2 correlated (or paired) ROC curves.36 Furthermore, the sensitivity and specificity of plasma p-tau217 and [18F]FDG-PET for AD biological were derived. We did not use multiple comparison corrections for our study's main outcome. Finally, statistical power (G*Power) was used to determine the minimum significant effect size. With an alpha of 0.05, 81 observations would provide 80% statistical power to detect an effect size of w = 0.31.
Data Availability
Data supporting the findings in this article will be shared through a material transfer agreement on request by qualified investigator.
Results
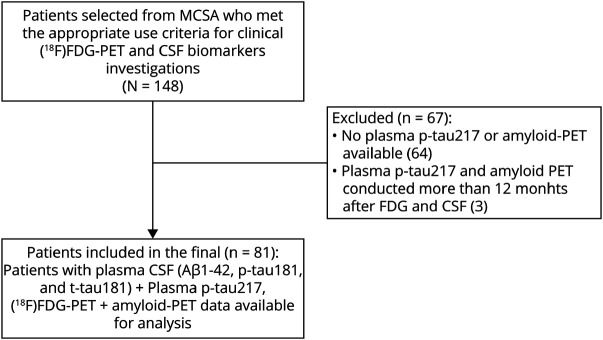
We identified a total of 148 patients with early-onset and/or atypical AD between 2018 and 2023 who were clinically assessed with CSF and [18F]FDG-PET based on appropriate use criteria11-13 at the McGill Centre for Studies in Aging. From 148 patients who were eligible for [18F]FDG-PET and CSF analysis based on appropriate use criteria, only 84 individuals had plasma p-tau217 assessments and amyloid-PET performed as part of the TRIAD study. Three of these patients were excluded as the time interval between blood draw, and [18F]FDG-PET assessment or lumbar puncture was greater than 12 months. The final sample size of our study was 81 participants.
A median time of 5 months (IQR = 3–8) was found between CSF biomarker measurements and [18F]FDG-PET measurements, similarly 5 months (IQR = 2–10) between CSF and plasma p-tau217 measurements, and 4 months (IQR = 2–8) between [18F]FDG-PET and plasma p-tau217. A flowchart of the study selection process is shown in Figure 1.
Figure 1. Flowchart Summarizing the Selection of Cases in Our Cross-Sectional Study.
We analyzed early onset and/or atypical Alzheimer disease (AD) cases who were clinically assessed with CSF and [18F]FDG-PET and also had plasma p-tau217 and amyloid-PET as part of TRIAD study. The gap between all assessments was lower than 12 months. MCSA = McGill University Research Center for Studies in Aging; TRIAD = Translational Biomarkers of Aging and Dementia cohort.
A summary of demographics and clinical information for the study sample is presented in Table 1. According to an established cutoff (ATI<1.0 and p-tau181 > 61 pg/mL) (Table 1), individuals were classified according to their CSF biomarker status as either non-AD (n = 47, 58%) or AD (n = 34, 42%). The AD and non-AD groups did not differ significantly based on sex, age, or years of education. However, when comparing MMSE and MoCA scores between the groups, statistically significant differences were found. In the AD group, MMSE scores were lower than in the non-AD group (p = 0.001). A similar pattern was observed for MoCA scores (p = 0.001) in the AD group as compared with the non-AD group. There was also an increase in the number of individuals in the AD group who carried at least 1 ApoE4 allele (68%) compared with individuals in the non-AD group (34%) (p = 0.002). Among participants with CSF biomarker evidence of AD, Aβ42 concentration, and ATI index were lower, and p-tau181 and t-tau levels were significantly higher compared with the participants in the CSF biomarker non-AD group. Data regarding an individual CSF biomarker according to [18F]FDG-PET and amyloid-PET are displayed in eFigures 1 and 2.
Table 1.
Demographics, Clinical Characteristics, and Biomarker Measures Grouped According to the CSF AD Profile
| Non-AD CSF profile | CSF AD profile | p Value | |
| No. of patients (81) | 47 | 34 | |
| Sex, n (%) | |||
| Male | 21 (44.7) | 12 (35.2) | 0.40 |
| Female | 26 (54.1) | 22 (64.7) | |
| Age median (IQR) | 65 (55.0–73.0) | 64 (56.8–69.3) | 0.59 |
| Education median (IQR) | 16 (12–17) | 15.5 (13–18) | 0.71 |
| MMSE median (IQR) | 27 (22–29) | 19 (18–26) | 0.001 |
| MoCA median (IQR) | 25 (17–27) | 16 (12–22) | 0.001 |
| CDR median (IQR) | 0.5 (0.0–0.5) | 1.0 (0.5–1.0) | 0.004 |
| APOE e4 status, n (%) | |||
| Carriers | 16 (34.0) | 23 (68.0) | 0.002 |
| Noncarriers | 31 (66.1) | 11 (32.4) | |
| CSF Aβ42 median (IQR) | 714.7 (528.7–938.2) | 431.7 (350.3–541.1) | <0.001 |
| CSF p-tau181 median (IQR) | 46.10 (32.9–52.6) | 86.05 (72.71–125.0) | <0.001 |
| CSF t-tau median (IQR) | 246.1 (176.2–318.9) | 608.7 (503.2–948.5) | <0.001 |
| ATI median (IQR) | 1.3 (1.01–1.7) | 0.4 (0.3–0.6) | <0.001 |
| Plasma p-tau217 median (IQR) | 0.1 (0.04–0.1) | 0.2 (0.2–0.4) | <0.001 |
| [18F]FDG-PET (+) | 14 | 25 | <0.001 |
| [18F]FDG-PET (−) | 33 | 9 |
Abbreviations: [18F]FDG = fluorine-18 fluorodeoxyglucose; AD = Alzheimer dementia; ATI = Aβ42 to t-tau index; Aβ = amyloid-β; CDR = Clinical Dementia Rating; IQR = interquartile range; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; non-AD = non–Alzheimer disease; p-tau181 = phosphorylated tau181; p-tau217 = phosphorylated-tau217; t-tau = total tau.
For categorical variables, numbers and percentages are reported, whereas for continuous variables, medians and interquartile ranges are reported. To test if there were any statistical differences between the non-AD and AD groups based on the CSF status profile, categorical variables were compared by the χ2 test and continuous variables were compared by Mann-Whitney U tests.
Patients included in the final (n = 75): A total of 75 participants had plasma CSF (Aβ 1–42, p-tau181 and t-tau181) + plasma p-tau217, [18F]FDG-PET + amyloid-PET data available for analysis.
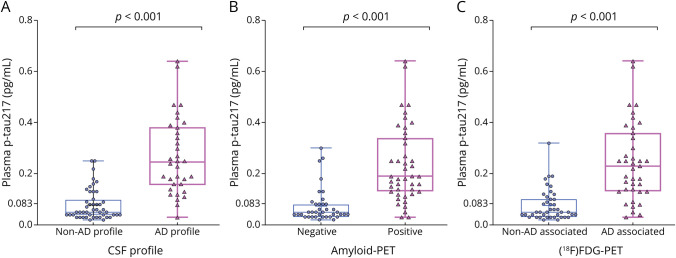
Significantly higher plasma p-tau217 levels were also observed in the CSF biomarker AD group compared with the non-AD CSF group (Figure 2A). The association between individual CSF biomarker concentrations and plasma p-tau217 levels is available in eFigure 3.
Figure 2. Concentration of p-tau217 in Plasma According to CSF Biomarker Profile, Amyloid-PET Status, and [18F]FDG-PET–Associated Metabolic Pattern.
There were statistically significant differences in plasma p-tau217 concentrations between AD and non-AD CSF biomarker profiles, between amyloid-PET–positive and amyloid-PET–negative individuals, as well as [18F]FDG-PET metabolic pattern visual rating (all p < 0.001). AD = Alzheimer dementia; amyloid-PET = AZD4694 and [18F]FDG = fluorine-18 fluorodeoxyglucose; non-AD = non–Alzheimer dementia; p-tau217 = phosphorylated tau 217.
Plasma concentrations of p-tau217 were also significantly higher in amyloid-PET+ individuals than in amyloid-PET individuals (Figure 2B). Similarly, plasma p-tau217 concentration was significantly higher in individuals whose [18F]FDG-PET–associated pattern indicated AD compared with those without an AD-associated pattern (Figure 2C).
Concordance and Discordance Between Plasma p-tau217 and Qualitative [18F]FDG-PET Assessment
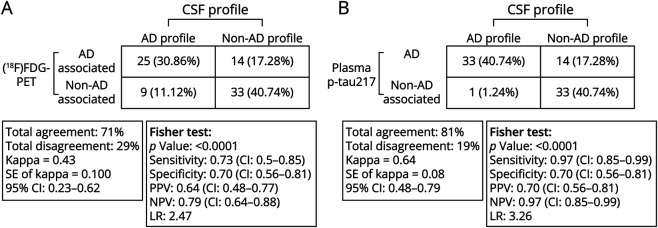
Figure 3, A and B, shows the discriminative accuracy and concordance and discrepancy of [18F]FDG-PET and plasma p-tau217 among non-AD and AD groups according to the CSF classification profile. The overall level of agreement between the results of CSF biomarkers and [18F]FDG-PET was 71% from 81 individuals with a calculated kappa coefficient of agreement of 0.43 (95% CI 0.23–0.62), indicating a moderate degree of agreement of [18F]FDG-PET with CSF AD biomarkers (Figure 3A). However, the overall agreement between plasma p-tau217 and CSF AD biomarkers was 81%, with a kappa coefficient of agreement of 0.64 (95% CI 0.48–0.79), showing a substantial degree of agreement between plasma p-tau217 and CSF AD biomarkers (Figure 3B).
Figure 3. Illustrating the Discriminative Accuracy of (A) [18F]FDG-PET and (B) Plasma ptau217 for AD and Non-AD CSF Biomarker Groups.
Plasma p-tau217 had higher agreement with the CSF biomarker profile than did [18F]FDG-PET–associated metabolic pattern. A CSF AD-positive profile was defined as an Amyloid-Tau Index (ATI) < 1.0 and a p-tau181 > 61 pg/mL. [18F]FDG-PET classification patterns are based on visual ratings from nuclear medicine physicians specializing in the diagnosis of neurodegenerative diseases. [18F]FDG = fluorine-18 fluorodeoxyglucose; AD = Alzheimer dementia; LR = likelihood ratio; non-AD = non–Alzheimer disease; NPV = negative predictive value; PPV = positive predictive value.
Among the 34 patients with a CSF profile consistent with AD, 25 had an [18F]FDG-PET pattern that was associated with AD (30.86% of total sample, Figure 3A), as compared with 33 who had plasma p-tau217 that was compatible with AD (40.74% of total sample, Figure 3B). Among these CSF AD profile groups, 9 (11.12%) participants had an [18F]FDG-PET scan not associated with AD, whereas only 1 (1.2%) participant who with a CSF AD biomarker profile was negative for plasma p-tau217. Both [18F]FDG-PET and plasma p-tau217 identified 33 of the 47 participants who had a non-AD CSF profile. The remaining 14 individuals (17.28% of total sample) had an [18F]FDG-PET pattern associated with AD (Figure 3A). Similarly, among these 47 patients with non-AD CSF profile, 14 individuals (17% of total sample) also had plasma levels of p-tau217 compatible with AD (Figure 3B).
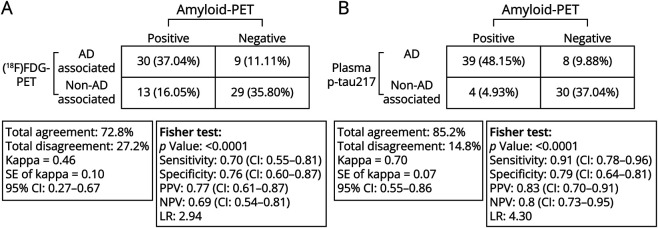
The concordance and discordance between [18F]FDG-PET and plasma p-tau217 in AD and non-AD groups according to the amyloid-PET classification profile were also evaluated (Figure 4, A and B). The overall agreement between amyloid-PET status and [18F]FDG-PET status results was 73% from 81 individuals with a calculated kappa coefficient of agreement of 0.46 (95% CI 0.27–0.67), indicating a moderate degree of agreement (Figure 4A). Of the 43 patients with a positive amyloid-PET pattern for AD, 30 also displayed an [18F]FDG-PET–associated pattern for AD (37.0% of overall the sample), while 13 individuals (16.1%) did not display an [18F]FDG-PET pattern compatible with AD. Of the 38 individuals whose amyloid-PET results were negative for AD, 29 (35.8%) individuals displayed an [18F]FDG-PET pattern that was not associated with AD. However, 9 individuals (11.1%) had an [18F]FDG-PET pattern that was compatible with AD. The overall agreement between amyloid-PET profile and plasma p-tau217 in AD and non-AD groups according to amyloid-PET classification was 85.2% (from 81 individuals), with a kappa coefficient of agreement of 0.70 (95% CI 0.55–0.86), meaning a substantial level of agreement (Figure 4B). Of the 43 patients with an amyloid-PET profile indicating AD, 39 also had plasma levels of p-tau217 associated with AD (48.2% of the overall sample); however, 4 did not display plasma p-tau217 compatible with AD. Among 38 participants with an amyloid-PET status profile that did not indicate AD, 8 patients (9.8%) showed plasma levels of p-tau217 associated with AD, while 30 patients with an amyloid-PET profile that was not associated with AD (37.0% of the overall sample) also did not show plasma p-tau217 compatible with AD.
Figure 4. Illustrating the Discriminative Accuracy of (A) [18F]FDG-PET and (B) Plasma p-tau217 for Amyloid-PET Positivity.
There was a greater agreement between plasma p-tau217 and the amyloid-PET status than with [18F]FDG-PET–associated metabolic pattern. Amyloid-PET positivity was defined based on a previously validated [18F]AZD4694 SUVR of 1.55 or greater, and [18F]FDG-PET classification patterns were based on visual ratings from nuclear medicine physicians specializing in the diagnosis of neurodegenerative diseases. Abbreviations: [18F]FDG = fluorine-18 fluorodeoxyglucose; AD = Alzheimer dementia; LR = likelihood ratio; non-AD = non–Alzheimer dementia; NPV = negative predictive value; PPV = positive predictive value.
Discriminative Performance of Plasma p-tau217 and [18F]FDG-PET to Identify Biological AD
Thirty-four (42.0%) individuals had a CSF biomarker profile consistent with AD, and 43 (53.1%) individuals were amyloid-PET–positive across the entire sample. ROC curves were used to assess the accuracy of plasma p-tau217 and [18F]FDG-PET in identifying positive AD biomarkers (CSF) and amyloid-PET positivity across the entire sample (Figures 3 and 4, eFigure 4).
Plasma p-tau217 showed a good performance in differentiating participants with a positive CSF biomarker test from individuals with negative CSF biomarker with an area under the curve (AUC) of 0.84 (95% CI 0.75–0.93), the sensitivity was 97% and the specificity was 70% (Figure 3B, eFigure 4A). Meanwhile, [18F]FDG-PET had an AUC of 0.72 (95% CI 0.60–0.83), the sensitivity was 73%, and the specificity was 70% (Figure 3A, eFigure 4A). Based on bootstrap testing, statistically significant difference was found between the AUCs of [18F]FDG-PET and plasma p-tau217 (p = 0.02) (eFigure 4A). We also compared the sensitivities and specificities of [18F]FDG-PET and plasma p-tau217 for a CSF AD biomarker profile. As determined by the McNemar test, the sensitivities of [18F]FDG-PET and plasma p-tau217 differed significantly (p = 0.01), while the specificities of [18F]FDG-PET and plasma p-tau217 were not significantly different (p = 0.1) (Figure 3, A and B, eFigure 4A). Similarly, plasma p-tau217 performed well in discriminating individuals with positive amyloid-PET scans from participants with negative amyloid-PET scans, with an AUC of 85% (95% CI 0.76–0.94), a sensitivity of 91%, and a specificity of 79% (Figure 4B, eFigure 4B). In turn, [18F]FDG-PET showed an AUC of 0.73 (95% CI 0.62–0.84), a sensitivity of 70%, and a specificity of 76% for identifying amyloid-PET positivity (Figure 4A, eFigure 4B). A significant difference was found when comparing the AUC of [18F]FDG-PET with plasma p-tau217 (p = 0.05) (eFigure 4B). On the basis of amyloid-PET profile, we compared the sensitivity and specificity of [18F]FDG-PET and plasma p-tau217. A significant (p = 0.006) difference was found when comparing the sensitivities between [18F]FDG-PET and plasma p-tau217. No statistical differences were found regarding specificities (p = 0.78) (Figure 4, A and B, eFigure 4B).
Accuracy of Plasma p-tau217 to Identify Amyloid-PET Positivity in Individuals With Non-AD [18F]FDG-PET Patterns
Of the patients with a positive plasma p-tau217 and [18F]FDG-PET visual reading not consistent with AD (n = 13), the [18F]FDG-PET visual readings were 8 patients with nonneurodegeneration pattern, 1 patient with frontotemporal lobar degeneration, 1 patient with semantic PPA, 1 patient with nonfluent aphasia, and 2 patients with LBD. Furthermore, 10 of these 13 patients were amyloid-PET–positive, and 9 of these 13 patients had a CSF biomarkers profile compatible with AD (eTable 1).
Discussion
This study compared the diagnostic accuracy of plasma p-tau217 with visual ratings of [18F]FDG-PET scans for the identification of AD in individuals who meet appropriate use criteria for AD biomarker assessments. According to our findings, p-tau217 had greater sensitivity and overall accuracy than [18F]FDG-PET scans for AD pathology as identified by CSF and amyloid-PET. Specificity for AD pathology was similar for both plasma p-tau217 and [18F]FDG-PET. Overall, our study provides evidence of a strong performance of plasma p-tau217 in assessing patients with suspected AD, but the specificity of 79% (meaning 21% false positives) for amyloid-PET positivity in our study calls for caution for possible use in clinical practice and for determining eligibility for disease-modifying treatments for AD,10 and calls for further research studies in real-life clinical practice settings.29
The development of ultrasensitive blood biomarkers promises to transform the clinical diagnostic assessment of individuals suspected of having AD and the surrounding research landscape.37 Owing to their superior accessibility and scalability over amyloid-PET, it is anticipated that blood biomarkers will allow for a greater number of individuals to access AD biomarker testing, particularly in areas of the globe where PET scans are rarely performed. Our study, combined with recent studies showing high agreement between plasma p-tau217 and core CSF biomarkers of AD,24,27,28 suggests that plasma biomarkers may contribute to more timely and equitable diagnoses, especially if used in a 2-cutoff approach to recognize patients at high and low risk for having brain amyloidosis, which would leave a smaller group in need of CSF or PET testing.38
Plasma p-tau217 had significantly higher sensitivity than [18F]FDG-PET visual assessments for identifying AD pathology identified using CSF or amyloid-PET positivity. Because brain hypometabolism occurs later in the natural history of AD than fluid p-tau abnormality,16-19 [18F]FDG-PET visual assessments may be less well-suited to identify early AD-related pathologic changes that have not yet resulted in detectable neurodegeneration.23 Therefore, it is possible that plasma p-tau217 is detecting biological AD in individuals with copathology where AD is not the driving cause of symptoms. Future efforts to stage AD severity using plasma biomarker panels that can detect early AD pathology (i.e., p-tau217) and later AD pathology (i.e., p-tau205 and/or MTBR-243) may be helpful in determining whether AD is a driving cause of clinical symptoms.16,39 However, in contrast to sensitivity, specificity of [18F]FDG-PET visual ratings by expert nuclear medicine physicians for AD pathologic changes was high and was not statistically different from plasma p-tau217. These findings lend support to the strong association between specific patterns of hypometabolism on [18F]FDG-PET as read by nuclear medicine experts with clinical manifestations of AD, even in atypical and early onset cases.4,5,21,33
It is essential to consider that [18F]FDG-PET assessment from expert raters provides far more detailed information than the mere presence or absence of AD.40 Specifically, [18F]FDG-PET is a highly sensitive measure used to identify abnormalities in brain metabolism and is incorporated in the criteria for many other neurodegenerative diseases (reviewed in 40), including when attempting to differentiate between late-onset psychiatric disease from neurodegenerative diseases such as behavioral variant frontotemporal dementia.41 Furthermore, [18F]FDG-PET patterns can be helpful for detecting hippocampal sclerosis cases in tau-negative amnestic dementia42 and identifying limbic-predominant age-related TDP-43 encephalopathy (LATE).43 Moreover, the cingulate island sign on [18F]FDG-PET is highly sensitive and moderately specific for Lewy body dementia.44 It is important that recent studies have suggested plasma biomarkers are effective in identifying abnormal amyloid-PET and tau-PET in patients with Lewy body dementia.45
Because of the close association between [18F]FDG-PET and clinical symptoms, the topography of [18F]FDG-PET scans likely provides valuable prognostic information about upcoming cognitive decline.4 Moreover, [18F]FDG-PET can inform on the presence of copathologies, which are highly prevalent among community-dwelling older adults with cognitive disability.8 [18F]FDG-PET is also be helpful for differential diagnosis within the frontotemporal lobar degeneration spectrum in individuals with a negative amyloid-PET.40 Therefore, we do not believe that plasma biomarkers should replace [18F]FDG-PET in the clinical evaluation of patients with neurodegenerative diseases of uncertain etiology because these biomarkers give complementary information. Instead, it is more likely that plasma p-tau217 can decrease the need for highly expensive and often inaccessible amyloid-PET and tau-PET scans, and reduce the need for invasive lumbar punctures. Future studies are needed to investigate the value of plasma p-tau217 and [18F]FDG-PET scans in the differential diagnosis of cognitive impairment, especially for atypical clinical syndromes.
Because plasma p-tau217 and [18F]FDG-PET provide complementary information, future studies may wish to determine clinical workflows that incorporate plasma biomarkers and [18F]FDG-PET in relation to the patient's clinical presentation. Owing to the high correspondence of plasma p-tau217 with amyloid-β abnormality,25-28 such a diagnostic workflow could be based on the recently proposed diagnostic algorithm that uses MRI, amyloid-PET, and [18F]FDG-PET in the differential diagnosis of patients with suspected neurodegenerative disease.40 However, caution is still necessary when interpreting plasma biomarkers at the individual level, especially in unselected populations. Caution is warranted when interpreting blood biomarker results in relation to clinical pretest probability46 and in relation to medical comorbidities.47 Our study does not suggest that p-tau217 is sufficient for an accurate diagnosis of AD, especially in light of recent recommendations on minimal acceptable diagnostic performance of plasma biomarkers.48 Overall, conducting prospective multicenter studies that assess the performance of the blood biomarkers in real-world clinical settings and assess changes in diagnostic management and confidence related to plasma AD biomarkers will help determine their clinical role in the future.
This study must be interpreted within the context of significant limitations. First, this study was limited to a single tertiary care memory center in which the sample size was relatively small. Furthermore, no replication cohort was available. Second, the interpretation of our study is limited by the lack of histopathologic assessments. Despite this limitation, individuals with a positive amyloid-PET scan nearly always have Braak III or higher neurofibrillary tangle pathology, and therefore, amyloid-PET positivity likely indicates moderate or severe AD neuropathologic changes.39 Third, as blood biomarker measurements are becoming potential candidates for clinical application, it is essential to gain a deeper insight into their performance within real-world clinical cohorts. Generally speaking, the performance of blood biomarker in real-world populations-based studies is promising.26 However, the effect of systemic medical disorders such as chronic kidney disease26 requires further investigation. Recent immunoprecipitation-mass spectrometry (IP-MS) studies suggest that in such cases, plasma p-tau217/tau ratios may offer superior performance,47 while other IP-MS studies found equal diagnostic performance to identify amyloid pathology and AD for plasma p-tau217/tau ratio and plasma p-tau217 by itself.49 Fourth, another limitation of our study is that CSF dynamics can be affected by some non-AD disorders. However, our use of amyloid-PET status as a reference standard in supplementary analyses helps attenuate this concern. Furthermore, because of the 100% agreement between quantitatively derived and visually derived amyloid-PET positivity classifications in our study, our results may have better generalizability because visually determined amyloid-PET positivity is the standard of care in clinical practice.12 Fifth, our study may also be limited by the absence of quantitative [18F]FDG-PET assessments. Comparing quantitative [18F]FDG-PET imaging with visual inspection, the quantitative [18F]FDG-PET imaging approaches have been shown to be more specific.50 However, in our study, [18F]FDG-PET assessments were performed as part of a standard clinical workup (not blinded interpretation), which has stronger ecological validity and represents the standard of care in clinical practice. Nonetheless, it is possible that novel [18F]FDG-PET classification methods have superior performance to visual assessments. Sixth, although the goal of our study was not to create clinical models with the optimal performance to identify amyloid-PET status, it is possible that clinical models incorporating age, sex, and ApoE4 status; MoCA; or MMSE score will result in higher accuracy of [18F]FDG-PET and/or plasma biomarkers to detect amyloid-PET status. In particular, plasma biomarkers may be at an advantage because determination of ApoE4 status also requires a blood sample. Finally, our study is not designed to demonstrate that plasma p-tau217, or any plasma biomarker, outperforms [18F]FDG-PET in the clinical assessment of individuals with suspected neurodegenerative diseases because [18F]FDG-PET has many uses outside of identifying the existence of AD pathology, including identifying copathologies and risk of near-term cognitive decline.40 Furthermore, this study did not demonstrate the role of plasma p-tau217 in the differential diagnosis of cognitive impairment, and future prospective studies evaluating changes in diagnosis and clinical management are needed for this purpose.
In conclusion, among cognitively impaired individuals who meet appropriate use criteria for AD biomarker investigations, plasma p-tau217 had greater agreement with CSF and amyloid-PET biomarker profiles than did [18F]FDG-PET visual assessments. Future work is necessary to determine patient eligibility for plasma p-tau217 assessments and where these fall in the diagnostic pathway about other biomarker studies. Nevertheless, the topographical information from [18F]FDG-PET may give complementary information to AD-specific biomarkers by providing information on non-AD neurodegenerative diseases, potential copathologies, and disease severity.
Glossary
- AD
Alzheimer dementia
- AUC
area under the curve
- FTLD
frontal temporal lobar degeneration
- IP-MS
immunoprecipitation-mass spectrometry
- IQR
interquartile range
- LBD
Lewy body
- MMSE
Mini Mental State Examination
- MoCA
Montreal Cognitive Assessment
- p-tau
phosphorylated tau
- ROC
receiver operating characteristics
- TRIAD
Translational Biomarkers of Aging and Dementia cohort
Author Contributions
K.M. Quispialaya: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. J. Therriault: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. A. Aliaga: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. A.L. Benedet: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. N.J. Ashton: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. T. Karikari: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. A. Cassa Macedo: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. N. Rahmouni: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. C. Tissot: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. J. Fernandez Arias: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. Y.-T.T. Wang: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. L. Trudel: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. S.A. Hosseini: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. T. Matsudaira: drafting/revision of the manuscript for content, including medical writing for content; study concept or design. T. Chan: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. T. Pascoal: drafting/revision of the manuscript for content, including medical writing for content. B Gilfix: drafting/revision of the manuscript for content, including medical writing for content. P. Vitali: drafting/revision of the manuscript for content, including medical writing for content. E.R. Zimmer: drafting/revision of the manuscript for content, including medical writing for content. K. Provost: drafting/revision of the manuscript for content, including medical writing for content. J.-P. Soucy: drafting/revision of the manuscript for content, including medical writing for content. S. Gauthier: drafting/revision of the manuscript for content, including medical writing for content. H. Zetterberg: drafting/revision of the manuscript for content, including medical writing for content. B.J. Jean-Claude: drafting/revision of the manuscript for content, including medical writing for content. K. Blennow: drafting/revision of the manuscript for content, including medical writing for content. P. Rosa-Neto: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data.
Study Funding
This research is supported by the Weston Brain Institute, Canadian Institutes of Health Research (CIHR) [MOP-11-51-31; RFN 152985, 159815, 162303], Canadian Consortium of Neurodegeneration and Aging (CCNA; MOP-11-51-31 -team 1), the Alzheimer's Association [NIRG-12-92090, NIRP-12-259245], Brain Canada Foundation (CFI Project 34874; 33397), the Fonds de Recherche du Québec - Santé (FRQS; Chercheur Boursier, 2020-VICO-279314). TAP, P.R-N and SG are members of the CIHR-CCNA Canadian Consortium of Neurodegeneration in Aging. Colin J. Adair Charitable Foundation.
Disclosure
K.M. Quispialaya reports no disclosures relevant to the manuscript. J. Therriault has served as a consultant for the Neurotorium educational platform and for Alzheon Inc. A. Aliaga, A.L. Benedet, N.J. Ashton, T.K. Karikari, A.C. Macedo, N. Rahmouni, C. Tissot, J. Fernandez-Arias, Y.-T. Wang, L. Trudel, S.A. Hosseini, T. Matsudaira, T. Chan, T.A. Pascoal, and B.M. Gilfix report no disclosures relevant to the manuscript. P. Vitali serves on the scientific advisory boards for NovoNordisc, Eisai, and Lilly, and received honoraria from IntelGenx Corp. E.R. Zimmer serves on the scientific advisory board of Next Innovative Therapeutics, and is cofounder and member of the scientific advisory board of Masima. K. Provost and J.-P. Soucy report no disclosures relevant to the manuscript. S. Gauthier serves on scientific advisory boards for Alzeon, AmyriAD, Advantage, Eisai Canada, Enigma USA, Lilly Canada, Medesis, Lundbeck Foundation, Novo-Nordisk Canada, Okutsa, and TauRx. H. Zetterberg serves on the scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). B.J. Jean-Claude reports no disclosures relevant to the manuscript. K. Blennow reports having served as a consultant and at advisory boards for AC Immune, Acumen, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. P. Rosa-Neto reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primer. 2021;7(1):33. doi: 10.1038/s41572-021-00269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Therriault J, Pascoal TA, Benedet AL, et al. Frequency of biologically defined Alzheimer disease in relation to age, sex, APOE ε4, and cognitive impairment. Neurology. 2021;96(7):e975-e985. doi: 10.1212/WNL.0000000000011416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi: 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townley RA, Graff-Radford J, Mantyh WG, et al. Progressive dysexecutive syndrome due to Alzheimer's disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2020;2(1):fcaa068. doi: 10.1093/braincomms/fcaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(Pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130(Pt 10):2636-2645. doi: 10.1093/brain/awm213 [DOI] [PubMed] [Google Scholar]
- 7.Therriault J, Pascoal TA, Savard M, et al. Intrinsic connectivity of the human brain provides scaffold for tau aggregation in clinical variants of Alzheimer's disease. Sci Transl Med. 2022;14(659):eabc8693. doi: 10.1126/scitranslmed.abc8693 [DOI] [PubMed] [Google Scholar]
- 8.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197-2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 9.Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286-1294. doi: 10.1001/jama.2019.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate Use recommendations. J Prev Alzheimers Dis. 2023;10(3):362-377. doi: 10.14283/jpad.2023.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw LM, Arias J, Blennow K, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2018;14(11):1505-1521. doi: 10.1016/j.jalz.2018.07.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the amyloid imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9(1):e1–e16. doi: 10.1016/j.jalz.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel on Neurological Imaging, Moonis G, Subramaniam RM, et al. ACR appropriateness criteria® dementia. J Am Coll Radiol. 2020;17(5S):S100-S112. doi: 10.1016/j.jacr.2020.01.040 [DOI] [PubMed] [Google Scholar]
- 14.Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD. The diagnosis of young-onset dementia. Lancet Neurol. 2010;9(8):793-806. doi: 10.1016/S1474-4422(10)70159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perini G, Rodriguez-Vieitez E, Kadir A, Sala A, Savitcheva I, Nordberg A. Clinical impact of 18F-FDG-PET among memory clinic patients with uncertain diagnosis. Eur J Nucl Med Mol Imaging. 2021;48(2):612-622. doi: 10.1007/s00259-020-04969-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therriault J, Schindler SE, Salvadó G, et al. Biomarker-based staging of Alzheimer disease: rationale and clinical applications. Nat Rev Neurol. 2024;20(4):232-244. doi: 10.1038/s41582-024-00942-2 [DOI] [PubMed] [Google Scholar]
- 17.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795-804. doi: 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therriault J, Pascoal TA, Lussier FZ, et al. Biomarker modeling of Alzheimer's disease using PET-based Braak staging. Nat Aging. 2022;2(6):526-535. doi: 10.1038/s43587-022-00204-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quispialaya KM, Therriault J, Aliaga A, et al. Discordance and concordance between cerebrospinal and [18F]FDG-PET biomarkers in assessing atypical and early-onset AD dementia cases. Neurology. 2022;99(22):e2428-e2436. doi: 10.1212/WNL.0000000000201198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones D, Lowe V, Graff-Radford J, et al. A computational model of neurodegeneration in Alzheimer's disease. Nat Commun. 2022;13(1):1643. doi: 10.1038/s41467-022-29047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobili F, Arbizu J, Bouwman F, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F‐fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: delphi consensus. Eur J Neurol. 2018;25(10):1201-1217. doi: 10.1111/ene.13728 [DOI] [PubMed] [Google Scholar]
- 24.Ashton NJ, Brum WS, Di Molfetta G, et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 2024;81(3):255-263. doi: 10.1001/jamaneurol.2023.5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therriault J, Vermeiren M, Servaes S, et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 2023;80(2):188-199. doi: 10.1001/jamaneurol.2022.4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28(7):1398-1405. doi: 10.1038/s41591-022-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772-781. doi: 10.1001/jama.2020.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therriault J, Servaes S, Tissot C, et al. Equivalence of plasma p‐tau217 with cerebrospinal fluid in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2023;19(11):4967-4977. doi: 10.1002/alz.13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18(12):2669-2686. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therriault J, Benedet AL, Pascoal TA, et al. Association of apolipoprotein E ε4 with medial temporal tau independent of amyloid-β. JAMA Neurol. 2020;77(4):470-479. doi: 10.1001/jamaneurol.2019.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Cho S-G, Song M, et al. Usefulness of 3-dimensional stereotactic surface projection FDG PET images for the diagnosis of dementia. Medicine (Baltimore). 2016;95(49):e5622. doi: 10.1097/MD.0000000000005622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ossenkoppele R, Pijnenburg YAL, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer's disease: clinical, neuroimaging and pathological features. Brain 2015;138(Pt 9):2732-2749. doi: 10.1093/brain/awv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Therriault J, Benedet AL, Pascoal TA, et al. Determining amyloid-β positivity using F-AZD4694 PET imaging. J Nucl Med. 2021;62(2):247-252. doi: 10.2967/jnumed.120.245209 [DOI] [PubMed] [Google Scholar]
- 35.Stock C. DTComPair: Comparison of Binary Diagnostic Tests in a Paired Study Design [online]; 2024. Accessed January 3, 2024. github.com/chstock/DTComPair [Google Scholar]
- 36.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 37.Karikari TK, Ashton NJ, Brinkmalm G, et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol. 2022;18(7):400-418. doi: 10.1038/s41582-022-00665-2 [DOI] [PubMed] [Google Scholar]
- 38.Brum WS, Cullen NC, Janelidze S, et al. A two-step workflow based on plasma p-tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nat Aging. 2023;3(9):1079-1090. doi: 10.1038/s43587-023-00471-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR, Andrews JS, Beach TG, et al. Revised criteria for diagnosis and staging of Alzheimer's disease: Alzheimer's Association Workgroup. Alzheimers Dement. 2024;20(8):5143-5169. doi: 10.1002/alz.13859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chételat G, Arbizu J, Barthel H, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer's disease and other dementias. Lancet Neurol. 2020;19(11):951-962. doi: 10.1016/S1474-4422(20)30314-8 [DOI] [PubMed] [Google Scholar]
- 41.Ducharme S, Dols A, Laforce R, et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain. 2020;143(6):1632-1650. doi: 10.1093/brain/awaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Botha H, Mantyh WG, Murray ME, et al. FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain. 2018;141(4):1201-1217. doi: 10.1093/brain/awy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grothe MJ, Moscoso A, Silva-Rodríguez J, et al. Differential diagnosis of amnestic dementia patients based on an FDG-PET signature of autopsy-confirmed LATE-NC. Alzheimers Dement. 2023;19(4):1234-1244. doi: 10.1002/alz.12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graff-Radford J, Murray ME, Lowe VJ, et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology. 2014;83(9):801-809. doi: 10.1212/WNL.0000000000000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diaz‐Galvan P, Przybelski SA, Algeciras‐Schimnich A, et al. Plasma biomarkers of Alzheimer's disease in the continuum of dementia with Lewy bodies. Alzheimers Dement. 2024;20(4):2485-2496. doi: 10.1002/alz.13653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Therriault J, Janelidze S, Benedet AL, et al. Diagnosis of Alzheimer's disease using plasma biomarkers adjusted to clinical probability. Nat Aging. 2024;4(11):1529-1537. doi: 10.1038/s43587-024-00731-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janelidze S, Barthélemy NR, He Y, Bateman RJ, Hansson O. Mitigating the associations of kidney dysfunction with blood biomarkers of Alzheimer disease by using phosphorylated tau to total tau ratios. JAMA Neurol. 2023;80(5):516-522. doi: 10.1001/jamaneurol.2023.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schindler SE, Galasko D, Pereira AC, et al. Acceptable performance of blood biomarker tests of amyloid pathology—recommendations from the Global CEO Initiative on Alzheimer's Disease. Nat Rev Neurol. 2024;20(7):426-439. doi: 10.1038/s41582-024-00977-5 [DOI] [PubMed] [Google Scholar]
- 49.Montoliu-Gaya L, Alosco ML, Yhang E, et al. Optimal blood tau species for the detection of Alzheimer's disease neuropathology: an immunoprecipitation mass spectrometry and autopsy study. Acta Neuropathol (Berl). 2023;147(1):5. doi: 10.1007/s00401-023-02660-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabinovici GD, Rosen HJ, Alkalay A, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77(23):2034-2042. doi: 10.1212/WNL.0b013e31823b9c5e [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings in this article will be shared through a material transfer agreement on request by qualified investigator.