Abstract
Background and Objectives
Infections, including infection with SARS-CoV-2 (COVID-19), could alter the course of multiple sclerosis (MS). Previous studies assessing the effects of COVID-19 on MS outcomes were small and had discordant findings. The study objective was to evaluate the association of COVID-19 infection with changes in the trajectory of MS symptoms and disability.
Methods
We used a controlled interrupted time series (ITS), a quasiexperimental study design using longitudinal data from the North American Research Committee on Multiple Sclerosis Registry. Participants who completed at least 3 surveys before and after their index survey were identified. Exposure of interest was COVID-19 infection based on confirmed diagnosis using an at-home or laboratory test, as reported by the participant. Symptoms were measured using the SymptoMScreen (SMSS), a self-report measure of symptom severity across 12 domains common in MS. Disability was measured using PDDS. Segmented regression was used to compare changes in outcomes over time between cohorts, before and after the exposure (COVID-19 infection).
Results
The spring 2023 survey response rate was 67.3%, and 4,787 participants completed the COVID-19 questions. Of those participants, 2,106 (44.0%) reported ever having confirmed COVID-19. The COVID-19 infection cohort included 796 participants with ≥3 surveys both before and after index survey. The uninfected cohort included 1,534 participants (32.0%) who reported never having COVID-19 nor other infections in the previous 6 months, of whom 1,336 had the requisite number of presurveys and postsurveys. After adjusting for participant characteristics, the SMSS score increased nominally over time in the COVID-19 and uninfected cohorts and this change over time did not differ between cohorts either before (0.005, 95% CI −0.025 to 0.035) or after (−0.0002, 95% CI −0.014 to 0.014) COVID-19 infection. The immediate effect of COVID-19 infection on the SMSS total score was minimal within the COVID-19 cohort and did not differ between cohorts (0.41, 95% CI −0.13 to 0.94). Findings were similar for disability.
Discussion
Our study using an ITS design found that COVID-19 infection was not associated with immediate changes in symptom severity or disability, nor did it change the trajectories of these outcomes over a median follow-up of 18 months. Although results may not generalize to younger people with MS, these findings enhance our general understanding of the consequences of infection in people with MS.
Introduction
The COVID-19 pandemic has focused attention on the consequences of infection on the course of disease. As of July 2024, at least 775 million people worldwide were infected with SARS-CoV-2 (hereinafter COVID-19).1 Infection has the potential for clinically significant consequences in people with multiple sclerosis (MS). MS is associated with an increased risk of infection and infection-related hospitalizations, including for viral infections.2–4 Infection-related hospitalizations are concerning because, in one study, hospitalizations were associated with greater disability accrual, regardless of the reason for admission.5
Several small and moderately sized studies assessed the effects of COVID-19 infection on MS outcomes with conflicting findings.6–11 Using a pre-post design examining 53 people with relapsing-remitting MS, relapse rates did not differ after COVID-19 infection nor did the frequency of 3-month sustained disability progression.12 Similarly, in a propensity-matched study, COVID-19 was not associated with an increased risk of relapses or disability worsening, but fatigue, dyspnea, and hyposmia were transiently more frequent.9 However, another study reported that after COVID-19 infection, one-third of people with MS and related disorders were neurologically worse than before infection because of pseudorelapses, relapses, worsening of preexisting symptoms, or new neurologic symptoms.10 Thus, the long-term effects of COVID-19 on symptoms and disability in people with MS remain uncertain.
The conflicting findings in the literature highlight how the assessment of the effects of COVID-19 on subsequent symptoms in people with MS may be complicated by several factors. First, many of the common symptoms after COVID-19 infection are nonspecific and overlap with symptoms of MS. For example, fatigue, impaired memory and concentration, sleep problems, and depression or anxiety are common COVID-19 symptoms,13 and these overlap with symptoms experienced by people with MS. Second, the pandemic adversely affected the psychological well-being of people with MS14 in addition to changing levels of physical activity, frequency of social interaction, and patterns of health care use,15,16 all of which may influence outcomes in MS.
The controlled interrupted time series (ITS) design is a quasiexperimental design, which compares the change in the outcome of interest over time between 2 groups, before and after the intervention or exposure of interest. The pre-post comparison within a group allows each participant to act as their own control, thereby controlling for both fixed measured and unmeasured factors. The comparison between groups controls for changes that may occur over time unrelated to the exposure, in this case those generally related to the pandemic period but not COVID-19 infection itself. Using an ITS design, we evaluated the association of COVID-19 infection with changes in the trajectory of MS symptoms and disability, hypothesizing that COVID-19 infection would not be associated with changes in these trajectories.
Methods
Study Design and Data Source
We used longitudinal data from the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry in a controlled ITS approach. Participants in the NARCOMS Registry self-report demographic and clinical information at enrollment and semiannually thereafter. The semiannual questionnaires update information regarding sociodemographic factors such as income and clinical information such as symptoms using online or paper questionnaires according to the preference of the participant. Registry surveys are conducted every 6 months in April and October.
Standard Protocols, Approvals, and Patient Consents
Participants agreed to the use of their deidentified information for research, and the registry was approved by the University of Texas Southwestern Institutional Review Board.
COVID-19 Infection
In spring 2023, survey participants reported whether they ever had COVID-19, the number of COVID-19 infections, and dates of infection (month and year). For participants with multiple infections, dates of their first and most recent infection were requested. The COVID-19 infection cohort and uninfected cohort were based on the responses to these questions. In addition, surveys through the index period (spring 2020 to fall 2022) also queried participants about COVID-19 infections and, when available, were used to confirm presence or absence of COVID-19 infection in these cohorts.
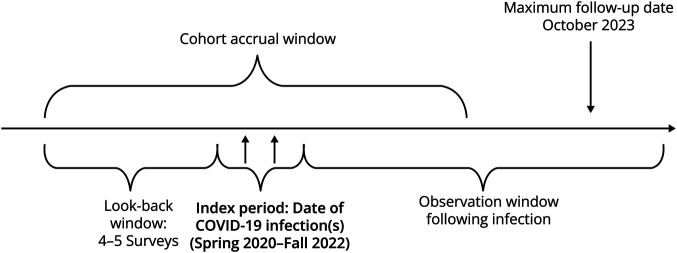
Participants
For this analysis, we included NARCOMS Registry participants who reported clinician-diagnosed MS, were aged 18 years or older, and US residents. Two cohorts were created. The COVID-19 infection cohort included participants who reported that they had a confirmed diagnosis of COVID-19 infection through a self-administered home or laboratory test during the period from spring 2020 to fall 2022. This index period was necessary to allow sufficient follow-up after infection (Figure 1). For the COVID-19 infection cohort, the index survey was the survey(s) when the participant reported having the COVID-19 infection. A follow-up survey was excluded if the survey was completed within 30 days after the reported COVID-19 infection. If multiple COVID-19 infections were reported, the index survey was the survey associated with the first COVID-19 infection and all surveys between the participant's first and most recent COVID-19 infection were excluded from the pre- and post-periods. The uninfected cohort included participants who reported never having a positive COVID-19 test during the period between spring 2020 and spring 2023. In addition, the uninfected cohort did not report having other infections for the 6-month period before the spring 2023 survey. Other infections included influenza, common cold, gastrointestinal tract infection, pneumonia, zoster (shingles), strep throat, urinary tract infection, skin infection, osteomyelitis, and joint infection. For the uninfected cohort, the index survey was assigned to the survey that met the eligibility criteria. When more than one survey met the eligibility criteria, we randomly assigned the index survey to the surveys completed between spring 2020 and fall 2022 to align with the distribution of index surveys in the COVID-19 infection cohort. Participants in each cohort were required to have completed at least 3 surveys before and after the index survey.
Figure 1. Study Design.
Outcomes
Our semiannual updates collected symptom and disability outcomes, including in the period before the index period, to examine whether COVID-19 infection altered the trajectories of symptom severity and disability. Comparisons with the control cohorts addressed potential confounding due to general pandemic effects.
Symptoms
The SymptoMScreen (SMSS) is a self-report measure of symptom severity across 12 domains common to MS including, walking, hand function/dexterity, spasticity and stiffness, bodily pain, sensation, bladder function, fatigue, vision, dizziness, cognitive function, depression, and anxiety.17 Each domain is rated on an ordinal scale ranging from 1 (not affected at all) to 7 (total limitation). The SMSS was validated against performance-based and clinician-assessed measures and showed strong criterion and construct validity and reliability.18 NARCOMS began collecting the SMSS in the fall 2017 survey. The SMSS total score, which is the sum of the 12 individual domain scores, was used, as well as that of each domain separately. The SMSS total score ranged from 0 to 72, where higher scores indicate more severe symptoms.
Disability
The patient-determined disease steps (PDDS) is a single-item measure of disability with 9 levels including 0 (normal), 1 (mild disability), 2 (moderate disability), 3 (gait disability), 4 (early cane), 5 (late cane), 6 (bilateral support), 7 (wheelchair/scooter), and 8 (bedridden).19,20 Scores on the PDDS are highly correlated with those from a physician-scored Expanded Disability Status Scale (EDSS; r = 0.73; 95% CI 0.66–0.79), where a score of 0 approximates an EDSS score of 0, a score of 3 represents early gait disability without needing an assistive device and approximates an EDSS score of 4.0–4.5, and scores of 4, 5, and 6 represent EDSS scores of 6–6.5.21,22
Covariates
From the enrollment questionnaire, we obtained information regarding date of birth, sex, race and ethnicity, level of education attained, and age at MS symptom onset. Age at symptom onset was used to calculate disease duration. We categorized race as White, African American/Black, and other; the number of participants reporting Hispanic ethnicity was too small for the analysis (<50). Education level was categorized as high school/General Educational Development Test and postsecondary education (associate's degree, bachelor's degree, postgraduate education, and technical degree).
From the index questionnaire, we obtained information regarding disease-modifying therapy use, annual household income, marital status, health behaviors (smoking, alcohol intake, physical activity), and comorbid conditions. Disease-modifying therapies (DMTs) from each survey in the preindex and postindex periods were included in the analysis (time varying) and categorized as none, S1P modulators, B-cell therapies, and other based on their previous association with severity of COVID-19 infection.23 Annual household income response options were <$15,000; $15,001-$30,000; $30,001-$50,000; $50,001-$100,000; >$100,000; and “I do not wish to answer.”
Analysis
We characterized the study population using descriptive statistics including mean (SD), median (interquartile range), and frequency (percentage). For the ITS analysis, we implemented segmented regression models to evaluate the following questions simultaneously: (1) Did the COVID-19 infection cause a change in the outcome for either cohort? (2) Was the preperiod to postperiod change in the outcome rates between the cohorts significant? (3) Was the immediate outcome change at the time of COVID-19 infection the same for both groups? The ITS model used generalized estimating equations and an autoregressive covariance structure to evaluate the immediate impact and change in trend on symptoms and disability associated with COVID-19 infection while controlling for ongoing changes related to MS or the pandemic over time.24,25 A Durbin-Watson test was used to investigate the presence of autocorrelation. The model for the SMSS total score used a normal distribution and model assumptions were verified using residuals, whereas the models for the PDDS and individual SMSS domains used a Poisson distribution. Contrast statements were used to explicitly evaluate each question in the model, and the estimate and standard error (SE) are reported for each contrast. Models were adjusted for age at index, sex, race, education level, disease duration at index, and DMT category at each survey.
Sensitivity analyses examined these questions in a cohort using a 90-day window in the COVID-19 infection cohort for excluding follow-up surveys and in a 30-day window propensity score–weighted cohort that included all covariates described above. Propensity score weighting included model covariates and annual household income, comorbidities (anxiety disorder, depression, autoimmune thyroid disease, diabetes, hypertension, hyperlipidemia, heart disease, chronic lung disease, irritable bowel syndrome, psoriasis, fibromyalgia, sleep apnea, stroke, and cancer), current smoking status, any physical activity, and alcohol intake. Stabilized inverse probability of treatment weights was used in the analysis, and plots and standardized differences were used to assess balance with the weighted propensity scores. An additional sensitivity analysis included disability level at index and vaccination status as an additional, time-varying covariate in the model. To control for multiple comparisons, we implemented a false discovery rate (FDR) correction of 5% with an adjusted significance level of 0.0001. Statistical analyses were conducted in SAS v9.4 (SAS Institute, Cary, NC) using PROC GEE.
Data Availability
Individual participant data that underlie the results reported in this article after deidentification will be made available for replication on request. The study survey, data dictionary, and analytic code will also be made available. Data will be available beginning 3 months and ending 5 years after article publication. Proposals should be directed to msregistry@narcoms.org; to gain access, data requestors will need to sign a data access agreement. Researchers who provide a methodologically sound proposal and rationale will be provided access to achieve aims in the approved proposal.
Results
Participants
The spring 2023 survey was distributed to 7,782 participants, of whom 5,239 (67.3%) responded. Of those, 344 participants were excluded for not reporting a clinician-diagnosed MS, being aged 18 years or older or a US resident, and an additional 108 did not complete the COVID-19 question. Of these 4,787 eligible participants, 2,106 participants (44.0%) reported having COVID-19 that was confirmed with a laboratory or at-home test. There were 796 participants with the requisite number of surveys before and after index in the COVID-19 infection cohort using a 30-day window around the COVID-19 infection. The median (25th, 75th) time from COVID-19 to index was 4 (1, 3) months. Using a 90-day window, there were 685 participants eligible for the analysis.
For the uninfected cohort, 1,534 (32.0%) reported never having a COVID-19 infection nor any other infection in the 6 months before the spring 2023 survey and 1,336 had at least 3 surveys in the pre- and post-periods. The COVID-19 infection cohort was younger and had a greater proportion of women, higher income, and level of education compared with the uninfected cohort (Table 1). They were also younger at symptom onset, with a lower median disability level at index, and they more frequently used anti-CD20 DMTs. The median [25th, 75th] number of survey participants in the COVID-19 infection cohort in the pre- and post-periods were 6 [5, 7] and 3 [3, 4], respectively while participants in the uninfected cohort had a similar number for the pre-period (6 [4, 7], p = 0.24) and a higher number for the post-period (4 [3, 4], p = 0.0004). Demographics of the 90-day window cohort were generally similar to the 30-day window cohort (eTable 1). Propensity score weights were balanced for the 2 groups (eFigure 1).
Table 1.
Participant Characteristics
| Total (N = 2,132) | Uninfected (unexposed) (N = 1,336) |
COVID-19 (exposed) (N = 796) |
p Value | |
| Age at index | 64.1 ± 9.4 | 65.7 ± 8.8 | 61.5 ± 9.9 | <0.001b |
| Femalea | 1710 (80.2) | 1,052 (78.8) | 658 (82.7) | 0.030c |
| Race | 0.093c | |||
| American Indian | 5 (0.23) | 4 (0.30) | 1 (0.13) | |
| Arab | 5 (0.23) | 3 (0.22) | 2 (0.25) | |
| Asian | 5 (0.23) | 5 (0.37) | 0 (0.0) | |
| Black/African American | 46 (2.2) | 37 (2.8) | 9 (1.1) | |
| Latino | 16 (0.75) | 11 (0.82) | 5 (0.63) | |
| Multiple | 23 (1.1) | 17 (1.3) | 6 (0.75) | |
| Other/unknown | 163 (7.6) | 97 (7.3) | 66 (8.3) | |
| White/Caucasian | 1869 (87.7) | 1,162 (87.0) | 707 (88.8) | |
| Education levela | 0.028c | |||
| High school/GED | 493 (24.7) | 339 (26.8) | 154 (21.0) | |
| Associates | 279 (14.0) | 172 (13.6) | 107 (14.6) | |
| Technical degree | 43 (2.2) | 23 (1.8) | 20 (2.7) | |
| Bachelor's | 643 (32.2) | 388 (30.7) | 255 (34.8) | |
| Post-bachelor’s | 538 (27.0) | 341 (27.0) | 197 (26.9) | |
| Annual income at indexa | <0.001c | |||
| Less than $15,000 | 65 (3.3) | 50 (4.0) | 15 (2.0) | |
| $15,000—$30,000 | 229 (11.5) | 170 (13.6) | 59 (8.0) | |
| $30,001—$50,000 | 287 (14.4) | 197 (15.7) | 90 (12.2) | |
| $50,001—$100,000 | 490 (24.6) | 293 (23.4) | 197 (26.7) | |
| Over $100,000 | 455 (22.8) | 225 (17.9) | 230 (31.1) | |
| I do not wish to answer | 467 (23.4) | 319 (25.4) | 148 (20.0) | |
| Marital status at indexa | <0.001c | |||
| Never married | 171 (8.5) | 125 (9.9) | 46 (6.2) | |
| Married | 1,311 (65.5) | 769 (61.1) | 542 (72.9) | |
| Divorced | 263 (13.1) | 187 (14.9) | 76 (10.2) | |
| Widowed | 170 (8.5) | 123 (9.8) | 47 (6.3) | |
| Separated | 13 (0.65) | 10 (0.79) | 3 (0.40) | |
| Cohabitating/domestic partner | 73 (3.6) | 44 (3.5) | 29 (3.9) | |
| Age at start of symptomsa | 31.8 ± 9.8 | 32.4 ± 9.8 | 30.9 ± 9.6 | 0.001b |
| PDDS at indexa | 3.0(1.00,6.0) | 4.0(1.00,6.0) | 3.0(1.00,5.0) | <0.001d |
| MS clinical course | <0.001c | |||
| Relapsing | 1,145 (53.7) | 662 (49.6) | 483 (60.7) | |
| Progressive | 766 (35.9) | 515 (38.5) | 251 (31.5) | |
| Unsure/not answered | 221 (10.4) | 159 (11.9) | 62 (7.8) | |
| DMT category at index | 0.009c | |||
| Anti-CD20 | 635 (29.8) | 373 (27.9) | 262 (32.9) | |
| S1P modulators | 158 (7.4) | 95 (7.1) | 63 (7.9) | |
| Other | 1,331 (62.4) | 860 (64.4) | 471 (59.2) | |
| None/not answered | 8 (0.38) | 8 (0.60) | 0 (0.0) | |
| Index survey | <0.001c | |||
| S20 | 30 (1.4) | 22 (1.7) | 8 (1.0) | |
| F20 | 99 (4.6) | 78 (5.8) | 21 (2.6) | |
| S21 | 321 (15.1) | 216 (16.2) | 105 (13.2) | |
| F21 | 325 (15.2) | 250 (18.7) | 75 (9.4) | |
| S22 | 429 (20.1) | 214 (16.0) | 215 (27.0) | |
| F22 | 928 (45.5) | 556 (41.6) | 372 (46.7) | |
| Before index time (mo), median (25th, 75th) | 42 (36, 54) | 42 (36, 54) | 42 (36, 48) | 0.96d |
| After index time (mo), median (25th, 75th) | 18 (12, 24) | 18 (12, 24) | 12 (12, 18) | <0.001d |
Abbreviations: DMT = disease-modifying therapy; MS = multiple sclerosis; PDDS = patient-determined disease steps.
Values are presented as mean ± SD, median (P25, P75), median (min, max), or N (column %).
Data not available for all participants. Missing values: sex = 1, education level = 136, annual income = 16, marital status = 131, age at start of symptoms = 125, and PDDS = 63.
ANOVA p values.
Pearson χ2 test p values.
Kruskal-Wallis test p values.
Symptoms
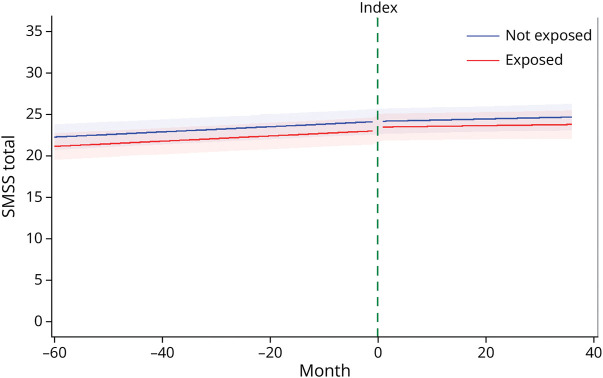
After adjusting for age at index, sex, race, education level, disease duration at index, and DMT, the slopes of the COVID-19 infection cohort and uninfected cohort did not differ in the change in the SMSS total score each month before or after the index period (p = 0.73, p = 0.98, respectively; Table 2, Figure 2). For each group, the SMSS score increased nominally by 0.02 points per month before to after index survey and this change did not differ between the groups (p = 0.75). The immediate effect of the COVID-19 infection on the SMSS score was minimal (less than half a point) within each group and did not differ between groups (p = 0.14). Results were similar when using the 90-day window, using the propensity score–weighted cohort, and including disability at index and vaccination status as covariates (eTables 2, 4, and 6).
Table 2.
Interrupted Time Series–Adjusted Estimates for Symptom and Disability
| SMSS | PDDS | |||
| Estimatea (SE) | p Value | Estimatea (SE) | p Value | |
| Difference in cohort trends (pre) | 0.005 (0.016) | 0.73 | −0.0003 (0.001) | 0.70 |
| Difference in cohort trends (post) | −0.0002 (0.007) | 0.98 | 0.0001 (0.0003) | 0.72 |
| Change in uninfected cohort trend (pre-post) | 0.02 (0.013) | 0.09 | −0.0004 (0.0007) | 0.54 |
| Change in COVID-19 cohort trend (pre-post) | 0.02 (0.011) | 0.13 | −0.00003 (0.0004) | 0.94 |
| Difference in the change in cohort trend (post-pre trend) | −0.01 (0.017) | 0.75 | 0.0004 (0.0008) | 0.62 |
| Difference in the immediate impact between cohorts | 0.41 (0.273) | 0.14 | 0.01 (0.013) | 0.37 |
| Immediate impact in uninfected cohort | −0.41 (0.218) | 0.06 | −0.007 (0.011) | 0.51 |
| Immediate impact in COVID-19 | 0.007 (0.165) | 0.97 | 0.004 (0.007) | 0.50 |
Abbreviation: DMT = disease-modifying therapy; PDDS = patient-determined disease steps; SE = standard error; SMSS = SymptoMScreen.
Estimates were adjusted for age at index, sex, race, education level, disease duration at index, and DMT category.
Figure 2. Trends in Symptoms Between and Within Cohorts.
Trends are presented for the following covariate levels: female, White, bachelor’s degree, not taking a disease-modifying therapy, 65 years of age, and a disease duration of 25 years. The index line represents the period of infection for the exposed (COVID-19) cohort and the matched index time assigned to the unexposed (uninfected) cohort.
Individual Symptom Domains
Findings for the domains of bodily pain, sensory, bladder control, fatigue, vision, dizziness, cognitive function, depression, and anxiety were similar to those for the SMSS total score (Table 3). A few domains showed changes in the COVID-19 cohort, although they were likely too small to be clinically meaningful and not statistically significant at the FDR-corrected level. Walking, hand function/dexterity, and spasticity and stiffness showed a small decrease in the change of the symptom severity in the COVID-19 infection cohort after infection compared with the preinfection period while the control cohort had a small increase in the symptom severity from pre- to post-periods. The results from the sensitivity analyses showed generally consistent results using the 90-day window, using the propensity score–weighted cohort, and including disability at index and vaccination status as covariates (eTables 3, 5, and 7).
Table 3.
Interrupted Time Series Estimates for Individual Symptom Domains
| Difference in cohort trends (pre) | Difference in cohort trends (post) | Change in uninfected cohort trend (pre-post) | Change in COVID-19 cohort trend (pre-post) | Difference in cohort trends (post) | Immediate impact in uninfected | Immediate impact in COVID-19 | Difference in immediate impact between cohorts | ||
| Walking | Estimate (SE)a | 0.002 (0.0009) | −0.0004 (0.0004) | 0.0016 (0.0009) | −0.0007 (0.0006) | −0.0023 (0.0011) | −0.0043 (0.0144) | −0.008 (0.0092) | −0.0037 (0.0171) |
| p Value | 0.04 | 0.36 | 0.07 | 0.26 | 0.03 | 0.77 | 0.38 | 0.83 | |
| Hand function/dexterity | Estimate (SE)a | 0.001 (0.0015) | −0.001 (0.0007) | 0.0028 (0.0013) | 0.001 (0.0010) | −0.0019 (0.0017) | 0.0123 (0.0219) | −0.007 (0.0162) | −0.019 (0.0272) |
| p Value | 0.53 | 0.19 | 0.03 | 0.35 | 0.25 | 0.57 | 0.66 | 0.48 | |
| Spasticity and stiffness | Estimate (SE)a | 0.001 (0.0014) | −0.0001 (0.0007) | 0.0034 (0.0013) | 0.002 (0.0010) | −0.001 (0.0016) | −0.006 (0.0213) | 0.018 (0.0159) | 0.024 (0.02655) |
| p Value | 0.38 | 0.88 | 0.01 | 0.05 | 0.39 | 0.79 | 0.25 | 0.37 | |
| Bodily pain | Estimate (SE)a | −0.0002 (0.002) | 0.0004 (0.0008) | −0.0004 (0.0016) | 0.0003 (0.0011) | 0.0007 (0.0019) | −0.0296 (0.0247) | 0.029 (0.0191) | 0.059 (0.03123) |
| p Value | 0.90 | 0.58 | 0.80 | 0.82 | 0.73 | 0.23 | 0.13 | 0.06 | |
| Sensory | Estimate (SE)a | −0.001 (0.0016) | −0.0003 (0.0008) | 0.0001 (0.0015) | 0.0011 (0.0011) | 0.001 (0.0018) | 0.004 (0.0253) | −0.006 (0.01756) | −0.009 (0.03078) |
| p Value | 0.45 | 0.72 | 0.93 | 0.32 | 0.60 | 0.89 | 0.74 | 0.76 | |
| Bladder control | Estimate (SE)a | −0.002 (0.0014) | −0.0001 (0.0007) | −0.0004 (0.0012) | 0.0017 (0.0010) | 0.0021 (0.0016) | −0.027 (0.0216) | −0.0001 (0.01512) | 0.027 (0.0264) |
| p Value | 0.11 | 0.88 | 0.76 | 0.08 | 0.18 | 0.21 | 1.00 | 0.31 | |
| Fatigue | Estimate (SE)a | −0.0002 (0.0011) | −0.0004 (0.0005) | 0.0008 (0.0010) | 0.0005 (0.0007) | −0.0002 (0.0012) | −0.011 (0.0171) | 0.012 (0.0107) | 0.023 (0.0202) |
| p Value | 0.85 | 0.40 | 0.44 | 0.44 | 0.85 | 0.52 | 0.28 | 0.26 | |
| Vision | Estimate (SE)a | 0.002 (0.0025) | 0.001 (0.0011) | 0.003 (0.0022) | 0.002 (0.0017) | −0.001 (0.0027) | −0.062 (0.0371) | −0.005 (0.0251) | 0.057 (0.0448) |
| p Value | 0.45 | 0.43 | 0.24 | 0.33 | 0.72 | 0.10 | 0.85 | 0.20 | |
| Dizziness | Estimate (SE)a | −0.0003 (0.0026) | −0.0003 (0.0012) | −0.0019 (0.0023) | −0.0019 (0.0018) | 0.00001 (0.0029) | −0.062 (0.0408) | −0.055 (0.0271) | 0.007 (0.0490) |
| p Value | 0.91 | 0.83 | 0.42 | 0.29 | 1.00 | 0.13 | 0.04 | 0.88 | |
| Cognitive function | Estimate (SE)a | −0.0002 (0.0016) | −0.001 (0.0008) | 0.002 (0.0014) | 0.002 (0.0011) | −0.001 (0.0018) | −0.012 (0.0234) | −0.015 (0.0171) | −0.003 (0.0290) |
| p Value | 0.91 | 0.30 | 0.09 | 0.13 | 0.73 | 0.61 | 0.38 | 0.92 | |
| Depression | Estimate (SE)a | 0.0007 (0.0025) | −0.0001 (0.0011) | 0.003 (0.0022) | 0.002 (0.0017) | −0.001 (0.0028) | −0.030 (0.0355) | 0.013 (0.0253) | 0.042 (0.0436) |
| p Value | 0.79 | 0.92 | 0.21 | 0.24 | 0.78 | 0.40 | 0.61 | 0.33 | |
| Anxiety | Estimate (SE)a | 0.002 (0.0027) | 0.001 (0.0012) | 0.004 (0.0024) | 0.003 (0.0019) | −0.001 (0.0031) | −0.059 (0.0395) | 0.014 (0.0287) | 0.073 (0.0489) |
| p Value | 0.56 | 0.67 | 0.14 | 0.20 | 0.73 | 0.14 | 0.63 | 0.14 |
Estimates were adjusted for age at index, sex, race, education level, disease duration at index, and disease-modifying therapy category.
Disability
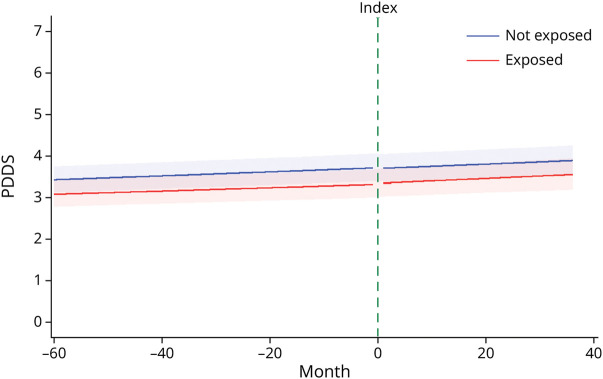
The change in disability, measured using the PDDS, did not differ between the groups in the pre- and post-periods (p = 0.70, p = 0.72, respectively; Table 2, Figure 3) after adjusting for demographic and clinical characteristics. A small increase in the rate of worsening over time was observed; however, for each group, the slopes before and after the index period changed little (uninfected, β [SE] = −0.00003 [0.0004]; COVID-19, β [SE] = −0.0004 [0.0007]) and were not different between the groups (p = 0.62). The immediate change in disability after the index period was not different between the cohorts (p = 0.37). Results were largely similar when using the 90-day window, using the propensity score–weighted cohort, and including disability at index and vaccination status as covariates (eTables 2, 4, and 6).
Figure 3. Trends in Disability Between and Within Cohorts.
Trends are presented for the following covariate levels: female, White, bachelor’s degree, not taking a disease-modifying therapy, 65 years of age, and a disease duration of 25 years. The index line represents the period of infection for the exposed (COVID-19) cohort and the matched index time assigned to the unexposed (uninfected) cohort. PDDS = patient-determined disease steps.
Discussion
In this large longitudinal study, we assessed the consequences of COVID-19 infection in people with MS regarding the trajectory of symptom severity and disability, making use of information collected before the pandemic to allow each participant to act as their own control. These within-cohort analyses accounted for fixed but unmeasured participant characteristics that could have affected the impact of COVID-19. In addition, we accounted for the potential effects of the COVID-19 pandemic such as changes in physical activity and social interactions by including a comparison group that had not experienced known COVID-19 infection. We found that compared with an uninfected control cohort, COVID-19 had no effect on symptom and disability trajectories in those people with MS who had a COVID-19 infection.
While our findings show an increase in the symptom severity over time, the increase was consistent with the rate of change before the COVID-19 infection and in the uninfected control cohort. One study reported neurologic worsening in 36.9% of those people with MS and related diseases with COVID-19 after an average (SD) of 17 (13.6) weeks of follow-up.10 Of those with neurologic worsening in that report, 58.5% had patient-reported symptom worsening. While the proportion of the people with MS reporting new or worsening symptoms in that study was high, the shorter duration of follow-up limited their ability to evaluate persistence of these symptoms and the overall effect on disease course. This is important, given that one national survey conducted throughout the pandemic reported deterioration of MS symptoms in almost half of respondents regardless of COVID-19 infection.26 We did not find an immediate effect of COVID-19 infection on symptom severity compared with those uninfected nor did we observe changes in symptom severity over time.
Within an individual symptom domain, few changes were detected between groups or because of the immediate effect of COVID-19. Within the COVID-19 cohort, most symptom trajectories changed little from their pre–COVID-19 rates. Symptoms such as fatigue and cognitive function, which are common in both MS and COVID-19, were consistent over time and compared with uninfected controls. Similarly, while the emotional well-being of people with MS was adversely affected during the pandemic,14 we noted minor changes in specific symptom domains compared with prepandemic trajectories in both those with COVID-19 and the uninfected cohort. These changes may be due to the instrument used in our study to assess these symptoms, being less sensitive compared with a specific scale for each domain.
Disability changes were similarly consistent after COVID-19 compared with before COVID-19 infection and the uninfected cohort. Three studies found no association of COVID-19 infection with disability progression compared with a control cohort.8,9,11 One case-control study observed higher disability scores using the EDSS after adjusting for age, sex, baseline EDSS scores, disease duration, and other factors. Two additional studies examining within-subject differences before and after COVID-19 (but no control cohort) found decreased rates of disability progression after COVID-19 compared with before. However, a study from a clinical cohort in Belgium found that COVID-19 was associated with disability worsening, measured using the EDSS and other paraclinical measures of disability.27 The mixture of findings highlights the complexity of assessing changes over time where the natural history includes progression and the influence of different study designs subject to biases.
Strengths of our study include the controlled ITS design with longitudinal data to rigorously examine symptom changes over time and comparisons with a control cohort not infected with COVID-19. Limitations of the study should also be considered. Participants in the NARCOMS Registry are volunteers and may impart some selection bias compared with the general MS population in the United States. While the NARCOMS Registry participants may not fully represent the general MS population, the average age of NARCOMS participants was 64 years in this study. While the peak age-specific prevalence of MS in the United States as of 2010 was 55–64,28 future studies investigating these associations in younger age ranges are warranted. Despite our large sample size with longitudinal data, it is possible that statistically significant differences could be detected with larger sample sizes. However, those differences would not be clinically meaningful based on the effect sizes observed. Our analyses considered participant-reported scales for symptoms and disability that may differ in their responsiveness to longitudinal change or need longer follow-up than clinician-assessed measures. While we limited the analysis to participants who reported a positive test for COVID-19 infection, these tests can produce incorrect results. Similarly, people may have contracted COVID-19 and not know it. We were unable to control for the severity of COVID-19 in our analyses. The within-person analysis controlled for fixed measured and unmeasured characteristics of the individual, but it did not control for unmeasured time-varying characteristics. However, the control group accounted for general underlying changes over time.
In summary, our study indicates that COVID-19 infection was not associated with immediate changes in symptom severity or disability, nor did it change the trajectories of these outcomes over the medium term. These findings enhance our general understanding of the consequences of infection in people with MS and are reassuring regarding the impact of COVID-19 on the course of MS.
Acknowledgment
NARCOMS is a project of the Consortium of Multiple Sclerosis Centers (CMSC) and the Foundation of the CMSC.
Glossary
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- FDR
false discovery rate
- ITS
interrupted time series
- MS
multiple sclerosis
- NARCOMS
North American Research Committee on Multiple Sclerosis
- PDDS
patient-determined disease steps
- SE
standard error
- SMSS
SymptoMScreen
Footnotes
Editorial, page e210272
Author contributions
A. Salter: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. S. Lancia: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. G.R. Cutter: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. R.J. Fox: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. R.A. Marrie: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data.
Study Funding
This investigation was supported by a Strategic Initiatives award (SI-2209-40362) from the National Multiple Sclerosis Society.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Accessed August 12, 2024. covid19.who.int/ [Google Scholar]
- 2.Wijnands JM, Kingwell E, Zhu F, et al. Infection-related health care utilization among people with and without multiple sclerosis. Mult Scler. 2017;23(11):1506-1516. doi: 10.1177/1352458516681198 [DOI] [PubMed] [Google Scholar]
- 3.Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol. 2013;20(8):1153-1160. doi: 10.1111/ene.12130 [DOI] [PubMed] [Google Scholar]
- 4.Marrie RA, Tan Q, Ekuma O, Marriott JJ. Development and internal validation of a disability algorithm for multiple sclerosis in administrative data. Front Neurol. 2021;12:754144. doi: 10.3389/fneur.2021.754144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garland A, Metz LM, Bernstein CN, Peschken CA, Hitchon CA, Marrie RA. Hospitalization is associated with subsequent disability in multiple sclerosis. Mult Scler Relat Disord. 2017;14:23-28. doi: 10.1016/j.msard.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Babtain F, Bajafar A, Nazmi O, et al. The disease course of multiple sclerosis before and during COVID-19 pandemic: a retrospective five-year study. Mult Scler Relat Disord. 2022;65:103985. doi: 10.1016/j.msard.2022.103985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahmani M, Moghadasi AN, Shahi S, et al. COVID-19 and its implications on the clinico-radiological course of multiple sclerosis: a case-control study. Med Clin (Engl Ed). 2023;160(5):187-192. doi: 10.1016/j.medcle.2022.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vercellino M, Bosa C, Alteno A, et al. SARS-CoV-2 pandemic as a model to assess the relationship between intercurrent viral infections and disease activity in multiple sclerosis: a propensity score matched case-control study. Mult Scler Relat Disord. 2023;74:104715. doi: 10.1016/j.msard.2023.104715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bsteh G, Assar H, Gradl C, et al. Long-term outcome after COVID-19 infection in multiple sclerosis: a nation-wide multicenter matched-control study. Eur J Neurol. 2022;29(10):3050-3060. doi: 10.1111/ene.15477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway SE, Healy BC, Zurawski J, et al. COVID-19 severity is associated with worsened neurological outcomes in multiple sclerosis and related disorders. Mult Scler Relat Disord. 2022;63:103946. doi: 10.1016/j.msard.2022.103946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montini F, Nozzolillo A, Tedone N, et al. COVID-19 has no impact on disease activity, progression and cognitive performance in people with multiple sclerosis: a 2-year study. J Neurol Neurosurg Psychiatry. 2024;95(4):342-347. doi: 10.1136/jnnp-2023-332073 [DOI] [PubMed] [Google Scholar]
- 12.Etemadifar M, Abhari AP, Nouri H, et al. Does COVID-19 increase the long-term relapsing-remitting multiple sclerosis clinical activity? A cohort study. BMC Neurol. 2022;22(1):64. doi: 10.1186/s12883-022-02590-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Coronavirus disease (COVID-19) post-covid-19 condition. who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition
- 14.Chiaravalloti ND, Amato MP, Brichetto G, et al. The emotional impact of the COVID-19 pandemic on individuals with progressive multiple sclerosis. J Neurol. 2021;268(5):1598-1607. doi: 10.1007/s00415-020-10160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling S, Moss B, Wang Z, Sullivan AB. Exploring the impact of the COVID-19 pandemic on social isolation and mental health in people with MS. Mult Scler Relat Disord. 2022;68:104186. doi: 10.1016/j.msard.2022.104186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Affinito G, Trama U, Palumbo L, et al. Impact of COVID-19 and system recovery in delivering healthcare to people with multiple sclerosis: a population-based Study. Neurol Sci. 2023;44(11):3771-3779. doi: 10.1007/s10072-023-07052-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green R, Kalina J, Ford R, Pandey K, Kister I. SymptoMScreen: a tool for rapid assessment of symptom severity in MS across multiple domains. Appl Neuropsychol Adult. 2017;24(2):183-189. doi: 10.1080/23279095.2015.1125905 [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald KC, Salter A, Tyry T, et al. Validation of the SymptoMScreen with performance-based or clinician-assessed outcomes. Mult Scler Relat Disord. 2019;29:86-93. doi: 10.1016/j.msard.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 19.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a longitudinal study comparing disease steps and EDSS to evaluate disease progression. Mult Scler. 1999;5:349-354. doi: 10.1177/135245859900500508 [DOI] [PubMed] [Google Scholar]
- 20.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45(2):251-255. doi: 10.1212/wnl.45.2.251 [DOI] [PubMed] [Google Scholar]
- 21.Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013;13:37. doi: 10.1186/1471-2377-13-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrie RA, Goldman M. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler. 2007;13(9):1176-1182. doi: 10.1177/1352458507078388 [DOI] [PubMed] [Google Scholar]
- 23.Simpson-Yap S, Pirmani A, Kalincik T, et al. Updated results of the COVID-19 in MS global data sharing initiative: anti-CD20 and other risk factors associated with COVID-19 severity. Neurol Neuroimmunol Neuroinflamm. 2022;9(6):e200021. doi. 10.1212/NXI.0000000000200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. doi: 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 25.Turner SL, Karahalios A, Forbes AB, et al. Creating effective interrupted time series graphs: Review and recommendations. Res Synth Methods. 2021;12(1):106-117. doi: 10.1002/jrsm.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meltzer E, Charron O, Wozny J, et al. Indirect impact of the COVID-19 pandemic on the care and outcomes of people with MS: a combined survey and insurance claims study. Mult Scler Relat Disord. 2023;80:105085. doi: 10.1016/j.msard.2023.105085 [DOI] [PubMed] [Google Scholar]
- 27.Peeters G, Van Remoortel A, Nagels G, Van Schependom J, D'Haeseleer M. Occurrence and severity of coronavirus disease 2019 are associated with clinical disability worsening in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10(3):e200089. doi: 10.1212/NXI.0000000000200089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi: 10.1212/WNL.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this article after deidentification will be made available for replication on request. The study survey, data dictionary, and analytic code will also be made available. Data will be available beginning 3 months and ending 5 years after article publication. Proposals should be directed to msregistry@narcoms.org; to gain access, data requestors will need to sign a data access agreement. Researchers who provide a methodologically sound proposal and rationale will be provided access to achieve aims in the approved proposal.